Abstract
The synovial membrane in osteoarthritis (OA) often exhibits inflammatory infiltrates, but the role of T cells in these infiltrates is not known. T-cell activation antigens were analyzed by immunohistochemistry, and T-cell cytokine transcripts were measured by competitive PCR in synovial membranes from patients with OA and rheumatoid arthritis (RA). Lymphoid cell aggregates, containing primarily CD3+ T lymphocytes, were found in 65% of patients with OA. Mononuclear cells expressing the activation antigens CD69, CD25, CD38, CD43, CD45RO, and HLA class II were present in both patient groups, although in higher numbers in patients with RA. Interleukin 2 (IL-2) transcripts were found in 10 of 18 patients with OA versus 12 of 13 patients with RA (P = 0.03). Gamma interferon (IFN-γ) transcripts were detected in 9 of 18 patients with OA versus 10 of 13 patients with RA (not significant), whereas IL-10 transcripts were found in nearly all patients. IL-4 and IL-5 were not detected in any patients. The levels of IFN-γ and IL-2 transcripts, normalized for T-cell number equivalents, were not statistically different between OA and RA, but the levels of IFN-γ, normalized for total cell number equivalents, were lower in OA than in RA (P = 0.01). Synovial membranes that expressed IL-2 and IFN-γ transcripts were more likely to have heavier infiltrations of T cells and cells bearing activation markers than synovial membranes that did not express these cytokines. The presence of activated T cells and TH1 cytokine transcripts in chronic joint lesions of patients with OA suggests that T cells contribute to chronic inflammation in a large proportion of these patients.
Osteoarthritis (OA), although a heterogeneous disease, is generally believed by rheumatologists to be primarily a disease of biomechanical alteration (18). However, apart from the relatively rare type of erosive inflammatory OA which clearly shows a strong inflammatory component, certain patients with OA exhibit inflammatory infiltrates in the synovial membrane (SM) (15, 17, 23, 28). These mononuclear infiltrates have not been fully characterized, and their possible role in the pathogenesis of the disease is not clearly understood. In certain patients with OA, mononuclear cell infiltrates in SM may resemble those found in rheumatoid arthritis (RA). In RA, significant evidence demonstrating that T cells play a significant role in the pathogenesis of the disease has accumulated (reviewed in reference 46). This evidence includes the amelioration of the disease by treatments directed against T cells, the association of the disease with certain HLA-DR4 alleles, and the presence in the SM of patients with RA of infiltrating T cells which express activation antigens, produce cytokines, and contain oligoclonal populations of T cells (reviewed in reference 46).
T-cell-derived cytokines are major determinants of the outcome of immune responses. TH1 cytokines (interleukin 2 [IL-2] and gamma interferon [IFN-γ]) are associated with macrophae activation, enhancement of cell-mediated cytotoxicity, delayed-type hypersensitivity responses, and effective responses to intracellular pathogens (38, 48, 62). TH2 cytokines (IL-4 and IL-5) are associated with allergic diseases, helminthic infections, and progressive infections by intracellular bacteria (38). A biased cytokine pattern is also found in animal models of autoimmune disease. For example, in experimental allergic encephalomyelitis, IL-2 and IFN-γ, but not IL-4, are expressed in the brain of rats at the peak of disease, whereas during recovery, the expression of IL-2 and IFN-γ decrease with the concomitant appearance of IL-4 (24). Also, in nonobese diabetic mice, IL-4 production is compromised, while administration of IL-4 to prediabetic mice prevents the development of diabetes (44).
Although several studies have examined the TH1/TH2 cytokine pattern in SM of patients with RA and have reported the prevalence of a TH1 pattern (9, 25, 33, 42, 47, 51, 58), the role of T cells and the pattern of TH1/TH2 cytokines in patients with OA are largely unknown. In this study, we employed (i) immunohistochemistry with a panel of monoclonal antibodies (MAbs) to antigens expressed on activated T cells to characterize the mononuclear cell infiltrates, and (ii) reverse transcriptase (RT) PCR and competitive PCR to detect and quantitate T-cell cytokine transcripts in SM from patients with OA.
MATERIALS AND METHODS
Patients.
Thirty patients with OA (37) (13 males, 17 females; age, 61.4 ± 11.5 [mean ± standard deviation {SD}]) were included in this study. All patients were seronegative for rheumatoid factor and were treated with nonsteroidal anti-inflammatory drugs (NSAIDs).
Thirteen patients with RA, diagnosed according to the 1987 criteria of the American College of Rheumatology (4) (3 males, 10 females; age, 61.8 ± 9.2 [mean ± SD]), were also included in the study. Their latest values of erythrocyte sedimentation rate were 56.6 ± 31 (mean ± SD), and four patients were seronegative for rheumatoid factor. All patients were treated with NSAIDs; in addition, three patients were treated with methotrexate, two patients were treated with hydroxychloroquine, and three patients were treated with gold injections.
Synovial tissue specimens.
Synovial tissue specimens were obtained during joint replacement or synovectomy (two RA patients). These were from knee (17 OA, 7 RA), hip (13 OA, 4 RA), and metacarpophalangeal (2 RA) joints. Specimens were divided in two and a portion was snap frozen in liquid nitrogen and kept at −70°C; the remaining portion was embedded in OCT (optimal cutting temperature) compound before being frozen for the immunohistochemical studies described below.
Preparation of PBMC.
Peripheral blood mononuclear cells (PBMC) from healthy donors were prepared by centrifugation on a Ficoll-Hypaque density cushion according to standard methods.
MAbs.
An anti-CD3 MAb (clone UCHT1; Novocastra, Newcastle upon Tyne, United Kingdom) was used as a marker of T cells. The following MAbs were used to identify activation markers: anti-CD69 (clone FN50; Pharmingen, San Diego, Calif.) (56), anti-CD25 (clone Tu69; Novocastra), anti-CD45RO (clone UCHL1; Novocastra) (1), anti-HLA class II (clone CR3/43; Dako, Carpinteria, Calif.), anti-CD38 (clone T16; Biomeda) (19), and anti-CD43 (clone DF-T1; Dako) (41). All MAbs were used at the optimal concentrations suggested by the suppliers.
Immunohistochemistry.
Six-micrometer-thick tissue sections were air dried for 2 h, fixed in cold acetone for 30 min, treated with cold methanol-H2O2 to block endogenous peroxidase activity, and stained with MAbs by the avidin-biotin complex immunoperoxidase method, according to the supplier’s instructions (Vector Laboratories, Burlingame, Calif.). Briefly, serial sections were first incubated with normal blocking serum for 1 h and then with a relevant MAb for 1 h at room temperature. An isotype-matched nonspecific mouse immunoglobulin G was used as a negative control. Next, sections were incubated with biotinylated anti-mouse antibody and subsequently with avidin-biotin peroxidase complex, each for 30 min. Between steps, sections were washed in phosphate-buffered saline (5 min). Finally, sections were developed with 3′,3′-diaminobenzidine as the chromogen and lightly counterstained with Meyer’s hematoxylin.
Grading of mononuclear cell infiltrates.
Infiltrating CD3+, CD45RO+, CD25+, CD69+, HLA class II+, CD38+, and CD43+ cells were determined independently by two observers, who were blinded to the identity of the specimens, at a magnification of ×400 (high-power field [HPF]) with a gridded ocular lens. The most heavily infiltrated HPFs were selected, and positive cells were counted in five different HPFs. Results from the two independent observers were averaged and were expressed as mean numbers of positive cells per HPF. The cell infiltration for each marker was graded on a relative scale of 0 to 4 (55), as follows. For CD3 and CD45RO antigens: 0, <2 cells; 1, 2 to 50 cells; 2, 51 to 100 cells; 3, 101 to 150 cells; 4: >150 cells. For CD25, CD69, CD38, and CD43 antigens: 0, <1 cell; 1, 1 to 20 cells; 2, 21 to 40 cells; 3, 41 to 60 cells; 4, >60 cells. For HLA class II antigens: 0, <20 cells; 1, 21 to 100 cells; 2, 101 to 200 cells; 3, 201 to 300 cells; 4, >300 cells. This grading system takes into account the fact that some of these activation antigens are expressed on more cells than others. Hematoxylin and eosin staining was also used on sequential sections to confirm cell morphologies.
Synthesis of cDNA.
Tissue specimens were homogenized with a tissue grinder (Kontes), and total RNA was prepared by a modification of the guanidinium-based method (6) with RNazol B (Tel-Test, Friendwood, Tex.). First-strand cDNA was synthesized from 5 μg of total RNA with avian myeloblastosis virus RT and 0.5 μg of oligo(dT) as a primer (Promega, Madison, Wis.). cDNA was heated at 94°C for 6 min to inactivate the RT, diluted 1:5, and kept at −20°C until needed.
PCR.
IL-2, IFN-γ, IL-4, IL-5, and IL-10, as well as β-actin and CD3δ transcripts, were amplified by PCR. The following primers (5′→3′) were used (62): IL-2, 5′-ACTCACCAGGATGCTCACAT and 3′-AGGTAATCCATCTGTTCAGA; IFN-γ, 5′-AGTTATATCTTGGCTTTTCA and 3′-ACCGAATAATTAGTCAGCTT; IL-4, 5′-CTTCCCCCTCTGTTCTTCCT and 3′-TTCCTGTCGAGCCGTTTCAG; IL-5, 5′-ATGAGGATGCTTCTGCATTTG and 3′-TCAACTTTCTATTATCCACTCGGTGTTCATTAC; IL-10, 5′-ATGCCCCAAGCTGAG AACCAAGACCCA and 3′-TCTCAAGGGGCTGGGTCAGCTATCCCA; CD3δ, 5′-CTGGACCTGGGAAAACGCATC and 3′-GTACTGAGCATCATCTCGATC; and β-actin, 5′-GTGGGGCGCCCCAGGCACCA and 3′-CTCCTTAATGTCACGCACGATTTC. Amplification primers were chosen to ensure that primer templates would span at least one intron. Any contaminating genomic DNA would result in a larger product than the cytokine transcript (62).
cDNA was amplified in a 40-cycle PCR, with each cycle at 94°C for 45 s, 55°C (76°C for IL-10, 65°C for β-actin, and 60°C for CD3δ) for 45 s, and 72°C (78°C for IL-10) for 90 s. For IL-4 and IL-5 amplification, two-phase cycles at 94°C for 45 s and 67°C (IL-4) or 60°C (IL-5) for 2.5 min were performed. Amplifications of cDNAs (50 ng of RNA equivalents) were carried out in a standard reaction mixture containing 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 1.5 mM MgCl2 (1.0 mM for IL-2 and IFN-γ), 200 μM (each) deoxynucleoside triphosphates, and 2.5 U of Taq DNA polymerase (Promega) in a Perkin-Elmer 480 thermocycler.
Quantitative PCR.
Competitive PCR, designated MIMIC PCR, was used to quantitate cytokine transcripts (14). Internal nonhomologous competitive fragments (MIMIC DNA) for each cytokine were constructed according to the instructions of the supplier (Clontech, Palo Alto, Calif.). Serial PCR mixtures containing a constant amount of sample cDNA (50 ng of RNA equivalents) were spiked with decreasing concentrations of MIMIC DNA. The PCR products were separated by electrophoresis through an ethidium bromide-stained 1.6% agarose gel and quantitated by comparing the intensity of the cytokine and MIMIC bands. When target cytokine DNA and MIMIC DNA product bands were equal in intensity, target DNA was equimolar to MIMIC DNA prior to amplification.
Statistical analysis.
Fisher’s exact, Mann-Whitney, Spearman correlation, and Student’s t tests were used as appropriate with two-tailed P values on Prism software.
RESULTS
Distribution of T cells and cells expressing activation antigens in the SM of patients with OA and RA.
Histology was evaluated in specimens from 30 patients with OA and 10 patients with RA. OA specimens exhibited varying degrees of mononuclear cell infiltration, with mild to moderate synovial cell hyperplasia. All 10 RA specimens variously exhibited typical changes of moderate to pronounced chronic synovitis. One specimen featured typical “rheumatoid nodules.” Overall, inflammatory changes in OA were less prominent than in RA. Histological findings and typical immunoreactivity profiles of serial sections from one OA specimen are shown in Fig. 1. Lymphocytic nodular aggregates, each containing >40 densely packed mononuclear cells, were found in 65% of SM specimens from patients with OA. These nodular aggregates were distributed around blood vessels as in RA, were primarily CD3+ T cells, and in some instances were indistinguishable from those found in RA. Cells positively stained for the activation antigens (CD69, CD25, HLA class II, CD38, CD43, and CD45RO) were localized to areas with CD3+ cells. Positive cells per HPF were counted by two observers independently with good interobserver agreement (κ = 0.8 [κ test; κ measures the interobserver agreement and ranges between 0, no agreement, and 1, complete agreement) (3). Variability of positive cell counts within the same specimen carried out by a single observer was 6%. A comparison of the numbers of positive cells per HPF (×400) for these antigens between OA and RA is shown in Table 1. Although similar immunoreactivity profiles were present in both patient groups, specimens from patients with OA were more likely to have fewer infiltrating CD3+ T cells (P = 0.01), CD69+ cells (P = 0.01), CD25+ cells (P = 0.004), HLA class II+ cells (P = 0.005), CD38+ cells (P = 0.003), CD43+ cells (P = 0.003), and CD45RO+ cells (P = 0.008) than patients with RA (Mann-Whitney test).
FIG. 1.
Avidin-biotin complex immunostaining with light hematoxylin counterstaining of the SM from a patient with OA (original magnification, ×400). Serial sections of the same field depicting nodular-perivascular mononuclear cell infiltrate were stained with anti-CD3, anti-CD69, anti-CD25, anti-CD45RO, anti-HLA class II, anti-CD38, and anti-CD43 MAbs and immunoglobulin G1 as a negative control. Immunoreactivity was more widespread with antibodies to late-activation antigens CD45RO and HLA class II than to early-activation antigens CD69 and CD25.
TABLE 1.
Comparison of numbers of cells positive for activation antigens between OA and RA
| Antigen | Median no. of cells per HPF (range)
|
Pa | |
|---|---|---|---|
| OA | RA | ||
| CD3 | 27.35 (0–831) | 92.3 (32.7–472.2) | 0.01 |
| CD45RO | 30.4 (1.8–662.2) | 147.6 (26–410.5) | 0.008 |
| CD69 | 7.7 (0–614.4) | 48.9 (0–212.9) | 0.01 |
| CD25 | 2.2 (0–220.5) | 18.4 (4–73) | 0.004 |
| HLA class II | 59.7 (0–980.2) | 152.9 (77–433.6) | 0.005 |
| CD38 | 6.2 (0–478.3) | 40 (12.8–124.8) | 0.003 |
| CD43 | 22.2 (0–235.1) | 91.7 (27.4–402.4) | 0.003 |
By Mann-Whitney test.
Next, the numbers of positive cells per HPF were expressed as relative grades 0 through 4 (as described in Materials and Methods), and the percentages of OA and RA patients in each grade are shown in Fig. 2. Except for two specimens, all OA specimens showed mild to heavy CD3+ T-cell infiltration. In particular, 17 of 30 OA specimens showed 2 to 50 CD3+ T cells, 5 of 30 specimens showed 51 to 100 T cells, 1 of 30 showed 101 to 150 T cells, and 5 of 30 OA specimens showed more than 150 T cells per HPF (×400). The respective figures in RA were 2 of 10, 3 of 10, 1 of 10, and 4 of 10. CD69 is an early-activation antigen (56), CD25 is an intermediate-activation antigen, CD45RO and HLA class II are late-activation antigens (1), CD38 is an intermediate-activation antigen (19), and CD43 antigen has diverse functions (41). Immunoreactivity was far more widespread with antibodies to late-activation antigens (CD45RO, HLA class II) than to early-activation antigen (CD69) in both patient groups (Fig. 1 and 2). CD38+ cells were commonly found at the periphery of nodular infiltrates in both OA and RA.
FIG. 2.
Degree of SM infiltration with mononuclear cells expressing activation markers in patients with OA and RA. Cell infiltration was graded on a relative scale of 0 to 4, based on the number of positive cells per HPF (×400). CD3 (A) and CD45RO (B) grading: 0, <2 cells; 1, 2 to 50 cells; 2, 51 to 100 cells; 3, 101 to 150 cells; 4, >150 cells. HLA class II (C) grading: 0, <20 cells; 1, 21 to 100 cells; 2, 101 to 200 cells; 3, 201 to 300 cells; 4, >300 cells. CD69 (D), CD25 (E), CD38 (F), and CD43 (G) grading: 0, <1 cell; 1, 1 to 20 cells; 2: 21 to 40 cells; 3, 41 to 60 cells; 4, >60 cells. Bars represent percentages of patients at each grade.
Detection of cytokine transcripts.
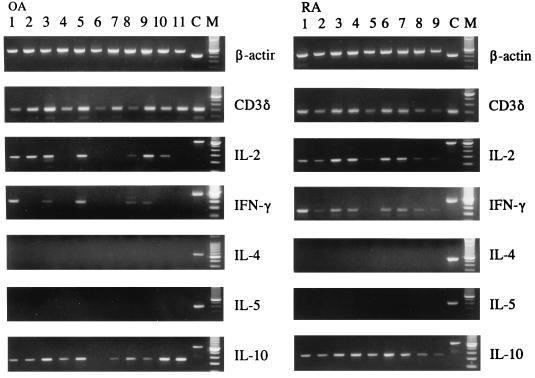
To assess the sensitivity of the method, detection of CD3δ transcripts from low numbers of PBMC was carried out in initial experiments. After 35 and 40 cycles of amplification, CD3δ transcripts were detected from as few as 50 and 5 PBMC equivalents, respectively (data not shown). Additionally, after 40 amplification cycles, IL-2 transcripts were detected from as few as 103 phytohemagglutinin-stimulated normal PBMC equivalents (data not shown). IL-2 transcripts were detected in the SM from 10 of 18 patients with OA and in the SM from 12 of 13 patients with RA (P = 0.03 [Fisher’s exact test]). Representative results from 7 OA and 9 RA patients are shown in Fig. 3. IFN-γ transcripts were detected in the SM of 9 of 18 patients with OA and in the SM of 10 of 13 patients with RA (P = 0.12 [not significant {NS}] [Fisher’s exact test]). In contrast, IL-10 was detected in nearly all patients in both groups (16 of 18 patients with OA and 12 of 13 patients with RA). IL-4 and IL-5 transcripts were not detected in any specimens.
FIG. 3.
Cytokine transcript profile in SM specimens from representative patients with OA and RA. The respective MIMIC DNAs or cDNA from phytohemagglutinin-stimulated normal PBMC were used as a positive control (C). A 100-bp DNA ladder was used as a molecular weight marker (M).
Quantitation of cytokine transcripts.
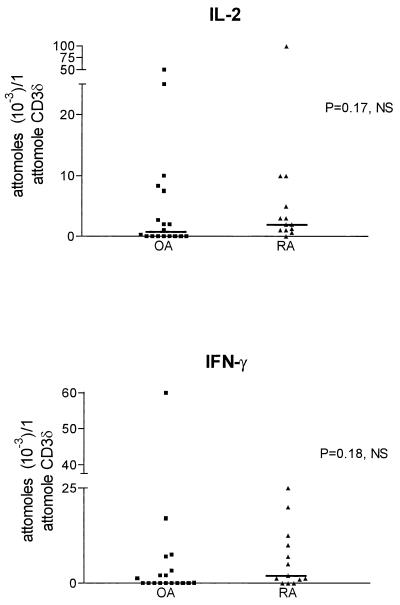
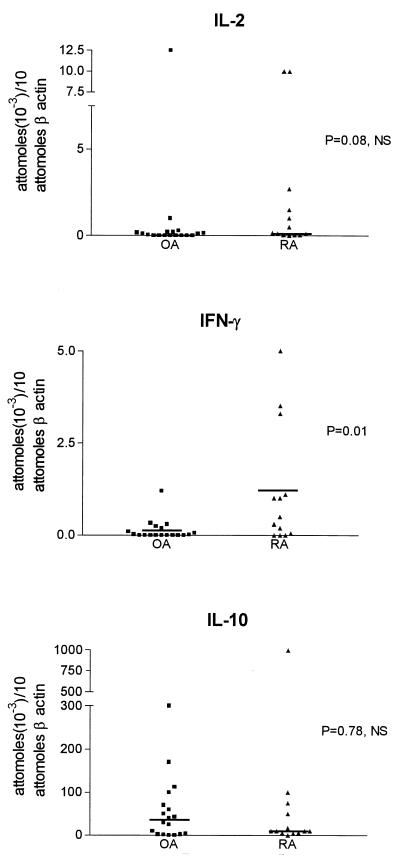
A representative example of cytokine (IL-10) transcript quantitation by MIMIC PCR is shown in Fig. 4. cDNA was normalized for β-actin (10 attomol of β-actin per 50 ng of RNA equivalent) as a measure of cell number equivalents. cDNA was also normalized for CD3δ as a measure of T-cell number equivalents (1 attomol of CD3δ per 50 ng of RNA equivalent) when T-cell cytokine transcripts (IL-2, IFN-γ) were analyzed. There was no difference in the levels of IL-2 and IFN-γ transcripts normalized for T-cell equivalents between the two groups of patients (Mann-Whitney t test) (Fig. 5). However, the levels of IFN-γ transcripts, normalized for the total cell number equivalent, were lower in OA than RA (P = 0.01 [Student’s t test]), in agreement with the significantly lower CD3+ cell number per HPF in OA. The levels of IL-10 transcripts were between 0 and 300 attomol in OA and between 10 and 1,000 attomol per 10 attomol of β-actin in RA (Fig. 6). IL-10 levels were 100- to 1,000-fold higher than those of IFN-γ (normalized for β-actin) in patients with RA and OA (Fig. 6). There was no statistical difference in IL-10 transcript levels between the two groups of patients (Mann-Whitney test).
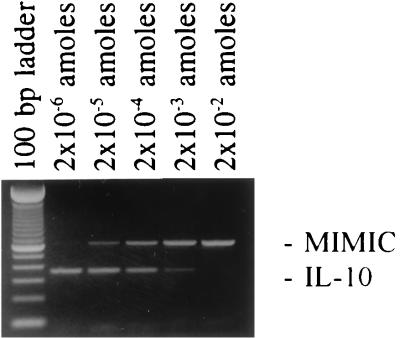
FIG. 4.
Example of cytokine (IL-10) transcript quantitation by MIMIC PCR. A constant amount of cDNA was amplified along with serial dilutions of a known quantity of IL-10 MIMIC DNA. As the intensity of the MIMIC DNA band was decreasing, the intensity of the IL-10 band was increasing. The lane with 1:1 ratio of MIMIC to IL-10 bands corresponds to 2 × 10−4 attomol (amoles) of MIMIC DNA input and shows an equimolar amount of IL-10 cDNA.
FIG. 5.
IL-2 and IFN-γ transcript levels in OA and RA SM quantitated by MIMIC PCR. Cytokine transcripts were normalized for CD3δ transcripts and therefore for T-cell number equivalents.
FIG. 6.
IFN-γ and IL-10 transcript levels in SM from patients with OA and RA normalized for β-actin cDNA and therefore for total cell number equivalents. IFN-γ transcript levels were higher in RA than in OA (P = 0.01). IL-10 transcript levels were 100- to 1,000-fold higher than those of IFN-γ in both patient groups.
SM specimens from patients with OA that expressed IL-2 and IFN-γ transcripts were more likely to have higher numbers of infiltrating T cells and cells expressing activation antigens (except for HLA class II) than specimens that did not express these cytokines (P < 0.001 [Mann-Whitney]).
DISCUSSION
This study revealed SM mononuclear cell infiltrates, mostly consisting of T cells and expressing early (CD69)-, intermediate (CD25, CD38)-, and late (CD45RO, HLA class II)-activation antigens, in the majority of patients with OA. These patients had advanced disease, since they required joint replacement. This is an interesting finding since OA is largely regarded as a noninflammatory disease. The presence of early-, intermediate-, and late-activation antigen in OA suggests active on-going inflammation in the SM. While the memory T cells (CD45RO+ cells) may extravasate into the SM from the peripheral blood, the presence of T cells expressing CD69 suggests activation occurring in situ. Interestingly, CD38 antigen mediates cell adhesion to vascular endothelial cells, whereas CD43 is a ligand for ICAM-1, which in turn is up-regulated on fibroblasts and macrophages by IFN-γ.
Others (15, 17, 23, 28, 32) have also reported the presence of mononuclear infiltrates containing CD3+ T cells in the SM of certain patients with OA, which in some cases resembled those of RA. In addition, the acute-phase proteins C-reactive protein and serum amyloid A have been reported to be elevated in OA, albeit to a lesser extent than found in RA (52), suggesting the presence of considerable inflammation in patients with OA. Furthermore, the normally HLA-DR-negative chondrocytes become positive in OA (32), suggesting that they may function as antigen-presenting cells. It has been suggested that the synovial inflammation in OA is a secondary phenomenon caused by fragments of cartilage or crystals (23). This view is contradicted by the finding that lymphoid follicles are present to a greater extent in primary OA than in mechanical or traumatic OA, and detritic fragments of bone, cartilage, or calcium pyrophosphate crystals do not correlate with inflammatory infiltrates (45). In a recent study, lymphocytic aggregates were found in 14% of patients with early OA and in 37% of patients with late OA at the time of joint replacement surgery (53). In our study, which was based on patients with advanced OA, lymphoid nodular aggregates containing >40 densely packed mononuclear cells were found in 65% of patients.
IL-2 and IFN-γ (TH1 cytokines) were detected in the SM in 50% of the patients with OA examined, whereas IL-4 and IL-5 (TH2 cytokines) were not detected in any patient. Limited information is available on T-cell-derived cytokines in OA and is derived mostly from small numbers of specimens and from studies where these specimens have been used as a control for RA (8, 11, 49, 59, 60). By in situ hybridization, which is less sensitive than the method employed in our study, IFN-γ transcripts were barely detectable (11) and IL-2 transcripts were undetectable (60) in OA and RA SM specimens. However, IFN-γ protein was detected in the SM of most patients with OA examined by immunohistochemistry (36). IFN-γ protein (21, 49) and IL-4 protein (49) were also detected in the synovial fluid. In our study, the levels of IFN-γ transcripts in OA were similar to those in RA when normalized for T-cell equivalents. This suggests that T cells infiltrating the SM of patients with OA produce levels of cytokines similar to patients with RA. This finding was surprising, since we expected higher IFN-γ levels in RA than in OA. One possible explanation is that our RA patients were late in the course of their disease, and rheumatoid disease may have subsided. IFN-γ transcript levels were lower in OA than in RA when normalized for total cell equivalents, a finding that reflected the lower numbers of infiltrating T cells in the SM in this disease.
Several studies have addressed the TH1/TH2 cytokine pattern in RA and demonstrated predominantly TH1 cytokines (5, 8, 9, 25, 33, 42, 47, 51, 58). In the rheumatoid SM, a clear TH1 pattern at the transcript level was found by some investigators (47, 51), whereas a mixed cytokine pattern was found by others (25, 58). A recent study addressed the question of cytokine mRNA patterns in different types of synovitis in RA (25). High levels of IFN-γ and IL-4 transcripts were associated with granulomatous synovitis, and low levels of IFN-γ and IL-4 transcripts were found in diffuse synovitis, whereas a clear TH1 pattern was present in follicular synovitis.
In agreement with these results (25), the RA specimens that we have examined in this study had mainly follicular histology and a clear TH1 pattern. IFN-γ was detected in 10 of 13 patients with RA, whereas IL-4 was not found in any specimen. Furthermore, IFN-γ was detected by immunostaining in all 10 patients with RA in one study (8). However, a TH0 (IFN-γ and IL-4) pattern was reported in another study (57). A TH1 pattern was also found in T-cell clones developed from rheumatoid SM (34). A clear TH1 cytokine pattern at the mRNA (5) and protein (8) levels was found in rheumatoid synovial fluid, as well as in the majority of T-cell clones developed from rheumatoid synovial fluid (43).
IL-10, produced by both T cells and monocytes in the SM (22), was frequently detected in addition to IFN-γ in nearly all patients with OA and RA. An anti-inflammatory TH2 cytokine in mice, IL-10 in humans inhibits the production of proinflammatory cytokines by monocytes (30) and RA synovial macrophages (22, 54). IL-10 production in OA and RA may be driven by IL-12, since IL-12 induces concomitant secretion of IFN-γ and IL-10 by T cells in humans (13, 61) and IL-12 is present in the SM in both diseases (47). Our findings that the transcript levels of IL-10 were higher than the levels of IFN-γ are consistent with previous observations that non-T-cell cytokines are abundant in the SM of patients with RA and OA (7, 39).
The abundance of macrophage cytokines in OA has recently led to speculation that cells of macrophage lineage are important in the pathogenesis of OA (40). These cells produce IL-1 and tumor necrosis factor alpha, which in turn stimulate the production of metalloproteinases and prostaglandins (40), leading to breakdown of the extracellular matrix of cartilage. The present study suggests that T cells may also be important in the pathogenesis of OA. For example, activated T cells may contribute to OA joint destruction by inducing the production of collagenase in SM (34). It is also known that TH1 cytokines (IFN-γ) induce increased adhesion of mononuclear cells to synovial fibroblasts (50), activate macrophages, up-regulate HLA class II expression in a variety of cell types, and thus contribute to ongoing inflammation in both OA and RA. IFN-γ also inhibits type II collagen synthesis and in this way contributes to cartilage damage (16).
The process that drives an immune response toward a TH1 or TH2 cytokine pattern has not been fully elucidated. Among the factors found to play a role are the binding affinity of antigenic peptides for the major histocompatibility complex (27), the antigen-presenting cells (10), and the local balance of IL-4 and IL-12, the former favoring the TH2 pattern and the latter favoring the TH1 pattern (31, 38). An inciting antigen(s), which has not yet been identified, might be the driving force for this TH1-cell activation. Proliferative responses of peripheral blood and synovial fluid T cells to chondrocyte membranes from patients with RA and OA have been reported (2, 36). IL-12, which has been found in the SM of patients with RA and OA (47) and is produced by macrophages during phagocytosis even of inert material (12), might drive the cytokine pattern toward TH1. Another mechanism of TH1-cell recruitment in SM may be through chemokines, possibly in conjunction with adhesion molecules. The chemokine MIP-1β (macrophage inflammatory protein 1β) has been shown to be up-regulated in the synovial fluid of patients with OA (26). MIP-1β is a ligand for the chemokine receptor CCR5, which is expressed in high levels in TH1 cells and acts as a chemoattractant for these cells (29, 43). This chemokine may be responsible, at least in part, for attracting TH1 lymphocytes in the SM of these patients.
Two important issues arise from this study. First, the presence of lymphoid aggregates, comprised primarily of T cells, and TH1 cytokines along with activated macrophages in OA indicates that a cell-mediated immune response takes place in a substantial proportion of patients with OA. This finding reemphasizes the notion that simple analgesics without anti-inflammatory properties may be inadequate treatment for this group of patients. Second, manipulation of the cytokine pattern may be a potential biological treatment not only for RA but also for OA. IL-4 alone (35) or in combination with IL-10 (54) resulted in a reduction of proinflammatory cytokine production in SM from patients with RA in vitro. IL-4 in combination with IL-10 suppressed collagen-induced arthritis in mice (20). Trials of IL-4 and IL-10 in patients with RA are awaited with interest.
ACKNOWLEDGMENTS
This work was supported in part by grants RO1 AR41003 and T32 AI07101 from the National Institutes of Health.
REFERENCES
- 1.Akbar A N, Terry L, Timms A, Beverley P C, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- 2.Alsalameh S, Mollenhauter J, Hain N, Stock K-P, Kalden J, Burmester G R. Cellular immune response toward human articular chondrocytes. T cell reactivities against chondrocyte and fibroblast membranes in destructive joint diseases. Arthritis Rheum. 1990;33:1477–1486. doi: 10.1002/art.1780331004. [DOI] [PubMed] [Google Scholar]
- 3.Altman D G. Practical statistics for medical research. London, England: Chapman & Hall; 1991. [Google Scholar]
- 4.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S, Healey L A, Kaplan S R, Liang M H, Luthra H S, Medger T A, Jr, Mitchell D M, Neustadt D H, Pinals R S, Schaller J G, Sharp J T, Wilder R L, Hunder G G. The American Rheumatism Association 1987 criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 5.Bucht A, Larsson P, Weisbrot L, Thorne C, Pisa P, Smedegaard G, Keystone E C, Gronberg A. Expression of interferon-gamma (IFN-γ), IL-10, IL-12 and transforming growth factor-beta (TGF-beta) mRNA in synovial fluid cells from patients in the early and late phases of rheumatoid arthritis. Clin Exp Immunol. 1996;103:357–367. doi: 10.1111/j.1365-2249.1996.tb08288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Chu C-Q, Field M, Feldman M, Maini R N. Localization of tumor necrosis factor α in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- 8.Dolhain R J, der Haar N T, Hoefakker S, Tak P P, de Ley M, Claassen E, Breedveld F C, Miltenburg A M. Increased expression of interferon (IFN)-gamma together with IFN-γ receptor in the rheumatoid synovial membrane compared with synovium of patients with osteoarthritis. Br J Rheumatol. 1996;35:24–32. doi: 10.1093/rheumatology/35.1.24. [DOI] [PubMed] [Google Scholar]
- 9.Dolhain R J, van der Heiden A N, der Haar N T, Breedveld F C, Miltenburg A M. Shift toward T lymphocytes with a helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–1969. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 10.Duncan D D, Swain S L. Role of antigen presenting cells in the polarized development of helper T cell subsets: evidence for differential cytokine production by Th0 cells in response to antigen presentation by B cells and macrophages. Eur J Immunol. 1994;24:2506–2514. doi: 10.1002/eji.1830241037. [DOI] [PubMed] [Google Scholar]
- 11.Firestein G S, Alvaro-Garcia J M, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–3353. [PubMed] [Google Scholar]
- 12.Fulton S, Johnsen J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli M T, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliland G, Perrin S, Blanchard K, Bunn H F. Analysis of cytokine mRNA and DNA detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci USA. 1990;87:2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldenberg D L, Egan M S, Cohen A S. Inflammatory synovitis in degenerative joint disease. J Rheumatol. 1982;9:204–209. [PubMed] [Google Scholar]
- 16.Goldring M B, Sandel L J, Stephenson M L, Krane S M. Immune interferon suppresses levels of procollagen in mRNA and type II collagen synthesis in cultured human articular and costal chondrocytes. J Biol Chem. 1986;261:9049–9056. [PubMed] [Google Scholar]
- 17.Haraoui B, Pelletier J P, Cloutier J M, Faure M P, Martel-Pelletier J. Synovial membrane histology and immunopathology in rheumatoid arthritis and osteoarthritis: in vivo effects of anti-rheumatic drugs. Arthritis Rheum. 1991;34:153–163. doi: 10.1002/art.1780340205. [DOI] [PubMed] [Google Scholar]
- 18.Howel D S, Pelletier J-P. Etiopathogenesis of osteoarthritis. In: McCarty D J, Koopman W J, editors. Arthritis and allied conditions. Philadelphia, Pa: Lea & Febiger; 1993. pp. 1723–1734. [Google Scholar]
- 19.Jackson D G, Bell J I. Isolation of a cDNA encoding the human CD38(T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990;144:2811–2815. [PubMed] [Google Scholar]
- 20.Joosten L A B, Lubberts E, Durez P, Helsen M M A, Jacobs M J M, Goldman M, van den Berg W B. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 21.Kahle P, Saal J G, Schaudt K, Zacher J, Fritz P, Pawelec G. Determination of cytokines in synovial fluids: correlation with diagnosis and histomorphological characteristics of synovial tissue. Ann Rheum Dis. 1992;51:731–734. doi: 10.1136/ard.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsikis P D, Chu C-Q, Brennan F M, Maini R N, Feldman M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994;179:1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy T D, Plater-Zyberk C, Partridge T A, Woodrow D F, Maini R N. Morphometric comparison of synovium from patients with osteoarthritis and rheumatoid arthritis. J Clin Pathol. 1988;41:847–852. doi: 10.1136/jcp.41.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoury S J, Hancock W W, Weiner H L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klimiuk P A, Goronzy J J, Bjornsson J, Beckenbaugh A, Weyand C M. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am J Pathol. 1997;151:1311–1319. [PMC free article] [PubMed] [Google Scholar]
- 26.Koch A E, Kunkel S L, Shah M R, Fu R, Mazarakis D D, Haines G K, Burdick M D, Pope R M, Strieter R M. Macrophage inflammatory protein-1β: a C-C chemokine in osteoarthritis. Clin Immunol Immunopathol. 1995;77:307–314. doi: 10.1006/clin.1995.1157. [DOI] [PubMed] [Google Scholar]
- 27.Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon γ by T cells. Proc Natl Acad Sci USA. 1995;92:9510–9514. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindblad S, Hedfors E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987;30:1081–1088. doi: 10.1002/art.1780301001. [DOI] [PubMed] [Google Scholar]
- 29.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 30.Malefyt W, Abrams J, Bennett B, Figdor C, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manetti R, Parronchi P, Giudizi M G, Piccinni M P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin-12) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matzkin, E., and R. Winchester. 1984. Heterogeneity of cell populations in osteoarthritis cartilage as detected by monoclonal antibodies. Arthritis Rheum. 27(Suppl. 4):S36.
- 33.Miltenburg A M M, van Laar J M, de Kuiper R, Daha M R, Breedveld F C. T cells cloned from human rheumatoid synovial membrane functionally represent the TH1 subset. Scand J Immunol. 1992;35:603–610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 34.Miltenburg A M M, Lacraz S, Welgus H G, Dayer J-M. Immobilized anti-CD3 antibody activates T cell clones to induce the production of interstitial collagenase, but not tissue inhibitor of metalloproteinases, in monocytic THP-1 cells and dermal fibroblasts. J Immunol. 1995;154:2655–2667. [PubMed] [Google Scholar]
- 35.Miossec P, Briolay J, Dechanet J, Wijdenes J, Martinez-Valdez A, Banchereau J. Inhibition of the production of pro-inflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid arthritis. Arthritis Rheum. 1992;35:874–880. doi: 10.1002/art.1780350805. [DOI] [PubMed] [Google Scholar]
- 36.Mollenhauer J, van der Mark K, Burmester G R, Gluckert K, Lutjen-Drecoll E, Brune K. Serum autoantibodies against chondrocyte cell surface proteins in osteoarthritis and rheumatoid arthritis. J Rheumatol. 1988;15:1811–1817. [PubMed] [Google Scholar]
- 37.Moskowitz R W. Clinical and laboratory findings in osteoarthritis. In: McCarty D J, Koopman W J, editors. Arthritis and allied conditions. Philadelphia, Pa: Lea & Febiger; 1993. pp. 1735–1760. [Google Scholar]
- 38.Paul W E, Seder R A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier J-P, DiBattista J A, Roughley P J, McCollum R, Martel-Pelletier J. Cytokines and inflammation in cartilage degradation. Rheum Dis Clin N Am. 1993;19:545–568. [PubMed] [Google Scholar]
- 40.Pelletier J-P, Martel-Pelletier J, Howel D S. Etiopathogenesis of osteoarthritis. In: Koopman W J, editor. Arthritis and allied conditions. Baltimore, Md: Williams & Wilkins; 1997. pp. 1969–1984. [Google Scholar]
- 41.Piller F, Le Deist F, Weinberg K I, Parkman R, Fukuda M. Altered O-glycan synthesis in lymphocytes from patients with Wiskott-Aldrich syndrome. J Exp Med. 1991;173:1501–1510. doi: 10.1084/jem.173.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quale A J, Chomarat P, Miossec P, Kjeldsen-Kragh J, Forre O, Natvig J B. Rheumatoid inflammatory T-cell clones express mostly Th1 but also Th2 and mixed (Th0-like) cytokine patterns. Scand J Immunol. 1993;38:75–82. doi: 10.1111/j.1365-3083.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 43.Quin S, Rottman J B, Myers P, Kassam N, Weiblatt M, Loetscher M, Koch A E, Moser B, Mackay C R. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Investig. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rapoport M J, Jaramillo A, Zipris A, Lazarus H, Serreze D V, Leiter E H, Cyopick P, Danska J S, Delovitch T L. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in non-obese diabetic mice. J Exp Med. 1993;178:87–95. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Revell P A, Mayston V, Lalor P, Mapp P. The synovial membrane in osteoarthritis: a histological study including characterization of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann Rheum Dis. 1988;47:300–307. doi: 10.1136/ard.47.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakkas L I, Chen P-F, Platsoucas C D. T-cell antigen receptors in rheumatoid arthritis. Immunol Res. 1994;13:117–138. doi: 10.1007/BF02918273. [DOI] [PubMed] [Google Scholar]
- 47.Sakkas L I, Johanson N A, Burkholder J, Mitra A, Scanzello C, Salgame P, Platsoucas C D. TH1/TH2 cytokines in synovial membranes of rheumatoid arthritis and osteoarthritis: IL-12 may influence TH1 pattern. FASEB J. 1996;10:A1353. [Google Scholar]
- 48.Salgame P, Abrams J S, Clayberger C, Goldstein H, Modlin R L, Bloom B R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 49.Schaak J F, Pfers I, Meyer zum Buschenfelde K H. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondyloarthropathies. Clin Exp Rheumatol. 1996;14:155–162. [PubMed] [Google Scholar]
- 50.Schlaak J F, Schwarting A, Knolle P, Meyer zum Buschenfelde K H, Mayer W. Effects of Th1 and Th2 cytokines on cytokine production and ICAM-1 expression on synovial fibroblasts. Ann Rheum Dis. 1995;54:560–565. doi: 10.1136/ard.54.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon A K, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci USA. 1994;91:8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sipe J D. Acute phase proteins in osteoarthritis. Semin Arthritis Rheum. 1995;25:75–86. doi: 10.1016/s0049-0172(95)80020-4. [DOI] [PubMed] [Google Scholar]
- 53.Smith M D, Triantafillou S, Parker A, Youssef P P, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 54.Sugiyama E, Kuroda A, Taki H, Ikemoto M, Hori T, Yamashita N, Maruyama M, Kobayashi M. Interleukin-10 co-operates with interleukin-4 to suppress inflammatory cytokine production by freshly prepared adherent rheumatoid synovial cells. J Rheumatol. 1995;22:2020–2026. [PubMed] [Google Scholar]
- 55.Tak P P, Thurkow E W, Daha M R, Kluin P M, Smeets T J M, Meinders A E, Breedveld F C. Expression of adhesion molecules in early rheumatoid synovial tissue. Clin Immunol Immunopathol. 1995;77:236–242. doi: 10.1006/clin.1995.1149. [DOI] [PubMed] [Google Scholar]
- 56.Testi R, Phillips J H, Lanier L L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 57.Ulfgren A-K, Lindblad S, Klareskog L, Andersson J, Andersson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54:654–661. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waalen K, Sioud M, Natvig J B, Forre O. Spontaneous in vivo gene transcription of interleukin-2, interleukin-3, interleukin-4, interleukin-6, interferon-gamma, interleukin-2 receptor (CD25) and proto-oncogene c-myc by rheumatoid synovial T lymphocytes. Scand J Immunol. 1992;36:865–873. doi: 10.1111/j.1365-3083.1992.tb03148.x. [DOI] [PubMed] [Google Scholar]
- 59.Wagner S, Fritz P, Einsele H, Sell S, Saal J G. Evaluation of synovial cytokine patterns in rheumatoid arthritis and osteoarthritis by quantitative reverse transcription polymerase chain reaction. Rheumatol Int. 1997;16:191–196. doi: 10.1007/BF01330295. [DOI] [PubMed] [Google Scholar]
- 60.Warren C J, Howell W M, Bhambhani M, Cawley M I, Smith J L. An investigation of T-cell subset phenotype and function in the rheumatoid synovium using in situ hybridization for IL-2 mRNA. Immunology. 1991;72:250–255. [PMC free article] [PubMed] [Google Scholar]
- 61.Windhagen A, Anderson D E, Carrizosa A, Williams R E, Hafler D A. IL-12 induces human T cells secreting IL-10 with IFN-γ. J Immunol. 1996;157:1127–1131. [PubMed] [Google Scholar]
- 62.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: lymphokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]