Abstract
Objective
Nowadays, due to globalisation, the likelihood that infectious diseases spread rapidly is extraordinarily high. SARS and COVID‐19 are two diseases of the Coronavirus family, which developed in China and then spread internationally, causing global public health emergencies. This study investigates the role that risk management and communication systems played in mitigating these emergencies, to establish how they should be improved in the future.
Methods
A narrative review was carried out to investigate different knowledge domains, such as risk management and communication, risk assessment and indicators, epidemiological and clinical data, diagnostic methods, vaccines, public health and social measures.
Results
On one side, risk management systems assess the main data, knowledge, and indicators on epidemiology, diagnostics, and vaccines (science‐based); on the other side, they apply public health and social measures (socially‐based). Decision‐makers, in fact, implement their actions by constantly balancing these two sides (policy‐based).
Conclusions
A correct crisis management approach should support the governance of pandemics, by harmonising the actual risks assessed by experts with those perceived by the general population. It should incorporate not only the biological, but even the environmental, social and economic aspects of virus emergencies, towards establishing a suitable framework to deal with possible future pandemics.
Keywords: COVID‐19, health planning, risk communication, risk management, SARS
Highlights
COVID‐19 and SARS enhanced rapid research on specific diagnostics and vaccines.
Public health and social measures remain the primary response to virus outbreaks.
Risk perception affects people's behaviour and public health measures' application.
Risk management and communication plans are crucial to cope with pandemics.
SARS and COVID‐19 experiences should help to deal with future virus emergencie.
1. INTRODUCTION
Virus pandemics are unforeseeable but repeated events that can have a noteworthy impact on health, behavioural and economic well‐being worldwide. Pandemic risk management helps to prevent and mitigate the consequences of a widespread contagion during all the main phases of the outbreak, by harmonising public health emergency plans with the disaster risk management already existing in many countries. 1 This approach is based on an accurate and timely assessment of the actual and perceived risks, to plan and implement evidence‐based decision‐making at national, regional, and local levels. International bodies ‐ such as the World Health Organization (WHO) and the Organization for Economic Co‐operation and Development (OECD)‐lead this approach to virus pandemic management worldwide. They also stimulate each Member State to develop flexible national risk assessment and governance plans, 1 grounded on indicators to evaluate risks, through examining epidemiological and clinical data as well as diagnostic, therapeutic, and vaccine options.
In the last decades, several weighty viral pandemics occurred, either actual or potential, requiring risk management systems at the international as well as at the national levels to be applied. Some of those emergencies have been caused by coronaviruses (CoVs), which represent a group of small RNA viruses that infect humans and other different species of vertebrate animals. Rarely, animal CoVs infect people and then spread ‐ such as SARS‐CoV, MERS‐CoV, and now SARS‐CoV‐2 ‐ causing severe respiratory illnesses with high fatality rates. Most CoVs in humans come from the bat reservoir. A high genetic similarity was found between SARS‐CoV‐2 and a bat SARS‐related coronavirus while a low similarity to SARS‐CoV (approximately 79%) or MERS‐CoV (about 50%). SARS‐CoV‐2 uses the same receptor, the angiotensin II converting enzyme, as SARS‐CoV. The development of new variations in functional sites in the receptor‐binding domain of the spike observed in SARS‐CoV‐2 is likely caused by natural selection in addition to recombination. 2
CoVs represent potential threats to global public health, 3 while the COVID‐19 pandemic is the largest global public health crisis of this generation, immediately causing a unique wave of research and data sharing with the goal of understanding the disease, tracking, and controlling its spread. An increasing stream of research claimed that ‐ besides the classic biological determinants ‐ a series of social, economic and environmental determinants should be evaluated and monitored within crisis management systems, in order to contain the transmission of SARS‐CoV‐2 as well as of other viruses in the future. 4 , 5 , 6 Among the environmental determinants, the air pollution, 7 , 8 , 9 , 10 , 11 as well as the population density and the social interactions 4 , 5 , 11 , 12 , 13 have been identified as very important in increasing the risk of transmission.
The first research question of this study regards how the actual international risk management and communication systems handled the emergency of the SARS‐CoV‐2 pandemic compared with the systems that handled the SARS‐CoV epidemic in 2003. The main aspects selected to be investigated within those systems are: on one side, the main data, knowledge, and indicators on epidemiology, diagnostics, and vaccines (science‐based factors); on the other side, public health measures and social measures (socially‐based factors). Risk management, in fact, constantly chooses to implement a series of actions by balancing these two sides, in order to contain the contagion (policy‐based factors). Following this logic, there is a constant reference to the organizations responsible for international public health and their role in promoting the control of contagion at national and sub‐national levels. The second research question regards how the existing international risk management and communication systems should be improved, consequently suggesting research challenges and policy implications to create a suitable framework that embraces also the environmental, social, and economic determinants of future pandemic disease emergencies.
2. STUDY DESIGN
In order to describe how pandemic evolution can be affected by accurate diagnosis of the infection, vaccine development, and contagion prevention, this study examines the literature covering epidemiology, clinical biochemistry, and social sciences. With the objective of catching the complexity of crisis management and communication systems during virus pandemics, a narrative review was performed, also on the basis of what was recommended by Greenhalgh and colleagues. 14 A narrative approach was preferred to a systematic one, since the main concern was not to address closely focussed issues and then summarise the data as it does in systematic reviews. The main scope was that of providing a wide framework of many aspects embraced by the risk management and communication systems, in a continuous comparison among SARS and COVID‐19 emergencies. This scope necessitated clarifications and insights, for which a more interpretative and descriptive synthesis of existing literature is needed, thus requiring the narrative review method. 14
The literature search was executed by the three review authors, which form a multidisciplinary team with expertise in health risk communication, public health and epidemiology, and biomedical diagnostic technologies. The search was conducted on MEDLINE/Pubmed and Google Scholar and was limited to documents published in the English language. In addition, the literature was collected through the search into the following institutional websites: US CDC‐Centres for Disease Control and Prevention (link: https://www.cdc.gov/), WHO‐World Health Organization (link: https://www.who.int/); FIND‐Foundation for Innovative New Diagnostics (https://www.finddx.org/); OECD‐Organisation for Economic Co‐operation and Development (https://www.oecd.org). All the document types were accepted (e.g., journal articles, books, chapters, institutional reports and guidelines, and websites of relevant organizations). The different knowledge domains of interest were: 1) risk management and communication, 2) risk assessment and indicators of severity, 3) epidemiological and clinical data, 4) diagnostic methods, 5) development of vaccines, 6) public health and social measures.
For each target knowledge domain, the specific keywords were combined with the terms “SARS”, “SARS‐CoV”, “COVID‐19” and “SARS‐CoV‐2”, by using the AND operator. Regarding SARS and SARS‐CoV, the search was filtered at the time of publication from 2003 to 2020, while for COVID‐19 and SARS‐CoV‐2 from 2019 to 2021.
3. RESULTS AND DISCUSSION
3.1. Risk management and communication systems for public health emergencies
The international outbreak crises challenge severely politicians and risk managers often due to an unpredictable large scale and transboundary nature, disclosing the necessity to improve the existing information and communication systems. 15 This necessity descends from a dichotomy among experts and the general public concerning risk perception. The traditional theory establishes that the public usually bases decisions on their perception of the risk, whereas institutions more often on the actual risk. 16 , 17 However, also other factors intermediate governments' decisions, for instance, the political, social, and economic demands of the public. 18 , 19 , 20 Based on the dominant psychometric paradigm, risk communication strategies are usually designed to help the public to achieve perceptions of risk more similar to those of experts. 21 This is one of the main objectives of crisis communication strategies, pursued to enhance risk understanding, trust, acceptance, and compliance with the necessary public health measures. 20 , 22 A risk management framework foresees a series of strategies, programs, and activities, to face a potential crisis during each of its typical phases. When a crisis is possible, the system acts as a “risk management system” and includes “risk communication” strategies and programs. When the crisis is ongoing, the system regards an actual “crisis management” and involves “crisis communication” strategies and plans. Although with different terminologies, current risk management systems embrace three phases: “preparedness” before the crisis, “response” to control and reduce damage during the crisis, and “recovery” after the crisis. 15 , 23 , 24 During the alert phase (high hazard, low outrage) ‐ the precautionary principle should address uncertainty, usually leading to an overestimation of risk. During the pandemic phase (high hazard, high outrage), the pessimistic public perception of risk requires continuous communication of the actual risk and the necessary adoption of health measures. During the recovery phase (low hazard, high outrage), post‐crisis communication manages outrage, reassures people, and addresses blame. In this last phase, governments can also stimulate people to debrief and think about long‐term preparedness. 17 Risk assessment is the first activity implemented by the political and technical leadership during the preparedness phase. An outbreak crisis obliges us to recognise, investigate and evaluate the key threats, hazards, and associated exposures. 15
Along with ensuring cooperation and effective decision‐making, leadership plays a key role in crisis communication during all the phases. Communicating via the mass and social media with the general public, to provide rationality regarding events, maintain trust in the emergency organizations and governments, as well as to transmit useful messages is a vital responsibility of leadership during the crisis. 15 Communication toolkits are developed to help institutions either to calm public concerns, if experts felt that these concerns are excessive, or to raise risk perception if experts feel that the public is inadequately worried about the severe risks. 25
The WHO plays a crucial role in creating and updating international risk management systems related to the epidemic and pandemic crises. 19 At their beginning, risk management systems focussed more on risk assessment and management than on risk communication strategies. In the last 20 years, the WHO has provided Member States with specific systems aimed to detect and respond to epidemic emergencies. Whenever those epidemics could evolve towards a significant international spread, the WHO activated a global surveillance and response mechanism. In 2000, it established the Global Outbreak Alert and Response Network (GOARN) with the technical expertise of about 120 partner institutions worldwide to be entailed in this scope. 26 During the SARS outbreak, eleven scientists from nine countries joined a network within the WHO, to identify the causal agent and generate a diagnostic device. 27
SARS represented such an exceptional emergency, as to provoke, for the first time in contemporary years, an important chance to experiment with the available international warning systems. 28 The international community reacted at an unexpected speed despite the Chinese government showing a denial attitude in the initial 5‐month period. Within two weeks since the Hong Kong emergency, in March 2003, WHO issued a global health alert concerning cases of abnormal pneumonia. 29 Consequently, various agencies produced many new websites, to disseminate information widely and quickly. 29 Thus, on one side SARS augmented the worries about globalisation, which reduces the levels to which people can control their destiny and increases their exposure to external threats. On the other side, it also demonstrated the positive facets of globalisation, by enabling scientists to share research information in real‐time and facilitating an international coordinated response. 19 During SARS, experts noticed that risk communication created an excessive risk perception in people concerning its low morbidity and mortality, 30 with a media coverage evaluated as occasionally inexact and distressing. 31
While during SARS outbreak an explicit risk communication system didn't exist within the risk management embodied by the GOARN, during the current COVID‐19 outbreak the institutions could make use of crisis management systems that explicitly include crisis communication. Currently, the possibility to adopt an integrated system to respond to the COVID‐19 outbreak does exist, at least at a theoretical level. Table 1 describes the evolution from GOARN to the actual Risk Management and Communication Systems, created at the international level by WHO and the U.S. Centres for Disease Control and Prevention (CDC). As underlined by the WHO in its risk management systems updated in 2013 and 2017, the global pandemic phases (inter‐pandemic, alert, pandemic, and transition phases) express the diffusion of the disease worldwide (Table 1). Nevertheless, as pandemic emerges, countries and local communities face different risks at different times. 1 , 24 CDC tried to fill in this gap with its pandemic guidelines, conceived for the local and individual alert levels. 23 , 32 , 33
TABLE 1.
Responding to pandemic outbreaks
| Name | Source | Scope | Description‐how it works |
|---|---|---|---|
| GOARN‐Global outbreak alert and response network | WHO (2003) | Risk management | It is a network of technical experts from 120 countries, to respond to global surveillance needs of each pandemic outbreak. |
| SARS risk assessment and preparedness framework | WHO (2004) | Risk assessment | It is a phased framework of activities, to assess the risk that SARS might reappear and to prepare suitable emergency plans at national and international levels. |
| Global pandemic six‐phases threat index | WHO (2005) | Risk assessment | It ponders only the geographical spread of the outbreak via a technical language, producing misunderstandings among the international organizations, the media and the general public. |
| Crisis communication best practices | Sandman (2006) | Crisis communication | Crisis communication strategies should change along the four phases of an outbreak, which are based on two factors: The hazard; the public outrage. |
| PSI‐pandemic severity index | CDC (2007) | Risk assessment | The pandemic severity index (PSI) considers the severity of the potential pandemic and decodes it to specific guidelines for individuals and communities. |
| Risk communication phases | Sandman (2007) | Risk assessment and communication | It complements the WHO six‐phase system. Besides the impact of the virus ‐ it takes into account also (a) the level of public concern, and (b) the location of the disease. |
| Revised pandemic phases | WHO (2013) | Risk management | WHO recommends a new four‐phase system, in order to stimulate national authorities to develop their local risk management plans. This new system works on two matching axes ‐ the global and the local risk‐based phases. |
| Intervals‐preparedness and response framework | Holloway et al. (2014) | Risk management | This system clarifies the link among the broad WHO phases and the in‐depth planning intervals. In addition, the intervals express precise transmission‐related indicators, as well as the detailed response activities that should occur. |
| Pandemic influenza risk management | WHO (2017) | Risk management and communication | WHO harmonises national and international pandemic influenza preparedness and response phases, encouraging member states to develop agile plans based on national risk assessment. It also describes and stimulates risk communication strategies and activities. |
| Preparedness and response framework, with CDC intervals and WHO phases | Qualls et al. (2017) | Risk management and communication | Each interval is associated with particular activities, such as: 1) active participation in implementing non‐pharmaceutical interventions (NPIs) during the initiation and acceleration intervals; and 2) coordinated discontinuation of community‐level NPIs during the deceleration interval. |
| Novel coronavirus (2019‐nCoV): Strategic preparedness and response plan. | WHO (2020a, 2020b) | Risk management and communication | The plan sketches the public health measures that the international community stands ready to support all countries to prepare for and respond to 2019‐nCoV. |
| Strategy update. | The subsequent strategy update document provides a guide for countries preparing for a transition from widespread transmission to a stable state of low‐level or no transmission. |
Note: The evolution from GOARN to the actual international Risk Management and Communication Systems.
Sandman provided risk communication phases, which are based on a risk assessment index and focus on a common language directly useable by media, by avoiding technical definitions and addressing diverse kinds of risk perception. 34 Following this index, the uncertainty of available knowledge on diagnostics, therapies, and vaccines during novel infectious disease outbreaks generates a dichotomy among optimistic and pessimistic messages, which need to be continuously balanced by appropriate crisis communication. 17
The necessity of an integrated model which encompasses all the frameworks proposed by WHO, CDC, and Sandman has been originally recommended within the EU Tell Me Project 35 and partially achieved by CDC. 23 Although the available knowledge would allow this integration, the actual international risk management frameworks designed to cope with the COVID‐19 emergency 36 , 37 do not fully match the technical assessment aspects and phases with the psychosocial dimensions of communication strategies. 38
During the alert phase, there was a late response to the emergency in many countries worldwide probably provoked by an absent collaboration between national health and risk management systems, as well as by a late communication by international organizations. 39 Throughout the pandemic phases, which it was necessary to make citizens aware of the correct adoption of public health measures, the positive phenomenon of the worldwide explosion of scientific studies and timely global information was accompanied by the negative phenomenon of infodemic and the resulting total confusion and misinformation. 40 , 41 This problem is continuing at the current stage, where it would be necessary to promote proper adherence to vaccination campaigns in the various States, 42 to be able to settle the final blows to the current danger of the virus and its variants.
3.2. Risk assessment and indicators of severity
Risk management is a policy‐based decision‐making process on adopting, boosting, or adjusting specific public health and social measures. 37 This should be based on risk assessment, which is a systematic science‐based process led with a validated methodology for gathering, evaluating and recording information, with the aim of assigning a level of risk. 43 Therefore, risk management aims to balance the risk of reducing public health and social measures with the following capabilities: identifying a reappearance of new cases, treating additional patients in health care or other structures, and reestablishing public health and social measures if needed. All the factors upon which risk management advisors decide to implement certain actions are called “indicators” and usually constitute the risk assessment structure (See Appendix A). WHO suggested similar indicators to control both SARS and COVID‐19 pandemics. 44 , 45 The main new indicators introduced to control COVID‐19 regard: a) the serological testing; b) the more detailed health care capacities; c) the public health rapid response teams to investigate suspect cases and clusters.
At an international level, pandemic risk assessment and risk management are separate responsibilities. 46 WHO carries out risk assessment activities in the form of study outcomes and recommendations. Member States may choose to directly accept WHO outcomes and recommendations or to designate their own risk assessment bodies that share their findings with those from WHO. 46 At a national level, risk management is a specific responsibility of State governments, although they can ask to autonomous expert bodies to generate recommendations. Since the transmission of a pandemic virus is usually uneven within a country, subnational and community‐level risk assessment structures should assist the national risk assessment system.
Some authors suggest that ECDC should continuously monitor transmission and mortality and work closely with WHO to provide guidelines, encourage control and prevention activities, and comprehend the effects of the lockdown on the containment of Covid‐19. 47 In this respect, one important challenge is the design of simple but effective multifactorial indexes to support appropriate strategies of prevention during the emergence of a pandemic, in order to reduce many negative effects on society. 48 , 49 Some scientists, in fact, observed that an excessive number of people belonging to certain minority populations got infected and died due to the COVID‐19. 5 They discovered that in those communities the risk for morbidity and mortality is exacerbated by social, economic and environmental determinants of health, which interconnect with biological factors (comorbidities, underlying genetics, host immunity, vitamin D levels, epigenetics). 5 Socio‐economic determinants regard the healthcare accessibility, the socio‐economic status, the employment, the public transport. 5 , 50 Environmental determinants include the residential isolation, 5 the population density and social interactions, 4 , 5 , 11 , 12 , 13 the air pollution, 7 , 8 , 9 , 10 , 11 the housing situations, the distance from alimentary and medical facilities, the violence. 5 Socio‐cultural determinants embrace the racism, the education, the health literacy, the trust in the healthcare system. 5 Finally, the development of new technologies should play a key role in advancing crisis management systems in the aspects of prevention, monitoring and control, 47 , 50 as well as in the adaptation of clinical trials during pandemics. 51
In the next paragraphs, some indicators regarding epidemiological and clinical factors, molecular and immune assays, serological tests and vaccines were analysed, with the purpose of helping to understand how these data drove decision‐making in each phase of SARS and COVID‐19 pandemics, within the existing risk management and communication framework.
3.3. Epidemiological and clinical data
The two crises due to SARS‐CoV and SARS‐CoV‐2 have both raised great concern for global public health, albeit with different epidemiological spreads. The previous epidemic of SARS infected between November 2002 and July 2003 over 8000 and killed 916 people in 32 countries. The disease appeared in the Guangdong province of southern China and spread mainly in Canada, Hong Kong, Singapore, and Vietnam. Most cases occurred in the health care setting, in the absence of adequate infection control precautions. Adoption of appropriate infection control measures brought the global outbreak to an end, and no cases of SARS have been reported since. 52
The similar epidemic caused by SARS‐CoV‐2 that occurred in early December 2019, produced a significantly more dramatic impact. Starting in Mainland China, in the city of Wuhan, Hubei, it became more widespread in the rest of the world than in China, so much that on 11 March 2020 the WHO announced a pandemic condition. From 7 December 2019 to the middle of July 2021, the outbreak resulted in an estimated 186 million worldwide, killing more than 4 million people with a global lethality of about 2.2 during three epidemic waves. In recent months, emerging variants of the SARS‐CoV‐2 virus have been documented (Alpha, Beta, Gamma, Delta) with an increased transmissibility. In particular, the Delta variant has been detected in at least 111 countries across the world in the last two months. 53
Overall, SARS‐CoV‐2 is less deadly than SARS, which had a fatality rate of 10% with a range from 0% to nearly 30% based on different ages of the infected subjects. According to WHO data relating only to SARS cases reported in China, there was no death among patients under the age of 20. The patients between 20 and 30 were less likely to die (less than 1%), whereas a higher percentage of mortality was observed between 70 and 79 years (28%). 52
COVID‐19 disease also shows a higher mortality gradient in older age groups. One of the most complete analyses conducted among patients with COVID‐19 in China reported on 44,672 confirmed cases with a fatality rate of 2.3%, with percentages close to zero for patients under the age of 50, while values of 8% among subjects aged 70% and 79% and 14.8% in subjects over 80 years old. 54
SARS and COVID‐19 present themselves as the typical viral pneumonia with similar pathogenic characteristics but with differences in clinical manifestations. The most prevalent clinical manifestations of both include fever, cough, headache, dyspnea, diarrhea, and myalgia. Additional evidence reports in COVID‐19 patients, symptoms such as taste or olfactory disorders or skin manifestations describing the potential involvement of other organs besides the respiratory and gastrointestinal tract. 55
The spectrum of clinical manifestations varies from the absence of symptoms to severe respiratory failure. While the percentage of asymptomatic subjects with SARS was between 7.5% and 13%, that of SARS‐CoV‐2 positive subjects was between 5% and 80%. 56
Critical cases often progress quickly, developing respiratory distress and needing intensive care. Approximately 20% of patients with SARS develop severe respiratory failure that required admission to ICU. 57 Almost a third of the patients affected by SARS‐CoV2 developed complications that included acute respiratory distress syndrome and were required to be treated in the ICU. 58 The main risk factors for a severe clinical course and worse outcomes in patients with SARS‐CoV‐2 were old age, male gender, and pre‐existing chronic conditions. 59
An increased risk of infection and worse clinical outcomes has been found in individuals from Black, Indigenous, Asian, and Latin communities and Ethnic Minorities, showing the need to implement national ethnic surveillance systems in order to create large‐scale international registries and studies for future public health interventions to promote health equity. 60 , 61 Additional predisposing factors may impact the risk of COVID‐19 infection and severe illness. For example, a recent meta‐analysis supported the role of the ABO blood group in the susceptibility to the infection, with a higher risk of infection in subjects with blood type A and a lower risk of blood type O. 62
Furthermore, strong evidence of causation between meteorological factors and COVID‐19 outcomes has also been reported. 63 Therefore, seasonality and other environmental factors determined by the change in how human society interacts with natural ecosystems should be considered when implementing adequate public health policies, timely vaccination campaigns, and other non‐pharmaceutical interventions to cope with recurring waves. 64
SARS seroprevalence studies have shown overall seroprevalence rates of 0.1% for the general population and 0.23% for health care workers (HCW). 65 In a meta‐analysis involving about 400,000 people from 23 countries, the SARS‐CoV‐2 seroprevalence in the general population varied from 0.37% to 22.1% with a pooled estimate of 3.38% and marked differences among geographic regions. 66 A further meta‐analysis including 49 studies and 127,480 HCW, reported an overall estimated SARS‐CoV‐2 antibody seroprevalence of 8.7%, with values ranging from 0% to 45.3% across the studies and the different geographic realities. The notable differences can be due to the different (a) sensitivity or specificity of the tests used for antibody detection, (b) lockdown and quarantine measures, and (c) time of data collection. 67
3.4. Diagnostic methods: Guidelines to the times of SARS and COVID‐19
On 16 April 2003, WHO announced the identification of the new pathogen responsible for SARS as the result of an unprecedented collaboration of 13 laboratories, from 10 countries, working to fulfill Koch's postulates, to establish the cause of the disease. 68
As summarised on the WHO website, 69 laboratory tests were based on the approaches described as it follows.
To detect the genetic material of the SARS‐CoV in various specimens (blood, stool, respiratory secretions, body tissues), primers for Polymerase Chain Reaction (PCR) had been made publicly available on the website by WHO network laboratories and a ready‐to‐use PCR test kit with relative controls had been developed. The availability of PCR protocols at http://www.bni.uni‐hamburg.de/ was reported to the WHO network on March 27 and ProMed mail [an international e‐mail notification service for infectious‐disease outbreaks] on March 30. Over time, diverse protocols were designed within the different testing approaches. Most PCR assays were designed with the Orf1b or nucleoprotein gene, with the latter gene being more copious in infected cells, and therefore of better sensitivity, although not verified in clinical studies. Quantitative Reverse Transcription Polymerase Chain Reaction (RT‐PCR) of the nasopharyngeal aspirate resulted in the most sensitive and rapid method for clinical diagnosis (sensitivity of 80%, good specificity), even if collected in the first 5 days from clinical signs onset. 70
A parallel strategy was based on a panel of three monoclonal antibodies, enzyme immunoassay (EIA) detection of the N protein was completed in a large study with more than 300 serum samples of SARS patients, at different time points of illness. As early as day 5 after the start of infection, EIA revealed a sensitivity of 94% and a specificity of 99.9%. 71 Antigen detection with EIA in non‐serum specimens resulted less sensitive than RT‐PCR due to the higher cutoff level aiming to overcome the background noise. However, most of these tests have never been exhaustively investigated in prospective cohort studies owing to the short‐lived epidemic. 70
Viruses in specimens (such as respiratory secretions, blood, or stool) from SARS patients can also be detected by inoculating cell cultures and growing the virus. Once isolated, the virus must be identified as the SARS one with further tests. Cell culture is a very demanding test and it only means to show the existence of a live virus.
On the other hand, to detect the antibodies produced in response to the SARS coronavirus infection the following tests were developed or were under optimization:
-
‐
ELISA (Enzyme Linked ImmunoSorbant Assay), searching for IgM and IgG antibodies in the serum of SARS patients produced positive results reliably at around day 21 of illness
-
‐
IFA (Immunofluorescence Assay), searching for IgM antibodies in serum of SARS patients produced positive readouts 10 days earlier 69
Subsequent studies have revealed that serum‐specific antibodies against SARS‐CoV whole virus from indirect immunofluorescence or neutralisation tests begin to appear around day 7, while IgM were undetectable after 2–3 months, and IgG maintained for over a year. 72
Overall, among Sars‐CoV diagnostic tests, in Table 2, only those rapidly optimised and published (within 2003) are reported, to frame the first technological response given during SARS pandemic in terms of developed assay type and performance. Considering comparable sample collection time (day 1–5 after onset of symptoms) and without weighing the number of clinical specimens analysed, the data reveal equivalent specificity (100%) for those molecular tests optimised on nasopharyngeal aspirate or nose throat swab as well as for anti‐N protein antibodies test performed on plasma samples. With respect to sensitivity, molecular tests reach the peak at day 2, while antibody tests performed on plasma collected in the range 1–5 days show the lower value, highlighting the importance of both the method and the specific timing of application. Over the years, new methods and relative data on analytical effectiveness were published.
TABLE 2.
SARS‐CoV, clinical evaluation of molecular diagnostic tests and antibody detection assays
| Diagnostic method and detection target | Clinical specimen | % Sensitivity* (no. of samples; day | % Specificity | Reference |
|---|---|---|---|---|
| In‐house RT‐PCR | NPA | 59.7 (72; 1–5) | 100 | Yam et al. 2003 |
| RNA pol | ||||
| In‐house RT‐PCR | Nose and throat swab | 61.1 (54; 1–5) | 100 | Yam et al. 2003 |
| RNA pol | ||||
| In‐house RT‐PCR | Blood (plasma) | 79.2 (24; 1–3) | ‐‐‐ | Grant et al. 2003 |
| Nested RNA pol | ||||
| In‐house quantitative PCR | NPA | 62.5 (8; 1) | 100 | Poon et al. 2003 |
| ORF 1b | ||||
| In‐house quantitative PCR | NPA | 87.5 (16; 2) | 100 | Poon et al. 2003 |
| ORF 1b | ||||
| In‐house quantitative PCR | NPA | 80.1 (26; 3) | 100 | Poon et al. 2003 |
| ORF 1b | ||||
| EIA | Blood (plasma) | 14.8 (27; 1–5) | 100 | Shi et al. 2003 |
| Anti‐N protein antibodies |
Note: Tests reported in this table have been selected among those optimised and published within 2003, with respect of sample collection time comparability (day 1–5 after onset of symptoms). *values are copies/ml.
Abbreviation: NPA, nasopharyngeal aspirate
Nearly two decades later, on 7 January 2020, the Chinese authorities isolated a new type of coronavirus, and on 12 January 2020, they shared its genetic sequence to be used in the development of specific diagnostic kits. 73 Actually, established laboratory technologies have facilitated researchers in the design of COVID‐19 diagnostics. Knowledge acquired from the 2002 SARS outbreak has directed the rapid identification of COVID‐19 and the development of detection tools. 74 Interim guidance was published online 75 regarding the following methods:
-
‐
Nucleic acid amplification tests (NAAT). Routine confirmation of cases of COVID‐19 is based on detection of unique sequences of virus RNA by real‐time RT‐PCR and additional confirmation by nucleic acid sequencing. The N, E, S, and RdRP viral genes are so far targeted.
-
‐
Antibody testing. At the date of the above cited WHO document, 75 only some studies with COVID‐19 serological data on clinical samples have been available. 76 , 77 Wide‐ranging serology immunoassays (IAs) have been developed; the most prominent IAs are automated chemiluminescent IA (CLIA), manual ELISA, and rapid lateral flow IA (LFIA), which detect the immunoglobulin M (IgM) and immunoglobulin G (IgG) produced in response to SARS‐CoV‐2 infection.
In the diverse periods of the pandemic, complementary serological analysis has at least a double intrinsic value: from i) estimating epidemiological variables useful in community transmission level evaluation, 78 to ii) individuals immunological response monitoring for plasma therapy strategy. 79
Viral sequencing. International databases (i.e. GISAID) for deposition of genetic sequence information are public access. In addition, to confirm the presence of the virus, systematic sequencing of a percentage of specimens from clinical cases can be exploited to monitor for viral genome mutations, to prevent issues on the performance of medical adopted countermeasures, including diagnostic tests.
Viral culture. Isolation of live viruses is not suggested as a routine approach.
Insufficient reagents and an unplanned laboratory testing organization have influenced the initial stages of the actual pandemic. Typically conducted in centralised laboratories, the routine for population‐scale testing is represented by the RT–qPCR. Cheap tests can be performed without specialised equipment through the isothermal nucleic acid amplification method. Moreover, SARS‐CoV‐2 emergent variant strains are assessed by the next‐generation sequencing (NGS) technology. By targeting SARS‐CoV‐2 spike protein or nucleocapsid protein on immunoaffinity lateral flow tests, rapid results are made available in 30 min at the point of care, with time and cost‐effectiveness, although missing in specificity and sensitivity than corresponding molecular assays. 80
Overall, to accelerate clinical decision‐making and to take some of the workloads off centralised test laboratories, rapid diagnostic tests (RDTs), at the Point of Care (POC), for use at the community level were identified by a WHO expert group as the first of eight research priorities.
At the beginning of the pandemic, based on the available data, 81 WHO limited the use of these new POC immunodiagnostics (antigen (Ag) and antibody (Ab) tests) only in research settings. Remarkably, on September 11, WHO published advice on the use of Ag‐RDTs during the COVID‐19 pandemic, reversing the previous one. 82 Despite the lower sensitivity than that molecular tests permit, antigen tests are now recognized by WHO as a chance of fast and cost‐effective detection of SARS‐CoV‐2 in persons with high viral loads (>106 genomic virus copies/mL), in the pre/early‐symptomatic phases of the illness, with an elevated risk of pathogen transmission. 82 , 83 As recommended, only Ag‐RDTs matching the performance criteria (minimum sensitivity and specificity respectively as 80% and 97% compared with a molecular reference assay) should be applied and conducted by trained operators, within the first 5–7 days from the onset of symptoms, in areas where standard molecular methods are congested. 82
Notably, aiming to support policymakers and healthcare providers with current information on tests for SARS‐CoV‐2, in partnership with WHO, the global non‐profit organization ‐ Foundation for Innovative New Diagnostics (FIND) is guiding independent evaluations of diagnostics, as part of the worldwide reaction to the COVID‐19 pandemic. On 19 February 2020, FIND launched the first expression of interest (EOI), with deadline fixed on 9 March 2020, for test developers of in vitro diagnostics (IVDs) revealing SARS‐CoV‐2 nucleic acid (molecular tests). On 13 March 2020, a new EOI process for immunoassays evaluation (i.e. manual ELISA, machine‐based or lateral flow, rapid tests specific for SARS‐CoV‐2 antigen or antibodies) was published, with deadline fixed on 20 March 2020. All the test submissions were reviewed according to their regulatory status and time to market; the manufacturing and distribution capacity of the supplier; and their clinical and analytical performance.
Regardless of the state of maturity of the kit (marketed or in development), a large number of companies voluntarily applied for the process on the FIND. To the end of May 2021, an increasing number of diagnostics are being proposed (more than 1000) on FIND database (https://www.finddx.org/covid‐19/pipeline/). Figure 1 summarises the commercialised tools, resulting in line with the declaration of conformity under the ‐ In Vitro Diagnostic Regulation (IVR)‐and those that have received at least the Emergency Use Authorisation (EUA). Even if of technical value, the tests intended for Research Use Only (RUO) were not considered.
FIGURE 1.
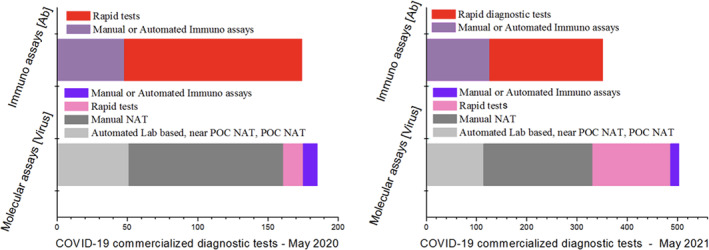
Distribution of COVID‐19 commercialised tests under FIND evaluation. On the left, distribution by number of test type, at May 2020; on the right, data are updated at May 2021. A considerable number of rapid test (both for Ab and for Ag) development is observable, with a marked increment of Ag‐RDTs from May 2020 to May 2021
3.5. Development of vaccine against SARS and COVID‐19
The absence of herd immunity and specific antiviral treatment make all individuals susceptible to new infection and can determine an exponential increase in the number of cases. This is what has happened for SARS and currently for COVID‐19. Therefore, vaccination represents one of the main approaches to prevent and control future emergencies, especially when ‐ as for COVID‐19 ‐ the high transmissibility occurs also by asymptomatic subjects.
SARS‐CoV and SARS‐CoV‐2 exhibit a very high genetic similarity; both include in their genome four main structural proteins: S, M, E, and N. The SARS‐CoV‐2 gene S has approximately 75% of the same nucleotide sequences as SARS‐CoV, and both viruses bind to host cells using the human angiotensin‐converting enzyme 2 receptors. 84 , 85 For SARS‐CoV, several vaccines were tested in animal models, including recombinant S‐protein‐based vaccines, attenuated and whole inactivated vaccines, and vectored vaccines, but no vaccine was developed. 86 Studies from SARS‐CoV have been a reference for the development of COVID‐19 vaccines for which multiple strategies have been adopted, most of which target the S protein. 87
The first two vaccines available, developed by BioNTech/Pfizer and Moderna Therapeutics, were mRNA vaccines that use messenger RNA strands encoding for S protein and encapsulated within lipid nanoparticles. 88
Moderna Therapeutics used the mRNA technology platform to develop the vaccine mRNA‐1273, a lipid‐encapsulated mRNA encoding for a prefusion stabilised full‐length, S protein. BioNTech/Pfizer has developed the BNT162b2 vaccine using the same technology as LNP‐encapsulated mRNA. A Phase III clinical trial of the Moderna vaccine demonstrated 94.1% efficacy after two doses at 1 and 28 in preventing COVID‐19. 30,420 participants were involved in the study and no safety concerns were observed. The most common adverse events (AE) were injection site pain, erythema, and induration and no difference was observed in the frequency of serious AE between participants in the vaccinated group and the controls. 89 The BioNTech/Pfizer Vaccine after the administration of two doses at 21 days intervals has shown the 95% efficacy. A total of 43,548 participants were enrolled in the trial and the most common local AE were transient mild‐moderate pain at the injection site and redness and swelling. A low incidence of systemic AE was reported and those more frequently observed were fatigue and headache, fever, and chills. Four serious AE were reported and two deaths, not associated with the vaccine. 90
A third vaccine was developed by Astra Zeneca with different technology. This vaccine uses ChAdOx1 chimpanzee adenovirus as a viral vector with a full SARs‐CoV‐2 spike insert. The efficacy was assessed following the administration of two doses with an interval of 4–12 weeks showing across 24,422 subjects recruited an efficacy of 66.7% more than 14 days after the second dose. The published results have shown that AE were mild‐moderate and included fever, headache, body pain, and malaise; the serious adverse effects were not related to the vaccine. 91 The Ad26.COV2.S vaccine by Janssen and Johnson & Johnson is based on the same design; the human adenovirus serotype 26 (Ad26) is used as a vector encoding the full‐length S protein. The efficacy evaluated at ≥14 days after vaccination, was 63.0% across all participants in the trial. 92 The most common local AE were injection‐site pain, redness and swelling, headache, fatigue and myalgia, and fever among the systemic ones. 93
At the time of writing, the BioNTech/Pfizer, Moderna, and Janssen COVID‐19 vaccines have received EUA from the FDA allowing use in individuals over 12 years of age for the first vaccine and over 18 years old for the other two. BioNTech/Pfizer and Moderna vaccines require a double administration, 3 weeks and 1 month apart, respectively; on the other hand, the Janssen COVID‐19 vaccine is a single dose. The FDA amended the EUA including the increased risks of myocarditis and pericarditis following vaccination for the BioNTech/Pfizer and Moderna vaccines, and a very rare and serious type of blood clot in people who receive the Janssen vaccine (https://www.fda.gov/emergency‐preparedness‐and‐response/coronavirus‐disease‐2019‐covid‐19).
Four vaccines have been authorised by the European Medicines Agency (EMA) for use in the European Union: BioNTech/Pfizer, Moderna, Janssen, and AstraZeneca vaccines (https://www.ema.europa.eu/en/human‐regulatory/overview/public‐health‐threats/coronavirus‐disease‐covid‐19/treatments‐vaccines/vaccines‐covid‐19/covid‐19‐vaccines‐authorised).
Currently, 18 vaccines are in use authorised by at least one national regulatory authority for public use, including RNA, with Vector (non‐replicating), Inactivated, and with Protein subunit vaccines. 94 On 20 July 2021, over 3,6 billion doses have been administered globally with the 26,3% of the world population has received at least one dose of a COVID‐19 vaccine and only 1% of people in low‐income countries. United Arab Emirates, Chile, Israel, and Bahrain present a higher percentage of people fully vaccinated. 95
A successful pandemic control should face a series of important issues, such as the efficacy against new variants, the extent of protection, and the ideal level of vaccination. Some studies have demonstrated the power of vaccination to reduce confirmed cases and negative social impact. 96 Some studies have demonstrated the high immune response induced by vaccination as well as the ability of vaccination to reduce confirmed cases and negative social impact. 96 , 97 A recent research based on data from 192 countries showed that the optimal level of vaccination is associated with the evolution of the pandemic wave; vaccination in the early phase of the pandemic wave requires an optimal lower dose level to reduce the number of infected individuals and thus the number of deaths globally compared to later stages of the pandemic. 98 Moreover, vaccine acceptance is a key alarm launched by the international public health community during the last decades. This concern is due to the dissemination of ambiguous, distorting information that is not based on scientific evidence, the spread of fake news, as well as to the public scepticism about vaccination programs. 99 , 100
Finally, some authors foresaw the necessity to offer guidelines for governments and healthcare professionals on how the vaccine supply chains can be threatened by new cyber risks caused by the use of the Internet of Things (IoT). 101 The COVID‐19 pandemic, in fact, boosted many chances associated with the application of digital technologies to medical services and devices. However, this also generated a variety of new cyber risks within the shared healthcare infrastructure. Those worries are usually not detectable by distinct health care departments and many organizations do not hold the needed cyber skills. Consequently, the Health Technology Assessment of new evolving cyber risks requires a new ethical awareness, transparency, and accountability before introducing IoT technologies into healthcare systems (Radanliev, 2021b). 102
3.6. Public health and social measures
Due to the lack of specific diagnostics testing, therapies and vaccines, early containment of various epidemic outbreaks can only rely on non‐pharmaceutical public health measures. 103 , 104 In the last decades, SARS is the first case for the use of traditional public health measures to contain an infectious disease outbreak. 19 Recently, the U.S. CDC recommends some of these measures ‐ all called “NonPharmaceutical Interventions” (NPIs) ‐ to prevent a respiratory virus transmission. During an influenza epidemic, for instance, public health institutions recommend the use of NPIs regardless of the severity of the contagion, 23 and whenever a new virus with a possible pandemic risk occurs. This requires local decisions on measures' selection and timing, based on the overall severity and contextual conditions 105 and can be modified as the epidemic advances and new knowledge comes to be accessible. All NPIs belong to three main categories, depending on what level they can be applied: individual, community, and environmental. 23
During the COVID‐19 outbreak, further measures arose and were highlighted by other experts. 104 , 106 , 107 , 108 , 109 , 110 , 111 In the Appendix A, the measures used during SARS are compared with those implemented during COVID‐19.
To face the COVID‐19 epidemic, the Chinese government as first activated for the whole State a public health emergency response called “Level I”, initiating wide‐ranging and severe prevention and control procedures for health and safety. 104 A team of professionals from the Chinese CDC and National Health Commission (NHC) implemented the related emergency plan. The full lockdown was adopted as an unprecedented measure‐previously used only during the plague of Saint Charles in Milan in the sixteenth century 114 ‐ and subsequently many countries decided to undertake similar containment actions. 55 , 104 Two measures‐absent during the SARS‐have been combined with lockdown (Appendix B): a) mandatory home isolation of “apparently healthy” household members; and b) closing of all unnecessary facilities and factories. Maintaining a safe distance between people has been also exceptionally emphasised during COVID‐19, not only by the authorities, but also by some studies investigating the best distance between people to avoid contagion. 112 , 113 Finally, the curfew has been implemented, although is a measure never tested against a virus, often used to face natural disasters or to keep the riots down. 109 The only measure that authorities used during the SARS and neglected during COVID‐19 is that of closing poorly maintained facilities, probably due to the prevalent adoption of the full lockdown. 110
All these public health and social measures belong to an overall strategy, which is the most traditional and widespread worldwide, based on social distancing of population, isolation, testing of persons with symptoms, and treatment of the infected individuals. Some studies demonstrated the effectiveness of this strategy in lowering the spread of contagion and the number of deaths. 110 , 115 The Swedish government‐which at the beginning ignored any mandatory measure by only promoting voluntary physical distancing‐admitted that its policy caused an excessive number of deaths compared to other countries. 116 Nevertheless, the negative effects of this strategy consist of the huge economic loss, 110 , 117 and of the mental and physical health damages expected and demonstrated by experts. 118 , 119 , 120 Governments should pay attention to physical and mental injuries as they seek to mitigate economic loss. Some authors propose that, in the event of future pandemics, alternative therapies for mental health could be incorporated into healthcare systems by means of using low‐cost home‐based technologies. 121
Finally, during the COVID‐19 emergency, the governments of South Korea and Singapore first adopted another exceptional approach, called the “TTT strategy”: testing, tracking infected people, and tracing their contacts. 122 In this approach, most of the tested people are not infected, asymptomatic, or paucisymptomatic. In order to be effective, the TTT strategy necessitates to trace a huge proportion of all cases (from 70% to 90%). This requires three demanding conditions: an enormous and speed testing capacity 123 ; strict measures to prevent potentially infectious people from breaking quarantine; rigorous contact tracing, provided that new approaches to digital tracing‐currently under development‐can overcome privacy concerns.
4. CONCLUSION AND HEALTH POLICY IMPLICATIONS
Public health scientific efforts should identify all the obstacles/limitations and challenges/policy implications that regard the current pandemic at the international and national level to improve the containment of possible future outbreaks. This narrative review‐based on a comparison among SARS and COVID‐19 outbreaks‐aimed to provide a risk management and communication framework by means of linking: the epidemiological and clinical aspects; the last updates on diagnostic and vaccine options; the international guidelines, indicators and plans; the public health measures and strategies to be adopted at national and subnational levels.
Nonetheless, both the crises demonstrated that whenever a new infection begins to spread in humans, the lacking primary prevention, diagnostic and therapeutic tools generate the need to implement early containment measures grounded on risk management and communication plans. Results from this review indicate that an ideal virus pandemic risk management system should also forecast all the necessary support to research structures during the inter‐pandemic phases, as well as to facilitate the acceleration of scientific outcomes on effective and specific treatments, diagnostic options, and vaccines in case of a potential pandemic. In the case of COVID‐19, the scientific knowledge needed to fight the virus surged in a rapid, impressive and unprecedented way. Despite this, and although the considerable improvements, the scientific results necessary to eradicate definitively the virus are still in progress at the time of writing. This demonstrates that, in the real world, at least effective plans regarding public health measures and rapid diagnostic testing should be equipped.
The most important limitation of this approach consists of the fact that the existing crisis management systems do not incorporate all the environmental, economic and social determinants of the pandemic risks. Another relevant limitation is the fact that the available technologies that could advance risk management were not included in the topics to be studied throughout this narrative review. However, all these aspects were discussed at the end of each paragraph regarding the results. Moreover, the main relevant solutions and challenges descending from those discussions concerning risk management and communication systems are recalled in the following health policy implications.
A suitable risk management system should embrace some key activities, as follows:
-
■
Regularly producing and stocking Personal Protective Equipments (PPEs) in a rational way and quantity at a national level, at least those PPEs that are important to protect health operators.
-
■
Planning the rapid organization of facilities to carry out diagnostic tests on a large number of citizens.
-
■
Establishing protocols for the rapid conversion of manufacture facilities to produce PPEs, diagnostic kits and reagents.
-
■
Maintaining and stimulating companies that have strategic productions, for instance, of the raw material for the intermediate layer of the masks, or of technologies to robotise and speed up the execution of diagnostic tests, or of technologies to rapidly produce vaccines.
-
■
Helping some strategic companies to rapidly expand their production in case of emergency, and/or forcing them to produce primarily for the national market.
-
■
Managing a global structural and permanent emergency fund, to support the lowest‐income nations, where hygienic conditions and health systems are weak and the emergence of variants is probable.
-
■
Provisionally waiving intellectual property protection on virus vaccines as well on drugs to allow more countries to produce the already validated formulations independently, whenever nations have fewer R&D resources or centralised production cannot be adequately guaranteed.
-
■
Regularly reviewing the national and international legal frameworks, in order to ensure their alignment with the most effective public health measures.
-
■
Adopting an intensive vaccination campaign in the early phases of the pandemic as the best political strategy in response to the adverse consequences of the crisis on the environmental and socio‐economic system.
-
■
Engaging people in vaccination campaigns to control the pandemic, by using vaccine confidence as the principal indicator.
-
■
Adopting new comparative performance systems for pandemic risks that should support public policies whenever they are highly reactive, adaptable, resistant, and scalable for decreasing the negative impacts of pandemic viruses.
-
■
Designing and using effective multifactorial indexes to support appropriate strategies of prevention in the early phases of a pandemic, in order to reduce many negative effects on society.
-
■
Incorporating into the existing crisis management systems‐besides the biological ones‐all the social, economic and environmental determinants of health, in order to assess the risk for morbidity and mortality and to adopt the related containment strategies.
Finally, the rapid spread of infectious disease does not necessarily become a great threat to the population, but the effect that an excessively pessimistic or optimistic risk communication strategy can have on the public perception and consequently on the economy could be a concrete danger. Thus, equilibrate risk communication should be an essential part of risk management plans, by balancing‐as suggested by the psychometric paradigm ‐ the actual hazard with the perceived risk during all the different phases of the emergency. In this regard, current knowledge reveals that:
-
■
An accurate, coordinated and integrated risk communication is essential for the vigorous engagement of the general public in applying health measures sustained by international and national authorities whenever knowledge of specific diagnostics, treatments and vaccines is under development.
-
■
Uncertainty is a fundamental part of risk management and so of risk communication. Therefore, expressing and acknowledging it is crucial in public engagement, and authorities should never deny or diminish the real risk.
-
■
Decision‐makers should consider and respect different perceptions of risk and concerns from different groups of citizens, through a continuous and open dialogue, even mediated by local opinion leaders.
-
■
Risk communication should address vaccine acceptance to contrast the dissemination of ambiguous, fake and distorting information that is not based on scientific evidence, as well as the consequent public scepticism about vaccination programs.
-
■
Risk communication experts should support risk management by critically monitoring the effects of the messages conveyed through the various traditional and social media.
-
■
Consequently, risk communication plans should guide a series of dynamic activities in order to suggest constant adjustments not only according to the various phases of the pandemic but also according to the reactions of the local population, aiming to obtain a constructive alliance.
Since the implementation of risk management systems by many countries as well as social configurations and healthcare systems are heterogeneous, even the consequent risk communication plans and mitigation measures are likely to generate different results. An interesting research challenge would be to investigate the different risk management and communication systems and their impact on the control of the COVID‐19 pandemic.
As a main result, this article highlights the relevance of improving the existing risk management systems, by incorporating risk communication processes. Moreover, risk management systems‐by adopting the most advanced available technologies‐should comprise all the environmental, economic and social aspects, in order to enhance the containment of the virus spread during the main phases of a pandemic emergency. These are remarkable necessities emphasised by both SARS‐COV‐1 and SARS‐COV‐2, but regarding any potential pandemic viruses in the future.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
There are no ethical concerns.
Supporting information
Supplementary Material S1
Supplementary Material S2
ACKNOWLEDGEMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Open Access Funding provided by Consiglio Nazionale delle Ricerche within the CRUI‐CARE Agreement.
Recchia V, Aloisi A, Zizza A. Risk management and communication plans from SARS to COVID‐19 and beyond. Int J Health Plann Mgmt. 2022;1‐22. 10.1002/hpm.3545
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. WHO World Health Organization . Pandemic Influenza Risk Management. WHO Interim Guidance. WHO; 2020. https://www.who.int/influenza/preparedness/pandemic/GIP_PandemicInfluenzaRiskManagementInterimGuidance_Jun2013.pdf [Google Scholar]
- 2. Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS‐CoV‐2. Natl Sci Rev. 2020;7(6):1012‐1023. 10.1093/nsr/nwaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coccia M. An index to quantify environmental risk of exposure to future epidemics of the COVID‐19 and similar viral agents: theory and practice. Environ Res. 2020;191:110155. 10.1016/j.envres.2020.110155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saini G, Swahn MH, Aneja R. Disentangling the coronavirus disease 2019 health disparities in african Americans: biological, environmental, and social factors. Open Forum Infect Dis. 2021;8(3):ofab064. 10.1093/ofid/ofab064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coccia M. The impact of first and second wave of the COVID‐19 pandemic in society: comparative analysis to support control measures to cope with negative effects of future infectious diseases. Environ Res. 2021;197:111099. 10.1016/j.envres.2021.111099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bashir MF, Ma BJ, Bilal, et al. Correlation between environmental pollution indicators and COVID‐19 pandemic: a brief study in Californian context. Environ Res. 2020;187:109652. 10.1016/j.envres.2020.109652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. How CM. (Un)sustainable environments are related to the diffusion of COVID‐19: the relation between coronavirus disease 2019, air pollution, wind resource and energy. Sustainability. 2020;12(22):9709. 10.3390/su12229709 [DOI] [Google Scholar]
- 9. Xu K, Cui K, Young L.‐H, et al. Impact of the COVID‐19 event on air quality in Central China. Aerosol Air Qual. Res. 2020;20(5):915‐929. 10.4209/aaqr.2020.04.0150 [DOI] [Google Scholar]
- 10. Srivastava A. COVID‐19 and air pollution and meteorology‐an intricate relationship: a review. Chemosphere. 2021;263:128297. 10.1016/j.chemosphere.2020.128297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rahimi NR, Fouladi‐Fard R, Aali R, et al. Bidirectional association between COVID‐19 and the environment: a systematic review. Environ Res. 2021;194:110692. 10.1016/j.envres.2020.110692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Askitas N, Tatsiramos K, Verheyden B. Estimating worldwide effects of non‐pharmaceutical interventions on COVID‐19 incidence and population mobility patterns using a multiple‐event study. Sci Rep. 2021;11(1):1972. Published 2021 Jan 21. 10.1038/s41598-021-81442-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diao Y, Kodera S, Anzai D, Gomez‐Tames J, Rashed EA, Hirata A. Influence of population density, temperature, and absolute humidity on spread and decay durations of COVID‐19: a comparative study of scenarios in China, England, Germany, and Japan. One Health. 2020;12:100203. Published 2020 Dec 11. 10.1016/j.onehlt.2020.100203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenhalgh T, Thorne S, Malterud K. Time to challenge the spurious hierarchy of systematic over narrative reviews? Eur J Clin Invest. 2018;48(6):e12931. 10.1111/eci.12931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baubion COECD. Risk management: strategic crisis management. OECD Work Pap Public Gov. 2013:23. 10.1787/5k41rbd1lzr7-en [DOI] [Google Scholar]
- 16. Fischhoff B, Bostrom A, Quadrel MJ. Risk perception and communication. In: Detels R, McEwen J, Beaglehole R, Tanaka H, eds. Oxford textbook of public health. Oxford University Press; 2002:1105‐1123. [Google Scholar]
- 17. Sandman PM. Crisis communication best practices: some quibbles and additions. J Appl Commun Res. 2006;34(3):257‐262. 10.1080/00909880600771619 [DOI] [Google Scholar]
- 18. Slovic P. The Perception of Risk. Earthscan; 2000:1‐511. [Google Scholar]
- 19. Smith RD. Responding to global infectious disease outbreaks: lessons from SARS on the role of risk perception, communication and management. Soc Sci Med. 2006;63(12):3113‐3123. 10.1016/j.socscimed.2006.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glik DC. Risk communication for public health emergencies. Annu Rev Publ Health. 2007;28(1):33‐54. 10.1146/annurev.publhealth.28.021406.144123 [DOI] [PubMed] [Google Scholar]
- 21. Covello VT, Peters RG, Wojtecki JG, Hyde RC. Risk communication, theWest Nile Virus epidemic: responding to the communication challenges posed by the intentional and unintentional release of a pathogen in an urban setting. J Urban Health Bull N Y Acad Med. 2001;78(2):382‐391. 10.1093/jurban/78.2.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruinen de Bruin Y, Lequarre AS, McCourt J, et al. Initial impacts of global risk mitigation measures taken during the combatting of the COVID‐19 pandemic. Saf Sci. 2020;128:104773. 10.1016/j.ssci.2020.104773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qualls N, Levitt A, Kanade N, et al. Community mitigation guidelines to prevent pandemic influenza ‐ United States. MMWR Recomm Rep. 2017;2017(66):1‐34. 10.15585/mmwr.rr6601a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. WHO World Health Organization . Pandemic Influenza Risk Management: A WHO Guide to Inform and Harmonize National and International Pandemic Preparedness and Response. WHO; 2017. Accessed 20 June 2020. https://apps.who.int/iris/bitstream/handle/10665/259893/WHO‐WHE‐IHM‐GIP‐2017.1‐eng.pdf?sequence=1%26isAllowed=y [Google Scholar]
- 25. Abraham T. Risk and outbreak communication: lessons from alternative paradigms. Bull World Health Organ. 2009;87(8):604‐607. 10.2471/BLT.08.058149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heymann DL, Rodier G. Global surveillance, national surveillance, and SARS. Emerg Infect Dis. 2004;10(2):173‐175. 10.3201/eid1002.031038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. WHO World Health Organization . WHO Multicentre Collaborative Network for Severe Acute Respiratory Syndrome Diagnosis. A multicentre collaboration to investigate the cause of severe acute respiratory syndrome. Lancet. 2003;361(9370):1730‐1733. 10.1016/s0140-6736(03)13376-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zambon M. Severe acute respiratory syndrome revisited. BMJ. 2003;326(7394):831‐832. 10.1136/bmj.326.7394.831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larkin M. Technology confronts SARS. Lancet Infect Dis. 2003;3(7):453. 10.1016/S1473-3099(03)00677-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau JT, Yang X, Pang E, Tsui HY, Wong E, Wing YK. SARS‐related perceptions in Hong Kong. Emerg Infect Dis. 2005;11(3):417‐424. 10.3201/eid1103.040675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rezza G, Marino R, Farchi F, Taranto M, Superiore di Sanita I. SARS Epidemic in the press. Emerg Infect Dis. 2004;10(2):381‐382. 10.3201/eid1002.030743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. CDC Centers for Disease Control and Prevention . Interim Pre‐pandemic Planning Guidance: Community Strategy for Pandemic Influenza Mitigation in the United States‐early, Targeted, Layered Use of Nonpharmaceutical Interventions. US Department of Health and Human Services, CDC; 2007. Accessed April 22, 2021. https://stacks.cdc.gov/view/cdc/11425 [Google Scholar]
- 33. Holloway R, Rasmussen SA, Zaza S, Cox NJ, Jernigan DB. The influenza pandemic framework workgroup. Updated preparedness and response framework for influenza pandemics. CDC MMWR Recomm Rep. 2014;63:1‐18. [PubMed] [Google Scholar]
- 34. Sandman PM. Start thinking in phases ‐ risk communication phases. CIDRAP business source weekly briefing and the CIDRAP business source website. 2007. Accessed May 12, 2021. https://www.cidrap.umn.edu/news‐perspective/2007/04/start‐thinking‐phases‐risk‐communication‐phases
- 35. European Union (EU) Tell me project . A new integrated pandemic threat index. 2015. Accessed April 24, 2020. https://www.tellmeproject.eu/sites/default/files/Tellmepolicybriefs.pdf
- 36. WHO World Health Organization . WHO; 2020. 2019 novel coronavirus (2019‐nCoV): strategic preparedness and response plan. Accessed June 20, 2020. https://www.who.int/docs/default‐source/coronaviruse/srp‐04022020.pdf
- 37. WHO World Health Organization . COVID‐19 Strategy Update. WHO; 2020. Accessed June 20, 2020. https://www.who.int/publications/i/item/covid‐19‐strategy‐update–‐14‐april‐2020 [Google Scholar]
- 38. Correia T. SARS‐CoV‐2 pandemics: the lack of critical reflection addressing short‐ and long‐term challenges. Int J Health Plann Manag. 2020;35(3):669‐672. 10.1002/hpm.2977 [DOI] [PubMed] [Google Scholar]
- 39. Palladino R, Bollon J, Ragazzoni L, Barone‐Adesi F. Excess deaths and hospital admissions for COVID‐19 due to a late implementation of the lockdown in Italy. Int J Environ Res Publ Health. 2020;17(16):5644. 10.3390/ijerph17165644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hua J, Shaw R. Coronavirus (covid‐19) ‘infodemic’ and emerging issues through a data lens: the case of China. Int J Environ Res Publ Health. 2020;17(7):2309. 10.3390/ijerph17072309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. WHO, UN, UNICEF, UNDP, UNESCO, UNAIDS , et al. Managing the COVID‐19 infodemic: promoting healthy behaviours and mitigating the harm from misinformation and disinformation. 2020. Accessed March 16, 2021. https://www.who.int/news/item/23‐09‐2020‐managing‐the‐covid‐19‐infodemic‐promoting‐healthy‐behaviours‐and‐mitigating‐the‐harm‐from‐misinformation‐and‐disinformation
- 42. Gesualdo F, Bucci LM, Rizzo C, Tozzi AE. Digital tools, multidisciplinarity and innovation for communicating vaccine safety in the COVID‐19 era. Hum Vaccines Immunother. 2021;18:1‐4. 10.1080/21645515.2020.1865048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. WHO World Health Organization . Rapid Risk Assessment of Acute Public Health Events. WHO; 2020. http://apps.who.int/iris/bitstream/10665/70810/1/WHO_HSE_GAR_ARO_2012.1_eng.pdf [Google Scholar]
- 44. WHO World Health Organization . WHO SARS Risk Assessment and Preparedness Framework. WHO; 2004. Accessed May 21, 2020. https://www.who.int/csr/resources/publications/CDS_CSR_ARO_2004_2.pdf [Google Scholar]
- 45. WHO World Health Organization . Considerations in adjusting public health and social measures in the context of COVID‐19 Interim guidance. WHO; 2020. Accessed June 20, 2020. https://apps.who.int/iris/bitstream/handle/10665/331773/WHO‐2019‐nCoV‐Adjusting_PH_measures‐2020.1‐eng.pdf?sequence=1%26isAllowed=y [Google Scholar]
- 46. Dowdle WR. Influenza pandemic periodicity, virus recycling, and the art of risk assessment. Emerg Infect Dis. 2006;12(1):34‐39. 10.3201/eid1201.051013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yuan J, Li M, Lv G, Lu ZK. Monitoring transmissibility and mortality of COVID‐19 in Europe. Int J Infect Dis. 2020;95:311‐315. 10.1016/j.ijid.2020.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coccia M. Comparative critical decisions in management. In: Farazmand A, ed. Global Encyclopedia of Public Administration, Public Policy, and Governance. Springer Nature, Cham; 2020. 10.1007/978-3-319-31816-5_3969-1 [DOI] [Google Scholar]
- 49. Coccia M. Preparedness of countries to face COVID‐19 pandemic crisis: strategic positioning and factors supporting effective strategies of prevention of pandemic threats. Environ Res. 2022;203:111678. 10.1016/j.envres.2021.111678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coccia M. Pandemic prevention: lessons from COVID‐19. Encycl. 2021;1(2):433‐444. 10.3390/encyclopedia1020036 [DOI] [Google Scholar]
- 51. Stansbury N, Barnes B, Adams A, et al. Risk‐based monitoring in clinical trials: increased adoption throughout 2020. Ther Innov Regul Sci. 2022;56(3):415‐422. 10.1007/s43441-022-00387-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. WHO World Health Organization . Update 95‐SARS: chronology of a serial killer. WHO; 2003. Accessed May 21, 2020. https://www.who.int/emergencies/disease‐outbreak‐news/item/2003_07_04‐en
- 53. WHO World Health Organization . Coronavirus Disease (COVID‐19) Situation Reports – Weekly Operational Update on COVID‐19. WHO; 2021. Accessed July 15, 2021. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19–‐13‐july‐2021 [Google Scholar]
- 54. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 55. Zizza A, Recchia V, Aloisi A, Guido M. Clinical features of COVID‐19 and SARS epidemics. A literature review. J Prev Med Hyg. 2021;62(1):E13‐E24. 10.15167/2421-4248/jpmh2021.62.1.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou X, Li Y, Li T, Zhang W. Follow‐up of asymptomatic patients with SARS‐CoV‐2 infection. Clin Microbiol Infect. 2020;26(7):957‐959. 10.1016/j.cmi.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Joynt GM, Yap HY. SARS in the intensive care unit. Curr Infect Dis Rep. 2004;6(3):228‐233. 10.1007/s11908-004-0013-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID‐19 patients: a review. Allergy. 2021;76(2):428‐455. 10.1111/all.14657 [DOI] [PubMed] [Google Scholar]
- 60. Nana‐Sinkam P, Kraschnewski J, Sacco R, et al. Health disparities and equity in the era of COVID‐19. J Clin Transl Sci. 2021;5(1):e99. 10.1017/cts.2021.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID‐19: a systematic review. EClinicalMedicine. 2020;23:100404. 10.1016/j.eclinm.2020.100404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Banchelli F, Negro P, Guido M, et al. The role of ABO blood type in patients with SARS‐CoV‐2 infection: a systematic review. J Clin Med. 2022;11(11):3029. 10.3390/jcm11113029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sarkodie SA, Owusu PA. Impact of meteorological factors on COVID‐19 pandemic: evidence from top 20 countries with confirmed cases. Environ Res. 2020;191:110101. 10.1016/j.envres.2020.110101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Coccia M. COVID‐19 pandemic over 2020 (withlockdowns) and 2021 (with vaccinations): similar effects for seasonality and environmental factors. Environ Res. 2022;208:112711. 10.1016/j.envres.2022.112711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leung GM, Lim WW, Ho LM, et al. Seroprevalence of IgG antibodies to SARS‐coronavirus in asymptomatic or subclinical population groups. Epidemiol Infect. 2006;134(2):211‐221. 10.1017/S0950268805004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rostami A, Sepidarkish M, Leeflang MMG, et al. SARS‐CoV‐2 seroprevalence worldwide: a systematic review and meta‐analysis. Clin Microbiol Infect. 2021;27(3):331‐340. 10.1016/j.cmi.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS‐CoV‐2 antibodies and associated factors in healthcare workers: a systematic review and meta‐analysis. J Hosp Infect. 2021;108:120‐134. 10.1016/j.jhin.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. WHO World Health Organization . (2003d April 16) Update 31‐Coronavirus never before seen in humans is the cause of SARS. WHO; 2003. Accessed May 21, 2020. https://apps.who.int/mediacentre/news/releases/2003/pr31/en/index.html
- 69. WHO World Health Organization . Emergencies preparedness, response. Severe acute respiratory syndrome (SARS): laboratory diagnostic tests. WHO; 2003. Accessed May 21, 2020.
- 70. Cheng VCC, Lau SKP, Woo PCY, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20(4):660‐694. 10.1128/CMR.00023-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Che XY, Hao W, Wang Y, et al. Nucleocapsid protein as early diagnostic marker for SARS. Emerg Infect Dis. 2004;10(11):1947‐1949. 10.3201/eid1011.040516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Woo PC, Lau SK, Wong BH, et al. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab Immunol. 2004;11(4):665‐668. 10.1128/CDLI.11.4.665-668.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. WHO World Health Organization . WHO, Novel Coronavirus (2019‐nCoV) Situation Report ‐ 1, 2020. WHO; 2020. Accessed May 20, 2020. https://apps.who.int/iris/bitstream/handle/10665/330760/nCoVsitrep21Jan2020‐eng.pdf?sequence=3%26isAllowed=y [Google Scholar]
- 74. Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID‐19: the disease and tools for detection. ACS Nano. 2020;14(4):3822‐3835. 10.1021/acsnano.0c02624 [DOI] [PubMed] [Google Scholar]
- 75. WHO World Health Organization . Laboratory Testing for Coronavirus Disease (COVID‐19) in Suspected Human Cases: Interim Guidance. WHO; 2020. Accessed March 30, 2020. https://apps.who.int/iris/bitstream/handle/10665/331501/WHO‐COVID‐19‐laboratory‐2020.5‐eng.pdf?sequence=1%26isAllowed=y [Google Scholar]
- 76. Shaoli B, Jianyun W, Desheng ZYY, Xiaomin G, Lingling L, Fan Y. Analysis of the first family epidemic situation of new coronavirus pneumonia in Gansu Province. Chin J Prev Med. 2020;54:E005. [Google Scholar]
- 77. Xiao S.‐Y, Wu Y, Li J. Evolving status of the 2019 novel coronavirus infections: proposal of conventional serologic assays for disease diagnostics and infection monitoring. J Med Virol. 2020;92(5):464‐467. 10.1002/jmv.25702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Winter AK, Hegde ST. The important role of serology for COVID‐19 control. Lancet Infect Dis. 2020;20(7):758‐759. 10.1016/S1473-3099(20)30322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis. 2020;20(4):398‐400. 10.1016/S1473-3099(20)30141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mercer TR, Salit M. Testing at scale during the COVID‐19 pandemic. Nat Rev Genet. 2021;22(7):415‐426. 10.1038/s41576-021-00360-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. WHO World Health Organization . Advice on the Use of Point‐Of‐Care Immunodiagnostic Tests for COVID‐19. WHO; 2020. Accessed April 20, 2020. https://apps.who.int/iris/bitstream/handle/10665/331713/WHO‐2019‐nCoV‐Sci_Brief‐POC_immunodiagnostics‐2020.1‐eng.pdf?sequence=1%26isAllowed=y [Google Scholar]
- 82. WHO World Health Organization . Antigen‐detection in the Diagnosis of SARS‐CoV‐2 Infection Using Rapid Immunoassays. WHO; 2020. Accessed October 20, 2020. https://apps.who.int/iris/bitstream/handle/10665/334253/WHO‐2019‐nCoV‐Antigen_Detection‐2020.1‐eng.pdf?sequence=1%26isAllowed=y [Google Scholar]
- 83. Peeling RW, Olliaro PL, Boeras DI, Fongwen N. Scaling up COVID‐19 rapid antigen tests: promises and challenges. Lancet Infect Dis. 2021;21(9):S1473‐S3099. 10.1016/S1473-3099(21)00048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS‐CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226‐236. 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhou Y.‐m, Yang R.‐q, Tao S.‐c, et al. The design and application of DNA chips for early detection of SARS‐CoV from clinical samples. J Clin Virol. 2005;33(2):123‐131. 10.1016/j.jcv.2004.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cyranoski D. This scientist hopes to test coronavirus drugs on animals in locked‐down Wuhan. Nature. 2020;577(7792):607. 10.1038/d41586-020-00190-6 [DOI] [PubMed] [Google Scholar]
- 87. Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID‐19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14(10):12522‐12537. 10.1021/acsnano.0c07197 [DOI] [PubMed] [Google Scholar]
- 88. Le TK, Paris C, Khan KS, Robson F, Ng WL, Rocchi P. Nucleic acid‐based technologies targeting coronaviruses. Trends Biochem Sci. 2021;46(5):351‐365. 10.1016/j.tibs.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Voysey M, Costa Clemens SA, Madhi SA, et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881‐891. 10.1016/S0140-6736(21)00432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Oliver SE, Gargano JW, Scobie H, et al. The advisory committee on immunization practices’ Interim recommendation for use of janssen COVID‐19 vaccine ‐ United States. MMWR Morb Mortal Wkly Rep. 2021;70(9):329‐332. 10.15585/mmwr.mm7009e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1‐2a trial of Ad26.COV2.S covid‐19 vaccine. N Engl J Med. 2021;384(19):1834‐1835. 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shrotri M, Swinnen T, Kampmann B, Parker E. An interactive website tracking COVID‐19 vaccine development. Lancet Global Health. 2021;9(5):e590‐e592. 10.1016/S2214-109X(21)00043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mathieu E, Ritchie H, Ortiz‐Ospina E, et al. A global database of COVID‐19 vaccinations. Nat Human Behav. 2021;5(7):947‐953. 10.1038/s41562-021-01122-8 [DOI] [PubMed] [Google Scholar]
- 96. Coccia M. High health expenditures and low exposure of population to air pollution as critical factors that can reduce fatality rate in COVID‐19 pandemic crisis: a global analysis. Environ Res. 2021;199:111339. 10.1016/j.envres.2021.111339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Panico A, Lobreglio G, Bagordo F, et al. Antibody response in healthcare workers before and after the third dose of anti‐SARS‐CoV‐2 vaccine: a pilot study. Vaccines. 2022;10(6):862. 10.3390/vaccines10060862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Coccia M. Optimal levels of vaccination to reduce COVID‐19 infected individuals and deaths: a global analysis. Environ Res. 2022;204(Pt C):112314. 10.1016/j.envres.2021.112314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Harrison EA, Wu JW. Vaccine confidence in the time of COVID‐19. Eur J Epidemiol. 2020;35(4):325‐330. 10.1007/s10654-020-00634-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. López‐García X, Costa‐Sánchez C. Vizoso Á. Journalistic fact‐checking of information in pandemic: stakeholders, hoaxes, and strategies to fight disinformation during the COVID‐19 crisis in Spain. Int J Environ Res Publ Health. 2021;18(3):1227. 10.3390/ijerph18031227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Radanliev P, De Roure D, Ani U, Carvalho G. The ethics of shared Covid‐19 risks: an epistemological framework for ethical health technology assessment of risk in vaccine supply chain infrastructures. Health Technol. 2021;11(5):1083‐1091. 10.1007/s12553-021-00565-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Radanliev P, De Roure D.Epistemological and bibliometric analysis of ethics and shared responsibility—health policy and IoT systems. Sustainability. 2021;13(15):8355. 10.3390/su13158355 [DOI] [Google Scholar]
- 103. Anderson RM, Fraser C, Ghani AC, et al. Epidemiology, transmission dynamics and control of SARS: the 2002‐2003 epidemic. Philos Trans R Soc Lond B Biol Sci. 2004;359(1447):1091‐1105. 10.1098/rstb.2004.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yang Y, Peng F, Wang R, et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. 10.1016/j.jaut.2020.102434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Barrios LC, Koonin M, Kohl KS, Cetron M. Selecting nonpharmaceutical strategies to minimize influenza spread: the 2009 influenza A (H1N1) pandemic and beyond. Publ Health Rep. 2012;127(6):565‐571. 10.1177/003335491212700606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. CDC Centers for Disease Control and Prevention . What Grocery and Food Retail Workers Need to Know about COVID‐19. National Center for Immunization and Respiratory Diseases (NCIRD); 2020. Accessed April 22, 2021. https://www.cdc.gov/coronavirus/2019‐ncov/community/organizations/grocery‐food‐retail‐workers.html
- 107. Cook TM. Personal protective equipment during the COVID‐19 pandemic ‐ a narrative review. Anaesthesia. 2020;75(7):920‐927. 10.1111/anae.15071 [DOI] [PubMed] [Google Scholar]
- 108. Dancer SJ. Controlling hospital‐acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014:27‐90. 10.1128/CMR.00020-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Di Domenico L, Sabbatini CE, Pullano G, Lévy‐Bruhl D, Colizza V. Impact of January 2021 curfew measures on SARS‐CoV‐2 B.1.1.7 circulation in France. Euro Surveill. 2021;26(15):pii=2100272. 10.2807/1560-7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Flaxman S, Mishra S, Gandy A, et al. Estimating the effects of non‐pharmaceutical interventions on COVID‐19 in europe. Nature. 2020;584(7820):257‐261. 10.1038/s41586-020-2405-7 [DOI] [PubMed] [Google Scholar]
- 111. Machida M, Nakamura I, Saito R, et al. Changes in implementation of personal protective measures by ordinary Japanese citizens: a longitudinal study from the early phase to the community transmission phase of the COVID‐19 outbreak. Int J Infect Dis. 2020;96(1):371‐375. 10.1186/s41182-020-00250-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chiu R. Functions of music making under lockdown: a trans‐historical perspective across two pandemics. Front Psychol. 2020;11:616499. 10.3389/fpsyg.2020.616499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR. Airborne or droplet precautions for health workers treating COVID‐19? J Infect Dis. 2020:jiaa189. 10.1093/infdis/jiaa189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Morawska L, Cao J. Airborne transmission of SARS‐CoV‐2: the world should face the reality. Environ Int. 2020;139:105730. 10.1016/j.envint.2020.105730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ghosal S, Bhattacharyya R, Majumderc M. Impact of complete lockdown on total infection and death rates: a hierarchical cluster analysis. Diabetes Metabol Syndr. 2020;14(4):707‐711. 10.1016/j.dsx.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Henley J. We should have done more, admits architect of Sweden's Covid‐19 strategy. Guard. 2020. Accessed June 5, 2020. https://www.theguardian.com/world/2020/jun/03/architect‐of‐sweden‐coronavirus‐strategy‐admits‐too‐many‐died‐anders‐tegnell [Google Scholar]
- 117. National Bureau of Statistics of China . The overall prevention and control of epidemic situation and economic and social development have achieved remarkable results. 2020. Accessed June 30, 2020. http://www.stats.gov.cn/tjsj/zxfb/202004/t20200417_1739327.html
- 118. Mackolil J, Mackolil J. Addressing psychosocial problems associated with the COVID‐19 lockdown. Asian J Psychiatr. 2020;51:102156. 10.1016/j.ajp.2020.102156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Thakur K, Kumar N, Sharma N. Effect of the pandemic and lockdown on mental health of children. Indian J Pediatr. 2020;12(7):1. 10.1007/s12098-020-03308-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Alfawaz H, Amer OE, Aljumah AA, et al. Effects of home quarantine during COVID‐19 lockdown on physical activity and dietary habits of adults in Saudi Arabia. Sci Rep. 2021;11(1):5904. 10.1038/s41598-021-85330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Radanliev P, De Roure D. Alternative mental health therapies in prolonged lockdowns: narratives from Covid‐19. Health Technol. 2021;11(5):1‐7. 10.1007/s12553-021-00581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. OECD Organisation for Economic Co‐operation and Development . (2020) Testing for COVID‐19: A Way to Lift Confinement Restrictions. OECD; 2020. Accessed June 20, 2021. https://www.oecd.org/coronavirus/policy‐responses/testing‐for‐covid‐19‐a‐way‐to‐lift‐confinement‐restrictions‐89756248/ [Google Scholar]
- 123. Abeysuriya RG, Delport D, Stuart RM, et al. Preventing a cluster from becoming a new wave in settings with zero community COVID‐19 cases. BMC Infect Dis. 2022;22(1):232. 10.1186/s12879-022-07180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Supplementary Material S2
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


