Abstract
Simple Summary
We conducted a large-scale population-based study with long-term follow-up to obtain a comprehensive assessment of death causes, especially cardiovascular disease death, among 80,042 older bladder cancer patients from a national cancer registry containing 44 years of data. To our knowledge, this was the first study to report the importance of CVD-related death as a competing risk among older patients with bladder cancer. CVD-related death surpassed BC as the leading cause of death 5–10 years after diagnosis among older BC patients, especially for patients with localized-stage and low-grade tumors. Furthermore, older BC patients had a higher risk of CVD-related death than the general population. Although BC management should be the primary focus of older BC patients, our results emphasized the importance of competing risks, the most prominent being CVD. Individual follow-up and management should focus not only on primary cancer but also on CVD-related death to minimize the risk of death in older patients with bladder cancer.
Abstract
Background: To identify the risk of death from cardiovascular disease (CVD) in older patients with bladder cancer (BC). Methods: This population-based study included 80,042 older BC patients (≥65 years) diagnosed between 1975 and 2018, with a mean follow-up of 17.2 years. The proportion of deaths, competing risk models, standardized mortality ratio (SMR), and absolute excess risk (AER) per 10,000 person-years were applied to identify the risk of CVD-related deaths among older BC patients. Results: For older patients with BC, CVD-related death was the chief cause of death, and cumulative CVD-related mortality also exceeded primary BC as the leading cause of death mostly 5–10 years after BC diagnosis, especially in localized-stage and low-grade subgroups. The risk of short- and long-term CVD-related death in older BC patients was higher than in the general older adult population (SMR = 1.30, 95% CI 1.28–1.32; AER = 105.68). The risk of sex-specific CVD-related deaths also increased compared to the general population of older adults, including heart disease, cerebrovascular diseases, hypertension without heart disease, atherosclerosis, aortic aneurysm and dissection, and other diseases of the arteries, arterioles, and capillaries. Conclusions: CVD-related death is an important competing risk among older BC patients and has surpassed primary BC as the chief cause of death, mainly 5–10 years after BC diagnosis. The risk of CVD-related death in older patients with BC was greater than in the general population. The management of older patients with BC should focus not only on the primary cancer but also on CVD-related death.
Keywords: bladder cancer, cardiovascular disease death, cardio-oncology, older patients, SEER
1. Introduction
Bladder cancer (BC) is one of the 10 most frequently diagnosed malignancies worldwide [1]. Given its prevalence and lengthy management, cancer care costs for BC patients are among the highest [2,3]. Individuals aged ≥65 years account for ~81% of BC cases [4]. The medical burden of older BC patients has increased with the aging population due to the growing proportion of older adults. Identifying the chief cause of death in older patients with BC is the key step in effectively improving prognosis.
Cardiovascular disease (CVD) is responsible for the most deaths worldwide [5]. Increasing concerns about CVD-related death among older patients with BC are fueled by the impact of aging but also by shared CVD risk factors and the cardiovascular toxicity of antitumor therapy [6,7]. However, the risk of CVD-related death in older individuals with BC is still unclear.
Previous studies have focused on the primary cancer cause of BC death but have overlooked the risk of CVD-related death in older BC patients [8]. Recent European Association of Urology Guidelines lack recommendations on the risk of CVD-related death in older patients with BC and focus only on cancer-related deaths [9,10]. Although current studies have reported higher proportions of noncancer deaths (including CVD-related deaths) among all BC patients [11,12,13,14,15,16] and nonmetastatic BC patients [17], such studies have not compared the transforming trend of the competing risks of CVD and BC-related death. Furthermore, because these studies lack stratification according to different age subgroups, their results may not be generalizable to older BC patients [11,12,17]. Most studies have simply delineated the composite CVD death outcome or specific CVD death outcomes, such as fatal heart disease [11,13]. These results on the transforming trend of the competing risk of CVD and BC death are controversial [11,13]. Whether CVD death exceeds BC death to become the leading cause of death in older BC patients is still unclear. Therefore, the identification and quantification of the CVD-related death risk among older BC patients are warranted.
Our study characterized the competing risk of CVD-related death among older patients with BC. We identified BC subgroups at high risk of CVD-related death and quantified the risk of short- and long-term CVD mortality for older BC patients compared with the general population. These findings could provide important population-level data to aid the follow-up and management of older BC patients.
2. Materials and Methods
2.1. Data Collection
Data from the Surveillance, Epidemiology, and End Results (SEER) database were used in our study. The SEER program is an authoritative source of high-quality cancer registries worldwide [18] and relies on systematic, standardized, and regular data collection procedures for quality assurance and the avoidance of surveillance bias [19,20] (Supplementary File S1: Supplementary Methods). Ethics committee approval was waived due to the use of de-identified data [21].
Older patients diagnosed with BC from 1975 to 2018 were included in our study (Supplementary Figure S1). Older BC patients refer to patients aged ≥65 years [4,22]. The inclusion criteria were (1) case selection (site and morphology, primary site-labeled) = “C67.0–9”; (2) histological diagnosis from 1975 to 2018; (3) active follow-up with the definite cause of death; and (4) only one primary cancer. The exclusion criteria were (1) unknown race; (2) age at diagnosis <65 years; and (3) follow-up <2 months. The BC patients less than 65 years is shown in the File S1.
2.2. Study Outcomes
The underlying causes of death in the SEER database were documented using the codes of the International Classification of Diseases 10 (ICD-10) of the National Cancer for Health Statistics [23,24]. The primary outcome was death from any cause, with the cause classified as primary neoplasm, CVD, other neoplasms, and other non-neoplasms, among which CVD and other non-neoplasm causes consisted of six and eight specific causes, respectively (Supplementary Table S1). The person-years of follow-up were cumulated from the initial diagnosis of BC until loss-to-follow-up, date of death, or final follow-up date (31 December 2018).
2.3. Statistical Analyses
Categorical variables in the baseline characteristics were evaluated using the chi-squared test [25]. To determine the most common cause of death, the proportion of deaths was defined as the number of a specific cause of death divided by the overall number of all deaths in the individuals with BC. To further assess the interaction between BC and CVD-related deaths, we calculated cumulative mortality using competing risk models [26]. The standardized mortality ratio (SMR) was applied to quantify the risk of CVD-related death in BC patients. The SMR was calculated as the ratio of observed deaths to the number of expected deaths [11,24]. Absolute excess risk (AER) was calculated as follows: AER = 10,000 ([number observed–number expected]/[person-years at risk]). CVD-related AER reflected the absolute increase in CVD death risk (i.e., the CVD death burden) in the population [27]. All statistical analyses were completed with R software (version 3.4.4; Vienna, Austria) and SEER*Stat (version 8.3.9, National Cancer Institute, USA). Statistical significance was defined by a p-value < 0.05 (File S1).
3. Results
3.1. Patient Characteristics
Overall, 80,042 older patients with BC diagnosed from 1975 to 2018 were enrolled (Table 1). Of these, 72.0% were male, 91.4% were white, 49.7% had non-muscle-invasive bladder cancer (NMIBC), 69.2% had a localized-stage tumor, 41.8% had low-grade tumors, and 95.2% had received surgery. Compared to BC patients aged 75–84 and >85 years at diagnosis, patients aged 65–74 years were more likely to be male, black, have localized-stage or low-grade tumors, and be diagnosed from 1975 to 1983. The average follow-up time was 17.2 years (SD 1.7 years) for older patients with BC.
Table 1.
Baseline characteristics of elderly patients with bladder cancer.
| Age at Diagnosis (n/%) | |||||
|---|---|---|---|---|---|
| Characteristic | Total | 65–74 Years | 75–84 Years | 85+ Years | p Value |
| Overall | 80,042 | 35,841 | 31,676 | 12,525 | |
| Sex | |||||
| Male | 57,634 (72.0) | 26,926 (75.1) | 22,724 (71.7) | 7984 (63.7) | <0.001 |
| Female | 22,408 (28.0) | 8915 (24.9) | 8952 (28.3) | 4541 (36.3) | |
| Race | |||||
| White | 73,151 (91.4) | 32,692 (91.2) | 29,017 (91.6) | 11,442 (91.4) | <0.001 |
| Black | 3320 (4.1) | 1611 (4.5) | 1239 (3.9) | 470 (3.8) | |
| Other * | 3571 (4.5) | 1538 (4.3) | 1420 (4.5) | 613 (4.9) | |
| Stage | |||||
| Localized | 55,374 (69.2) | 25,195 (70.3) | 21,808 (68.8) | 8371 (66.8) | <0.001 |
| Regional | 15,829 (19.8) | 6504 (18.1) | 6519 (20.6) | 2806 (22.4) | |
| Distant | 2702 (3.4) | 1300 (3.6) | 1072 (3.4) | 330 (2.6) | |
| Unknown | 6137 (7.7) | 2842 (7.9) | 2277 (7.2) | 1018 (8.1) | |
| Grade | |||||
| Low | 33,495 (41.8) | 16,072 (44.8) | 12,982 (41.0) | 4441 (35.5) | <0.001 |
| High | 35,222 (44.0) | 14,619 (40.8) | 14,375 (45.4) | 6228 (49.7) | |
| Other # | 50 (0.1) | 14 (0.0) | 23 (0.1) | 13 (0.1) | |
| Unknown | 11,275 (14.1) | 5136 (14.3) | 4296 (13.6) | 1843 (14.7) | |
| Subtype | <0.001 | ||||
| NMIBC | 39,791 (49.7) | 17,670 (49.3) | 15,973 (50.4) | 6148 (49.1) | |
| MMIBC | 12,249 (15.3) | 4793 (13.4) | 5127 (16.2) | 2329 (18.6) | |
| Unknown | 28,002 (35.0) | 13,378 (37.3) | 10,576 (33.4) | 4048 (32.3) | |
| Years of diagnosis | |||||
| 1975–1983 | 12,457 (15.6) | 6027 (16.8) | 4787 (15.1) | 1643 (13.1) | <0.001 |
| 1984–1993 | 16,459 (20.6) | 7902 (22.0) | 6379 (20.1) | 2178 (17.4) | |
| 1994–2003 | 17,991 (22.5) | 7500 (20.9) | 7671 (24.2) | 2820 (22.5) | |
| 2004–2018 | 33,135 (41.4) | 14,412 (40.2) | 12,839 (40.5) | 5884 (47.0) | |
| Surgery | |||||
| Yes | 76,202 (95.2) | 34,192 (95.4) | 30,221 (95.4) | 11,789 (94.1) | <0.001 |
| No | 3445 (4.3) | 1481 (4.1) | 1287 (4.1) | 677 (5.4) | |
| Unknown | 395 (0.5) | 168 (0.5) | 168 (0.5) | 59 (0.5) | |
* Other includes American Indian/Alaska Native and Asian/Pacific Islander. # Other includes B-cell, pre-B, B-precursor, and B-cell. Abbreviations: NMIBC, non–muscle-invasive bladder cancer; MMIBC, muscle-invasive and metastatic bladder cancer.
3.2. Proportion of CVD-Related versus Primary Cancer-Related Deaths
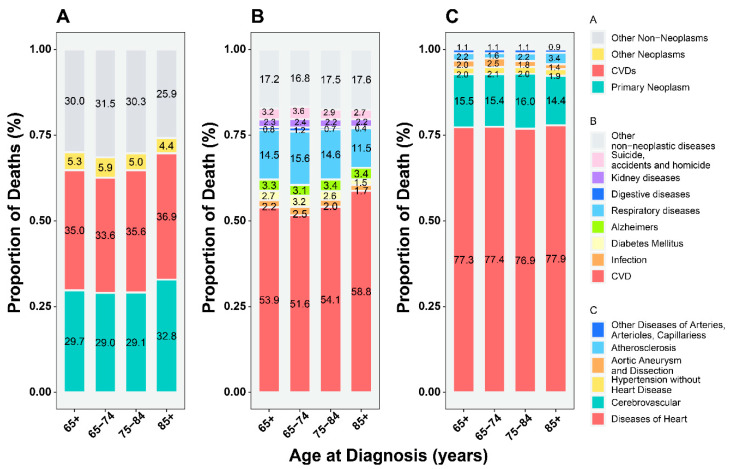
Older BC patients were likely to die from causes other than BC, chief among which was CVD. When classified by specific CVD, heart disease was the leading cause of death (~70%), while CVD was the main cause of death among noncancer deaths (~50%) (Figure 1). Death from CVD was more common than primary BC among older patients (Figure 1), especially among patients with localized-stage, low-grade tumors and NMIBC (Supplementary Figures S2 and S3A), while the proportion of CVD-related deaths was lower than BC-related deaths in patients <65 years (Supplementary Figure S4).
Figure 1.
The proportion of deaths among older patients (≥65 years) with bladder cancer. (A) All causes of deaths; (B) all causes of non-cancer deaths; (C) all causes of CVD-related deaths. Abbreviations: CVD, cardiovascular disease.
Stratifying patients by age at diagnosis (Supplementary Figure S5), the proportion of CVD deaths increased with age (65–74, 75–84, and >85 years) and exceeded that of the primary BC-related deaths in men, surgical interventions, white, localized-stage, low-grade, and NMIBC subgroups (Supplementary Figures S3B and S5).
In the follow-up time subgroups (Supplementary Figure S6), the proportion of CVD-related deaths increased markedly with time and exceeded that of primary BC to become the main cause of death 5–10 years after diagnosis in most subgroups, except for the distant-stage subgroup. This transition was observed earlier, 1–5 years after diagnosis, in the localized-stage, low-grade, and NMIBC subgroups (Supplementary Figures S3C and S6).
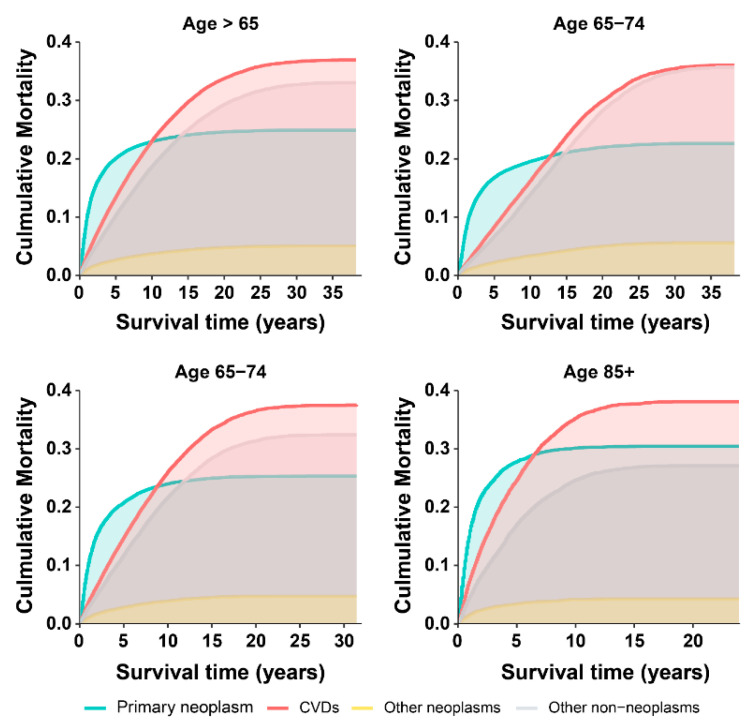
3.3. Cumulative Mortality from the CVD versus Primary Cancer
Cumulative CVD mortality increased steadily with survival time and exceeded primary BC mortality to become the leading cause of death 5–10 years after diagnosis in older BC patients (Figure 2), including the white, other race, localized-stage, surgery, male, low grade, and NMIBC subgroups (Supplementary Figures S3D and S7). No higher incidence of CVD-related deaths was observed other than BC-related deaths in patients <65 years (Supplementary Figure S4). However, this transition was observed earlier (1–5 years after diagnosis) in patients with localized-stage, low-grade tumors and NMIBC (Supplementary Figures S3D and S7), while it was delayed (15 years after diagnosis) in females and the subgroup including other races (Supplementary Figure S7).
Figure 2.
Cumulative mortality among older patients (≥65 years) with bladder cancer.
To identify patients with a high risk of CVD-related death, further subgroup analyses were conducted, stratifying patients by the age at diagnosis (65–74, 75–84, >85 years) (Supplementary Figure S8). BC patients aged between 65 and 84 years showed higher cumulative mortality from CVD compared with those from primary BC belonging to the following subgroups: males, females, white, other race, localized-stage, low-grade tumor, and surgical intervention. In BC patients aged >85, this transition was observed in the male, white, localized-stage, low-grade tumor, and surgical intervention groups, while the cumulative CVD mortality was close but did not exceed the cumulative BC mortality among females and other races (Supplementary Figure S8). These results were not observed in the other subgroups (Supplementary Figure S9).
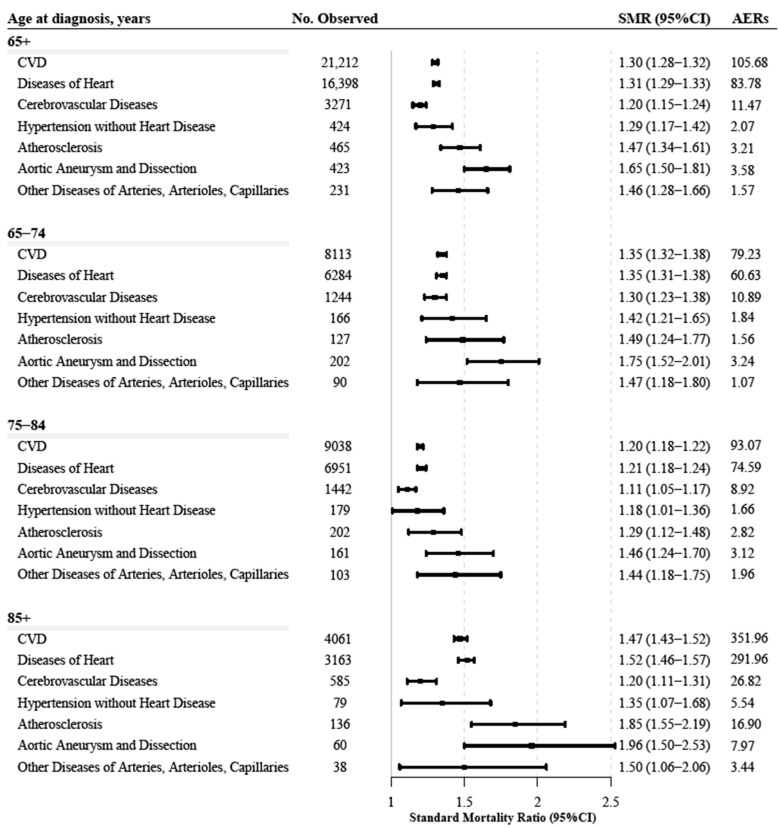
3.4. CVD Mortality Compared to the General Population
Compared with the general population, older BC patients had a higher risk of CVD death (SMR = 1.30, 95% CI 1.28–1.32; AER = 105.68), specifically from heart disease, cerebrovascular diseases, atherosclerosis, aortic aneurysm and dissection, hypertension without heart disease, and other diseases of the arteries, arterioles, capillaries. Similar patterns were observed for patients aged >65 years of age at diagnosis (Figure 3). NMIBC and muscle-invasive and metastatic BC (MMIBC) both had higher CVD-related SMR (Supplementary Table S2).
Figure 3.
The risk of cardiovascular death among older patients (≥65 years) with bladder cancer.
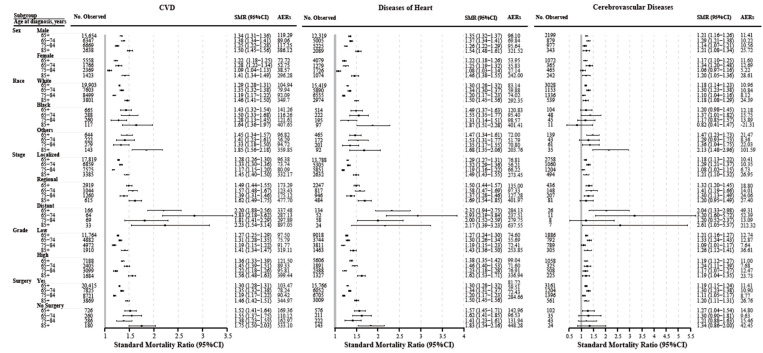
In individualized subgroups, the risks of CVD-related death, heart disease, and cerebrovascular disease were also higher in BC patients aged >65 years compared to the general population (Figure 4). The other four outcomes (atherosclerosis, aortic aneurysm and dissection, hypertension without heart disease, and other diseases of the arteries, arterioles, and capillaries) showed similar trends (Supplementary Figures S10–S13).
Figure 4.
The risk of cardiovascular death among older patients (≥65 years) with bladder cancer according to age at diagnosis. Abbreviations: CVD, cardiovascular disease.
In terms of the risk of CVD death according to short-term and long-term follow-up, CVD-related death risk, including the six specific outcomes mentioned above in older-aged BC patients, was greater than in the general population (Table 2; Supplementary Table S3).
Table 2.
Standardized mortality ratios for cardiovascular death in elderly patients with bladder cancer based on years after diagnosis.
| Years After Diagnosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–5 | 5–10 | 10–15 | 15+ | ||||||
| Cause of Death | Obs | Smr (95% CI) |
Obs | Smr (95% CI) |
Obs | Smr (95% CI) |
Obs | Smr (95% CI) |
Obs | Smr (95% CI) |
| 65+ years | ||||||||||
| CVD | 2686 | 1.57 | 7207 | 1.23 | 5805 | 1.28 | 3244 | 1.3 | 2270 | 1.34 |
| (1.51–1.63) | (1.20–1.26) | (1.25–1.32) | (1.25–1.34) | (1.28–1.39) | ||||||
| Diseases of the Heart | 2124 | 1.62 | 5615 | 1.25 | 4483 | 1.29 | 2472 | 1.29 | 1704 | 1.32 |
| (1.55–1.69) | (1.21–1.28) | (1.25–1.33) | (1.24–1.35) | (1.25–1.38) | ||||||
| Cerebrovascular Diseases | 380 | 1.34 | 1075 | 1.1 | 906 | 1.19 | 523 | 1.22 | 387 | 1.33 |
| (1.21–1.48) | (1.04–1.17) | (1.11–1.27) | (1.12–1.33) | (1.20–1.46) | ||||||
| Hypertension without Heart Disease | 40 | 1.4 | 104 | 1 | 113 | 1.25 | 81 | 1.41 | 86 | 1.82 |
| (1.00–1.90) | (0.82–1.21) | (1.03–1.50) | (1.12–1.76) | (1.46–2.25) | ||||||
| Atherosclerosis | 67 | 1.83 | 161 | 1.34 | 123 | 1.42 | 74 | 1.68 | 40 | 1.46 |
| (1.42–2.32) | (1.14–1.56) | (1.18–1.69) | (1.32–2.11) | (1.05–1.99) | ||||||
| Aortic Aneurysm and Dissection | 46 | 1.55 | 172 | 1.72 | 123 | 1.71 | 53 | 1.49 | 29 | 1.47 |
| (1.14–2.07) | (1.48–2.00) | (1.42–2.04) | (1.12–1.95) | (0.99–2.12) | ||||||
| Other Diseases of the Arteries, Arterioles, and Capillaries | 29 | 1.78 | 80 | 1.44 | 57 | 1.3 | 41 | 1.64 | 24 | 1.38 |
| (1.19–2.56) | (1.14–1.79) | (0.99–1.69) | (1.17–2.22) | (0.89–2.06) | ||||||
| 65–74 years | ||||||||||
| CVD | 632 | 1.94 | 2073 | 1.51 | 2090 | 1.4 | 1628 | 1.19 | 1690 | 1.18 |
| (1.79–2.10) | (1.45–1.58) | (1.34–1.46) | (1.13–1.25) | (1.12–1.24) | ||||||
| Diseases of the Heart | 506 | 1.92 | 1637 | 1.5 | 1652 | 1.42 | 1230 | 1.17 | 1259 | 1.15 |
| (1.76–2.10) | (1.43–1.57) | (1.35–1.49) | (1.11–1.24) | (1.09–1.21) | ||||||
| Cerebrovascular Diseases | 87 | 2.01 | 286 | 1.47 | 297 | 1.25 | 276 | 1.19 | 298 | 1.21 |
| (1.61–2.48) | (1.30–1.65) | (1.11–1.40) | (1.05–1.34) | (1.08–1.36) | ||||||
| Hypertension without Heart Disease | 6 | 1.4 | 23 | 1.19 | 32 | 1.3 | 43 | 1.48 | 62 | 1.56 |
| (0.52–3.06) | (0.76–1.79) | (0.89–1.84) | (1.07–2.00) | (1.20–2.00) | ||||||
| Atherosclerosis | 11 | 2.97 | 34 | 2.01 | 32 | 1.57 | 23 | 1.06 | 27 | 1.19 |
| (1.48–5.32) | (1.39–2.80) | (1.07–2.22) | (0.67–1.59) | (0.78–1.73) | ||||||
| Aortic Aneurysm and Dissection | 10 | 1.22 | 69 | 2.04 | 64 | 1.92 | 35 | 1.52 | 24 | 1.41 |
| (0.59–2.25) | (1.58–2.58) | (1.48–2.45) | (1.06–2.11) | (0.91–2.10) | ||||||
| Other Diseases of the Arteries, Arterioles, and Capillaries | 12 | 3.6 | 24 | 1.71 | 13 | 0.85 | 21 | 1.5 | 20 | 1.36 |
| (1.86–6.30) | (1.10–2.55) | (0.45–1.45) | (0.93–2.29) | (0.83–2.11) | ||||||
| 75–84 years | ||||||||||
| CVD | 1140 | 1.59 | 3179 | 1.08 | 2733 | 1.07 | 1426 | 1.33 | 560 | 2.16 |
| (1.50–1.69) | (1.04–1.12) | (1.03–1.11) | (1.27–1.41) | (1.98–2.35) | ||||||
| Diseases of the Heart | 894 | 1.63 | 2462 | 1.1 | 2077 | 1.07 | 1089 | 1.34 | 429 | 2.18 |
| (1.53–1.74) | (1.05–1.14) | (1.02–1.12) | (1.26–1.42) | (1.98–2.40) | ||||||
| Cerebrovascular Diseases | 169 | 1.37 | 502 | 0.99 | 462 | 1.05 | 223 | 1.21 | 86 | 1.88 |
| (1.17–1.60) | (0.91–1.08) | (0.96–1.16) | (1.06–1.38) | (1.51–2.33) | ||||||
| Hypertension without Heart Disease | 18 | 1.62 | 40 | 0.76 | 61 | 1.12 | 36 | 1.35 | 24 | 3.25 |
| (0.96–2.57) | (0.55–1.04) | (0.85–1.43) | (0.94–1.87) | (2.08–4.84) | ||||||
| Atherosclerosis | 23 | 1.67 | 67 | 1.08 | 56 | 1.02 | 44 | 2.08 | 12 | 2.66 |
| (1.06–2.51) | (0.83–1.37) | (0.77–1.33) | (1.51–2.80) | (1.38–4.65) | ||||||
| Aortic Aneurysm and Dissection | 24 | 1.71 | 70 | 1.44 | 47 | 1.42 | 15 | 1.27 | 5 | 1.88 |
| (1.10–2.55) | (1.12–1.81) | (1.04–1.88) | (0.71–2.09) | (0.61–4.39) | ||||||
| Other Diseases of the Arteries, Arterioles, and Capillaries | 12 | 1.75 | 38 | 1.38 | 30 | 1.26 | 19 | 1.82 | 4 | 1.52 |
| (0.90–3.05) | (0.98–1.90) | (0.85–1.80) | (1.09–2.84) | (0.41–3.89) | ||||||
| 85+ years | ||||||||||
| CVD | 914 | 1.38 | 1955 | 1.27 | 982 | 2.01 | 190 | 3.04 | 20 | 4.39 |
| (1.29–1.47) | (1.21–1.33) | (1.89–2.14) | (2.63–3.51) | (2.68–6.78) | ||||||
| Diseases of the Heart | 724 | 1.45 | 1516 | 1.3 | 754 | 2.04 | 153 | 3.24 | 16 | 4.67 |
| (1.34–1.56) | (1.24–1.37) | (1.89–2.19) | (2.75–3.80) | (2.67–7.59) | ||||||
| Cerebrovascular Diseases | 124 | 1.05 | 287 | 1.06 | 147 | 1.72 | 24 | 2.17 | 3 | 3.63 |
| (0.87–1.25) | (0.94–1.19) | (1.45–2.02) | (1.39–3.23) | (0.75–10.61) | ||||||
| Hypertension without Heart Disease | 16 | 1.21 | 41 | 1.27 | 20 | 1.78 | 2 | 1.24 | 0 | - |
| (0.69–1.96) | (0.91–1.72) | (1.09–2.75) | (0.15–4.49) | - | ||||||
| Atherosclerosis | 33 | 1.72 | 60 | 1.45 | 35 | 3.01 | 7 | 5.45 | 1 | 12.9 |
| (1.19–2.42) | (1.11–1.87) | (2.10–4.19) | (2.19–11.23) | (0.33–71.88) | ||||||
| Aortic Aneurysm and Dissection | 12 | 1.62 | 33 | 1.92 | 12 | 2.28 | 3 | 4.63 | 0 | - |
| (0.84–2.83) | (1.32–2.70) | (1.18–3.98) | (0.96–13.53) | |||||||
| Other Diseases of the Arteries, Arterioles, and Capillaries | 5 | 0.83 | 18 | 1.28 | 14 | 3.09 | 1 | 1.63 | 0 | - |
| (0.27–1.93) | (0.76–2.03) | (1.69–5.18) | (0.04–9.07) | - | ||||||
Abbreviations: CVD, cardiovascular disease; CI, confidence interval; Obs, observed; SMR, standardized mortality ratio.
4. Discussion
This large-scale, population-based, long-term follow-up study performed a comprehensive assessment of the risk of CVD-related death among 80,042 older BC patients using a national cancer registry comprising 44 years of data. To our knowledge, this was the first study to report the importance of CVD-related death as a competing risk among older BC patients. CVD-related death surpassed BC as the leading cause of death 5–10 years after diagnosis among older BC patients, especially for patient subgroups of localized-stage and low-grade tumors. Furthermore, older BC patients had a greater risk of CVD-related death than the general population.
CVD-related deaths surpassed BC-related deaths as the chief cause of death among older BC patients, while the main cause of death in BC patients aged <65 years remained BC. These results were confirmed in terms of the proportion of deaths and by a competing risk model. The proportion of CVD-related deaths was significantly higher than that of BC-related deaths in older patients. Furthermore, the competing risk model analyzing the interaction between CVD-related and BC-related deaths [26] revealed that the cumulative CVD mortality exceeded BC mortality in patients 5–10 years after the BC diagnosis. Our results are supported by the heterogeneity at the cancer site and heterogeneous outcomes in older patients (Figure 1 and Supplementary Figure S4) [28]. In contrast, some studies found that the greatest risk of CVD appeared in the first year after cancer diagnosis [29] and the long-term risk of CVD death might not exceed that of the primary cancer [11]. Previous studies have reported CVD deaths in BC patients; however, these studies did not analyze long-term trends during follow-up and provided a rough analysis of composite outcomes in the general population of BC patients [11,12]. Similar to our results, a population-based study also found that the proportion of noncancer deaths in BC patients gradually exceeded that of cancers with increasing calendar years, although the study did not stratify cases according to the type of CVD-related death or into older-aged subgroups [12]. In partial contrast to our study, a population-based study including all BC patients found a higher incidence of CVD-related deaths than BC-related deaths only in the proportion of deaths analysis but not in the competitive risk model [11], which could be attributed to the rough analysis of the general BC population masking information on important subgroup characteristics specific to older populations.
Considering that analyses including the general population of older BC could mask information about subgroup characteristics, we conducted subgroup analyses among older-aged BC patients. Subgroups with a higher risk of CVD-related deaths than BC-related deaths included the older-aged groups (65–74, 75–84, and >85), white race, other races, male or female, localized-stage, low-grade tumors, surgery, and NMIBC subgroups. Remarkably, in the localized-stage and low-grade subgroup, the higher risk of CVD-related mortality surpassed that of BC-related mortality shortly after BC diagnosis, which was confirmed by the analysis of the proportion of deaths and the competing risk model. Our findings are consistent with a population-based cohort study in which cumulative mortality from noncancer deaths exceeded deaths from cancer in older patients with early-stage Hodgkin lymphoma [27]. Similarly, a breast cancer cohort study also supported our results, finding that CVD competed with breast cancer as the chief cause of death in early-stage and older patients [30]. In contrast, a study using the Cox regression model found that BC patients with localized-stage and low-grade tumors had a lower risk of CVD-related death [14]. This difference might be attributed to differences in the statistical approach used, as indicated by two other studies from the United States [13,31] showing a higher risk of death from heart disease and stroke in cancer patients with localized-stage and low-grade tumors by logistic regression, whereas the results from Cox proportional hazards regression showed the opposite findings. Neither study adequately accounted for competing risks and resulted in biased conclusions, while our competing risk model provided a more reliable framework to analyze the interaction between BC and CVD, as it correctly estimated the marginal probability of an event in the presence of competing events [26]. Further supporting evidence comes from another study using the competing risk model that found that the risk of CVD-related death is higher than cancer-related death in older patients with early-stage breast cancer [32]. Interestingly, aging is a well-known risk factor for CVD and cancer, but current guidelines remain controversial about defining the age cutoff for high-risk CVD death in cancer patients. The European Society of Cardiology (ESC) guidelines proposed that cancer patients >65 years are at high risk of CVD [33], while the consensus of the European Society for Medical Oncology (ESMO) emphasizes that the age cutoff is 75 years [34]. Our findings support this controversy. BC patients ≥65 years were all at higher risk of CVD-related death than BC-related death, but this relationship was not observed in BC patients aged <65 years. In fact, consistent with most previous studies, individuals ≥65 years were defined as the older population and were associated with a high risk of CVD [28]. Moreover, patients ≥65 years formed the largest group of BC patients [4]. Therefore, our results provide a scientific rationale for considering 65 years as the cutoff point for high-risk CVD-related death in older-aged BC patients.
Was the elevated CVD risk in older BC patients influenced by aging or by the direct and indirect effects of BC? The increase in CVD-SMR partially reflects the direct and indirect CVD effects on BC, due to SMR adjusted by age, sex, and ethnicity to the general population at the same time; thus, the results of the SMR largely excluded the effects of aging [13,31]. Our study quantified the risk of CVD death in older BC patients by SMR and found that compared to the general population, older BC patients had a higher risk of CVD-related death, which was also confirmed in clinical subgroups, follow-up time subgroups, and specific CVD types. According to a previous community cohort study, cancer patients had a higher risk of CVD than noncancer controls, but the risk of CVD death was not evaluated [35]. The heterogeneity of the specific causes of CVD deaths was revealed in older patients with BC. Heart disease accounts for the highest proportion of CVD deaths (~70%), followed by cerebrovascular disease (~20%), while the highest CVD-SMR was observed in aortic aneurysm and dissection, followed by atherosclerosis and other diseases of the arteries, arterioles, and capillaries. Similarly, a UK cohort study found that compared to cancer-free controls, BC patients had a 1.18–1.31-fold risk of heart disease and a 1.14-fold risk of stroke [36], offering a possible explanation for our findings.
Our study offered new insights into the interaction between CVD death and BC death to advance the understanding of cardio-oncology. Although the management of BC should be the primary focus of older BC patients [1,9], our results emphasized the importance of competing risks, chief among which is CVD. The time points associated with a higher risk of CVD-related death than BC-related should guide a multidisciplinary team to focus on CVD-related death risk in older BC patients. Multidisciplinary teams, including a urological surgeon, cardiologist, and cardio-oncologist, could work together to manage and mitigate CVD-related death risk in older BC patients.
The causes of increased CVD-related death risk in older BC patients are diverse, but our analysis suggests that, besides aging, the indirect and direct effects of BC (shared risk factors, anticancer cardiotoxicity, and cancer itself) on CVD likely play an important role. First, aging is associated with a higher risk of CVD-related deaths. Second, shared risk factors for CVD and cancer, such as obesity and smoking, promote the risk of CVD-related death [6]. Third, the fatal cardiotoxicity of chemotherapy and immunotherapy [7] also increases the risk of CVD in older BC patients. Finally, increasing evidence from clinical studies and basic research suggests that cancer may directly damage the cardiovascular system. A new cancer diagnosis is independently related to increased CVD-related death risk [37]. BC can induce arterial and venous thromboembolism, increasing the risk of cardiovascular events, such as cancer-associated stroke [38,39,40]. Cancer itself could damage the heart in treatment-naive individuals with cancer [41]. Cancer-induced neutrophil extracellular traps (NETs) accumulate in the systemic vasculature and heart, resulting in vascular and cardiac dysfunction [42,43]. Nevertheless, the causative contribution of BC itself to cardiovascular disease still needs to be further explored.
The strengths of our study were its large-scale multicenter population and long-term follow-up. Ours is one of the largest studies evaluating the risk of CVD-related death in older BC patients. The size of our study population allowed a comprehensive, stratified analysis by patient characteristics and specific outcomes. Long-term follow-up allowed us to quantify the short- and long-term risk of CVD-related death.
Study Limitations. Some limitations to the study should be considered. Despite the larger population size, only a limited number of deaths occurred in some categories (e.g., hypertension without heart disease), as reflected in the imprecise SMR estimates. The SEER database did not provide detailed treatment information, and we could not evaluate the risk of CVD death according to chemotherapeutic agents or immunotherapy. The lack of cardiovascular comorbidities, cardiovascular risk factors, and symptoms such as hematuria [44,45,46] in the SEER database prevents the further exploration of their impact on the risk of CVD death. Nonetheless, the key result in our study is a phenomenal description but not a causal result, while anticancer therapy, cardiovascular risk factors, and comorbidities were majorly associated with the causes of the phenomenon. The SEER database did not have data on residual disease and positive surgical margins’ locations, and we could not further explore their impact on CVD death risk in older BC patients [47,48].
5. Conclusions
CVD-related death is an important competing risk among older BC patients and surpasses BC as the leading cause of death 5–10 years after BC diagnosis. The risk of CVD-related death in older patients was higher than in the general older population. Cardio-oncology care, including strategies to prevent, screen, monitor, and treat CVD-related death, is needed for the growing population of older BC patients.
Acknowledgments
We thank the research staff from SEER and the National Cancer Institute in the US.
Abbreviations
| AER | Absolute excess risk |
| BC | Bladder cancer |
| CVD | Cardiovascular disease |
| CI | Confidence interval |
| ESC | The European Society of Cardiology |
| ESMO | The European Society for Medical Oncology |
| SMR | Standardized mortality ratio |
| SEER | Surveillance, Epidemiology, and End Result database |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14194572/s1, reference [11,18,19,24,26,27,49]. File S1: Supplementary Methods. Figure S1: Selection of eligible patients and study design; Figure S2: The proportion of death in older patients (≥65 years) with bladder cancer in different subgroups; Figure S3: The risk of CVD-related deaths among older patients with different subtypes of bladder cancer; Figure S4: The proportion of deaths and cumulative mortality in bladder cancer patients under 65 years; Figure S5: The proportion of deaths in elderly patients with bladder cancer based on age at diagnosis; Figure S6: The proportion of deaths among older patients (≥65 years) with bladder cancer based on the years after diagnosis; Figure S7: Cumulative mortality among older patients (≥65 years) with bladder cancer in different subgroups; Figure S8: The cumulative mortality in older patients (≥65 years) with bladder cancer based on age at diagnosis (high competing risk subgroups); Figure S9: The cumulative mortality in older patients (≥65 years) with bladder cancer based on age at diagnosis (low competing risk subgroups); Figure S10: The death risk of hypertension without heart disease in older patients (≥65 years) with bladder cancer based on age at diagnosis; Figure S11: The death risk of atherosclerosis in older patients (≥65 years) with bladder cancer based on age at diagnosis; Figure S12: The death risk of aortic aneurysm and dissection in older patients (≥65 years) with bladder cancer based on age at diagnosis; Figure S13: The death risk of other diseases of the arteries, arterioles, and capillaries in older patients (≥65 years) with bladder cancer based on age at diagnosis; Table S1: The classifications about non-neoplasm causes of death; Table S2: Standardized mortality ratios for cardiovascular death in older patients with different subtypes of bladder cancer; Table S3: Absolute excess risks for cardiovascular death in older patients (≥65 years) with bladder cancer.
Author Contributions
Conceptualization, T.G.; data curation, T.G., M.S., Z.L. (Zehao Luo) and W.P.; formal analysis, T.G., M.S., Z.L. (Zehao Luo), Z.L. (Zhenxing Lu) and W.L.; funding acquisition, C.O. and M.C.; investigation, T.G., M.S., Z.L. (Zehao Luo) and W.P., Y.T., M.F. and Y.J. methodology, T.G., M.S., Z.L. (Zehao Luo) and Ruoyun Zhou; project administration, C.O. and M.C.; resources, T.G., M.S., Z.L. (Zehao Luo) and W.P.; software, T.G., M.S., Z.L. (Zehao Luo) and W.P.; supervision, C.O. and M.C.; validation, T.G., M.S., Z.L. (Zehao Luo), Y.T., M.F., W.L. and Y.J. visualization, T.G., M.S. and Z.L. (Zehao Luo); writing—original draft, T.G., M.S., Z.L. (Zehao Luo), W.P., R.Z. and M.F.; writing—review and editing, T.G., M.S., Z.L. (Zehao Luo), Z.L. (Zhenxing Lu) and W.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
As the data are publicly available, ethical approval was unnecessary for this study.
Informed Consent Statement
As the data are publicly available, informed consent was unnecessary for this study.
Data Availability Statement
The datasets analyzed in this study are publicly available from the SEER database (http://seer.cancer.gov) (accessed on 16 June 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by the National Natural Science Foundation of China (Nos. 81971765, 31771060, 31671025, 81871504, 32171355, and 82172103).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Babjuk M., Burger M., Capoun O., Cohen D., Compérat E.M., Dominguez Escrig J.L., Gontero P., Liedberg F., Masson-Lecomte A., Mostafid A.H., et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ) Eur. Urol. 2022;81:75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Sloan F.A., Yashkin A.P., Akushevich I., Inman B.A. The Cost to Medicare of Bladder Cancer Care. Eur. Urol. Oncol. 2020;3:515–522. doi: 10.1016/j.euo.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Wong V.K., Ganeshan D., Jensen C.T., Devine C.E. Imaging and Management of Bladder Cancer. Cancers. 2021;13:1396. doi: 10.3390/cancers13061396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., Jemal A., Kramer J.L., Siegel R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 5.Roth G.A., Forouzanfar M.H., Moran A.E., Barber R., Nguyen G., Feigin V.L., Naghavi M., Mensah G.A., Murray C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D.Y., Salem J.E., Cohen J.V., Chandra S., Menzer C., Ye F., Zhao S., Das S., Beckermann K.E., Ha L., et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efstathiou J.A., Mouw K.W., Gibb E.A., Liu Y., Wu C.L., Drumm M.R., da Costa J.B., du Plessis M., Wang N.Q., Davicioni E., et al. Impact of Immune and Stromal Infiltration on Outcomes Following Bladder-Sparing Trimodality Therapy for Muscle-Invasive Bladder Cancer. Eur. Urol. 2019;76:59–68. doi: 10.1016/j.eururo.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witjes J.A., Bruins H.M., Cathomas R., Comperat E.M., Cowan N.C., Gakis G., Hernandez V., Linares Espinos E., Lorch A., Neuzillet Y., et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 10.Babjuk M., Burger M., Compérat E.M., Gontero P., Mostafid A.H., Palou J., van Rhijn B.W.G., Rouprêt M., Shariat S.F., Sylvester R., et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Sturgeon K.M., Deng L., Bluethmann S.M., Zhou S., Trifiletti D.M., Jiang C., Kelly S.P., Zaorsky N.G. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019;40:3889–3897. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaorsky N.G., Churilla T.M., Egleston B.L., Fisher S.G., Ridge J.A., Horwitz E.M., Meyer J.E. Causes of death among cancer patients. Ann. Oncol. 2017;28:400–407. doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoltzfus K.C., Zhang Y., Sturgeon K., Sinoway L.I., Trifiletti D.M., Chinchilli V.M., Zaorsky N.G. Fatal heart disease among cancer patients. Nat. Commun. 2020;11:2011. doi: 10.1038/s41467-020-15639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong J., Diao X., Diao F., Fan X., Zheng J., Yan D., Huang J., Qin H., Lin T. Causes of death in long-term bladder cancer survivors: A population-based study. Asia-Pac. J. Clin. Oncol. 2019;15:e167–e174. doi: 10.1111/ajco.13156. [DOI] [PubMed] [Google Scholar]
- 15.Scosyrev E., Wu G., Golijanin D., Messing E. Non-bladder cancer mortality in patients with urothelial cancer of the bladder. Urol. Oncol. 2013;31:656–663. doi: 10.1016/j.urolonc.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Noon A.P., Albertsen P.C., Thomas F., Rosario D.J., Catto J.W. Competing mortality in patients diagnosed with bladder cancer: Evidence of undertreatment in the elderly and female patients. Br. J. Cancer. 2013;108:1534–1540. doi: 10.1038/bjc.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai M., Tang C., Li M., Chen X., Jin Y., Ying X., Tang Z., Wang X., Wu Y., Sun C., et al. Short-term mortality risks among patients with non-metastatic bladder cancer. BMC Cancer. 2020;20:1148. doi: 10.1186/s12885-020-07655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institute About the SEER Program. [(accessed on 16 June 2021)]; Available online: https://seer.cancer.gov/about/
- 19.Park H.S., Lloyd S., Decker R.H., Wilson L.D., Yu J.B. Overview of the Surveillance, Epidemiology, and End Results database: Evolution, data variables, and quality assurance. Curr. Probl. Cancer. 2012;36:183–190. doi: 10.1016/j.currproblcancer.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute Casefinding Studies—SEER Quality Improvement. [(accessed on 16 June 2021)]; Available online: https://seer.cancer.gov/qi/
- 21.Wang L., Wang F., Chen L., Geng Y., Yu S., Chen Z. Long-term cardiovascular disease mortality among 160 834 5-year survivors of adolescent and young adult cancer: An American population-based cohort study. Eur. Heart J. 2021;42:101–109. doi: 10.1093/eurheartj/ehaa779. [DOI] [PubMed] [Google Scholar]
- 22.Chow R., Lage D.E., Williams G.R., Sedrak M.S., Greer J.A., Temel J.S., Nipp R.D. Representation and Outcomes of Older Adults in Practice-Changing Oncology Trials in the Era of Novel Therapies: A Guideline Appraisal. J. Natl. Compr. Cancer Netw. 2022;20:37–44. doi: 10.6004/jnccn.2021.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention: National Center for Health Statistics. [(accessed on 22 August 2022)]; Available online: https://www.cdc.gov/nchs.
- 24.Sung H., Hyun N., Leach C.R., Yabroff K.R., Jemal A. Association of First Primary Cancer with Risk of Subsequent Primary Cancer among Survivors of Adult-Onset Cancers in the United States. JAMA. 2020;324:2521–2535. doi: 10.1001/jama.2020.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng W., Li Z., Guo W., Fan X., Zhou F., Zhang K., Ou C., Huang F., Chen M. Association between Fasting Glucose Variability in Young Adulthood and the Progression of Coronary Artery Calcification in Middle Age. Diabetes Care. 2020;43:2574–2580. doi: 10.2337/dc20-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin P.C., Lee D.S., Fine J.P. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dores G.M., Curtis R.E., Dalal N.H., Linet M.S., Morton L.M. Cause-Specific Mortality Following Initial Chemotherapy in a Population-Based Cohort of Patients with Classical Hodgkin Lymphoma, 2000–2016. J. Clin. Oncol. 2020;38:4149–4162. doi: 10.1200/JCO.20.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carioli G., Malvezzi M., Bertuccio P., Hashim D., Waxman S., Negri E., Boffetta P., La Vecchia C. Cancer mortality in the elderly in 11 countries worldwide, 1970–2015. Ann. Oncol. 2019;30:1344–1355. doi: 10.1093/annonc/mdz178. [DOI] [PubMed] [Google Scholar]
- 29.Fang F., Fall K., Mittleman M.A., Sparén P., Ye W., Adami H.O., Valdimarsdóttir U. Suicide and cardiovascular death after a cancer diagnosis. N. Engl. J. Med. 2012;366:1310–1318. doi: 10.1056/NEJMoa1110307. [DOI] [PubMed] [Google Scholar]
- 30.Patnaik J.L., Byers T., DiGuiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaorsky N.G., Zhang Y., Tchelebi L.T., Mackley H.B., Chinchilli V.M., Zacharia B.E. Stroke among cancer patients. Nat. Commun. 2019;10:5172. doi: 10.1038/s41467-019-13120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H., Lin W., Chen D., Wang K., Tu W., Lin H., Li K., Ye S., Guan T., Chen Y. Cardiovascular and Other Competing Causes of Death in Male Breast Cancer Patients: A Population-Based Epidemiologic Study. Clin. Interv. Aging. 2021;16:1393–1401. doi: 10.2147/CIA.S314689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., Aboyans V., Asteggiano R., Galderisi M., Habib G., Lenihan D.J., Lip G.Y.H., Lyon A.R., et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 34.Curigliano G., Lenihan D., Fradley M., Ganatra S., Barac A., Blaes A., Herrmann J., Porter C., Lyon A.R., Lancellotti P., et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armenian S.H., Xu L., Ky B., Sun C., Farol L.T., Pal S.K., Douglas P.S., Bhatia S., Chao C. Cardiovascular Disease among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strongman H., Gadd S., Matthews A., Mansfield K.E., Stanway S., Lyon A.R., dos-Santos-Silva I., Smeeth L., Bhaskaran K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394:1041–1054. doi: 10.1016/S0140-6736(19)31674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson D.I., Wiebe N., Cheung W.Y., Mackey J.R., Pituskin E., Reiman A., Tonelli M. Incident Cardiovascular Disease among Adults with Cancer: A Population-Based Cohort Study. JACC CardioOncol. 2022;4:85–94. doi: 10.1016/j.jaccao.2022.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzbach C.J., Schaefer A., Ebert A., Held V., Bolognese M., Kablau M., Hennerici M.G., Fatar M. Stroke and cancer: The importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43:3029–3034. doi: 10.1161/STROKEAHA.112.658625. [DOI] [PubMed] [Google Scholar]
- 39.Navi B.B., Reiner A.S., Kamel H., Iadecola C., Okin P.M., Elkind M.S.V., Panageas K.S., DeAngelis L.M. Risk of Arterial Thromboembolism in Patients with Cancer. J. Am. Coll. Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker A.J., Card T.R., West J., Crooks C., Grainge M.J. Incidence of venous thromboembolism in patients with cancer—A cohort study using linked United Kingdom databases. Eur. J. Cancer. 2013;49:1404–1413. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Cedervall J., Herre M., Dragomir A., Rabelo-Melo F., Svensson A., Thålin C., Rosell A., Hjalmar V., Wallén H., Lindman H., et al. Neutrophil extracellular traps promote cancer-associated inflammation and myocardial stress. Oncoimmunology. 2022;11:2049487. doi: 10.1080/2162402X.2022.2049487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cedervall J., Zhang Y., Huang H., Zhang L., Femel J., Dimberg A., Olsson A.K. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015;75:2653–2662. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- 43.Döring Y., Libby P., Soehnlein O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ. Res. 2020;126:1228–1241. doi: 10.1161/CIRCRESAHA.120.315931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khadhouri S., Gallagher K.M., MacKenzie K.R., Shah T.T., Gao C., Moore S., Zimmermann E.F., Edison E., Jefferies M., Nambiar A., et al. Developing a Diagnostic Multivariable Prediction Model for Urinary Tract Cancer in Patients Referred with Haematuria: Results from the IDENTIFY Collaborative Study. Eur. Urol. Focus. 2022 doi: 10.1016/j.euf.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Khadhouri S., Gallagher K.M., MacKenzie K.R., Shah T.T., Gao C., Moore S., Zimmermann E.F., Edison E., Jefferies M., Nambiar A., et al. The IDENTIFY study: The investigation and detection of urological neoplasia in patients referred with suspected urinary tract cancer—A multicentre observational study. BJU Int. 2021;128:440–450. doi: 10.1111/bju.15483. [DOI] [PubMed] [Google Scholar]
- 46.Iseki K., Konta T., Asahi K., Yamagata K., Fujimoto S., Tsuruya K., Narita I., Kasahara M., Shibagaki Y., Moriyama T., et al. Higher cardiovascular mortality in men with persistent dipstick hematuria. Clin. Exp. Nephrol. 2021;25:150–156. doi: 10.1007/s10157-020-01971-z. [DOI] [PubMed] [Google Scholar]
- 47.Laukhtina E., Boehm A., Peyronnet B., Bravi C.A., Batista Da Costa J., Soria F., D’Andrea D., Rajwa P., Quhal F., Yanagisawa T., et al. Urethrectomy at the time of radical cystectomy for non-metastatic urothelial carcinoma of the bladder: A collaborative multicenter study. World J. Urol. 2022;40:1689–1696. doi: 10.1007/s00345-022-04025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Claps F., van de Kamp M.W., Mayr R., Bostrom P.J., Boormans J.L., Eckstein M., Mertens L.S., Boevé E.R., Neuzillet Y., Burger M., et al. Risk factors associated with positive surgical margins’ location at radical cystectomy and their impact on bladder cancer survival. World J. Urol. 2021;39:4363–4371. doi: 10.1007/s00345-021-03776-5. [DOI] [PubMed] [Google Scholar]
- 49.Surveillance Research Program, National Cancer Institute SEER*Stat Software, Version 8.3.9.2. [(accessed on 16 June 2021)]; Available online: https://seer.cancer.gov/seerstat/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in this study are publicly available from the SEER database (http://seer.cancer.gov) (accessed on 16 June 2021).