Abstract
Background
Whether the association between pulse pressure (PP) and mortality varies with systolic blood pressure (SBP) in ischaemic heart failure (HF) with left ventricular systolic dysfunction (LVSD) is unknown.
Objective
To evaluate the association between PP and all-cause mortality in ischaemic HF patients with SBP status at admission.
Patients and methods
This prospective cohort study included 1581 ischaemic HF patients with LVSD. A total of 23.3% (n = 368) and 22.2% (n = 351) of the participants had SBP <110 mmHg and SBP >140 mmHg, respectively, with more than 80% of participants being male. Restricted cubic spline was performed to determine whether a nonlinear relationship existed between PP and all-cause mortality risk. A multivariable Cox proportional hazards model was used to assess the association between PP and all-cause mortality.
Results
After a median of follow-up of 3.0 years, 257 events (16.4%) were observed in the cohort. There was a J-shaped relationship between PP and all-cause mortality (P value for nonlinearity = 0.020), with a risk nadir of approximately 46–49 mmHg. All-cause mortality risk varied with SBP status. Higher PP was associated with worse prognosis when the SBP was ≥110 mmHg, whereas the relationship did not reach statistical significance when the SBP was <110 mmHg.
Conclusion
A J-shaped relationship between PP and all-cause mortality was observed in ischaemic HF patients with LVSD, and higher PP was associated with worse prognosis only in those with SBP ≥110 mmHg. Further studies are needed to corroborate these findings.
KEY MESSAGES
A J-shaped relationship between pulse pressure and all-cause mortality was observed in ischaemic heart failure patients with left ventricular systolic dysfunction, with a risk nadir of approximately 46–49 mmHg.
All-cause mortality risk varied with systolic blood pressure status, and higher pulse pressure was associated with worse prognosis when systolic blood pressure was above 110 mmHg.
Keywords: Pulse pressure, blood pressure, ischaemic heart failure, left ventricular systolic dysfunction
Introduction
Elevated pulse pressure (PP), a traditional indicator of aortic stiffening, has been regarded as a marker of poor outcome not only in healthy individuals [1,2] but also in those with hypertension and other comorbidities [3–6]. The prognostic value of PP in patients with heart failure (HF) is inconsistent. In HF with preserved ejection fraction (HFpEF) patients, both low and high PP were associated with adverse outcomes, suggesting a J-shaped relationship [7–9]. In HF with reduced EF (HFrEF) patients, PP showed a linear inverse relationship with mortality in clinical studies [9–11] and meta-analyses [12], whereas other studies displayed a J-shaped relationship [13]. Both of these findings were in contrast to findings of studies decades ago [14,15]. A potential explanation was that low PP, a marker of left ventricular systolic dysfunction (LVSD) and low stroke volume rather than aortic stiffening [10], was associated with worse outcomes in those with HFrEF. However, it has been noted that some HFrEF patients have systolic blood pressure (SBP) within the normal range despite reduced left ventricular ejection fraction (LVEF) [12], whereas others have a low SBP due to low stroke volume.
We herein hypothesize that the relationship between PP and mortality may vary with SBP status in HFrEF patients. In this study, we assessed the association between PP and all-cause mortality according to SBP status in ischaemic HF patients with LVSD.
Methods
Study design and participants
This was a single-centre, prospective cohort study including ischaemic HF patients with LVSD as described previously [16]. In brief, ischaemic aetiology of HF was defined based on coronary angiography, a prior history of myocardial infarction (MI), or prior revascularization including percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). LVEF was assessed during the index hospitalization using echocardiogram and those with LVEF <45% were eligible for this study. Participants aged >18 years who were hospitalized in the Department of Cardiology, Guangdong Provincial People’s Hospital from December 2015 to June 2019 were screened. Patients with HF due to non-ischaemic aetiology (e.g. idiopathic dilated cardiomyopathy, valvular heart disease) or patients who were lost to follow-up since discharge were excluded. This study was approved by the Clinical Research Ethics Committee of Guangdong Provincial People’s Hospital (approval reference number: GDREC2017172H) and was performed in accordance with the Declaration of Helsinki. Before enrolment, all patients provided written informed consent.
Study procedure
Baseline data of interest were extracted from the electronic medical records of Guangdong Provincial People’s Hospital, including demographics, the reason for admission, vital signs at admission, comorbid conditions, echocardiographic parameters and medical therapy at discharge. As reported previously [16], fasting venous blood was drawn to evaluate lipid parameters and haemoglobin A1c (HbA1c) levels on the second day after admission. Venous blood at admission was drawn to evaluate creatinine, high-sensitivity C-reactive protein (hs-CRP), high-sensitivity cardiac troponin-T (hs-cTnT) and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels. The estimated glomerular filtration rate (eGFR) was calculated using the modified diet of renal disease formula using serum creatinine [17], and an eGFR <60 ml/min/1.73 m2 was defined as chronic kidney disease (CKD).
Blood pressure measurement
After the participants had rested and sat quietly for 5 min, brachial BP was measured at admission by experienced physicians or nurses using electronic sphygmomanometers, and PP was defined as the difference between the SBP and diastolic BP (DBP). Patients were classified into three groups based on the SBP at admission: the low SBP group (<110 mmHg), normal SBP group (110–140 mmHg) and high SBP group (>140 mmHg).
Echocardiographic examination
Transthoracic echocardiography was performed by experienced sonographers when the patient’s clinical condition was stable. The LVEF was measured using the modified biplane Simpson’s rule from the apical 2- and 4- chamber view. Left atrial and left ventricular sizes were measured by 2D echocardiography. Mitral inflow E- and A-wave velocity was assessed using pulsed wave Doppler from the apical 4-chamber view. Peak early diastolic tissue velocity (e′) was measured from the septal aspect of the mitral annulus and estimated left ventricular (LV) filling pressure was calculated using the E/e′ ratio [18].
Follow-up and clinical outcomes
Follow-up was performed through phone call interviews. Due to the unavailability of assessment for the exact reason of death, all-cause mortality was used in the current study. The follow-up time was calculated as the date of death or last follow-up minus the date of discharge.
Statistical analysis
Continuous variables were expressed as the mean value ± standard deviation (SD) or median (interquartile range), and categorical variables were presented as numbers and proportions. Student’s t-test or the Kruskal–Wallis H-test was used to compare continuous variables, and the Chi-square test was used to compare categorical variables. Differences in baseline characteristics were examined among the three SBP groups.
To assess whether there was a nonlinear relationship between PP and all-cause mortality, restricted cubic spline was performed. The rate of all-cause mortality according to quintiles of PP was displayed in a dot plot.
The cumulative incidence of all-cause mortality was plotted with the Kaplan–Meier (KM) curve and was compared between low and high PP subgroups stratified by 3 SBP groups with the log-rank test. The hazard ratio (HR) and 95% confidence interval (CI) were computed using the Cox proportional-hazards analyses, with stepwise adjustment for covariates, including age, sex, the reason for admission, New York Heart Association (NYHA) class, smoking status, diabetes, dyslipidemia, eGFR, low-density lipoprotein cholesterol (LDL-C), log10 hs-cTNT, log10 NT-proBNP, left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), LVEF, left atrial (LA) diameter, the E/e′ ratio, the use of intravenous diuretics and intravenous inotropic agents. Missing values for hs-cTnT, NT-proBNP, eGFR, LDL-C and LA diameter were imputed with the median, whereas covariates with >10% missing data (e.g. hs-CRP) were excluded.
Two-sided p values < .05 were considered statistically significant. All analyses were performed using Stata version 15.1 (StataCorp LLC, College Station, TX, USA) and R version 4.1.1 (The R Project for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
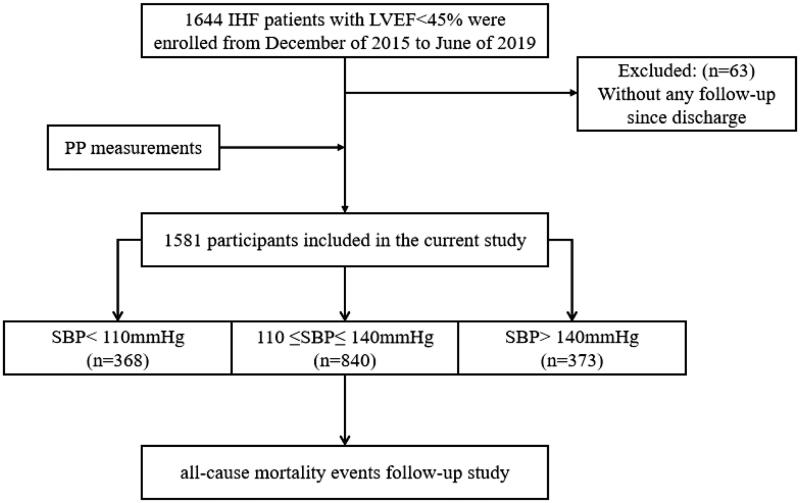
A total of 1644 patients with ischaemic HF between December 2015 and June 2019 were recruited, and 1581 were enrolled in this study (Figure 1). Table 1 shows the baseline differences among the three SBP groups. Briefly, patients with SBP >140 mmHg were older, had higher DBP, PP and LVEF values and were less likely to present with ST segment elevation myocardial infarction (STEMI). Patients with SBP <110 mmHg had higher hs-cTnT and NT-proBNP levels and were more likely to receive intravenous inotropic agents and diuretics. The comorbidities (except for anaemia and stroke/transient ischaemic attack, TIA) were comparable between the three SBP groups.
Figure 1.
Study flowchart. IHF: ischaemic heart failure; LVEF: left ventricular ejection fraction; PP: plus pressure; SBP: systolic blood pressure.
Table 1.
Baseline characteristics comparison between systolic blood pressure group.
| Variables | SBp < 110 mmHg (n = 368) | 110 ≤ SBp ≤ 140 mmHg (n = 862) | >140 mmHg (n = 351) | p-Value |
|---|---|---|---|---|
| Age (years) | 61.1 ± 11.4 | 63.3 ± 10.9 | 65.7 ± 10.3 | <.001 |
| Male, n (%) | 310 (84.2) | 750 (87.0) | 287 (81.8) | .055 |
| Vital sign at admission | ||||
| Systolic blood pressure (mm Hg)* | 102 (96–106) | 124 (118–131) | 150 (145–158) | <.001 |
| Diastolic blood pressure (mm Hg)* | 64 (58–69) | 74 (69–81) | 85 (77–92) | <.001 |
| PP (mmHg)* | 37 (32–42) | 49 (42–57) | 67 (58–77) | <.001 |
| Heart rate (beat per minute)* | 79 (70–91) | 78 (70–88) | 78 (70–90) | .198 |
| Reason for admission | ||||
| STEMI, n (%) | 83 (22.6) | 119 (13.8) | 30 (8.6) | <.001 |
| Non-STEMI, n (%) | 32 (8.7) | 58 (6.7) | 30 (8.6) | .366 |
| Unstable angina, n (%) | 178 (48.4) | 454 (52.7) | 196 (55.8) | .130 |
| Acute heart failure, n (%) | 136 (37.0) | 329 (38.2) | 141 (40.2) | .668 |
| NYHA Class III–IV, n (%) | 99 (26.9) | 218 (25.3) | 97 (27.6) | .658 |
| Comorbidities | ||||
| Current smoker, n (%) | 84 (22.8) | 211 (24.5) | 78 (22.2) | .650 |
| Diabetes mellitus, n (%) | 128 (34.8) | 278 (32.3) | 125 (35.6) | .456 |
| Dyslipidemia, n (%) | 229 (62.2) | 564 (65.4) | 236 (67.2) | .353 |
| Chronic heart failure, n (%) | 152 (41.3) | 348 (40.4) | 144 (41.0) | .947 |
| Anaemia, n (%) | 98 (26.6) | 146 (16.9) | 78 (22.2) | <.001 |
| CKD, n (%) | 84 (22.8) | 198 (23.0) | 94 (26.8) | .326 |
| COPD, n (%) | 28 (7.6) | 58 (6.7) | 31 (8.8) | .440 |
| Atrial fibrillation, n (%) | 17 (4.6) | 42 (4.9) | 12 (3.4) | .536 |
| Prior stroke/TIA, n (%) | 15 (4.1) | 68 (7.9) | 43 (12.3) | <.001 |
| Prior MI, n (%) | 140 (38.0) | 321 (37.2) | 105 (29.9) | .032 |
| Prior PCI, n (%) | 225 (61.1) | 500 (58.0) | 187 (53.3) | .099 |
| Prior CABG, n (%) | 4 (1.1) | 19 (2.2) | 7 (2.0) | .416 |
| Laboratory | ||||
| Haemoglobin (g/L)* | 130 (117–143) | 134 (122–146) | 134 (119–145) | .012 |
| Total cholesterol (mmol/L)* | 4.10 (3.32–5.00) | 4.20 (3.52–5.00) | 4.30 (3.60–5.10) | .022 |
| LDL-C (mmol/L)* | 2.67 (2.14–3.33) | 2.73 (2.20–3.33) | 2.79 (2.25–3.41) | .140 |
| HDL-C (mmol/L)* | 0.87 (0.73–1.03) | 0.92 (0.79–1.06) | 0.96 (0.81–1.11) | <.001 |
| Triglyceride (mmol/L)* | 1.27 (0.95–1.76) | 1.28 (1.00–1.81) | 1.31 (0.98–1.77) | .559 |
| Lp (a) (mg/dL)* | 21.8 (10.4–46.2) | 18.5 (9.8–39.0) | 22.2 (10.2–43.6) | .194 |
| Creatinine (µmol/L)* | 93.9 (80.5–108.0) | 90.9 (77.7–108.9) | 91.0 (75.1–111.2) | .572 |
| eGFR (ml/min/1.73 m2)* | 74.3 (61.2–88.3) | 75.5 (61.3–91.3) | 74.1 (57.9–89.3) | .264 |
| HbA1c (%)* | 6.2 (5.7–7.4) | 6.3 (5.8–7.5) | 6.4 (5.8–7.6) | .222 |
| FPG (mmol/L)* | 5.31 (4.61–6.89) | 5.35 (4.64–6.74) | 5.49 (4.62–6.82) | .814 |
| Hs-CRP (mg/L)* | 5.56 (1.40–19.26) | 5.30 (1.33–16.30) | 4.61 (1.63–11.4) | .492 |
| Hs-cTNT (pg/mL)* | 58.3 (22.7–430.8) | 34.6 (17.6–188.3) | 29.3 (16.5–100.8) | <.001 |
| NT-proBNP (pg/mL)* | 2163 (991–4447) | 1410 (551–3488) | 1473 (549–3291) | <.001 |
| Echocardiographic index | ||||
| LA diameter (mm)* | 40 (36–45) | 40 (36–44) | 40 (37–44) | .557 |
| LVESD (mm)* | 48 (41–54) | 47 (41–53) | 46 (40–52) | .054 |
| LVEDD (mm)* | 59 (53–65) | 59 (53–64) | 58 (53–63) | .332 |
| LVEF (%)* | 35 (29–39) | 36 (30–41) | 38 (32–41) | <.001 |
| E/e* | 16.7 (12.5–25.0) | 16.2 (12.1–22.3) | 16.3 (12.8–22.0) | .182 |
| Coronary angiography | ||||
| LM, n (%) | 88 (23.9) | 218 (25.3) | 90 (25.6) | .841 |
| LAD, n (%) | 331 (90.0) | 793 (92.0) | 323 (92.0) | .463 |
| LCX, n (%) | 253 (68.8) | 635 (73.7) | 268 (76.4) | .062 |
| RCA, n (%) | 277 (75.3) | 664 (77.0) | 278 (79.2) | .454 |
| Single vessel, n (%) | 49 (13.3) | 105 (12.2) | 45 (12.8) | .850 |
| Two vessels, n (%) | 76 (20.7) | 173 (20.1) | 64 (18.2) | .687 |
| Three vessels, n (%) | 220 (59.8) | 547 (63.5) | 232 (66.1) | .208 |
| In-hospital treatment | ||||
| IV inotropic agents, n (%) | 80 (21.7) | 100 (11.6) | 26 (7.4) | <.001 |
| IV diuretics, n (%) | 160 (43.5) | 292 (33.9) | 123 (35.0) | .005 |
| Coronary stenting, n (%) | 245 (66.6) | 586 (68.0) | 235 (67.0) | .870 |
| Medications at discharge | ||||
| Aspirin, n (%) | 317 (86.1) | 767 (89.0) | 311 (88.6) | .357 |
| Clopidogrel, n (%) | 264 (71.7) | 632 (73.3) | 267 (76.1) | .409 |
| Ticagrelor, n (%) | 58 (15.8) | 114 (13.2) | 33 (9.4) | .038 |
| Statins, n (%) | 345 (93.8) | 827 (95.9) | 330 (94.0) | .171 |
| Betablocker, n (%) | 311 (84.5) | 727 (84.3) | 297 (84.6) | .992 |
| RASi, n (%) | 207 (56.3) | 625 (72.5) | 280 (79.8) | <.001 |
| ARNI, n (%) | 10 (2.7) | 24 (2.8) | 12 (3.4) | .811 |
| MRA, n (%) | 213 (57.9) | 439 (50.9) | 158 (45.0) | .003 |
| Loop diuretic, n (%) | 200 (54.4) | 405 (47.0) | 160 (45.6) | .030 |
| Digoxin, n (%) | 33 (9.0) | 71 (8.2) | 14 (4.0) | .018 |
| CCB, n (%) | 4 (1.1) | 68 (7.9) | 86 (24.5) | <.001 |
| Insulin, n (%) | 31 (8.4) | 55 (6.4) | 25 (7.1) | .437 |
| Oral anti-diabetics, n (%) | 121 (32.9) | 259 (30.1) | 106 (30.2) | .596 |
| Oral anticoagulants, n (%) | 35 (9.5) | 63 (7.3) | 24 (6.8) | .325 |
PP: pulse pressure; STEMI: ST segment elevation myocardial infarction; non-STEMI: non-ST segment elevation myocardial infarction; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; TIA: transient ischaemic attack; MI: myocardial infarction; CABG: coronary artery bypass grafting; LDL-C: low density lipoprotein-cholesterol; HDL-C: high density lipoprotein cholesterol; Lp(a): lipoprotein(a); eGFR: estimated glomerular filtration rate; HbA1c: glycated haemoglobin A1c; FPG: fasting plasma glucose; Hs-CRP: high sensitivity C reactive protein; Hs-cTnT: high sensitivity cardiac troponin-T; NT-proBNP: N-terminal pro B-type natriuretic peptide; LA: left atrial; LVESD: left ventricular end systolic diameter; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction; IV: intravenous; LM: left main coronary artery; LAD: left anterior descending coronary artery; LCX: left circumflex artery; RCA: right coronary artery; RASi: renin-angiotensin-system inhibitor; ARNI: angiotensin receptor-neprilysin inhibitor; MRA: mineralocorticoid receptor antagonist; CCB: Calcium channel blocker; *presented as median (interquartile range).
Relationship of pulse pressure with all-cause mortality
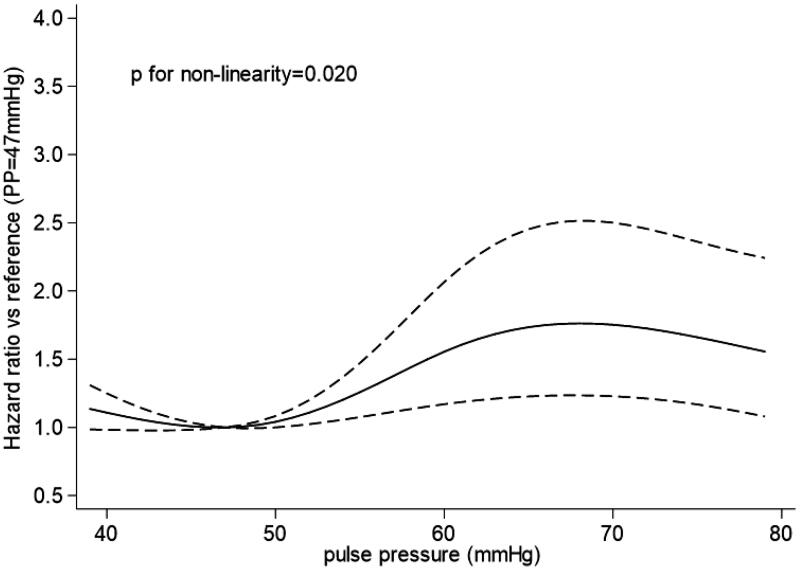
The crude all-cause mortality rate was 16.4% (n = 259). Restricted cubic spline showed a nonlinear (J-shaped) relationship between PP and all-cause mortality (p value for nonlinearity = 0.020), with a risk nadir of approximately 46–49 mmHg (Figure 2). The rate of all-cause mortality according to the quintiles of PP is shown in Figure 3. Both PP >50 mmHg and <46 mmHg were associated with an increased risk of all-cause mortality.
Figure 2.
Association between pulse pressure and risk of all-cause mortality.
Figure 3.
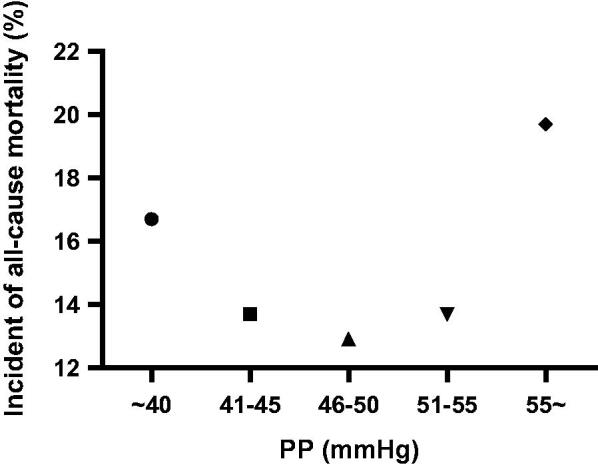
Incident rate of all-cause death events according to groups defined by quintiles of pulse pressure.
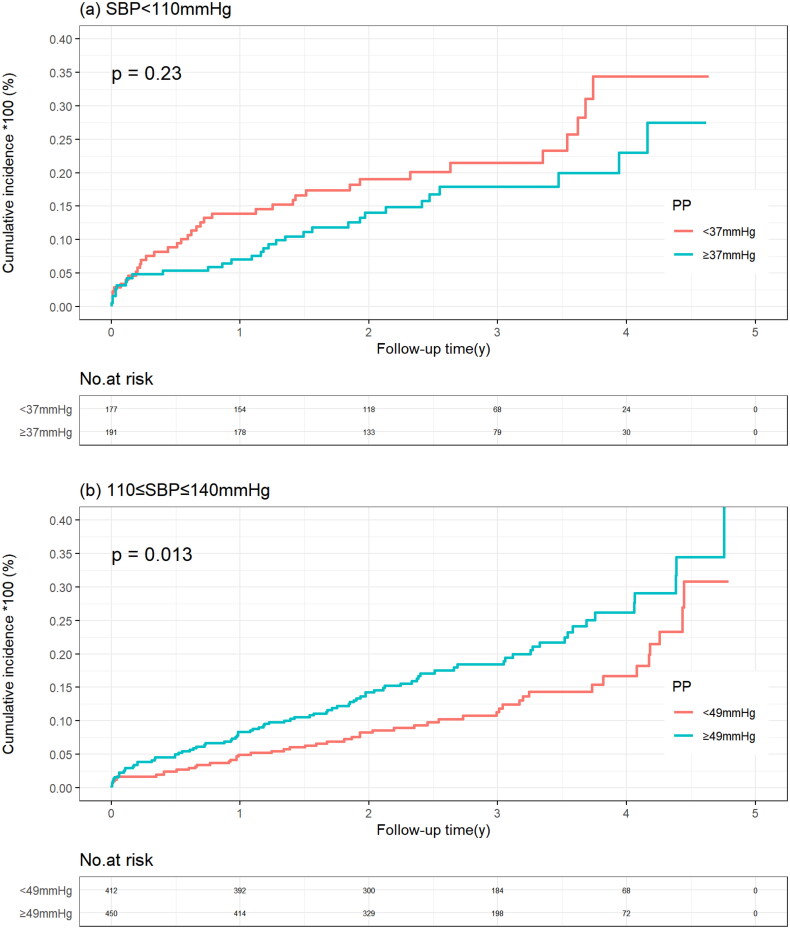
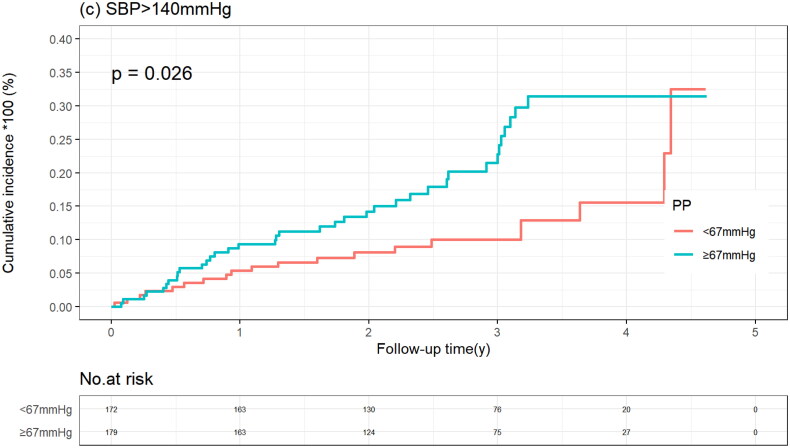
All-cause mortality by median PP value according to SBP
In the SBP <110 mmHg group, the cumulative incidence of all-cause mortality was similar in patients with a PP below and above the median PP (20.3% vs. 15.7%; p = .230), which was consistent when PP was considered a continuous variable. In the SBP between 110 and 140 mmHg group and in the SBP >140 mmHg group, patients with PP above the median had a higher all-mortality risk, with adjusted HRs of 1.54 (95%CI 1.08–2.20) and 2.00 (95% CI 1.15–3.48), respectively. When PP was considered a continuous variable, in the SBP of 110 to 140 mmHg group, all-cause mortality risk increased with increased PP ((HR 1.20 and 95% CI 1.03–1.40) per 10-mmHg increase in PP), whereas in the SBP >140 mmHg group, there was a trend towards increased all-cause mortality risk with increased PP (p = .097) (Table 2, Figure 4).
Table 2.
PP level-associated all-cause mortality risk according to SBP groups.
| Groups | Events/total (%) | HR (95% CI) | p Value | Adjusted HR (95% CI) | p-Value |
|---|---|---|---|---|---|
| SBP <110 mmHg | 66/368 (17.9) | ||||
| Per 10-mmHg PP increase | 66/368 (17.9) | 0.85 (0.62–1.15) | .283 | / | / |
| PP <37 mmHg | 36/177 (20.3) | Reference | .230 | Reference | / |
| PP ≥37 mmHg | 30/191 (15.7) | 0.74 (0.46–1.20) | / | ||
| 110 ≤ SBP ≤140 mmHg | 136/862 (15.8) | ||||
| Per 10-mmHg PP increase | 136/862 (15.8) | 1.18 (1.02–1.38) | .027 | 1.20 (1.03–1.40) | .019 |
| PP <49 mmHg | 51/412 (12.4) | Reference | .013 | Reference | .018 |
| PP ≥49 mmHg | 85/450 (18.9) | 1.56 (1.10–2.21) | 1.54 (1.08–2.20) | ||
| SBP >140 mmHg | 57/351 (16.2) | ||||
| Per 10-mmHg PP increase | 57/351 (16.2) | 1.22 (0.99–1.56) | .068 | 1.34 (0.97–1.76) | .097 |
| PP <67 mmHg | 20/172 (11.6) | Reference | .026 | Reference | .014 |
| PP ≥67 mmHg | 37/179 (20.7) | 1.84 (1.07–3.32) | 2.00 (1.15–3.48) |
Multivariable Cox proportional hazards regression model was adjusted for age, sex, reason for admission, NYHA class, smoking status, diabetes, dyslipidemia, eGFR, LDL-C, log10 Hs-cTNT, log10 NT-proBNP, LVEDD, LVESD, LVEF, LA diameter, E/e′, intravenous diuretics, intravenous inotropic agents.
Figure 4.
Kaplan–Meier curve of all-cause mortality stratified by systolic blood pressure according to pulse pressure level. (a) SBP >140 mmHg group. (b) 110 ≤ SBP ≤140 mmHg group. (c) SBP <110 mmHg group.
Discussion
The results of this study demonstrated a J-shaped relationship between PP and all-cause mortality in ischaemic HF patients with LVSD, and this relationship varied by SBP status. Specifically, when the SBP was ≥110 mmHg, an increased PP was associated with a higher all-cause mortality risk, whereas when the SBP was <110 mmHg, the association between increased PP and all-cause mortality was nonsignificant.
Consistent with a previous report [13], our current study also showed a J-shaped relationship between PP and all-cause mortality in ischaemic HF patients with LVSD. Interestingly, the relationship between PP and all-cause mortality risk varied by baseline SBP status in the present study. PP has been considered a complex marker in the HF population [19], and the explanations for the present study are speculative. Previous studies have shown that a low PP was associated with increased mortality in advanced HF patients, while the trend was opposite in asymptomatic HFrEF patients [20]. In the current study, patients in the SBP <110 mmHg group had higher NT-proBNP values and were more likely to receive intravenous diuretics and inotropic agents than their counterparts in the SBP ≥110 mmHg group, suggesting that patients in the low SBP group (SBP <110 mmHg) might be more likely to have advanced HF. This might explain why high PP was related to death in the normal (110–140 mmHg) and high (>140 mmHg) SBP groups. However, no association was observed between PP and all-cause mortality in the low SBP group, which was inconsistent with previous study [20]. This discrepant finding might be due to the small sample size or different clinical characteristics of the participants in our current study.
Notably, SBP is associated with left ventricular systolic function [21]. We speculated that a high PP was mainly due to arteriosclerosis in the normal SBP group, leading to a high mortality risk [22,23], whereas a low PP in the low SBP group, which reflected the severity of LV systolic dysfunction, played a critical role in the prognosis [10]. Importantly, the median PP in the low SBP group in the current study was far below than that in other study of the relationship between PP and atherosclerosis [24]. Previous studies have also confirmed that aortic stiffness is more common in individuals with hypertension than in the normotensive population [25,26], suggesting that hypertensive patients with a high PP are at a higher mortality risk due to arterial stiffness [22].
In accordance with the MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) analysis in HFrEF patients [12], the current study also showed that when patients had SBP >140 mmHg, higher PP was associated with a worse prognosis. The results of our study extend prior studies to assess the relationship between PP and all-cause mortality in ischaemic HF patients with normal SBP. The findings suggest that the all-cause mortality risk increases at a higher PP level even in those with controlled SBP. Combined with the recent study [24], it is considered that PP should be taken into consideration not only in patients with concomitant hypertension, but also in those who are at target SBP.
In contrast to the present study, Bonapace S and co-workers found that the risk of all-cause mortality at 1 year decreased with high PP in acute HFrEF patients with SBP between 100 and 140 mmHg [9]. The explanation is as follows. First, participants involved in their study were de novo and worsening HF patients, including 88% of patients who were in NYHA class III or IV [9], which was higher than our current study. As discussed above, advanced HF patients with low PP usually have poor prognosis [20]. Second, the prevalence of atrial fibrillation in Bonapace S’ study was much higher than that in ours. This may be another reason to explain the discrepancy because loss of atrial contribution to LV filling caused an obvious reduction in stroke volume [27].
Study limitations
The current study has several limitations. First, this was a single-centre cohort study so the conclusions of the study may not be generalizable to all ischaemic HF populations. Second, observation studies have inherent limitations, including missing values and incomplete information, the collection of nonrandomized data, and potential unknown confounding factors. Third, we only evaluated PP at admission, and the alterations in PP at discharge or during follow-up were unknown. Nevertheless, PP amplitude varies by time [28], and data for PP collected early at admission were related to poor prognosis [29]. Whether variation in PP affects prognosis in ischaemic HF populations should be examined in future studies. Fourth, all-cause mortality was used as the endpoint because we were unable to ascertain the cause of death through phone call interviews. The association between PP and cardiovascular mortality or HF hospitalization in the ischaemic HF population should be further studied.
Conclusion
There was a J-shaped association between PP and all-cause mortality in ischaemic HF patients with LVSD, and the risk of mortality varied with SBP status. A higher PP was associated with worse prognosis in those with SBP > 110 mmHg, whereas the relationship did not reach statistical significance in those with low SBP. Further large randomized clinical trials are warranted to confirm our current results.
Funding Statement
The current study was supported by Guangdong Provincial People’s Hospital Clinical Research Fund [Y012018085].
Ethical approval
This study was approved by the Clinical Research Ethics Committee of Guangdong Provincial People’s Hospital (approval reference number: GDREC2017172H) and performed in accordance with the Declaration of Helsinki. All patients included in the current study provided written informed consent.
Author contributions
Weida Qiu contributed to the data collection, analysis and drafted the manuscript. Xiaoju Xiao contributed to the data collection and contributed to telephonic interviews. Anping Cai revised the manuscript. Zhiping Gao and Liwen Li contributed to the conception and design of the work. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The deidentified participant data will be shared on a request basis. Please directly contact the corresponding author to request data sharing.
Permission to reproduce material from other sources
All the authors permit this manuscript has not been published or presented elsewhere in part or in entirety and not under consideration by another journal.
References
- 1.Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation. 1999;100(4):354–360. [DOI] [PubMed] [Google Scholar]
- 2.Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30(6):1410–1415. [DOI] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Aznaouridis K, Stefanadis C.. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. [DOI] [PubMed] [Google Scholar]
- 4.Haider AW, Larson MG, Franklin SS, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham heart study. Ann Intern Med. 2003;138(1):10–16. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160(8):1085–1089. [DOI] [PubMed] [Google Scholar]
- 6.Bergmark BA, Scirica BM, Steg PG, et al. Blood pressure and cardiovascular outcomes in patients with diabetes and high cardiovascular risk. Eur Heart J. 2018;39(24):2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tokitsu T, Yamamoto E, Hirata Y, et al. Clinical significance of pulse pressure in patients with heart failure with preserved left ventricular ejection fraction. Eur J Heart Fail. 2016;18(11):1353–1361. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Claggett B, Minamisawa M, et al. Pulse pressure, prognosis, and influence of sacubitril/valsartan in heart failure with preserved ejection fraction. Hypertension. 2021;77(2):546–556. [DOI] [PubMed] [Google Scholar]
- 9.Bonapace S, Rossi A, Laroche C, et al. Brachial pulse pressure in acute heart failure. Results of the heart failure registry. ESC Heart Fail. 2019;6(6):1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regnault V, Lagrange J, Pizard A, et al. Opposite predictive value of pulse pressure and aortic pulse wave velocity on heart failure with reduced left ventricular ejection fraction: insights from an eplerenone post-acute myocardial infarction heart failure efficacy and survival study (EPHESUS) substudy. Hypertension. 2014;63(1):105–111. [DOI] [PubMed] [Google Scholar]
- 11.Petrie CJ, Voors AA, Robertson M, et al. A low pulse pressure predicts mortality in subjects with heart failure after an acute myocardial infarction: a post-hoc analysis of the CAPRICORN study. Clin Res Cardiol. 2012;101(1):29–35. [DOI] [PubMed] [Google Scholar]
- 12.Jackson CE, Castagno D, Maggioni AP, et al. Differing prognostic value of pulse pressure in patients with heart failure with reduced or preserved ejection fraction: results from the MAGGIC individual patient meta-analysis. Eur Heart J. 2015;36(18):1106–1114. [DOI] [PubMed] [Google Scholar]
- 13.Laskey WK, Wu J, Schulte PJ, et al. Association of arterial pulse pressure with Long-Term clinical outcomes in patients with heart failure. JACC Heart Fail. 2016;4(1):42–49. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell GF, Moyé LA, Braunwald E, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and ventricular enlargement. Circulation. 1997;96(12):4254–4260. [DOI] [PubMed] [Google Scholar]
- 15.Domanski MJ, Mitchell GF, Norman JE, et al. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33(4):951–958. [DOI] [PubMed] [Google Scholar]
- 16.Cai A, Wu Z, Xu L, et al. Association of anaemia and all‐cause mortality in patients with ischemic heart failure varies by renal function status. ESC Heart Failure. 2021;8(3):2270–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. [DOI] [PubMed] [Google Scholar]
- 19.Naka KK, Ikonomidis I.. Brachial pulse pressure in heart failure: simple to measure but complex to interpret. Eur Heart J. 2019;40(26):e8–e10. [DOI] [PubMed] [Google Scholar]
- 20.Lam CS, Teng TH.. Minding the gap in heart failure: understanding the pulse pressure in reduced versus preserved ejection fraction. JACC Heart Fail. 2016;4(1):50–54. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Lacolley P, Protogerou AD, et al. Arterial stiffness in hypertension and function of large arteries. Am J Hypertens. 2020;33(4):291–296. [DOI] [PubMed] [Google Scholar]
- 22.Selvaraj S, Steg PG, Elbez Y, et al. Pulse pressure and risk for cardiovascular events in patients with atherothrombosis: from the REACH registry. J Am Coll Cardiol. 2016;67(4):392–403. [DOI] [PubMed] [Google Scholar]
- 23.Harbaoui B, Nanchen D, Lantelme P, et al. Prognostic value of pulse pressure after an acute coronary syndrome. Atherosclerosis. 2018;277:219–226. [DOI] [PubMed] [Google Scholar]
- 24.Bohm M, Schumacher H, Teo KK, et al. Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120–140 mmHg) and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Eur Heart J. 2018;39(33):3105–3114. [DOI] [PubMed] [Google Scholar]
- 25.Smulyan H, Lieber A, Safar ME.. Hypertension, diabetes type II, and their association: Role of arterial stiffness. Am J Hypertens. 2016;29(1):5–13. [DOI] [PubMed] [Google Scholar]
- 26.Avolio AP, Van Bortel LM, Boutouyrie P, et al. Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension. 2009;54(2):375–383. [DOI] [PubMed] [Google Scholar]
- 27.Zimetbaum P. Atrial fibrillation. Ann Intern Med. 2017;166(5):ITC33–ITC48. [DOI] [PubMed] [Google Scholar]
- 28.Sung SH, Yu WC, Cheng HM, et al. Pulsatile hemodynamics and clinical outcomes in acute heart failure. Am J Hypertens. 2011;24(7):775–782. [DOI] [PubMed] [Google Scholar]
- 29.Aronson D, Burger AJ.. Relation between pulse pressure and survival in patients with decompensated heart failure. Am J Cardiol. 2004;93(6):785–788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified participant data will be shared on a request basis. Please directly contact the corresponding author to request data sharing.