Abstract
Simple Summary
Competitive endogenous RNAs (ceRNAs) can regulate gene expression at the posttranscriptional level by competitively binding to microRNAs. In cancer, ceRNA activity is dysregulated. The imbalanced ceRNA-related regulatory network has been theorized to play an important role in cancer progression. In this review, we summarize the biological functions and clinical implications of long noncoding RNAs and circular RNAs as ceRNAs in nasopharyngeal carcinoma (NPC).
Abstract
Nasopharyngeal carcinoma (NPC) is a kind of head-and-neck malignant tumor, and distant metastasis treatment resistance is the leading cause of patient death. In-depth understanding of NPC progression and treatment failure remains to be explored. Long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs) are noncoding RNAs that play key regulatory role in shaping tumor cell activities. Recent studies have revealed that lncRNA and circRNA function as competitive endogenous RNAs (ceRNAs) by regulating the posttranscriptional expression of genes as miRNA baits. The imbalanced ceRNA networks derived from lncRNA/circRNA-miRNA-mRNA interaction are widely found to contribute to NPC development. Herein, we summarize typical examples of lncRNA/circRNA-associated ceRNAs in recent years, which involved the potential molecular mechanisms in the regulation of proliferation, apoptosis, treatment resistance and metastasis of NPC, and discuss their potential clinical significance in the prognosis and treatment of NPC. Interpreting the involvement of ceRNAs networks will provide new insight into the pathogenesis and treatment strategies of NPC. However, ceRNA regulatory mechanism has some limitations currently. Screening the most effective ceRNA targets and the clinical application of ceRNA still has many challenges.
Keywords: long noncoding RNA, circular RNA, microRNA, competing endogenous RNA, regulatory network, nasopharyngeal carcinoma
1. Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy with high incidence in China that develops on the top and lateral wall of the nasopharynx cavity [1]. The incidence and mortality rate of NPC are 1.7/104 and 1.0/104 in males and 0.7/104 and 0.4/104 in females, respectively [2]. Epstein–Barr virus (EBV) is a causative factor of NPC and participates in the multistage process of NPC [3]. The occurrence of NPC may also be closely related to diet and environment [4,5]. Owing to the complex anatomy of the nasopharynx, the occult nature of the site of NPC, and the atypical early symptoms, approximately 70% of NPC patients already have locally advanced or metastatic disease at the time of diagnosis [6]. Currently, the most effective treatment of NPC is radiotherapy-based comprehensive treatment, but distant metastasis and the weak radiation sensitivity of the tumors may hinder the success of treatment [7,8]. In addition, multidrug resistance (MDR) is the major cause of chemotherapy failure in locally advanced NPC [9]. Cisplatin, paclitaxel, and 5-FU are commonly used chemotherapy drugs [10] that can significantly improve treatment efficiency, but their use in large doses often causes serious cytotoxic reactions and thus induces tumors to develop MDR [11,12,13,14]. Although immune checkpoint inhibitor (ICI) therapy is regarded as a novel standard of care in multiple malignancies like NPC, only a minority of patients benefit from it at present [1]. Hence, finding sensitive and specific biomarkers is a key to improving the cure rate of NPC, achieving an excellent prognosis, and predicting recurrence and metastasis.
Long noncoding RNAs (lncRNAs) are noncoding RNAs (ncRNAs) longer than 200 bp. When initially discovered, lncRNAs were considered to have no biological function [15]. However, recent studies have shown that nuclear lncRNA can interact with DNA [16], RNA [16], protein [17] and other molecules, regulate the chromosome structure [16,17,18] and participate in chromatin reconstruction. Completely processed lncRNAs are transported to the cytoplasm or other organelles, and cytoplasmic lncRNAs act as posttranscriptional regulators to directly target mRNA transcripts to regulate their stability or inhibit mRNA through a competitive endogenous RNA (ceRNA) mode [19,20]. Extensive studies have confirmed that lncRNAs, as ceRNAs, are widely involved in the occurrence and development of NPC. This review summarizes this aspect next.
As a newly discovered type of noncoding RNA molecule, circular RNA (circRNA) forms a closed loop by backsplicing to protect it from ribonuclease R and it is stably expressed [21]. Based on the origin of its sequence, circRNAs can be divided into three categories: exonic circRNAs, intronic circRNAs, and exon–intron circRNAs [22]. In the past, circRNA was thought to be a useless by-product of incorrect splicing [23]. Recent studies have found that circRNA is an important participant in the development of cancer. Some circRNAs contain open reading frames (ORFs) and have protein-coding functions [24]. CircRNAs usually contain several microRNA (miRNA) binding sites, and some contain one or multiple RNA binding sites, which can be used as sponges for miRNA. As lncRNAs, circRNAs are also able to function as ceRNA via miRNA response elements in them. Increasing evidence has shown that circRNAs participate in proliferation, metastasis, and invasion and even affect chemotherapy resistance and radiosensitivity of NPC by acting as ceRNAs [25,26,27].
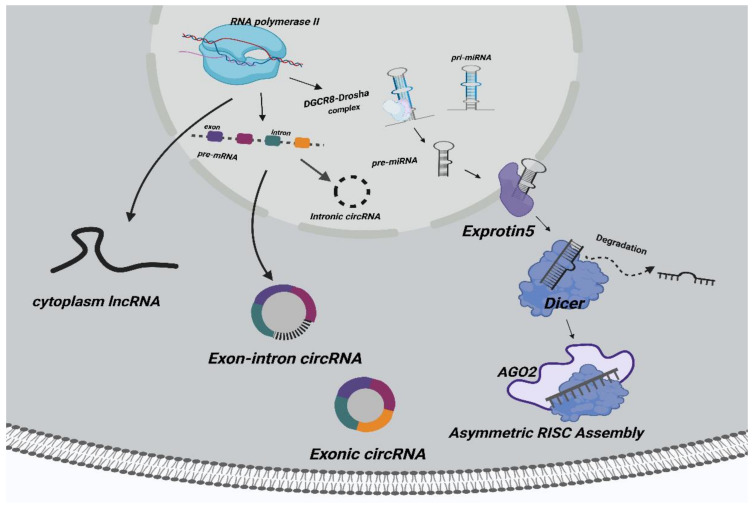
In eukaryotes, some small RNA molecules are often used to guide gene silencing, such as small interfering RNA (siRNA) and miRNA, a mechanism known as RNA interference (RNAi) [28]. MiRNA is also a kind of noncoding RNA with a length of about 20 bp, which is a highly conserved gene family [29]. Studies have shown that the RNA-induced silencing complex (RISC) is the key to miRNA function, and it is comprised of siRNA, the Dicer enzyme, Argonaut protein (AGO), and other biological macromolecules [30]. Based on its complementary pairing form with mRNA, there are two functions of miRNA: complete complementary pairing, where RISC degrades the mRNA, and incomplete complementary pairing, which inhibits mRNA translation [31]. Researchers have confirmed that miRNAs play biological roles in tumors by negatively regulating downstream genes [32,33,34]. MiRNAs are considered to be promising targets for cancer therapy. Given that miRNAs are key points in the ceRNA networks, lncRNAs and circRNAs serving as ceRNAs may therefore serve as potential therapeutic targets. The synthesis process of noncoding RNAs (lncRNAs, circRNAs, miRNAs) is shown in Figure 1.
Figure 1.
The synthetic pathways of noncoding RNAs. Cytoplasmic lncRNAs are transcribed from its corresponding parent gene. CircRNAs are reverse-spliced from precursor mRNAs and form three types, namely, exon circRNAs, intron circRNAs, and exon–intron circRNAs. MiRNAs are transcribed by RNA polymerase II to generate pre-miRNA and then cut into pre-miRNA through the DGCR8–Drosha enzyme complex. Exprotin 5 transports pre-miRNA out of the nucleus. Dicer and other enzymes cut pre-miRNA into unstable double-stranded RNA outside of the nucleus. One single-stranded miRNA is degraded, and the other is integrated into RISC (ribonuclein complex containing members of AGO histones).
The ceRNA hypothesis was proposed by the Pier Paolo Pandolfi research group of Harvard Medical College in 2011 [20]. There are one or more mRNA binding sites on miRNAs that degrade target genes or inhibit translation through RISC [35]. However, some lncRNAs/circRNAs form complementary pairing with miRNAs as ceRNAs to competitively bind miRNAs, thereby preventing miRNAs from inhibiting their target genes. The ceRNA hypothesis has attracted wide attention since it was first proposed, and it may explain the biological role of some lncRNAs and circRNAs. Significantly, many studies have confirmed that the ceRNA mode exists in a variety of cancers [36,37,38]. In recent years, emerging studies have showed that lncRNA- and circRNA-mediated ceRNA networks play an important role in NPC progression.
2. Methods
We searched several databases to identify relevant studies: Google Scholar, PubMed, and Medline. Search terms such as “ceRNA,” “NPC,” “non-coding RNA,” “lncRNA,” and “circRNA” were used in various combinations to obtain relevant information. We collected research papers on the ceRNA mechanism involved in NPC progression and integrated information to determine the content of the narrative review. Date restrictions (published ≥2017) were only used to obtain and summarize evidence of popular literature.
3. Biological Functions of LncRNA/CircRNA-Mediated CeRNA Networks in NPC
Accumulating evidence from studies has shown that lncRNA and circRNA participate in the occurrence and development of various cancers by adsorbing miRNAs to inhibit the expression of target genes. As a novel mode of gene regulation, ceRNA reveals the potential function of ncRNAs. In this section, we review lncRNA/circRNA-miRNA-target gene networks regulating biological functions of NPC.
3.1. Regulation of NPC Cell Proliferation
Tumor cells have self-sufficient proliferative capacity, even without external stimulation. The ceRNA mechanism is one of the ways to maintain proliferation signal transduction in NPC cells. Almost all lncRNAs/circRNAs function as ceRNAs, which can promote the proliferation of NPC tumor cells. We focus on ceRNA networks that maintain the proliferation signal transduction ability of NPC tumor cells. Their specific molecular mechanisms are shown in Table 1 and Table 2.
Table 1.
CeRNA networks of lncRNA-miRNA-mRNA involved in NPC proliferation and apoptosis.
| LncRNA | miRNA | mRNA | Function | Reference |
|---|---|---|---|---|
| ZFAS1 | miR-7-5p | ENO2 | Proliferation, apoptosis, radiation resistance | [39] |
| SNHG5 | miR-1179 | HMGB3 | Proliferation, migration and invasion | [40] |
| SNHG7 | miR-514-5p | ELAVL1 | Proliferation, migration | [41] |
| DRAIC | miR-122 | SATB1 | Proliferation, migration and invasion | [42] |
| SOX2-OT | miR-146b-5p | HNRNPA2B | Proliferation, apoptosis, migration, invasion and metastasis | [43] |
| XIST | miR-148a-3p | ADAM17 | Proliferation, apoptosis, migration, invasion, EMT and metastasis | [44] |
| FAM225A | miR-590-3p miR-1275 |
ITGB3 | Proliferation, migration, invasion, metastasis and FAK/PI3K/AKT pathway | [45] |
| CYTOR | miR-613 | ANXA2 | Proliferation, migration, invasion and metastasis | [46] |
| LINC02570 | miR-4649-3p | SREBP1 | Proliferation, invasion, and migration | [47] |
| HOXC13-AS | miR-383-3p | HMGA2 | Proliferation, invasion, and migration | [48] |
| SMAD5-AS1 | miR-106a-5p | SMAD5 | Proliferation, invasion, migration and EMT | [49] |
| PTPRG-AS1 | miR-194-3p | PRC1 | Proliferation, apoptosis, invasion, migration, metastasis and radiosensitivity | [50] |
| PTPRC-AS1 | miR-124-3p | LHX2 | Proliferation, apoptosis and radiosensitivity | [51] |
| FOXD3-AS1 | miR-185-3p | FOXD3 | Proliferation, invasion, migration and cell stemness | [52] |
| MEG3 | miR-21 | PTEN | Apoptosis and autophagy | [53] |
| NEAT1 | miR-129 | Bcl-2 | Apoptosis in SAHA tolerance NPC cell lines | [54] |
Table 2.
CeRNA networks of circRNA-miRNA-mRNA involved in NPC proliferation and apoptosis.
| CircRNA | miRNA | mRNA | Function | Reference |
|---|---|---|---|---|
| CircCTDP1 | miR-320b | HOXA10 | Proliferation, invasion, migration, apoptosis and TGFβ2 pathway | [55] |
| CircRNA_000543 | miR-9 | PDGFRB | Proliferation, apoptosis and radiosensitivity | [26] |
| CircHIPK3 | miR-4288 | ELF3 | Proliferation, invasion, and migration | [56] |
| CircTGFBR2 | miR-107 | TGFBR2 | Proliferation, invasion, migration, EMT, TGF-β and PI3K/Akt pathway | [57] |
| CircITCH | miR-214 | PTEN | Proliferation, migration and invasion | [58] |
For instance, tumor cells increase de novo fatty acid synthesis to promote proliferation. Oncogenic LNC02570 is upregulated in advanced stage NPC patients. High expression of LINC02570 significantly promotes NPC proliferation. As a ceRNA, LINC02570 upregulates the expression level of the key gene SREBP1 in the lipid biosynthesis pathway by adsorbing miR-4649-3p [47]. SREBP1 simultaneously activates FASN (downstream gene) expression. In previous studies, EBV-LMP1 mediated the activation of the SREBP1-FASN pathway, an important mechanism for promoting NPC proliferation [59]. LINC02570, as a ceRNA, plays the same function as LMP1. Although the correlation between LMP1 and LINC02570 has not been reported, LINC02570 shows a new function of ceRNA in the activation of lipogenesis, an effect that accelerates NPC proliferation and tumor development. In tumor cells, lipogenesis is highly active and promotes the rapid proliferation of tumors. Hence, targeting lncRNA or key genes for lipogenesis in ceRNA networks may effectively inhibit the proliferation of NPC cells.
The atypical cell cycle process caused by abnormal activation of the PI3K/Akt signaling pathway is considered a basic feature of many malignant tumors, including NPC. Excessive activation of this signaling pathway leads to abnormal cell proliferation. CeRNA networks at least partially promote NPC proliferation by inhibiting PTEN expression. PTEN is a tumor suppressor of NPC and a key negative regulatory gene of PI3K/Akt signaling pathway [60,61].
Low expression of PTEN predicts poor prognosis and shorter progression-free survival in NPC patients [61]. Overexpression of circITCH significantly inhibits NPC cell proliferation [58]. In vitro, circITCH blocks miR-214-mediated inhibition of PTEN, suggesting that circITCH, as a ceRNA, may inhibit the PI3K/Akt signaling pathway [58] and NPC proliferation. PTEN can also be upregulated by lncRNA MEG3/miR-21 and promote autophagy and apoptosis in NPC cells [53]. Notably, circITCH and lncRNA MEG3, as ceRNAs, jointly regulate PTEN cells and promote proliferation in HK-1 cells.
Therefore, lncRNA/circRNA-associated ceRNA cross talk may be a potential therapeutic target for NPC. Understanding the specific mechanism of the ceRNA network involved in the regulation of proliferation-related genes and signaling pathways in NPC is of great significance for the development and treatment of NPC.
3.2. Regulation of NPC Cell Apoptosis
Apoptosis is a programmed death, which plays a vital role in the development of organisms and defense against intracellular infection factors. Contrary to the effect of cell proliferation, most ceRNA-mediated abnormal expression of apoptosis-related genes inhibits apoptosis of NPC tumor cells and promotes their survival. B cell lymphoma 2 (Bcl-2) is an antiapoptotic protein. It inhibits apoptosis by regulating mitochondrial outer membrane and preventing the release of proapoptotic molecules into the cytoplasm. Bcl-2 is the main target of apoptosis molecular mechanism research, and is also the target gene of lymphoma and other cancer treatment. Some researchers have confirmed that Bcl-2 plays an important role in the antiapoptotic activity of EBV latent proteins (such as BHRF1, BARF1, and EBNA-3C) in the development of NPC. Xue et al. confirmed that the abnormal expression of Bcl-2 induced by lncRNA NEAT1/miR-129 was the main reason for suberoylanilide hydroxamic acid (SAHA)-induced apoptosis [54]. Inhibition of lncRNA NEAT1 and restoration of miR-129 levels are potential therapeutic targets for NPC and strategies for overcoming SAHA resistance. In lncRNA/circRNA mediated ceRNA networks, other apoptosis-related genes such as caspase, Fas and p53 have not been reported.
3.3. Modulating NPC Chemosensitivity
Improving the radiosensitivity of NPC is a challenge clinically. CircRNA_000543 increased in radiation-resistant NPC tissue, and the overall survival of patients was poor [26]. CircRNA_000543/miR-9/PDGFRB is a ceRNA involved in NPC radiation resistance, and any element imbalance will affect radiation resistance [26]. Notably, imatinib, a PDFGRB inhibitor used to treat chronic myeloid leukemia (CML), can effectively increase the radiosensitivity of NPC [26]. Elements in circRNA_000543-mediated ceRNA model are potential targets for NPC therapy.
The Gene Expression Omnibus (GEO) database was used to identify lncRNAs with abnormally high PTPRG-AS1 expression. LncRNA PTPRG-AS1 promotes radiosensitivity of NPC cells as a ceRNA [51]. LncRNA PTPRG-AS1 adsorbs miR-194-3p and miR-124-3p negatively regulate PRC1 and LHX2, respectively [50,51]. LHX2 activates the Notch pathway and reduces radiosensitivity of NPC cells. Although the specific mechanism of LncRNA PTPRG-AS1 as a ceRNA involved in radiation resistance of NPC remains unclear, these two studies provided potential biomarkers for evaluating the results of radiation resistance in NPC treatment. Moreover, lncRNA ZFAS1/miR-7-5p regulates ENO2 and participates in NPC radioresistance [39]. Studies have confirmed that ENO2 shapes the hypoxic environment of tumors by regulating HIF-1 signaling, which may be the reason for ZFAS1 as a ceRNA to promote NPC radiation resistance [39]. Furthermore, the lncRNA XIST/miR-381-3p/NEK5 network promotes glycolysis and NPC progression under hypoxic conditions. Oxygen-enriched tumor cells are more sensitive to radiotherapy than hypoxic tumor cells. The above studies show that inhibiting lncRNA-mediated ceRNA networks can effectively regulate the glycolysis and HIF-1 signal of NPC tumor cells to resist low radiosensitivity under hypoxia.
Recent studies have confirmed that lncRNA/circRNA-mediated ceRNA networks play an important role in regulating drug resistance of NPC cells (Table 3). The treatment of recurrent NPC is mainly multidrug chemotherapy containing cisplatin. Cisplatin drugs promote tumor cell death by blocking DNA polymerase and inhibiting gene replication. Compared with parental NPC cells, lncRNA HOXA11-AS is upregulated and miR-454-3p downregulated in cisplatin-resistant NPC cells [62]. Liu et al. confirmed that HOXA11-AS as a ceRNA enhanced cisplatin resistance of NPC cells by adsorbing miR-454-3p [62]. At the same time, the downstream c-Met/Akt/mTOR pathway was activated by HOXA11-AS/miR-454-3p, thereby promoting the cisplatin resistance of NPC cells [62]. Combined use of Si-HOXA11-AS and cisplatin may improve the treatment of drug-resistant NPC. As a ceRNA, lncRNA MAGI2-AS3 confers cisplatin resistance to NPC cells by regulating miR-218-5p/GDPD5 [63]. Furthermore, circNRIP1 was identified as the oncogene of multiple tumors [64,65]. It was highly expressed in sera of chemotherapy-resistant NPC patients [27,64]. The knockdown of CircNRIP1 significantly enhanced the sensitivity of NPC cells to 5-Fu and cisplatin by competitive binding with miR-515-5p [27,64]. Targeted inhibition of circNRIP1-mediated ceRNA model is a potential therapeutic strategy for drug-resistant NPC patients [27,64].
Table 3.
CeRNA networks of lncRNA/circRNA-miRNA-mRNA involved in NPC chemosensitivity.
| LncRNA/CircRNA | miRNA | mRNA | Function | Reference |
|---|---|---|---|---|
| CCAT1 | miR-181a | CPEB2 | Paclitaxel resistance | [66] |
| MAGI2-AS3 | miR-218-5p | GDPD5 SEC61A1 |
Cisplatin resistance and EMT Proliferation and migration |
[63] |
| CircNRIP1 | miR-515-5p | IL-25 | 5-Fu and cisplatin resistance | [27] |
| CircCRIM1 | miR-422a | FOXQ1 | Docetaxel chemosensitivity, invasion, migration, metastasis and EMT | [38] |
| XIST | miR-381-3p | NEK5 | Glycolysis, migration, invasion and metastasis under hypoxic conditions | [67] |
| HOXA11-AS | miR-454-3p | c-Met | Cisplatin resistance, C-Met/AKT/mTOR pathway | [62] |
Paclitaxel is also one of the commonly used chemotherapy drugs for recurrent NPC. It inhibits tumor cell division by promoting tubulin polymerization and inhibiting its depolymerization. CPEB2 is an RNA-binding protein, and its role in tumors is contradictory. The identical CPEB2A of CPEB2 is a tumor suppressor and inhibits the translation of target mRNA, while the identical CPEB2 can competitively bind to the target RNA of CPEB2A, thus allowing subsequent translation. CPEB2B is a tumor promoter [68,69]. LncRNA CCAT1, as a ceRNA, regulates CPEB2 at posttranscriptional level. In NPC, it seems to be a tumor-promoting factor. The specific mechanism of CPEB2 involved in promoting paclitaxel resistance in NPC cells is worthy of further study, which provides guidance for the potential regulatory function of ceRNA. Paclitaxel is sensitive to G2/M cells and significantly promotes the radiosensitivity of tumor cells. Targeted inhibition of a CCAT1-mediated ceRNA model may promote the therapeutic effect of radiotherapy and chemotherapy in paclitaxel resistant NPC patients.
3.4. Modulating NPC Metastasis
After chemoradiotherapy, 20–30% of NPC patients still face metastasis [70]. Their median overall survival (OS) time was shortened to 10–20 months [71]. Therefore, effective molecular targets are urgently needed for the treatment of metastatic NPC. Some lncRNAs/circRNAs act as ceRNAs and are involved in the regulation of NPC cell invasion, migration and metastasis, which were also verified in vivo experiments (Table 4). These lncRNAs/circRNAs may provide promising cancer biomarkers for early diagnosis and prognosis in patients with metastatic NPC.
Table 4.
CeRNA networks of lncRNA/circRNA-miRNA-mRNA involved in NPC metastasis.
| LncRNA/CircRNA | miRNA | mRNA | Function | Reference |
|---|---|---|---|---|
| AATBC | miR-1237-3p | PNN | Migration, invasion, EMT and metastasis | [72] |
| AFAP1-AS1 | miR-423-5p | FOSL2 RAB11B LASP1 |
Invasion, migration, metastasis and Rho/Rac pathway, invasion | [73] |
| CircSETD3 | miR-615-5p miR-1538 |
MAPRE1 | Invasion, migration and metastasis | [25] |
| Circ_0046263 | miR-133a-5p | IGFBP3 | Proliferation, invasion, EMT and metastasis | [74] |
| EBV-encoded CircRPMS1 | miR-203 miR-31 miR-451 |
Proliferation, invasion, EMT and metastasis | [75] |
Epithelial–mesenchymal transition (EMT) is a process in which epithelial cells lose their characteristics and acquire mesenchymal characteristics. In cancer, EMT is considered to be the key mechanism in metastasis. EMT is often regarded as a binary process of epithelial cells and mesenchymal cells [76].
TGF-β is considered to be the most important signaling pathway of EMT induced by tumor cells. TGF-β-mediated EMT can occur in two ways. One is TGF-β signaling activates Smad2 and Smad3, and then forms a complex with Smad4 to transfer into the nucleus and mediates the inhibition and activation of target genes with EMT-related transcription factors (TF). Snail, Slug, and ZEB1 were identified as the main transcription activators of EMT. The loss of epithelial gene E-cadherin is the marker of EMT, and the upregulation of mesenchymal gene N-cadherin is also the key phenomenon of EMT [77]. In addition, TGF-β can induce EMT together with Notch, Wnt, and other signaling pathways [78,79].
Recent studies have shown that lncRNA/circRNA-mediated ceRNA networks induce tumor EMT formation by activating TGF-β signals, e.g., lncRNA AATBC regulates PNN by adsorbing miR-1237-3p. The interaction between PNN and ZEB1 can also promote EMT of NPC [72]. Circ_0046263 promotes lymph-node metastasis of NPC cells in vivo [74]. The potential regulatory mechanism is to relieve the inhibition of miR-133a-5p on IGFBP3 expression and promote IGFBP3-mediated TGF-β activation [74].
In addition, the reconstruction of the cytoskeleton can also trigger EMT formation. CircRNA participates in the assembly of the dynamic cytoskeleton through a ceRNA mechanism, thereby promoting metastasis in NPC. CircSETD3 competitively adsorbs miR-615-5p and miR-1538, and ultimately upregulates MAPRE1 [25].The upregulation of MAPRE1 prevents α-tubulin acetylation, thereby affecting NPC cell motility, migration and EMT [25]. CircSETD3 was once considered a tumor suppressor; however, ceRNA mechanism conferred it an oncogene function [80]. It provides a new potential biomarker for metastatic NPC.
Virus ncRNA can also be used as miRNA decoys to participate in ceRNA network regulation. This regulation pattern has been defined as “competitive viruses and host RNA” (cvhRNAs) [81]. This novel interaction was first described in the hepatitis B virus (HBV) ncRNA and later in human papilloma (HPV) [82,83]. EBV-encoded circRNAs can also serve as ceRNAs by adsorbing host miRNAs, thereby hindering the targeted inhibition of miRNAs on target genes and accelerating the progression of NPC. The EBV-encoded gene RPMS1 exons 2–4 form circRPMS1 through backsplicing from exon 4 to exon 2 (circRPMS1_E4_E2). In EBV-positive NPC, circRPMS1 is activated and sponges multiple miRNAs (miR-203, miR-31, miR-451) to promote the EMT process of NPC and play a role in promoting cancer [57]. Notably, there are very few studies on the involvement of EBV-encoded lncRNAs and circRNAs in the cvhRNA hypothesis. In-depth study of the specific molecular mechanisms of EBV-encoded lncRNAs/circRNAs-mediated ceRNA networks contributes to virus-targeted therapy of NPC.
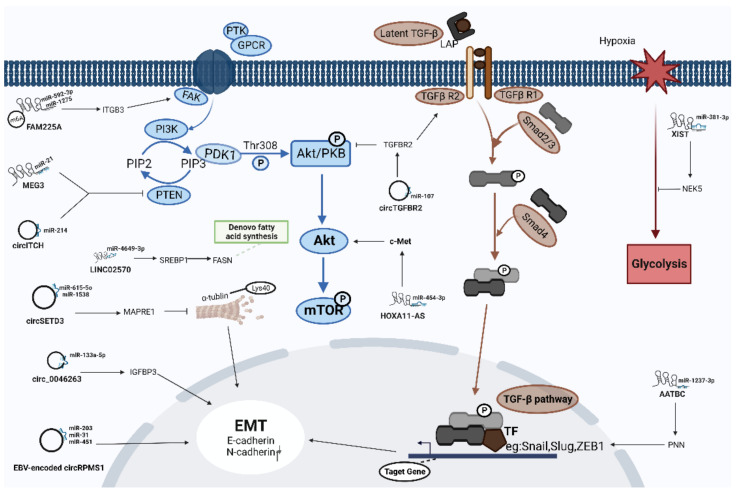
Understanding the ceRNA networks mediated by lncRNAs and circRNAs can provide valuable insights into the molecular mechanisms underlying the pathogenesis of a variety of human malignancies, including NPC (Figure 2).
Figure 2.
The specific regulatory mechanism of some representative lncRNA/circRNA-mediated ceRNA networks in NPC progression.
4. Implications of LncRNA/CircRNA-Associated ceRNAs as Diagnostic Markers or Therapeutic Targets in NPC
The TNM (tumor–lymph node–metastasis) staging system is a general guideline for cancer treatment. In clinical practice, it cannot accurately identify the risk of recurrence or distant metastasis in NPC patients. Stage II patients are generally considered at low risk. Final recurrence occurs in 15–20% of patients without adjuvant chemotherapy. Conversely, more than half of patients in stage III and IV were cured only by chemoradiation without adjuvant chemotherapy [84]. Thus, novel prognostic biomarkers are needed. The ceRNA spectrum in cancer cells is different from that in normal cells. Some functional ceRNAs are deactivated in tumor cells and may serve as diagnostic or therapeutic markers in the future. For example, lncRNA FOXD3-AS1 as a ceRNA promotes FOXD3 gene expression. The upregulation of these two genes was associated with TNM stage and histological type of NPC [52]. LncRNA FAM225A was identified as an oncogenic gene in NPC. FAM225A, as a ceRNA, effectively activates the FAK/PI3K/Akt signaling pathway by upregulating ITGB3, thereby promoting NPC proliferation and metastasis [45]. The combination of TNM staging and FAM225A expression is expected to become an effective prognostic indicator.
CircCRIM1, as a ceRNA, plays an important prognostic role in NPC metastasis and chemotherapy resistance [38]. Hong et al. combined circCRIM1 expression with N staging to construct a prognostic prediction model [38]. Patients with low CircCRIM1 expression or early-stage N were sensitive to systemic induction chemotherapy of docetaxel, while patients with high CircCRIM1 expression or late-stage N did not benefit from systemic induction chemotherapy of docetaxel [38]. These findings are used clinically to avoid unnecessary drug toxicity.
Knockdown of oncogenic lncRNAs/circRNAs may be an effective strategy to interfere with the progression of NPC, but how to deliver therapeutic nucleic acids accurately into cells based on the ceRNA model is a challenge. Based on the CRISPR/Cas system, the precision of lipid nanoparticles and polymer hydrogel nanoparticles to treat NPC may be a potential solution [85,86].
In a word, miRNAs are considered promising targets for cancer therapy. Given that miRNAs are key points in ceRNA networks, lncRNAs and circRNAs serving as ceRNAs may therefore serve as potential therapeutic targets.
5. Conclusions
Since lncRNAs and circRNAs combine noncoding RNAs with protein-coding RNAs through complex ceRNA networks, they play indispensable regulatory roles in cancers like NPC. Exploring the specific molecular mechanism of lncRNAs/circRNAs as ceRNAs could provide targeted molecular therapy and clinical prevention strategies in NPC. Emerging studies have indicated that a variety of dysregulated lncRNA/circRNA-mediated ceRNAs may form networks to regulate multitudinous biological functions in NPC, including tumor cell proliferation, apoptosis, invasion, migration, metastasis, and treatment resistance.
In fact, any transcript containing miRNA seed matches can function as a ceRNA. However, in the complex ceRNA networks, how to select an effective ceRNA as a target and how to specifically manipulate the target ceRNA without affecting other ceRNAs for the treatment of NPC? This is a challenging task for clinical applications of ceRNA. Moreover, the occurrence of NPC is closely related to EBV infection. Although some individual EBV-encoded circRNAs have been confirmed as ceRNAs to accelerate NPC progression, research on EBV-encoded lncRNAs/circRNAs as ceRNAs is still rare in NPC. Targeting EBV-encoded genes is also a specific therapeutic strategy for NPC. All in all, further understanding of the regulatory networks of lncRNA/circRNA-mediated ceRNAs originating from both tumor cells and EBV will provide more novel insight into the pathogenesis and therapy of NPC.
Author Contributions
Conceptualization, S.Z, Y.L., S.X., L.Y., M.J. and J.L.; writing—original draft preparation, S.Z.; writing—review and editing, S.Z., Y.X., Y.W., J.Y. and J.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundations of China (81974427) and Graduate Research and Innovation Projects of Central South University (2022ZZTS0872).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen Y.-P., Chan A.T.C., Le Q.-T., Blanchard P., Sun Y., Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 2.Tang L.-L., Chen W.-Q., Xue W.-Q., He Y.-Q., Zheng R.-S., Zeng Y.-X., Jia W.-H. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374:22–30. doi: 10.1016/j.canlet.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Wong K.C.W., Hui E.P., Lo K.-W., Lam W.K.J., Johnson D., Li L., Tao Q., Chan K.C.A., To K.-F., King A.D., et al. Nasopharyngeal carcinoma: An evolving paradigm. Nat. Rev. Clin. Oncol. 2021;18:679–695. doi: 10.1038/s41571-021-00524-x. [DOI] [PubMed] [Google Scholar]
- 4.Jia W.-H., Qin H.-D. Non-viral environmental risk factors for nasopharyngeal carcinoma: A systematic review. Semin. Cancer Biol. 2012;22:117–126. doi: 10.1016/j.semcancer.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Tsao S.W., Yip Y.L., Tsang C.M., Pang P.S., Lau V.M.Y., Zhang G., Lo K.W. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014;50:330–338. doi: 10.1016/j.oraloncology.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Li C., Jing B., Ke L., Li B., Xia W., He C., Qian C., Zhao C., Mai H., Chen M., et al. Development and validation of an endoscopic images-based deep learning model for detection with nasopharyngeal malignancies. Cancer Commun. 2018;38:59. doi: 10.1186/s40880-018-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X.-S., Li X.-Y., Chen Q.-Y., Tang L.-Q., Mai H.-Q. Future of Radiotherapy in Nasopharyngeal Carcinoma. Br. J. Radiol. 2019;92:20190209. doi: 10.1259/bjr.20190209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Z., Xiao L., Tang M., Bai F., Li J., Li L., Shi F., Li N., Li Y., Du Q., et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics. 2018;8:2329–2347. doi: 10.7150/thno.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan S., Wei J., Huang L., Wu L. Chemotherapy and chemo-resistance in nasopharyngeal carcinoma. Eur. J. Med. Chem. 2020;207:112758. doi: 10.1016/j.ejmech.2020.112758. [DOI] [PubMed] [Google Scholar]
- 10.Liu S.-L., Sun X.-S., Li X.-Y., Chen Q.-Y., Lin H.-X., Wen Y.-F., Guo S.-S., Liu L.-T., Xie H.-J., Tang Q.-N., et al. Liposomal paclitaxel versus docetaxel in induction chemotherapy using Taxanes, cisplatin and 5-fluorouracil for locally advanced nasopharyngeal carcinoma. BMC Cancer. 2018;18:1279. doi: 10.1186/s12885-018-5192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H., Qi B., Guo X., Tang L.-Q., Chen Q.-Y., Zhang L., Guo L., Luo D.-H., Huang P.-Y., Mo H.-Y., et al. Genetic variations in radiation and chemotherapy drug action pathways and survival in locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy. PLoS ONE. 2013;8:e82750. doi: 10.1371/journal.pone.0082750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce J.P., To K.-F., Lui V.W.Y., Chung G.T.Y., Chan Y.-Y., Tsang C.M., Yip K.Y., Ma B.B.Y., Woo J.K.S., Hui E.P., et al. Whole-genome profiling of nasopharyngeal carcinoma reveals viral-host co-operation in inflammatory NF-κB activation and immune escape. Nat. Commun. 2021;12:4193. doi: 10.1038/s41467-021-24348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M., Luo R., Liu Y., Gao L., Fu Z., Fu Q., Luo X., Chen Y., Deng X., Liang Z., et al. miR-3188 regulates nasopharyngeal carcinoma proliferation and chemosensitivity through a FOXO1-modulated positive feedback loop with mTOR-p-PI3K/AKT-c-JUN. Nat. Commun. 2016;7:11309. doi: 10.1038/ncomms11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qing X., Tan G.-L., Liu H.-W., Li W., Ai J.-G., Xiong S.-S., Yang M.-Q., Wang T.-S. LINC00669 insulates the JAK/STAT suppressor SOCS1 to promote nasopharyngeal cancer cell proliferation and invasion. J. Exp. Clin. Cancer Res. 2020;39:166. doi: 10.1186/s13046-020-01674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridges M.C., Daulagala A.C., Kourtidis A. LNCcation: LncRNA localization and function. J. Cell Biol. 2021;220:e202009045. doi: 10.1083/jcb.202009045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Leary V.B., Ovsepian S.V., Carrascosa L.G., Buske F.A., Radulovic V., Niyazi M., Moertl S., Trau M., Atkinson M.J., Anastasov N. PARTICLE, a Triplex-Forming Long ncRNA, Regulates Locus-Specific Methylation in Response to Low-Dose Irradiation. Cell Rep. 2015;11:474–485. doi: 10.1016/j.celrep.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Holdt L.M., Hoffmann S., Sass K., Langenberger D., Scholz M., Krohn K., Finstermeier K., Stahringer A., Wilfert W., Beutner F., et al. Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks. PLoS Genet. 2013;9:e1003588. doi: 10.1371/journal.pgen.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isoda T., Moore A.J., He Z., Chandra V., Aida M., Denholtz M., Piet van Hamburg J., Fisch K.M., Chang A.N., Fahl S.P., et al. Non-coding Transcription Instructs Chromatin Folding and Compartmentalization to Dictate Enhancer-Promoter Communication and T Cell Fate. Cell. 2017;171:103–119.e18. doi: 10.1016/j.cell.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statello L., Guo C.-J., Chen L.-L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denzler R., Agarwal V., Stefano J., Bartel D.P., Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristensen L.S., Andersen M.S., Stagsted L.V.W., Ebbesen K.K., Hansen T.B., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 22.Chen L., Wang C., Sun H., Wang J., Liang Y., Wang Y., Wong G. The bioinformatics toolbox for circRNA discovery and analysis. Brief. Bioinform. 2021;22:1706–1728. doi: 10.1093/bib/bbaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obi P., Chen Y.G. The design and synthesis of circular RNAs. Methods. 2021;196:85–103. doi: 10.1016/j.ymeth.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L.-L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020;21:475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 25.Tang L., Xiong W., Zhang L., Wang D., Wang Y., Wu Y., Wei F., Mo Y., Hou X., Shi L., et al. circSETD3 regulates MAPRE1 through miR-615-5p and miR-1538 sponges to promote migration and invasion in nasopharyngeal carcinoma. Oncogene. 2021;40:307–321. doi: 10.1038/s41388-020-01531-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen L., Zhou H., Guan Z. CircRNA_000543 knockdown sensitizes nasopharyngeal carcinoma to irradiation by targeting miR-9/platelet-derived growth factor receptor B axis. Biochem. Biophys. Res. Commun. 2019;512:786–792. doi: 10.1016/j.bbrc.2019.03.126. [DOI] [PubMed] [Google Scholar]
- 27.Lin J., Qin H., Han Y., Li X., Zhao Y., Zhai G. CircNRIP1 Modulates the miR-515-5p/IL-25 Axis to Control 5-Fu and Cisplatin Resistance in Nasopharyngeal Carcinoma. Drug Des. Dev. Ther. 2021;15:323–330. doi: 10.2147/DDDT.S292180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pushparaj P.N., Aarthi J.J., Manikandan J., Kumar S.D. siRNA, miRNA, and shRNA: In vivo applications. J. Dent. Res. 2008;87:992–1003. doi: 10.1177/154405910808701109. [DOI] [PubMed] [Google Scholar]
- 29.Kilikevicius A., Meister G., Corey D.R. Reexamining assumptions about miRNA-guided gene silencing. Nucleic Acids Res. 2022;50:617–634. doi: 10.1093/nar/gkab1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwakawa H.-O., Tomari Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell. 2022;82:30–43. doi: 10.1016/j.molcel.2021.11.026. [DOI] [PubMed] [Google Scholar]
- 31.Kawamata T., Tomari Y. Making RISC. Trends Biochem. Sci. 2010;35:368–376. doi: 10.1016/j.tibs.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Zheng X., Qin Z., Wei L., Lu Y., Peng Q., Gao Y., Zhang X., Zhang X., Li Z., et al. Epstein-Barr virus miR-BART3-3p promotes tumorigenesis by regulating the senescence pathway in gastric cancer. J. Biol. Chem. 2019;294:4854–4866. doi: 10.1074/jbc.RA118.006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo L., Xie Y., Tang J., Xin S., Liu L., Zhang S., Yan Q., Zhu F., Lu J. Targeting Exosomal EBV-LMP1 Transfer and miR-203 Expression via the NF-κB Pathway: The Therapeutic Role of Aspirin in NPC. Mol. Ther. Nucleic Acids. 2019;17:175–184. doi: 10.1016/j.omtn.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong X., Duan Y., Sang Y., Li Y., Zhang H., Liang Y., Liu Y., Zhang N., Yang Q. LncRNA-CDC6 promotes breast cancer progression and function as ceRNA to target CDC6 by sponging microRNA-215. J. Cell Physiol. 2019;234:9105–9117. doi: 10.1002/jcp.27587. [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Huo X., Yang X.-R., He J., Cheng L., Wang N., Deng X., Jin H., Wang N., Wang C., et al. STAT3-mediated upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer metastasis by regulating SOX4. Mol. Cancer. 2017;16:136. doi: 10.1186/s12943-017-0680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong X., Liu N., Liang Y., He Q., Yang X., Lei Y., Zhang P., Zhao Y., He S., Wang Y., et al. Circular RNA CRIM1 functions as a ceRNA to promote nasopharyngeal carcinoma metastasis and docetaxel chemoresistance through upregulating FOXQ1. Mol. Cancer. 2020;19:33. doi: 10.1186/s12943-020-01149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng J., Liu F., Zheng H., Wu Q., Liu S. IncRNA ZFAS1 contributes to the radioresistance of nasopharyngeal carcinoma cells by sponging hsa-miR-7-5p to upregulate ENO2. Cell Cycle. 2021;20:126–141. doi: 10.1080/15384101.2020.1864128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D., Wang Y., Zhao Y., Gu X. LncRNA SNHG5 promotes nasopharyngeal carcinoma progression by regulating miR-1179/HMGB3 axis. BMC Cancer. 2020;20:178. doi: 10.1186/s12885-020-6662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu W., Li H., Wang S. LncRNA SNHG7 promotes the proliferation of nasopharyngeal carcinoma by miR-514a-5p/ELAVL1 axis. BMC Cancer. 2020;20:376. doi: 10.1186/s12885-020-06775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao B., Wang Z., Zhu Y., Wang M., Liu Y. Long noncoding RNA DRAIC acts as a microRNA-122 sponge to facilitate nasopharyngeal carcinoma cell proliferation, migration and invasion via regulating SATB1. Artif. Cells Nanomed. Biotechnol. 2019;47:3585–3597. doi: 10.1080/21691401.2019.1656638. [DOI] [PubMed] [Google Scholar]
- 43.Zhang E., Li X. LncRNA SOX2-OT regulates proliferation and metastasis of nasopharyngeal carcinoma cells through miR-146b-5p/HNRNPA2B1 pathway. J. Cell Biochem. 2019;120:16575–16588. doi: 10.1002/jcb.28917. [DOI] [PubMed] [Google Scholar]
- 44.Shi J., Tan S., Song L., Song L., Wang Y. LncRNA XIST knockdown suppresses the malignancy of human nasopharyngeal carcinoma through XIST/miRNA-148a-3p/ADAM17 pathway in vitro and in vivo. Biomed. Pharmacother. 2020;121:109620. doi: 10.1016/j.biopha.2019.109620. [DOI] [PubMed] [Google Scholar]
- 45.Zheng Z.-Q., Li Z.-X., Zhou G.-Q., Lin L., Zhang L.-L., Lv J.-W., Huang X.-D., Liu R.-Q., Chen F., He X.-J., et al. Long Noncoding RNA FAM225A Promotes Nasopharyngeal Carcinoma Tumorigenesis and Metastasis by Acting as ceRNA to Sponge miR-590-3p/miR-1275 and Upregulate ITGB3. Cancer Res. 2019;79:4612–4626. doi: 10.1158/0008-5472.CAN-19-0799. [DOI] [PubMed] [Google Scholar]
- 46.Chen W., Du M., Hu X., Ma H., Zhang E., Wang T., Yin L., He X., Hu Z. Long noncoding RNA cytoskeleton regulator RNA promotes cell invasion and metastasis by titrating miR-613 to regulate ANXA2 in nasopharyngeal carcinoma. Cancer Med. 2020;9:1209–1219. doi: 10.1002/cam4.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu F., Wei J., Hao Y., Lan J., Li W., Weng J., Li M., Su C., Li B., Mo M., et al. Long intergenic non-protein coding RNA 02570 promotes nasopharyngeal carcinoma progression by adsorbing microRNA miR-4649-3p thereby upregulating both sterol regulatory element binding protein 1, and fatty acid synthase. Bioengineered. 2021;12:7119–7130. doi: 10.1080/21655979.2021.1979317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao C., Lu W., Lou W., Wang L., Xu Q. Long noncoding RNA HOXC13-AS positively affects cell proliferation and invasion in nasopharyngeal carcinoma via modulating miR-383-3p/HMGA2 axis. J. Cell Physiol. 2019;234:12809–12820. doi: 10.1002/jcp.27915. [DOI] [PubMed] [Google Scholar]
- 49.Zheng Y.-J., Zhao J.-Y., Liang T.-S., Wang P., Wang J., Yang D.-K., Liu Z.-S. Long noncoding RNA SMAD5-AS1 acts as a microRNA-106a-5p sponge to promote epithelial mesenchymal transition in nasopharyngeal carcinoma. FASEB J. 2019;33:12915–12928. doi: 10.1096/fj.201900803R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi L., Ouyang L., Wang S., Li S.-S., Yang X.-M. Long noncoding RNA PTPRG-AS1 acts as a microRNA-194-3p sponge to regulate radiosensitivity and metastasis of nasopharyngeal carcinoma cells via PRC1. J. Cell Physiol. 2019;234:19088–19102. doi: 10.1002/jcp.28547. [DOI] [PubMed] [Google Scholar]
- 51.Shen Z., Wu Y., He G. Long non-coding RNA PTPRG-AS1/microRNA-124-3p regulates radiosensitivity of nasopharyngeal carcinoma via the LIM Homeobox 2-dependent Notch pathway through competitive endogenous RNA mechanism. Bioengineered. 2022;13:8208–8225. doi: 10.1080/21655979.2022.2037364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu J., Pan J., Luo Z., Duan Q., Wang D. Long non-coding RNA FOXD3-AS1 silencing exerts tumor suppressive effects in nasopharyngeal carcinoma by downregulating FOXD3 expression via microRNA-185-3p upregulation. Cancer Gene Ther. 2021;28:602–618. doi: 10.1038/s41417-020-00242-z. [DOI] [PubMed] [Google Scholar]
- 53.Lin L., Liu X., Lv B. Long non-coding RNA MEG3 promotes autophagy and apoptosis of nasopharyngeal carcinoma cells via PTEN up-regulation by binding to microRNA-21. J. Cell Mol. Med. 2021;25:61–72. doi: 10.1111/jcmm.15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue F., Cheng Y., Xu L., Tian C., Jiao H., Wang R., Gao X. LncRNA NEAT1/miR-129/Bcl-2 signaling axis contributes to HDAC inhibitor tolerance in nasopharyngeal cancer. Aging. 2020;12:14174–14188. doi: 10.18632/aging.103427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H., You J., Xue H., Tan X., Chao C. CircCTDP1 promotes nasopharyngeal carcinoma progression via a microRNA-320b/HOXA10/TGFβ2 pathway. Int. J. Mol. Med. 2020;45:836–846. doi: 10.3892/ijmm.2020.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ke Z., Xie F., Zheng C., Chen D. CircHIPK3 promotes proliferation and invasion in nasopharyngeal carcinoma by abrogating miR-4288-induced ELF3 inhibition. J. Cell Physiol. 2019;234:1699–1706. doi: 10.1002/jcp.27041. [DOI] [PubMed] [Google Scholar]
- 57.Li W., Lu H., Wang H., Ning X., Liu Q., Zhang H., Liu Z., Wang J., Zhao W., Gu Y., et al. Circular RNA TGFBR2 acts as a ceRNA to suppress nasopharyngeal carcinoma progression by sponging miR-107. Cancer Lett. 2021;499:301–313. doi: 10.1016/j.canlet.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Wang L., Sang J., Zhang Y., Gao L., Zhao D., Cao H. Circular RNA ITCH attenuates the progression of nasopharyngeal carcinoma by inducing PTEN upregulation via miR-214. J. Gene Med. 2022;24:e3391. doi: 10.1002/jgm.3391. [DOI] [PubMed] [Google Scholar]
- 59.Lo A.K.-F., Lung R.W.-M., Dawson C.W., Young L.S., Ko C.-W., Yeung W.W., Kang W., To K.-F., Lo K.-W. Activation of sterol regulatory element-binding protein 1 (SREBP1)-mediated lipogenesis by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) promotes cell proliferation and progression of nasopharyngeal carcinoma. J. Pathol. 2018;246:180–190. doi: 10.1002/path.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li H.-L., Deng N.-H., He X.-S., Li Y.-H. Small biomarkers with massive impacts: PI3K/AKT/mTOR signalling and microRNA crosstalk regulate nasopharyngeal carcinoma. Biomark. Res. 2022;10:52. doi: 10.1186/s40364-022-00397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu G., Huang W., Xu J., Li W., Wu Y., Yang Q., Liu K., Zhu M., Balasubramanian P.S., Li M. Dynamic contrast-enhanced MRI predicts PTEN protein expression which can function as a prognostic measure of progression-free survival in NPC patients. J. Cancer Res. Clin. Oncol. 2022;148:1771–1780. doi: 10.1007/s00432-021-03764-7. [DOI] [PubMed] [Google Scholar]
- 62.Lin F.-J., Lin X.-D., Xu L.-Y., Zhu S.-Q. Long Noncoding RNA HOXA11-AS Modulates the Resistance of Nasopharyngeal Carcinoma Cells to Cisplatin via miR-454-3p/c-Met. Mol. Cells. 2020;43:856–869. doi: 10.14348/molcells.2020.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao C., Zhou S., Hu J. Long noncoding RNA MAGI2-AS3/miR-218-5p/GDPD5/SEC61A1 axis drives cellular proliferation and migration and confers cisplatin resistance in nasopharyngeal carcinoma. Int. Forum Allergy Rhinol. 2020;10:1012–1023. doi: 10.1002/alr.22562. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X., Wang S., Wang H., Cao J., Huang X., Chen Z., Xu P., Sun G., Xu J., Lv J., et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol. Cancer. 2019;18:20. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Ambrosi S., Visser A., Antunes-Ferreira M., Poutsma A., Giannoukakos S., Sol N., Sabrkhany S., Bahce I., Kuijpers M.J.E., Oude Egbrink M.G.A., et al. The Analysis of Platelet-Derived circRNA Repertoire as Potential Diagnostic Biomarker for Non-Small Cell Lung Cancer. Cancers. 2021;13:4644. doi: 10.3390/cancers13184644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q., Zhang W., Hao S. LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle. 2017;16:795–801. doi: 10.1080/15384101.2017.1301334. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Zhao C.H., Bai X.F., Hu X.H. Knockdown of lncRNA XIST inhibits hypoxia-induced glycolysis, migration and invasion through regulating miR-381-3p/NEK5 axis in nasopharyngeal carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2505–2517. doi: 10.26355/eurrev_202003_20518. [DOI] [PubMed] [Google Scholar]
- 68.D’Ambrogio A., Nagaoka K., Richter J.D. Translational control of cell growth and malignancy by the CPEBs. Nat. Rev. Cancer. 2013;13:283–290. doi: 10.1038/nrc3485. [DOI] [PubMed] [Google Scholar]
- 69.Tordjman J., Majumder M., Amiri M., Hasan A., Hess D., Lala P.K. Tumor suppressor role of cytoplasmic polyadenylation element binding protein 2 (CPEB2) in human mammary epithelial cells. BMC Cancer. 2019;19:561. doi: 10.1186/s12885-019-5771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin M., Zhang X.-L., You R., Yang Q., Zou X., Yu K., Liu Y.-P., Zou R.-H., Hua Y.-J., Huang P.-Y., et al. Neoantigen landscape in metastatic nasopharyngeal carcinoma. Theranostics. 2021;11:6427–6444. doi: 10.7150/thno.53229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee V., Kwong D., Leung T.-W., Lam K.-O., Tong C.-C., Lee A. Palliative systemic therapy for recurrent or metastatic nasopharyngeal carcinoma—How far have we achieved? Crit. Rev. Oncol. Hematol. 2017;114:13–23. doi: 10.1016/j.critrevonc.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 72.Tang T., Yang L., Cao Y., Wang M., Zhang S., Gong Z., Xiong F., He Y., Zhou Y., Liao Q., et al. LncRNA AATBC regulates Pinin to promote metastasis in nasopharyngeal carcinoma. Mol. Oncol. 2020;14:2251–2270. doi: 10.1002/1878-0261.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lian Y., Xiong F., Yang L., Bo H., Gong Z., Wang Y., Wei F., Tang Y., Li X., Liao Q., et al. Long noncoding RNA AFAP1-AS1 acts as a competing endogenous RNA of miR-423-5p to facilitate nasopharyngeal carcinoma metastasis through regulating the Rho/Rac pathway. J. Exp. Clin. Cancer Res. 2018;37:253. doi: 10.1186/s13046-018-0918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin L., Chen J., Ma C., Pei S., Du M., Zhang Y., Feng Y., Yin R., Bian X., He X., et al. Hsa_circ_0046263 functions as a ceRNA to promote nasopharyngeal carcinoma progression by upregulating IGFBP3. Cell Death Dis. 2020;11:562. doi: 10.1038/s41419-020-02785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Q., Shuai M., Xia Y. Knockdown of EBV-encoded circRNA circRPMS1 suppresses nasopharyngeal carcinoma cell proliferation and metastasis through sponging multiple miRNAs. Cancer Manag. Res. 2019;11:8023–8031. doi: 10.2147/CMAR.S218967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nieto M.A., Huang R.Y.-J., Jackson R.A., Thiery J.P. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 77.Pastushenko I., Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J., Tian X.-J., Xing J. Signal Transduction Pathways of EMT Induced by TGF-β, SHH, and WNT and Their Crosstalks. J. Clin. Med. 2016;5:41. doi: 10.3390/jcm5040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brabletz S., Bajdak K., Meidhof S., Burk U., Niedermann G., Firat E., Wellner U., Dimmler A., Faller G., Schubert J., et al. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu L., Feng X., Hao X., Wang P., Zhang Y., Zheng X., Li L., Ren S., Zhang M., Xu M. CircSETD3 (Hsa_circ_0000567) acts as a sponge for microRNA-421 inhibiting hepatocellular carcinoma growth. J. Exp. Clin. Cancer Res. 2019;38:98. doi: 10.1186/s13046-019-1041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li C., Hu J., Hao J., Zhao B., Wu B., Sun L., Peng S., Gao G.F., Meng S. Competitive virus and host RNAs: The interplay of a hidden virus and host interaction. Protein Cell. 2014;5:348–356. doi: 10.1007/s13238-014-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gunasekharan V., Laimins L.A. Human papillomaviruses modulate microRNA 145 expression to directly control genome amplification. J. Virol. 2013;87:6037–6043. doi: 10.1128/JVI.00153-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li C., Wang Y., Wang S., Wu B., Hao J., Fan H., Ju Y., Ding Y., Chen L., Chu X., et al. Hepatitis B virus mRNA-mediated miR-122 inhibition upregulates PTTG1-binding protein, which promotes hepatocellular carcinoma tumor growth and cell invasion. J. Virol. 2013;87:2193–2205. doi: 10.1128/JVI.02831-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Au K.H., Ngan R.K.C., Ng A.W.Y., Poon D.M.C., Ng W.T., Yuen K.T., Lee V.H.F., Tung S.Y., Chan A.T.C., Sze H.C.K., et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study) Oral Oncol. 2018;77:16–21. doi: 10.1016/j.oraloncology.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Samaridou E., Heyes J., Lutwyche P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020;154–155:37–63. doi: 10.1016/j.addr.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 86.Mukherjee A., Waters A.K., Kalyan P., Achrol A.S., Kesari S., Yenugonda V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019;14:1937–1952. doi: 10.2147/IJN.S198353. [DOI] [PMC free article] [PubMed] [Google Scholar]