Abstract
Simple Summary
Climate change and overexploitation of natural resources drive the need for innovative food production within a sustainability corridor. Aquaponics, combining the technology of recirculation aquaculture systems (RAS) and hydroponics in a closed-loop network, could contribute to addressing these problems. Aquaponic systems have lower freshwater demands than agriculture, greater land use efficiency, and decreased environmental impact combined with higher fish productivity. Rainbow trout is one of the major freshwater fish cultured worldwide, which, however, has not yet been commercially developed in aquaponics. Nevertheless, research conducted so far indicates that the trout species represents a good candidate for aquaponics.
Abstract
The impact of climate change on both terrestrial and aquatic ecosystems tends to become more progressively pronounced and devastating over the years. The sector of aquaculture is severely affected by natural abiotic factors, on account of climate change, that lead to various undesirable phenomena, including aquatic species mortalities and decreased productivity owing to oxidative and thermal stress of the reared organisms. Novel innovative technologies, such as aquaponics that are based on the co-cultivation of freshwater fish with plants in a sustainable manner under the context of controlled abiotic factors, represent a promising tool for mitigating the effect of climate change on reared fish. The rainbow trout (Oncorhynchus mykiss) constitutes one of the major freshwater-reared fish species, contributing to the national economies of numerous countries, and more specifically, to regional development, supporting mountainous areas of low productivity. However, it is highly vulnerable to climate change effects, mainly due to the concrete raceways, in which it is reared, that are constructed on the flow-through of rivers and are, therefore, dependent on water’s physical properties. The current review study evaluates the suitability, progress, and challenges of developing innovative and sustainable aquaponic systems to rear rainbow trout in combination with the cultivation of plants. Although not commercially developed to a great extent yet, research has shown that the rainbow trout is a valuable experimental model for aquaponics that may be also commercially exploited in the future. In particular, abiotic factors required in rainbow trout farming along, with the high protein proportion required in the ratios due to the strict carnivorous feeding behavior, result in high nitrate production that can be utilized by plants as a source of nitrogen in an aquaponic system. Intensive farming of rainbow trout in aquaponic systems can be controlled using digital monitoring of the system parameters, mitigating the obstacles originating from extreme temperature fluctuations.
Keywords: aquaponics, hydroponics, climate change, sustainability, rainbow trout, intelligent aquaculture, controlled system
1. Introduction
The ecological crisis and the disruption of the ecological balance of aquatic ecosystems are the main and primary issues negatively influencing both marine and freshwater basins, as well as their inhabitants [1]. They derive from a major impact of climate change, causing alterations in a combination of abiotic factors, such as increased temperatures, rainfall reduction, oxygen availability, and pollution [2]. In this context, temperature, nutrient availability, and oxygen distribution in water levels tend to become a major challenge for global aquaculture [1,3]. Fresh- and seawater temperatures increase due to global warming, and it is reflected in the physiology and welfare of aquatic species through a loss in productivity that occasionally may lead to severe mortalities [3]. Freshwater and wetland ecosystems are, thus, facing extreme impacts owing to the increase in frequency and magnitude of temperature fluctuations, threatening the health of aquatic ectothermic organisms and causing economic losses to aquaculture [4]. In addition, climate change tends to affect human well-being because ecosystem services, such as access to fresh water and the production of aquaculture food, are under threat.
More specifically, the freshwater aquaculture sector is occasionally extremely vulnerable to climate change effects [5,6], which are even more intense in freshwater fish farming, due to concrete raceways built on the flow-through rivers. One of the major freshwater fish reared in this form of aquaculture is the rainbow trout (Oncorhynchus mykiss) (Walbaum, 1792), which plays an important role in regional development. Since this fish is mostly reared in raceways [7], it is exposed to a great extent to abiotic factors and is, therefore, expected to be severely affected by climate change [8,9,10].
In recent years, research has been conducted on the development of novel production systems, such as aquaponics, generally characterized as less sensitive to climate change effects. Aquaponic systems combine the co-cultivation of plants, along with freshwater fish farming, in a recycling sustainable system [11,12]. Specifically, the implementation and development of such innovative and sustainable systems are crucial in order to avoid environmental fluctuations that can harm the fish and minimize their production, as well as for the adequate production of aquaculture products with reduced environmental impact.
This study constitutes a literature review of the attempts conducted so far to rear rainbow trout in aquaponic systems, as well as to evaluate the limitations and perspectives. The temperature increase tends to disturb the homeostasis of rainbow trout. Keeping this in mind, the current review further examines the rapid rise in temperature due to climate change and its effect on rainbow trout farming.
2. Rainbow Trout Farming, Biological Aspects and Effects of Climate Change
2.1. Rainbow Trout Farming Systems and Production Trends
O. mykiss is the main freshwater fish species bred in Europe, which possesses significant market value [13]. A large proportion of the rainbow trout farming sector is operated by family businesses throughout Europe, with many of them focusing on primary production, while others hold integrated production facilities performing filleting, smoking, and preparation of various edible products [14,15].
Rainbow trout cultivation comprises mainly grow-out techniques of farming that depend on physical water supplies. For instance, cage farming in water reservoirs (lakes, seawater, and ponds) has gained popularity over the last decade [16]. Although this technique can demonstrate advantages, such as larger cultivating space and bigger production, climate change limits this advantage through acute changes in abiotic factors. Additionally, there are farming units in which water supply is available through springs or rivers, such as flow-through systems in concrete raceways (Figure 1), which are considered to be ideal, exploiting the clarity of the optimal conditions of incoming water and exporting the final wastes back to the riverbanks. Although this technique is common in many countries, many others have established legislation in order to minimize waste exportation to water reservoirs [17]. Additionally, flow-through systems, especially from rivers, have been proven to be dangerous for each other when they co-exist on the same riverbank and the transfer of waste occurs from one to another through the water supply.
Figure 1.
Concrete raceways built on the flow through of a small river in Pella, Greece, utilized in rainbow trout farming.
However, it should be pointed out that at the highest rate of approximately 70% of the production, farmed rainbow trout is traditionally raised in flow-through raceways built on the route of mountain streams, whereas only approximately 10% of rainbow trout is grown in aquaculture in a water recirculation system (RAS), mainly in countries, such as Denmark [18,19]. They are grown to a size of between 350 and 600 g, which corresponds also to the marketable size, with the following forms of disposal and maintenance: (a) live trout, (b) fresh trout, (c) frozen trout, (d) fresh trout fillets, (e) smoked trout, including fillets, (f) prepared trout [14,15]. Occasionally, the rainbow trout is also bred for its eggs in countries, such as France.
Economic value for rainbow trout starts at 5.6 EUR/kilo in Poland, reaching up to 14.25–22.22 EUR/kg in France, Italy, and Denmark, while even higher market prices of rainbow trout are observed in Austria and Finland [14,15,20]. The main producers of rainbow trout are Iran (164,000 tons), the European Union (183,819 tons), Turkey (103,000 tons), and Peru (55,000 tons). Additionally, a significant amount of rainbow trout is produced in Indonesia, India, Vietnam, Bangladesh, Philippines, Korea, Japan, Egypt, Norway, and Chile. It should be also noted that in some Mediterranean countries, such as Greece and Portugal, despite the numerous existing farming units, the price is relatively low, mainly on account of the consumers’ preference for marine fish. However, in various member states of the European Union (e.g., France, Denmark), rainbow trout is strongly recognized by consumers as a healthy and organic product [21,22].
In Greece, approximately 60 units of rainbow trout operate today, with an annual production yield that reaches 1900 tons, worth 6,270,000 million EUR, and four hatcheries [22,23]. The produced fresh rainbow trout is available in two sizes: (a) average weight of 200–500 grams and (b) average weight of 400–500 grams at a price of 2.7 EUR/kg [24].
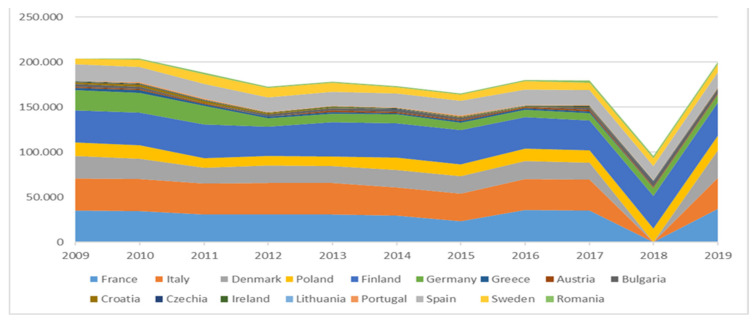
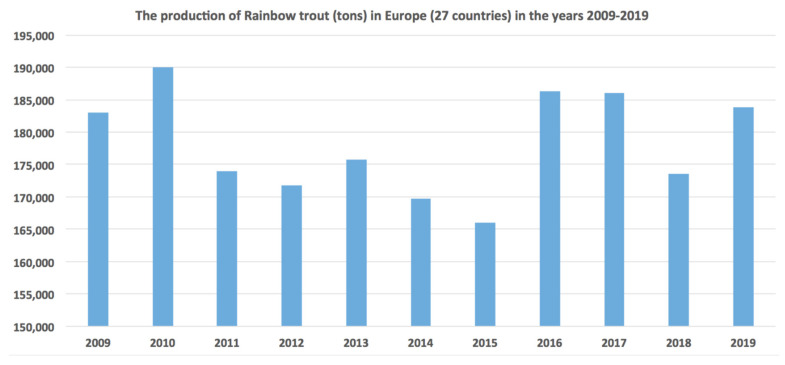
Interestingly, there has been a sharp decline in the production rates of rainbow trout in recent years in all producing countries (Figure 2), as well as a general fluctuation in the European Union, probably reflecting the physical conditions in combination with the consumers’ annual demand (Figure 3).
Figure 2.
Popular countries with significant production of rainbow trout (tons) in the years 2009–2019 (EUMOFA 2020–2021; EUROSTAT; FEAP 2014–2019).
Figure 3.
The production of rainbow trout (tons) in Europe (27 countries) in the years 2009–2019.
It should be also underlined that, in 2018, rainbow trout was the second most produced and marketable species of fish in EU countries, while in 2019, rainbow trout reached the first most produced and marketable species of fish in EU countries, overpassing Atlantic salmon [15,20], emphasizing the high value of the sector for many national economies.
2.2. Biological Aspects of Rainbow Trout
O. mykiss is native to the shores of the Pacific Ocean of North America and Asia [25,26]. However, it has been introduced and is farmed in at least 82 other countries [27,28,29,30] where it exhibits positive results from an economic and sustainability point of view, as it tolerates a wide range of environmental conditions. This species presents rapid growth rates (1000 grams in 14–16 months), leading to profitable and sustainable farming [31,32].
O. mykiss was introduced to Europe in the late 19th century and is now cultivated in almost all European countries [33]. It is usually bred mainly in semi-extensive raceway concrete tanks in most countries inside or outside the EU [25,27,32]. Some rainbow trout populations spend the mature phase of their lives in the ocean, similar to the Salmo species [34].
Wild rainbow trout is a psychrophilic species, which usually inhabits the upper reaches of rivers and cold fresh waters [35]. In addition, O. mykiss spawns in small to moderately large, well-oxygenated, shallow rivers with gravel bottoms [35]. Rainbow trouts can reach 50 pounds, but farmed trout usually weigh only a few pounds when they are harvested. Rainbow trout is a desirable fish for anglers and consumers, and it is also preferred for farming because of its high-quality flesh, high survival, ability to be fed on pellets, and tolerance to temperatures as high as 21 °C [34]. Additionally, it is capable of being stocked in high raceway/tank densities and possesses great growth rates since it reaches a marketable size in less than a year [36].
Since trouts are carnivorous, their feed should contain high levels of protein, which is a more expensive higher carbon footprint than the pellets appropriate for omnivorous fish [34]. Often, in trout aquaculture, the phenomenon of cannibalism is observed (Figure 4). Cannibalism is an aggressive behavior, which can be caused by stress induced by various population and environmental factors and may occur in two main forms: early larval and late juvenile or adult trout [37]. Farmed rainbow trout requires certain environmental factors (temperature, pH, and dissolved oxygen) for its normal growth and welfare [38,39]. With the intensity of climate change, in recent years, these environmental factors have gradually shifted, affecting the welfare and health of rainbow trout [40,41]. For this reason, researchers chose to use the rainbow trout as an experimental model against climate change based on its capability to tolerate a wide range of environments and handling procedures [40,41].
Figure 4.
Rainbow trout juvenile after cannibalism observed in an experimental farm.
Moreover, the various anthropogenic interventions in the natural environment, mirrored in the climate change crisis, may have also contributed to the reduction in the existing genetic diversity of the rainbow trout populations. Trout populations are intensively affected by water quality parameters, showing genetic and phenotypic differences when there are changes in water temperature, pH, and oxygen levels [13].
The rapid variation of environmental conditions as a result of climate change may affect the genetic diversity of rainbow trouts with an impact on conservation of the species [42]. Loss of genetic variability influences the long-term survival of populations associated with reduced fitness and an eventual higher risk of extinction. For instance, in a small population of rainbow trout, in successive generations and without gene flow, the probability of mating with closely related individuals is very high, leading to inbreeding and specifically a decrease in the population structure [43,44,45,46,47]. The assessment of reproductive values in all generations showed that multi-leaf selection (with a low rate of inbreeding) has brought an average of 7% genetic gain per generation in the development of rainbow trout of market size [48].
The reduced physical condition of the offspring of closely related individuals is fundamentally associated with the concept of heterozygocity, since the offspring of these species of couples are, necessarily, less heterozygous in their entire genome than extragamous individuals. This lower heterozygocity (i.e., low genetic diversity) makes small populations of rainbow trout more susceptible to low juvenile survival, reduced population growth, small body size, and a decreased number of rainbow trouts reaching adulthood [49]. Genetic diversity plays a crucial role in determining the probability of survival of a population from environmental changes, new pathogens, as well as the average suitability of a population for successive generations [50].
Concerning abiotic factors, water clarity is vital to the efficiency of finding food. Water should not contain harmful solids or harmful gaseous wastes produced during metabolism and respiration. A rainbow trout prefers water temperatures of 13–18 °C [34] and survives in a range of 9–20 °C [19]. Temperature plays a decisive role in a rainbow trout’s growth and metabolic rate, in the duration of egg incubation, in its resistance to various diseases, and in its ability to retain dissolved oxygen from water. Furthermore, water with low temperatures during the summer months (less than 10 °C) should not be used for the cultivation of rainbow trout since its growth rate is significantly slowed down [51].
Furthermore, rainbow trout tolerates adverse pH conditions depending on its life stages. The optimal and acceptable pH ranges of the breeding water also differ. For the development of offspring, the optimal pH range is wide, ranging between 6.5 and 8 [51]. Therefore, it can reach the optimum growth rate in well-aerated, freshwaters with a pH level of 6.7–8.2 [52]. In general, alkaline waters are more suitable for rainbow trout farming in an aquaponic system, being rich in Ca and Mg, elements necessary for the growth of fish bones and the growth of various aquatic organisms offering a better-structured ecosystem [51]. When the pH value of water is more than 9.0 and less than 5.5, then the water is unsuitable for rainbow trout breeding [51].
Water alkalinity also plays an important role in fish production. To ensure rainbow trout’s welfare, alkalinity must be at least 5 ppm. Great growth rates of rainbow trout are usually observed in waters with an alkalinity equal to 150–200 ppm. The lower alkalinity level for trout farming should be 20–50 ppm [51].
The dissolved oxygen (DO) in water ensures the respiration of various aquatic plants and animals. At high water temperatures, the DO content is lower and vice versa. [39]. Specifically, when the temperature rises, the oxygen content of the water generally decreases, while the oxygen consumption of the fish increases. Climate change leads to a reduction in the minimum oxygen consumption (5mg/l) and a decrease in the pH value of water (pH 3) [41,53,54]. This implies that the fish cannot feed effectively and, therefore, extremely high temperatures of the water will block the growth rate of the fish and cause health problems [54].
Apart from the above, optimal and acceptable oxygen concentrations in the water vary depending on the stage of the fish’s development [51]. For the breeding stage, the optimal oxygen content of the breeding water should be close to saturation. More specifically, dissolved oxygen (DO) ranges between 5 and 6 mg/L during egg incubation and in the early stages of pre-larval development [51]. In the adult stages, the acceptable limit of DO content in water may be approximately 4–5 mg/L [55]. During and after feeding, the oxygen consumption of fish increases significantly. During these periods, the demand for oxygen temporarily increases. In short, DO ranges from 5 to 8 ppm and contributes to the growth of plants, fish, and bacteria [55]. Oxygen is among the most important growth regulators in rainbow trout and fish capacity of tanks. The value of the DO in the water for the survival of the rainbow trout shall not be less than 5.5 ppm and >6 mg/L [51]. The lower value of the DO content of water for the survival of the trout is significantly influenced by the dissolved carbon dioxide content of the water. Therefore, at 16.5 °C the rainbow trout can live for 24 h in water with an oxygen content of 2 ppm, while on the contrary, it may not survive an hour or even less if the carbon dioxide content of the water reaches 15 ppm [51]. Higher carbon dioxide content indicates either contamination by organic substances or lack of oxygen [51]. Water hardness for optimal growth of rainbow trout should be between 50 ppm and 200 ppm [51]. Regarding water conductivity, it is well established that it should be between 400 and 450 / [51]. Water with conductivity values below 100 /cm is considered poor for large-scale O. mykiss production, while it is optimal for incubation [51].
Usually, wastewater exists in the form of ammonia ( NH₄⁺) and is released as solid waste (feces). In particular, the amount of unionized ammonia for the survival of the rainbow trout should be <0.001 mg/L, while the number of nitrates should be <0.8 mg/L. [51]. The free form of ammonia is toxic to rainbow trout even at a content of 0.012 ppm because it causes damage to the gills and reduces their growth. Nitrites () are very toxic to rainbow trout when they are at a content of 0.10 ppm. Nitrates () when found in large quantities in the water, create suitable conditions for the growth of aquatic plants. However, the content of nitrates in the rainbow trout farm water should not exceed the value of 100–150 ppm, since above this value, the water becomes toxic [56]. Ideally, in rainbow trout farming, the nitrate concentration should be less than 75 mg/L [56].
2.3. Climate Change as a Huge Challenge for Rainbow Trout Aquaculture
Aquaculture is a fast-growing food production sector contributing to global food security. According to FAO, aquaculture food production needs to increase in order to meet future global demand in 2050 [57,58]. Undoubtedly, aquaculture production depends on farming conditions which may be influenced by climate change. The impact of climate change on aquaculture may vary both in type (e.g., water temperature) and extent, depending on climatic zones (temperate, tropical, or Mediterranean), geographical areas (indoor, marine, or coastal aquaculture), types of aquaculture production systems, and aquatic species reared [41]. However, global warming that leads to water temperature increase, will inevitably affect farmed O. mykiss species which require lots of oxygen and high-quality water, such as the cold, pure water in the streams where they inhabit and bred in semi-intensive aquaculture systems [41].
Climate change contributes to water quality degradation. Water temperature fluctuations can affect the optimal aquatic conditions of rainbow trout farming and increase the costs demands for keeping temperature stable under farming conditions [59]. Extreme weather incidents impose risks in aquaculture production. For example, the increasing frequency and intensity of storms will complicate work, especially, in the cultivation of open water ages and will affect the choice of feeding methods and equipment [54]. Moreover, because nowadays, aquaculture has an increasing and important role in the global production of aquatic animals and food [60,61], aquaculture activities with an increased carbon footprint due to logistics, transport, input power, and feed production contribute to greenhouse gas emissions [54]. The reckless and inefficient use of resources for animal feed, water, and land are deteriorating climate change effects with serious impacts on aquaculture and the bioeconomy [54,59].
Hence, climate change affects and alters environmental factors influencing the welfare of rainbow trout (O. mykiss) and, therefore, problems in the growth rate and welfare of the fish may occur [54]. In the waters of the Southern Mediterranean (e.g., shallow, and semi-enclosed bays), during summer, temperatures can exceed 32 °C [62,63]. On the contrary, in the northern part of the Mediterranean, extreme winter temperatures ranging between 10 °C and -20 °C are often observed [64,65,66]. In recent decades, there have been extreme heat waves in Bangladesh due to climate change and global warming [67,68]. In addition, there is an extreme rise in temperature in Vietnam, Australia, and Hawaii [69,70,71]. Moreover, a 2% increase in surface water temperatures in the summer months is expected in Germany, England, and Denmark, and a prolonged increase in temperature by 6% within the period 2040–2059 [9]. During this period, increased summer rainfall is expected in the above countries, increasing the risk of flooding [72]. The impact of the climate is of the utmost importance for the above countries because they constitute the largest part of rainbow trout production and the main export markets. In addition, climate change has a significant impact on rainbow trout aquaculture in north-western European countries. Such effects are warming, deoxygenation, and acidification. Studies have shown that climate change affects non-EU states (e.g., Canada, Turkey, and Japan) by the means of reducing the growth rate of rainbow trout populations [9]. The induction of heat stress of freshwater aquaculture fish (e.g., rainbow trout) in parts of Asia [73], the Mediterranean [74,75], and Australia [76,77,78] during summer (heatwaves) and winter (extreme cold weather events), modifies the energy costs for normal homeostasis, osmotic, and ionic regulation [41,79].
For a homeostasis balance, fish must compensate for a significant amount of energy, which ultimately affects growth, immunity, and resistance to diseases [80,81,82]. Thus, essentially, climate change tends to affect aquaculture-producing rainbow trout in two ways. Firstly, it causes changes in homeostasis [80,81,82] and behavior of both wild and farmed rainbow trout [41,83,84,85]. Secondly, by facilitating the emergence of new pathogens, such as parasites, with adverse effects on fish health.
Climate Change and Physiological Homeostasis
Thermal tolerance of rainbow trout (which is sensitive both at extremely high and low temperatures) depends on the genotype, age, stages of development, physical condition, and the history of previous thermal exposure [82,86,87,88,89]. Extreme changes and frequent fluctuations in the temperature of the water affect the endocrine, antioxidant, molecular, immune, and hemato-biochemical functions of rainbow trout [41], thus, having consequences on the growth of fish, reproduction, metabolism, hemato-physiology, and immunology [89,90,91,92] (Figure 5).
Figure 5.
Schematic representation of the major climate change stressors in rainbow trout farming.
Fluctuations outside of the preferable temperature range (7–18 °C) cause a reduction in fish appetite and food utilization [51]. Eventually, fish stop feeding at very low or very high water temperatures. The feeding of rainbow trout intensifies as the water temperature rises. Thus, it will consume food intensively at 18 °C. On the other hand, the digestion process will be decreased at this temperature. Rainbow trout grows better in relation to food consumption when water temperature ranges from 13°C to 15 °C. Optimal, acceptable, and lethal water temperatures also vary depending on the fish’s developmental stage [51]. Therefore, fish mortality is caused at temperatures much lower or higher than ideal [93,94,95].
When fish are exposed to several stress factors, such as climate change effects, levels of catecholamines, corticosteroids, cortisol, glycogen, and lactic acid increase [41]. Thermal stress responses lead to the release of hormones into the blood and tissues, thus, resulting in changes in energy mobilization, ionic balance, immunological responses, cardiac activity, physical performance, growth, and reproduction [96,97,98,99]. Reproductive performance of fish, and in particular, sexual maturity and sex differentiation are affected [100,101,102,103]. Specifically, temperature changes either promote or delay gametogenesis [100,101,102,103]. Extreme high temperatures reduce gene steroidogenesis [101,104,105,106,107], inhibit sperm production, and affect sperm motility in rainbow trout [108]. Moreover, global warming causes changes in the nerve tissues (neuroendocrine function) of the rainbow trout and affects the normal functioning of the central nervous system, which is a major threat to aquaculture production [109,110,111]. Temperature affects gills plasticity through impairments of Na, K, and ATPase activities at 24 °C, therefore, resulting in changes in ionic balance [112,113,114].
In addition, extreme warming contributes to the release of heat shock proteins (HSPs) and activates the genes associated with the immune system [115,116,117,118,119,120,121,122,123,124]. Moreover, the exposure of rainbow trout to extremely high temperatures affects the number of blood cells and cell morphology, causing significant cellular damage and abnormalities [109,110,112]. Therefore, climate change is exposing rainbow trout to thermal stress, affecting fish physiology and behavior [82,88,125]. Moreover, thermal stress affects the redox status of O. mykiss. Specifically, temperature-induced oxidative stress leads to significant variability in the antioxidant response of fish, causing cellular and protein damage, which can affect the production yield of rainbow trout [117,126,127]. Chronic thermal and oxidative stress affect the metabolic rate of rainbow trout with extreme aggravation from high respiratory rhythms and oxygen consumption [92,126,128,129,130]. Specifically, a three-fold increase in rainbow trout’s metabolic rate is observed when the water temperature increases by 10 °C [131,132,133]. Under extremely cold conditions, glucose levels in the blood of the fish increase [119,134,135]. More specifically, a sharp decrease in temperature from 11°C to 1 °C during 7 days of extreme cold exposure results in an increase in the blood plasma levels of rainbow trout [136]. Moreover, cold stress decreases enzymatic activity by moderating the lactic acid content and substituting energy [41].
In addition, warming and terrestrial wastewater runoff because of climate change tend to increase the toxicity effects of pollutants on the wildlife of rainbow trout [137]. Additionally, due to global warming, rainbow trout are becoming more sensitive to metal toxicity (e.g., Cu), worsening mitochondrial function (ATP production) [138,139,140,141,142]. Fish exposure to high concentrations of such metals is an important stressor, affecting their biological activities [143,144].
The magnitude of the response of the rainbow trout to stress depends on the rate of temperature variation towards the upper and lower limits and the duration of exposure. Therefore, the increase in the temperature of the water can cause thermal stress on the fish, burdening their immune systems and leading to greater susceptibility to diseases [145,146,147,148,149].
More specifically, continuous temperature fluctuations above 15 °C increase the sensitivity of the rainbow trout to the pathogenic parasite Tetracapusloides bryosalmonae, which causes proliferative kidney disease (PKD) [9,150]. In addition, temperature rise contributes to the development of more resistant pathogens, such as the Rhabdoviridae virus that causes the disease IHN (infectious hematopoietic necrosis virus) [9] and the parasite T. Bryosalmonae. The above diseases cause mass mortality in rainbow trout aquaculture [150].
In short, climate change increases the risk of exposure of O. mykiss to infections due to thermal stress. It has been reported that, at 21 °C, pathological changes in liver tissue and inflammation resulting from thermal stress have been observed [151]. Additionally, a significant decrease in immune function in rainbow trout and serious tissue injuries at temperatures above 24 °C was observed [152,153]. In addition, findings support that thermal stress leads to increased levels of cortisol secretion from fish kidneys [41].
3. The Potential of Rainbow Trout Farming in Aquaponics
Nowadays, rapidly elevated levels of carbon dioxide (CO₂) have threatened rainbow trout aquaculture and global food security. More specifically, increased CO₂ levels can negatively impact the cardiovascular, metabolic, and homeostatic balance of O. mykiss [154]. To increase the global aquaculture production, despite climate change, an expansion of sustainable aquaculture systems (e.g., aquaponic systems, biofloc systems, etc.) is needed in the context of a circular economy. Aquaponics, in combination with selective breeding and thermal acclimation, are promising management strategies that may contribute to the development of a new form of rainbow trout farming [47,48,155,156,157]. Nevertheless, climate change remains a difficult obstacle for examining the genetic diversity of rainbow trout populations, a species sensitive to climate change.
3.1. Aquaponics as a Novel Technology
So far, aquaponics research is limited to studies with small populations of fish individuals, mainly focusing on plant features or abiotic and economic parameters. Therefore, it cannot be accurately evaluated whether fish raised in aquaponics systems can overcome the challenges of climate change. However, it can be estimated that various species of fish will be greatly or slightly affected by the effects of climate change in the near future. Specifically, the mainly affected species are freshwater fish of the Salmonidae family, which include rainbow trout (Oncorhynchus mykiss) and salmon (Salmo salar) [158]. On the other hand, fish of the Cichlidae family, such as tilapia (Oreochromis spp.), carps, and catfish, are more resistant to climate change [158]. Additionally, tropical ornamental fish, such as Paracheirodon axelrodi, are more vulnerable to climate change than temperate fish. Tropical ornamental species have a limited acclimatization capacity, as they thrive in a limited thermal range [158].
Morocco’s inland aquacultures are a prime example of the perception of the effects of climate change in rearing major families with good economic potential [159], such as, for example, some species of trout, i.e., Salmo akairos, Salmo multipunctata, and Salmo pallaryi [159]. The blue barbell Pterocapoeta maroccana (Günther, 1902) is a native species of Cyprinidae family, only found in Morocco, included in the IUCN category of endangered species since 2006. Pterocapoeta maroccana is the only representative of the genus, threatened by climate change, that species needs to be reared in order to repopulate its habitat, as a freshwater species, with aquaponics and aquaculture potential [159].
As far as salmonoids are concerned, the prevalence of diseases in salmon and trout aquaculture increases as the temperature of the water in cold-water aquaculture rises. Rainbow trout (Oncorhynchus mykiss) has been deliberately developed to resist the disease of cold water caused by the bacterium Flavobacterium psychrophilum, using genetic improvement [160]. For this reason, it is worth estimating rainbow trout aquaculture in aquaponics systems.
Due to the fact that global food demand will increase by 70–100% by 2050 [161,162] and the key role of the agricultural sector in food security [163], one of the greatest issues of the 21st century is to find a way to produce more food using fewer resources and minimizing environmental impacts [164]. Among the systems currently used by the agricultural sector, aquaculture seems to be the most suitable and convenient for counteracting deficiencies in food production [54]. Modern freshwater aquaculture relies mainly on closed aquaculture systems (RAS), which reuse the same volume of water [165]. In these systems, the rate of water reuse ranges between 80 and 99%, therefore reducing water requirements and the environmental impact of aquaculture [166,167]. The unification of closed aquaculture systems (RAS) and hydroponics—known as aquaponics—improves sustainability and ensures food sufficiency, providing various significant economic and social benefits [52,168]. These innovative sustainable aquaculture systems, already implemented, are characterized as integrated multi-trophic aquaculture (IMTA) or polycultures [169,170].
Specifically, an aquaponic system can be defined as a production system that combines the hydroponic co-cultivation of plants (vegetables, flowers, or herbs) with the rearing of fish in closed greenhouse-type cultivation systems [171]. It is carried out in a closed-circuit aquaculture (recycled farming systems) properly modified, without the addition of chemicals, and requires a minimum volume of water exchange (about 10%) [172]. The addition of water is very low and is only required due to evaporation or operational work [172]. Essentially, the aquaponic system provides by-products, including waste from fish, as inputs (organic fertilizers) to the plants. Aquaponics is excellently applied in collaboration with RAS [14,173], where water is constantly circulating in fish tanks with the help of pipes to the corresponding filters to be filtered (conversion of ammonia and nitrites into nitrates), ending up in plants and then returning cleanly back to fish tanks [174]. Consequently, aquaponic systems maintain better control of natural conditions [175] without requiring large volumes of water, fertile land use [52,176] and increased operating costs due to the non-use of fertilizers [177].
Stocking density and fish species are key factors in aquaponics systems that can significantly affect plant yield and quality, aspects that both farmers and consumers need to be aware of [178]. According to several studies, different plants and fish will have different optimal ratios and largely depend on factors, such as fish stocking density, aquaponics type, plant species, planting density, hydroponic or soil crop production system type, water flow rate, and external factors [179]. Therefore, the density of plants that will be grown in a system of aquaponics depends on the species and the density of the fish [180].
Research by Addler et al. (2000) examined the cultivation of lettuce and basil in hydroponic cultivation in NFT at densities of approximately 6 plants/ using the waste of rainbow trout (Oncorhynchus mykiss). Approximately 109 of trout effluent is produced daily. Basil (Ocimum basilicum L.) and lettuce (Lactuca sativa L.) removed p to 0.003 mg/ and < 0.001 mg/, respectively, from an influent concentration > 0.5 mg/ [181].
In addition, research by McMurty et al. (1990) used aquaponic cultivation in sand bed bush beans (Phaseolus vulgaris L. cv. Blue Lake 274), cucumbers (Cucumis sativus L. cv Burpee hybrid II), and tomatoes (Lycopersicon esculentum Mill. Cv. Champion) in various densities with parallel aquaculture of blue tilapia (Sarotherodon aureus L.). Tilapia was bred at a density of 1.68 kg, while the crop densities of bush beans were 12,5 plants/, 16.7 plants/ and 20 plants/. For cucumbers, the densities were 1.8 plants/, 2.6 plants/, 4 plants/ and for tomato it was 6.7 plants/ [182].
Another research by McMurty et al. (1997) was done on floating hydroponic/aquaponic systems with tomato cultivation (solanum lycopersicum) and cucumber (Cucumis sativus) and parallel aquaculture of two species of tilapia (Oreochromis mossambicus and Oreochromis niloticus). They tested four different plant densities of 3.83 plants/, 4 plants/, 3.94 plants/, and 4.022 plants/, based on the biofilter volume tank to fish tank ratio. Plant growth was adequate. Certain nutrients (N, S, K, P, Ca, Mg, and B) fell below sufficiency standards but were above deficiency levels [183].
Aquaponics can be more productive and sustainable under specific circumstances, especially when arable land and water are limited [184]. Thus, aquaponics is more suitable in high altitudes, which are characterized by little water supply and soils with organic matter deficiency. Significant amounts of food can be produced in locations where soil-based agriculture is difficult or impossible [185]. Deserts and arid areas, sandy islands, and urban gardens are the most suitable locations because of reduced water supply [184]. It offers supportive and collaborative methods of producing vegetables and fish and can grow significant amounts of food in locations and situations where soil-based agriculture is difficult or impossible [185].
From an environmental point of view, aquaponics represents a novel technology that improves production efficiency while mitigating environmental impacts (pollution load, including greenhouse gas emissions), diversifying fish production, animal welfare in aquaculture systems, climate change studies, soil depletion, technologies that mitigate the emergence of animal diseases or parasites, and reducing the use of antibiotics, chemical fertilizers, new feed ingredients, and carbon footprint reductions. It prevents the release of aquaculture waste that pollutes water bodies (eutrophication) [169,170,186], allowing greater control of water and production, which makes food safer against possible residues [187]. Therefore, aquaponics is characterized as an alternative method for food production to agricultural practice and sustainability [166]. It applies techniques and procedures aimed at producing biosecurity food, trying to meet the nutritional needs of the population while significantly minimizing the environmental impact by reducing the environmental load [186,187].
Nevertheless, aquaponic systems are still at an early stage of development, and although many new aquaponic companies are starting up in Europe, only a few of them are currently reaching an economically viable minimum production size [188]. The EU countries that have already developed aquaponic farms (commercial or not) are France, Hungary, Belgium, Germany, Ireland, Italy, Slovenia, Spain, the Netherlands, the United Kingdom, the Czech Republic, Denmark, Finland, Romania, and Sweden, with other ones being at the stage of development [188]. There is a marketing opportunity, as consumers are willing to pay more for antibiotic-free products, pesticides, and herbicides and purchase them from local producers. Commercial aquaculture farms could boost their profit by taking advantage of these marketing aspects (quality and local food) [188]. The controlled supply of nutrients, water, and environmental modifications (due to climate change) under greenhouse conditions is one major reason why aquaponics is going to be very successful [189]. In Greece, the technology of aquaponics is at an early stage and is limited to small domestic and research efforts [185,190,191].
The most popular herbs and spices with beneficial properties for culinary purposes that are intended to be grown in aquaponic systems are basil, coriander, chives, parsley, slippery, and mint [192].
However, the most widespread cultivated herbs in aquaponics systems, in which studies are carried out are basil, thyme, coriander, spearmint, and sage [193]. They are the most prevalent in aquaponics systems owing to their fast harvest rate, higher application of these plants in various sectors (cosmetics, pharmaceutical, and food industries), and their significant economic value worldwide [193].
According to several studies, thyme (Thymus vulgaris) and basil (Ocimum basilicum var. Aristotle) are among the most appreciated aromatic plants by consumers as culinary herbs, but also with multiple uses in the pharmaceutical, cosmetic, or food industries for meat bio-preservation [194,195,196,197].
In the research of Cretu et al. [193], the developmental performance of basil and thyme in hydroponic systems and in an aquaponics system with parallel aquaculture of goldfish (Carassius auratus) were compared. Based on the results, it was evaluated that the production of thyme and basil was more profitable and favorable in the aquaponics system, since at the same time a profitable production of fish was achieved [193].
In another research by Abdel-Rahim et al. [198], who cultivated the aromatic and medicinal plants rosemary (Rosmarinus officinalis L.), mint (Mentha spicata L.), marjoram (Origanum majorana L.), and thyme (Thymus vulgaris) with parallel tilapia aquaculture (Oreochromis niloticus), remarkable results of plant yield and fish growth were observed [198], with the only drawback being the low consumer demand for tilapia in comparison to other fish species.
Another study is mentioned, where spearmint (Mentha spicata) was cultivated in an aquaponic system with parallel aquaculture of African catfish (Clarias gariepinus), giving very good yield results of mint, which gave it great commercial importance [199].
To the best of our knowledge, no study has been conducted to report the contribution of rainbow trout to aquaponic cultivation of aromatic and medicinal plants.
3.2. Description and Operation of an Aquaponic System
Due to the fact that aquaponics depends on the nutrient cycle [200], the metabolism and excretory products of fish increase water nutrients (excrement and food debris), which are thereafter used by plants at the right proportions for their best possible growth [52,201,202]. Fish wastewater is distributed at plants’ roots, providing the necessary nutrients for plant growth [52]. Then, the plants act as a natural filter, and therefore, the water can be reused by the fish. This creates a small-scale symbiotic ecosystem, where fish and plants can grow [202]. Fish waste (liquids and solids) is filtered with the support of mechanical and biological filters to keep the water clean [52,201,202]. If water stops circulating through the system, the most immediate result will be a reduction in DO oxygen and the accumulation of nutrients in the fish tank [52]. The water in the plant tanks will become anoxic, and the system will collapse [52]. Diseases, mortality, and nutritional deficiencies are the result of an unbalanced system [55]. The proper functioning of an aquaponic system requires the regular control of the system’s balance, through the control of nitrogen levels at the points of entry and exit of water from the hydroponic plant cultivation tank and the aquaculture pool [187,203].
Specifically, the basic function of an aquaponic system is based on the metabolic products of fish as well as the residues of their food, which through the biochemical process of nitrification are oxidized into non-toxic derivatives used/consumed by plants [204,205]. For this reason, aquaponics is also proposed as an alternative way of wastewater management in a closed agricultural fish system with fully controlled conditions [206]. More specifically, ammonia is released from fish as a metabolic product through the gills at a rate of 80% and becomes toxic in high concentrations of fish [164,202,207]. Toxic metabolites (ammonia and nitrite ions) are converted into nitrates, as they are not harmful to fish and are digested at a rate of 97% by plants to meet their requirements for nutrients [55]. Water containing nitrates enters the hydration system and hydroponic growing plants, which are placed on a substrate with water [52,208]. The water is enhanced by nitrates derived from fish waste [185,202,208]. Their roots bind nitrates as well as phosphate ions so that nutrient-free water is reused and is not a restrictive growth factor for fish [206,209]. Thus, the roots of plants are suspended through the substrate in water full of nutrients [210]. Therefore, the water used by a hydroponic system is enriched with nutrients (nitrates, phosphate ions, etc.), and through its constant recirculation, it is absorbed by the roots of plants, acting as a natural fertilizer [188]. According to Lennard (2006), this process consists of a practical technique used to denitrify water in fish tanks in a closed system of recycled fish farming (RAS) [211].
As such, aquaculture waste is diverted through plants and not released into the environment, while at the same time, nutrients for plants are provided in a sustainable, cost-effective, and non-chemical way [168,201,210,212]. In addition, aquaponics has shown that plant and fish production are comparable to those of hydroponics and closed-circuit aquaculture systems [166]. In an aquaponic system, the plant growth is comparable to conventional hydroponics despite the small concentration of nutrients existing in aquaculture water [212,213,214]. Additionally, the production can be higher compared to the production from the soil [215] attributed to increased concentrations of in the air and changes occurring around the roots [213,214,216]. Moreover, the amount of plant biomass produced, in relation to the volume of water used, is 5–10 times greater compared to classical agriculture [217].
The mezzanine method using substrate (MBT—media bed technique) is the most common method applied to small-scale hydration systems [200], while the resources of the filter provide support to plants but also act as a mechanical and biological filter [52]. The mechanical filter is responsible for removing the solid waste of fish (feces) and food remains [52]. It is considered necessary, since the accumulation of waste releases harmful gases, clogs the pipes, changes the flow of water, and therefore is involved in the collapse of the system [52]. On the other hand, the biological filter oxidizes ammonia and nitrites to nitrates through certain Nitrosomonas sp. and Nitrobacter sp. bacteria [52,207]. These bacteria in an aquaponic system are used to biologically regulate the system, creating the right environment for fish and plants [52]. The system is constantly “fed” ammonia from fish waste that binds to bacteria for the growth and regulation of the biological filter [52,218]. Conversely, the nutrient film technique (NFT) and the deep-water culture (DWC) aquaponic method can be used for commercial aquaponic systems, but both types require a mechanical and a biological filter [200]. The water runoff, assessed by gravity, is concentrated in the filter where the submersible pump is located [200]. Organisms that coexist in an aquaponic system have different requirements, and therefore caution is needed to achieve a balance in the farming system [55]. Fish, plants, and bacteria are characterized by different needs and optimal levels of environmental factors [55,183]. On the other hand, the successful growth of the plant depends on the daily rate of ammonia production and varies depending on the species and nutritional requirements of the plants that will be placed in the cultivation system [186]. The tank should be composed of low-cost materials that ensure the lowest exposure of the fish to potential risks of toxicity (i.e., fiberglass, polyethylene, and stainless steel) [219]. Round tanks with a conical bottom are those recommended due to better water circulation and transportation of solid waste to the center of the tank [52,219].
3.3. Perspectives and Requirements of Rainbow Trout Farming in an Aquaponic System
Various fish species exhibit excellent performance, welfare, and growth rates in aquaponic systems [34]. However, the rainbow trout, although it can survive in the cold temperatures required in aquaponic production systems [51], has not been commercially reared so far in such systems. It should be noted that, keeping in mind the abiotic factors generally needed for trout welfare (Table 1), this fish species may represent an optimum candidate for an aquaponic system [34].
Table 1.
| Requirements | Values |
|---|---|
| Dissolved oxygen (DO) | >5.0–5.5 ppm |
| Water quality | Good flow, clean freshwater |
| pH | 6.7–8.2 |
| Growth rate | Rapid |
| Marketable size | 350 gr up to one kilo in less than a year |
| Protein content | High (50%) |
| Circular tank containing | 300 gallons of water |
| Density | 7.26–20 kg/m3 |
| Temperature | 9–21 °C |
| Nitrates | <75 mg/L |
Aquaponics can be integrated into raceway rainbow trout cultivation systems, enabling additional revenues (growing vegetables, insects) as well as improvements in wastewater quality [220]. The integration of the two aquaculture systems makes them largely non-consumer and non-polluting users of the water resources [221]. According to already performed experiments, rainbow trout in aquaponics is bred in round plastic or stainless-steel tanks [36]. Additionally, tanks should be covered with a net to prevent fish escape and exogenous factors’ entrance (i.e., leaves) and for protection from various predators [52] (Figure 6).
Figure 6.
Round plastic tanks covered by a fish protection grid in an experimental research of rainbow trout aquaculture in an aquaponic system in Greece.
O. mykiss is one of the most well-adapted domesticated fish, cultivated for centuries and the first to be tested in a closed farming system [36,222]. It is a carnivorous fish characterized by high protein demands, consuming approximately 40–50% of their diet. High protein levels release significant amounts of ammonia, nitrates, and biomass [36,217]. Thus, freshwater fish species, such as rainbow trout, are ideal for integrating into a hydroponic crop due to their viability, ease of nutrition, and their effective conversion of food into biomass in combination with their sufficient market value [209].
It is noteworthy to mention that, as the water temperature increases, fish metabolic rates, enzymatic activity, and blood circulation increase, leading to higher oxygen consumption, while at the same time, at the bottom of the tank, more feces and unused food accumulate [55]. Feces and unused food decomposition require oxygen consumption, resulting in a decrease in DO in the water [55]. Remarkably, in cases of high fish stocking density and high temperature of the water, the DO content may significantly reduce, threatening the survival of the rainbow trout [222,224].
Due to its high growth rate and metabolism, rainbow trout has an increased percentage of waste in plants, which leads to a rapid rate of plant growth [223]. The water temperature range for trout aquaculture is optimal for plants that will thrive in cool temperatures, such as leafy greens, e.g., lettuce, beets, carrots, spinach, cabbage, peas, chards, and more [34,210,225]. Secondly, plants with higher nutrient demands, such as tomatoes, cucumbers, peppers, eggplants, broccoli, as well as fruits, such as strawberries and herbs, such as basil, represent also a good option for combined co-cultivation with rainbow trout [52]. For instance, Velichkova et al. (2019) found increased lettuce production (Lactusa sativa) in a small-scale rainbow trout NFT and MBT aquaponic system [223].
From an environmental point of view and according to Bordignon et al., (2022), high density stocks of fish tend to determine a lower environmental impact per kg for an increase in fish production, as it regards global warming, cumulative energy demand, and freshwater ecotoxicity [226]. Replacing conventional energy sources with renewable sources, such as solar energy from photovoltaic panels, can significantly reduce the environmental impact resulting from electricity use [226]. Only 3% of the water of traditional raceway aquaculture, which is commonly applied in rainbow trout farming, is used in aquaponics, resulting in significantly reduced demands for water [222]. Thus, according to Addler et al. (2000), the integration of RAS with hydroponic plant cultivation can bring profit by reducing water use and significantly reducing the discharge of unwanted nutrients into the environment [222]. Nevertheless, the effects of water depletion should be carefully assessed from the moment of extraction of the raw materials to the gate of the farm (from production to consumption) [226]. For example, an incubator can significantly increase the water consumption required to produce fish [226].
The balance between the rate of absorption of plants and the amount of food administered to fish in terms of nitrate and potassium budgets is significant [227]. Fish have an upward tendency to retain phosphorus, while plants have different rates of nutrient absorption, more stable in the case of calcium, because they have different periods of evolution in their lifetimes [227]. In addition, conventional treatment alternatives for nitrogen and phosphorus in wastewater, whether using chemical precipitation, physical removals, or land application technologies, represent significant additional costs for the owner of an aquaculture farm [222].
Additionally, for an economically and environmentally sustainable aquaponic system, it is recommended to replace fish feed with a high carbon footprint with alternative feeds while maintaining entirely a green circular economy. Such feeds may be insect meals or extracts from certain plant species. Bordignon et al. (2022) found that the breeding of fish of high commercial value in a system of aquaponics combined with the substitution of their diet with insect meal (Hermetia illucens) can contribute to enhancing the competitiveness and attractiveness of aquaponics products, provided that the cost of insect meals is reduced [228]. Moreover, the extract from plants, Achillea millefolium and Acorus calamus, used as a supplement in the diet of a rainbow trout enhances its growth by improving the food conversion rate (FCR) and its physiological state without affecting the fish flesh quality and productivity of the aquaponic system with water recirculation [227,228].
Based on the research of Petrea S. M. et al. (2013), it was found that the growth of plants in a system of aquaponics is similar to that of conventional, traditional cultivation if appropriate plant densities are used [229]. However, water temperature has shown a significant effect on the growth of both plants and fish in conventional farming systems and in aquaponics. The temperature at 21 °C has been proved to be more suitable for growing in aquaponics, as the growth of fish and plants was higher at this temperature [230]. In contrast, the temperature at 11 °C had a negative effect on the uptake of nutrients by plants, the metabolism of fish, the effectiveness of the biological filter, and the microbial parameters of the system [230]. Based on the same research, it was found that the selection of fish feed and the optimization of nutrients must meet the requirements of plants at low water temperatures in order to produce high-quality plants and fish. With only 39% of the total fixed costs and 63% of the total annual variable costs, the aquaponics system generates 67% of the annual revenue for the combined multi-trophic system [220]. At the same time, revenues from the greenhouse also helped to compensate for the operating losses of the first year of the fish system. The economics of the fish system can be improved by increasing the scale of production [221] and by using fish species with great commercial value and high market prices, e.g., O. mykiss. However, the added technical complexity, marketing work, production and market risks, fish and plant diseases, mechanical failures, regulatory changes, and market fluctuations can significantly reduce projected returns [221].
To sum up, the treatment of fishing wastewater with the parallel use of plant hydroponics represents a potentially profitable additional business for fish farmers [221]. Aquaponics is becoming a cost-effective and environmentally friendly system that will encourage classical aquaculture facilities to adapt to the environmental management of their wastewater [231,232]. The breeding of rainbow trout in an aquaponic system with water recirculation is a promising alternative to common flow systems, especially in view of minimizing the depletion of water resources [226].
3.4. Comparative Yields of Rainbow Trout with Other Fish Reared in Aquaponics Systems
Among freshwater fish, rainbow trout occupies one of the highest market values, possessing the highest commercial interest in the world. Nevertheless, aquaponics using this fish is performed in a small percentage due to the special climatic conditions it requires for its welfare and proper development.
In 2014, a study in 44 countries around the world found that the most common fish species in aquaponics were tilapia (55%), followed by ornamental fish (koi, goldfish, and tropical fish) (48%) [233]. Similarly, Love et al. reported tilapia (69%), ornamental fish (43%), catfish (25%), and other aquatic animals, including perch (16%), bluegill (15%), trout (10%), and bass (7%) [234]. Villarroel et al. (2016) reported that tilapia (27%), catfish (10%), ornamental fish (8%), trout (7%), bass (4%), and perch (2%) are the most commonly farmed species in Europe [235].
Several ‘easy-to-produce’ fish species, such as blue tilapia (Oreochromis aureus), Nile tilapia (Oreochromis niloticus), common carp (Cyprinus carpio), tench (Tinca tinca), and African catfish (Clarias gariepinus) have been successfully reared in aquaponic farming [187,236]. Most of these inferences are focused on warm-water fish species, whereas, to our knowledge, no data are available on cold-water species, such as rainbow trout (Oncorhynchus mykiss).
One of the most commonly used species in aquaponics is tilapia. This is due to its omnivorous nature, rapid reproduction, and rapid growth [216]. Unlike rainbow trout, this species of fish, together with catfish and cyprinoids, is very resistant and tolerant to a wide range of water parameters, such as the wide temperature range (15–30 °C) and the concentration of free ammonia , which ranges from 0.2–3.0 mg/L [192].
However, it should not be omitted that the choice of fish species depends upon its economic value, the market demand, and the geographic localization of the production system [210].
Therefore, in the development of aquaponics, the demand of the consumer public plays an important role. Rainbow trout is a globally recognized edible fish in gastronomy, with a high protein content, nutritional value, and good flesh quality compared to tilapia and carp.
Based on the results of Birollo et al. [36], rainbow trout can be successfully grown in a low-tech water-ponic system up to a final density of approximately 17 kg/ without adverse effects on flesh growth and quality. At the same time, the increased yield and marketability of lettuce is confirmed [36]. More specifically, on average, the carcass yield was 89.0% of the slaughter weight. Meanwhile, the weight and yield of the fillet were 163 g and 49.1% of the slaughter weight, respectively. The marketable lettuce yield was 2.82 ± 0.64 kg/ at the end of the first cycle and 1.89 ± 0.18 kg/ at the end of the second cycle [36].
In aquaponics, Palm et al. reported a specific growth rate (SGR) of 0.71% for the Nile tilapia (Oreochromis niloticus) (174 g initial weight, 5.62 kg/ initial stocking density) and 0.65% for the African catfish (Clarias gariepinus) (initial weight 480 g, initial rearing density of 6.72 kg/), which are almost similar to those observed in the Maucieri study (0.74% on average in systems, however, both species used in the aforementioned study showed a lower FCR (1.02 on average) compared to the values in the Maucieri tilapia survey (1.71 on average) [237]. Aquaponics at 1.4 kg/ of baseline stocking density for a 45-d test showed an SGR similar to those observed in the tilapia study [238] (0.82% on average), but dissimilar to those observed in the FCR (2.31 on average) [239].
The mortality rate was very low (3.3% on average), as previously observed for Cyprinus carpio bred in the same aquaponic at similar densities (baseline values: 2.5 and 4.6 kg/, final values: 6.9 and 11.5 kg/) [238]. To our knowledge, only one study is available on rainbow trout bred in aquaponic systems focused on lettuce micro-biological analysis [240], while commercial aquaponics systems successfully operate in Colombia, Chile, and Canada; other authors found that the mortality rate increased simultaneously when the initial stocking density increased, both in Cyprinus carpio var. Koi (from 2.1 to 2.8 kg/) [241] and in tilapia Oreochromis niloticus (from 0.5 to 1.6 kg/) [242] grown in aquaponics. However, the reaction to different stocking densities can vary dramatically between fish species [243] and is greatly influenced by the biological requirements of fish and water quality.
The carp breeding density influenced the performance of the tested aquaponic system, with better results, in terms of water quality and vegetable production, achieved with an initial rearing density of 2.5 kg/ [238]. At the end of the trial, fish weighed 446 g on average, reaching a specific growth rate (SGR) of 0.74% . The feed conversion ratio for the whole period was 1.71, and the total biomass produced was 5.66 kg/, on average [238].
Although possible fish-plant combinations are quite high, the main fish and plant crops used are tilapia and herbs. Growers producing more than 1000 kg of fish developed tilapia in addition to other species and always used agglomerated feed. This coincides with the idea of producing fish in greenhouses, and therefore with warmer water [235].
4. Conclusions
Climate change may affect the production of the rainbow trout aquaculture sector in the near future, whereas it should be noted that a decline has already been observed in several countries. Climate change highlights the need for innovative food production within a sustainable corridor. Aquaponics, combining the technology of recirculation aquaculture systems (RAS) and hydroponics in a closed-loop network, may contribute to addressing these problems. In particular, rainbow trout farming, usually carried out within flow-through raceways, is vulnerable to increased temperature and oxygen availability. On the other hand, rainbow trout is a product of high quality and market value, characterized by high growth rates, thus, presenting a very promising candidate for rearing in aquaponics. The intensification of aquaponic systems makes it possible to control the production system, fish, and environmental factors using intelligent, innovative technologies. Aquaponics achieves automatic and digital monitoring of aquaculture systems by controlling and mitigating abiotic factors. Although rainbow trout farming has not been commercially developed on a large scale yet, the research conducted so far indicates that it may represent a promising fish candidate with various potential benefits to be reared in aquaponics.
Author Contributions
Conceptualization, C.V. and I.A.G.; software, M.V.A. and I.C.; validation, K.F., A.L. and D.K.P.; formal analysis, D.K.P. and G.L.; investigation, I.G.; resources, G.K.N.; data curation, G.K.N.; writing—original draft preparation, C.V. and M.V.A.; writing—review and editing, A.L., K.F. and I.A.G.; visualization, M.R.; supervision, G.K.N. and I.A.G.; project administration, G.K.N.; funding acquisition, G.K.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-00756).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Georgoulis I., Feidantsis K., Kouvas D., Lattos A., Delis G.A., Theodoridis A., Michaelidis B., Giantsis I.A. The effect of seawater physical parameters in bivalve farming: Could systematic monitoring and early warning prevent negative impacts: A review focused on Vistonikos Gulf, North Aegean Sea. Int. J. Agric. Resour. Gov. Ecol. 2022;18:22–37. doi: 10.1504/IJARGE.2022.124639. [DOI] [Google Scholar]
- 2.Kalaitzidou M.P., Alvanou M.V., Papageorgiou K.V., Lattos A., Sofia M., Kritas S.K., Petridou E., Giantsis I.A. Pollution Indicators and HAB-Associated Halophilic Bacteria Alongside Harmful Cyanobacteria in the Largest Mussel Cultivation Area in Greece. Int. J. Environ. Res. Public Health. 2022;19:5285. doi: 10.3390/ijerph19095285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feidantsis K., Giantsis I.A., Vratsistas A., Makri S., Pappa A.Z., Drosopoulou E., Anestis A., Mavridou E., Exadactylos A., Vafidis D., et al. Correlation between intermediary metabolism, Hsp gene expression, and oxidative stress-related proteins in long-term thermal-stressed Mytilus galloprovincialis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020;319:264–281. doi: 10.1152/ajpregu.00066.2020. [DOI] [PubMed] [Google Scholar]
- 4.Georgoulis I., Feidantsis K., Giantsis I.A., Kakale A., Bock C., Pörtner H.O., Sokolova I.M., Michaelidis B. Heat hardening enhances mitochondrial potential for respiration and oxidative defence capacity in the mantle of thermally stressed Mytilus galloprovincialis. Sci. Rep. 2021;11:17098. doi: 10.1038/s41598-021-96617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochrane K., De Young C., Soto D., Bahri T., editors. Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge. FAO; Rome, Italy: 2009. 212p FAO Fisheries and Aquaculture Technical Paper. No. 530. [Google Scholar]
- 6.Gitz V., Meybeck A., Lipper L., Young C.D., Braatz S. Climate Change and Food Security: Risks and Responses. Volume 110. FAO; Rome, Italy: 2016. pp. 2–4. Food and Agriculture Organization of the United Nations (FAO) Report. [Google Scholar]
- 7.Pirozzi I., Southgate P.C., Lucas J.S. Aquaculture Systems Design. In: Lucas J.S., Southgate P.C., Pirozzi I., Tucker S.C., editors. Aquaculture: Farming Aquatic Animals and Plants. 3rd ed. John Wiley & Sons Ltd; Hoboken, NJ, USA: 2019. pp. 41–62. [Google Scholar]
- 8.Abdulhai H., Ghomi M.K.S. Rainbow trout culture in Iran: Development and concerns. Aquac Asia. 2005;10:34–38. [Google Scholar]
- 9.Climate change and European aquatic resources (CERES) European Union’s Horizon 2020 Research and Innovation Program. CERES; Hamburg, Germany: 2019. Case study: Rainbow trout in north-west Europe. No 678193. [Google Scholar]
- 10.Wenger S.J., Isaak D.J., Luce C.H., Neville H.M., Fausch K.D., Dunham J.B., Dauwalter D.C., Young M.K., Elsner M.M., Rieman B.E., et al. Flow regime, temperature, and biotic interactions drive differential declines of trout species under climate change. Proc. Natl. Acad. Sci. USA. 2011;108:14175–14180. doi: 10.1073/pnas.1103097108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David L.H., Pinho S.M., Agostinho F., Costa J.I., Portella M.C., Keesman K.J., Garcia F. Sustainability of urban aquaponics farms: An emergy point of view. J. Clean. Prod. 2022;333:129896. doi: 10.1016/j.jclepro.2021.129896. [DOI] [Google Scholar]
- 12.Farrant D.N., Frank K.L., Larsen A.E. Reuse and recycle: Integrating aquaculture and agricultural systems to increase production and reduce nutrient pollution. Sci. Total Environ. 2021;785:146859. doi: 10.1016/j.scitotenv.2021.146859. [DOI] [PubMed] [Google Scholar]
- 13.Martsikalis P., Gkafas G.A., Apostolidis A.P., Exadactylos A. Genetic Structure Profile of Rainbow Trout (Oncorhynchus mykiss) Farmed Strains in Greece. Turk. J. Fish. Aquat. Sci. 2014;14:749–757. doi: 10.4194/1303-2712-v14_3_17. [DOI] [Google Scholar]
- 14.European Market Observatory for Fisheries and Aquaculture Products (EUMOFA) Freshwater Aquaculture in Europe. Publications Office of the European Union; Luxembourg: 2021. pp. 1–83. [Google Scholar]
- 15.European Market Observatory for Fisheries and Aquaculture Products (EUMOFA) Portion Trout in the Europ. Publications Office of the European Union; Luxembourg: 2021. pp. 1–62. [Google Scholar]
- 16.Bozoglu M., Ceyhan V., Cinemre A.H., Demiryürek K., Kilic O. Evaluation of different trout farming systems and some policy issues in the Black Sea region, Turkey. J. Appl. Sci. 2006;6:2882–2888. doi: 10.3923/jas.2006.2882.2888. [DOI] [Google Scholar]
- 17.D’Orbcastel E.R., Blancheton J.P., Boujard T., Aubin J., Moutounet Y., Przybyla C., Belaud A. Comparison of two methods for evaluating waste of a flow through trout farm. J. Aquac. 2008;274:72–79. doi: 10.1016/j.aquaculture.2007.10.053. [DOI] [Google Scholar]
- 18.Cowx I.G., Nunn A.D., Harvey J.P. Quantitative sampling of 0-group fish populations in large lowland rivers: Point abundance sampling by electric fishing versus micromesh seine netting. Arch. Für Hydrobiol. 2001;151:369–382. doi: 10.1127/archiv-hydrobiol/151/2001/369. [DOI] [Google Scholar]
- 19.FAO . In: Oncorhynchus Mykiss: Cultured Aquatic Species Information Programme. Cowx I.G., editor. Fisheries and Aquaculture Division; Rome, Italy: 2022. [Google Scholar]
- 20.European Market Observatory for Fisheries and Aquaculture Products (EUMOFA) The European Fish Market. Publications Office of the European Union; Luxembourg: 2021. pp. 1–111. [Google Scholar]
- 21.European Aquaculture Production Report (FEAP) Fish Farming Production in Europe, 2014–2019. FEAP; Brussels, Belgium: 2020. pp. 2–48. [Google Scholar]
- 22.Eurostat Database. [(accessed on 13 August 2022)]. Available online: https://ec.europa.eu/eurostat/data/database.
- 23.Cai A., Yan J.N.X., Leung P.S. Benchmarking Species Diversification in Global Aquaculture. Volume 605 Food & Agriculture Organization; Rome, Italy: 2022. [Google Scholar]
- 24.Apc advanced planning—Consulting . Report on the Marketing of Aquaculture Species Produced in GREECE, Part A. European Union; Athens, Greece: 2016. pp. 1–91. [Google Scholar]
- 25.Crawford S.S., Muir A.M. Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Fish Biol. Fish. 2008;18:313–344. doi: 10.1007/s11160-007-9079-1. [DOI] [Google Scholar]
- 26.Stanković D., Crivelli A.J., Snoj A. Rainbow Trout in Europe: Introduction, Naturalization, and Impacts. Rev. Fish. Sci. Aquac. 2015;23:39–71. doi: 10.1080/23308249.2015.1024825. [DOI] [Google Scholar]
- 27.Framian B.V. Review of the EU aquaculture sector and results of costs and earnings survey. Final Rep. 2009;6:69. [Google Scholar]
- 28.Economidis P.S., Dimitriou E., Pagoni R., Michaloudi E., Natsis L. Introduced and translocated fish species in the inland waters of Greece. Fish. Manag. Ecol. 2000;7:239–250. doi: 10.1046/j.1365-2400.2000.00197.x. [DOI] [Google Scholar]
- 29.Copp G.H., Garthwaite R., Gozlan R.E. Risk identification and assessment of non-native freshwater fishes: Concepts and perspectives on protocols for the UK. J. Appl. Ichthyol. 2005;21:371–373. doi: 10.1111/j.1439-0426.2005.00692.x. [DOI] [Google Scholar]
- 30.Welcomme R.L., Bartley D.M. Current approaches to the enhancement of fisheries. Fish. Manag. Ecol. 1998;5:351–382. doi: 10.1046/j.1365-2400.1998.550351.x. [DOI] [Google Scholar]
- 31.Behnke R.J. Comment: First Documented Case of Anadromy in a Population of Introduced Rainbow Trout in Patagonia, Argentina. Trans. Am. Fish. Soc. 2002;131:582–585. doi: 10.1577/1548-8659(2002)131<0582:CFDCOA>2.0.CO;2. [DOI] [Google Scholar]
- 32.FAO . Management of the Aquaponic Systems. FAO; Rome, Italy: 2015. [Google Scholar]
- 33.Gall G.A.E., Crandell P.A. The Rainbow trout. Aquaculture. 1992;100:1–10. doi: 10.1016/0044-8486(92)90333-G. [DOI] [Google Scholar]
- 34.Stout M. Aquaponic Gardening: Discover the Dual Benefits of Raising Fish and Plants Together (Idiot’s Guides) Illustrated ed. Alpha Publishing; New York, NY, USA: 2013. pp. 1–352. [Google Scholar]
- 35.Appleford P., Lucas S.J., Southgate C.P. In: Aquaculture: Farming Aquatic Animals and Plants. Lucas S.J., Southgate C.P., editors. Blackwell Publishing Ltd.; Hoboken, NJ, USA: 2012. pp. 18–50. Second Condition. [Google Scholar]
- 36.Birolo M., Bordignon F., Trocino A., Fasolato L., Pascual A., Godoy S., Nicoletto C., Maucieri C., Xiccato G. Effects of stocking density on the growth and flesh quality of rainbow trout (Oncorhynchus mykiss) reared in a low-tech aquaponic system. Aquaculture. 2020;529:735653. doi: 10.1016/j.aquaculture.2020.735653. [DOI] [Google Scholar]
- 37.Naumowicz K., Pajdak J., Terech-Majewska E., Szarek J. Intracohort cannibalism and methods for its mitigation in cultured freshwater fish. Rev Fish Biol Fish. 2017;27:193–208. [Google Scholar]
- 38.Molony B. Environmental Requirements and Tolerances of Rainbow Trout (Oncorhynchus mykiss) and Brown Trout (Salmo trutta) with Special Reference to Western Australia: A Review. Volume 130. Department of Fisheries Perth, Department of Fisheries, Government of Western Australia; Perth, Australia: 2001. pp. 1–28. [Google Scholar]
- 39.Avkhimovich D. Bachelor’s Thesis. Mikkely University of Applied Sciences; Mikkely, Finland: 2013. Effect of Water Quality on Rainbow Trout Performance Water Oxygen Level in Commercial Trout Farm. [Google Scholar]
- 40.Möck A., Peters G. Lysozyme activity in rainbow trout, Oncorhynchus mykiss (Walbaum), stressed by handling, transport and water pollution. J. Fish Biol. 1990;37:873–885. doi: 10.1111/j.1095-8649.1990.tb03591.x. [DOI] [Google Scholar]
- 41.Islam J.M., Kunzmann A., Slater J.M. Responses of aquaculture fish to climate change induced extreme temperatures: A review. J. World Aquac. Soc. 2021;53:314–366. doi: 10.1111/jwas.12853. [DOI] [Google Scholar]
- 42.Leberg P.L. Influence of genetic variability on population growth: Implications for conservation. J. Fish Biol. 1990;37:193–195. doi: 10.1111/j.1095-8649.1990.tb05036.x. [DOI] [Google Scholar]
- 43.Gjerde B., Gunnes K., Gjerdem T. Effect of inbreeding on survival and growth in rainbow trout. Aquaculture. 1983;34:327–332. doi: 10.1016/0044-8486(83)90212-0. [DOI] [Google Scholar]
- 44.Su G.S., Liljedahl L.E., Gall G.A.E. Effects of inbreeding on growth and reproductive traits in rainbow trout (Oncorhynchus mykiss) Aquaculture. 1996;142:139–148. doi: 10.1016/0044-8486(96)01255-0. [DOI] [Google Scholar]
- 45.Wang S., Hard J.J., Utter F. Salmonid inbreeding: A review. Rev. Fish Biol. Fish. 2002;11:301–319. doi: 10.1023/A:1021330500365. [DOI] [Google Scholar]
- 46.Frankham R. Genetics and extinction. Biol. Conserv. 2005;126:131–140. doi: 10.1016/j.biocon.2005.05.002. [DOI] [Google Scholar]
- 47.Paul K., D’ambrosio J., Phocas F. Temporal and region-specific variations in genome-wide inbreeding effects on female size and reproduction traits of rainbow trout. Evol. Appl. 2022;15:645–662. doi: 10.1111/eva.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kause A., Ritola O., Paananen T., Wahlroos H., Mäntysaari E.A. Genetic trends in growth, sexual maturity and skeletal deformations, and rate of inbreeding in a breeding programme for rainbow trout (Oncorhynchus mykiss) Aquaculture. 2005;247:177–187. doi: 10.1016/j.aquaculture.2005.02.023. [DOI] [Google Scholar]
- 49.Ferguson M.M., Drahushchak L.R. Disease resistance and enzyme heterozygosity in rainbow trout. Heredity. 1990;64:413–417. doi: 10.1038/hdy.1990.52. [DOI] [PubMed] [Google Scholar]
- 50.Frankham R. Stress and adaptation in conservation genetics. J. Evol. Biol. 2005;18:750–755. doi: 10.1111/j.1420-9101.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 51.Papageorgiou N. Trout and Its Rearing. University Studios Press; Thessaloniki, Greece: 1985. pp. 10–50. (in Greek) [Google Scholar]
- 52.FAO . Small-Scale Aquaponic Food Production—Integrated Fish and Plant Farming. FAO; Rome, Italy: 2014. [Google Scholar]
- 53.Ultsch G.R., Ott M.E., Heisler N. Standard metabolic rate, critical oxygen tension, and aerobic scope for spontaneous activity of trout (Salmo gairdneri) and carp (Cyprinus carpio) in acidified water. Comp. Biochem. Physiol. 1980;67:329–335. doi: 10.1016/S0300-9629(80)80004-1. [DOI] [Google Scholar]
- 54.Sae-Lim P., Kause A., Mulder H.A., Olesen I. Breeding and genetics symposium: Climate change and selective breeding in aquaculture. J. Anim. Sci. 2017;95:1801–1812. doi: 10.2527/jas2016.1066. [DOI] [PubMed] [Google Scholar]
- 55.Yildiz Y.H., Robaina L., Pirhonen J., Mente E., Domínguez D., Parisi G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water. 2017;9:13. doi: 10.3390/w9010013. [DOI] [Google Scholar]
- 56.Davidson J., Good C., Barrows F.T., Welsh C., Kenney P.B., Summerfelt S.T. Comparing the effects of feeding a grain- or a fish meal-based diet on water quality, waste production, and rainbow trout Oncorhynchus mykiss performance within low exchange water recirculating aquaculture systems. Aquac. Eng. 2014;52:45–57. doi: 10.1016/j.aquaeng.2012.08.001. [DOI] [Google Scholar]
- 57.Gjedrem T., Robinson N., Rye M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture. 2012;350–353:117–121. doi: 10.1016/j.aquaculture.2012.04.008. [DOI] [Google Scholar]
- 58.United Nations [(accessed on 16 November 2016)];The World Population Prospects: 2015 Revision. 2015 Available online: http://www.un.org/en/development/desa/publications/world-population-prospects-2015-revision.html.
- 59.De Silva S.S., Soto D. Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge. FAO; Rome, Italy: 2009. Climate change and aquaculture: Potential impacts, adaptation and mitigation; pp. 151–212. FAO Fisheries and Aquaculture Technical paper 530. [Google Scholar]
- 60.Kutty M.N. Would Food Crisis, FAO Alert and India. World Aquac. 2010;41:6–7. [Google Scholar]
- 61.FAO . FAO Yearbook 2014: Fishery and Aquaculture Statistics. FAO; Rome, Italy: 2016. pp. 1–105. [Google Scholar]
- 62.Dülger N., Kumlu M., Türkmen S., Ölçülü A., Tufan Eroldoĝan O., Asuman Yilmaz H., Öçal N. Thermal tolerance of European Sea Bass (Dicentrarchus labrax) juveniles acclimated to three temperature levels. J. Therm. Biol. 2012;37:79–82. doi: 10.1016/j.jtherbio.2011.11.003. [DOI] [Google Scholar]
- 63.Reid G.K., Gurney-Smith H.J., Marcogliese D.J., Knowler D., Benfey T., Garber A.F., Forster I., Chopin T., Brewer-Dalton K., Moccia R.D., et al. Climate change and aquaculture: Considering biological response and resources. Aquac. Environ. Interact. 2019;11:569–602. doi: 10.3354/aei00332. [DOI] [Google Scholar]
- 64.Aranda I., Castro L., Alía R., Pardos J.A., Gil L. Low temperature during winter elicits differential responses among populations of the Mediterranean evergreen cork oak (Quercus suber) Tree Physiol. 2005;25:1085–1090. doi: 10.1093/treephys/25.8.1085. [DOI] [PubMed] [Google Scholar]
- 65.Besson M., Vandeputte M., van Arendon J.A.M.A.M., Aubin J., de Boer I.J.M.J.M., Quillet E., Komen H. Influence of water temperature on the economic value of growth rate in fish farming: The case of sea bass (Dicentrarchus labrax) cage farming in the Mediterranean. J. Aquac. 2016;462:47–55. doi: 10.1016/j.aquaculture.2016.04.030. [DOI] [Google Scholar]
- 66.Llorente I., Luna L. The competitive advantages arising from different environmental conditions in seabream, Sparus aurata, production in the Mediterranean Sea. J. World Aquac. Soc. 2013;44:611–627. doi: 10.1111/jwas.12069. [DOI] [Google Scholar]
- 67.Choi Y.W., Campbell D.J., Aldridge J.C., Eltahir E.A.B. Near-term regional climate change over Bangladesh. Clim. Dyn. 2021;57:3055–3073. doi: 10.1007/s00382-021-05856-z. [DOI] [Google Scholar]
- 68.Dastagir M.R. Modeling recent climate change induced extreme events in Bangladesh: A review. Weather Clim. Extrem. 2015;7:49–60. doi: 10.1016/j.wace.2014.10.003. [DOI] [Google Scholar]
- 69.Thuy N.T.T., Giang N.T.H., Hoai H.T.T., Van Dan T. Impact of climate change on aquaculture in Phu Vang district, Thua Thien Hue province, Vietnam. Southeast Asian Regional Center for Graduate Study and Research in Agriculture (SEARCA); Los Baños, Philippines: 2017. pp. 1–51. (Agriculture and Development Discussion Paper Series 2017-3). [Google Scholar]
- 70.Hobday A.J., Spillman C.M., Eveson J.P., Hartog J.R., Zhang M.X., Brodie S. A framework for combining seasonal forecasts and climate projections to aid risk management for fisheries and aquaculture. Front. Mar. Sci. 2018;5:137. doi: 10.3389/fmars.2018.00137. [DOI] [Google Scholar]
- 71.McCoy D., McManus M.A., Kotubetey K., Kawelo A.H., Young C., D’Andrea B., Ruttenberg K.C., Alegado R.A. Large-scale climatic effects on traditional Hawaiian fishpond aquaculture. PLoS ONE. 2017;12:e0187951. doi: 10.1371/journal.pone.0187951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamon K.G. Report on Minimising Economic Losses, Opportunities and Challenges for Aquaculture in EUROPE; CERES Deliverable 4.2. 2019. [(accessed on 29 August 2022)]. pp. 1–151. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5c7cfc5cc&appId=PPGMS.
- 73.Roychowdhury P., Aftabuddin M., Pati M.K. Thermal stress altered growth performance and metabolism and induced anaemia and liver disorder in Labeo rohita. Aquac. Res. 2020;51:1406–1414. doi: 10.1111/are.14486. [DOI] [Google Scholar]
- 74.Rosa R., Marques A., Nunes M.L. Impact of climate change in Mediterranean aquaculture. Rev. Aquac. 2012;4:163–177. doi: 10.1111/j.1753-5131.2012.01071.x. [DOI] [Google Scholar]
- 75.Rosa R., Marques A., Nunes M.L. The Mediterranean Sea: Its history and Present Challenges. Volume 9789400767. Springer; Dodrecht, The Netherlands: 2014. Mediterranean aquaculture in a changing climate; pp. 605–616. [Google Scholar]
- 76.Doubleday Z.A., Clarke S.M., Li X., Pecl G.T., Ward T.M., Battaglene S., Frusher S., Gibbs P.J., Hobday A.J., Hutchinson N., et al. Assessing the risk of climate change to aquaculture: A case study from south-east Australia. Aquac. Environ. Interact. 2013;3:163–175. doi: 10.3354/aei00058. [DOI] [Google Scholar]
- 77.Roberts S.D., Van Ruth P.D., Wilkinson C., Bastianello S.S., Bansemer M.S. Marine heatwave, harmful algae blooms and an extensive fish kill event during 2013 in South Australia. Front. Mar. Sci. 2019;6:610. doi: 10.3389/fmars.2019.00610. [DOI] [Google Scholar]
- 78.Rountrey A.N., Coulson P.G., Meeuwig J.J., Meekan M. Water temperature and fish growth: Otoliths predict growth patterns of a marine fish in a changing climate. Glob. Change Biol. 2014;20:2450–2458. doi: 10.1111/gcb.12617. [DOI] [PubMed] [Google Scholar]
- 79.Vornanen M. The temperature dependence of electrical excitability in fish hearts. J. Exp. Biol. 2016;219:1941–1952. doi: 10.1242/jeb.128439. [DOI] [PubMed] [Google Scholar]
- 80.Barton B.A., Schreck C.B., Ewing R.D., Hemmingsen A.R., Patiño R. Changes in plasma cortisol during stress and smoltification in Coho Salmon, Oncorhynchus kisutch. Gen. Comp. Endocrinol. 1985;59:468–471. doi: 10.1016/0016-6480(85)90406-X. [DOI] [PubMed] [Google Scholar]
- 81.Heath A.G., Iwama G.K., Pickering A.D., Sumpter J.P., Schreck C.B. Fish stress and health in aquaculture. Estuaries. 1998;21:501. doi: 10.2307/1352849. [DOI] [Google Scholar]
- 82.Schreck C.B., Tort L. Fish Physiology. Volume 35. Academic Press; Cambridge, MA, USA: 2016. The concept of stress in fish; pp. 1–34. [Google Scholar]
- 83.Crawshaw L.I. Physiological and behavioral reactions of fishes to temperature change. J. Fish. Res. Board Can. 1977;34:730–734. doi: 10.1139/f77-113. [DOI] [Google Scholar]
- 84.Li A.J., Leung P.T.Y., Bao V.W.W., Lui G.C.S., Leung K.M.Y. Temperature-dependent physiological and biochemical responses of the marine medaka Oryzias melastigma with consideration of both low and high thermal extremes. J. Therm. Biol. 2015;54:98–105. doi: 10.1016/j.jtherbio.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Van den Burg E.H., Peeters R.R., Verhoye M., Meek J., Flik G., Van der Linden A. Brain responses to ambient temperature fluctuations in fish: Reduction of blood volume and initiation of a whole-body stress response. J. Neurophysiol. 2005;93:2849–2855. doi: 10.1152/jn.01113.2004. [DOI] [PubMed] [Google Scholar]
- 86.Alfonso S., Gesto M., Sadoul B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021;98:1496–1508. doi: 10.1111/jfb.14599. [DOI] [PubMed] [Google Scholar]
- 87.Faught E., Hernandez-Perez J., Wilson J.M., Vijayan M.M. Climate Change and Non-Infectious Fish Disorders. CABI; Wallingford, UK: 2020. Stress in response to environmental changes; pp. 136–162. [Google Scholar]
- 88.Feidantsis K., Pörtner H.O., Giantsis I.A., Michaelidis B. Advances in understanding the impacts of global warming on marine fishes farmed offshore: Sparus aurata as a case study. J. Fish Biol. 2021;98:1509–1523. doi: 10.1111/jfb.14611. [DOI] [PubMed] [Google Scholar]
- 89.Little A.G., Loughland I., Seebacher F. What do warming waters mean for fish physiology and fisheries. J. Fish Biol. 2020;97:328–340. doi: 10.1111/jfb.14402. [DOI] [PubMed] [Google Scholar]
- 90.Vandeputte M., Clota F., Sadoul B., Blanc M.O., Blondeau-Bidet E., Bégout M.L., Cousin X., Geffroy B. Low temperature has opposite effects on sex determination in a marine fish at the larval/postlarval and juvenile stages. Ecol. Evol. 2020;10:13825–13835. doi: 10.1002/ece3.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Islam M.J., Kunzmann A., Slater M.J. Extreme winter cold-induced osmoregulatory, metabolic, and physiological responses in European seabass (Dicentrarchus labrax) acclimatized at different salinities. Sci. Total Environ. 2021;771:145202. doi: 10.1016/j.scitotenv.2021.145202. [DOI] [PubMed] [Google Scholar]
- 92.Tort L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011;35:1366–1375. doi: 10.1016/j.dci.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 93.Dockray J.J., Reid S.D., Wood C.M. Effects of elevated summer temperatures and reduced pH on metabolism and growth of juvenile rainbow trout (Oncorhynchus mykiss) on unlimited ration. Can. J. Fish. Aquat. Sci. 1996;53:2752–2763. doi: 10.1139/f96-232. [DOI] [Google Scholar]
- 94.Linton T.K., Reid S.D., Wood C.M. The metabolic costs and physiological consequences to juvenile rainbow trout of a simulated summer warming scenario in the presence and absence of sublethal ammonia. Trans. Am. Fish. Soc. 1997;126:259–272. doi: 10.1577/1548-8659(1997)126<0259:TMCAPC>2.3.CO;2. [DOI] [Google Scholar]
- 95.Morgan I.J., McDonald D.G., Wood C.M. The cost of living for freshwater fish in a warmer, more polluted world. Glob. Change Biol. 2001;7:345–355. doi: 10.1046/j.1365-2486.2001.00424.x. [DOI] [Google Scholar]
- 96.Barton B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002;42:517–525. doi: 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- 97.Bonga W., Sjoerd E. The stress response in fish. Physiol. Rev. 1977;77:591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- 98.Reid S.G., Bernier N.J., Perry S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. —C Pharmacol. Toxicol. Endocrinol. 1998;120:1–27. doi: 10.1016/S0742-8413(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 99.Weyts F.A.A., Cohen N., Flik G., Verburg-van Kemenade B.M.L. Interactions between the immune system and the hypothalamo-pituitary-interrenal axis in fish. Fish Shellfish Immunol. 1999;9:1–20. doi: 10.1006/fsim.1998.0170. [DOI] [Google Scholar]
- 100.Chang C.H., Huang J.J., Yeh C.Y., Tang C.H., Hwang L.Y., Lee T.H. Salinity effects on strategies of glycogen utilization in livers of euryhaline milkfish (Chanos chanos) under hypothermal stress. Front. Physiol. 2019;9:81. doi: 10.3389/fphys.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pörtner H.O., Berdal B., Blust R., Brix O., Colosimo A., De Wachter B., Giuliani A., Johansen T., Fischer T., Knust R., et al. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: Developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus) Cont. Shelf Res. 2001;21:1975–1997. doi: 10.1016/S0278-4343(01)00038-3. [DOI] [Google Scholar]
- 102.Seebacher F., Post E. Climate change impacts on animal migration. Clim. Change Responses. 2015;2:5. doi: 10.1186/s40665-015-0013-9. [DOI] [Google Scholar]
- 103.Servili A., Canario A.V.M., Mouchel O., Muñoz-Cueto J.A. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020;291:113439. doi: 10.1016/j.ygcen.2020.113439. [DOI] [PubMed] [Google Scholar]
- 104.King H., Pankhurst N. Ovarian growth and plasma sex steroid and vitellogenin profiles during vitellogenesis in Tasmanian female Atlantic salmon (Salmo salar) J. Aquac. 2003;219:797–813. doi: 10.1016/S0044-8486(02)00647-6. [DOI] [Google Scholar]
- 105.King H.R., Pankhurst N.W. Effect of short-term temperature reduction on ovulation and LHRHa responsiveness in female Atlantic salmon (Salmo salar) maintained at elevated water temperatures. J. Aquac. 2004;238:421–436. doi: 10.1016/j.aquaculture.2004.05.011. [DOI] [Google Scholar]
- 106.Pankhurst N.W., King H.R. Temperature and salmonid reproduction: Implications for aquaculture. J. Fish Biol. 2010;76:69–85. doi: 10.1111/j.1095-8649.2009.02484.x. [DOI] [PubMed] [Google Scholar]
- 107.Zarski D., Horváth A., Bernáth G., Krejszeff S., Radoczi J., Palinska-Zarska K., Bokor Z., Kupren K., Urbányi B. Controlled Reproduction of Wild Eurasian Perch. Springer; Cham, Switzerland: 2017. Stimulation of ovulation and spermiation; pp. 33–40. [Google Scholar]
- 108.Dadras H., Dzyuba V., Golpour A., Xin M., Dzyuba B. In vitro antioxidant enzyme activity and sperm motility at different temperatures in sterlet Acipenser ruthenus and rainbow trout Oncorhynchus mykiss. Fish Physiol. Biochem. 2019;45:1791–1800. doi: 10.1007/s10695-019-00675-w. [DOI] [PubMed] [Google Scholar]
- 109.Amiel J.J., Bao S., Shine R. The effects of incubation temperature on the development of the cortical forebrain in a lizard. Anim. Cogn. 2017;20:117–125. doi: 10.1007/s10071-016-0993-2. [DOI] [PubMed] [Google Scholar]
- 110.O’Donnell S. The neurobiology of climate change. Sci. Nat. 2018;105:11. doi: 10.1007/s00114-017-1538-5. [DOI] [PubMed] [Google Scholar]
- 111.Pallotta M.M., Turano M., Ronca R., Mezzasalma M., Petraccioli A., Odierna G., Capriglione T. Brain gene expression is influenced by incubation temperature during leopard gecko (Eublepharis macularius) development. J. Exp. Zool. —Part B Mol. Dev. Evol. 2017;328:360–370. doi: 10.1002/jez.b.22736. [DOI] [PubMed] [Google Scholar]
- 112.Chadwick J.G., McCormick S.D. Upper thermal limits of growth in brook trout and their relationship to stress physiology. J. Exp. Biol. 2017;220:3976–3987. doi: 10.1242/jeb.161224. [DOI] [PubMed] [Google Scholar]
- 113.Giffard-Mena I., Lorin-Nebel C., Charmantier G., Castille R., Boulo V. Adaptation of the sea-bass (Dicentrarchus labrax) to fresh water: Role of aquaporins and Na+/K+-ATPases. Comp. Biochem. Physiol. —Part A Mol. Integr. Physiol. 2008;150:332–338. doi: 10.1016/j.cbpa.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 114.Vargas-Chacoff L., Regish A.M., Weinstock A., McCormick S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018;93:550–559. doi: 10.1111/jfb.13683. [DOI] [PubMed] [Google Scholar]
- 115.Smith S., Bernatchez I., Beheregaray I.B. RNA-seq analysis reveals extensive transcriptional plasticity to temperature stress in a freshwater fish species. BMC Genom. 2013;14:375. doi: 10.1186/1471-2164-14-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Madeira D., Narciso L., Cabral H.N., Vinagre C., Diniz M.S. HSP70 production patterns in coastal and estuarine organisms facing increasing temperatures. J. Sea Res. 2012;73:137–147. doi: 10.1016/j.seares.2012.07.003. [DOI] [Google Scholar]
- 117.Maulvault A.L., Barbosa V., Alves R., Custodio A., Anacleto P., Repolho T., Pousão Ferreira P., Rosa R., Marques A., Diniz M. Ecophysiological responses of juvenile seabass (Dicentrarchus labrax) exposed to increased temperature and dietary methylmercury. Sci. Total Environ. 2017;586:551–558. doi: 10.1016/j.scitotenv.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 118.Reyes-Lopez F.E., Aerts J., Vallejos-Vidal E., Ampe B., Dierckens K., Tort L., Bossier P. Modulation of innate immune-related genes and glucocorticoid synthesis in gnotobiotic full-sibling European sea bass (Dicentrarchus labrax) larvae challenged with Vibrio anguillarum. Front. Immunol. 2018;9:914. doi: 10.3389/fimmu.2018.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Donaldson M.R., Cooke S.J., Patterson D.A., Macdonald J.S. Cold shock and fish. J. Fish Biol. 2008;73:1491–1530. doi: 10.1111/j.1095-8649.2008.02061.x. [DOI] [Google Scholar]
- 120.Nakano K., Iwama G.K. The 70-kDa heat shock protein response in two intertidal sculpins, Oligocottus maculosus and O. snyderi: Relationship of hsp70 and thermal tolerance. Comp. Biochem. Physiol. —Part A Mol. Integr. Physiol. 2002;133:79–94. doi: 10.1016/S1095-6433(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 121.Werner I., Viant M.R., Rosenblum E.S., Gantner A.S., Tjeerdema R.S., Johnson M.L. Cellular responses to temperature stress in steelhead trout (Onchorynchus mykiss) parr with different rearing histories. Fish Physiol. Biochem. 2006;32:261–273. doi: 10.1007/s10695-006-9105-6. [DOI] [Google Scholar]
- 122.Werner I., Smith T.B., Feliciano J., Johnson M.L. Heat shock proteins in juvenile steelhead reflect thermal conditions in the Navarro river watershed, California. Trans. Am. Fish. Soc. 2005;134:399–410. doi: 10.1577/T03-181.1. [DOI] [Google Scholar]
- 123.Sopinka N.M., Donaldson M.R., O’Connor C.M., Suski C.D., Cooke S.J. Stress indicators in fish. Fish Physiol. 2016;35:405–462. [Google Scholar]
- 124.Yamashita M., Yabu T., Ojima N. Stress protein HSP70 in fish. Aqua-BioScience Monogr. 2010;3:111–141. doi: 10.5047/absm.2010.00304.0111. [DOI] [Google Scholar]
- 125.Eissa N., Wang H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016;8:61–88. doi: 10.1111/raq.12081. [DOI] [Google Scholar]
- 126.Almroth B.C., Asker N., Wassmur B., Rosengren M., Jutfelt F., Gräns A., Sundell K., Axelsson M., Sturve J., Sturve J. Warmer water temperature results in oxidative damage in an Antarctic fish, Bald notothen. J. Exp. Mar. Biol. Ecol. 2015;468:130–137. doi: 10.1016/j.jembe.2015.02.018. [DOI] [Google Scholar]
- 127.Madeira D., Vinagre C., Diniz M.S. Are fish in hot water? Effects of warming on oxidative stress metabolism in the commercial species Sparus aurata. Ecol. Indic. 2016;63:324–331. doi: 10.1016/j.ecolind.2015.12.008. [DOI] [Google Scholar]
- 128.Benítez-Dorta V., Caballero M.J., Betancor M.B., Manchado M., Tort L., Torrecillas S., Zamorano M.J., Izquierdo M., Montero D. Effects of thermal stress on the expression of glucocorticoid receptor complex linked genes in Senegalese sole (Solea senegalensis): Acute and adaptive stress responses. Gen. Comp. Endocrinol. 2017;252:173–185. doi: 10.1016/j.ygcen.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 129.Araújo J.E., Madeira D., Vitorino R., Repolho T., Rosa R., Diniz M. Negative synergistic impacts of ocean warming and acidification on the survival and proteome of the commercial sea bream, Sparus aurata. J. Sea Res. 2018;139:50–61. doi: 10.1016/j.seares.2018.06.011. [DOI] [Google Scholar]
- 130.Kyprianou T.D., Pörtner H.O., Anestis A., Kostoglou B., Feidantsis K., Michaelidis B. Metabolic and molecular stress responses of gilthead seam bream Sparus aurata during exposure to low ambient temperature: An analysis of mechanisms underlying the winter syndrome. J. Comp. Physiol. B. 2010;180:1005–1018. doi: 10.1007/s00360-010-0481-y. [DOI] [PubMed] [Google Scholar]
- 131.Kamunde C., Sappal R., Melegy T.M. Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PLoS ONE. 2019;14:e0219792. doi: 10.1371/journal.pone.0219792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu Y., Liu J., Ye S., Bureau D.P., Liu H., Yin J., Mou Z., Lin H., Hao F. Global metabolic responses of the lenok (Brachymystax lenok) to thermal stress. Comparative Biochemistry and Physiology—Part D Genom. Proteom. 2019;29:308–319. doi: 10.1016/j.cbd.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 133.Volkoff H., Rønnestad I. Effects of temperature on feeding and digestive processes in fish. Temperature. 2020;7:307–320. doi: 10.1080/23328940.2020.1765950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nie X., Zhang F., Wang T., Zheng X., Li Y., Huang B., Zhang C. Physiological and morphological changes in Turbot (Psetta maxima) gill tissue during waterless storage. J. Aquac. 2019;508:30–35. doi: 10.1016/j.aquaculture.2019.04.060. [DOI] [Google Scholar]
- 135.Pickering A.D.D. Growth and stress in fish production. Genet. Aquac. 1993;111:51–63. doi: 10.1016/0044-8486(93)90024-S. [DOI] [Google Scholar]
- 136.Barton B.A., Peter R.E. Plasma cortisol stress response in fingerling rainbow trout, Salmo gairdneri Richardson, to various transport conditions, anaesthesia, and cold shock. J. Fish Biol. 1982;20:39–51. doi: 10.1111/j.1095-8649.1982.tb03893.x. [DOI] [Google Scholar]
- 137.Sappal R., MacDougald M., Stevens D., Fast M.D., Kamunde C. Copper alters the effect of temperature on mitochondrial bioenergetics in rainbow trout, Oncorhynchus mykiss. Arch. Environ. Contam. Toxicol. 2014;66:430–440. doi: 10.1007/s00244-013-9985-2. [DOI] [PubMed] [Google Scholar]
- 138.Portner H.O. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2002;132:739–761. doi: 10.1016/S1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 139.Portner H.O., Peck M.A. Climate change impacts on fish and fisheries: Towards a cause and effect understanding. J. Fish Biol. 2010;77:1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- 140.Iftikar F.I., Hickey A.J. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE. 2013;8:e64120. doi: 10.1371/journal.pone.0064120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blier P.U., Lemieux H., Pichaud N. Holding our breath in our modern world: Will mitochondria keep the pace with climate changes. Can. J. Zool. 2014;92:591–601. doi: 10.1139/cjz-2013-0183. [DOI] [Google Scholar]
- 142.Guderley H. Mitochondria and temperature. In: Farrell A.P., editor. Encyclopedia of Fish Physiology: Energetics, Interactions with the Environment, Lifestyles, and Applications. Academic Press; New York, NY, USA: 2011. pp. 1709–1716. [Google Scholar]
- 143.Ficke A.D., Myrick C.A., Hansen L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007;17:581–613. doi: 10.1007/s11160-007-9059-5. [DOI] [Google Scholar]
- 144.Doney S.C., Ruckelshaus M., Duffy J.E., Barry J.P. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012;4:11–37. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- 145.Makrinos D.L., Bowden T.J. Natural environmental impacts on teleost immune function. Fish Shellfish Immunol. 2016;53:50–57. doi: 10.1016/j.fsi.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 146.Wright R.K., Cooper E.L. Temperature effects on ectotherm immune responses. Dev. Comp. Immunol. 1981;5:117–122. doi: 10.1016/0145-305X(81)90016-1. [DOI] [Google Scholar]
- 147.Bowden T.J. Modulation of the immune system of fish by their environment. Fish Shellfish Immunol. 2008;25:373–383. doi: 10.1016/j.fsi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 148.Pascoli F., Lanzano G.S., Negrato E., Poltronieri C., Trocino A., Radaelli G., Bertotto D. Seasonal effects on hematological and innate immune parameters in sea bass Dicentrarchus labrax. Fish Shellfish Immunol. 2011;31:1081–1087. doi: 10.1016/j.fsi.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 149.Yada T., Tort L. Stress and disease resistance: Immune system and Immunoendocrine interactions. Fish Physiol. 2016;35:365–403. [Google Scholar]
- 150.Bailey C., Segner H., Casanova-Nakayama A., Wahli T. Who needs the hotspot? The effect of temperature on the fish host immune response to Tetracapsuloides bryosalmonae the causative agent of proliferative kidney disease. Fish Shellfish Immunol. 2017;63:424–437. doi: 10.1016/j.fsi.2017.02.039. [DOI] [PubMed] [Google Scholar]
- 151.Huang J., Li Y., Liu Z., Kang Y., Wang J. Transcriptomic responses to heat stress in rainbow trout Oncorhynchus mykiss head kidney. Fish Shellfish Immunol. 2018;82:32–40. doi: 10.1016/j.fsi.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 152.Xia B.P., Liu Z., Zhou Y.J., Wang Y.J., Huang J.Q., Li Y.J., Kang Y.J., Wang J.F., Liu X.X. Effects of Chronic Heat Stress on Part of Serum Non-specific Immunity Parameters in Rainbow Trout (Oncorhynchus mykiss) J. Agric. Biotechnol. 2017;25:1078–1085. [Google Scholar]
- 153.Zhou T., Gui L., Liu M., Li W., Hu P., Duarte D.F.C., Niu H., Chen L. Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2019;84:1145–1156. doi: 10.1016/j.fsi.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 154.Hafs A.W., Mazik P.M., Kenney P.B., Silverstein J.T. Impact of carbon dioxide level, water velocity, strain, and feeding regimen on growth and fillet attributes of cultured rainbow trout (Oncorhynchus mykiss) J. Aquac. 2012;350–353:46–53. doi: 10.1016/j.aquaculture.2012.04.020. [DOI] [Google Scholar]
- 155.Yáñez J.M., Xu P., Carvalheiro R., Hayes B. Genomics applied to livestock and aquaculture breeding. Evol. Appl. 2022;15:517. doi: 10.1111/eva.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Adams O.A., Zhang Y., Gilbert M.H., Lawrence C.S., Snow M., Farrell A.P. An unusually high upper thermal acclimation potential for rainbow trout. Conserv. Physiol. 2022;10:coab101. doi: 10.1093/conphys/coab101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mulder H.A., Sae-Lim P., Kause A., Olesen I. Selective breeding in aquaculture for future environments under climate change; Proceedings of the FAO International Symposium on “The Role of Agricultural Biotechnologies in Sustainable Food Systems and Nutrition”; Rome, Italy. 15–17 February 2016; pp. 45–46. [Google Scholar]
- 158.Abisha R., Krishnani K.K., Sukhdhane K., Verma A.K., Brahmane M., Chadha N.K. Sustainable development of climate-resilient aquaculture and culture-based fisheries through adaptation of abiotic stresses: A review. J. Water Clim. Change. 2022;13:2671–2689. doi: 10.2166/wcc.2022.045. [DOI] [Google Scholar]
- 159.Rharrhour H., Wariaghli F., Goddek S., Sadik M., El A. Towards sustainable food productions in Morocco: Aquaponics. E3S Web Conf. 2022;337:03004. doi: 10.1051/e3sconf/202233703004. [DOI] [Google Scholar]
- 160.Wiens G.D., LaPatra S.E., Welch T.J., Evenhuis J.P., Rexroad III C.E., Leeds T.D. On-farm performance of rainbow trout (Oncorhynchus mykiss) selectively bred for resistance to bacterial cold water disease: Effect of rearing environment on survival phenotype. Aquaculture. 2013;388:128–136. doi: 10.1016/j.aquaculture.2013.01.018. [DOI] [Google Scholar]
- 161.Godfray H.C.J., Beddington J.R., Crute I.R., Haddad L., Lawrence D., Muir J.F., Pretty J., Robinson S., Thomas S.M., Toulmin C. Food security: The challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 162.World Development Report . Agriculture for Development. World Bank; Washington, DC, USA: 2008. [Google Scholar]
- 163.Rivera-Ferre M., Ortega-Cerdà M., Baumgärtner J. Rethinking. Study and Management of Agricultural Systems for Policy Design. Sustainability. 2013;5:3858–3875. doi: 10.3390/su5093858. [DOI] [Google Scholar]
- 164.Velten S., Leventon J., Jager N., Newig J. What Is Sustainable Agriculture: A Systematic Review. Sustainability. 2015;7:7833–7865. doi: 10.3390/su7067833. [DOI] [Google Scholar]
- 165.Ebeling J.M., Timmons M.B. Recirculating Aquaculture. Cayuga Aqua Ventures; Ithaca, NY, USA: 2010. [Google Scholar]
- 166.Ebeling J.M., Timmons M.B. Recirculating Aquaculture Systems. In: Tidwell J.H., editor. Aquaculture Production Systems. John Willey& Sons; Hoboken, NJ, USA: 2012. [Google Scholar]
- 167.Kingler D., Naylor R. Searching for Solutions in Aquaculture: Charting a Sustainable Course. Annu. Rev. Environ. Resour. 2012;37:247–276. [Google Scholar]
- 168.Tyson R.C., Treadwell D.D., Simonne E.H. Opportunities and Challenges to Sustainability in Aquaponic Systems. HorTechnology. 2011;21:6–13. doi: 10.21273/HORTTECH.21.1.6. [DOI] [Google Scholar]
- 169.Ridler N., Wowchuk M., Robinson B., Barrington K., Chopin T., Robinson S., Page F., Reid G., Szemerda M., Sewuster J., et al. Integrated Multi-Trophic Aquaculture (IMTA): A potential strategic choice for farmers. Aquac. Econ. Manag. 2007;11:1–13. doi: 10.1080/13657300701202767. [DOI] [Google Scholar]
- 170.Reid G.K., Liutkus M., Robinson S.M.C., Chopin T.R., Blair T., Lander T., Mullen J., Page F., Moccia R.D. A review of the biophysical properties of salmonid faeces: Implications for aquaculture waste dispersal models and integrated multi-trophic aquaculture. Aquac. Res. 2009;40:257–273. doi: 10.1111/j.1365-2109.2008.02065.x. [DOI] [Google Scholar]
- 171.Ecolife Conservation . Introduction to Aquaponics. Ecolife Conservation; Escondido, CA, USA: 2017. pp. 2–24. [Google Scholar]
- 172.Kaltsis I. Bachelor’s Thesis. Faculty of Agricultural Technology and Food and Nutrition Technology, Department of Fisheries and Aquaculture Technology; Mesologgi, Greece: 2014. Aquaculture a Perfect Ecosystem for the Development of Ornamental Fish and Plants. [Google Scholar]
- 173.Martins C.I.M., Edinga E.H., Verdegema M.C.J., Heinsbroeka L.T.N., Schneider O., Blanchetond J.P., Roque d’Orbcastel E., Verretha J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Agric. Eng. 2010;43:83–93. doi: 10.1016/j.aquaeng.2010.09.002. [DOI] [Google Scholar]
- 174.Nelson R.L., Pade J.S. Aquaponic equipment the clarifier. Aquaponics J. 2007;4:30–31. [Google Scholar]
- 175.Tesi R. Colture Protette: Ortoflorovivaismo in Ambiente Mediterraneo. Il sole 24 ore Edagricole; Bologna, Italy: 2008. [Google Scholar]
- 176.Malorgio F., Incrocci L., Pardossi A. La Tecnica della Coltivazione Fuori Suolo. Universita di Pisa; Pisa, Italy: 2005. pp. 1–143. [Google Scholar]
- 177.Hochmuth G.J., Hanlon E.A. Commercial Vegetable Fertilization Principles. University of Florida, Soil Water Science Department; Gainesville, FL, USA: 2010. [Google Scholar]
- 178.Maucieri C., Nicoletto C., Zanin G., Xiccato G., Borin M., Sambo P. Composition and quality traits of vegetables grown in a low-tech aquaponic system at different fish stocking densities. J. Sci. Food Agric. 2020;100:4310–4318. doi: 10.1002/jsfa.10475. [DOI] [PubMed] [Google Scholar]
- 179.Al Tawaha A.R., Wahab P.E.M., Binti Jaafar H., Zuan A.T.K., Hassan M.Z. Effects of Fish Stocking Density on Water Quality, Growth Performance of Tilapia (Oreochromis niloticus) and Yield of Butterhead Lettuce (Lactuca sativa) Grown in Decoupled Recirculation Aquaponic Systems. J. Ecol. Eng. 2021;22:8–19. doi: 10.12911/22998993/128692. [DOI] [Google Scholar]
- 180.Maucieri C., Nicoletto C., Zanin G., Birolo M., Xiccato G., Sambo P., Borin M. Nitrogen budget in recirculating aquaponic systems with different fish stocking density. Ital. J. Agron. 2020;15:239–245. doi: 10.4081/ija.2020.1639. [DOI] [Google Scholar]
- 181.Adler P.R., Harper J.K., Takeda F., Summerfelt S.T. Economic analysis of an aquaponic system for the integrated production of rainbow trout and plants. Int. J. Recirc. Aquac. 2000;1:15–34. doi: 10.21061/ijra.v1i1.1359. [DOI] [Google Scholar]
- 182.McMurtry M.R., Nelson P.V., Sanders D.C., Hodges L. Sand culture of vegetables using recirculated aquacultural effluents. Appl. Agric. Res. 1990;5:280–284. [Google Scholar]
- 183.McMurtry M.R., Sanders D.C., Cure J.D., Hodson R.G. Effects of biofilter/culture tank volume ratios on productivity of a recirculating fish/vegetable co-culture system. J. Appl. Aquac. 1997;7:33–51. doi: 10.1300/J028v07n04_03. [DOI] [Google Scholar]
- 184.Goddek S., Joyce A., Kotzen B., Burnell G.M. Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future. Springer Nature; Berlin/Heidelberg, Germany: 2019. 619p [Google Scholar]
- 185.Papoutsoglou S.E. Fish Diet. Publications Stamoulis A; Athens, Greece: 2008. 976p [Google Scholar]
- 186.Olesen I., Myhr A.I., Rosendal G.K. Sustainable aquaculture: Are we getting there? Ethical perspectives on salmon farming. J. Agric. Environ. Ethics. 2011;24:381–408. doi: 10.1007/s10806-010-9269-z. [DOI] [Google Scholar]
- 187.Somerville C., Cohen M., Pantanella E., Stankus A., Lovatelli A. FAO Fisheries and Aquaculture. FAO; Rome, Italy: 2014. Small-scale aquaponic food production. Integrated fish and plant farming.262p Technical Paper. No. 589. [Google Scholar]
- 188.Miličić V., Thorarinsdottir R., Dos Santos M., Turnšek Hančić M. Commercial Aquaponics Approaching the European Market: To Consumers’ Perceptions of Aquaponics Products in Europe. Water. 2017;9:80. doi: 10.3390/w9020080. [DOI] [Google Scholar]
- 189.Al-Hafedh Y.S., Alam A., Beltagi M.S. Food production and water conservation in a recirculating aquaponic system in Saudi Arabia at different ratios of fish feed to plants. J. World Aquac. Soc. 2008;39:510–520. doi: 10.1111/j.1749-7345.2008.00181.x. [DOI] [Google Scholar]
- 190.Pappa V.A., Kapsis P., Mente E., Berillis P. Aquaponics Software in Greece. J. Fish. Sci. 2017;11:1–4. doi: 10.21767/1307-234X.1000123. [DOI] [Google Scholar]
- 191.Stathopoulou P., Berillis P., Levizou E., Sakellariou-Makrantonaki M., Kormas A.K., Aggelaki A., Kapsis P., Vlahos N., Mente E. Aquaponics: A mutually beneficial relationship of fish, plants and bacteria; Proceedings of the 3rd International Congress on Applied Ichthyology & Aquatic Environment; Volos, Greece. 8–11 November 2018; pp. 8–11. [Google Scholar]
- 192.Krastanova M., Sirakov I., Ivanova-Kirilova S., Yarkov D., Orozova P. Aquaponic systems: Biological and technological parameters. Biotechnol. Biotechnol. Equip. 2022;36:305–316. doi: 10.1080/13102818.2022.2074892. [DOI] [Google Scholar]
- 193.Cretu M., Dediu L., Coadă M.T., Rîmniceanu C., Plăcintă S., Stroe M.D., Vasilean I. Comparative study on the growth and development of thyme and basil herbs in aquaponic system and hydroponic system. Anim. Sci. 2022;1:573–589. [Google Scholar]
- 194.Mihailovic-Stanojevic N., Belščak-Cvitanović A., Grujić-Milanović J., Ivanov M., Jovović D., Bugarski D., Miloradović Z. Antioxidant and antihypertensive activity of extract from Thymus serpyllum L. in experimental hypertension. Plant Foods Hum. Nutr. 2013;68:235–240. doi: 10.1007/s11130-013-0368-7. [DOI] [PubMed] [Google Scholar]
- 195.Grespan R., Aguiar R.P., Giubilei F.N., Fuso R.R., Damião M.J., Silva E.L., Mikcha J.G., Hernandes L., Amado C.B., Cuman R.K.N. Hepatoprotective effect of pretreatment with Thymus vulgaris essential oil in experimental model of acetaminophen-induced injury. Evid. -Based Complementary Altern. Med. 2014;2014:954136. doi: 10.1155/2014/954136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fratianni F., De Martino L., Melone A., De Feo V., Nazzaro F., Coppola R. Preservation of Chicken Breast Meat Treated with Thyme and Balm Essential Oils. J. Food Sci. 2010;75:528–535. doi: 10.1111/j.1750-3841.2010.01791.x. [DOI] [PubMed] [Google Scholar]
- 197.Sandulachi E., Macari A., Ghendov-Mosanu A., Cojocari D., Sturza R. Antioxidant and Antimicrobial Activity of Basil, Thyme and Tarragon 580 Used in Meat Products. Adv. Microbiol. 2021;11:591–606. doi: 10.4236/aim.2021.1111043. [DOI] [Google Scholar]
- 198.Abdel-Rahim M.M., Awad Y.M., Abdallah Y.A., Radwan S.M. Effects of four medicinal plants on the bioeconomic analysis and water-use efficiency of Nile tilapia, Oreochromis niloticus fry nursed under a small-scale aquaponics system. Aquac. Aquar. Conserv. Legis. 2019;12:851–866. [Google Scholar]
- 199.Knaus U., Wenzel L.C., Appelbaum S., Palm H.W. Aquaponics (sl) Production of spearmint (Mentha spicata) with African catfish (Clarias gariepinus) in Northern Germany. Sustainability. 2020;12:8717. doi: 10.3390/su12208717. [DOI] [Google Scholar]
- 200.Körner O., Gutzmann E., Kledal P.R. A dynamic model simulating the symbiotic effects in aquaponic systems; Proceedings of the International Symposium on New Technologies and Management for Greenhouses-GreenSys2015; Evora, Portugal. 19–23 July 2015; pp. 309–316. [Google Scholar]
- 201.Rakocy J.E. Aquaponics-Integrating Fish and Plant Culture. Aquac. Prod. Syst. 2012;1:343–386. [Google Scholar]
- 202.Siapatis K., Galanis I. Diploma Thesis. Department of Agricultural Science and Aquaculture, University of Thessaly; Volos, Greece: 2018. Aquaponic System: The Combination of Fish Culture and Hydroponic of Plants; pp. 2–50. [Google Scholar]
- 203.Schmautz Z., Loeu F., Liebisch F., Graber A., Mathis A., Bulc T.G., Junge R. Tomato productivity and quality in aquaponics: Comparison of three hydroponic methods. Water. 2016;8:533. doi: 10.3390/w8110533. [DOI] [Google Scholar]
- 204.Alessio G., Allegrucci G., Angle G., Arlati G., Baldrati R., Ballestrazzi P., Belvedere C.P., Beraldo D., Berton C., Boglione P., et al. Acquacoltura Responsabile: Verso le Produzioni Acquatiche del Terzo Millennio. FAO; Rome, Italy: 2001. [Google Scholar]
- 205.Fronte B., Galliano G., Bibbiani C. From freshwater to marine aquaponic: New opportunities for marine fish species production; Proceedings of the Conference VIVUS—On Agriculture, Environmentalism, Horticulture and Floristics, Food Production and Processing and Nutrition. With Knowledge and Experience to New Entrepreneurial Opportunities; Biotechnical Centre Naklo, Slovenia. 20–21 April 2016; pp. 514–521. [Google Scholar]
- 206.Boroujerdnia M., Ansari N.A. Effect of Different Levels of Nitrogen Fertilizer and Cultivars on Growth, Yield and Yield Components of Romaine Lettuce (Lactuca sativa L.) Middle East. Russ. J. Plant Sci. Biotechnol. 2007;1:47–53. [Google Scholar]
- 207.Rakocy J.E., Masser M.P., Losordo T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture. Southern Regional Aquaculture Center; Stoneville, MS, USA: 2006. Publication no. 454. [Google Scholar]
- 208.Maucieri C., Nicoletto C., Junge R., Schmautz Z., Sambo P., Borin M. Hydroponic systems and water management in aquaponics: A review. Ital. J. Agron. 2018;13:1012. doi: 10.4081/ija.2017.1012. [DOI] [Google Scholar]
- 209.Walker R.L., Burns I.G., Moorby J. Responses of plant growth rate to nitrogen supply: A comparison of relative addition and N interruption treatments. J. Exp. Bot. 2001;52:309–317. doi: 10.1093/jexbot/52.355.309. [DOI] [PubMed] [Google Scholar]
- 210.Forchino A.A., Lourguioui H., Brigolin D., Pastres R. Aquaponics and sustainability: The comparison of two different aquaponic techniques using the Life Cycle Assessment (LCA) Aquac. Eng. 2017;77:80–88. doi: 10.1016/j.aquaeng.2017.03.002. [DOI] [Google Scholar]
- 211.Lennard W.A. Ph.D. Thesis. School of Applied Sciences, Department of Biotechnology and Environmental Biology, Royal Melbourne Institute of Technology; Melbourne, VIC, Australia: 2006. Aquaponic Integration of Murray Cod (Maccullochella peelii peelii) Aquaculture and Lettuce (Lactuca sativa) Hydroponics. [Google Scholar]
- 212.Pantanella J.E. Ph.D. Thesis. Università degli studi della Tuscia; Viterbo, Italy: 2012. Nutrition and Quality of Aquaponic Systems. [Google Scholar]
- 213.Graber A., Junge R. Aquaponic systems: Nutrient recycling from fish wastewater by vegetable production. Desalination. 2009;246:147–156. doi: 10.1016/j.desal.2008.03.048. [DOI] [Google Scholar]
- 214.Bittsanszky A., Uzinger N., Mathis A., Gunlai G. Nutrient supply of plants in aquaponic systems. Ecocycles. 2016;2:17–20. doi: 10.19040/ecocycles.v2i2.57. [DOI] [Google Scholar]
- 215.Delaide B., Goddek S., Gott J., Soyeurt H., Haissam Jijakli M., Lalman J., Junge R. Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water. 2016;8:467. doi: 10.3390/w8100467. [DOI] [Google Scholar]
- 216.Rakocy J.E., Shultz R.C., Bailey D.S., Thoman E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system; Proceedings of the South Pacific Soilless Culture Conference-SPSCC 648; Palmerston North, New Zealand. 10–13 February 2003; pp. 63–69. [Google Scholar]
- 217.Licamele J. Ph.D. Thesis. Department of Agriculture and Biosystems Engineering, University of Arizona; Tuscon, AZ, USA: 2009. Biomass Production and Nutrient Dynamics in an Aquaponics System. [Google Scholar]
- 218.Liedl B.E., Cummins M., Young A., Williams M.L. Hydroponic lettuce production using liquid effluent from poultry waste bioremediation as a nutrient source; Proceedings of the VII International Symposium on Protected Cultivation in Mild Winter Climates: Production, Pest Management and Global Competition 659; Kissimmee, FL, USA. 23–27 March 2004; pp. 721–728. [Google Scholar]
- 219.Calfo A., Williams C. Book of Coral Propagation. 2nd ed. Volume 1. Reading Trees; Oxford, UK: 2007. 250p Reef Gardering for Aquarist. [Google Scholar]
- 220.Buzby K.M., West T.P., Waterland N.L., Lin L.S. Remediation of flow-through trout raceway effluent via aquaponics. N. Am. J. Aquac. 2017;79:53–60. doi: 10.1080/15222055.2016.1221010. [DOI] [Google Scholar]
- 221.Velichkova K., Sirakov I., Stoyanova S., Staykov Y. Cultivation of lettuce (Lactuca sativa L.) and rainbow trout (Oncorhynchus mykiss W.) in the aquaponic recirculation system. J. Cent. Eur. Agric. 2019;20:967–973. doi: 10.5513/JCEA01/20.3.2223. [DOI] [Google Scholar]
- 222.Endut A., Jusoh A., Ali N., Wan Nik W.B. Nutrient removal from aquaculture wastewater by vegetable production in aquaponics recirculation system. Desalination Water Treat. 2011;32:422–430. doi: 10.5004/dwt.2011.2761. [DOI] [Google Scholar]
- 223.Khater E.S.G., Bahnasawy A.H., Shams A.E.H.S., Hassaan M.S., Hassan Y.A. Utilization of effluent fish farms in tomato cultivation. Ecol. Eng. 2015;83:199–207. doi: 10.1016/j.ecoleng.2015.06.010. [DOI] [Google Scholar]
- 224.Bordignon F., Sturaro E., Trocino A., Birolo M., Xiccato G., Berton M. Comparative life cycle assessment of rainbow trout (Oncorhynchus mykiss) farming at two stocking densities in a low-tech aquaponic system. Aquaculture. 2022;556:738264. doi: 10.1016/j.aquaculture.2022.738264. [DOI] [Google Scholar]
- 225.Petrea Ş.M., Cristea V., Dediu L., Contoman M., Cretu M., Antache A., Coadă M.T., Bandi A.C. A Study of Phosphorus and Calcium Dynamics in an Integrated Rainbow Trout and Spinach (Nores variety) Aquaponic System with Different Crop Densities. Sci. Pap. Anim. Sci. Biotechnol. 2014;47:196–206. [Google Scholar]
- 226.Bordignon F., Gasco L., Birolo M., Trocino A., Caimi C., Ballarin C., Bortoletti M., Nicoletto C., Maucieri C., Xiccato G. Performance and fillet traits of rainbow trout (Oncorhynchus mykiss) fed different levels of Hermetia illucens meal in a low-tech aquaponic system. Aquaculture. 2022;546:737279. doi: 10.1016/j.aquaculture.2021.737279. [DOI] [Google Scholar]
- 227.Sirakov I., Velichkova K., Slavcheva-Sirakova D. The effect of yarrow (Achillea millefolium) supplemented diet on growth performance, biochemical blood parameters and meat quality of rainbow trout (Oncorhynchus mykiss W.) and growth of lettuce (Lactuca sativa) cultivated in aquaponic recirculation system. J. Hyg. Eng. Des. 2019;28:28–32. [Google Scholar]
- 228.Velichkova K., Sirakov I., Valkova E. The effect of sweet flag (Acorus calamus L.) supplemented diet on growth performance, biochemical blood parameters and meat quality of rainbow trout (Oncorhynchus mykiss W.) and growth of lettuce (Lactuca sativa L.) cultivated in aquaponic recirculation system. Aquac. Aquar. Conserv. Legis. 2020;13:3840–3848. [Google Scholar]
- 229.Petrea Ş.M., Cristea V., Dediu L., Contoman M., Lupoae P., Mocanu M. Vegetable Production in an Integrated Aquaponic System with Rainbow Trout and Spinach. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2013;70:45–54. [Google Scholar]
- 230.Khalil S. Growth performance, nutrients and microbial dynamic in aquaponics systems as affected by water temperature. Eur. J. Hortic. Sci. 2018;83:388–394. doi: 10.17660/eJHS.2018/83.6.7. [DOI] [Google Scholar]
- 231.Zainal A.G., Yulianto H., Yanfika H. Financial benefits of the environmentally friendly aquaponic media system; Proceedings of the IOP Conference Series: Earth and Environmental Science; Changsha, China. 18–20 September 2020; Bristol, UK: IOP Publishing; 2020. pp. 1–8. [Google Scholar]
- 232.Kaiser F., Harbach H. InnoFish-innovative adaptation of integrated aquaculture in an established extensive fish farm. AACL Bioflux. 2022;15:873–877. [Google Scholar]
- 233.Love D.C., Fry J.P., Genello L., Hill E.S., Frederick J.A., Li X., Semmens K. An international survey of aquaponics practitioners. PLoS ONE. 2014;9:e102662. doi: 10.1371/journal.pone.0102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Love D.C., Fry J.P., Li X., Hill E.S., Genello L., Semmens K., Thompson R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture. 2015;435:67–74. doi: 10.1016/j.aquaculture.2014.09.023. [DOI] [Google Scholar]
- 235.Villarroel M., Junge R., Komives T., König B., Plaza I., Bittsánszky A., Joly A. Survey of aquaponics in Europe. Water. 2016;8:468. doi: 10.3390/w8100468. [DOI] [Google Scholar]
- 236.Goddek S., Delaide B., Mankasingh U., Ragnarsdottir K.V., Jijakli H., Thorarinsdottir R. Challenges of sustainable and commercial aquaponics. Sustainability. 2015;7:4199–4224. doi: 10.3390/su7044199. [DOI] [Google Scholar]
- 237.Palm H.W., Bissa K., Knaus U. Significant factors affecting the economic sustainability of closed aquaponic systems. Part II: Fish and plant growth. Aquac. Aquar. Conserv. Legis. 2014;7:162–175. [Google Scholar]
- 238.Maucieri C., Nicoletto C., Zanin G., Birolo M., Trocino A., Sambo P., Borin M., Xiccato G. Effect of stocking density of fish on water quality and growth performance of European Carp and leafy vegetables in a low-tech aquaponic system. PLoS ONE. 2019;14:e0217561. doi: 10.1371/journal.pone.0217561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hussain T., Verma A.K., Tiwari V.K., Prakash C., Rathore G., Shete A.P., Saharan N. Effect of water flow rates on growth of Cyprinus carpio var. Koi (Cyprinus carpio L., 1758) and spinach plant in aquaponic system. Aquac. Int. 2015;23:369–384. doi: 10.1007/s10499-014-9821-3. [DOI] [Google Scholar]
- 240.Alcarraz Q.E.W., Tapia L.O., Alcarraz Q.Y.M. Microbiological analysis of lettuce (Lactuca sativa L.) grown in an aquaponic and hydroponic system. Net J. Agric. Sci. 2019;7:50–55. [Google Scholar]
- 241.Hussain T., Verma A.K., Tiwari V.K., Prakash C., Rathore G., Shete A.P., Nuwansi K.K.T. Optimizing koi carp, Cyprinus carpio var. Koi (Linnaeus, 1758), stocking density and nutrient recycling with spinach in an aquaponic system. J. World Aquac. Soc. 2014;45:652–661. doi: 10.1111/jwas.12159. [DOI] [Google Scholar]
- 242.Rayhan M.Z., Rahman M.A., Hossain M.A., Akter T., Akter T. Effect of stocking density on growth performance of monosex tilapia (Oreochromis niloticus) with Indian spinach (Basella alba) in a recirculating aquaponic system. Int. J. Environ. Agric. Biotechnol. 2018;3:239073. doi: 10.22161/ijeab/3.2.5. [DOI] [Google Scholar]
- 243.Ashley P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007;104:199–235. doi: 10.1016/j.applanim.2006.09.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.