Abstract
Purpose
A primary treatment option for lung cancer patients is surgical resection. Patients who have poor lung function prior to surgery are at increased risk of developing serious and life‐threatening complications after surgical resection. Surgeons use nuclear medicine ventilation‐perfusion (VQ) scans along with pulmonary function test (PFT) information to assess a patient's pre‐surgical lung function. The nuclear medicine images and pre‐surgery PFTs are used to calculate percent predicted postoperative (%PPO) PFT values by estimating the amount of functioning lung tissue that would be lost with surgical resection. Nuclear medicine imaging is currently considered the standard of care when evaluating the amount of ventilation that would be lost due to surgery. A novel lung function imaging modality has been developed in radiation oncology that uses 4‐Dimensional computed tomography data to calculate ventilation maps (4DCT‐ventilation). Compared to nuclear medicine, 4DCT‐ventilation is cheaper, does not require a radioactive contrast agent, provides a faster imaging procedure, and has improved spatial resolution. In this work we perform a retrospective study to assess the use of 4DCT‐ventilation as a pre‐operative surgical lung function evaluation tool. Specifically, the purpose of our study was to compare %PPO PFT values calculated with 4DCT‐ventilation and %PPO PFT values calculated with nuclear medicine ventilation‐perfusion imaging.
Methods
The study included 16 lung cancer patients that had undergone 4DCT imaging, nuclear medicine imaging, and had Forced Expiratory Volume in 1 second (FEV 1) acquired as part of a standard PFT. The 4DCT datasets, spatial registration, and a density‐change‐based model were used to compute 4DCT‐ventilation maps. Both 4DCT‐ventilation and nuclear medicine images were used to calculate %PPO FEV 1. The %PPO FEV 1 was calculated by scaling the pre‐surgical FEV 1 by (1‐fraction of total resected ventilation); where the resected ventilation was determined using either the 4DCT‐ventilation or nuclear medicine imaging. Calculations were done assuming both lobectomy and pneumonectomy resections. The %PPO FEV 1 values were compared between the 4DCT‐ventilation‐based calculations and the nuclear medicine‐based calculations using correlation coefficients, average differences, and Receiver Operating Characteristic (ROC) analysis.
Results
Overall the 4DCT‐ventilation derived %PPO FEV 1 values agreed well with nuclear medicine‐derived %PPO FEV 1 data with correlations of 0.99 and 0.81 for lobectomy and pneumonectomy, respectively. The average differences between the 4DCT‐ventilation and nuclear medicine‐based calculation for %PPO FEV 1 were less than 5%. ROC analysis revealed predictive accuracy that ranged from 87.5% to 100% when assessing the ability of 4DCT‐ventilation to predict for nuclear medicine‐based %PPO FEV 1 values.
Conclusions
4DCT‐ventilation is an innovative technology developed in radiation oncology that has great potential to translate to the surgical domain. The high correlation results when comparing 4DCT‐ventilation to the current standard of care provide a strong rationale for a prospective clinical trial assessing 4DCT‐ventilation in the clinical setting. 4DCT‐ventilation can reduce the cost and imaging time for patients while providing improved spatial accuracy and quantitative results for surgeons.
Keywords: CT Ventilation, functional imaging, lung cancer, quantitative imaging, surgical evaluation
1. Introduction
A primary treatment option for early stage lung cancer patients is surgical resection. Lobectomy, pneumonectomy, and wedge resection surgeries are often the first salvage therapies considered for lung cancer patients. The complication with resectional methods for lung cancer is that patients may not have sufficient lung function to tolerate surgery. Patients that have poor lung function prior to surgery are at increased risk of developing serious and life‐threatening complications after surgical resection. As part of a standard pre‐surgery work‐up, surgeons use nuclear medicine ventilation‐perfusion (VQ) scans along with pulmonary function test (PFT) information to assess a patient's lung function.1 The nuclear medicine images and pre‐surgery PFTs are used to calculate percent predicted postoperative (%PPO) PFT values by estimating the amount of functioning lung tissue that would be lost with surgical resection. In other words, the %PPO metric presents pre‐surgical PFT values that are scaled by the amount of ventilation being lost due to surgery where the amount of ventilation lost to surgery is determined using nuclear medicine imaging. The %PPO values are considered an essential part of the pre‐surgery evaluation process because they were found to be an independent predictor of long‐term survival after lung resection for lung cancer patients.2
A novel lung function imaging modality has been developed in radiation oncology that uses 4‐Dimensional Computed Tomography (4DCT)3, 4 data along with imaging processing techniques to calculate lung ventilation maps5, 6 (4DCT‐ventilation). 4DCT‐ventilation has been an attractive research topic in thoracic radiation oncology because 4DCTs are typically acquired as standard of care for lung cancer patients treated with radiation therapy. Therefore, calculating lung function from 4DCT data enables the physician to evaluate spatial lung function without added monetary or dosimetric cost to the patient. In the radiation therapy setting, 4DCT‐ventilation has been validated against nuclear medicine imaging,7, 8, 9 other forms of lung function imaging,10, 11 and PFTs8, 12 with promising results. The two most suggested clinical uses of 4DCT‐ventilation have been to evaluate lung function throughout and after the completion of radiation therapy13, 14 and for functional avoidance. Functional avoidance radiation therapy implies sparing functional portions of the lung (as measured by 4DCT‐ventilation) in favor of irradiation through less‐functioning lung tissue.15, 16, 17, 18 The idea is that by sparing functional lung the rate of complications after radiation therapy would be reduced. Clinical trials are underway19, 20 to prospectively use 4DCT‐ventilation imaging in radiation therapy to spare functional lung.
In this work we evaluate the concept of using 4DCT‐ventilation in the surgical setting, specifically for the purposes of spatial lung function evaluation for lung cancer patients being assessed for resectional surgery. The current lung function imaging modality considered standard of care to calculate %PPO PFT metrics is nuclear medicine planar perfusion imaging.21, 22 However, studies have shown that spatial lung function assessment with nuclear medicine planar imaging can be inaccurate23, 24 and produce errors in predicting post‐surgical lung function.25 4DCT‐ventilation offers some potentially attractive advantages over nuclear medicine imaging in the surgical setting. Compared to nuclear medicine imaging, 4DCT‐ventilation is cheaper, does not require a radioactive contrast agent, does not suffer from an aerosol deposition artifact, provides a faster imaging procedure, has improved spatial resolution, and is an imaging modality that by definition provides anatomical (4DCT) and functional (4DCT‐ventilation) information. We perform a retrospective study to assess the use of 4DCT‐ventilation as a pre‐operative surgical lung function evaluation tool. Specifically, the purpose of our work was to compare %PPO PFT values calculated with 4DCT‐ventilation and %PPO PFT values calculated with nuclear medicine ventilation‐perfusion imaging.
2. Methods
2.1. Patient population
16 lung cancer patients being considered for lung surgery or radiotherapy were retrospectively reviewed. Clinical parameters for the patient cohort are presented in Table 1.
Table 1.
Patient and clinical characteristics for the patient population used for the study
| Parameter | Median (range) or number (%) |
|---|---|
| Age (y) | 68.5 (45–87) |
| Gender | |
| Female | 8 (50) |
| Male | 8 (50) |
| COPDa | |
| Yes | 8 (50) |
| No | 8 (50) |
| Tumor location | |
| Right | 9 (56) |
| Left | 7 (44) |
| Stage | |
| I | 4 (25) |
| II | 3 (19) |
| III | 8 (50) |
| IV | 1 (6) |
COPD = chronic obstructive pulmonary disease.
The study cohort consisted of 25% stage I, 19% stage II, 50% stage III, and 6% stage IV lung cancer patients. Eligible patients had undergone nuclear medicine imaging (both the ventilation and perfusion scans were acquired) and PFTs as part of a standard pre‐surgical evaluation and 4DCT imaging as part of the standard radiotherapy treatment planning process. Nine patients had all 3 procedures (nuclear medicine, PFTs, and 4DCT imaging) done within 50 d, 15/16 patients had all 3 procedures done within 100 d, and 1 patient had 150 d between their nuclear medicine and 4DCT imaging. Patients were excluded from the study if they had any significant medical interventions (surgery or radiotherapy for example) between the PFT, nuclear medicine imaging, and 4DCT imaging.
2.2. Nuclear medicine imaging
The nuclear medicine scans were acquired on Siemens scanners (Siemens ECAM or Siemens Symbia T16, Siemens, Malvern, PA, USA) in planar mode using 1.0 mCi Tc‐99 m diethylene triamine pentaacetic acid (DTPA) aerosol for ventilation and intravenous injection of a radioactive technetium macro aggregated albumin (MAA) for perfusion. Immediately after radioactive contrast administration, 8 planar view images were acquired: anterior, posterior, 4 oblique, and 2 lateral projections. Images were acquired with pixel resolution of 3.3 mm. The surgical guidelines suggest to use the perfusion component of the nuclear medicine scan,1, 21 however; the ventilation component has also been used for resection assessment.26, 27 We performed analysis using both the perfusion and ventilation components of the nuclear medicine scan.
2.3. Pulmonary function testing
PFTs use spirometry to measure air flow and are an established way of measuring lung function. For each patient we noted the Forced Expiratory Volume in 1 s (FEV1) which is a standard PFT metric. The FEV1 was reported as percentage of predicted value which is based on healthy subjects with the same anthropomorphic characteristics (height, age, gender, and others) as the patient being tested.28
2.4. 4DCT‐ventilation image generation
Each patient underwent a standard 4DCT in the department of radiation oncology. Patients were scanned on a Philips Brilliance Big Bore CT scanner (Philips Healthcare, Andover, MA, USA) under free breathing conditions using the gated lung protocol along with the bellows belt to track the patient's breathing trace. The 4DCT images were generated with a spatial resolution of 1 mm × 1 mm × 3 mm voxels. Each 4DCT image was reviewed for artifacts and patients were excluded if their 4DCT included substantial volume averaging around the diaphragm.
A 4DCT‐ventilation image was calculated using the patient's 4DCT imaging data. The Hounsfield Unit (HU) calculation technique previously described5, 13, 29 was used to calculate ventilation. Briefly, the first step was to segment the lungs in the end‐inhalation and end‐exhalation image. Deformable image registration was then performed to link lung voxel elements from inhale to exhale.30 The spatial accuracy of the deformable registration algorithm used for the study was evaluated and found to be on the order of 1.25 mm for thoracic registration.30 Each deformation field was manually reviewed for discontinuities or anomalies. Once the inhale and exhale voxels were linked, a density‐change‐based equation was applied to calculate ventilation:
| (1) |
where V in and V ex are the inhale and exhale volumes and HU in and HU ex are the inhale and exhale Hounsfield units of the individual lung voxels. Eq. (1) calculates the local change in air content for each voxel and produces a 3D map of ventilation function. Figure 1 shows a 4DCT‐ventilation image example for a patient with a ventilation defect in the right lung due to a central mass occluding an airway (with the bright colors representing functional lung and areas of blue representing ventilation defect regions). Each 4DCT‐ventilation image was manually reviewed for image artifacts, particularly in regions of lung‐tissue interphases. As previously described, there were few negative ventilation values in the generated 4DCT‐ventilation images.5 All negative ventilation values were converted to zeroes.
Figure 1.
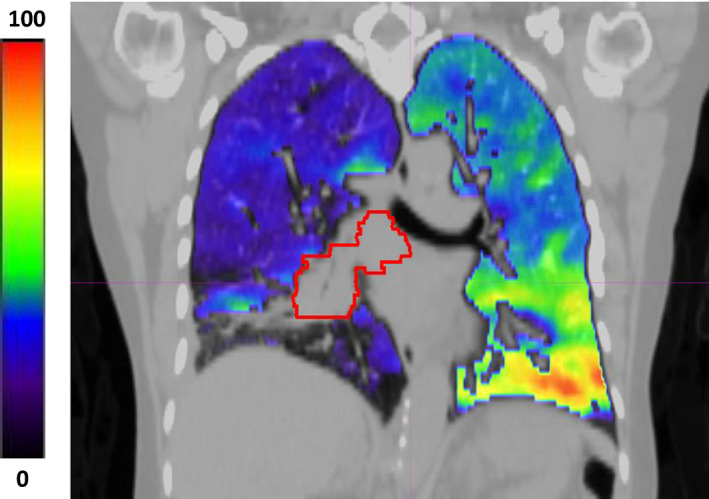
An example of a 4DCT‐ventilation image overlaid with a standard CT. The bright colors represent functional lung while the shades of blue and darker colors represent regions of ventilation defects. The 4DCT‐ventilation image was normalized by converting the raw image data to percentile values. The displayed patient presents with a central right mass (contoured in red) that is causing a ventilation defect in the right lung. [Color figure can be viewed at wileyonlinelibrary.com]
2.5. Percent predicted postoperative (%PPO) metrics
The 4DCT‐ventilation and nuclear medicine images were used to calculate %PPO FEV1 values. The first step was to calculate the percent ventilation in each lung third for both the 4DCT‐ventilation and the nuclear medicine imaging. The percent ventilation in each lung third is a standard metric used in nuclear medicine imaging for surgical evaluation7, 31 and is intended to geometrically approximate the ventilation in each lobe. For the nuclear medicine images the percent ventilation in each lung third was calculated using commercially available software used in our clinic (GE Xeleris v2.1753, GE, Milwaukee, WI, USA). For the 4DCT‐ventilation imaging, we used custom‐written software to mimic the data provided from the nuclear medicine scans. The software divided the lung into equal superior‐inferior geometrical thirds and calculated the percent ventilation in each third.
The percent ventilation in each lung third was used to calculate the PPO FEV1 using equations provided in standard physiologic evaluation guidelines for lung resection.21 Calculations were done assuming both pneumonectomy and lobectomy resection. A pneumonectomy is a resectional surgery where the surgeon removes an entire lung due to spread of the disease (to the mediastinum) while a lobectomy is a procedure where only one lobe is removed (typically done for early stage lung cancer patients).
For both pneumonectomy and lobectomy, we used the equation
where the total ventilation of resected lung is determined from the lung function imaging. The idea behind the equation is to scale the preoperative FEV1 by the amount of functioning lung tissue that would be lost with surgical resection. For pneumonectomy, the ventilation of resected lung was taken as the ventilation of the entire lung to be resected. For lobectomy, we used previously published methods,32 where the ventilation of resected lung was calculated by assigning each geometrical third to its accompanying lobe (the right upper third was assigned to the right upper lobe for example). For the left lung (which contains 2 lobes), the upper two‐thirds comprised the left upper lobe and the lower third comprised the left lower lobe.
%PPO FEV1 values were calculated and compared using 4DCT‐ventilation imaging and nuclear medicine imaging. For nuclear medicine imaging, the calculations were done using both the ventilation (V) and perfusion (Q) components of the scan. The comparison between the %PPO FEV1 metrics calculated with 4DCT‐ventilation imaging and nuclear medicine imaging was done using correlation coefficients and scatter plots. In addition, the 4DCT‐ventilation‐based PPO FEV1 values were subtracted from the nuclear medicine‐based calculations and descriptive statistics of the difference results [means, standard deviations, and 95% confidence intervals (CI)] are provided.
Additional analysis was performed by applying PPO FEV1 thresholds. These are thresholds where certain values of PPO FEV1 are used to make clinical decisions. Our analysis tested whether the decision would be the same using either nuclear medicine or 4DCT‐ventilation. The two noted thresholds in the guidelines for preoperative physiologic assessment are 60% and 30%. Thirty percent is used to indicate an increased risk of perioperative death and cardiopulmonary complications and 60% is a threshold that indicates additional testing (a low technology exercise test for example).21 We used 30% and 60% as thresholds and applied Receiver Operating Characteristic (ROC) analysis to assess the specificity, sensitivity, and accuracy of using the 4DCT‐ventilation‐based PPO FEV1 versus the nuclear medicine‐based PPO FEV1. In other words, it was assessed whether there were cases where one method of calculating PPO FEV1 (nuclear medicine) produced results on one side of the threshold and the other method (4DCT‐ventilation) produced results on the other side of the threshold. The ROC analysis enables the assessment of the ability of 4DCT‐ventilation to produce clinically congruent results to nuclear medicine imaging.
In summary, the PPO FEV1 values were calculated with 4DCT‐ventilation imaging and compared to calculations done with nuclear medicine‐ventilation imaging as well as nuclear medicine‐perfusion imaging. For each comparison, calculations were done assuming both a pneumonectomy and lobectomy resections.
3. Results
A representative patient example is shown in Fig. 2 with good agreement between the 4DCT‐ventilation and nuclear medicine ventilation images. Qualitatively, both imaging modalities demonstrate a significant ventilation defect in the left lung. Quantitatively, the percent ventilation in each lung and lung third shows good agreement between the two imaging modalities. The percent ventilation in the right and left lung were 93.8% and 6.2%, respectively, with 4DCT‐ventilation and 91.3% and 8.7% with nuclear medicine imaging for the example patient. The representative patient presented in Fig. 2 had a pre‐treatment FEV1 of 51%. Assuming a pneumonectomy of the left lung, the patient in Fig. 2 would have a %PPO FEV1 of 47.8% calculated with 4DCT‐ventilation, 48.5% calculated with nuclear medicine ventilation, and 46.5% calculated with nuclear medicine perfusion. An example patient with poor agreement between the 4DCT‐ventilation and nuclear medicine scans is displayed in Fig. 3. The 4DCT‐ventilation image for the patient presented in Fig. 3 shows lower ventilation in the right lung while the nuclear medicine ventilation and perfusion images display lower function in the left lung.
Figure 2.
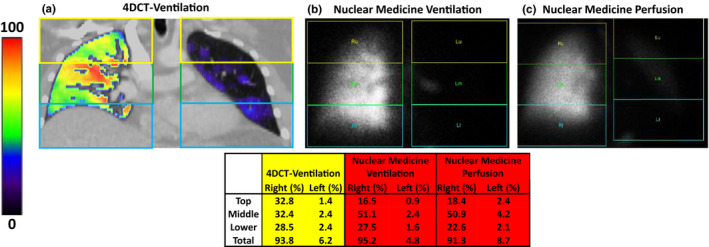
An example patient displaying good agreement between a 4DCT‐ventilation image (a), a nuclear medicine ventilation image (b), and a nuclear medicine perfusion image (c). The 4DCT‐ventilation and nuclear medicine demonstrate good agreement with both images showing a non‐functional left lung. The division of each lung into individual lung third is schematically presented for each imaging modality and the quantitative results for each lung and lung third are shown in the table. The quantitative results show good agreement between 4DCT‐ventilation and nuclear medicine. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.
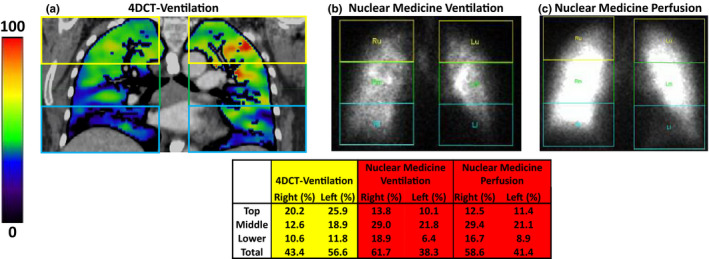
An example patient displaying poor agreement between a 4DCT‐ventilation image (a), a nuclear medicine ventilation image (b), and a nuclear medicine perfusion image (c). The 4DCT‐ventilation image demonstrates lower ventilation in the right lung while the nuclear medicine ventilation and perfusion images demonstrate lower function in the left lung. The division of each lung into individual lung third is schematically presented for each imaging modality and the quantitative results for each lung and lung third are shown in the table. [Color figure can be viewed at wileyonlinelibrary.com]
Table 2 presents the quantitative results comparing the PPO FEV1 values calculated with 4DCT‐ventilation imaging and nuclear medicine imaging for both the pneumonectomy and lobectomy calculations. Overall the PPO FEV1 values calculated with 4DCT‐ventilation and nuclear medicine demonstrated good agreement. The correlation coefficient between PPO FEV1 values calculated with 4DCT‐ventilation and nuclear medicine for the pneumonectomy calculation was on the order of 0.8 (0.81 for nuclear medicine‐ventilation and 0.78 for nuclear medicine‐perfusion). For the lobectomy calculations the correlation coefficient was 0.99 comparing the 4DCT‐ventilation and nuclear medicine‐based PPO FEV1 values. The average difference for both the pneumonectomy and lobectomy calculations between the 4DCT‐ventilation‐based %PPO FEV1 values and the nuclear medicine‐based %PPO FEV1 values were less than 5% (Table 2). Maximum difference for any individual patient between 4DCT‐ventilation and nuclear medicine‐based calculation was 24.2% and 10.7% for pneumonectomy and lobectomy, respectively. Scatter plots visually demonstrate the comparison between PPO FEV1 calculated with 4DCT‐ventilation and both nuclear medicine ventilation and nuclear medicine perfusion (Fig. 4).
Table 2.
Comparison of %PPO FEV1 values calculated with 4DCT‐ventilation and nuclear medicine imaging (results for both nuclear‐medicine‐ventilation and nuclear medicine‐perfusion are presented). The lobectomy %PPO FEV1 values were calculated using the geometrical approximations of each lung third
| Nuclear medicine ventilation | Nuclear medicine perfusion | |
|---|---|---|
| Correlation coefficient pneumonectomy | 0.81 | 0.78 |
| Correlation coefficient lobectomy | 0.99 | 0.99 |
| Average difference pneumonectomya | 4.1% ± 8.5% | 4.2% ± 10.7% |
| [−0.1% to 2.9%] | [−1.0% to 9.5%] | |
| Average difference lobectomya | 2.9% ± 3.0% | 2.3% ± 3.1% |
| [1.4% to 4.4%] | [0.8% to 3.8%] | |
| Maximum difference pneumonectomy | 24.2% | 23.6% |
| Maximum difference lobectomy | 9.7% | 10.7% |
Average differences are presented as mean ± standard deviation [95% confidence intervals].
Figure 4.
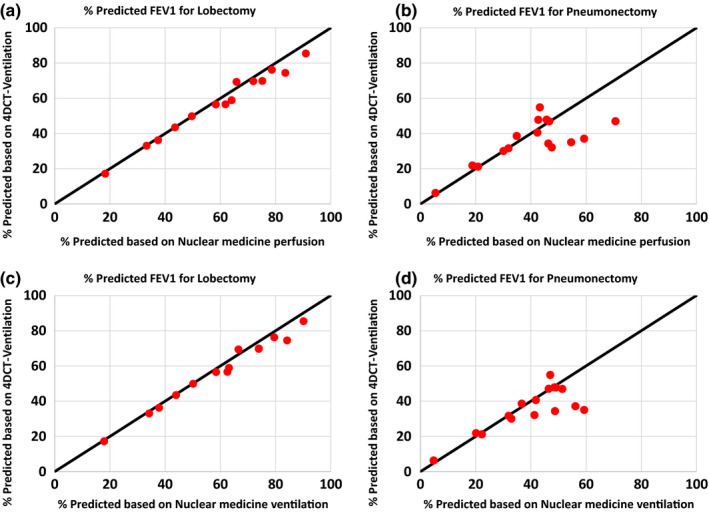
A scatter plot showing a comparison between the 4DCT‐ventilation calculated %PPO FEV1 values (y‐axis) and the nuclear medicine calculated %PPO FEV1 values (x‐axis). The 4DCT‐ventilation comparisons are shown against nuclear medicine perfusion for lobectomy (a), nuclear medicine perfusion for pneumonectomy (b), nuclear medicine ventilation for lobectomy (c), nuclear medicine ventilation for pneumonectomy (d). The line y = x (black) is provided as a reference for perfect agreement on each plot. [Color figure can be viewed at wileyonlinelibrary.com]
We applied thresholds of 30% and 60% and used ROC methods to calculate the sensitivity, specificity, and accuracy of calculating PPO FEV1 with 4DCT‐ventilation against calculating PPO FEV1 with nuclear medicine imaging. For the 30% threshold all comparisons (comparing 4DCT‐ventilation to both nuclear medicine ventilation and perfusion, using both lobectomy and pneumonectomy calculations) yielded sensitivity, specificity and accuracy of 100%. In other words, there were no cases where the nuclear medicine PPO FEV1 produced values on one side of the 30% threshold and the 4DCT‐ventilation %PPO FEV1 produced values on the other side of the threshold. For the 60% threshold, the lobectomy calculation produced an accuracy of 87.5% (14/16) and the pneumonectomy calculation produced an accuracy of 93.8% (15/16). In all the disagreement cases in the ROC analysis, the 4DCT‐ventilation‐based calculation produced PPO FEV1 values less than the 60% threshold while the nuclear medicine‐based calculations produced values greater than the 60% threshold.
4. Discussion
Overall, our correlation values (range 0.79 to 0.99) and scatter plot results demonstrate good agreement between the 4DCT‐ventilation‐based %PPO FEV1 values and the nuclear medicine‐based %PPO FEV1 values. Our results are in line with previous work which report correlations of 0.80 to 0.88 for Magnetic Resonance Imaging (MRI) and quantitative CT,33, 34, 35 and correlations of 0.96 comparing CT‐ventilation against Positron Emission Tomography.36 The ROC analysis demonstrated similarly promising results with sensitivity, specificity and accuracy on the order of 90% to 100%; demonstrating the ability of 4DCT‐ventilation to produce clinically congruent results to nuclear medicine imaging. The maximum difference results of 24.2% (pneumonectomy) and 10.7% (lobectomy) demonstrate that 4DCT‐ventilation and nuclear medicine can produce different %PPO results for individual patients.
Previous work by Eslick et al. presented an evaluation of CT‐based ventilation in the surgical setting. The study used breath‐hold CTs to calculate CT‐based ventilation36 and compared CT‐based ventilation to positron emission tomography (PET)‐based ventilation. Both the CT‐based ventilation and PET‐based ventilation were also compared to the segment counting method of calculating %PPO FEV1. The study found that CT‐based and PET‐based ventilation correlated well with each other and that both imaging modalities had low correlations with the segment counting method.36 There are several differences between our study and the work done by Eslick et al.36 Our study compared CT‐based ventilation to nuclear medicine ventilation‐perfusion using DTPA and MAA radio‐contrast agents while Eslick et al. compared their results to PET‐based Galligas ventilation imaging.36 Ventilation imaging with PET‐Galligas has improved image quality over nuclear medicine ventilation‐perfusion imaging with DTPA and MAA due to the smaller particle size of Galligas. On the other hand, PET‐Galligas is not widely available and is not considered the standard of care imaging modality for surgical assessment. In addition, PET‐Galligas provides the ventilation component of lung function, while our work was able to evaluate both the ventilation and perfusion components. There were also differences in the method of CT‐based calculation between our work and Eslick et al. Eslick et al.36 calculated CT‐based ventilation using inhale and exhale breath‐hold CTs while we performed ventilation calculations using 4DCTs. In addition, Eslick et al. used a modified version of Eq. (1) to calculate ventilation by adding a density scaling factor.8, 11, 36 Using the CT portions of each imaging modality, Eslick et al. calculated %PPO metrics using contours of lung lobes rather than relying on geometrical approximation. Although there are some differences between our work and the study presented by Eslick et al.,36 both studies suggest promise for CT‐based ventilation to be used as a functional imaging modality for surgical resection evaluation.
The current standard of practice to calculate %PPO metrics is nuclear medicine imaging.21 4DCT‐ventilation offers several potential image quality advantages over using nuclear medicine to calculate %PPO metrics. 4DCT‐ventilation theoretically provides finer spatial resolution than the spatial resolution of a nuclear medicine scan. Nuclear medicine suffers from an aerosol deposition artifact where for patients with Chronic Obstructive Pulmonary Disease (COPD) the aerosol can get stuck in the airway and produce false hotspots toward the center of the lung that distort the entire image [example shown in Fig. 2(b) and 2(c)]. 4DCT‐ventilation does not suffer from an aerosol deposition artifact because there is no contrast requirement. In addition, 4DCT‐ventilation provides an imaging modality that by definition presents anatomical (4DCT) and functional information (4DCT‐ventilation). The anatomical and functional information can enable physicians to contour the lung lobe and provide anatomically accurate ventilation values for each lung lobe rather than relying on a surrogate of dividing the lung into geometrical thirds. Although nuclear medicine imaging has been a clinically established method, studies have shown that spatial lung function estimation with nuclear medicine can be inaccurate23, 26 and that prediction errors may be large.25 The improved spatial resolution, no aerosol artifact, and multi‐modality information with 4DCT‐ventilation can potentially improve assessment of pre‐surgical lung function and prediction of post‐surgical function. In terms of patient and practical considerations, 4DCTs are generally cheaper than nuclear medicine scans, have a faster acquisition time, and do not require the production of a radioactive aerosol or any contrast agent. With respect to imaging radiation dose to the patient, 4DCT generally provides a higher imaging dose than nuclear medicine imaging.37, 38
There have been other, more experimental methods proposed to generate functional information to calculate %PPO metrics including dynamic contrast‐enhanced MRI as well as contrast‐enhanced quantitative CT.33, 34, 35, 39 4DCT‐ventilation has advantages over both of these methods in that a contrast agent is not required. Compared to contrast‐enhanced MRI, 4DCT‐ventilation is able to provide a finer slice thickness (generally 2.5 mm for 4DCT‐ventilation compared to 10 mm for the MRI images). The contrast‐enhanced quantitative CT methods previously presented35 use thresholding techniques to extract lung parenchyma and do not provide functional information; whereas the presented 4DCT‐ventilation method is able to provide direct functional information by calculating the amount of air movement in a given voxel. Another imaging modality that has been proposed in the surgical evaluation setting is Single‐photon emission computed tomography (SPECT) imaging and particularly SPECT‐CT.40 SPECT‐CT imaging addresses some of the limitations of a planar VQ scan with 3D imaging data and anatomical information but still suffers from many of the same image quality issues as planar imaging including limited spatial resolution and radioaerosol clumping artifacts.41 In addition, SPECT‐CT scans are currently not standard of care for31 lung function assessment. There have been studies which have evaluated SPECT and SPECT‐CT in the thoracic surgical setting with inconclusive results.32, 35, 41 One study cited a difference between using SPECT‐CT and planar VQ imaging32 for surgical evaluation and other studies noted little improvement in using SPECT over planar VQ imaging for surgical assessment.41 Future clinical trials can help elucidate some of the differences between 4DCT‐ventilation, SPECT‐CT, and planar nuclear medicine VQ imaging.
An important question to address is whether the ventilation (V) or perfusion (Q) component is important for evaluation in the surgical setting. Current image processing techniques can only produce ventilation maps from 4DCT data. The surgical guidelines suggest to use the perfusion component of the VQ scan,1, 21 however; the ventilation component has also been used for resection assessment,26, 27 and studies have found little difference between using ventilation or perfusion for pre‐surgical lung function assessment.42 In clinical practice, either the perfusion component is acquired or both the perfusion and ventilation portions are acquired for each patient. Large mismatches of ventilation and perfusion information is generally expected for patients with pulmonary embolism,43 which is unlikely in the lung cancer population we evaluated. In the case of lung cancer, the data assessing whether there are clinically significant differences between the ventilation and perfusion components is limited and generally inconclusive.44, 45, 46 One advantages of nuclear medicine perfusion over ventilation is that perfusion images are less prone to aerosol deposition artifacts than ventilation images. Future prospective clinical trials will have to be conducted to provide conclusive answers on whether it will be sufficient to only evaluate the ventilation component using 4DCT‐ventilation for surgical resection or if it will be necessary to also include the perfusion aspect.
The promising results provide a strong rationale for a prospective clinical trial to evaluate 4DCT‐ventilation in the surgical setting. The clinical trial would use pre‐surgery PFT and 4DCT‐ventilation imaging to calculate %PPO PFTs and directly compare the results to post‐surgical PFTs (which can be considered the comparison gold standard). A prospective clinical trial would enable us to overcome some of the uncertainties associated with the current retrospective work including a small patient dataset, a larger time interval between imaging datasets and incomplete PFT information [missing Diffusing capacity of the lungs for carbon monoxide (DLCO) for example].
Although there are potential advantages to using 4DCT‐ventilation in the surgical setting; 4DCT‐ventilation also has some limitations. While 4DCTs are common in thoracic radiation oncology, breathing‐based, 4DCTs are not common in radiology. Most gated (4D) CTs acquired in radiology are gated by the cardiac signal47 or are dynamically synced with contrast injection.48 The calculation techniques described by Eslick et al.36 do not require a full 4DCT but rather breath‐hold images, while our calculation are based on an entire 4DCT dataset. Breath‐hold CT images are generally less prone to motion artifacts than 4DCT while 4DCT images provide more flexibility in terms of ventilation image calculation options. Future work will have to evaluate whether it is sufficient to calculate CT‐based ventilation images from breath‐hold CTs or whether 4DCTs are necessary. The calculation techniques of 4DCT‐ventilation are still being optimized. 4DCT‐ventilation image quality can be affected by the quality of the 4DCT,49 deformable image registration algorithm,50 calculation technique,11 and normalization methods.51 We attempted to mitigate the impact of 4DCT and deformable image registration quality by manually reviewing the 4DCT images and the registration results. With improved segmentation, registration, and ventilation image processing techniques we believe the quality of 4DCT‐ventilation for surgical assessment could be further improved. To facilitate a direct comparison between 4DCT‐ventilation and the current standard of care methods with nuclear medicine imaging, we used methods previously presented32 and calculated %PPO FEV1 values using geometrical thirds rather anatomically segmenting the lung lobes. Previous work has found that contouring the lung lobes and using functional imaging to calculate anatomically accurate %PPO FEV1 can produce results that differ statistically from the geometrical methods32, 36 and produce more accurate post‐operative prediction when compared to true, post‐operative PFTs.33, 35 In future work, we will aim to contour the lung lobes to calculate 4DCT‐ventilation‐based %PPO FEV1 values and specifically assess the differences between anatomical methods of calculating PPO FEV1 and geometrical methods.
5. Conclusion
Our study is one of the first to provide data comparing 4DCT‐ventilation, to the current standard in surgical evaluation imaging. The correlation coefficients of 0.81 and 0.99 and high sensitivity, specificity and accuracy results demonstrate good agreement between %PPO FEV1 calculated with 4DCT‐ventilation and nuclear medicine. The data presented in the current work provide a strong rationale for a prospective clinical trial to assess 4DCT‐ventilation as a lung function imaging modality for surgical resection evaluation. 4DCT‐ventilation is an innovative technology developed in radiation oncology that has great potential to translate to the surgical domain. 4DCT‐ventilation can reduce the cost and imaging time for patients and while providing improved spatial accuracy and quantitative results for thoracic surgeons.
Conflicts of interest
The authors have no relevant conflicts of interest to disclose.
Acknowledgments
This work was partially funded by grant R01CA200817 (YV, LS, MM, BK, EC, RC, TG), 1K01‐CA‐181292‐01 (RC), and State of Colorado grant (YV).
References
- 1. Beckles MA, Spiro SG, Colice GL, Rudd RM. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest J. 2003;123:105S–114S. [DOI] [PubMed] [Google Scholar]
- 2. Ferguson MK, Watson S, Johnson E, Vigneswaran WT. Predicted postoperative lung function is associated with all‐cause long‐term mortality after major lung resection for cancer. Eur J Cardio‐Thorac Surg. 2014;45:660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rietzel E, Pan TS, Chen GTY. Four‐dimensional computed tomography: image formation and clinical protocol. Med Phys. 2005;32:874–889. [DOI] [PubMed] [Google Scholar]
- 4. Vedam SS, Keall PJ, Kini VR, Mostafavi H, Shukla HP, Mohan R. Acquiring a four‐dimensional computed tomography dataset using an external respiratory signal. Phys Med Biol. 2003;48:45. [DOI] [PubMed] [Google Scholar]
- 5. Guerrero T, Sanders K, Castillo E, et al. Dynamic ventilation imaging from four‐dimensional computed tomography. Phys Med Biol. 2006;51:777–791. [DOI] [PubMed] [Google Scholar]
- 6. Reinhardt JM, Ding K, Cao K, Christensen GE, Hoffman EA, Bodas SV. Registration‐based estimates of local lung tissue expansion compared to xenon CT measures of specific ventilation. Med Image Analy. 2008;12:752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vinogradskiy Y, Koo PJ, Castillo R, et al. Comparison of 4‐dimensional computed tomography ventilation with nuclear medicine ventilation‐perfusion imaging: a clinical validation study. Int J Radiat Oncol Biol Phys. 2014;89:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamamoto T, Kabus S, Lorenz C, et al. Pulmonary ventilation imaging based on 4‐dimensional computed tomography: comparison with pulmonary function tests and SPECT ventilation images. Int J Radiat Oncol Biol Phys. 2014;90:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castillo R, Castillo E, McCurdy M, et al. Spatial correspondence of 4D CT ventilation and SPECT pulmonary perfusion defects in patients with malignant airway stenosis. Phys Med Biol. 2012a;57:1855–1871. [DOI] [PubMed] [Google Scholar]
- 10. Mathew L, Wheatley A, Castillo R, et al. Hyperpolarized He‐3 magnetic resonance imaging: comparison with four‐dimensional X‐ray computed tomography imaging in lung cancer. Acad Radiol. 2012;19:1546–1553. [DOI] [PubMed] [Google Scholar]
- 11. Kipritidis J, Siva S, Hofman MS, Callahan J, Hicks RJ, Keall PJ. Validating and improving CT ventilation imaging by correlating with ventilation 4D‐PET/CT using 68Ga‐labeled nanoparticles. Med Phys. 2014;41:011910. [DOI] [PubMed] [Google Scholar]
- 12. Brennan D, Schubert L, Diot Q, et al. Clinical validation of 4‐dimensional computed tomography ventilation with pulmonary function test data. Int J Radiat Oncol Biol Phys. 2015;92:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vinogradskiy YY, Castillo R, Castillo E, Chandler A, Martel MK, Guerrero T. Use of weekly 4DCT‐based ventilation maps to quantify changes in lung function for patients undergoing radiation therapy. Med Phys. 2012;39:289–298. [DOI] [PubMed] [Google Scholar]
- 14. Bayouth J, Du K, Christensen G, Smith B, Buatti J, Reinhardt J. Establishing a relationship between radiosensitivity of lung tissue and ventilation. Int J Radiat Oncol Biol Phys. 2012;84:S31–S32. [Google Scholar]
- 15. Vinogradskiy Y, Castillo R, Castillo E, et al. Use of 4‐dimensional computed tomography‐based ventilation imaging to correlate lung dose and function with clinical outcomes. Int J Radiat Oncol Biol Phys. 2013;86:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yaremko BP, Guerrero TM, Noyola‐Martinez J, et al. Reduction of normal lung irradiation in locally advanced non‐small‐cell lung cancer patients, using ventilation images for functional avoidance. Int J Radiat Oncol Biol Phys. 2007;68:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto T, Kabus S, von Berg J, Lorenz C, Keall PJ. Impact of four‐dimensional computed tomography pulmonary ventilation imaging‐based functional avoidance for lung cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2011a;79:279–288. [DOI] [PubMed] [Google Scholar]
- 18. Huang T‐C, Hsiao C‐Y, Chien C‐R, Liang J‐A, Shih T‐C, Zhang GG. IMRT treatment plans, functional planning with functional lung imaging from 4D‐CT for thoracic cancer patients. Oncol Radiat 2013;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoover DA, Capaldi DPI, Sheikh K, et al. Functional lung avoidance for individualized radiotherapy (FLAIR): study protocol for a randomized, double‐blind clinical trial. BMC Cancer. 2014;14:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamoto T, Kabus S, Bal M, Keall P, Benedict S, Daly M. The first patient treatment of computed tomography ventilation functional image‐guided radiotherapy for lung cancer. Radiother Oncol. 2016;118:227–231. [DOI] [PubMed] [Google Scholar]
- 21. Brunelli A, Kim AW, Berger KI, Addrizzo‐Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest J. 2013;143:e166S–e190S. [DOI] [PubMed] [Google Scholar]
- 22. The A.C.R. A.B.R. W. Group . ACR/ABR clinical statement on credentialing and privileging of radiologists for diagnostic nuclear medicine, including multimodality hybrid imaging. J Am Coll Radiol. 2011;8:617–621. [DOI] [PubMed] [Google Scholar]
- 23. Brunelli A, Refai M, Salati M, Xiumé F, Sabbatini A. Predicted versus observed FEV 1 and DLCO after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg. 2007;83:1134–1139. [DOI] [PubMed] [Google Scholar]
- 24. Giordano A, Calcagni ML, Meduri G, Valente S, Galli G. Perfusion lung scintigraphy for the prediction of postlobectomy residual pulmonary function. Chest J. 1997;111:1542–1547. [DOI] [PubMed] [Google Scholar]
- 25. Ladurie ML, Ranson‐Bitker B. Uncertainties in the expected value for forced expiratory volume in one second after surgery. Chest J. 1986;90:222–228. [DOI] [PubMed] [Google Scholar]
- 26. Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139:902–910. [DOI] [PubMed] [Google Scholar]
- 27. Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced‐based clinical practice guidelines. Chest J. 2007;132:161S–177S. [DOI] [PubMed] [Google Scholar]
- 28. Tafuro F, Corradi M, Mutti A. Interpretative strategies of lung function tests: obstructive pattern. Med Lav. 2014;105:197–213. [PubMed] [Google Scholar]
- 29. Castillo R, Castillo E, Martinez J, Guerrero T. Ventilation from four‐dimensional computed tomography: density versus Jacobian methods. Phys Med Biol. 2010;55:4661–4685. [DOI] [PubMed] [Google Scholar]
- 30. Castillo E, Castillo R, White B, Rojo J, Guerrero T. Least median of squares filtering of locally optimal point matches for compressible flow image registration. Phys Med Biol. 2012b;57:4827–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parker JA, Coleman RE, Grady E, et al. SNM practice guideline for lung scintigraphy 4.0. J Nucl Med Technol. 2012;40:57–65. [DOI] [PubMed] [Google Scholar]
- 32. Toney LK, Wanner M, Miyaoka RS, Alessio AM, Wood DE, Vesselle H. Improved prediction of lobar perfusion contribution using technetium‐99m–labeled macroaggregate of albumin single photon emission computed tomography/computed tomography with attenuation correction. J Thorac Cardiovasc Surg. 2014;148:2345–2352. [DOI] [PubMed] [Google Scholar]
- 33. Ohno Y, Koyama H, Nogami M, et al. State‐of‐the‐art radiological techniques improve the assessment of postoperative lung function in patients with non‐small cell lung cancer. Eur J Radiol. 2011;77:97–104. [DOI] [PubMed] [Google Scholar]
- 34. Ohno Y, Hatabu H, Higashino T, et al. Dynamic perfusion MRI versus perfusion scintigraphy: prediction of postoperative lung function in patients with lung cancer. Am J Roentgenol. 2004;182:73–78. [DOI] [PubMed] [Google Scholar]
- 35. Ohno Y, Koyama H, Nogami M, et al. Postoperative lung function in lung cancer patients: comparative analysis of predictive capability of MRI, CT, and SPECT. Am J Roentgenol. 2007;189:400–408. [DOI] [PubMed] [Google Scholar]
- 36. Eslick EM, Bailey DL, Harris B, et al. Measurement of preoperative lobar lung function with computed tomography ventilation imaging: progress towards rapid stratification of lung cancer lobectomy patients with abnormal lung function. Eur J Cardio‐Thorac Surg. 2016;49:1075–1082. [DOI] [PubMed] [Google Scholar]
- 37. Parker JA, Coleman RE, Siegel BA, Sostman HD, McKusick KA, Royal HD. Procedure guideline for lung scintigraphy: 1.0. J Nucl Med. 1996;37:1906–1910. [PubMed] [Google Scholar]
- 38. Mori S, Ko S, Ishii T, Nishizawa K. Effective doses in four‐dimensional computed tomography for lung radiotherapy planning. Med Dosim. 2009;34:87–90. [DOI] [PubMed] [Google Scholar]
- 39. Wu M‐T, Pan H‐B, Chiang AA, et al. Prediction of postoperative lung function in patients with lung cancer: comparison of quantitative CT with perfusion scintigraphy. Am J Roentgenol. 2002;178:667–672. [DOI] [PubMed] [Google Scholar]
- 40. Delbeke D, Coleman RE, Guiberteau MJ, et al. Procedure guideline for SPECT/CT imaging 1.0. J Nucl Med. 2006;47:1227–1234. [PubMed] [Google Scholar]
- 41. Piai DB, Quagliatto R, Toro I, Neto CC, Etchbehere E, Camargo E. The use of SPECT in preoperative assessment of patients with lung cancer. Eur Respir J. 2004;24:258–262. [DOI] [PubMed] [Google Scholar]
- 42. Wernly JA, DeMeester TR, Kirchner PT, Myerowitz PD, Oxford DE, Golomb HM. Clinical value of quantitative ventilation‐perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg. 1980;80:535–543. [PubMed] [Google Scholar]
- 43. Pioped I. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990;263:2753. [DOI] [PubMed] [Google Scholar]
- 44. Seppenwoolde Y, Muller SH, Theuws J, et al. Radiation dose‐effect relations and local recovery in perfusion for patients with non–small‐cell lung cancer. Int J Radiat Oncol Biol Phys. 2000;47:681–690. [DOI] [PubMed] [Google Scholar]
- 45. Theuws JCM, Seppenwoolde Y, Kwa SLS, et al. Changes in local pulmonary injury up to 48 months after irradiation for lymphoma and breast cancer. Int J Radiat Oncol Biol Phys. 2000;47:1201–1208. [DOI] [PubMed] [Google Scholar]
- 46. Bell J, McGivern D, Bullimore J, Hill J, Davies ER, Goddard P. Diagnostic imaging of post‐irradiation changes in the chest. Clin Radiol. 1988;39:109–119. [DOI] [PubMed] [Google Scholar]
- 47. Ohnesorge B, Flohr T, Becker C, et al. Cardiac imaging by means of electrocardiographically gated multisection spiral CT: initial experience 1. Radiol. 2000;217:564–571. [DOI] [PubMed] [Google Scholar]
- 48. Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. Am J Roentgenol. 2001;176:667–673. [DOI] [PubMed] [Google Scholar]
- 49. Yamamoto T, Kabus S, Lorenz C, et al. 4D CT lung ventilation images are affected by the 4D CT sorting method. Med Phys. 2013;40:101907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamamoto T, Kabus S, Klinder T, et al. Four‐dimensional computed tomography pulmonary ventilation images vary with deformable image registration algorithms and metrics. Med Phys. 2011b;38:1348–1358. [DOI] [PubMed] [Google Scholar]
- 51. Du K, Reinhardt JM, Christensen GE, Ding K, Bayouth JE. Respiratory effort correction strategies to improve the reproducibility of lung expansion measurements. Med Phys. 2013;40:123504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vinogradskiy Y, Schubert L, Diot Q, et al. Regional lung function profiles of stage I and III lung cancer patients: an evaluation for functional avoidance radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]


