Abstract
We have previously described the cloning and sequencing of a gene portion coding for the terminal part of a 34-kDa protein of Mycobacterium avium subsp. paratuberculosis, the etiological agent of Johne’s disease (P. Gilot, M. De Kesel, L. Machtelinckx, M. Coene, and C. Cocito, J. Bacteriol. 175:4930–4935, 1993). The recombinant polypeptide (a362) carries species-specific B-cell epitopes which do not cross-react with other mycobacterial pathogens (M. De Kesel, P. Gilot, M.-C. Misonne, M. Coene, and C. Cocito, J. Clin. Microbiol. 31:947–954, 1993). The present work describes the preparation of polyclonal and monoclonal antibodies directed against a362 and the use of these immunoglobulins for histopathological diagnosis of Johne’s disease. The new immunohistological procedures herewith detailed proved to be able to identify M. avium subsp. paratuberculosis antigens in the intestinal tissues and lymph nodes of cattle affected by either the paucibacillary or pluribacillary form of the disease. They yielded negative responses not only with healthy animals but also with those affected by tuberculosis (Mycobacterium bovis). Both immunohistological procedures proved to be as sensitive as or more sensitive than Ziehl-Neelsen staining and, in addition, to be endowed with species specificity.
Paratuberculosis (Johne’s disease) is the infectious process with the highest epidemiologic incidence in veterinary medicine. It is characterized by chronic enteritis, with fecal shedding of large numbers of mycobacteria (Mycobacterium avium subsp. paratuberculosis, the etiological agent of this disease) and rapid propagation to neighboring animals. Paratuberculosis develops through three clinical stages, differently expressing bacterial excretion and clinical symptoms. In the first stage (asymptomatic with undetectable excretion), the infectious process develops without appreciable shedding of bacteria. In the second stage, (the asymptomatic excretory phase), microbial shedding and granulomatous reactions in the intestinal mucosa steadily increase. The final stage is characterized by the appearance of clinical manifestations of increasing severity (intractable chronic diarrhea, decreased milk production, emaciation, and anorexia) (10, 12, 13).
It is only through the convergence of different approaches that the control of Johne’s disease can be envisaged and its successful eradication planned. Different indirect procedures exploring the immunoglobulin titers or cellular immune status of infected cattle are now available (6, 8, 14, 17, 21, 30, 38–40, 45, 49). However, direct characterization of M. avium subsp. paratuberculosis in biopsy materials or in fecal matter is presently unattainable. Microscopic examination after Ziehl-Neelsen staining does not allow distinction among different mycobacterial species (37, 41). Culture, though more sensitive, is a slow (2 months for positivity) and exacting procedure because of the long generation time (about 36 h, depending on growth conditions) of M. avium subsp. paratuberculosis (26, 29, 36, 41). Radiometric fecal culture (16) is reported to be faster and more sensitive than conventional fecal culture, but it requires expensive equipment and the use of radioisotopes. Amplification and detection of specific sequences (IS900, F57) proved to be more sensitive (15, 23, 24, 34, 35, 40, 42, 43). An alternative strategy would be the development of immunohistochemical procedures (20, 25, 41, 48) based on species-specific immunoglobulins.
We previously identified an immunodominant protein (P34) of M. avium subsp. paratuberculosis carrying species-specific B-cell epitopes (18). This protein is a component of A36, the major antigenic complex of M. avium subsp. paratuberculosis. A portion of the gene coding for P34 was cloned, and the expression product (a362) was a 13.6-kDa polypeptide containing B-cell epitopes present in the 9 M. avium subsp. paratuberculosis strains tested but not in 16 other mycobacterial species, including many strains of the M. avium-M. intracellulare-M. scrofulaceum group, M. bovis, M. tuberculosis, M. phlei, M. gordonae, M. smegmatis, and M. fortuitum (19, 22). This recombinant polypeptide, which represents the carboxyl-terminal portion of P34, was used as a reagent for an enzyme-linked immunosorbent assay (ELISA) (45). This test proved to be able to detect paratuberculous cattle at all stages of the disease (45).
The aim of the present investigation was to develop species-specific immunohistological assays for detection of M. avium subsp. paratuberculosis in biopsy materials. For this purpose, polyclonal and monoclonal antibodies directed against a362 have been produced and characterized; they were then used to identify this specific mycobacterial antigen in the tissues of paratuberculous cattle (infected by M. avium subsp. paratuberculosis). Tissues of healthy and tuberculous (M. bovis-infected) animals were used as negative controls. The present work provides histopathologists with new species-specific immunodiagnostic procedures for Johne’s disease.
MATERIALS AND METHODS
Microorganisms.
The following mycobacterial strains were used: M. avium subsp. paratuberculosis 2E (from F. Saxegaard, National Veterinary Institute, Oslo, Norway) and M. bovis BCG (from M. Weckx, Pasteur Institute, Brussels, Belgium).
Tissue samples.
Sixteen biopsy samples of intestine and mesenteric lymph nodes from paratuberculous cows were obtained from slaughterhouses in Belgium and Florida. Biopsy samples of prescapular lymph nodes from three paratuberculous cows were taken as controls.
Fourteen samples of tuberculous cow tissues (8 of pulmonary lymph nodes, 3 of mesenteric lymph nodes, and 3 segments of the ileal tract), 24 samples of healthy cow tissues (8 of mesenteric lymph nodes, 8 segments of ileum, and 8 of colon), and 1 specimen of mesenteric lymph nodes from a horse with M. avium infection were also analyzed. Samples were Formol fixed and paraffin embedded, according to current histological techniques.
Clinical and microbiological analysis.
Diagnoses of health and disease were made by conventional medical criteria (diarrhea, decreased milk production, emaciation, and anorexia are symptoms of paratuberculosis). Clinical diagnoses were confirmed by microbiological identification of the etiological agent. The Ziehl-Neelsen reagent was used to stain mycobacteria in tissues.
Preparation of the mycobacterial antigen a362.
The recombinant polypeptide a362 (a patented product of Innogenetics, Ghent, Belgium) was synthesized as a fusion protein with the first 25 amino acids of mouse tumor necrosis factor alpha (TNF-α) and was purified on a metal chelate adsorbent, as described previously (22).
Preparation of anti-a362 polyclonal rabbit serum.
Recombinant a362 polypeptide (500 μg) emulsified with complete Freund’s adjuvant was subcutaneously inoculated into rabbits twice, at a 2-week interval. The rabbit serum was titered by the a362 ELISA and revealed with peroxidase-labelled rat anti-rabbit immunoglobulin (Ig) monoclonal antibodies (LO-RG-1).
Preparation of anti-a362 monoclonal rat antibodies.
LOU/C rats (2) were immunized with the recombinant a362 polypeptide (50 μg) by the footpad route (1) three times at 2-week intervals. Popliteal, mesenteric, and cardiac lymph nodes were harvested, and lymphocytes were fused with the rat myeloma cell line IR983F (4). Anti-a362 reactivity of hybridoma supernatants was assayed by ELISA on TNF-α- and a362-coated plates. Positive hybridomas reacting specifically with a362 and not with TNF-α (the recombinant polypeptide being the fusion product of the two polypeptides) were used to inoculate intraperitoneally congenic LOU/C rats. Hybridoma ascitic fluids were purified by affinity chromatography (5), and isotyping of the resulting preparation [LO-ptb(a362)-2] was done by ELISA as previously described (3, 28).
ELISA.
Multiwell microtiter plates (high binding capacity) (Microwell module F16; Nunc, Roskilde, Denmark) were coated overnight at 4°C with either the recombinant a362 polypeptide or TNF-α (0.5 μg in 100 μl of 0.05 M sodium carbonate buffer [pH 9.6] per well). The wells were rinsed with PBST buffer (0.15 M NaCl, 0.005% Tween 80, 0.02 M sodium phosphate buffer [pH 7.2]) and saturated for 1 h at 37°C with bovine serum albumin (BSA) (0.1% BSA in 0.15 M NaCl). After a rinse, 100 μl of hybridoma supernatants per well were added for 1 h at 37°C. After a wash with PBST, peroxidase-labelled mouse anti-rat κ light-chain monoclonal antibodies (MARK-1) were added (100 μl of a 1/50 dilution in PBST containing 0.1% albumin per well for 1 h at 37°C). Excess reagent was removed by a wash with PBST, and peroxidase reagent (100 μl of 17 mM sodium citrate buffer [pH 6.3] containing 0.2% O-phenylenediamine and 0.015% hydrogen peroxide per well) was added for 30 min at 37°C in the dark. The reaction was stopped by the addition of 100 μl of 2 M H2SO4 per well, and samples were spectrophotometrically measured (492 nm) in a colorimetric plate reader (SLT 210; Kontron, Watford, United Kingdom).
Immunolocalization with anti-a362 polyclonal antiserum.
Dewaxed and rehydrated tissue sections were submitted to endogenous peroxidase inactivation (3% H2O2 for 20 min). After a wash with phosphate-buffered saline (PBS), sections were saturated with PBS supplemented with 0.02% goat serum and 1% BSA (20 min at 37°C). After incubation with anti-a362 polyclonal serum (1/500 dilution in PBS supplemented with 0.1% albumin for 1 h at 37°C), antigen-antibody complexes were detected with the anti-rabbit ABC Perox kit (Vector Labs, Burlingame, United Kingdom). Peroxidase activity was revealed by the benzidine-peroxide substrate DAB (3-3′-diaminobenzidine tetrahydrochloride [Aldrich] and H2O2). Sections were counterstained with Mayer’s hematoxylin, rinsed, and mounted (DPX 36029 4H; BDH, Poole, England) for histological examination. The following negative control experiments were performed in parallel: (i) replacement of the antiserum by PBS, (ii) use of a nonimmune rabbit serum, and (iii) immunoneutralization of the anti-a362 serum (overnight incubation with 0.8 mg of a362 per ml of diluted serum) before immunostaining.
Immunolocalization with anti-a362 monoclonal antibodies.
Dewaxed and rehydrated tissue sections, after endogenous peroxidase inactivation (see above), were incubated with anti-a362 monoclonal antibodies [300 μl of a solution containing 70 μg of LO-ptb(a362)-2 per ml of PBS supplemented with 1% (wt/vol) BSA for 70 min at room temperature]. After three washes with PBS-albumin, sections were incubated for 60 min with a cocktail of biotin-labelled mouse anti-rat Ig monoclonal antibodies (20 μg [each] of anti-IgG2a [MARG-2a-1] and anti-κ chain [MARK-1] per ml). Washed sections were incubated in the dark with streptavidin-peroxidase reagent diluted at 1/250 (S-5512; Sigma, St. Louis, Mo.). After washes, peroxidase activity was revealed by AEC substrate addition (3-amino-9-ethylcarbazol [K0697; Dako, Copenhagen Denmark] mixed with 0.1% H2O2 for 10 min in the dark, leading to formation of a red precipitate). Sections rinsed with water were counterstained with Mayer’s hematoxylin, rinsed, and mounted with an aqueous medium (Faramount S 3025; Dako). The following negative control experiments were performed in parallel: (i) omission of the first antibody or (ii) its replacement by a mouse IgG2a monoclonal antibody preparation recognizing baboon IgM (LOBM-2) and (iii) immunoneutralization of LO-ptb(a362)-2 with a362 (see above) before immunostaining.
Western blot analysis.
Mycobacterial soluble sonic extracts (30 μg protein/sample) were fractionated by electrophoresis on 12% polyacrylamide gels in the presence of sodium dodecyl sulfate, as previously described (18), in parallel with molecular weight markers (Sigma). Fractionated components were electrophoretically transferred to nitrocellulose membranes (BAA85; Macherey-Nagel, Dueren, Germany) in a transblot unit (217 Multiphor 2; LKB, Bromma, Sweden). Membranes saturated with TBST buffer (0.5 M NaCl and 0.023 M Tris-HCl [pH 7.5] containing 1% [wt/vol] gelatin and 0.05% Tween 20) were incubated either with anti-a362 polyclonal serum (1/500 dilution in TBST for 1 h at 37°C) or with LO-ptb(a362)-2 (1/50 dilution in TBST for 14 h at 37°C). Bound polyclonal Ig were identified with peroxidase-labelled anti-rabbit Ig swine serum (Dako), and bound monoclonal antibodies were revealed with peroxidase-labelled anti-rat Ig rabbit serum (Dako).
Statistical analysis.
Agreement between Ziehl-Neelsen staining and immunohistological methods was evaluated by using Cohen’s K coefficient; a value lower than 75% was considered indicative of low agreement between methods (Fleiss). Discordance between methods was assessed by using the binomial test of symmetry in disagreement; a value lower than 0.05 was considered significant.
RESULTS
Preparation and characterization of anti-a362 monoclonal antibodies.
Seven rat hybridomas reacting solely with a362 and not with TNF-α (the recombinant polypeptide preparation is an a362–TNF-α fusion product) were isolated. One of them [LO-ptb(a362)-2] was selected because of its strong reactivity with the antigen and its stability; it was then used as a reagent for the entire work. By isotyping, this hybridoma was found to secrete anti-a362 IgG2a.
Evaluation of the specificity of polyclonal and monoclonal Ig.
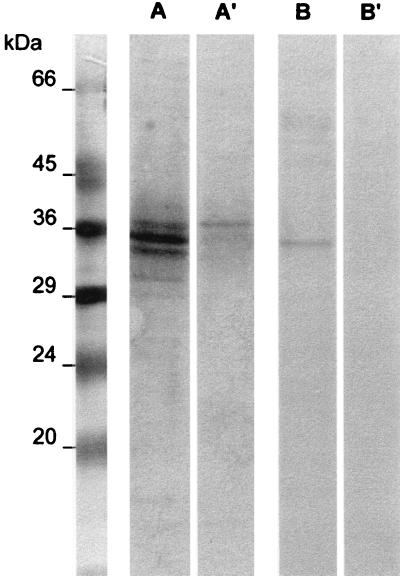
The anti-a362 reactivity of the LO-ptb(a362)-2 monoclonal antibodies and of the anti-a362 polyclonal serum was tested by Western blot analysis on electrophoresed soluble components of M. avium subsp. paratuberculosis and M. bovis BCG. Both Ig preparations recognized a major component of 34 kDa from M. avium subsp. paratuberculosis (Fig. 1). A minor band of lower molecular weight was invariably present in the preparations tested with the polyclonal antiserum. In M. bovis blots, a 38-kDa component was weakly stained by the polyclonal antiserum but not by the monoclonal antibodies.
FIG. 1.
Evaluation of antibody specificity by Western blotting. Soluble sonic extracts (50 μg of protein/sample) of M. paratuberculosis (lanes A and B) and M. bovis (lanes A′ and B′) were fractionated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and reacted with either anti-a362 polyclonal serum (lanes A and A′) or anti-a362 monoclonal antibodies (lanes B and B′). Antigen-antibody complexes were revealed by peroxidase-labelled secondary antibodies.
Immunohistological analysis of tissues from infected animals.
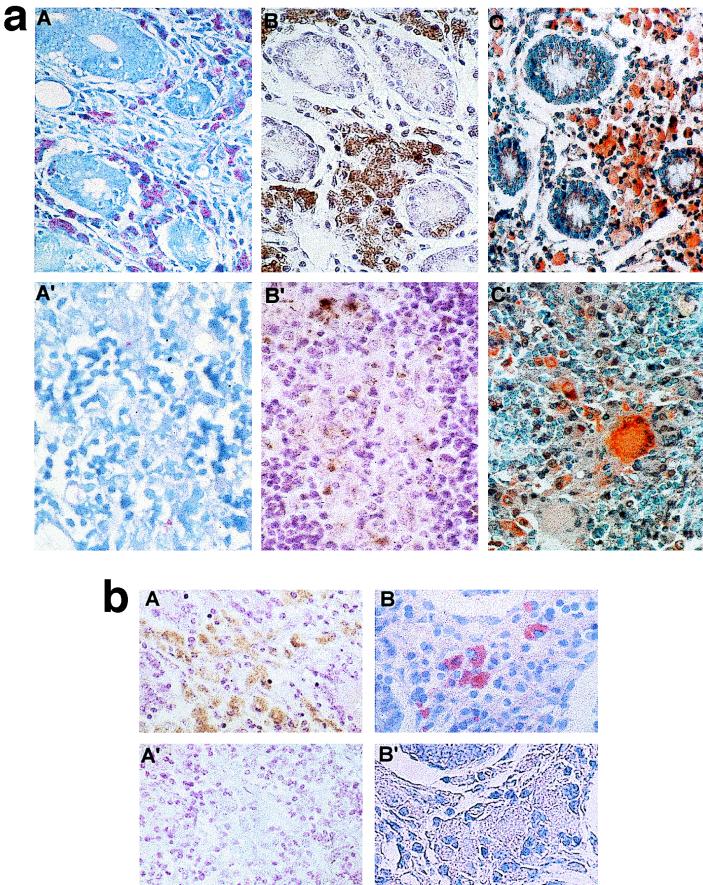
Paratuberculous cattle have been classified in pluribacillary and paucibacillary forms according to clinical criteria, types of lesions, and mycobacterial loads in intestinal or mesenteric lymph node biopsies (Table 1). In the pluribacillary form, the lamina propria of the intestinal mucosa appeared to be thickened and to contain numerous macrophage-infiltrated granulomas. Multinucleated giant cells were also observed in reduced numbers, mainly in the submucosa. Similar lesions were seen in mesenteric lymph nodes. In the paucibacillary form, only focal lesions were detected. After Ziehl-Neelsen staining, many macrophages contained clumps of reddish rods (Fig. 2a, panel A). Positively stained macrophages were less numerous or absent in the tissues of the paucibacillary cases (Fig. 2a, panel A′), whereas giant cells were invariably unstained.
TABLE 1.
Comparative analysis of paratuberculous tissue sections by Ziehl-Neelsen staining and a362-based immunohistological procedures
| Type of specimen | Clinical form | Result ofa:
|
||
|---|---|---|---|---|
| ZiehlNeelsen staining | Polyclonal procedure | Monoclonal procedure | ||
| Mesenteric lymph node | Pluribacillary | + | + | + |
| Ileum | Pluribacillary | + | + | + |
| Ileum | Pluribacillary | + | + | + |
| Ileum | Pluribacillary | + | + | + |
| Ileum | Pluribacillary | + | + | + |
| Colon | Pluribacillary | + | + | + |
| Mesenteric lymph node | Paucibacillary | + | + | + |
| Mesenteric lymph node | Paucibacillary | − | + | + |
| Mesenteric lymph node | Paucibacillary | − | − | + |
| Mesenteric lymph node | Paucibacillary | − | − | − |
| Ileum | Paucibacillary | − | + | + |
| Ileum | Paucibacillary | + | + | + |
| Ileum | Paucibacillary | − | + | + |
| Ileum | Paucibacillary | − | − | + |
| Ileum | Paucibacillary | − | − | − |
| Colon | Paucibacillary | − | + | + |
Agreement between methods (Cohen’s K coefficient): K1 (Ziehl-Neelsen and the polyclonal procedure), 44%; K2 (Ziehl-Neelsen and the monoclonal procedure), 25%; K3 (polyclonal and monoclonal procedures), 60%. Discordance between methods (binomial test): P1 (Ziehl-Neelsen and the polyclonal procedure), 0.062; P2 (Ziehl-Neelsen and the monoclonal procedure), 0.016; P3 (polyclonal and monoclonal procedures), 0.25.
FIG. 2.
(a) Ziehl-Neelsen staining and immunohistological detection of M. paratuberculosis in infected tissues. Sections of the ileocecal mucosa from a pluribacillary form of paratuberculosis (A, B, and C) and sections of a mesenteric lymph node from a paucibacillary form of paratuberculosis (A′, B′, and C′) were subjected to the following histological procedures: Ziehl-Neelsen staining (A and A′) and immunohistochemical detection with anti-a362 polyclonal serum (B and B′) and anti-a362 monoclonal Ig (C and C′). Note that the number of positive cells is higher in panel B′ than in panel A′. The chromogen used in panels B and B′ was DAB (yielding brown staining in numerous macrophages), whereas the chromogen in panels C and C′ was AEC (yielding red staining). Note the presence in panel C′ of two multinucleated giant cells, one positively stained and the other negative. (b) Specificity testing of immunohistological procedures. Sections of the ileocecal mucosa from a paratuberculous cow were incubated with either anti-a362 polyclonal serum (A and A′) or anti-a362 monoclonal Ig (B and B′), either before (A and B) or after (A′ and B′) immunoneutralization of anti-a362 antibodies with a preparation of the a362 recombinant polypeptide. Note the negative reaction occurring after immunoneutralization.
Clumps of brownish rods were stained in macrophage cytoplasm by the polyclonal anti-a362 antiserum (Fig. 2a, panels B and B′). To test the sensitivity of the polyclonal immunostaining procedure, serial sections of a paucibacillary sample were comparatively stained with Ziehl-Neelsen stain and anti-a362 serum. Within the same granulomas, immunolabelling was stronger than Ziehl-Neelsen staining, allowing better identification of infected macrophages. Thus, for instance, four biopsy samples were found to be positive by the polyclonal procedure and negative by Ziehl-Neelsen staining (Table 1).
The results obtained with the monoclonal anti-a362 antibodies were similar to those with the polyclonal antiserum in both intestinal tissues and lymph nodes (Fig. 2a, panels C and C′). Moreover, our immunohistological procedures proved to be significantly more sensitive than chemical staining, as indicated by Cohen’s agreement (K1, 44%; K2, 25%) and the binomial test of symmetry in disagreement (P1, 0.062 [marginally significant]; P2, 0.016). Indeed, the a362 antigen in two supplementary cows was immunologically detected (Table 1), and giant cells not stained by Ziehl-Neelsen stain were labelled by the anti-a362 monoclonal antibodies (Fig. 2a, panel C′).
Specificity of immunostaining with anti-a362 polyclonal serum and monoclonal antibodies was demonstrated in two ways: (i) absence of labelling upon replacement of the first antibody by PBS or by unrelated antibodies and (ii) preabsorption of polyclonal antiserum and monoclonal antibodies on a362 preparations (Fig. 2b). In the intestinal biopsy samples from some infected cows, a low background reaction was observed on collagen and smooth muscular cells. Similar reactions were obtained with nonimmune rabbit antiserum and anti-a362 polyclonal serum. This was considered a nonspecific staining artifact, and in fact it did not occur with monoclonal antibodies.
Microbiological specificity was demonstrated by the absence of labelling in 24 biopsy samples from healthy cows, 14 biopsy samples from tuberculous cows, and the prescapular lymph nodes of three paratuberculous cows. However, in two cows with tuberculosis, few macrophages and giant cells were positively stained with monoclonal antibodies. Also, lesions observed in biopsies from an M. avium-infected horse contained macrophages positively labelled with both the polyclonal anti-a362 serum and the monoclonal anti-a362 antibodies.
DISCUSSION
Because of the extensive shedding of mycobacterial organisms in advanced stages of Johne’s disease and the high infectivity of the etiological agent, early detection of infected animals is essential to avoid spreading of the disease. However, direct identification of M. avium subsp. paratuberculosis in biopsy samples by conventional methods is difficult (29, 36, 37, 41). In fact, the Ziehl-Neelsen reagent, which stains mycobacteria regardless of species, is unable to distinguish M. avium subsp. paratuberculosis not only from M. bovis but also from ubiquitous mycobacteria present in soil, water, and the intestinal tract. Moreover, only mycobacteria with intact walls are stained by the Ziehl-Neelsen reagent. These problems were recently addressed by PCR amplification of nucleic acid sequences specific for single mycobacterial species (15, 23, 24, 34, 35, 40, 42, 43, 47). A serious handicap of PCR, an otherwise good diagnostic procedure, is the difficulty of mycobacterial DNA purification owing to the peculiar structure of the cell wall of these microorganisms. Moreover, in infected animals, mycobacteria have an intracellular location, and several tissue components inhibiting PCR are difficult to remove prior to the amplification step.
The chosen strategy relies on previous cloning and characterization of a specific polypeptide sequence (a362) recognized by the immune system of the host (19, 22). In our work, polyclonal and monoclonal Ig directed against the recombinant polypeptide a362 were purified and used as reagents for immunohistological assays. In Western blot analysis of M. avium subsp. paratuberculosis components, the polyclonal antiserum was able to detect a major band of the corresponding (34-kDa) molecular weight. However, a minor band of 31 kDa was always present (see Results). The latter could be either a degradation product or a cross-reacting smaller protein. On the contrary, a single band of 34 kDa was stained by the anti-a362 monoclonal antibodies. Unlike PCR, the immunohistological approach allows the intracellular localization of infecting M. avium subsp. paratuberculosis organisms in tissues sections. In addition, this technique is not restricted to fresh tissues (some of our samples had been fixed in Formol for months or years before being successfully processed), hence the possibility of retrospective analysis.
The first immunohistological method described in this paper is based on anti-a362 polyclonal antiserum, while the second employs one of the seven isolated monoclonal antibodies directed against a362, which was chosen for its good reactivity and stability. According to our data, these procedures were able to identify the etiological agent in the majority of tissues from animals with pluribacillary and paucibacillary forms of the disease. Some cows with negative acid-fast staining were immunologically identified as paratuberculous animals (Table 1). Such apparently discordant results can be accounted for by the fact that the acid-fast method detects only intact organisms, whereas immunohistochemical techniques identify also free mycobacterial antigens, cell fragments, and altered microorganisms with defective cells walls. This is also the likely explanation for the observed immunological staining of infected giant cells that are usually negative by Ziehl-Neelsen staining (Fig. 2a, panels C and C′).
In a previous work (19), the species specificity of a362 epitopes was verified with respect to different mycobacterial species. However, as the main practical problem in veterinary medicine is the distinction between tuberculous and paratuberculous cattle, in the present work antibody specificity was verified only with respect to M. bovis. The weak reaction observed in the Western blot (Fig. 1) apparently does not alter the specificity of the anti-a362 polyclonal serum used in our immunohistochemical procedures: indeed, no appreciable reaction occurred with tuberculous specimens.
Macrophages and giant cells in the tissues of two tuberculous cows were positively stained by the monoclonal anti-a362 Ig. The simplest explanation for this finding is coinfection of these tuberculous cows with M. avium subsp. paratuberculosis, a quite frequent event accounted for by the very high epidemiological incidence of paratuberculosis in ruminants (10, 13). In agreement with such an interpretation is the usually negative response to a362 immunostaining of macrophages in granulomas from M. bovis-infected cows. However, the possibility of cross-reactivity between the P34 protein of M. avium subsp. paratuberculosis and some M. bovis components (perhaps a 38-kDa protein) cannot be rigorously excluded, thus leaving the double-infection hypothesis still questionable.
The immunohistological procedures described in this paper are interesting in several respects. They allow the species-specific diagnosis of paratuberculosis, which is essential for control of the disease, and the retrospective examination of fixed-mounted specimens stored in veterinary pathology libraries. In addition, histopathological examination of biopsies of mesenteric lymph nodes obtained from the region of the terminal ileum of infected cows proved to be a very accurate diagnostic method, in agreement with previous observations from other laboratories (7, 33).
It is generally assumed that M. avium subsp. paratuberculosis cells, introduced by feeding on contaminated pastures, penetrate the M cells of the dome epithelium covering the ileal Peyer’s patches, multiply intracellularly in phagocytosing macrophages, and then infect contiguous tissues (31). But another clinical picture, which involves early spreading of the infecting organism by the hematogenous route from the site of entry to all tissues and organs, is possible. This hypothesis better accounts for the diffusion of granulomatous reactions to large portions of the intestinal tract, the forward involvement of the lymphatic tissues, and the pronounced biochemical alterations (edema, anemia, and emaciation) indicating early impairment of several organs and tissues. Our limited knowledge of the routes of propagation of the infectious process and of tissue involvement is due to the lack of sensitive and species-specific staining techniques able to trace the etiological agent in different organs of the host. Similar technical difficulties were encountered, in an experimental infection approach, in following the fate of the etiological agent introduced by the parenteral route. The new procedures herewith described might thus help to clarify the pathogenesis of Johne’s disease.
Since recent works indicate that a relevant fraction of Crohn’s disease cases in humans have a mycobacterial etiology (9, 11, 27, 32, 44, 46, 48), the nutritional use of meat from paratuberculous cattle becomes questionable as potentially unsafe. Histopathological analysis of biopsy material taken at the slaughterhouse would allow safety controls.
We have previously described the development of the a362 ELISA for paratuberculosis (45). In addition to such a direct procedure (antibody capture on antigen-coated microplates), the possibility emerges from the present work of indirect ELISAs based on anti-a362 polyclonal serum and monoclonal Ig.
In conclusion, the present work describes two new procedures of diagnostic and scientific interest for veterinary medicine; these procedures use polyclonal and monoclonal Ig and are now also being applied to human medicine for a study of Crohn’s disease.
ACKNOWLEDGMENTS
We thank A. Robert (University of Louvain) for help in statistical analysis. Polypeptide a362 was a gift from Innogenetics (Ghent, Belgium).
This work was supported by grant 3-4506-96 from the Belgian Funds for Medical Research (FRSM).
REFERENCES
- 1.Ackermans F, Nisol F, Bazin H. Immunization of rats. In: Bazin H, editor. Rat hybridomas and rat monoclonal antibodies. Boca Raton, Fla: CRC Press; 1990. pp. 75–85. [Google Scholar]
- 2.Bazin H, Deckers C, Beckers A, Heremans J F. Transplantable immunoglobulin-secreting tumours in rats. I. General features of LOU-WS1 strain rat immunocytomas and their monoclonal proteins. Int J Cancer. 1972;10:568–580. doi: 10.1002/ijc.2910100316. [DOI] [PubMed] [Google Scholar]
- 3.Bazin H, Beckers A, Querinjean P. Three classes and four (sub)classes of rat immunoglobulins: IgM, IgA, IgE and IgG1, IgG2a, IgG2b, IgG2c. Eur J Immunol. 1974;4:44–48. doi: 10.1002/eji.1830040112. [DOI] [PubMed] [Google Scholar]
- 4.Bazin H. Production of rat monoclonal antibodies with the LOU rat non-secreting IR983F myeloma cell line. In: Peeters H, editor. Protides of the biological fluids. New York, N.Y: Pergamon Press; 1982. pp. 615–618. [Google Scholar]
- 5.Bazin H, Cormont F, De Clercq L. Rat monoclonal antibodies. II. A rapid and efficient method for purification from ascitic or serum. J Immunol Methods. 1984;71:9–16. doi: 10.1016/0022-1759(84)90200-x. [DOI] [PubMed] [Google Scholar]
- 6.Bech-Nielsen S, Jorgensen J B, Arhens P, Feld N C. Diagnostic accuracy of a Mycobacterium phlei-absorbed serum enzyme-linked immunosorbent assay for diagnosis of bovine paratuberculosis in dairy cows. J Clin Microbiol. 1992;30:613–618. doi: 10.1128/jcm.30.3.613-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedictus G, Haagsma J. The efficacy of mesenteric lymph node biopsy in the eradication of paratuberculosis from an infected dairy farm. Vet Q. 1986;8:5–11. doi: 10.1080/01652176.1986.9694012. [DOI] [PubMed] [Google Scholar]
- 8.Billman J, Carrigan M, Cockram F, Corner L, Gill I, Hill J, Jessep T, Milner A, Wood P. A comparison of the interferon gamma assay with the absorbed ELISA for the diagnosis of Johne’s disease in cattle. Aust Vet J. 1992;69:25–28. doi: 10.1111/j.1751-0813.1992.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiodini R J. Crohn’s disease and the mycobacterioses: a review and comparison of two disease entities. Clin Microbiol Rev. 1989;2:90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiodini R J, Van Kruiningen H J, Merkal R S. Ruminant paratuberculosis (Johne’s disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 11.Chiodini R J, Van Kruiningen H J, Thayer W R, Coutu J A. The spheroplastic phase of mycobacteria isolated from patients with Crohn’s disease. J Clin Microbiol. 1986;24:357–363. doi: 10.1128/jcm.24.3.357-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke C J. The pathology and pathogenesis of paratuberculosis in ruminants and other species. Review. J Comp Pathol. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 13.Cocito C, Gilot P, Coene M, De Kesel M, Poupart P, Vannuffel P. Paratuberculosis. Clin Microbiol Rev. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colgrove G S, Thoen C O, Blackburn B O, Murphy C D. Paratuberculosis in cattle: a comparison of three serologic tests with results of fecal culture. Vet Microbiol. 1989;19:183–187. doi: 10.1016/0378-1135(89)90083-7. [DOI] [PubMed] [Google Scholar]
- 15.Collins D M, Gabric D M, De Lisle G W. Identification of a repetitive DNA sequence specific to Mycobacterium paratuberculosis. FEMS Microbiol Lett. 1989;60:175–178. doi: 10.1016/0378-1097(89)90503-x. [DOI] [PubMed] [Google Scholar]
- 16.Collins M T, Kenefick K B, Sockett D C, Lambrecht R S, McDonald J, Jorgensen J B. Enhanced radiometric detection of Mycobacterium paratuberculosis using filter-concentrated bovine fecal specimens. J Clin Microbiol. 1990;28:2514–2519. doi: 10.1128/jcm.28.11.2514-2519.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins M T, Sockett D C, Ridge S, Cox J. Evaluation of a commercial enzyme-linked immunosorbent assay for Johne’s disease. J Clin Microbiol. 1991;29:272–276. doi: 10.1128/jcm.29.2.272-276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Kesel M, Gilot P, Coene M, Cocito C. Composition and immunological properties of the protein fraction of A36, a major antigen complex of Mycobacterium paratuberculosis. Scand J Immunol. 1992;36:201–212. doi: 10.1111/j.1365-3083.1992.tb03092.x. [DOI] [PubMed] [Google Scholar]
- 19.De Kesel M, Gilot P, Misonne M-C, Coene M, Cocito C. Cloning and expression of portions of the 34-kilodalton-protein gene of Mycobacterium paratuberculosis: its application to serological analysis of Johne’s disease. J Clin Microbiol. 1993;31:947–954. doi: 10.1128/jcm.31.4.947-954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisel O, Netter F, Hermanns W. Specificity of the immunohistochemical demonstration of mycobacterial antigens. J Vet Med. 1994;41:548–553. doi: 10.1111/j.1439-0450.1994.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 21.Gilot P, De Kesel M, Coene M, Cocito C. Induction of cellular immune reactions by A36, an antigen complex of Mycobacterium paratuberculosis. Comparison of A36 and johnin components. Scand J Immunol. 1992;36:811–821. doi: 10.1111/j.1365-3083.1992.tb03143.x. [DOI] [PubMed] [Google Scholar]
- 22.Gilot P, De Kesel M, Machtelinckx L, Coene M, Cocito C. Isolation and sequencing of the gene coding for an antigenic 34-kilodalton protein of Mycobacterium paratuberculosis. J Bacteriol. 1993;175:4930–4935. doi: 10.1128/jb.175.15.4930-4935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green E P, Tizard M L V, Moss M T, Thompson J, Winterbourne D J, McFadden J J, Hermon-Taylor J. Sequence and characteristics of IS900, an insertion element identified in a human Crohn’s disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 1989;17:9063–9073. doi: 10.1093/nar/17.22.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hance A J, Grandchamp B, Lévy-Frébault V, Lecossier D, Rauzier J, Bocart D, Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989;3:843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 25.Hines S A, Buergelt C D, Wilson J H. Disseminated Mycobacterium paratuberculosis infection in a cow. J Am Vet Assoc. 1987;190:681–682. [PubMed] [Google Scholar]
- 26.Lambrecht R S, Carriere J F, Collins M T. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl Environ Microbiol. 1988;54:910–916. doi: 10.1128/aem.54.4.910-916.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisby G, Andersen J, Engbaek K, Blinder V. Mycobacterium paratuberculosis in intestinal tissue from patients with Crohn’s disease demonstrated by a nested primer polymerase chain reaction. Scand J Gastroenterol. 1994;29:923–929. doi: 10.3109/00365529409094864. [DOI] [PubMed] [Google Scholar]
- 28.Manouvriez P, Nisol F, Delaunay T, Bazin H. Isotyping of rat monoclonal antibodies. In: Bazin H, editor. Rat hybridomas and rat monoclonal antibodies. Boca Raton, Fla: CRC Press; 1990. pp. 127–137. [Google Scholar]
- 29.Merkal R S, Curran B. Growth and metabolic characteristics of Mycobacterium paratuberculosis. Appl Microbiol. 1974;28:276–279. doi: 10.1128/am.28.2.276-279.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molina A, Morera L, Llanes D. Enzyme-linked immunosorbent assay for detection of antibodies against Mycobacterium paratuberculosis in goats. Am J Vet Res. 1991;52:863–868. [PubMed] [Google Scholar]
- 31.Momotani E, Whipple D, Thiermann A, Cheville N. Role of M cells and macrophage in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer’s patches in calves. Vet Pathol. 1988;25:131–137. doi: 10.1177/030098588802500205. [DOI] [PubMed] [Google Scholar]
- 32.Moss M T, Sanderson J D, Tizard M L V, Hermon-Taylor J, El-Zaatari F A K, Markesich D C, Graham D Y. Polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp silvaticum in long term cultures from Crohn’s disease and control tissues. Gut. 1992;33:1209–1213. doi: 10.1136/gut.33.9.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pemberton D H. Diagnosis of Johne’s disease in cattle using mesenteric lymph node biopsy: accuracy in clinical suspects. Aust Vet J. 1979;55:217–219. doi: 10.1111/avj.1979.55.5.217. [DOI] [PubMed] [Google Scholar]
- 34.Plante Y, Remenda B W, Chelack B J, Haines D M. Detection of Mycobacterium paratuberculosis in formalin-fixed paraffin-embedded tissues by polymerase chain reaction. Can J Vet Res. 1996;60:115–120. [PMC free article] [PubMed] [Google Scholar]
- 35.Poupart P, Coene M, Van Heuverswyn H, Cocito C. Preparation of a specific RNA probe for detection of Mycobacterium paratuberculosis and diagnosis of Johne’s disease. J Clin Microbiol. 1993;31:1601–1605. doi: 10.1128/jcm.31.6.1601-1605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridge S E. Cultivation of Mycobacterium paratuberculosis from bovine fecal samples by using elements of the Roche MB Check system. J Clin Microbiol. 1993;31:400–405. doi: 10.1128/jcm.31.2.400-405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ris D R, Hamel K L, Ayling J M. The detection of Mycobacterium paratuberculosis in bovine faeces by isolation and the comparison of isolation with the examination of stained smears by light microscopy. N Z Vet J. 1988;36:112–114. doi: 10.1080/00480169.1988.35503. [DOI] [PubMed] [Google Scholar]
- 38.Sockett D C, Conrad T, Thomas C, Collins M T. Evaluation of four serological tests for bovine paratuberculosis. J Clin Microbiol. 1992;30:1134–1139. doi: 10.1128/jcm.30.5.1134-1139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugden E A, Brooks B W, Young N M, Watson D C, Neilsen K H, Corner A H, Turcotte C, Michaelides A, Stewart R B. Chromatographic purification and characterization of antigens A and D from Mycobacterium paratuberculosis and their use in enzyme-linked immunosorbent assay for diagnosis of paratuberculosis in sheep. J Clin Microbiol. 1991;29:1659–1664. doi: 10.1128/jcm.29.8.1659-1664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thoen C O, Haagsma J. Molecular techniques in the diagnosis and control of paratuberculosis in cattle. J Am Vet Med Assoc. 1996;209:734–737. [PubMed] [Google Scholar]
- 41.Thoresen O F, Falk K, Evensen O. Comparison of immunohistochemistry, acid-fast staining, and cultivation for detection of Mycobacterium paratuberculosis in goats. J Vet Diagn Investig. 1994;6:195–199. doi: 10.1177/104063879400600210. [DOI] [PubMed] [Google Scholar]
- 42.Van der Giessen J W B, Eger A, Haagsma J, Haring R M, Gaastra W, van der Zeijst B A M. Amplification of 16S rRNA sequences to detect Mycobacterium paratuberculosis. J Med Microbiol. 1992;36:255–263. doi: 10.1099/00222615-36-4-255. [DOI] [PubMed] [Google Scholar]
- 43.Van der Giessen J W B, Haring R M, Vauclare E, Eger A, Haagsma J, van der Zeijst B A M. Evaluation of the abilities of three diagnostic tests based on the polymerase chain reaction to detect Mycobacterium paratuberculosis in cattle: application in a control program. J Clin Microbiol. 1992;30:1216–1219. doi: 10.1128/jcm.30.5.1216-1219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vannuffel P, Dieterich C, Naerhuyzen B, Gilot P, Coene M, Fiasse R, Cocito C. Occurrence, in Crohn’s disease, of antibodies directed against a species-specific recombinant polypeptide of Mycobacterium paratuberculosis. Clin Diagn Lab Immunol. 1994;1:241–243. doi: 10.1128/cdli.1.2.241-243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vannuffel P, Gilot P, Limbourg B, Naerhuyzen B, Dieterich C, Coene M, Machtelinckx L, Cocito C. Development of species-specific enzyme-linked immunosorbent assay for diagnosis of Johne’s disease in cattle. J Clin Microbiol. 1994;32:1211–1216. doi: 10.1128/jcm.32.5.1211-1216.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wall S, Kunze Z M, Saboor S, Soufleri I, Seechurn P, Chiodini R, McFadden J J. Identification of spheroplast-like agents isolated from tissues of patients with Crohn’s disease and control tissues by polymerase chain reaction. J Clin Microbiol. 1993;31:1241–1245. doi: 10.1128/jcm.31.5.1241-1245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wards B J, Collins D M, de Lisle G W. Detection of Mycobacterium bovis in tissues by polymerase chain reaction. Vet Microbiol. 1995;43:227–240. doi: 10.1016/0378-1135(94)00096-f. [DOI] [PubMed] [Google Scholar]
- 48.Wiley E L, Mulhollan T J, Beck B, et al. Polyclonal antibodies raised against Bacillus Calmette-Guerin, Mycobacterium duvalii and Mycobacterium paratuberculosis used to detect mycobacteria in tissues with the use of immunohistochemical techniques. Am J Clin Pathol. 1990;94:307–312. doi: 10.1093/ajcp/94.3.307. [DOI] [PubMed] [Google Scholar]
- 49.Woodruff T S, Shulaw W P, Bech-Nielsen S, Hoffsis G F, Spangler E, Heider L E. Serodiagnosis of bovine paratuberculosis by use of a dot enzyme-linked immunosorbent assay. Am J Vet Res. 1991;52:217–221. [PubMed] [Google Scholar]