Highlights
-
•
A climate service for cyanobacteria blooms in the Baltic Sea is proposed.
-
•
Satellite remote sensing successfully detected cyanobacteria blooms.
-
•
The duration and extent of cyanobacteria blooms in the Bothnian Sea has increased.
-
•
Microscope based data showed low resemblance with satellite-based data.
-
•
Phycocyanin fluorescence synchronized with both satellite and microscopy-based data.
Keywords: Harmful blooms, Filamentous cyanobacteria, Climate change, Remote sensing, Ferrybox
Abstract
Dense blooms of filamentous cyanobacteria are recurrent phenomena in the Baltic Sea, with occasional negative effects on the surrounding ecosystem, as well as on tourism, human health, aquaculture, and fisheries. Establishing a climate service is therefore suggested; including multi-method observations of cyanobacteria biomass, biodiversity, and biogeography, in correspondence to biotic and abiotic factors. Three different approaches were compared for determination of spatial and temporal variability and trends of the blooms; 1) microscopy-based long-term data, 2) satellite remote sensing, and 3) phycocyanin fluorescence mounted on a merchant vessel. Firstly, microscopy-based data on cyanobacteria biomass from the period 2000–2020 showed that the toxin producing genus Nodularia and non-toxic Aphanizomenon both had summer means of 15 µg C L−1, while Dolichospermum was less dominant with a mean of 8 µg C L−1. Some years also the Kattegat was affected by cyanobacteria blooms, likely transported here by ocean currents. Secondly, the satellite remote sensing time series for the period 2002–2020 indicated that near surface blooms were most frequent in the Northern Baltic Proper and that near surface blooms have increased in the Bothnian Sea, starting later in the season than in the Baltic Proper. The largest extents (i.e., total area covered) were observed in 2005, 2008, and 2018. Thirdly, phycocyanin fluorescence from a flow through sensor mounted on a merchant vessel was used as a proxy for cyanobacteria biomass and correlated to cyanobacteria biomass estimated by microscopy. However, the satellite remote sensing data on surface accumulations showed little resemblance to the data on cyanobacteria biomass based on water sampling and microscopy, interpreted as an effect of methods. Sensors on satellites mainly detect surface accumulations of cyanobacteria while the microscopy data was based on samples 0–10 m, thereby comprising a larger community. Data from satellite remote sensing of cyanobacteria was correlated to the phycocyanin fluorescence indicating that similar bio-optical properties are observed. Finally, results from a downscaled ocean climate model (NEMO—Nordic) were used to produce future scenarios for temperature and salinity, which directly affects cyanobacteria blooms in the Baltic Sea, supposedly by increasing in abundance and change in species composition. Short-term forecasts can be used together with observations for early warning of cyanobacteria blooms, and we suggest an internationally coordinated cyanobacteria observation and warning system for the Baltic Sea area.
1. Introduction
Filamentous cyanobacteria are important primary producers in the Baltic Sea. Microscopy-based observations indicate that at least 120 species are present representing 43 genera (HELCOM-PEG (Olenina et al., 2006)). This may be an underestimate considering data from molecular methods (Hu et al., 2016). Only a few genera of filamentous cyanobacteria frequently form high biomass blooms in the Baltic Sea, dominated by Aphanizomenon sp., Dolichospermum spp. and Nodularia spumigena (Klawonn et al., 2016). Their ability to perform nitrogen fixation is likely key to their success during nitrogen limited summers (Walve and Larsson, 2007) when forming surface accumulations during calm weather (Suikkanen et al., 2021). Cyanobacteria blooms in the Baltic Sea are not new phenomena (Finni et al., 2001), the first description of surface accumulations known to the authors of this publication is from 1854 (Lindström, 1855).
The above-mentioned filamentous cyanobacteria can utilise dissolved dinitrogen gas as nitrogen source, i.e., they are nitrogen fixers (diazotrophic), which gives them a competitive advantage compared to phytoplankton in general during conditions when nitrate or ammonium are limiting factors (Andersson et al., 2015; Moisander et al., 2007; Olofsson et al., 2016; Suikkanen et al., 2021). This means that in phosphate replete waters the nitrogen fixers can keep on growing when nitrate and ammonium are depleted (Olofsson et al., 2016), and some of the groups can even use organic phosphorus (Schoffelen et al., 2018). Another effect is that nitrogen fixing cyanobacteria contribute significantly to the internal nitrogen budget in some areas (Adam et al., 2016; Karlson et al., 2015; Niemisto et al., 1989; Olofsson et al., 2021).
Anthropogenic effects, like increased nutrient input from agriculture during recent decades, have resulted in an increase of surface accumulations since 1979 (Kahru and Elmgren 2014; Elmgren, 2021). However, quantitative observations of cyanobacteria blooms in the Baltic Sea did not commence until the late 1970′s or later. In a study compiling ∼40 years of microscope data the biovolumes of the toxic N. spumigena did not change in any of the sub-basins during the period. On the other hand, the biovolume of the mainly non-toxic Aphanizomenon sp. and the potentially toxic Dolichospermum spp. increased in the northern parts of the Baltic Sea, along with the decreased salinity and elevated temperatures (Olofsson et al., 2020). Extended monitoring is pivotal to further project future bloom situations in the locally variable Baltic Sea basins.
There are several ways to monitor cyanobacteria blooms where satellite observations of ocean colour have been used as a proxy for biomass of near surface blooms (Kahru, 1997; Kahru and Elmgren, 2014; Kahru et al., 2020, 2007). In a study compiling data from different satellites and sensors Kahru and Elmgren (2014) concluded that the average fraction with cyanobacteria accumulations was significantly higher for the period 1997–2013 than for 1979–1996. However, this did not seem to represent a long-term trend but decadal-scale oscillations. There are also indications that a 3-year cycle is present based on ocean colour observations (Kahru et al., 2018). To this comes relatively strong multidecadal variability in salinity and temperature (e.g., Kniebusch et al. (2019)) that may contribute to variability in cyanobacteria bloom magnitude. This highlights the need of continuous monitoring for several subsequent decades to understand ongoing trends in cyanobacteria blooms. amongst the cyanobacteria observed in the Baltic Sea 14 species are toxin producers (Karlson et al., 2021) out of a global total of 42 species of cyanobacteria that are on the Intergovernmental Oceanographic Commission (IOC) list of harmful algae (Churro and Moestrup, 2021). The most critical one in the Baltic Sea, N. spumigena, produces the hepatotoxic nodularin (Lehtimaki et al., 1997), a cyclic pentapeptide. Nodularin can accumulate in organisms at different trophic levels (Karjalainen et al., 2008), e.g., in blue mussels (Mytilus edulis) and fish such as three-spined stickleback (Gasterosteus aculeatus L.), herring (Clupea harengus L.), salmon (Salmo salar L.), cod (Gadus morhua) and round goby (Neogobius melanostomus Pallas) (Mazur‐Marzec et al., 2007; Sipia et al., 2001, 2007; Van Buynder et al., 2001; Złoch et al., 2018). Dogs drinking water contaminated with nodularin show acute signs of intoxication, such as vomiting and diarrhoea, as a consequence of hepatic and renal failure, and intoxication often leads to death (Algermissen et al., 2011; Harding et al., 1995; Nehring, 1993; Simola et al., 2012), while humans swimming in a bloom may get a rash. Cyanobacteria accumulating on beaches is a nuisance and affect leisure activities around the Baltic Sea and when dense blooms sink down, they enhance anoxia in the bottom waters as they decompose (Conley et al., 2009).
The aim of this article is to suggest a climate service for cyanobacteria blooms in the Baltic Sea. This includes an evaluation of different methods for observations (microscopy, satellites, and phycocyanin fluorescence), a risk assessment and a suggestion for a system for observing cyanobacteria blooms and environmental variables to build a climate service for the area. The climate service also includes results from a downscaled ocean climate model for the Baltic Sea area (NEMO—Nordic).
2. Methods
2.1. Phytoplankton long-term monitoring program
The Baltic Sea is a brackish semi-enclosed area embracing several sub-basins with different characteristics in salinity and nutrient loads. In this study we used data from the Swedish National Marine Monitoring Program that includes monthly sampling of phytoplankton diversity, abundance, and biomass. Sampling was more frequent, ∼24 times per year, at stations Anholt E, B1 and BY31. We include data from the period 2000–2020 and from twelve monitoring stations (Fig. 1). Note that data from station B3 also includes data from station B7 located very close to B3. Sampling at station M1V1 started in 2007. Phytoplankton samples were collected using a closing tube covering depths of 0–10 m for most stations and 0–20 m for stations B1 and BY31. Phytoplankton analyses was made using the sedimentation chamber method (Edler and Elbrächter, 2010; Utermöhl, 1931, 1958). Biomass was calculated based on cell numbers and filament sizes (Olenina et al., 2006) updated by HELCOM Phytoplankton Expert Group 2021 available at: https://www.ices.dk/data/Documents/ENV/PEG_BVOL.zip. Biomass, provided as carbon, was calculated using equations based on cultures of phytoplankton (Menden-Deuer and Lessard, 2000). The phytoplankton data are hosted by the Swedish National Oceanographic Data Centre at the Swedish Hydrological and Meteorological Institute (SMHI) and are available via open access at https://sharkweb.smhi.se. Phytoplankton biomass were compiled to determine the spatial and seasonal distribution of the three diazotrophic filamentous cyanobacteria Nodularia spumigena, Aphanizomenon sp., and Dolichospermum spp., and Nostocales represents the sum of their abundance.
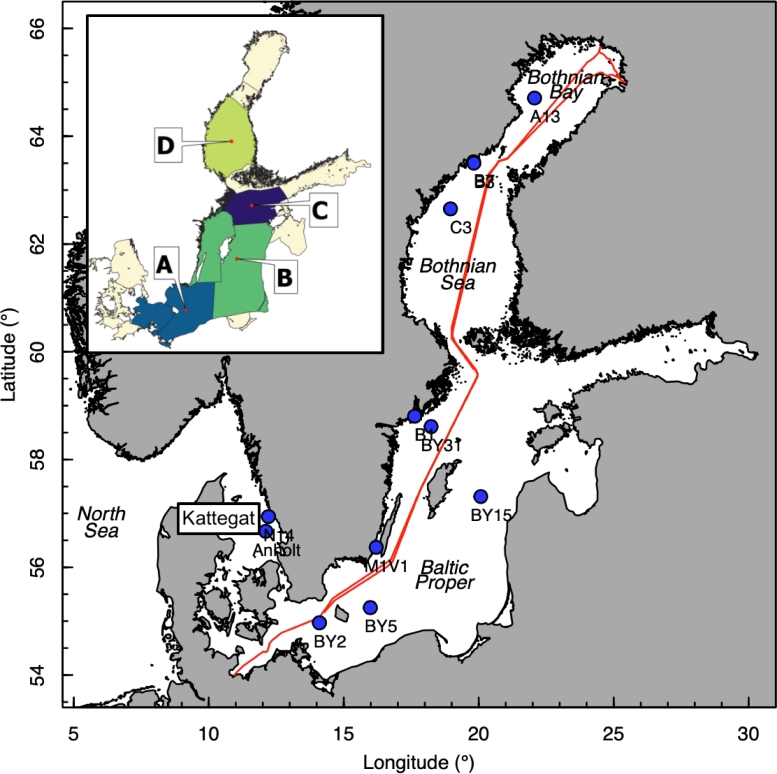
Fig. 1.
Map of the Baltic Sea area with blue dots representing sampling locations for long-term Swedish national phytoplankton monitoring. Station labelled B3 represents two stations (B3 and B7) located close to each other. The red line represents the route of the merchant vessel TransPaper (renamed Tavastland in 2017) where underway measurements of phycocyanin fluorescence were performed and additional phytoplankton samples were collected. Inset map indicates sea areas for which satellite data were averaged: A = Southern Baltic Proper, B = Central Baltic Proper, C = Northern Baltic Proper, and D = Bothnian Sea.
2.2. Data from FerryBox system
Automated sampling was carried out in the Baltic Sea during 2011–2020 onboard the merchant vessel TransPaper (new name Tavastland since 2017) between May and September (route in Fig. 1), using a FerryBox system. Water was sampled at ∼3 m dept and ∼25 m from the bow (total length 191 m). The ships movement throught the water causes tubulence and mixing of the surface water. This means that the sampled water represents a mix of approximately the upper 5 m. The Ferrybox system includes sensors for automated detection of water temperature, conductivity, chlorophyll fluorescence, and phycocyanin fluorescence. Phycocyanin fluorescence was used as a proxy for the biomass of phycocyanin rich cyanobacteria (Seppala et al., 2007). The sensor for phycocyanin fluorescence had an excitation wavelength of 620 nm and emission wavelength of 655 nm (model Microflu blue, TriOS Mess- und Datentechnik GmbH, Germany, www.trios.de). Range was 0.1 – 100 μg L−1 and sensitivity 0.1 μg L−1 according to the manufacturer. The data were collected every 20 s. which represents a data point approximately every 200 m, depending on ship speed. Quality flags defined by Copernicus Marine Service, CMEMS (https:/marine.copernicus.eu) were applied. Data from harbour areas was excluded in the analysis. The Ferrybox also included automated sampling devices for collecting water samples, triggered at predefined latitudes, and Lugol's iodine, was added to the sample containers beforehand, which meant that the phytoplankton was preserved when entering the containers. The FerryBox system is described in detail in Karlson et al. (2016). Fluorescence data and phytoplankton biomass, analysed the same way as described above, was compared for May-September of 2013 and 2015. Data is available at https://sharkweb.smhi.se.
2.3. Satellite observations of cyanobacteria
The satellite data were collected using the Moderate Resolution Imaging Spectroradiometer (MODIS) (Masuoka et al., 1998) on the NASA-satellites Aqua and Terra for the years 2002–2020. Level 2 data were downloaded from NASA's OceanColor Web https://oceancolor.gsfc.nasa.gov and processed using PyTROLL software available open source at https://pytroll.github.io/. Data was masked, and bad data removed, using the standard quality flags ATMFAIL, LAND, HIGLINT, HILT, HISATZEN, STRAYLIGHT, CLDICE, HISOLZEN, LOWLW, CHLFAIL, MAXAERITER, ATMWARN (https://oceancolor.gsfc.nasa.gov/atbd/ocl2flags/). In addition, areas near the coastline with water depth < 10 m were excluded from the analysis, since there is a high risk that suspended matter and vegetation on the sea floor affect results. In known highly turbid areas i.e., the Bay of Gdansk, the Gulf of Riga and the innermost part of the Gulf of Finland, data from depth less than 30 m were excluded. The Bothnian Bay, the northernmost basin of the Baltic Sea, was excluded from the analysis due to absence, or very low, surface accumulations of cyanobacteria (Olofsson et al., 2020). The analysis of ocean colour data was based on an algorithm described by Kahru et al. (2007). Surface accumulations of cyanobacteria were defined as when ocean colour band 13 (part of the red spectra) with a centre wavelength at 667 nm exceeded or was equivalent to 0.0012 sr−1. High reflectance from this band in open waters during summer time can be associated with large amount of backscatter from the cyanobacteria gas vacuoles (Kahru and Elmgren, 2014). In addition to the detection from band 13, band 12 (centre wavelength: 555 nm) was used in order to detect cyanobacterial sub-surface accumulations, as light from this wavelength penetrates deeper into the water column compared to band 13. A threshold of 0.00435 sr−1 was used.
The normalised extent (A) of the blooms were estimated as the area that had one observation of cyanobacteria during the investigated time period, plus the area with two observations, plus the area with three observations and so on. This value was divided with the sum of days with cyanobacteria observations. The normalised duration (T) was estimated as the area that has one observation of cyanobacteria during the investigated time period, plus the area with two observations, plus the area with three observations and so on. This value was divided with the total area resulting in length of time. Intensity is A x T. The time period was June-August each year. The following equations, originally described Hansson and Hakansson (2007), describes the calculations:
A = Normalized extent of cyanobacteria bloom
Normalized duration of cyanobacteria bloom
Normalized intensity of cyanobacteria bloom
ai is equal to the extent that is covered by surface accumulations of cyanobacteria during (i) number of days.
In addition to the long-term data from Aqua/Terra-MODIS a smaller data set for July 2019 emanating from European Space Agency satellites Sentinel 3A and 3B was used. Data from the Ocean and Land Colour Imager (OLCI) was downloaded from https://coda.eumetsat.int. Data from the channel representing water leaving reflectance centred at 560 nm was used for a comparison with in situ phycocyanin fluorescence.
2.4. Software and statistics
In addition to the PyTROLL software described above, R (R_Core_Team, 2021) was used for processing data. Components of Tidyverse (Wickham et al., 2019) were used for filtering data, aggregating data, and for investigating Pearson correlations using libraries ggplot and ggpmisc. Pearson correlations were used to determine inter-method relationships, with values >0.8 considered very strong, 0.6–0.8 strong, 0.4–0.6 moderate, and 0–2–0.4 weak. The library ggplot with geom_box was used for producing box and whisker plots. The upper whisker extends from the hinge to the largest value no further than 1.5 * IQR from the hinge (where IQR is the inter-quartile range, or distance between the first and third quartiles). The lower whisker extends from the hinge to the smallest value at most 1.5 * IQR of the hinge. Data beyond the end of the whiskers are called "outlying" points and are plotted individually. PBSmapping (Schnute et al., 2021) was used for producing maps using coastlines from Wessel and Smith (1996).
3. Results
3.1. Distribution of filamentous cyanobacteria based on microscopy data
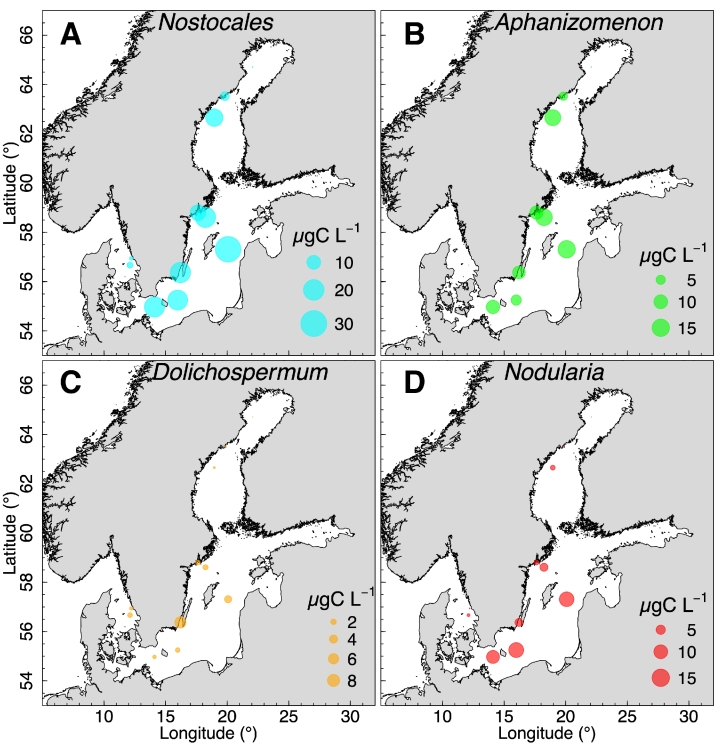
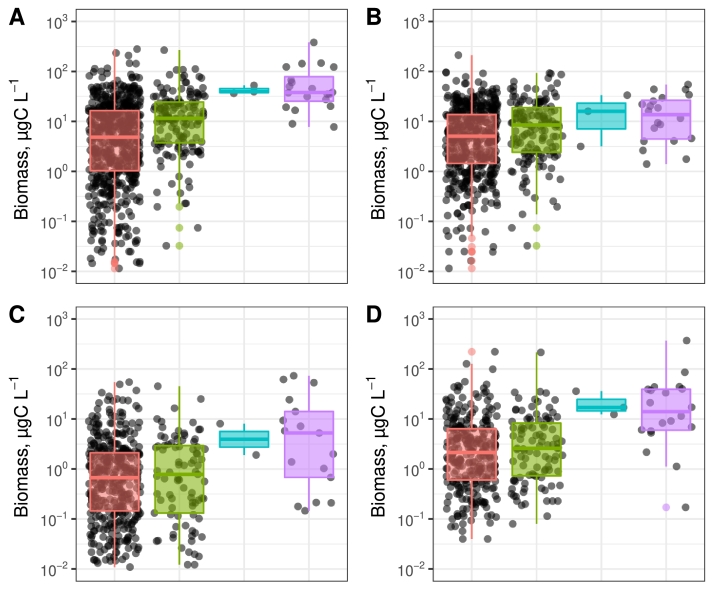
The long-term data set from the period 2000 – 2020 was used to investigate spatial distribution of bloom forming cyanobacteria. Aphanizomenon and Nodularia had a mean summer (June-August) biomass of up to 15 µg C L−1, while Dolichospermum had a maximum mean biomass of 8 µg C L−1 (Fig. 2). All the three genera were basically absent in the Bothnian Bay while Aphanizomenon dominated in the Bothnian Sea. In the Baltic Proper were all three genera observed with the highest biomass of Aphanizomenon and Nodularia. In the Kattegat low biomasses of all three genera were observed. Nostocales, defined as the sum of the three genera, had a mean biomass of 10–30 µg C L−1 during June-August in the Baltic Proper and in the Bothnian Sea.
Fig. 2.
The spatial distribution of biomass of three cyanobacteria genera (B-D) and the sum of these (A). The size of the circles represents mean biomass in carbon for June–August for the period 2000–2020.
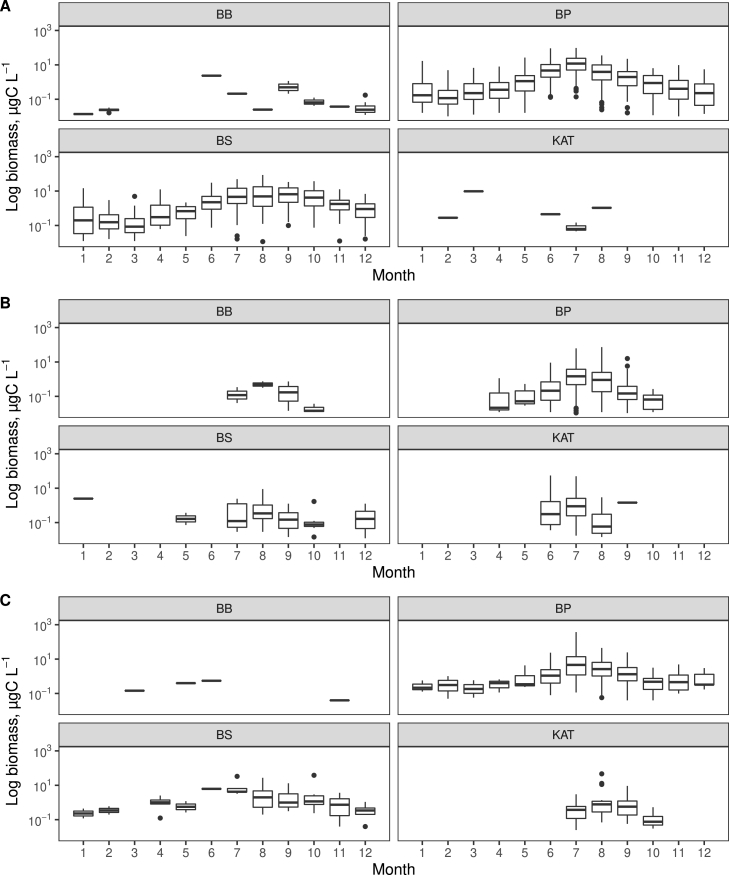
The peak biomass of Aphanizomenon was observed in July in the Baltic Proper while August was the month of peak biomass in the Bothnian Sea (Fig. 3). The biomass of Dolichospermum was in general much lower than Aphanizomenon and the observations were restricted to a shorter time period centred around summer months. The highest biomasses of Nodularia were observed in July in the Baltic Proper. The long-term data were also used to investigate temporal trends. No trends (Pearson correlation) in summer carbon biomass for the three cyanobacteria genera or for the sum of these were detected for the time period 2000–2020 at the basin level. The exception was Dolichsopermum spp. that showed a weak increase in the Baltic Proper (Supplementary material, Fig. S1).
Fig. 3.
The seasonal distribution of biomass of three cyanobacteria genera. A Aphanizomenon, B. Dolichospermum and C. Nodularia. Acronyms for sea basins: BB = Bothnian Bay, BS = Bothnian Sea (including the sea basin Northern Quark), BP = Baltic Proper and KAT = Kattegat. The details about box and whisker plots are described in Material and methods.
3.2. Distribution of filamentous cyanobacteria based on satellite data
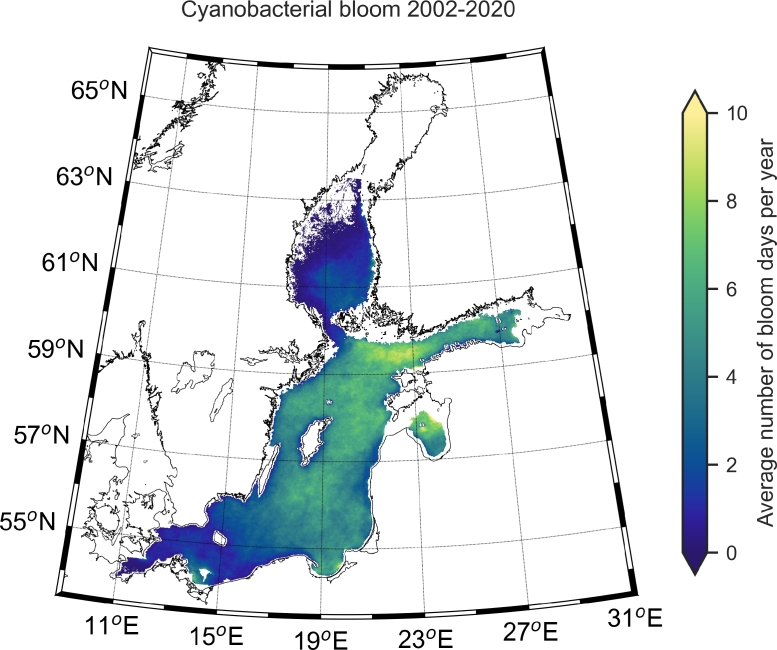
The cumulative distribution of satellite observations of cyanobacteria bloom varied largely between sub-basins (Fig. 4). Overall, the highest cumulative number of days of cyanobacteria observations were observed at the entrance of the Gulf of Finland with up to 10 days year−1 on average for the period 2002–2020 in contrast to ∼1–2 days in the Bothnian Sea. A large area of the Baltic Proper had an average of 6–8 days per year of cyanobacteria observations during the period 2002–2020. The annual distribution as number of days per year also indicated a spatial variation (Fig. 5), with local extremes of up to 20 days per year, but commonly from lower values of a few days per year up to about 5–10 in most of the central Baltic Proper.
Fig. 4.
The map illustrates the mean number of days per year with observations of near surface accumulations of filamentous cyanobacteria in summer (June–August) from satellite during the period 2002–2020. Sea areas in white were excluded from the analysis, see Material and methods for details. Maps of each year within the period are presented in Supplementary material, Fig. S2.
Fig. 5.
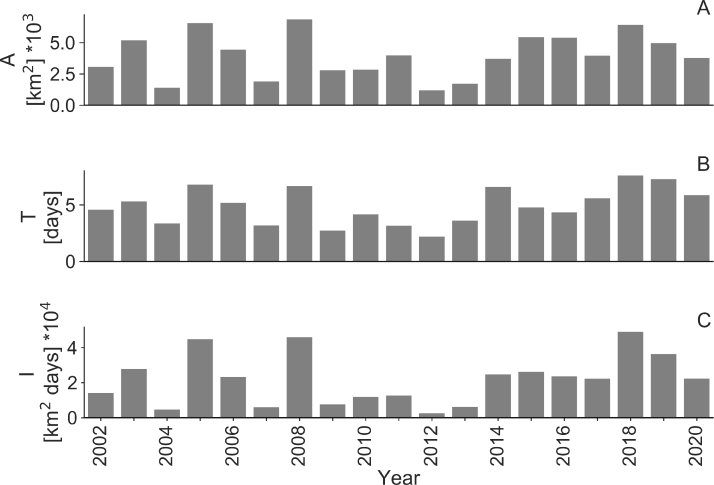
Summary of satellite observations of near surface cyanobacteria accumulations. The area analysed is the same as for Fig. 4. Subpanels: a) the extent of surface accumulations of cyanobacteria (km2, normalised) b) indicates the duration of the blooms (days, normalised), and c) the intensity of the blooms (km2 days) during summer (June-August) for the period 2002–2020. Results from sub-basin shown in inset map in Fig. 1 are presented in Supplementary material Fig. S3.
There was also a large inter-annual variation of bloom extent, duration and intensity determined from the satellite images (Fig. 4). Intensity was calculated from extent and duration, see Materials and methods. The largest extents (i.e., total area covered) and intensity of the blooms were observed in 2005, 2008, 2018. Splitting the satellite data into basins and sub-basins according to inset map in Fig. 1 the results were slightly different (Supplementary material, Fig. S3). In the Southern Baltic Proper year 2006 had the largest extent and intensity. In the central Baltic Proper years 2002, 2003, 2005 and 2008 had the largest bloom intensities. In the Northern Baltic Proper year 2019 had the longest duration and intensity, with an overall moderate increase in duration (R = 0.47). The Bothnian Sea had the longest duration and highest intensity in 2020 with strong increases in extent, duration, and intensity (Supplementary material, Fig. S3, R values 0.66, 0.61 and 0.67) during the period investigated.
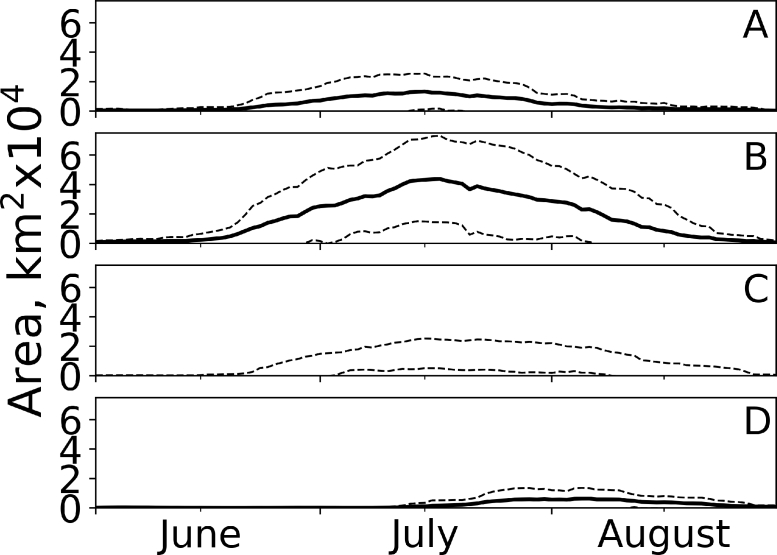
The satellite observations indicated a large variation in bloom start, end, length, and timing of the annual peak in surface accumulation of filamentous cyanobacteria between sea basins (Fig. 6). The observations of near surface cyanobacteria accumulations on average starts ∼20 June and ends in late August with a peak ∼10 July in the Baltic Proper areas, and in the Bothnian Sea, the blooms starts ∼10 July with a peak in early August.
Fig. 6.
Seasonal variation in area of surface accumulation of cyanobacteria based on satellite observations. Solid lines represent mean extent 2002–2020 and the dashed lines represent standard deviations. A = Southern Baltic Proper, B = Central Baltic Proper, C = Northern Baltic Proper and D = Bothnian Sea. Basins is in Fig. 1.
3.3. Comparison of satellite observation and microscopy-based data
Microscopy based data from 2002 to 2020 were extracted for summer (June-August) and satellite observations from corresponding dates and locations were extracted from the Aqua/Terra-MODIS data. In the high- resolution data from Aqua/Terra-MODIS one pixel represent an area of 250 × 250 m. In this study we pooled data from 3 × 3 pixels including the location where the water sample was collected. Note that cloud cover results in no observations of ocean colour from MODIS and that the scale of satellite data has thresholds, it is not continuous. Results indicate that satellite remote sensing only detected a minority of the samples in which biomass of cyanobacteria was identified using microscopy. The results are not surprising since the sampling for microscopy covers 0–10 m depth (0–20 m at two stations) while satellites mainly detect surface presence. Wind mixing would influence satellite observations of cyanobacteria by disrupting surface accumulation (Simis et al., 2021). Looking at Nostocales, the sum of the three cyanobacteria genera in focus, there seems to be a threshold at ∼10 µg C L−1 (Fig. 7).
Fig. 7.
A comparison of satellite observations of near surface blooms and biomass of cyanobacteria as observed by microscopy (2002–2020, June-August). A. Nostocales, B. Aphanizomenon, C. Dolichospermum and D. Nodularia. Red: cloud free, no bloom detected using satellite, green: cloud cover, blue: subsurface bloom detected and violet: surface accumulation detected. Number of samples = 917.
3.4. Phycocyanin fluorescence as a proxy for filamentous cyanobacteria
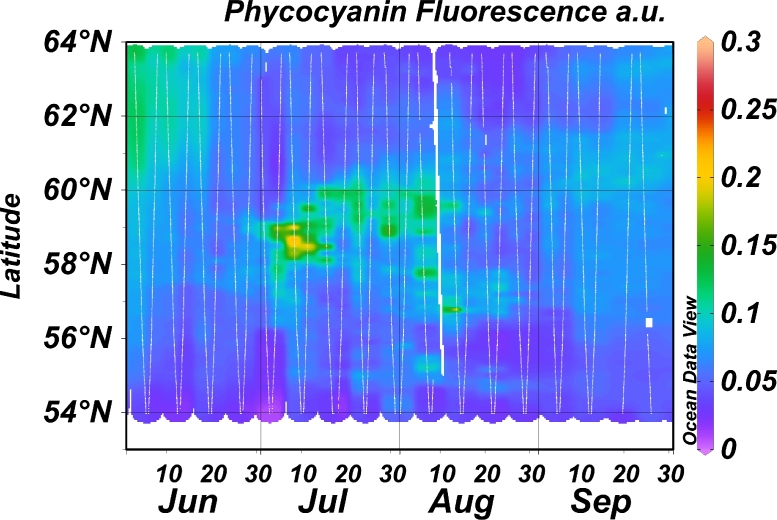
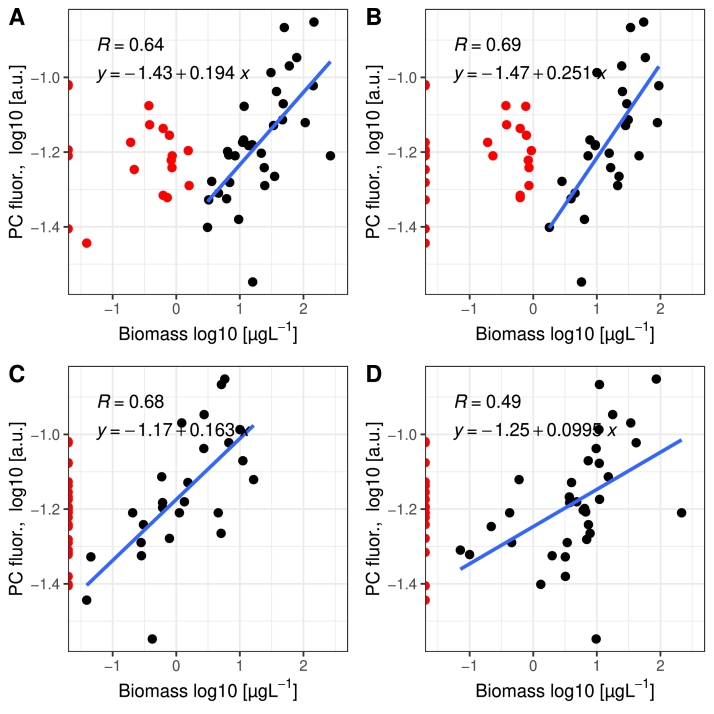
Phycocyanin fluorescence was evaluated as a proxy for the biomass of phycocyanin-rich cyanobacteria. An example of results from the Ferrybox on the route Lübeck-Kemi is shown in Fig. 8. The highest phycocyanin fluorescence values were observed early July at latitude 58–59° N. Later in July the high phycocyanin fluorescence indicate cyanobacteria blooms at latitude 59–60° N. Phycocyanin fluorescence was high also at latitudes 61–64° N in early June indicating that water constituents other than cyanobacteria may cause this fluorescence, since microscopy-based biomass are not yet abundant here (Figs. 3, 6). To evaluate if phycocyanin fluorescence is a useful proxy for the biomass of phycocyanin rich genera samples for microscopy analyses of cyanobacteria were collected in years 2013 and 2015 using a water sampling device which is part of the Ferrybox system. Results show moderate or strong correlations (Pearson) when values in the assumed linear parts of the graphs are used (Fig. 9).
Fig. 8.
Phycocyanin fluorescence June-September 2015 along route of ship TransPaper with phycocyanin fluorescence sensor in underway Ferrybox system.
Fig. 9.
Phycocyanin fluorescence (PC) vs biomass of three genera of cyanobacteria and Nostocales defined here as the sum of the three genera. A. Nostocales, B. Aphanizomenon, C. Dolichospermum and E. Nodularia. The phytoplankton samples analysed by microscopy were collected using an automated sampling device on ship TransPaper in June-September 2013 and 2015 along the route of ship in the Baltic Proper and the Bothnian Sea. n = 51. All data were log10 transformed. Blue lines indicate linear model fitted to the black data points assumed to represent a linear part of the relationship. Pearson R values and linear equations are shown.
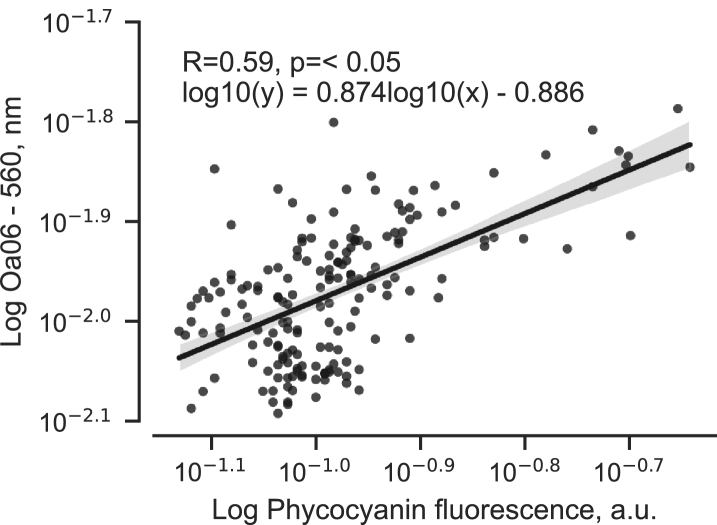
Phycocyanin fluorescence was also compared with satellite observations of water leaving reflectance at 560 nm to investigate possible correlations. Data from the OLCI sensor on satellites Sentinel 3A and 3B collected during a cyanobacteria bloom in year 2019 show a moderate correlation with phycocyanin fluorescence (Pearson, R = 0.59) indicating that both methods measure similar bio-optical properties (Fig. 10).
Fig. 10.
Phycocyanin fluorescence vs observations using the OLCI sensor on satellites Sentinel 3A and 3B Data were collected during cyanobacteria bloom for July 11–28, 2019. Phycocyanin fluorescence was observed using Ferrybox system on merchant vessel TransPaper. Reflectance values < 0 were excluded and data north of 60°N.
3.5. Model results
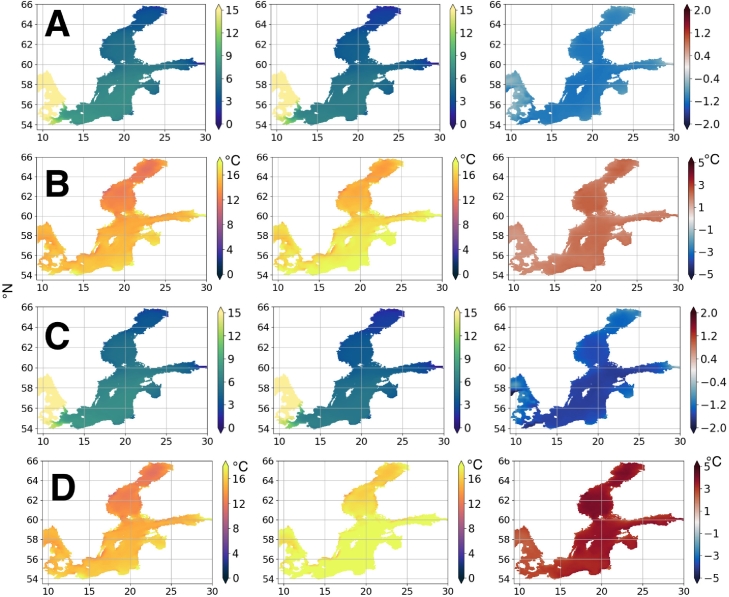
The downscaled ocean climate model NEMO—Nordic (Gröger et al., 2019) was used to produce scenarios of future salinity and temperature conditions in the Baltic Sea area. Two different Representative Concentration Pathway (RCP) were used. RCP 4.5 is an intermediate scenario and in RCP 8.5 emissions continue to rise throughout the 21st century (Gröger et al., 2019 and references therein). Results indicate an increase in summer temperatures of 3–5 °C and a decrease in salinity of 0.5–2 in the Baltic Proper when comparing the period 1970–1999 with 2070–2099 (Fig. 11).
Fig. 11.
Climate scenarios for the Baltic Sea area based on (Gröger et al., 2019). A. Salinity RCP4.5, B. Temperature RCP4.5, C. Salinity RCP8.5 and D. Temperature RCP8.5. Left 1970–1999, Middle 2070–2099 and right difference between the two periods.
4. Discussion
4.1. Evaluating methods for observing cyanobacteria
Three different methods were compared for observing cyanobacteria: (1) Microscopy based analysis of water samples, (2) satellite remote sensing using method based on (Kahru et al., 2007) using a consistent dataset emanating from MODIS sensor on satellites Aqua and Terra and (3) measurement of phycocyanin fluorescence in a flow through (Ferrybox) system mounted on a merchant vessel passing the Baltic Sea north to south and vice versa once per week. The microscopy data and the satellite data show little resemblance (Fig. 7). A reason for this may be the different nature of the samples and also that the data was collected during the same day, but not simultaneously. Water samples were collected from research vessels using a tube covering 0–10 m, in some cases 0–20 m. The satellite data include an area of 250 × 250 m. The depth from which the ocean colour integrates the signal varies depending on the biomass of phytoplankton and also due to water constituent like Coloured Dissolved Organic Matter (CDOM). In highly turbid waters only near surface cyanobacteria would have been detected by satellite. Another issue is the vertical migration of cyanobacteria. Some cyanobacteria have the capability of controlling their position in the water column utilising gas vesicles (Hajdu et al., 2007; Walsby et al., 1995, 1997). Near surface observations of these cyanobacteria during different times of the day may give different results. However, the larger patterns were synchronized between the methods, with bloom peaks in July for the Baltic Proper while in August for the Bothnian Sea (Figs. 3 and 6).
Phycocyanin fluorescence was compared with samples analysed by microscopy collected using the automated sampling device on the merchant vessel TransPaper in years 2013 and 2015. There was a strong correlation with log-transformed data when values from assumed linear parts of the graphs were used. A problem with the phycocyanin fluorescence data is calibration of the instrument. In this publication arbitrary units of phycocyanin fluorescence were used. A consistent method for calibrating the phycocyanin sensor was not available making comparisons between years difficult. Satellite observations of water leaving reflectance at 560 nm and phycocyanin fluorescence observed using the FerryBox system during a cyanobacteria bloom in July 2019 showed a moderate correlation (Fig. 10) indicating that both methods measure bio-optical properties related to the biomass of cyanobacteria.
Regular monitoring using tube-sampling will always provide the most detailed results integrated over a defined depth range, but with high regional variation. Microscopy provides details about species composition and biomass at the species level. On the other hand, both FerryBox data and satellite images will provide large-scale estimates of the distribution of blooms. Satellite images are limited by depth distribution and cloud coverage. From a combination of methods, we can attain detailed information to learn about cyanobacteria bloom dynamics and development. This information is important in ecosystem management and also for recreation purposes as dense bloom formations can disturb tourism in coastal areas.
4.2. Environmental factors and modelling cyanobacteria blooms
There are several different approaches for modelling harmful algal blooms (Flynn and McGillicuddy, 2018). To produce scenarios for the future these HAB models may be combined with downscaled ocean climate models (Gröger et al., 2019). Our results indicate an increase in summer temperatures of 3–5 °C and a decrease in salinity of 0.5–2 in the Baltic Proper when comparing the period 1970–1999 with 2070–2099 (Fig. 11). High temperature favours the growth of cyanobacteria in general (Paerl and Huisman, 2008). This applies to many phytoplankton but to cyanobacteria in particular, which is important to consider due to the ongoing changes in the climate. Salinity restricts the distribution and growth of cyanobacteria in the Baltic Sea area (Olofsson et al., 2020; Rakko and Seppälä, 2014), and with decreasing salinities, the composition of cyanobacteria may shift (Wulff et al., 2018) and their abundance locally increase (Olofsson et al., 2020). Changes in temperature and salinity in the Baltic Sea are likely according to future scenarios based on downscaled climate models forcing ocean models (Gröger et al., 2019), and has been demonstrated for several of the Baltic Sea basins when looking back nearly four decades (Olofsson et al., 2020). This means that monitoring of these basic parameters is important in a climate service for cyanobacteria.
Wind stress affects mixing of the water column and the vertical distribution of cyanobacteria. Wind speed has direct effect on satellite observations of cyanobacteria. High wind stress results in a breakup of cyanobacteria surface accumulations resulting in fewer observations of cyanobacteria by satellite ocean colour. In the Baltic Sea biogeochemical models, that include one, or a few, different types of phytoplankton using a bottom-up approach where nutrient and light conditions are important forcing variables, have been used to model cyanobacteria growth and biomass (Hense et al., 2013; Neumann and Schernewski, 2008; Saraiva et al., 2018). Biological interactions and the diversity in cyanobacteria and variability in growth rates etc. are in general missing in these models. A cyanobacteria life cycle was introduced in one of the model combinations, where the cyanobacteria seasonality was improved as compared to previous attempts (Hieronymus et al., 2021). A similar model combination could be used for future predictions based on climate scenarios. There is a high potential in developing more advanced models to describe cyanobacteria bloom development. The ecological niche approach may be applied as described for the harmful alga Osteropsis ovata in the Mediterranean Sea (Drouet et al., 2021). All these model combinations, however, need monitoring data with good coverage in time and space for validation, which is a benefit for the Baltic Proper with a long monitoring tradition and history of multi-stressors on the environment (Reusch et al., 2018), but needs improvement in the southern and central Bothnian Sea where blooms of filamentous cyanobacteria might be an increasing issue (Olofsson et al., 2020).
To make short term predictions on the advection of cyanobacteria blooms an atmospheric 3D model forcing a 3D physical oceanographic model can be used together with models for particle tracking and models describing the behaviour of the cyanobacteria. Individual based Models (IBM) is one option that has been applied to dinoflagellates (Gillibrand et al., 2016). Popular physical oceanographic models include NEMO (Hordoir et al., 2019a; Madec et al., 2017) and ROMS (Nagy et al., 2021; Shchepetkin and McWilliams, 2005). The velocity fields that they output can be used as input for particle drift models such as PADM (Particle Advection and Dispersion Model) (Liungman and Mattsson, 2011) and OpenDrift (Dagestad et al., 2018) to simulate advection of cyanobacteria. It is possible to give properties or traits to the particles to simulate cyanobacteria behaviour, e.g., buoyancy.
4.3. A suggested climate service for cyanobacteria blooms
4.3.1. Existing monitoring
Monitoring of cyanobacteria and related environmental parameters in the Baltic Sea area are currently mainly based on requirements from the Convention on the Protection of the Marine Environment of the Baltic Sea Area (Helsinki Convention) of 1974 and the Revised Convention of 1992 (Ehlers, 1994). Bottom-up control based on supply of inorganic nutrients is a strong focus. Sampling and analyses are consistent (Anonymous, 2017), but in general not very frequent, but where a strength of the monitoring program is that methods are consistent. In addition to the HELCOM-monitoring mainly ships of opportunity (e.g., Karlson et al., 2016) and satellite remote sensing (e.g., Kahru et al., 2020) are used for observing cyanobacteria. In general climate change is not included in the current monitoring approaches.
4.3.2. The climate service concept
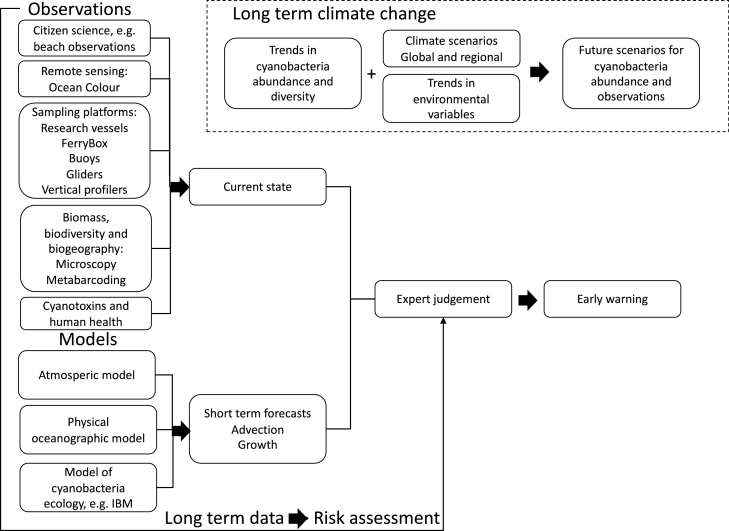
By combining observations and modelling a climate service can be created, conceptualized in Fig. 12. The climate service should include long term observations for risk assessment to address basic questions like: (1) What is the phenology, the temporal and spatial distribution of different species and genera? (2) What is the distribution of cyanobacteria toxins? (3) Is human health affected? (4) Are there long-term changes? (5) What abiotic and biotic factors are influencing the distribution of harmful cyanobacteria and frequency of cyanobacteria blooms? The observations can also describe the current state if data is available shortly after collection. If the current state is known and combined with short term modelling of the advection (Arneborg et al., 2017; Hordoir et al., 2019b) and growth of cyanobacteria (Hieronymus et al., 2021) early warnings of cyanobacteria blooms can be issued. Expert judgement based on long term observations and risk assessment need to be applied before issuing warnings. The climate service should also include effects of long-term climate change. For this, the long-term monitoring data are used together with downscaled global climate models forcing ocean models (Fig. 11 and Gröger et al. (2019)).
Fig. 12.
A concept for a climate service for harmful cyanobacteria. IBM = Individual Based Model.
4.3.2. End users and stakeholder needs
End users of a climate service for cyanobacteria include the public, coastal management, tourism industry, aquaculture industry, fisheries, food health managers, desalination plant operations, and ocean health. The direct target of products from a cyanobacteria early warning system may be ocean information offices that cover a specific region or coastline. In Sweden there are three marine information offices covering the Gulf of Bothnia, the Baltic Proper and the Kattegat-Skagerrak. The information offices have the responsibility to distribute information to media and to end users in a form fit for purpose. Web sites with information and weekly bulletins is one way to distribute information. In Sweden the Swedish Meteorological and Hydrological Institute provide information about cyanobacteria blooms to the public and to the information offices through www.smhi.se. A new initiative from the University of Gothenburg is Algal Blooms Sweden https://www.gu.se/en/research/algal-blooms-sweden. An example from the USA is the Lake Erie Harmful Algal Bloom Forecast: https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-forecasts/lake-erie/
4.3.3. Risk assessment
Nodularin, the toxin produced by N. spumigena, pose a threat to human and animal health. Results based on microscopy (Fig. 4) show that N. spumigena had the highest biomass in the Baltic Proper when comparing with adjacent sea basins. N. spumigena was most abundant in July in the Baltic Proper which is a high-risk period. This species also occurred in the Bothnian Sea but in lower abundances and with a peak in August. The risk for N. spumigena blooms in the Bothnian Bay was very low. In some years N. spumigena did occur in the Kattegat posing a risk for human and animal health in this area, especially in August. Aphanizomenon flosaquae is not considered as a toxin producer in the Baltic Sea (Karlson et al., 2021) but the species is a known toxin producer elsewhere (Churro and Moestrup, 2021). Thus, there is a risk for toxin production by A. flosaquae also in the Baltic Sea area, and monitoring of toxin concentration in the water should be collected to learn more about this potential threat. At least five representatives of the genus Dolichospermum present in the Baltic Sea are producers of cyanotoxins (e.g., anatoxin-a, Churro and Moestrup, 2021) which means that they constitute a risk to human and animal health as well. There are also other toxin-producing cyanobacteria in the Baltic Sea, e.g. Microcystis spp., but these are outside the scope of this study.
The satellite data on ocean colour (Figs. 4 and 5) and the Ferrybox data on phycocyanin fluorescence (Fig. 8) provide a different view on the distribution of cyanobacteria. The temporal and spatial coverage is larger, but the level of detail lower. The Northern Baltic Proper had the highest incidence of near surface blooms as observed by ocean colour (Fig. 4), but the interannual variability was substantial (Fig. 5). Some years the Southern Baltic Proper was the basin most affected by cyanobacteria surface accumulations (Supplementary material Figs. 2, 3). An increase in observations of cyanobacteria via satellite in the Bothnian Sea was noted (Supplementary material Fig. 3). Here the risk for harmful effects is increasing. According to the present study, filamentous cyanobacteria have spread further north during recent decades. This increase was also supported by previous satellite studies (Kahru and Elmgren, 2014), as well as monitoring studies (Olofsson et al., 2020), suggested due to favourable nutrient conditions (Andersson et al., 2015; Jaanus et al., 2011; Kuosa et al., 2017; Rolff and Elfwing, 2015).
5. Conclusions
Cyanobacteria blooms occur frequently in the Baltic Sea area with peaks in late summer. Cumulative satellite observations from 2002 showed that near surface accumulations were most frequent in the northern Baltic Proper and satellite remote sensing indicated an increase in cyanobacteria blooms in the Bothnian Sea. The risk for blooms of toxic species is highest in the Baltic Proper while lower in the Bothnian Bay. Climate change through increased temperatures and decreased salinities is very likely to affect the distribution, composition, and frequency of blooms. We propose a climate service based on multi method observations and modelling of cyanobacteria and environmental factors affecting blooms. The service would need to be internationally coordinated and should also include early detection and early warning of cyanobacteria blooms.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks to phytoplankton identification specialists analysing phytoplankton samples by microscopy in the Swedish National Marine Monitoring programme. This work was part of project CoCliME: Co-development of Climate Services for adaptation to changing Marine Ecosystems supported by ERA4CS, an ERA-NET initiated by JPI Climate and funded by EPA (IE), ANR (FR), BMBF (DE), UEFISCDI (RO), RCN (NO) and FORMAS (SE), with co-funding by the European Union (Grant 690462). Formas grant number was 2017–01737. The work was partly supported by the European Union Horizon 2020 project JERICO—NEXT (Joint European Research Infrastructure network for Coastal Observatory – Novel European eXpertise for coastal observaTories) grant agreement no. 654410.
Edited by Uwe John.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.hal.2022.102291.
Appendix. Supplementary materials
Data Availability
Links to data sources are included in material and methods. Contact the Swedish National Oceanographic Data Centre at SMHI if additional information is needed.
References
- Adam B., Klawonn I., Sveden J.B., Bergkvist J., Nahar N., Walve J., Littmann S., Whitehouse M.J., Lavik G., Kuypers M.M.M., Ploug H. N2-fixation, ammonium release and N-transfer to the microbial and classical food web within a plankton community. ISME J. 2016;10(2):450–459. doi: 10.1038/ismej.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algermissen D., Mischke R., Seehusen F., Göbel J., Beineke A. Lymphoid depletion in two dogs with nodularin intoxication. Vet. Rec.-English Edition. 2011;169(1):15. doi: 10.1136/vr.d1019. [DOI] [PubMed] [Google Scholar]
- Andersson A., Höglander H., Karlsson C., Huseby S. Key role of phosphorus and nitrogen in regulating cyanobacterial community composition in the northern Baltic Sea. Estuar. Coast Shelf Sci. 2015;164:161–171. [Google Scholar]
- Anonymous. HELCOM; Helsinki: 2017. Manual For Marine Monitoring in the COMBINE Programme of HELCOM.http://www.helcom.fi/Documents/Action%20areas/Monitoring%20and%20assessment/Manuals%20and%20Guidelines/Manual%20for%20Marine%20Monitoring%20in%20the%20COMBINE%20Programme%20of%20HELCOM.pdf [Google Scholar]
- Arneborg L., Höglund A., Axell L., Lensu M., Liungman O., Mattsson J. Oil drift modeling in pack ice–sensitivity to oil-in-ice parameters. Ocean Eng. 2017;144:340–350. [Google Scholar]
- Churro, C., Moestrup, Ø., 2021. Cyanobacteria, in IOC-UNESCO taxonomic reference list of harmful micro algae. Available online at http://www.marinespecies.org/hab. Accessed on 2021-12-12.
- Conley D.J., Björck S., Bonsdorff E., Carstensen J., Destouni G., Gustafsson B.G., Hietanen S., Kortekaas M., Kuosa H., Markus Meier H. Hypoxia-related processes in the Baltic Sea. Environ. Sci. Technol. 2009;43(10):3412–3420. doi: 10.1021/es802762a. [DOI] [PubMed] [Google Scholar]
- Dagestad K.-.F., Röhrs J., Breivik Ø., Ådlandsvik B. OpenDrift v1. 0: a generic framework for trajectory modelling. Geosci. Model Dev. 2018;11(4):1405–1420. [Google Scholar]
- Drouet K., Jauzein C., Herviot-Heath D., Hariri S., Laza-Martinez A., Lecadet C., Plus M., Seoane S., Sourisseau M., Lemée R. Current distribution and potential expansion of the harmful benthic dinoflagellate Ostreopsis cf. siamensis towards the warming waters of the Bay of Biscay, North-East Atlantic. Environ. Microbiol. 2021 doi: 10.1111/1462-2920.15406. [DOI] [PubMed] [Google Scholar]
- Edler L., Elbrächter M. In: Microscopic and Molecular Methods For Quantitative Phytoplankton Analysis. Karlson B., Cusack C., Bresnan E., editors. UNESCO; Paris: 2010. The Utermöhl method for quantitative phytoplankton analysis; pp. 13–20. [Google Scholar]
- Ehlers P. The Baltic Sea area: convention on the protection of the marine environment of the Baltic Sea area (Helsinki Convention) of 1974 and the revised convention of 1992. Mar. Pollut. Bull. 1994;29(6):617–621. [Google Scholar]
- Elmgren R. Vol. 50. AMBIO; 2021. pp. 739–741. (Assessing Human Effects On the Baltic Sea Ecosystem). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finni T., Kononen K., Olsonen R., Wallström K. The history of cyanobacterial blooms in the Baltic Sea. AMBIO. 2001;30(4):172–178. [PubMed] [Google Scholar]
- Flynn K.J., McGillicuddy D.J. Modeling marine harmful algal blooms: current status and future prospects. Harmful Algal Blooms. 2018:115–134. [Google Scholar]
- Gillibrand P.A., Siemering B., Miller P.I., Davidson K. Individual-based modelling of the development and transport of a Karenia mikimotoi bloom on the North-west European continental shelf. Harmful Algae. 2016;53:118–134. doi: 10.1016/j.hal.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Gröger M., Arneborg L., Dieterich C., Höglund A., Meier H. Summer hydrographic changes in the Baltic Sea, Kattegat and Skagerrak projected in an ensemble of climate scenarios downscaled with a coupled regional ocean–sea ice–atmosphere model. Clim. Dyn. 2019;53(9–10):5945–5966. [Google Scholar]
- Hajdu S., Höglander H., Larsson U. Phytoplankton vertical distributions and composition in Baltic Sea cyanobacterial blooms. Harmful Algae. 2007;6(2):189–205. [Google Scholar]
- Hansson M., Hakansson B. The Baltic algae watch system-a remote sensing application for monitoring cyanobacterial blooms in the Baltic Sea. J. Appl. Remote Sens. 2007;1(1) [Google Scholar]
- Harding W., Rowe N., Wessels J., Beattie K., Codd G. Death of a dog attributed to the cyanobacterial (blue-green algal) hepatotoxin nodularin in South Africa. J. S. Afr Vet. Assoc. 1995;66(4):256–259. [PubMed] [Google Scholar]
- Hense I., Meier H.E.M., Sonntag S. Projected climate change impact on Baltic Sea cyanobacteria Climate change impact on cyanobacteria. Clim. Change. 2013;119(2):391–406. [Google Scholar]
- Hieronymus J., Eilola K., Olofsson M., Hense I., Meier H.E.M., Almroth-Rosell E. Modeling cyanobacteria life cycle dynamics and historical nitrogen fixation in the Baltic Proper. Biogeosciences. 2021;18(23):6213–6227. doi: 10.5194/bg-18-6213-2021. [DOI] [Google Scholar]
- Hordoir R., Axell L., Höglund A., Dieterich C., Fransner F., Gröger M., Liu Y., Pemberton P., Schimanke S., Andersson H. Nemo-Nordic 1.0: a NEMO-based ocean model for the Baltic and North seas–research and operational applications. Geosci. Model Dev. 2019;12(1):363–386. [Google Scholar]
- Hordoir R., Axell L., Höglund A., Dieterich C., Fransner F., Gröger M., Liu Y., Pemberton P., Schimanke S., Andersson H., Ljungemyr P., Nygren P., Falahat S., Nord A., Jönsson A., Lake I., Döös K., Hieronymus M., Dietze H., Löptien U., Kuznetsov I., Westerlund A., Tuomi L., Haapala J. Nemo-Nordic 1.0: a NEMO-based ocean model for the Baltic and North seas – research and operational applications. Geosci. Model Dev. 2019;12(1):363–386. [Google Scholar]
- Jaanus, A., Andersson, A., Olenina, I., Toming, K., Kaljurand, K., 2011. Changes in phytoplankton communities along a north–south gradient in the Baltic Sea between 1990 and 2008.
- Hu Y.O.O., Karlson B., Charvet S., Andersson A.F. Diversity of Pico- to Mesoplankton along the 2000 km Salinity Gradient of the Baltic Sea. Front. Microbiol. 2016;7:17. doi: 10.3389/fmicb.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahru M. Using satellites to monitor large-scale environmental change: a case study of the cyanobacteria blooms in the Baltic Sea. Monitoring algal blooms: new techniques for detecting large-scale environmental change. Landes Biosci. 1997 Using Satellites to Monitor Large-Scale Environmental Change: A case study of the Cyanobacteria Blooms in the Baltic Sea. Monitoring algal blooms: New techniques for detecting large-scale environmental change. Landes Bioscience. [Google Scholar]
- Kahru M., Elmgren R. Multidecadal time series of satellite-detected accumulations of cyanobacteria in the Baltic Sea. Biogeosciences. 2014;11(13):3619. [Google Scholar]
- Kahru M., Elmgren R., Di Lorenzo E., Savchuk O. Unexplained interannual oscillations of cyanobacterial blooms in the Baltic Sea. Sci. Rep. 2018;8(1):1–5. doi: 10.1038/s41598-018-24829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahru M., Elmgren R., Kaiser J., Wasmund N., Savchuk O. Cyanobacterial blooms in the Baltic Sea: correlations with environmental factors. Harmful Algae. 2020;92 doi: 10.1016/j.hal.2019.101739. [DOI] [PubMed] [Google Scholar]
- Kahru M., Savchuk O., Elmgren R. Satellite measurements of cyanobacterial bloom frequency in the Baltic Sea: interannual and spatial variability. Mar. Ecol. Prog. Ser. 2007;343:15–23. [Google Scholar]
- Karjalainen M., Paakkonen J.P., Peltonen H., Sipia V., Valtonen T., Viitasalo M. Nodularin concentrations in Baltic Sea zooplankton and fish during a cyanobacterial bloom. Mar. Biol. 2008;155(5):483–491. [Google Scholar]
- Karlson A.M.L., Duberg J., Motwani N.H., Hogfors H., Klawonn I., Ploug H., Barthel Svedén J., Garbaras A., Sundelin B., Hajdu S., Larsson U., Elmgren R., Gorokhova E. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. Ambio. 2015;44(3):413–426. doi: 10.1007/s13280-015-0660-x. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson B., Andersen P., Arneborg L., Cembella A., Eikrem W., John U., West J.J., Klemm K., Kobos J., Lehtinen S., Lundholm N., Mazur-Marzec H., Naustvoll L., Poelman M., Provoost P., De Rijcke M., Suikkanen S. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae. 2021;102 doi: 10.1016/j.hal.2021.101989. [DOI] [PubMed] [Google Scholar]
- Karlson B., Andersson L.S., Kaitala S., Kronsell J., Mohlin M., Seppala J., Wranne A.W. A comparison of FerryBox data vs. monitoring data from research vessels for near surface waters of the Baltic Sea and the Kattegat. J. Mar. Syst. 2016;162:98–111. [Google Scholar]
- Klawonn I., Nahar N., Walve J., Andersson B., Olofsson M., Sveden J.B., Littmann S., Whitehouse M.J., Kuypers M.M.M., Ploug H. Cell-specific nitrogen- and carbon-fixation of cyanobacteria in a temperate marine system (Baltic Sea) Environmental Microbiology. 2016;18(12):4596–4609. doi: 10.1111/1462-2920.13557. [DOI] [PubMed] [Google Scholar]
- Kniebusch M., Meier H.E.M., Neumann T., Börgel F. Temperature variability of the Baltic Sea since 1850 and attribution to atmospheric forcing variables. J. Geophys. Res. 2019;124(6):4168–4187. [Google Scholar]
- Kuosa H., Fleming-Lehtinen V., Lehtinen S., Lehtiniemi M., Nygård H., Raateoja M., Raitaniemi J., Tuimala J., Uusitalo L., Suikkanen S. A retrospective view of the development of the Gulf of Bothnia ecosystem. J. Mar. Syst. 2017;167:78–92. [Google Scholar]
- Lehtimaki J., Moisander P., Sivonen K., Kononen K. Growth, nitrogen fixation, and nodularin production by two Baltic sea cyanobacteria. Appl. Environ. Microbiol. 1997;63(5):1647–1656. doi: 10.1128/aem.63.5.1647-1656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström, G., 1855. Bidrag till kännedom om Östersjöns invertebratfauna. Öfversigt af Kongl. Vetenskaps-Akademiens Förhandlingar 12(2), 49–73.
- Liungman, O., Mattsson, J., 2011. Scientific documentation of seatrack web; physical processes, algorithms and references. [Online] https://www.smhi.se/.
- Madec, G., Bourdallé-Badie, R., Bouttier, P.-.A., Bricaud, C., Bruciaferri, D., Calvert, D., Chanut, J., Clementi, E., Coward, A., Delrosso, D., 2017. NEMO ocean engine.
- Masuoka E., Fleig A., Wolfe R.E., Patt F. Key characteristics of MODIS data products. IEEE Trans. Geosci. Remote Sens. 1998;36(4):1313–1323. [Google Scholar]
- Mazur-Marzec H., Tymińska A., Szafranek J., Pliński M. Accumulation of nodularin in sediments, mussels, and fish from the Gulf of Gdańsk, southern Baltic Sea. Environ. Toxicol. 2007;22(1):101–111. doi: 10.1002/tox.20239. [DOI] [PubMed] [Google Scholar]
- Menden-Deuer S., Lessard E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000;45(3):569–579. [Google Scholar]
- Moisander P.H., Paerl H.W., Dyble J., Sivonen K. Phosphorus limitation and diel control of nitrogen-fixing cyanobacteria in the Baltic Sea. Mar. Ecol. Prog. Ser. 2007;345:41–50. [Google Scholar]
- Nagy H., Pereiro D., Yamanaka T., Cusack C., Nolan G., Tinker J., Dabrowski T. The Irish Atlantic CoCliME case study configuration, validation and application of a downscaled ROMS ocean climate model off SW Ireland. Harmful Algae. 2021 doi: 10.1016/j.hal.2021.102053. [DOI] [PubMed] [Google Scholar]
- Nehring S. Mortality of dogs associated with a mass development of Nodularia spumigena (Cyanophyceae) in a brackish lake at the German North Sea coast. J. Plankton Res. 1993;15(7):867–872. [Google Scholar]
- Neumann T., Schernewski G. Eutrophication in the Baltic Sea and shifts in nitrogen fixation analyzed with a 3D ecosystem model. J. Mar. Syst. 2008;74(1–2):592–602. [Google Scholar]
- Niemisto, L., Rinne, I., Melvasalo, T., Niemi, A., 1989. Blue-green algae and their nitrogen fixation in the Baltic Sea in 1980, 1982 and 1984.
- Olenina I., Hajdu S., Edler L., Andersson A., Wasmund N., Busch S., Göbel J., Gromisz S., Huseby S., Huttunen M., Jaanus A., Kokkonen P., Ledaine I., Niemkiewicz E. Baltic Marine Environment Protection Commission; Helsinki: 2006. Biovolumes and Size-Classes of Phytoplankton in the Baltic Sea. [Google Scholar]
- Olofsson M., Egardt J., Singh A., Ploug H. Inorganic phosphorus enrichments in Baltic Sea water have large effects on growth, carbon fixation, and N2 fixation by Nodularia spumigena. Aquat. Microb. Ecol. 2016;77(2):111–123. [Google Scholar]
- Olofsson M., Klawonn I., Karlson B. Vol. 50. AMBIO; 2021. pp. 203–214. (Nitrogen Fixation Estimates For the Baltic Sea Indicate High Rates For the Previously Overlooked Bothnian Sea). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson M., Suikkanen S., Kobos J., Wasmund N., Karlson B. Basin-specific changes in filamentous cyanobacteria community composition across four decades in the Baltic Sea. Harmful Algae. 2020;91 doi: 10.1016/j.hal.2019.101685. [DOI] [PubMed] [Google Scholar]
- Paerl H.W., Huisman J. Blooms like it hot. Science. 2008;320(5872):57–58. doi: 10.1126/science.1155398. [DOI] [PubMed] [Google Scholar]
- R_Core_Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ URL. [Google Scholar]
- Rakko A., Seppälä J. Effect of salinity on the growth rate and nutrient stoichiometry of two Baltic Sea filamentous cyanobacterial species. Estonian J. Ecol. 2014;63(2) [Google Scholar]
- Reusch T.B., Dierking J., Andersson H.C., Bonsdorff E., Carstensen J., Casini M., Czajkowski M., Hasler B., Hinsby K., Hyytiäinen K. The Baltic Sea as a time machine for the future coastal ocean. Sci. Adv. 2018;4(5):eaar8195. doi: 10.1126/sciadv.aar8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff C., Elfwing T. Vol. 44. AMBIO; 2015. pp. 601–611. (Increasing Nitrogen Limitation in the Bothnian Sea, Potentially Caused By Inflow of Phosphate-Rich Water from the Baltic Proper). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva S., Meier H., Andersson H., Höglund A., Dieterich C., Gröger M., Hordoir R., Eilola K.J. Uncertainties in projections of the Baltic Sea ecosystem driven by an ensemble of global climate models. Front. Earth Sci. 2018;6:244. [Google Scholar]
- Schnute, J.T., Boers, N., Haigh, R., Couture-Beil, A., Chabot, D., Grandin, C., Johnson, A., Wessel, P., Antonio, F., Lewin-Koh, N.J., Bivand, R., 2021. PBSmapping: mapping fisheries data and spatial analysis tools. R package version 2.73.
- Schoffelen N.J., Mohr W., Ferdelman T.G., Littmann S., Duerschlag J., Zubkov M.V., Ploug H., Kuypers M.M. Single-cell imaging of phosphorus uptake shows that key harmful algae rely on different phosphorus sources for growth. Sci. Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-35310-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala J., Ylostalo P., Kaitala S., Hallfors S., Raateoja M., Maunula P. Ship-of-opportunity based phycocyanin fluorescence monitoring of the filamentous cyanobacteria bloom dynamics in the Baltic Sea. Estuar. Coastal Shelf Sci. 2007;73(3–4):489–500. [Google Scholar]
- Shchepetkin A.F., McWilliams J.C. The regional oceanic modeling system (ROMS): a split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean Model. 2005;9(4):347–404. [Google Scholar]
- Simis S.G.H., Bresciani M., Duan H., Giardino C., Hu C., Kutser T., Ma R., Matta E., Matthews M.W. In: Observation of Harmful Algal Blooms with Ocean Colour Radiometry. Bernard S., Kudela R.M., Robertson Lain L., Pitcher G., editors. International Ocean Colour Coordinating Group; Dartmouth, Canada: 2021. Remote Sensing of Cyanobacterial Blooms; pp. 73–98. [Google Scholar]
- Simola O., Wiberg M., Jokela J., Wahlsten M., Sivonen K., Syrjä P. Pathologic findings and toxin identification in cyanobacterial (Nodularia spumigena) intoxication in a dog. Vet. Pathol. 2012;49(5):755–759. doi: 10.1177/0300985811415703. [DOI] [PubMed] [Google Scholar]
- Sipia V., Kankaanpaa H., Lahti K., Carmichael W.W., Meriluoto J. Detection of Nodularin in flounders and cod from the Baltic Sea. Environ. Toxicol. 2001;16(2):121–126. doi: 10.1002/tox.1015. [DOI] [PubMed] [Google Scholar]
- Sipia V., Kankaanpaa H., Peltonen H., Vinni M., Meriluoto J. Transfer of nodularin to three-spined stickleback (Gasterosteus aculeatus L.), herring (Clupea harengus L.), and salmon (Salmo salar L.) in the northern Baltic Sea. Ecotox. Environ. Safe. 2007;66(3):421–425. doi: 10.1016/j.ecoenv.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Suikkanen S., Uusitalo L., Lehtinen S., Lehtiniemi M., Kauppila P., Mäkinen K., Kuosa H. Food Webs; 2021. Diazotrophic Cyanobacteria in Planktonic Food Webs; p. e00202. [Google Scholar]
- Utermöhl H. Neue wege in der quantitativen erfassung des planktons (mit besonderer berücksichtigung des ultraplanktons) Verh. int. Ver. theor. angew. Limnol. 1931;5(2):567–596. [Google Scholar]
- Utermöhl H. Zur Vervollkomnung der quantitativen. Phytoplankton-Methodik. Mitt. Int. Ver. Ther. Angew. Limnol. 1958;9:1–38. [Google Scholar]
- Van Buynder P.G., Oughtred T., Kirkby B., Phillips S., Eaglesham G., Thomas K., Burch M. Nodularin uptake by seafood during a cyanobacterial bloom. Environ. Toxicol. 2001;16(6):468–471. [PubMed] [Google Scholar]
- Walsby A.E., Hayes P.K., Boje R. The gas vesicles, buoyancy and vertical distribution of cyanobacteria in the Baltic Sea. Eur. J. Phycol. 1995;30(2):87–94. [Google Scholar]
- Walsby A.E., Hayes P.K., Boje R., Stal L.J. The selective advantage of buoyancy provided by gas vesicles for planktonic cyanobacteria in the Baltic Sea. New Phytol. 1997;136(3):407–417. doi: 10.1046/j.1469-8137.1997.00754.x. [DOI] [PubMed] [Google Scholar]
- Walve J., Larsson U. Blooms of Baltic Sea Aphanizomenon sp (cyanobacteria) collapse after internal phosphorus depletion. Aquat. Microb. Ecol. 2007;49(1):57–69. [Google Scholar]
- Wessel P., Smith W.H. A global, self-consistent, hierarchical, high-resolution shoreline database. J. Geophys. Res. 1996;101(B4):8741–8743. [Google Scholar]
- Wickham H., Averick M., Bryan J., Chang W., McGowan L.D.A., François R., Grolemund G., Hayes A., Henry L., Hester J. Welcome to the tidyverse. J. Open Source Software. 2019;4(43):1686. [Google Scholar]
- Wulff A., Karlberg M., Olofsson M., Torstensson A., Riemann L., Steinhoff F.S., Mohlin M., Ekstrand N., Chierici M. Ocean acidification and desalination: climate-driven change in a Baltic Sea summer microplanktonic community. Mar. Biol. 2018;165(4):1–15. doi: 10.1007/s00227-018-3321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Złoch I., Hebel A., Mazur-Marzec H. Effect of crude extracts from Nodularia spumigena on round goby (Neogobius melanostomus) Mar. Environ. Res. 2018;140:61–68. doi: 10.1016/j.marenvres.2018.05.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Links to data sources are included in material and methods. Contact the Swedish National Oceanographic Data Centre at SMHI if additional information is needed.