Abstract
Eosinophilic meningoencephalitis due to the nematode Angiostrongylus cantonensis, which is endemic to Cuba, occurs in children and is due to accidental contact with soil snails. The course is less often fatal than in adult patients in southeastern Asia. Cerebrospinal fluid (CSF) and serum samples from 24 pediatric patients were analyzed and evaluated in CSF/serum quotient diagrams (Reiber graphs) to characterize the neuroimmunological response and the blood-CSF barrier dysfunction that occur in the course of the disease. At the time of the first diagnostic lumbar puncture, together with eosinophilic pleocytosis (1,920 ± 400 cells/μl), intermediate blood-CSF barrier dysfunction (i.e., an increased CSF/serum albumin quotient) with no intrathecal immunoglobulin G (IgG), IgA, and IgM class response was observed in all cases. Seven days later, at the time of early clinical recovery, the blood-CSF barrier dysfunction was normalized in 75% of the patients, but meanwhile, intrathecal immunoglobulin synthesis emerged in all cases, as either a two-class response (IgG and IgA in 85% of the patients) or a three-class response (IgG, IgA, and IgM; 30%). The fraction of eosinophilic cells (40%) remained large despite a decreasing total cell count. The neuroimmunological pattern of this inflammatory response to the parasite and its toxins is discussed with regard to the CSF patterns of other infectious diseases caused by bacteria or viruses.
Eosinophilic meningitis or meningoencephalitis induced by the nematode Angiostrongylus cantonensis is a disease with a poor prognosis commonly seen in southeastern Asia (6, 16), where fatal and chronic cases frequently occur. It was first recorded in Cuba in 1981 (1) and later in Puerto Rico (2). Most of the cases reported involved children with clinical manifestations different from those of adults (3), with less severe complications. In the majority, the anamnesis showed a history of accidental contact between soil snails and children living in rural and semirural areas. Infective third-stage larvae of the nematode develop in slugs and snails. Humans are infected due to ingestion of an infected intermediate host (1, 3, 6). In Cuba and other Caribbean countries, there is no tradition of eating raw snails, in contrast to the countries in southern Asia. This is why children are the primary victims of this disease in the Caribbean. The disease still continues to occur endemically in Cuba.
The clinical symptoms could confuse physicians because of the initial similarity to viral meningoencephalitis. The presence of eosinophilia in blood and cerebrospinal fluid (CSF) alerts the medical staff to suspect this disease. The best confirmation of the diagnosis is detection of A. cantonensis larvae surrounded by a cluster of eosinophilic cells in CSF (3). The neuroimmunological response pattern has not been previously reported. The characterization of disease-related immunoglobulin patterns (11, 12) in quotient diagrams as described by Reiber (8–10) is a widely accepted tool for diagnosis of neurological diseases (7, 11–13). In particular, this was done by introduction of the hyperbolic discrimination line in Reiber graphs to discriminate a brain-derived protein fraction from a blood-derived protein fraction (e.g., of immunoglobulins) in CSF. This is the physiological basis for the identification of pathological intrathecal synthesis of, e.g., immunoglobulin G (IgG) besides a change in the blood-derived fraction due to a blood-CSF barrier dysfunction.
The intrathecal immune response patterns and consequences for blood-CSF barrier function caused by parasites have not been described previously and deserve attention for diagnostic and theoretical, pathophysiological reasons.
MATERIALS AND METHODS
Patients.
This prospective study included 24 pediatric patients (18 males and 6 females aged 3 to 14 years; mean age, 7.2 years) with acute meningoencephalitis who underwent lumbar puncture on suspicion of CNS infection. Informed consent for the lumbar puncture was given by the parents. The incubation period was 15 days. The clinical symptoms in all of the cases indicated meningoencephalitis. The most common symptom was fever (92%), followed by vomiting and headache. Detailed descriptions of the clinical symptoms and course of the disease were given in references 3 and 4. All of the cases in this study involved peripheral leukocytosis and eosinophilia (above 10%). The CSF cell differentiation showed 8 to 42% lymphocytes and 30 to 90% eosinophils. The frequency of worm detection in the lumbar CSF by an enrichment method previously described (3) was 30%.
The control group (n = 15) contained pediatric patients punctured after febrile convulsions to exclude an inflammatory process.
Samples.
Serum and CSF were obtained simultaneously immediately after admission to the clinic during the acute phase, and a second puncture was done routinely 7 days later, at the time of clinical recovery.
Protein analysis.
Albumin, IgG, IgA, and IgM were measured in serum and CSF by radial immunodiffusion (NOR and LC Partigen immunodiffusion plates; Behringwerke AG, Marburg, Germany). The sensitivity of radial immunodiffusion for detection of albumin and IgG is, at 5 mg/liter, sufficient to detect normal values in the CSF of young patients. The much lower IgA and, in particular, IgM concentrations in normal CSF are below the detection limit of radial immunodiffusion, at 5 mg/liter for these molecules, but for typical clinical situations with pathologically increased IgM and IgA concentrations in CSF, the method is sufficient. The advantage of this method is the minimal technical equipment necessary, compared to the much more sensitive automated nephelometric assays or enzyme immunoassays (11).
After calculation of CSF/serum concentration quotients (4), intrathecal synthesis of individual immunoglobulins was calculated by the improved hyperbolic function of Reiber (9). For graphical representation, Reiber graphs (10) were used (Fig. 1). The improved diagrams (9, 10) are more sensitive at lower albumin quotients, which is particularly relevant for protein concentrations in the CSF of children, compared to an earlier report (8). An explanation of how to read the graphs is given in the legend to Fig. 1. Calculation of the intrathecal fractions in percent is done by the equation IgIF = [1 − QLim/QIg] · 100% with QIg and QAlb, the empirical values of an individual patient, QLim of IgG = 0.93 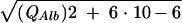 − 1.7 · 10−3, QLim of IgA = 0.77
− 1.7 · 10−3, QLim of IgA = 0.77 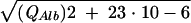 − 3.1 · 10−3, and QLim of IgM = 0.93
− 3.1 · 10−3, and QLim of IgM = 0.93 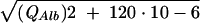 − 7.1 · 10−3.
− 7.1 · 10−3.
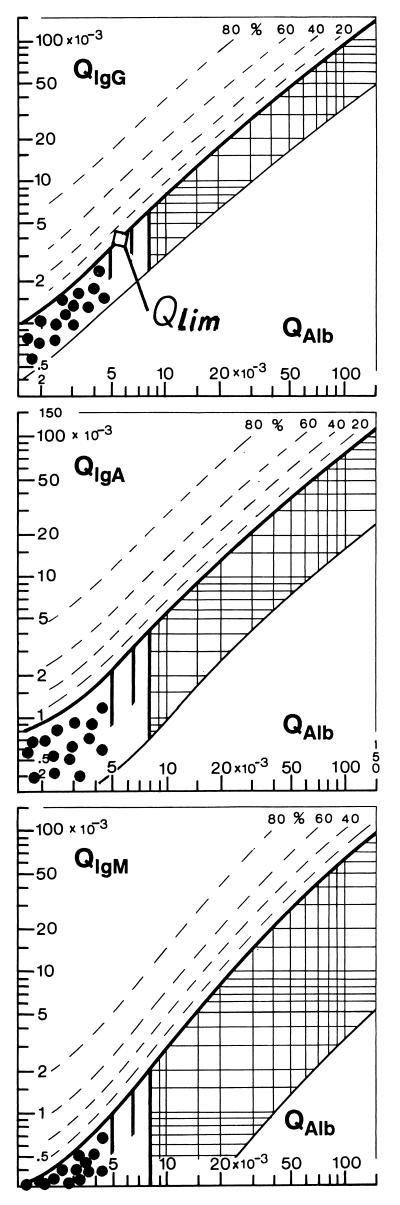
FIG. 1.
CSF/serum quotient diagrams for IgG, IgA, and IgM (Reiber graphs). The reference range of blood-derived IgG, IgA, and IgM concentrations in CSF include 99% (±3 s) of the group investigated (9). The upper hyperbolic curves (thick lines) represent the discrimination lines between brain-derived and blood-derived immunoglobulin fractions. Values above these upper discrimina- tion lines represent intrathecal IgG, IgA, or IgM synthesis. The dashed lines indicate the extent of intrathecal synthesis as intrathecal fractions (IgGIF, IgAIF, or IgMIF) with 20, 40, 60, and 80% of the measured total immunoglobulin concentration in CSF, with reference to the discrimination line as 0% intrathecal synthesis. The limit of the reference range for QAlb between normal and increased CSF protein concentrations due to blood-CSF barrier dysfunction is indicated by the age-dependent vertical lines at QAlb = 5 · 10−3 (up to 15 years), at QAlb = 6.5 · 10−3 (up to 40 years), and at QAlb = 8 · 10−3 (up to 60 years). The diagrams depict the following ranges: 1, normal; 2, blood-CSF barrier dysfunction (i.e., reduced CSF turnover); 4, intrathecal immunoglobulin synthesis with no change in CSF turnover; 3, intrathecal immunoglobulin synthesis with reduced CSF turnover. Values below the lower hyperbolic line in range 5 indicate a methodological fault. The data of 15 control patients (•) are representative of the age-related normal range with normal blood-CSF barrier function and no intrathecal immunoglobulin synthesis.
Isoelectric focusing to detect oligoclonal IgG in CSF and serum after protein staining was performed by the method reported in reference 10. The method is sensitive enough to detect oligoclonal IgG in CSF of 98% of multiple sclerosis patients and was evaluated in accordance with the international consensus (references cited in references 11 and 12).
RESULTS
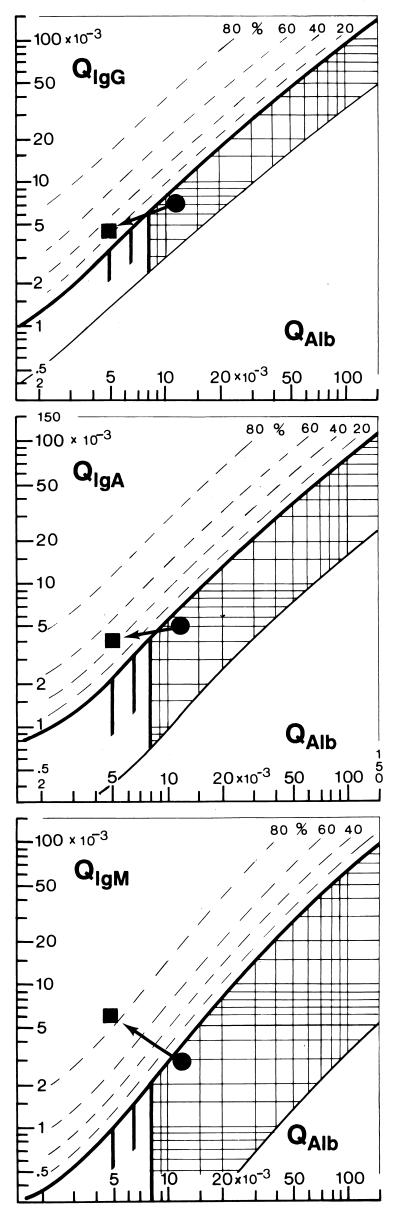
In Fig. 2, we report the representative changes in the protein patterns in the CSF of a 12-year-old male patient suffering from A. cantonensis meningoencephalitis. For comparison, Fig. 1 shows the representative data of normal controls in the Reiber graph. At the time of the first diagnostic puncture in the acute phase of the disease, a slight blood-CSF barrier dysfunction, but no humoral intrathecal immune response, was observed (Fig. 2). The absence of oligoclonal IgG, shown by isoelectric focusing, confirmed this result. At that time, the patient had 325 cells/μl of CSF with a large percentage (35%) of eosinophils. Eight days later, at the time of recovery, the same patient showed an almost normal albumin quotient, but then a humoral intrathecal immune response of all three immunoglobulin classes was detected (Fig. 2). The brain-derived intrathecal IgM fraction was dominant at 86% compared to an intrathecal IgA fraction of 42% and an IgG fraction of 22%. At that time, the CSF cell count was reduced to 87/μl with a still-large fraction of eosinophils (40%).
FIG. 2.
Time course of the intrathecal immune response in eosinophilic meningitis shown as patterns in Reiber graphs (9, 10). Filled circles represent the data of a patient with eosinophilic meningoencephalitis during the acute phase at the time of the first, diagnostic puncture within 3 days after the symptoms began. Slight blood-CSF barrier dysfunction, i.e., increased QAlb, but no intrathecal IgG, IgA, or IgM synthesis, was observed (points are below the hyperbolic discrimination line). Filled squares represent the data of the same patient 7 days later, at the time of clinical recovery. The albumin quotient is almost normal (QAlb, <5 · 10−3), but an intrathecal three-class immune response is observed (values above the discrimination lines) with dominance of the intrathecal IgM fraction (86%) over the intrathecal brain-derived IgA fraction (42%) and the IgG fraction (22%). For an explanation of the graph, see the legend to Fig. 1.
As a general result, we found that at time of the first, diagnostic puncture, all 24 patients had increased albumin quotients but none showed an intrathecal humoral immune response. The mean cell count in CSF was 1,920 ± 400 cells/μl at the time of the first diagnostic lumbar puncture. The percentages of eosinophilic cells were 35 to 60%. The lactate concentration in CSF was normal (<1.9 mmol/liter).
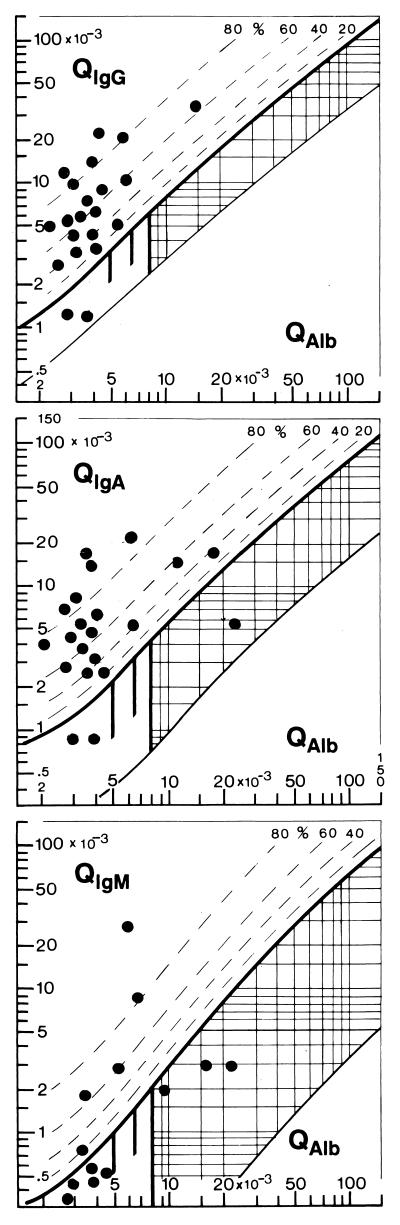
In the quotient diagrams of Fig. 3, the data of 21 patients at the time of recovery (second puncture, 7 days after first, diagnostic puncture) were collected. Seventy-five percent already had normal blood-CSF barrier function. The mean cell count was still 525 ± 40 cells/μl with a dominant fraction of eosinophilic cells. All patients had a humoral intrathecal immune response. Intrathecal IgG synthesis was detectable in the quotient diagram in 19 of 21 cases (Fig. 3) and as oligoclonal IgG by isoelectric focusing in the rest.
FIG. 3.
Patterns of the intrathecal immune response (IgG, IgA, and IgM classes) in eosinophilic meningitis at the time of clinical recovery. The data of 21 patients refer to the second puncture routinely done 7 days after the first, diagnostic puncture done at the time of admittance to the hospital. All of the patients showed an intrathecal humoral immune response; in 18 of 21 cases, it was a two-class (IgG and IgA) response, and in 5 of 13 cases, it was a three-class (IgG, IgA, and IgM) response. In the eight patients missing in the IgM diagram, IgM concentrations in CSF were below the detection limit of the assay.
A two-class (IgG and IgA) response was found in 18 of 21 cases, and a three-class response (IgG, IgA, and IgM) in CNS was observed) in 5 of 13 cases. The detection of the IgM class response was somewhat handicapped, as in eight cases, the IgM values in CSF were below the sensitivity of the detection method. Therefore, the number of cases with a three-class response might be somewhat larger than that shown in Fig. 3. However, the intrathecal IgM response is definitely less frequent than the intrathecal IgG or IgA response. The local intrathecal synthesis of IgG and IgA antibodies in CNS is an important characteristic of the CSF patterns of these patients, whose the lumbar punctures were performed at least 3 days after the start of their clinical symptoms. This time course and the pattern of the intrathecal immunoglobulin response are different from those of viral infections of the brain (12), with an intrathecal immune response starting as late as 7 to 12 days after the first clinical symptoms (5) and then most frequently representing an isolated IgG response.
DISCUSSION
A. cantonensis is the most common cause of eosinophilic meningitis in Cuba. Although, the diagnosis of the disease is usually made clinically, serologic methods can be helpful (15). Patients with meningitis living in an area of endemicity, having exposure to an intermediate host (snails, slugs, or molluscs), and presenting eosinophilic pleocytosis in blood should be considered for this diagnosis. In CSF, eosinophilic pleocytosis is the major laboratory finding at the time of the first diagnostic puncture, together with a blood-CSF barrier dysfunction (Fig. 2). Lactate values are normal. Occasionally, living larvae can be identified histologically in the CSF.
Regarding the neuroimmunological response in these patients suffering from A. cantonensis meningoencephalitis, we found characteristics in common with other infectious diseases. In the acute phase, during the first 3 days after the start of clinical symptoms, we found a blood-CSF barrier dysfunction, usually due to a reduced CSF flow rate (9), and pleocytosis without a humoral immune response. This is the usual pattern of the acute phase of an inflammatory disease (11, 12) caused by either a virus or a bacterium. The intermediate cell count and normal lactate concentrations in CSF would correspond better to a viral disease, but the intrathecal synthesis of IgG, IgA, and IgM appears rather early after initial clinical symptoms. This faster time course of the intrathecal immunoglobulin synthesis response corresponds better to neurological diseases caused by bacteria.
It is well known that dissociated bacterial cell debris acts as toxins in bacterial meningitis and causes many pathophysiological reactions (14). In eosinophilic meningitis, central nervous system damage caused by the motile worm, inflammatory responses to foreign antigens, and the possible toxicity of substances from the worm work in concert to produce the pathology and clinical picture of the disease. The immune response in this disease, as detected in the CSF, shows this influence of noninfectious agents very clearly. Thus, this response to a parasite allows discrimination between the primary and secondary influences of infectious agents in neurological diseases.
Regarding the pattern of the intrathecal immunoglobulin response, we have confirmed also for this disease that the intrathecal IgM response is not a sign preceding the early acute disease and cannot be interpreted on its own. It contributes to the differential diagnosis, just as part of a disease-related pattern (11, 12). This is in contrast to the meaning of the intrathecal IgA response.
Intrathecal IgA synthesis is seen in many acute and chronic neurologic diseases. Intrathecal IgA synthesis with an infectious cause is observed as an initial event in a bacterial infection, at the time of a diagnostic puncture in tuberculous meningitis, or in a brain abscess. It can be an isolated event (e.g., in pneumococcal or meningococcal meningitis [11, 12]) or part of a two- or three-class immunoglobulin response (e.g., in neuroborreliosis [13]). In viral infections, a transient IgA and IgM response is seen in few cases only at a much later time in the course of the disease (12). IgA synthesis without any cellular response in the CSF was also observed in a metabolic disease, adrenoleukodystrophy (7). This large spectrum of origins of intrathecal IgA synthesis has raised several questions regarding a common mechanism. Our report on eosinophilic meningitis adds another facet to this spectrum of causes of intrathecal IgA synthesis, in particular, as a consequence of a noninfectious agent.
REFERENCES
- 1.Aguiar P H, Morera O, Pascual J. First record of Angiostrongylus cantonensis in Cuba. Am J Trop Med Hyg. 1981;30:966–968. doi: 10.4269/ajtmh.1981.30.963. [DOI] [PubMed] [Google Scholar]
- 2.Andersen E, Gubler D J, Sorensen K, Beddorf J, Ash L R. First report of Angiostrongylus cantonensis in Puerto Rico. Am J Trop Med Hyg. 1986;35:319–322. doi: 10.4269/ajtmh.1986.35.319. [DOI] [PubMed] [Google Scholar]
- 3.Dorta-Contreras A J, Ferrá Valdés M, Plana-Bauly R, Díaz-Martínez A G, Gonzáles-García N, Escobar-Pérez X. Meningoencephalitis eosinofílica por Angiostrongylus cantonensis (Chen 1935). Estudio inmunológico. Rev Esp Pediatr. 1987;43:379–385. [Google Scholar]
- 4.Dorta-Contreras A J, Ferrá Valdés M, Díaz-Martínez A G, Gonzáles-García N, Escobar-Pérez X, Martín-Echenique G. Estudio inmunológico longitudinal en meningoencefalitis 1984–1986. Rev Cubana Pediatr. 1988;60:69–81. [Google Scholar]
- 5.Felgenhauer K, Reiber H. The diagnostic significance of antibody specificity indices in multiple sclerosis and herpes virus induced diseases of the nervous system. Clin Invest. 1992;70:28–37. doi: 10.1007/BF00422934. [DOI] [PubMed] [Google Scholar]
- 6.Hwang K P, Chen E R. Eosinophilic meningitis due to Angiostrongylus cantonensis. Epidemiol Bull Repub China. 1986;2:21–26. [Google Scholar]
- 7.Korenke G C, Reiber H, Hunemann D H, Hanefeld F. Intrathecal IgA synthesis in X-linked cerebral adrenoleukodystrophy. J Child Neurol. 1997;12:314–320. doi: 10.1177/088307389701200505. [DOI] [PubMed] [Google Scholar]
- 8.Reiber H, Felgenhauer K. Protein transfer at the blood-CSF barrier and the quantitation of humoral immunoresponse within the central nervous system. Clin Chem. 1987;163:319–328. doi: 10.1016/0009-8981(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 9.Reiber H. Flow rate of cerebrospinal fluid (CSF): a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122:189–203. doi: 10.1016/0022-510x(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 10.Reiber H. External quality assessment in clinical neurochemistry: survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin Chem. 1995;41:256–263. [PubMed] [Google Scholar]
- 11.Reiber H. Die diagnostische Bedeutung neuroimmunologischer Reaktionsmuster im Liquor cerebrospinalis. Lab Med. 1995;19:444–462. [Google Scholar]
- 12.Reiber H. Evaluation of blood-CSF barrier function and quantification of the humoral immune response within the CNS. In: Thompson E J, Trojano M, Livrea P, editors. Cerebrospinal fluid analysis in multiple sclerosis—1996. Milan, Italy: Springer-Verlag; 1996. pp. 51–72. [Google Scholar]
- 13.Tumani H, Nölker G, Reiber H. Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology. 1995;45:1663–1670. doi: 10.1212/wnl.45.9.1663. [DOI] [PubMed] [Google Scholar]
- 14.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;15:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 15.Yen C M, Chen E R. Detection of antibodies to Angiostrongylus cantonensis in serum and cerebrospinal fluid of patients with eosinophilic meningitis. Int J Parasitol. 1991;21:17–21. doi: 10.1016/0020-7519(91)90116-o. [DOI] [PubMed] [Google Scholar]
- 16.Yii C Y. Clinical observation on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis in Taiwan. Am J Trop Med Hyg. 1976;75:233–249. doi: 10.4269/ajtmh.1976.25.233. [DOI] [PubMed] [Google Scholar]