Abstract
Simple Summary
We analyzed the influence of the neutrophil to lymphocyte ratio (NLR) and its change during therapy as a potential prognostic marker in metastatic prostate cancer patients treated with 223Radium (223Ra) and patients treated with docetaxel. We found that a low NLR at baseline as well as at 12 weeks of treatment was associated with a better overall survival only in patients treated with 223Ra but not in patients treated with Docetaxel. Patients with a baseline NLR ≤ 5 that remained low (NLR ≤ 5) at 12 weeks of treatment had significantly longer median survivals compared to patients whose NLR was low (<5) at baseline and that converted at 12 weeks to >5. The prognostic value of NLR at baseline and 12 weeks will need to be validated in larger prospective cohorts.
Abstract
The neutrophil to lymphocyte ratio (NLR) at baseline has been shown to have prognostic value in metastatic prostate cancer. Little is known about the importance of a change in the NLR during treatment in patients treated with Radium-223 (223Ra). We investigated the prognostic value of the NLR at baseline and during therapy in patients with metastatic prostate cancer treated with 223Ra and also in patients treated with Docetaxel. We reviewed all patients treated with 223Ra in our center and randomly chosen patients treated with Docetaxel. Patients were stratified according to NLR ≤ 5 and >5 at baseline and at 12 weeks of therapy. The relationship between NLR measured at baseline and at 12 weeks and overall survival (OS) were evaluated. A total of 149 patients treated with 223Ra and 170 with Docetaxel were evaluated. For patients treated with 223Ra, overall survival was significantly better in patients that had both an NLR ≤ 5 at baseline and at 12 weeks. No such effect of NLR was found in patients treated with Docetaxel. In the present study, NLR at baseline and after 12 weeks of therapy was found to be prognostic factor in patients treated with 223Ra but not in those treated with Docetaxel.
Keywords: metastatic prostate cancer, Radium-223, neutrophil to lymphocyte ratio, prognostic factor
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer in men in the western world and the fifth leading cause of death worldwide [1]. In metastatic prostate cancer, especially in metastatic castration resistant prostate cancer (mCRPC), several life-extending therapeutic options are now available, such as androgen-signaling-targeted inhibitors, chemotherapy, and Radium-223 [2]. Despite, the therapeutic progress made recently, life expectancy remains poor. A better understanding of the predicting treatment response is needed to optimize the use and the sequencing of available therapies [3]. Lymphocytes and neutrophils have been implicated in host immune response to cancer including prostate cancer, either as a factor of host defense or tolerance [4]. Independently, the lymphocyte and neutrophil count depends on many factors and can be influenced by systemic therapy such as chemotherapy or immunotherapy. The neutrophil to lymphocyte ratio (NLR) is thought to reflect systemic inflammation and host response. The NLR, a simple measure of the absolute neutrophil count (ANC) divided by the absolute lymphocyte count (ALC) has been shown to be such a prognostic factor in many cancers as well as in urological cancers including prostate cancer [5,6]. NLR at baseline had a significant prognostic value in oncological outcomes [7,8]. Moreover, recent studies showed in kidney and lung cancers that a change of the NLR from a favorable ratio at baseline to an unfavorable ratio during treatment with immunotherapy was a negative prognostic factor of overall survival [9,10].
The bone selective, calcium mimetic Radium-223 dichloride (223Ra) was approved in mCRPC after docetaxel administration or if docetaxel is contraindicated [11]. It is a short-range, alpha particle emitter that might influence antigen presentation and could have a direct effect on the bone marrow. One could therefore expect a direct influence of 223Ra on the NLR while cytotoxic chemotherapy with docetaxel may have a different effect on the lymphocytes and neutrophil count [12].
The aim of the study was to analyze the influence of NLR and its change during therapy and its prognostic value in patients with metastatic prostate cancer treated with 223Ra and patients treated with docetaxel.
2. Materials and Methods
2.1. Study Population
All patients with a metastatic castration-resistant prostate cancer treated with 223Ra at Centre Hospitalier de l’Université de Montréal (CHUM) between October 2013 and March 2022 were included. Inclusion criteria were adult patients with histologically proven metastatic prostate cancer. As recommended by provincial guidelines, Radium-223 was offered to patients with castration-resistant prostate cancer with symptomatic bone metastases if they had been treated priorly with docetaxel or if docetaxel was contraindicated or not suitable for them. Patients treated with Docetaxel were randomly chosen among the large number of patients treated in our institution. Docetaxel was administered for either de novo metastatic castration-sensitive or metastatic castration-resistant prostate cancer. Patients were excluded if they had less than 3 cycles of 223Ra and less than 4 cycles of Docetaxel. Eligible patients were included in our institutional database. The current study was conducted according to the Declaration of Helsinki and approved by the institutional ethics committee’s (CER 21.328).
2.2. Study Variables and Outcomes
At baseline, patients’ age, Eastern Cooperative Group (ECOG) performance status, prostate specific antigen (PSA) level, hemoglobin level (Hb), full blood count (including absolute neutrophil and lymphocyte counts), and total alkaline phosphatase (tALP) levels were obtained. Before each cycle of 223Ra and docetaxel, all laboratory values were repeated. The neutrophil-to-lymphocyte ratio (NLR) was calculated as the ratio of the absolute neutrophil count (ANC) divided by the absolute lymphocyte count (ALC). We measured the baseline values and the values before the 3rd cycle of 223Ra and 4th cycle of Docetaxel, corresponding in both cases to 12 weeks after the beginning of therapy. Patients were stratified according to NLR in low NLR (NLR ≤ 5) and high NLR (NLR > 5). This cut-off was chosen because it had been shown to have prognostic value in previous studies in melanoma and lung cancer [13,14].
The NLR was recalculated at 12 weeks. Combining the results of baseline and at 12 weeks, a NLR score was calculated. Patients were stratified in “good” score recorded as a patient who had both a baseline and 12 weeks NLR of ≤5, an “intermediate” score those with NLR ≤ 5 at baseline and >5 at 12 weeks. Furthermore, a “poor” score was given to patients that had NLR > 5 both at baseline and at 12 weeks of treatment. The primary endpoint was overall survival (OS) defined as the time between the treatment onset (223Ra or Docetaxel) and death from any cause.
2.3. Statistical Analysis
Descriptive statistics were reported as median and interquartile range (IQR) for continuous variables, and as frequencies and percentages for categorical variables. Median and proportions were compared by the chi-square test or Fisher exact test, depending on the type of variable. OS was estimated by the Kaplan–Meier method and compared with the Log Rank test. Follow-up was calculated using the reverse Kaplan–Meier method. The association of clinical and biological variables with OS was first estimated with univariate Cox proportional hazard models, then with multivariable Cox regression model if parameters had p < 0.10 or was clinically relevant. Statistical analyses were performed using R Version 3.4.3 (The R foundation, Vienna, Austria). All tests were two-sided at a level of significance of p < 0.05 was used.
3. Results
3.1. Descriptive Characteristics of the Study Population
A total of 319 patients were analyzed, 149 (46.71%) were treated with 223Ra and 170 (54.29%) with Docetaxel. Baseline characteristics of 223Ra and Docetaxel are presented in Table 1a,b. Median age was 72 years (interquartile range, IQR 65–79) and 67 (IQR 59–74) years in 223Ra-treated patients and Docetaxel-treated patients, respectively. The median follow-up was 68.1 (95% CI 20.4-Not reached) months in 223Ra-treated patient and 77.8 (95% CI 68.8-Not reached) months in docetaxel-treated patients.
Table 1.
(a): Baseline characteristics of patients treated with 223Ra tabulated according to NLR. All values are median (IQR) or frequencies (%). (b): Baseline characteristics of patients treated with Docetaxel tabulated according to NLR. All values are median (IQR) or frequencies (%).
| (a) | ||||
|
Whole Sample
(n = 149) (%) |
NLR Low (
≤
5)
(n = 105) (%) |
NLR High (>5)
(n = 23) (%) |
p -Value | |
| Prior Docetaxel treatment | 0.21 | |||
| No | 65 (43.6%) | 42 (40%) | 6 (26.1%) | |
| Yes | 84 (56.4%) | 63 (60%) | 17 (73.9%) | |
| Age (years) | 72 (65; 79) | 71 (64; 77) | 74 (66; 79.5) | 0.83 |
| Missing | 1 | 1 | 0 | |
| ECOG | 0.04 | |||
| 0 | 50 (36.8%) | 42 (43.8%) | 4 (17.4%) | |
| 1 | 68 (50%) | 45 (46.9%) | 15 (65.2%) | |
| 2 | 18 (13.2%) | 9 (9.4%) | 4 (17.4%) | |
| Missing | 13 | 9 | 0 | |
| PSA levels (ng/mL) | 53.2 (14.8; 182) | 38.3 (10.1; 122.5) | 86.2 (23.8; 307.6) | 0.79 |
| Missing | 7 | 1 | 0 | |
| tALP (IU/L) | 106 (69.5; 198.5) | 96 (67; 156) | 86.2 (23.8; 307.6) | 0.1 |
| Missing | 18 | 6 | 0 | |
| Hb (g/L) | 122 (113.8; 133) | 96 (67; 156) | 158 (92; 267) | 0.07 |
| Missing | 9 | 0 | 0 | |
| White blood count (109/L) | 6.5 (5.3; 7.9) | 6.2 (5.2; 7.9) | 7.7 (6.8; 9) | 0.01 |
| Neutrophils (109/L) | 4.4 (3.5; 5.6) | 4.1 (3.3; 5.1) | 5.7 (5.3; 6.6) | 0.001 |
| Lymphocytes (109/L) | 3.5 (2.5; 4.6) | 1.4 (1.1; 1.9) | 1 (0.7; 1.1) | <0.001 |
| NLR | 3.5 (2.5; 4.6) | 3.2 (2.3; 4) | 5.8 (5.4; 7) | |
| Missing | 21 | 0 | 0 | |
| Platelets (109/L) | 239 (186.8; 287) | 237 (186; 285) | 246 (209; 322) | 0.16 |
| Missing | 9 | 0 | 0 | |
| (b) | ||||
|
Whole Sample
(n = 170) (%) |
NLR Low (
≤
5)
(n = 76) (%) |
NLR High (>5)
(n = 16) (%) |
p -Value | |
| Age | 67 (59; 74) | 69 (59.5; 75.5) | 66.5 (59.8; 69.2) | 0.15 |
| Missing | 1 | 1 | 0 | |
| Cancer Type | ||||
| Hormone-sensitive | 20 (12%) | 14 (18.7%) | 3 (18.8%) | 1 |
| Castration resistant | 147 (88%) | 61 (81.3%) | 13 (81.2%) | |
| Missing | 3 | 1 | 0 | |
| ECOG | 0.25 | |||
| 0 | 55 (43.7%) | 25 (58.1%) | 4 (36.4%) | |
| 1 | 57 (45.2%) | 12 (27.9%) | 6 (54.5%) | |
| 2 | 14 (11.1%) | 12 (27.9%) | 1 (9.1%) | |
| Missing | 44 | 33 | 5 | |
| PSA levels (ng/mL) | 29.4 (5.8; 115.7) | 20.4 (5.6; 94.3) | 121.4 (16.6; 567.8) | 0.09 |
| Missing | 34 | 0 | ||
| tALP (IU/L) | 84.5 (63.8; 168.2) | 86 (67; 167) | 90 (68.8; 292) | 0.1 |
| Missing | 74 | 7 | 0 | |
| Hb (g/L) | 130 (119; 143) | 132 (120.8; 143.2) | 122.5 (111.5; 134) | 0.14 |
| Missing | 77 | 0 | 0 | |
| White blood count (109/L) | 6.9 (5.5; 8.0) | 6.8 (5.5; 7.8) | 7.6 (5.8; 8.7) | 0.16 |
| Neutrophils (109/L) | 4.2 (3.4; 5.3) | 4.0 (3.2; 5) | 6.0 (4.6; 6.9) | 0.003 |
| Lymphocytes (109/L)) | 1.5 (1.1; 1.9) | 1.6 (1.3; 2) | 0.8 (0.7; 1) | <0.001 |
| NLR | 2.6 (2.1; 4.2) | 2.4 (1.9; 3) | 7.9 (5.6; 8; 7) | |
| Missing | 78 | 0 | 0 | |
| Platelets (109/L) | 227 (183.8; 264) | 230 (183; 261) | 200 (175.2; 270.8) | 0.29 |
| Missing | 78 | 0 | 0 | |
Eastern Cooperative Group (ECOG) performance status, prostate specific antigen (PSA) level, hemoglobin level (Hb), total alkaline phosphatase (tALP), and Neutrophil-to-lymphocyte ratio (NLR) was calculated as the ratio of the absolute neutrophil count (ANC) divided by the absolute lymphocyte count (ALC).
There was no significant difference between patients with a baseline NLR ≤ 5 or >5 for 223Ra and docetaxel regarding PSA, tALP, hemoglobin and platelets. However, in the NLR > 5 group patients treated with 223Ra had a significantly higher white blood count (7.7 (6.8; 9)) than the NLR ≤ 5 group (6.2 (5.2; 7.9)). This difference in white cell count was not seen in the Docetaxel group.
3.2. Primary Outcome within Radium-Treated Population
3.2.1. Association of NLR at Baseline with Overall Survival
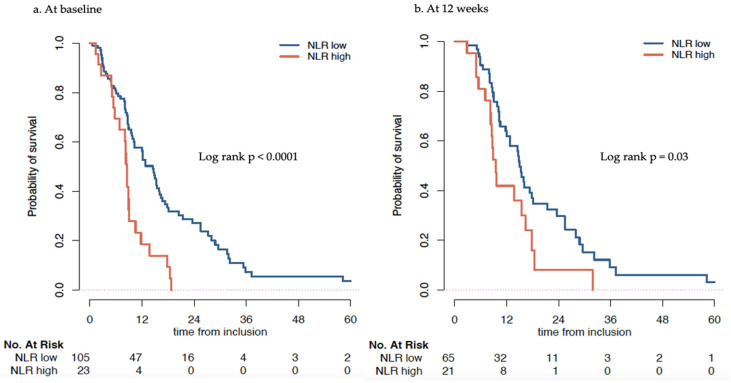
In the 223Ra cohort, low NLR ≤ 5 at baseline was associated with better overall survival: 14.5 months (95% CI 10.2–16.4) vs. 8.5 months (95% CI 6.8–10.5) in the high NLR > 5 group, log rank p < 0.0001 (Figure 1a). In multivariable analysis, adjusted on prior docetaxel treatment, previous prostatectomy, ECOG, PSA level, Hemoglobin, tALP, NLR at baseline was an independent predictive factor (HR 1.7, 95% CI 1.00–2.90, p = 0.05) (Table 2, Supplementary Table S1).
Figure 1.
Overall survival (months) of patients treated with 223Ra estimated by Kaplan–Meier stratified by Neutrophil to Lymphocyte ratio (NLR) low (≤5) and NLR high (>5) calculated at baseline (a) and at 12 weeks (b).
Table 2.
Median overall survival (months) of patients treated with 223Ra according to Neutrophil to Lymphocyte ratio (NLR).
| NLR Low (≤5) | NLR High (>5) | HR (95% CI) * | p-Value | |
|---|---|---|---|---|
| baseline | 14.5 (10.2–16.4) | 8.5 (6.8–10.5) | 1.7 (1.00–2.90) | 0.05 |
| at 12 weeks | 15.0 (12.7–21.4) | 9.5 (8.3–18.4) | 1.88 (1.07–3.29) | 0.03 |
* Hazard Ratio (HR) adjusted on docetaxel treatment, previous prostatectomy, Eastern Cooperative Group (ECOG) performance status, prostate specific antigen (PSA) level, hemoglobin level (Hb), total alkaline phosphatase (tALP).
3.2.2. Association of Change of NLR at 12 Weeks with OS
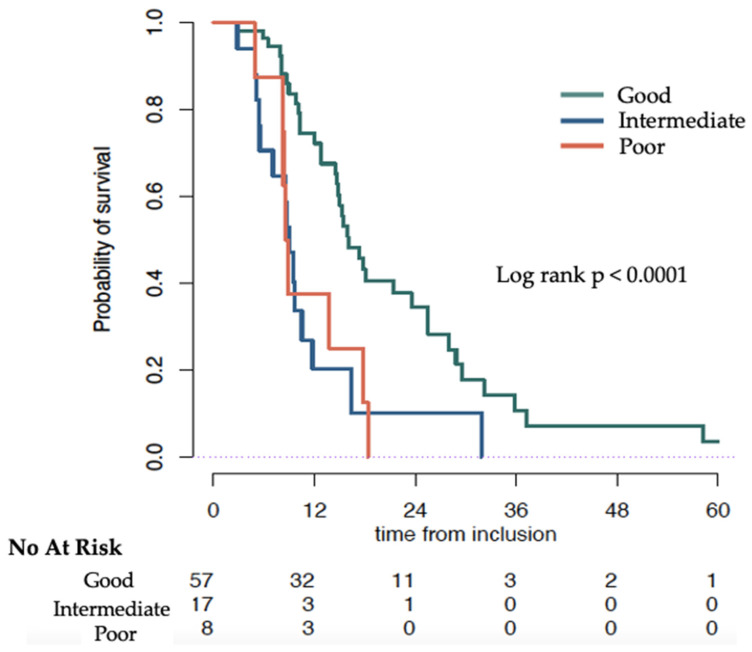
We then investigated if a change in NLR during treatment affected overall survival. At 12 weeks, NLR ≤ 5 was associated with higher OS. Patients with NLR ≤ 5 had a longer median survival (15.0 months, 95% CI 12.7–21.4), compared to patients with NLR > 5 after 12 weeks (9.5 months, 95% CI 8.3–18.4), p = 0.03 (Figure 1b). Patients who had a NLR ≤ 5 at baseline and maintained a NLR < 5 after 12 weeks showed a significantly (p = 0.001) better OS with a median of 16.0 months (95% CI 14.8–25.5) compared with a NLR that changed from ≤5 to >5 at 12 weeks, (median OS: 9.1 months, 95% CI 7.1–NR). OS was similarly low in patients who had NLR >5 at baseline and at 12 weeks ((median 8.7 months, 95% CI 8.3–NR) and patients whose NLR changed from ≤5 to >5 at 12 weeks (Table 3 and Figure 2). When investigating absolute change of the NLR, we found that the higher the increase in NLR between baseline and 12 weeks the larger the hazard ratio (Supplementary Figure S1).
Table 3.
Median overall survival of patients treated with 223Ra according to NLR score.
| Good | Intermediate | Poor | p-Value | |
|---|---|---|---|---|
| MedianOS Months (95% CI) |
16.0 (14.8–25.5) | 9.1 (7.1–NR) | 8.7 (8.3–NR) | Log rank 0.0001 |
| HR (95% CI) | Ref | 3.04 (1.62–5.68) | 2.88 (1.31–6.32) | Cox model 0.001 |
Hazard Ratio (HR), Not Reached (NR), Overall Survival (OS), Reference (Ref), Neutrophil to Lymphocyte Rate (NLR) Score: Good: NLR baseline ≤ 5 Furthermore, NLR at 12 week ≤ 5, Intermediate: NLR at baseline ≤ 5 and at 12 week > 5, Poor: NLR at baseline > 5 and NLR at 12 week > 5.
Figure 2.
Overall survival (months) of patients treated with 223Ra estimated by Kaplan–Meier stratified by Neutrophil to Lymphocyte ratio (NLR) score: Good (NLR ≤ 5 at baseline and ≤5 at 12 weeks), Intermediate (NLR ≤ 5 at baseline and >5 at 12 weeks) and Poor (NLR > 5 at baseline and at 12 weeks).
3.2.3. Evolution of ANC and ALC during Therapy
In general, during therapy the ANC and ALC tended to decrease as the treatment cycles progressed (Supplementary Figure S2).
3.3. Primary Outcome within Docetaxel Treated Population
3.3.1. Association of NLR at Baseline with Overall Survival
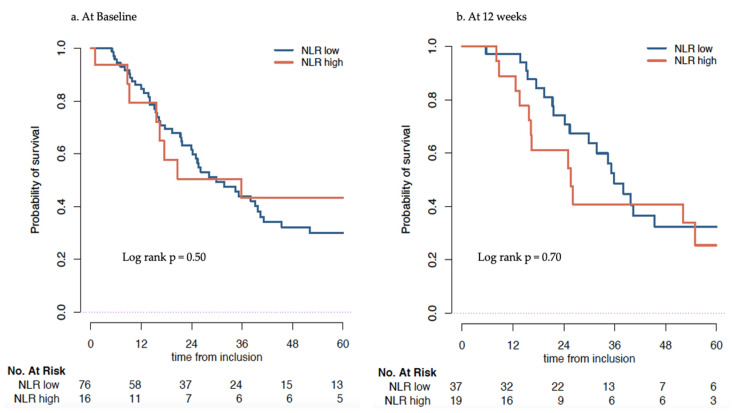
In the docetaxel cohort, the median OS was 29.9 (24.2–41.1) months at baseline for NLR ≤ 5 patients and 35.8 (16.4-Not Reached (NR)) months for patients with a NLR > 5. No association was found between the NLR and overall survival (p = 0.50) Likewise, after 12 weeks of docetaxel, the median OS was 35.8 (29.9–NR) months for low NLR and 25.6 (16.2–NR) months for high NLR. In multivariable analysis, a low NLR was not significantly associated with a better OS, p = 0.51 (Table 4, Figure 3 and Supplementary Table S2).
Table 4.
Median overall survival (months) of patients treated with Docetaxel according to NLR.
| NLR Low (≤5) | NLR High (>5) | HR (95% CI) * | p-Value | |
|---|---|---|---|---|
| Baseline | 29.9 (24.2–41.1) | 35.8 (16.8–NR) | 0.78 (0.37–1.6) | 0.50 |
| At 12 weeks | 35.8 (29.9–NR) | 25.6 (16.2–NR) | 1.27 (0.62–2.61) | 0.51 |
* Hazard Ratio (HR) adjusted on docetaxel treatment, previous prostatectomy, Eastern Cooperative Group (ECOG) performance status, prostate specific antigen (PSA) level, hemoglobin level (Hb), total alkaline phosphatase (tALP).
Figure 3.
Overall survival (months) of patients treated with Docetaxel estimated by Kaplan–Meier stratified by Neutrophil to Lymphocyte ratio (NLR) low (≤5) and NLR high (>5) calculated at baseline (a) and at 12 weeks (b).
3.3.2. Association of Change of NLR at 12 Weeks with OS
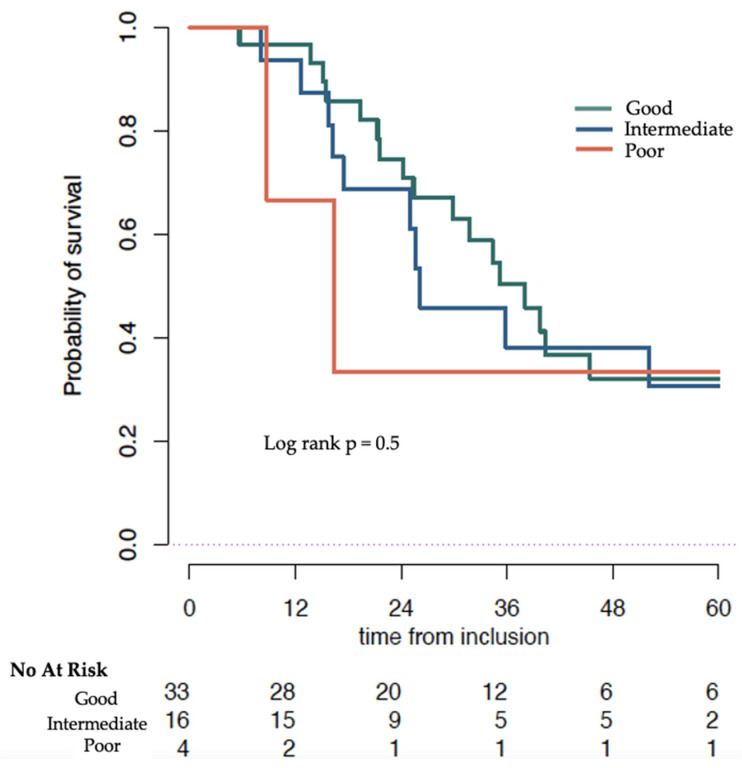
Patients who had a NLR ≤ 5 at baseline and maintained a NLR < 5 at 12 weeks had a median OS of 38.1 months (95% CI 30.0–NR). If the NLR changed from ≤ 5 to > 5 at 12 weeks, median OS was 26.1 months, 95% CI 17.1–NR). In patients with NLR > 5 at baseline whose NLR remained >5 at 12 weeks median OS was 16.4 months, 95% CI 8.8–NR. We were unable to find a significant difference in OS when the NLR changed from <5 to >5 during treatment (p = 0.86) (Table 5 and Figure 4).
Table 5.
Median overall survival of patients treated with Docetaxel according to NLR score.
| Good | Intermediate | Poor | p-Value | |
|---|---|---|---|---|
| MedianOS months (95% CI) | 38.1 (30.0–NR) | 26.1 (17.4–NR) | 16.4 (8.8–NR) | Log rank 0.3 |
| HR (95% CI) | Ref | 1.11 (0.51–2.42) | 1.49 (0.34–6.47) | Cox model 0.86 |
Hazard Ratio (HR), Not Reached (NR), Overall Survival (OS), Reference (Ref), Neutrophil to Lymphocyte Rate (NLR), Score: Good: NLR baseline ≤ 5 and NLR at 12 weeks ≤ 5, Intermediate: NLR at baseline < 5 and at 12 weeks > 5, Poor: NLR at baseline > 5 and NLR at 12 weeks > 5.
Figure 4.
Overall survival (months) of patients treated with Docetaxel estimated by Kaplan–Meier stratified by Neutrophil to Lymphocyte ratio (NLR) score: Good (NLR ≤ 5 at baseline and ≤5 at 12 weeks), Intermediate (NLR ≤ 5 at baseline and >5 at 12 weeks) and Poor (NLR > 5 at baseline and at 12 weeks).
3.3.3. ANC and ALC Evolution during Therapy
During therapy, unlike patient treated by 223Ra, the ANC and ALC tended to remain stable as treatment progressed (Supplementary Figure S3).
4. Discussion
In this study, we found that a low NLR at baseline as well as at 12 weeks after the start of treatment was associated with better overall survival in patients treated with 223Ra but not in patients treated with docetaxel. Patients with a baseline NLR ≤ 5 as well as low (≤5) NLR at 12 weeks of treatment had a significantly longer median survival (16.0 months) than patients whose NLR was low (≤5) at baseline and increased to >5 at 12 weeks (9.1 months). Regarding overall survival, in our study, 223Ra-treated patients with a baseline NLR ≤ 5 had 14.5 months (95% CI 10.2–16.4) median overall survival. These results are consistent with the phase 3 ALSYMPCA study in which patients treated with 223Ra had a median overall survival of 14 months [11].
The fact that a high NLR at baseline is associated with poor OS in many metastatic cancers is well documented [5,6,7,8]. In our study, after multivariable analysis, adjusted on prior docetaxel treatment, previous prostatectomy, ECOG, PSA level, Hemoglobin, tALP, a low NLR (≤5) at baseline was associated with a better prognosis in 223Ra-treated patients. These results are consistent with the available literature regarding 223Ra-treated patients. Indeed, the fact that the baseline NLR is a prognostic factor in 223Ra-treated patients is well recognized in most studies on the subject including the post hoc analysis of the ALSYMPCA trial by Meisel et al. [11,15] and other trials [16,17]. Our results are unique in finding that not only baseline NLR but also the change of the NLR at 12 weeks is a predictive factor and could help selecting patients who could benefit from changing their systemic treatment. Lorente et al. found in mCRPC patients treated with cabazitaxel that not only a favorable baseline NLR (<3) but also a conversion to a favorable NLR at 12 weeks was a significant predictive factor of improved OS (HR 0.66, 95% CI 0.51–0.85, p = 0.001) [18]. These results are consistent with Lalani et al. who found that in patients treated with multiple regimens of an immune checkpoint blockade for metastatic renal cell cancer a higher NLR at 6 weeks was a significantly stronger predictor of all analyzed outcomes than the baseline value. More precisely, a change of ≥25% from baseline to 6 weeks was associated with worse outcomes compared to a decrease in NLR by ≥25% [9]. The same results were obtained, in a cohort of more than a thousand patients with lung cancer. Mezquita et al. explored the derived neutrophil to leucocyte ratio (dNLR) predictive impact in patients treated with immune checkpoint inhibitors for non-small cell lung cancer. Again, the change in dNLR was prognostic [10].
In the docetaxel-treated group, we found that the NLR at baseline and at 12 weeks was not associated with OS. Even if our findings are consistent with Sumbul et al. and Linton et al. [19,20], many other studies have shown, for different NLR cut-offs, that the NLR ratio was associated with longer OS in chemotherapy-treated patients [3,18,21,22,23]. This difference in our patients could be explained by a lack of statistical power and the fact that prostate cancer is considered an immunologically cold cancer [24].
Moreover, we found that during treatment with 223Ra the ANC and ALC tended to decrease. This could help explain the mechanism of the relationship between increased NLR and poor OS in 223Ra-treated patients. Lymphocytes have been known to play a key role in maintaining tissue homeostasis, and therefore prevent uncontrolled tumor cells growth. Among various cancer defense mechanisms, cytotoxic CD8 T-cells are responsible for mediating cancer cells destruction, while T-regs cells allow for a control of the inflammatory environment [25]. Ionizing radiation agents have been used for years in cancer treatment for their direct cytocidal effect on tumor cells, but they are also known to cause inflammation and modulate the host’s immune response to cancer [25,26]. Several studies have shown the hematological toxicity of 223Ra [11,27]. Furthermore, Pike et al. have shown that lymphopenia induced by prolonged extracranial radiotherapy treatment can decrease OS in patients receiving immune checkpoint inhibitors [28]. High-linear energy transfer (LET) particles such as 223Ra are commonly used for the treatment of castration-resistant prostate cancer (CRPC) patients with symptomatic bone metastases. It has been shown that alpha-emitting atoms from a 223Ra source induce a systemic inflammatory response [29]. This effect could potentially at the same time decrease its efficacy through its lymphotoxic effect, as has been shown in our study. In the ALSYMPCA trial, 223Ra caused any grade thrombocytopenia in 12% and neutropenia in 5% of patients [11].
Our study is not without some limitations. Although there was no significant baseline difference for other clinical factors between the low and high NLR groups for each treatment independently, the 223Ra-treated patients and docetaxel-treated patients were not comparable. Several patients in the 223Ra group had received docetaxel before treatment with 223Ra and 223Ra was in general given as the last or before-last treatment line. It’s also important to underline the retrospective nature of our study. Thus, we did not have the data on the use of corticoids or hematopoietic growth factor in the chemotherapy group which could change neutrophil count and change the NLR. Finally, we use a cut off of 5 to determine low and high NLR. However, there is no consensus regarding the optimal cut off. The Neutrophil to Lymphocyte ratio remains a low-cost and minimally invasive easily measured biomarker. It can certainly be used as risk stratification for metastatic PCa in clinical trials or daily practice. More research into risk stratification using the NLR variable as a biomarker is needed to find the optimal cut off.
5. Conclusions
The NLR at baseline and at 12 weeks of treatment appears to have prognostic value for patients with metastatic prostate cancer patients treated with 223Ra but this was not found to be the case for patients treated with docetaxel. Statistically, significantly better OS was observed in patients with a NLR ≤ 5 both at baseline and at 12 weeks. A high NLR at baseline and at 12 weeks of therapy is associated with poor OS as well as those patients with NLR ≤ 5 at baseline that converted to a NLR > 5 at 12 weeks. With its low-cost and the fact that it is easily measurable, we believe that the change in NLR during treatment with 223Ra has the potential as a prognostic biomarker to guide clinical decisions. Further validating studies are needed to confirm these results as well as to assess the factors that influence NLR in metastatic prostate cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14194606/s1, Table S1: Adjustment variables in multivariate Cox proportional hazards regression model predicting overall survival in Radium-223 treated patients; Table S2: Adjustment variables in multivariate Cox proportional hazards regression model predicting overall survival in Docetaxel treated patients. Figure S1: Relation between absolute change of Neutrophil to lymphocyte ratio and Hazard Ratio for overall survival for Radium-223 treated patients and Docetaxel treated patients. Figure S2: Evolution of absolute neutrophil count and absolute lymphocyte count during therapy with Radium-223 treated patients. Figure S3: Evolution of absolute neutrophil count and absolute lymphocyte count during therapy in docetaxel treated patients.
Author Contributions
Conceptualization, K.K., D.T., F.S. and E.A.; methodology, K.K., D.T., F.S. and E.A.; software, E.A.; validation, K.K., D.T., F.S. and E.A.; formal analysis, K.K., D.T. and E.A.; investigation, K.K. and D.T.; resources, D.T. and F.S.; data curation, D.T.; writing—original draft preparation, K.K., J.D. and D.T.; writing—review and editing, K.K., D.T., F.S., C.C.-D., G.D., L.B. and E.A.; visualization, K.K., D.T., F.S. and E.A. supervision, D.T. and F.S.; project administration, D.T. and F.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The current study was conducted in accordance with the Declaration of Helsinki and approved by Ethics Committee of Centre Hospitalier de l’Université de Montréal (CHUM) on date 17 March 2022 (CER 21.328).
Informed Consent Statement
Patient consent was waived because our study was retrospective, and the data were processed anonymously.
Data Availability Statement
Data presented is contained within the article; for additional information, data sets are also available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rawla P. Epidemiology of Prostate Cancer. World J. Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaeffer E., Srinivas S., Antonarakis E.S., Armstrong A.J., Bekelman J.E., Cheng H., D’Amico A.V., Davis B.J., Desai N., Dorff T., et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021;19:134–143. doi: 10.6004/jnccn.2021.0008. [DOI] [PubMed] [Google Scholar]
- 3.de Wit R., Wülfing C., Castellano D., Kramer G., Eymard J.-C., Sternberg C.N., Fizazi K., Tombal B., Bamias A., Carles J., et al. Baseline Neutrophil-to-Lymphocyte Ratio as a Predictive and Prognostic Biomarker in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Cabazitaxel versus Abiraterone or Enzalutamide in the CARD Study. ESMO Open. 2021;6:100241. doi: 10.1016/j.esmoop.2021.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Templeton A.J., McNamara M.G., Šeruga B., Vera-Badillo F.E., Aneja P., Ocaña A., Leibowitz-Amit R., Sonpavde G., Knox J.J., Tran B., et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 6.Cupp M.A., Cariolou M., Tzoulaki I., Aune D., Evangelou E., Berlanga-Taylor A.J. Neutrophil to Lymphocyte Ratio and Cancer Prognosis: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. BMC Med. 2020;18:360. doi: 10.1186/s12916-020-01817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mjaess G., Chebel R., Karam A., Moussa I., Pretot D., Abi Tayeh G., Sarkis J., Semaan A., Peltier A., Aoun F., et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio (NLR) in Urological Tumors: An Umbrella Review of Evidence from Systematic Reviews and Meta-Analyses. Acta Oncol. 2021;60:704–713. doi: 10.1080/0284186X.2021.1886323. [DOI] [PubMed] [Google Scholar]
- 8.Guo J., Fang J., Huang X., Liu Y., Yuan Y., Zhang X., Zou C., Xiao K., Wang J. Prognostic Role of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Prostate Cancer: A Meta-Analysis of Results from Multivariate Analysis. Int. J. Surg. 2018;60:216–223. doi: 10.1016/j.ijsu.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Lalani A.-K.A., Xie W., Martini D.J., Steinharter J.A., Norton C.K., Krajewski K.M., Duquette A., Bossé D., Bellmunt J., Van Allen E.M., et al. Change in Neutrophil-to-Lymphocyte Ratio (NLR) in Response to Immune Checkpoint Blockade for Metastatic Renal Cell Carcinoma. J. Immunother. Cancer. 2018;6:5. doi: 10.1186/s40425-018-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mezquita L., Preeshagul I., Auclin E., Saravia D., Hendriks L., Rizvi H., Park W., Nadal E., Martin-Romano P., Ruffinelli J.C., et al. Predicting Immunotherapy Outcomes under Therapy in Patients with Advanced NSCLC Using DNLR and Its Early Dynamics. Eur. J. Cancer. 2021;151:211–220. doi: 10.1016/j.ejca.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Parker C., Nilsson S., Heinrich D., Helle S.I., O’Sullivan J.M., Fosså S.D., Chodacki A., Wiechno P., Logue J., Seke M., et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 12.Pienta K.J. Preclinical Mechanisms of Action of Docetaxel and Docetaxel Combinations in Prostate Cancer. Semin. Oncol. 2001;28:3–7. doi: 10.1016/S0093-7754(01)90148-4. [DOI] [PubMed] [Google Scholar]
- 13.Peng L., Wang Y., Liu F., Qiu X., Zhang X., Fang C., Qian X., Li Y. Peripheral Blood Markers Predictive of Outcome and Immune-Related Adverse Events in Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors. Cancer Immunol. Immunother. 2020;69:1813–1822. doi: 10.1007/s00262-020-02585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capone M., Giannarelli D., Mallardo D., Madonna G., Festino L., Grimaldi A.M., Vanella V., Simeone E., Paone M., Palmieri G., et al. Baseline Neutrophil-to-Lymphocyte Ratio (NLR) and Derived NLR Could Predict Overall Survival in Patients with Advanced Melanoma Treated with Nivolumab. J. Immunother. Cancer. 2018;6:74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meisel A., Parker C., Kühne R., Sartor O., Stenner-Liewen F. 637P The Prognostic Value of the Baseline Neutrophil-to-Lymphocyte Ratio (NLR) in Patients with Metastatic Castration-Resistant Prostate Cancer (MCRPC) Receiving Radium-223 (Ra-223): A Post-Hoc Analysis of the ALSYMPCA Phase-III Trial. Ann. Oncol. 2020;31:S524–S525. doi: 10.1016/j.annonc.2020.08.896. [DOI] [Google Scholar]
- 16.Bauckneht M., Rebuzzi S.E., Signori A., Frantellizzi V., Murianni V., Lodi Rizzini E., Mascia M., Lavelli V., Donegani M.I., Ponzano M., et al. The Prognostic Power of Inflammatory Indices and Clinical Factors in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223 (BIO-Ra Study) Eur. J. Nucl. Med. Mol. Imaging. 2022;49:1063–1074. doi: 10.1007/s00259-021-05550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauckneht M., Rebuzzi S.E., Ponzano M., Borea R., Signori A., Frantellizzi V., Lodi Rizzini E., Mascia M., Lavelli V., Miceli A., et al. Prognostic Value of the BIO-Ra Score in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Radium-223 after the European Medicines Agency Restricted Use: Secondary Investigations of the Multicentric BIO-Ra Study. Cancers. 2022;14:1744. doi: 10.3390/cancers14071744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorente D., Mateo J., Templeton A.J., Zafeiriou Z., Bianchini D., Ferraldeschi R., Bahl A., Shen L., Su Z., Sartor O., et al. Baseline Neutrophil–Lymphocyte Ratio (NLR) Is Associated with Survival and Response to Treatment with Second-Line Chemotherapy for Advanced Prostate Cancer Independent of Baseline Steroid Use. Ann. Oncol. 2015;26:750–755. doi: 10.1093/annonc/mdu587. [DOI] [PubMed] [Google Scholar]
- 19.Sümbül A.T., Sezer A., Abalı H., Köse F., Gültepe I., Mertsoylu H., Muallaoğlu S., Özyılkan Ö. Neutrophil-to-Lymphocyte Ratio Predicts PSA Response, but Not Outcomes in Patients with Castration-Resistant Prostate Cancer Treated with Docetaxel. Int. Urol. Nephrol. 2014;46:1531–1535. doi: 10.1007/s11255-014-0664-7. [DOI] [PubMed] [Google Scholar]
- 20.Linton A., Pond G., Clarke S., Vardy J., Galsky M., Sonpavde G. Glasgow Prognostic Score as a Prognostic Factor in Metastatic Castration-Resistant Prostate Cancer Treated with Docetaxel-Based Chemotherapy. Clin. Genitourin. Cancer. 2013;11:423–430. doi: 10.1016/j.clgc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Z.-G., Liao S.-G. Baseline Neutrophil-Lymphocyte Ratio Is Associated with Outcomes in Patients with Castration-Resistant Prostate Cancer Treated with Docetaxel in South China. Medicine. 2021;100:e27361. doi: 10.1097/MD.0000000000027361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Soest R.J., Templeton A.J., Vera-Badillo F.E., Mercier F., Sonpavde G., Amir E., Tombal B., Rosenthal M., Eisenberger M.A., Tannock I.F., et al. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Men with Metastatic Castration-Resistant Prostate Cancer Receiving First-Line Chemotherapy: Data from Two Randomized Phase III Trials. Ann. Oncol. 2015;26:743–749. doi: 10.1093/annonc/mdu569. [DOI] [PubMed] [Google Scholar]
- 23.Buttigliero C., Pisano C., Tucci M., Vignani F., Bertaglia V., Iaconis D., Guglielmini P., Numico G., Scagliotti G.V., Di Maio M. Prognostic Impact of Pretreatment Neutrophil-to-Lymphocyte Ratio in Castration-Resistant Prostate Cancer Patients Treated with First-Line Docetaxel. Acta Oncol. 2017;56:555–562. doi: 10.1080/0284186X.2016.1260772. [DOI] [PubMed] [Google Scholar]
- 24.Runcie K.D., Dallos M.C. Prostate Cancer Immunotherapy—Finally in from the Cold? Curr. Oncol. Rep. 2021;23:88. doi: 10.1007/s11912-021-01084-0. [DOI] [PubMed] [Google Scholar]
- 25.Demaria S., Formenti S.C. Role of T Lymphocytes in Tumor Response to Radiotherapy. Front. Oncol. 2012;2:95. doi: 10.3389/fonc.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride W.H., Chiang C.-S., Olson J.L., Wang C.-C., Hong J.-H., Pajonk F., Dougherty G.J., Iwamoto K.S., Pervan M., Liao Y.-P. A Sense of Danger from Radiation. Radiat. Res. 2004;162:1–19. doi: 10.1667/RR3196. [DOI] [PubMed] [Google Scholar]
- 27.Turner P.G., Jain S., Cole A., Grey A., Mitchell D., Prise K.M., Hounsell A.R., McGarry C.K., Biggart S., O’Sullivan J.M. Toxicity and Efficacy of Concurrent Androgen Deprivation Therapy, Pelvic Radiotherapy, and Radium-223 in Patients with de Novo Metastatic Hormone-Sensitive Prostate Cancer. Clin. Cancer Res. 2021;27:4549–4556. doi: 10.1158/1078-0432.CCR-21-0685. [DOI] [PubMed] [Google Scholar]
- 28.Pike L.R.G., Bang A., Mahal B.A., Taylor A., Krishnan M., Spektor A., Cagney D.N., Aizer A.A., Alexander B.M., Rahma O., et al. The Impact of Radiation Therapy on Lymphocyte Count and Survival in Metastatic Cancer Patients Receiving PD-1 Immune Checkpoint Inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 2019;103:142–151. doi: 10.1016/j.ijrobp.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Keisari Y., Kelson I. The Potentiation of Anti-Tumor Immunity by Tumor Abolition with Alpha Particles, Protons, or Carbon Ion Radiation and Its Enforcement by Combination with Immunoadjuvants or Inhibitors of Immune Suppressor Cells and Checkpoint Molecules. Cells. 2021;10:228. doi: 10.3390/cells10020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented is contained within the article; for additional information, data sets are also available upon request from the corresponding author.