Abstract
Parkinson’s Disease (PD) is a chronic progressive neurodegenerative disease. Current treatments for PD are symptomatic and only increase striatal dopamine levels. Proactive neuroprotective approaches that slow the progression of PD and maintain appropriate dopamine neuron populations are needed to treat the disease. One suggested mechanism contributing to the pathology of PD involves the binding of cyclin-dependent kinase 5 (Cdk5) to p25, creating a hyperactivated complex to induce cell death. The objective of this study is to investigate the neuroprotective and neurorestorative properties of Truncated Peptide 5 (TP5), a derivative of the p35 activator involved in Cdk5 regulation, via the inhibition of Cdk5/p25 complex function. SH-SY5Y cell line and the nematode Caenorhabditis elegans were exposed to paraquat (PQ), an oxidative stressor, to induce Parkinsonian phenotypes. TP5 was administered prior to PQ exposure to determine its neuroprotective effects and, in further experiments, after PQ exposure to examine its neurorestorative effects. In the SH-SY5Y cell line, TP5 was found to have neuroprotective effects using a cell viability assay and demonstrated neuroprotective and neurorestorative effects in C. elegans by examining dopaminergic neurons and dopamine-dependent behaviour. TP5 decreased elevated Cdk5 activation in worms that were exposed to PQ. TP5’s inhibition of Cdk5/p25 hyperactivity led to the protection of dopamine neurons in these PD models. This suggests that TP5 can act as a potential therapeutic drug towards PD.
Keywords: Cdk5/p25, TP5, Paraquat, Parkinson’s disease, SH-SY5Y cells, Caenorhabditis elegans, Dopaminergic neurons
Graphical abstract
Highlights
-
•
Truncated Peptide 5 (TP5) is tested in SH-SY5Y culture cells and the worm C. elegans.
-
•
TP5 protects and/or restores dopaminergic neurons in both Parkinson’s disease models.
-
•
TP5 shows promising therapeutic effects in the worm system.
-
•
Beneficial effects of TP5 are likely due to the reduced Cdk5/p25 hyperactivity.
1. Introduction
Parkinson’s disease (PD) is a chronic progressive neurodegenerative disease that affects approximately 41 people out of 100 000 over the age of 40, and 1900 people out of 100 000 over 80, making PD the second most common neurodegenerative disease after Alzheimer’s disease (AD) (Cacabelos, 2017). This demonstrates the need for a therapeutic treatment as there are currently no available treatments that can slow the progression or prevent the pathology of this neurodegenerative disease. Existing treatments, such as dopamine agonists, levodopa, and monoamine oxidase type B inhibitors, focus on alleviating symptoms by increasing levels of striatal dopamine or stimulating dopamine receptors, which only provide temporary relief and can possibly lead to adverse effects (Cacabelos, 2017; Ellis and Fell, 2017; Miyazaki and Asanuma, 2008). A disease modifying drug that can affect the underlying pathophysiology of Parkinson’s Disease is required to alleviate the symptoms from worsening without adverse effects.
Truncated Peptide 5 (TP5), a novel synthetic peptide, has shown to be disease modifying in other studies of neurodegeneration (Zheng et al., 2010). This synthetic peptide contains an 11 amino acid sequence derived from the transactivator of transcription (TAT) protein that is conjugated at the C-terminus. The TAT protein not only penetrates plasma membranes but facilitates the passage of the blood brain barrier as well. TP5 has led to promising results as it was tested initially in AD models, specifically the prevention of increased tau hyperphosphorylation and cell apoptosis in cortical neurons treated with β-amyloid, a marker of AD (Zheng et al., 2010). When mice with AD pathology were pretreated with TP5, cellular dysfunctions, such as neuroinflammation and increased oxidative stress, were also inhibited (Shukla et al., 2013). In addition, behavioural tests demonstrated that mice with AD pathology had improvement in working memory. TP5 has also led to neuroprotective effects in mesencephalic primary cultures and in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to generate PD’s pathology (Binukumar et al., 2015). Although the MPTP model has been widely used in the PD field, its findings cannot be generally applied to other forms of PD due to the idiopathic nature of this disease. Therefore, different models are necessary to determine the underlying pathology and validate the effectiveness of therapeutic treatments for PD.
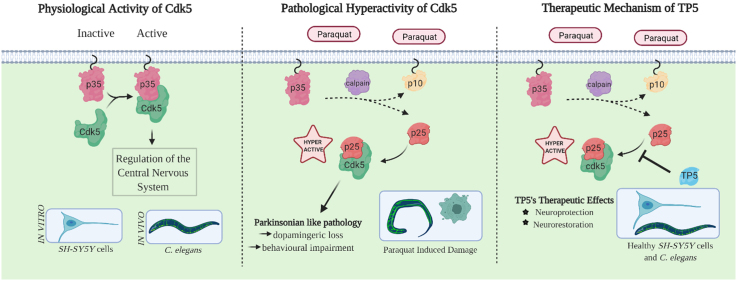
We chose 1,1′-dimethyl- 4,4′-bipyridium (PQ) as our toxin-induced model of PD due to its clinical and biochemical phenotypes that were characteristic to PD. The exposure of this herbicide has been attributed to the symptoms of idiopathic PD (Nandipati and Litvan, 2016). PQ’s main cellular toxicity mechanism consists of mitochondrial dysfunction and increased oxidative stress (Dinis-Oliveira et al., 2006). Studies have demonstrated that mice exposed to PQ develop reduced motor activity due to a loss of dopaminergic neurons (Brooks et al., 1999; Fernagut et al., 2007). PQ has also been found to induce alpha-synuclein aggregation by specifically accelerating the fibril formation rate in vitro and in vivo (Manning-Bog et al., 2002). The clinical and pathological characteristics of PD exhibited in these models validate the use of PQ to study PD in a laboratory setting. A pathological characteristic of PD, present in many neurodegenerative diseases, is an aberrant cyclin-dependent kinase 5 (Cdk5) activity that remains to be investigated in PQ models of PD.
Cyclin-dependent kinase 5 (Cdk5) is a proline directed serine/threonine kinase that is activated when bound to its regulatory partners, such as p35 or p39; this complex is essential for the proper regulation of the central nervous system (Lopes and Agostinho, 2011). However, when multiple neurotoxic signals are present, such as increased oxidative stress or mitochondrial dysfunction, a Ca2+ influx sensor, calpain, cleaves p35 into p25 and p10. High levels of Cdk5/p25 lead to pathological consequences, resulting in neurodegenerative diseases (Wilkaniec et al., 2016). Post-mortem brain tissues of PD patients displayed elevated levels of calpain-related proteolytic formation of p25 as well as an increase in the p25/p35 immunoreactivity ratio (Alvira et al., 2008); thus, signifying the importance of Cdk5/p25 to give rise to further consequences such as Lewy body (LB) formation and dopaminergic degeneration (Avraham et al., 2007; Smith et al., 2003). Regulation of this pathway has been suggested to prevent these detrimental consequences. TP5, derived from the activator p35, has a stronger binding affinity towards Cdk5 than p25, allowing TP5 to be an effective inhibitor against the hyperactivated Cdk5/p25 complex.
In this study, we have investigated the effects of TP5 using in vitro and in vivo PQ-induced models of PD. We examined if TP5 can act as a Cdk5/p25 inhibitor against PQ that induces Parkinsonian-like properties. The SH-SY5Y catecholaminergic neuroblastoma cell line was selected as an in vitro model. The cell line was originally derived from SK-H-SH cells, that were taken from a bone marrow biopsy of a metastatic neuroblastoma patient (Biedler et al., 1978). This in vitro model is representative of PD when differentiated with all-trans retinoic acid, leading to high expression of genes that control dopamine synthesis and degradation, and dopamine transporter thereby confirming that this is an adequate model to mimic dopaminergic neurons. (Korecka et al., 2013). The nematode, Caenorhabditis elegans, was chosen as an in vivo model due to few dopaminergic neurons and the ease by which neurons can be visualized in live animals. Furthermore, C. elegans shows high genetic and neurobiological conservation with humans and pre-clinical models of PD (Ma et al., 2018). The dat-1p::YFP transgenic strain chosen in this study has been used in several other studies to confirm dopaminergic degeneration (for example see the review by Maulik et al., 2017). Additionally, this strain was used in a previous study by our group to investigate dopaminergic neurodegeneration in various toxin-treated animals (Salam et al., 2013). Overall, both models have allowed us to determine the pathology of PD and further validate the effectiveness of TP5 as a drug. This report is the first to demonstrate the effect of PQ on Cdk5/p25 activity and that TP5 inhibits the Cdk5/p25 complex function in the pathology of Parkinson’s Disease.
2. Materials and methods
2.1. Cell culture conditions
SH-SY5Y cells were obtained from American type culture collection (ATCC) and maintained in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM:F12), supplemented with heat-inactivated fetal bovine serum (FBS) (10%v/v), L-glutamine (1%v/v), and pencillin-strepomycin (1%v/v) at 37 °C in 5% C02.
2.2. Drug treatments
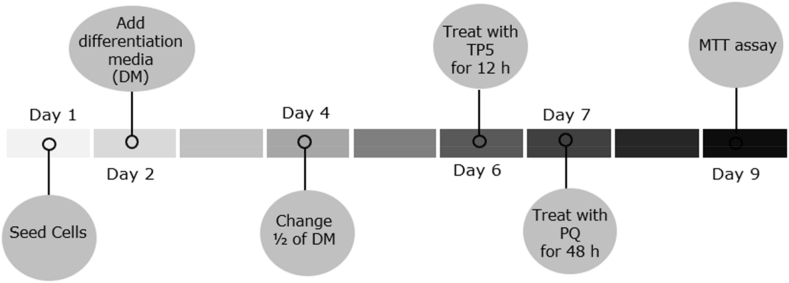
Cells were seeded at a density of 1.5 × 105 cells/ml in a 96 well plate and the experimental timeline is shown in Fig. 1. The following day, cultures were differentiated with all-trans retinoic acid (10 μM) and FBS (1%v/v). On the sixth day, cultures were treated with TP5, dissolved in ddH20, at 12.5 μM for 12 h. Then cultures were treated with PQ at 250 μM, dissolved in differentiation media, for 48 h. Cell viability assay was performed after treatment. Scrambled TP5, which consisted of the same amino acids in TP5 but organized in a different sequence, was used as a negative control. TP5 peptide and scrambled TP5 peptide were synthesized by GenScript (Piscataway, NJ).
Fig. 1.
Timeline of experimental procedure for SH-SY5Y cells.
2.3. Cell viability assay
Cell viability was determined using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay. 20 μl of MTT powder, dissolved in differentiation media, was added into each well and incubated at 37 °C for 3–4 h. MTT is metabolically converted into formazan, a precipitate, by the mitochondrial dehydrogenases of living cells. The solution was replaced with dimethyl sulfoxide (DMSO) to dissolve the precipitate and absorbance was measured at 570 nm with a microplate reader (Spectromax).
2.4. Strain and culture conditions
C. elegans worms were cultured on standard Nematode Growth Medium (NGM) agar containing plates with Escherichia coli strain OP50 as a food source (Brenner, 1974). The strain used in the study was DY328 unc-119; bhEx120[unc-119(+) + pGLC72(Cel-dat-1 5’UTR::YFP)]. The dat-1p::YFP plasmid pGLC72 was made by amplifying a 710 bp fragment of dat-1 5′ genomic region using primers GL563 (5′ AGGAAGCTTCCAGTTTTCACTAAAACGACCTCATACACTTCTC-3′) and GL564 (5′-ATGGGTACCGGCACCAACTGCATGGCTAAAAATTGTTGAG-3′). The resulting PCR product was digested with HindIII and KpnI and subcloned into pPD 136.64 (Fire lab vector, www.addgene.com). pGLC72 was injected into unc-119(ed4) animals to generate stable transgenic lines. Age-synchronized cultures were obtained by treating the strain with sodium hypochlorite and sodium hydroxide (3:2 ratio of NaOCl:NaOH). The animals were maintained at 20 °C. Day 1 adult worms were used for drug treatments as detailed below.
2.5. Drug treatments
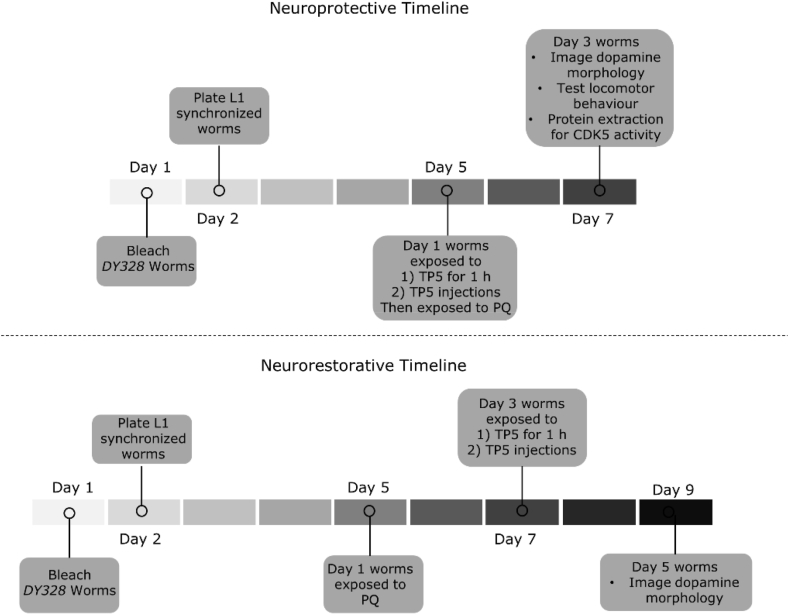
Experimental timelines of the neuroprotective and neurorestorative conditions can be seen in Fig. 2. For the neuroprotective experiment, Day 1 age synchronized adult worms were treated with 10 μM of TP5 then placed into an agar plate containing 250 μM of PQ for 48 h. There were two different techniques of administering TP5: 1) worms were suspended in 500 μL of TP5 (10 μM) inside an Eppendorf tube and placed in a rotator for 1 h; 2) picolitres of TP5 (10 μM) were microinjected into the anterior body cavity of the worm close to the terminal pharyngeal bulb but away from the region where dopaminergic neurons are located. Different routes of administration for TP5 were tested to determine which route was most effective and non-lethal for worms. After exposure to PQ for 48 h, worms were tested for dopamine-dependent locomotor behaviour, imaged for neurodegeneration, and harvested for Cdk5 kinase activity. PQ exposed worms were treated with PQ from Day 1 to Day 3, then tested for dopamine-dependent locomotor behaviour, imaged for neurodegeneration, and harvested. Two negative controls were made: worms were given ddH20 or scrambled TP5 before exposure to PQ.
Fig. 2.
Timeline of experimental procedure for C. elegans. Neuroprotective (top) and neurorestorative conditions (bottom).
For the neurorestorative experiment, Day 1 age synchronized adult worms were placed on an agar plate containing 250 μM of PQ for 48 h. Two days later (Day 3), adult worms were exposed to 1 mM of TP5. There were two different techniques of administering TP5: 1) worms were suspended in 500 μL of TP5 (1 mM) inside an Eppendorf tube and placed in a rotator for 1 h; 2) picolitres of TP5 (1 mM) were microinjected into the anterior body cavity of the worm close to the terminal pharyngeal bulb but away from the region where dopaminergic neurons are located. Different routes of administration for TP5 were tested to determine which route was most effective and non-lethal for worms. After TP5 treatment, worms were transferred to a standard NGM agar plate to recover for two days. Day 5 worms were imaged for neurodegeneration. PQ exposed worms were treated with PQ from Day 1 to Day 3, then transferred onto a standard seeded agar plate for two days to be tested for neurodegeneration. Two negative controls were made: worms were given ddH20 or scrambled TP5 after exposure to PQ.
2.6. Dopamine dependent locomotion assay
Assay plates were prepared as described (Sawin et al., 2000). The treatments groups in the neuroprotective experiment (Fig. 2, top) were examined using this assay. One condition consisted of seeded plates of Escherichia coli HB101 as a food source and another (control) without HB101. Young adults were washed off of NGM agar culture plates and placed in the center of HB101 plates, one worm at a time. After 5 min of recovery, sinusoidal body bends were counted for 20 s in three different trials. Control experiments were carried out in a similar way on plates devoid of bacteria. In all conditions, body bends were counted blindly in each treatment group.
2.7. Microscopy
Nematodes were mounted on 2% agar pad with a glass coverslip, anesthetized using 30 mM sodium azide. GFP fluorescence was visualized using a Zeiss Observer Z1 microscope equipped with an Apotome 2 and X-Cite R 120LED fluorescence illuminator.
2.8. Dopaminergic neurodegeneration
The detailed methods of scoring neurodegeneration have been described, with modifications, in (Richman et al., 2018, Taylor et al., 2021). Briefly, neuronal phenotype was visually scored by counting cell bodies of dopaminergic neurons and observing dendritic morphologies under a Zeiss Observer Z1 microscope. Typically, wild-type animals have three pairs of dopaminergic neurons with smooth dendritic/axonal projections visible in the head region. Defects in neurons result in fewer cell bodies and/or dendritic projections showing abnormal phenotypes including blebbing, punctate pattern, deformed shape, faint appearance, and complete absence. Multiple batches of animals were scored on different days and from different culture plates to ensure that results were consistent and unbiased.
Animals having defects either in cell bodies or projections were considered abnormal. Neurons were scored in day 1, 3 and 5 old adult animals. Our neuronal scoring has taken both these factors into consideration (Yin et al., 2014).
2.9. Protein extraction
Approximately 100 worms from each condition in Fig. 2 (top) were harvested for protein extraction. Initially, worms were washed with phosphate-buffered saline (PBS) three times then resuspended with 400 μL of M-per lysis buffer (Thermo Fisher), along with protease and phosphatase inhibitors. Next, worms were sonicated to create a lysate and then centrifuged to create a supernatant.
2.10. Immunoprecipitation and kinase assays
Kinase assays were performed as described previously, with modification (Binukumar et al., 2014). Briefly, Cdk5 was immunoprecipitated with polyclonal C8 antibody for 2 h at 4 °C and protein A–Sepharose beads were used to isolate immunoglobulin. Immunoprecipitates were washed three times with lysis buffer and once with 1X kinase buffer. 1X kinase buffer contained 5 mM MOPS, pH 7.4, 2.5 mM β-glycerophosphate, 1 mM EGTA, 0.4 mM EDTA, and 5 mM MgCl2. Samples were added to the reaction mix containing kinase buffer, 50 μM ATP, 20 μg of histone H1, and 0.1 mCi of [32P]ATP containing 0.1 mm DTT and 1X Halt protease and phosphatase inhibitor (Thermo Fisher) and incubated at 30 °C for 1 h. Reactions were stopped by adding Laemmli sample loading buffer, and samples were electrophoresed on 12% SDS–PAGE gels. Histone bands were visualized by Coomassie blue staining and gels were autoradiographed and were scanned on a PhosphorImager. Radioactive band density was analyzed using ImageJ.
2.11. Statistical analysis
Statistical analyses were performed using GraphPad Prism 7. Groups were analyzed by using the One-way Analysis of Variance (ANOVA) Test with Tukey Multiple Comparisons Test. Outliers were removed using a Grubbs outlier test calculator where indicated. All bars are considered to be standard error of the mean (SEM) unless indicated. P values smaller than 0.05 are considered statistically significant.
3. Results
3.1. TP5 protects differentiated SH-SY5Y cells against PQ cell induced toxicity
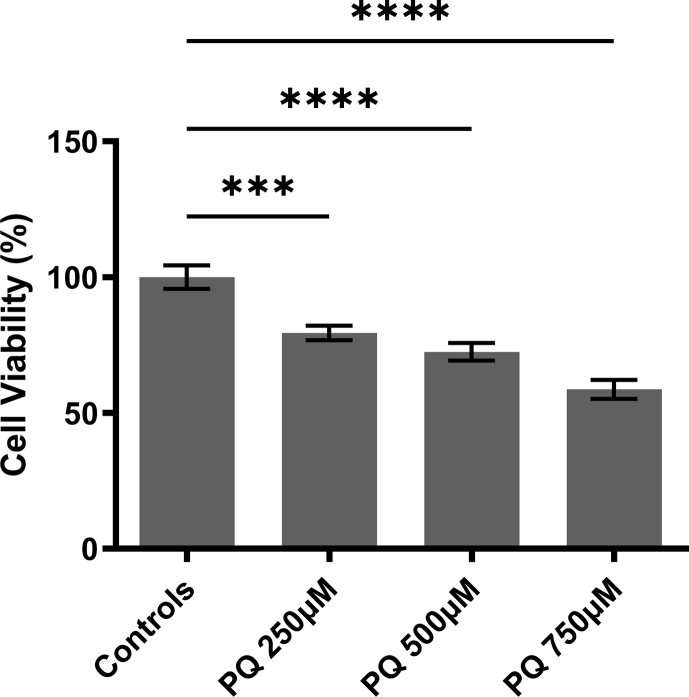
Studies that have examined PQ as a potential PD model have performed a variety of cell viability assays to determine PQ’s toxicity (Yang and Tiffany-Castiglioni, 2005). Using the MTT assay, a widely used tool to determine cell viability in vitro (Peng et al., 2004), PQ caused a significant decrease in cell viability at 48 h in a concentration-dependent manner (p < 0.001) (Fig. 3). We chose to use 250 μM of PQ in subsequent in vitro experiments to evaluate neuroprotection of TP5 in differentiated neuroblastoma cells.
Fig. 3.
Paraquat inducesconcentrationdependent cell toxicity in differentiated human neuroblastoma cells. SH-SY5Y cells were treated with increasing concentrations of PQ for 48 h then subjected to MTT assays. Cell viability is demonstrated as the percentage of healthy living cells that are normalized to controls. Results are expressed as mean ± SEM and pooled from two independent experiments, with each experiment having at least five different triplicate wells per condition. Data were analyzed using one-way ANOVA with Dunnett’s post hoc tests (∗∗∗∗p < 0.0001; ∗∗∗p < 0.001).
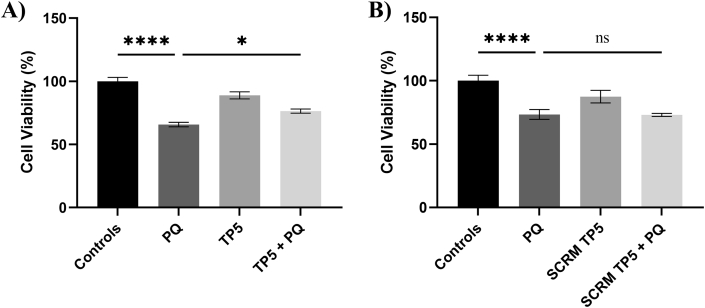
Although TP5 has been tested in mesencephalic primary cultures and mice models (Binukumar and Pant, 2016), we wanted to ensure that there were no toxicity effects with TP5 in this differentiated neuronal culture. We found 12.5 μM to be the optimal concentration of TP5’s therapeutic effect and to have no toxicity (Fig. S1). Next, we decided to treat neuronal cultures with TP5 before exposure to PQ. This sequential order was to investigate whether TP5’s mechanism of action will protect against PQ induced cell death. TP5, in combination with PQ, were found to have a significantly increased cell viability compared to cells exposed to PQ alone (p < 0.05) (Fig. 4A). Scrambled TP5, a negative control, was tested against PQ to confirm that the amino acid sequence of TP5 was indeed essential for this neuroprotective effect. As expected, scrambled TP5, in combination with PQ, had no significant differences against cells exposed to PQ alone (Fig. 4B). This result confirms that the specific sequence of TP5 and the resultant binding to Cdk5 can protect differentiated SH-SY5Y cells against PQ.
Fig. 4.
TP5 inhibits PQ-induced cellular toxicity in differentiated human neuroblastoma cells. SH-SY5Y cells were pretreated with A) TP5 (12.5 μM) or B) scrambled TP5 (12.5 μM) for 12 h then further co-treated with PQ (250 μM) for 48 h. The cultures were then subjected to MTT assays. Cell viability is demonstrated as the percentage of healthy living cells that are normalized to controls. Results are expressed as mean ± SEM and pooled from two independent experiments, each experiment having at least seven different triplicate wells per condition. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗∗∗p < 0.0001; ∗∗p < 0.01; ∗p < 0.05). A) There is a significant difference between the PQ exposed group compared to the TP5 group treated with PQ (∗p < 0.05). B) There are no significant differences between the PQ exposed group compared to the scrambled TP5 treated with PQ (p > 0.999).
3.2. TP5 protects dopamine function of Caenorhabditis elegans against PQ
The dopaminergic system of C. elegans is essential for responding to environmental stimuli, specifically bacteria as their source of food. This is an adaptive mechanism that allows worms to increase their time spent in the presence of bacteria. It has been shown that upon encountering bacteria, worms exhibit a slower movement. This behaviour, termed ‘basal slowing response,’ allows animals to maximize their feeding time (Sawin et al., 2000). Ablation of dopaminergic neurons abolishes the basal slowing response. This method has been used in other studies to demonstrate how toxins, such as lead, affect dopamine dependent behaviour (Akinyemi et al., 2019; Maulik et al., 2017). We investigated the neuroprotective effect of TP5 in worms following PQ-induced dopaminergic neurodegeneration by investigating C. elegans’ locomotor activity using the basal slowing response assay.
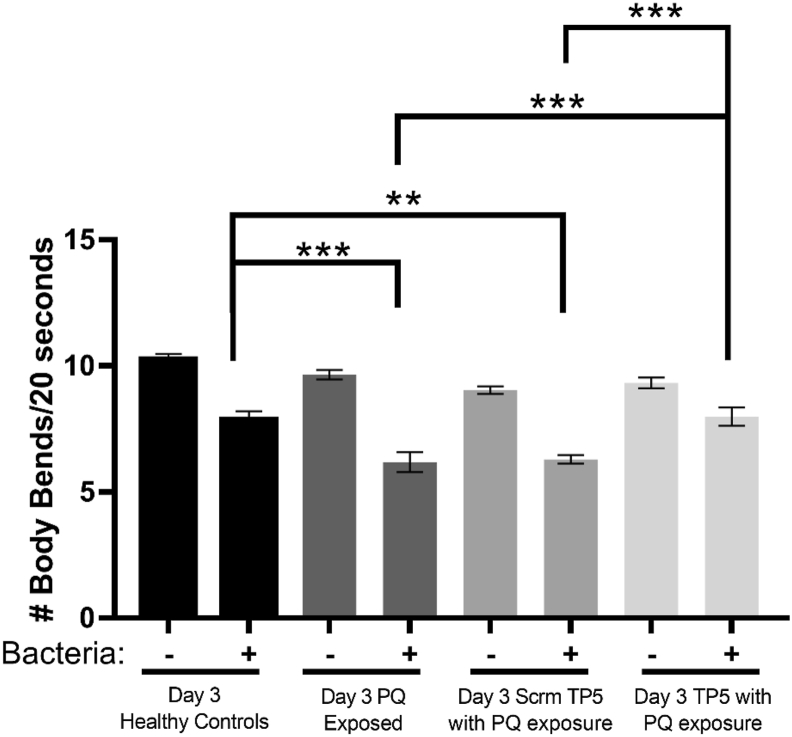
First, we confirmed if PQ can impair dopaminergic functioning in C. elegans. Day 1 adult worms were exposed to 250 μM of PQ for 48 h until they reached Day 3 of adulthood. While this treatment did not affect the locomotor activity in the absence of food, animals exhibited a significantly decreased movement response when exposed to food (p < 0.001) (Fig. 5).
Fig. 5.
TP5 protects dopamine dependent locomotor ofDY328 C. elegans against PQ. Four groups of well-fed DY328 worms were transferred to plates with (+) or without (−) bacteria and the locomotion of each worm was recorded. Groups (starting from left to right) consisted of 1) Day 3 control worms; 2) Day 3 worms that were exposed to PQ for 48 h prior; 3) Day 3 worms that were previously treated with scrambled TP5 (10 μM) for 1 h then exposed to PQ for 48 h; 4) Day 3 worms that were previously treated with TP5 (10 μM) for 1 h then exposed to PQ for 48 h. Each worm was recorded for 20 s for an average of three trials. The number of body bends are demonstrated as the locomotor activity of the worms for 20 s. Results are expressed as mean ± SEM and pooled from five independent experiments, each experiment having at least ten worms per condition. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗∗p < 0.001; ∗∗p < 0.01).
To determine if TP5 can protect dopaminergic function in C. elegans’ behaviour against PQ, we pretreated Day 1 adults with TP5 (10 μM) or scrambled TP5 (10 μM) for 1 h in the liquid environment and then transferred them to 250 μM of PQ for 48 h. The results showed that scrambled TP5 treatment caused no difference between non-PQ and PQ exposed groups. However, worms treated with TP5 followed by PQ exposure had a significant increase in their locomotor activity compared to two types of control groups consisting of PQ exposed animals, one of which were not treated with TP5 (p < 0.001) and the other were treated with scrambled TP5 (p < 0.05) (Fig. 5). Specifically, we found that TP5 treatment had a protective effect against PQ exposure such that the basal slowing response of animals was comparable to wild-type untreated controls, suggesting that TP5 protects against PQ-induced dopaminergic toxicity.
3.3. TP5 protects and restores dopaminergic neurons in C. elegans exposed to PQ
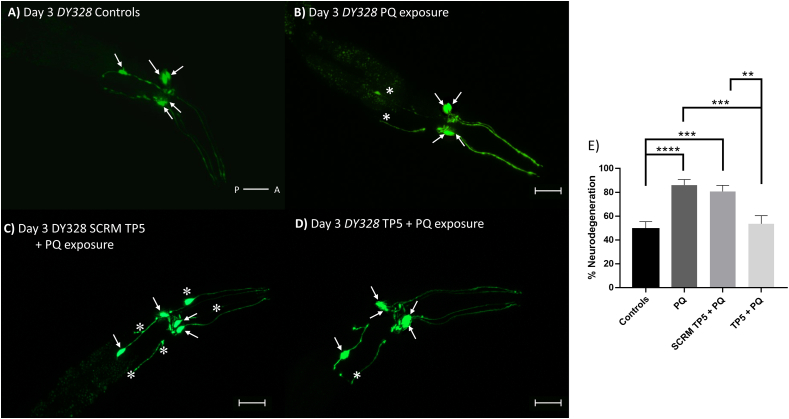
Although we have confirmed that TP5 protects the adaptive mechanism of worms against PQ through dopaminergic function, it is essential to validate whether the morphology of the dopaminergic neurons is also protected. This was done by investigating the cell bodies and trajectories of dopaminergic neurons in live animals using a dat-1p::YFP (dopamine transporter) transgenic strain (Fig. 6A–D).
Fig. 6.
TP5 provides neuroprotection against PQ in DY328 worms. Confocal images of dopaminergic neurons in Day 3 DY328 adults of control (A), PQ exposed (B), pretreated scrambled TP5 then PQ exposure (C), and pretreated with TP5 then PQ exposure (D) worms. Arrows indicate neuronal cell bodies of the CEPs and ADEs and asterisks represent any abnormalities in the neuron. Scale bar = 50 μM. E) Quantification of dopaminergic neuronal defects from groups A to D. Results are expressed as mean ± SEM and pooled from four different batches, each batch had at least 15 worms per condition. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗∗∗p < 0.0001; ∗∗∗p < 0.001; ∗∗p < 0.01).
Worms exposed to PQ solely, as well as those pre-treated with scrambled TP5 in liquid before PQ exposure, exhibited neuronal defects indicated by the asterisks in Fig. 6B and C, respectively, suggesting neurodegeneration. By contrast, controls (i.e., no treatment group) and pre-treatment with liquid TP5 followed by PQ exposure, respectively, exhibited little to no neuronal defects in Fig. 6A and D. The quantification of neuronal phenotypes in Day 3 animals showed that TP5 had a significant neuroprotective effect (Controls vs PQ-alone control: p < 0.0001, Controls vs scrambled TP5 + PQ: p < 0.001, TP5 vs PQ-alone control: p < 0.001, TP5 vs scrambled TP5 + PQ: p < 0.01) (Fig. 6E). We repeated this experiment using the injection protocol (see Methods) to determine if the trends were consistent. TP5 injected worms, which were later exposed to PQ, demonstrated significantly decreased neurodegeneration compared to control groups (PQ only p < 0.001, injected ddH20 p < 0.01) (Fig. S2). In summary, both methods demonstrate that TP5 can protect the morphology of dopaminergic neurons against PQ toxicity.
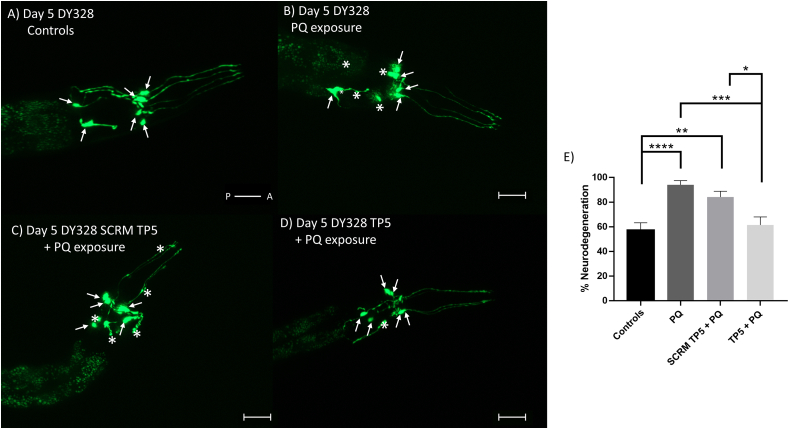
Our results have so far revealed that TP5 can protect secondary cell lines in vitro as well as the morphology and function of dopaminergic neurons in vivo against PQ. Next, we wanted to examine if TP5 has the potential for neuronal restoration. This was done to determine if TP5 can be used in clinical settings to protect dopaminergic neurons. To this end, we made a neurorestorative model to analyze dopaminergic morphology (Fig. 2, bottom). Worms exposed to PQ solely, as well as those post-treated with scrambled TP5 in a liquid environment following PQ, exhibited neuronal defects indicated by the asterisks in Fig. 7B and C, respectively, suggesting neurodegeneration. Controls (i.e., no treatment group) and those post-treated with TP5 following PQ exhibited no neuronal defects (Fig. 7A and D). The quantification of neuronal morphology in Day 5 adults demonstrated that TP5 has significant neurorestorative properties (Fig. 7E) (Controls vs PQ-alone control: p < 0.0001, Controls vs PQ + post-scrambled TP5: p < 0.01, PQ + post TP5 vs PQ-alone control: p < 0.001, PQ + post-TP5 vs PQ + post-scrambled TP5: p < 0.05). We repeated this experiment by injecting TP5 or ddH20 to determine if the trends were similar. Surprisingly, worms injected with TP5 did not show a significant decrease in neurodegeneration compared to those exposed to both PQ and ddH20 (p = 0.0604) (Fig. S3). We think that this might be due a combination of PQ exposure and injection-induced stress. PQ toxicity results in the vulnerability of animals, so the addition of injections may cause the worms to become more distressed, which is supported by a higher proportion of deaths in batches that were performed. In summary, our liquid exposure data demonstrates that 1 h TP5 exposure is an effective method for restoring the morphology of dopaminergic neuron damage caused by PQ.
Fig. 7.
TP5 induces neurorestoration against PQ in DY328 worms. Confocal images of dopaminergic neurons in Day 5 DY328 adults of control (A), PQ exposed (B), pretreated with PQ then exposed to scrambled TP5 (1 mM) for 1 h (C), and pretreated with PQ then exposed to TP5 (1 mM) for 1 h (D) worms. Arrows indicate neuronal cell bodies of the CEPs and ADEs and asterisks represent any abnormalities in neurons. Scale bar = 50 μM. E) Quantification of dopaminergic neuronal defects from groups A to D. Results are expressed as mean ± SEM and are pooled from four different batches, each batch had at least 15 worms per condition. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗∗∗p < 0.0001; ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05).
3.4. TP5 blocks Cdk5/p25 activity in C. elegans following exposure to PQ
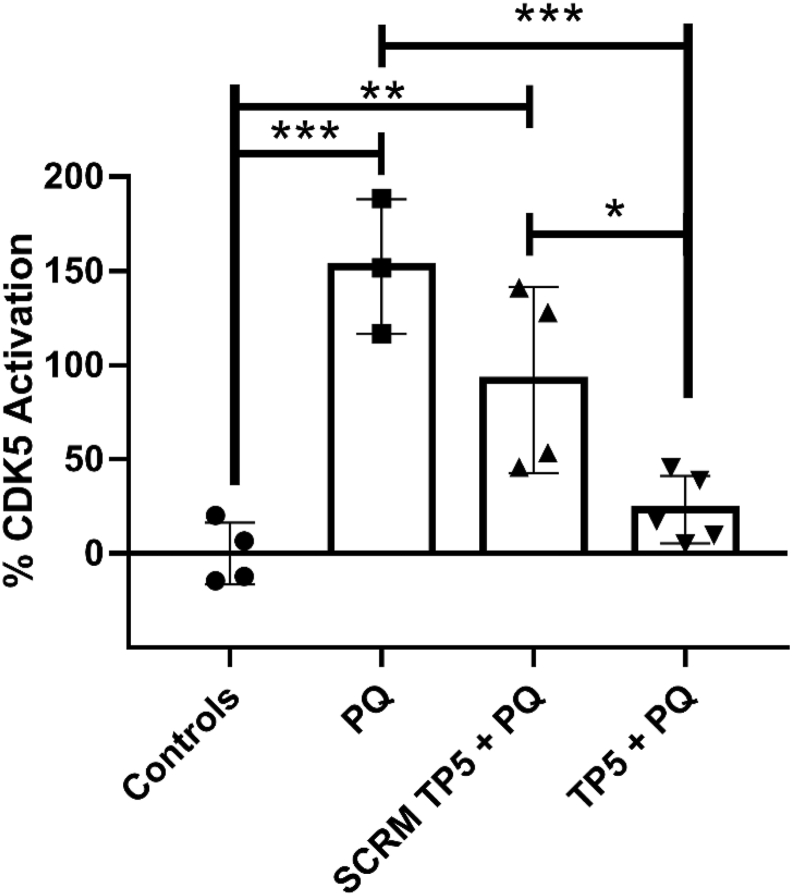
Research has shown that blocking the cleavage of p35 to p25 has a neuroprotective effect on dopaminergic neurons and causes improved locomotor activity in a PD mice model (Smith et al., 2003). Therefore, after confirming TP5’s neuroprotective effects against PQ using cellular and behavioural assays, the mechanism of this effect needs to be validated by observing Cdk5/p25 levels through the hyperactive pathway by using the immunoprecipitation and kinase assay that has been established with TP5 (Binukumar et al., 2014, 2015). This is also the first study that examines the direct relationship of PQ elevating Cdk5/p25 levels. Although TP5 has been shown to inhibit this hyperactivation in an MPTP mice model (Binukumar et al., 2015), we wanted to confirm TP5’s mechanism of action since the peptide has shown to be both neuroprotective and neurorestorative in PQ exposed worms. C. elegans in the neuroprotective timeline in Fig. 2 (top) were treated and subjected to the kinase assay. Fig. 8 demonstrates that PQ caused a significant increase in Cdk5/p25 levels compared to controls (p < 0.001). As expected, TP5 exposure in combination with PQ was found to significantly decrease the levels of Cdk5/p25 compared to worms exposed to PQ alone (p < 0.001). These results implicate TP5 having a protective effect on cellular and behavioural processes due to its ability to directly inhibit the elevated levels of Cdk5/p25 caused by PQ.
Fig. 8.
TP5 blocks elevated Cdk5/p25 levels exhibited by PQ. Conditions are demonstrated in Fig. 2 (top) and Fig. 6A–D. Cdk5 activation is demonstrated as percentage of Cdk5 activation normalized to controls. Results are expressed as mean ± SD and are pooled from four different batches, each batch had at least 100 worms per condition. One outlier was removed from the PQ group. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05).
4. Discussion
Parkinson’s Disease is hard to detect in early stages; even more so, it cannot be cured once diagnosed and there are currently no disease modifying treatments that exist. Current treatments, such as levodopa, only help patients temporarily. Long-term usage of levodopa can lead to motor and non-motor fluctuations and dyskinesia, which was demonstrated in over 80% of patients using levodopa for over 10 years (Ellis and Fell, 2017). In addition, since many patients can develop PD through familial or idiopathic causes, it is difficult to treat all patients in the same manner. This implicates the importance of toxin-induced models to investigate the pathophysiology of PD.
Many different toxins, such as MPTP, 6-hydroxydopamine (6-OHDA), and rotenone, have been used to model PD (Bové and Perier, 2012). MPTP induces some features that are characteristic of PD, such as impaired dopaminergic function; however, post-mortem samples of individuals exposed to this toxin did not show Lewy bodies, revealing an incomplete representation of the disease (Langston et al., 1999). 6-OHDA and rotenone also led to inconsistencies in PD hallmarks (Bové and Perier, 2012; Drolet et al., 2009). Thus, MPTP and other toxins demonstrate a wide variety of idiopathic cases of PD. Studies by Manning-Bog et al. (2002) and Langston et al. (1999) demonstrated that PQ led to dopaminergic cell death and increased aggregation of alpha-synuclein, the classical hallmarks of PD, further validating why this toxin was used in this study. Furthermore, the toxicity mechanism of PQ also suggests that PQ induces the hyperactivation of Cdk5/p25 mechanism. The Cdk5/p25 pathway is of great interest since this pathway diverges into further downstream effects that can exacerbate the pathology and symptomology.
Our study has shown that TP5 is an effective peptide against in vitro and in vivo PQ-induced PD-like symptoms and pathology. This is the first study that demonstrates both neuroprotective effects and neurorestorative effects in a PD model, further confirming TP5 as a potential therapeutic drug towards PD. Also, we have shown the therapeutic effects of TP5 in different models of PD, namely SH-SY5Y human neuroblastoma cultures and C. elegans that are commonly used to investigate the effects of toxins and drugs for neurodegenerative diseases.
Differentiated SH-SY5Y human neuroblastoma cultures are an effective model to study morphological, biochemical, and cytotoxic parameters related to PD. Many toxins have been tested in this cell line for PD research due to its dopaminergic properties, including high expression of genes that regulate processes such as dopamine synthesis and degradation (Korecka et al., 2013). PQ has been used in this cell line to examine its cellular mechanism (Yang and Tiffany-Castiglioni, 2008). The presence of PQ decreasing mitochondrial complex I activity and inducing apoptotic markers caspase 9 and 3 coincide with our decreased cell viability results in vitro. Specifically, TP5 effectively inhibited PQ’s toxic effect suggesting that TP5 is able to protect the SH-SY5Y cells by interfering with PQ-induced processes that cause cell death.
Although cell cultures are a great tool to study biochemical and cytotoxic parameters, they can only tell one part of the story. Most cell cultures have a monolayer structure that represents a very simple microenvironment and does not permit observation of the overall behaviour of an organism. C. elegans, with a well-defined nervous system with 302 neurons, eight of which are dopaminergic, represents a good model to study PD in vivo (Chase and Koelle, 2007). Furthermore, C. elegans possesses conserved genes involved in dopamine synthesis, transport, and signaling. As dopaminergic neurons are susceptible to PQ, we can examine dopamine-dependent behaviour such as movement in the presence of food. The transparent body of the worm also makes it possible to visually examine dopaminergic neurons in live animals for the effect of TP5 on neuroprotection and neurorestoration.
The behavioural research of Sawin et al. (2000) showed that dopaminergic neurons mediate the response towards environmental cues, which allows animals to slow down and spend longer time in the presence of food. However, the locomotion speed is higher in the absence of dopaminergic signaling. Interestingly, we observed that worms exposed to PQ had decreased speed. This might be due to differences in protocols. While Sawin et al. (2000) selectively ablated dopaminergic neurons, PQ treatment in our case does not completely remove the entire set of the neurons. Additionally, PQ may have other effects on dopaminergic signaling. For example, it was demonstrated that increased oxidative stress and mitochondrial dysfunction, induced by PQ, can interfere with locomotion (Wu et al., 2018; Taylor et al., 2021); therefore, worms exposed to PQ exhibit reduced velocity and body bends, which are consistent with our results. The main takeaway from published studies and those described in this paper, is that TP5 is able to protect against impaired dopamine-dependent behaviour.
In addition to behavioural experiments, we also used a YFP-tagged reporter for in vivo examination of neurons. It was shown earlier that PQ causes dose-dependent degeneration of dopaminergic neurons (González-Hunt et al., 2014). Similar detrimental effects have also been reported for other toxins, such as 6-OHDA (Nass and Blakely, 2003). We have demonstrated that TP5 protects the neurons against PQ exposure. Additionally, our work shows that there is clinical potential for TP5 treatment based on the neurorestoration of dopaminergic morphology against PQ.
Studies have confirmed the primary toxicity mechanism of PQ; however, the direct correlation of PQ leading to the hyperactivation of Cdk5/p25 levels has not been demonstrated (Yang and Tiffany-Castiglioni, 2005). In PQ’s induced cell toxicity, increased oxidative stress and mitochondrial dysfunction will lead to the presence of cell death signals, such as caspase 9 and 3 markers (Yang and Tiffany-Castiglioni, 2008). Neuronal death is induced by triggering apoptosis through increased activation of calpain with caspase 9 and 3, which led to the abnormal activation of Cdk5/p25 (Cagnon and Braissant, 2008). Our results are consistent with these findings and demonstrate that PQ elevates abnormal Cdk5/p25 levels in C. elegans likely through activated caspases and calpain. Furthermore, our data validate the use of PQ to generate a PD model as this toxin expresses the hallmarks of PD (Brooks et al., 1999; Fernagut et al., 2007, & Manning-Bog et al., 2002). Finally, we have shown that TP5 can inhibit the increase in Cdk5/p25 levels due to PQ exposure, which coincides with the research of Binukumar et al. (2015). Binukumar et al. (2015) examined the expression of Cdk5, p35 and p25 in Western blots and concluded that Cdk5 and p25 levels were elevated following treatment of MPTP, which has a similar toxicity mechanism to PQ. The addition of TP5 decreased p25 expressio, confirming that TP5 selectively inhibits the activity of Cdk5/p25 without affecting Cdk5/p35 as there was no change in p35 when TP5 was introduced in vivo and in vitro (Binukumar et al., 2014). Thus, we conclude that TP5 can target the aberrant activation Cdk5/p25 in our PD model, validating the neuroprotective effects of TP5 at the cellular and behavioural level.
The pathophysiological markers of PD, i.e., dopaminergic neuron degeneration and Cdk5/p25 level, were inhibited by TP5. Overall, we have demonstrated the therapeutic potential of TP5 against Parkinsonian-like symptoms, through the protection of dopaminergic neurons and inhibition of elevated Cdk5/p25 levels. Further experiments should investigate other hallmarks of PD to validate the potential of TP5 as a treatment or neuroprotective agent towards PD.
Author roles
J. Tran designed the in vitro experimental design with R. Mishra and H. Pant and in vivo experimental design with B. Gupta, S. Taylor, and A. Gupta. J. Tran conducted the in vitro experiments. J. Tran, S. Taylor and A. Gupta conducted the in vivo experiments consisting of the behavioural work and imaging. B. Gupta performed the microinjections. N. Amin performed the Cdk5 immunoreactivity. J. Tran completed all statistical analyses and wrote the manuscript. All authors were involved in reviewing and editing the manuscript.
Funding
This research was supported by Canadian Institute of Health Research grant #126004 to Ram Mishra and Natural Sciences and Engineering Research Council (NSERC) Discovery grants to Ram Mishra and Bhagwati Gupta.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
A Peer Review Overview and (sometimes) Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.crneur.2021.100006.
Contributor Information
Judith Tran, Email: tranj27@mcmaster.ca.
Shane K.B. Taylor, Email: taylos49@mcmaster.ca.
Anika Gupta, Email: anikausa22@gmail.com.
Niranjana Amin, Email: aminn@ninds.nih.gov.
Harish Pant, Email: panth@ninds.nih.gov.
Bhagwati P. Gupta, Email: guptab@mcmaster.ca.
Ram K. Mishra, Email: mishrar@mcmaster.ca.
Peer Review Overview and Supplementary data. Supplementary data
A Peer Review Overview and (sometimes) Supplementary data associated with this article:
Dose response curve of TP5 in differentiated human neuroblastoma cells. SH-SY5Y cells were treated with increasing doses of TP5 for 12 h then subjected to MTT assays. Cell viability is demonstrated as the percentage of healthy living cells that are normalized to controls. Results are expressed as mean ± SEM with at least five different triplicate wells per condition. Data were analyzed using one-way ANOVA with Dunnett’s post hoc tests (∗∗∗∗p < 0.0001; ∗∗p < 0.01; ∗p < 0.05).
Injection of TP5 induces neuroprotection against PQ in DY328 worms. TP5 (10 μM) was injected into the distal gonads of the worms prior to PQ exposure. DdH20 was used as a negative control to the injections. Quantification of dopaminergic neuronal defects in Day 3 DY328 adults of control, PQ exposed, ddH20 injected then PQ exposure, and TP5 injected then PQ exposure worms. Results are expressed as mean ± SEM and pooled from four different batches, each batch had at least 15 worms per condition. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗∗∗p < 0.0001; ∗∗∗p < 0.001; ∗∗p < 0.01).
Injection of TP5 induces neurorestoration against PQ in DY328 worms. TP5 (1 mM) was injected into the distal gonads of the worm’s prior exposure to PQ. DdH20 was used as a negative control to the injections. Quantification of dopaminergic neuronal defects in Day 5 DY328 adults of control (n = 69), PQ exposed (n = 50), PQ exposure then ddH20 injected (n = 43), and PQ exposure then TP5 injected worms (n = 33). Results are expressed as mean ± standard deviation (SD) and pooled from four different batches. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗p < 0.01).
References
- Akinyemi A.J., Miah M.R., Ijomone O.M., Tsatsakis A., Soares F., Tinkov A.A., Skalny A.V., Venkataramani V., Aschner M. Lead (Pb) exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans: involvement of the dopamine transporter. Toxicology reports. 2019;6:833–840. doi: 10.1016/j.toxrep.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvira D., Ferrer I., Gutierrez-Cuesta J., Garcia-Castro B., Pallàs M., Camins A. Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson’s disease. Park. Relat. Disord. 2008;14(4):309–313. doi: 10.1016/J.PARKRELDIS.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Avraham E., Rott R., Liani E., Szargel R., Engelender S. Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J. Biol. Chem. 2007;282(17):12842–12850. doi: 10.1074/jbc.M608243200. [DOI] [PubMed] [Google Scholar]
- Biedler J.L., Roffler-Tarlov S., Schachner M., Freedman L.S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38(11 Pt 1):3751–3757. [PubMed] [Google Scholar]
- Binukumar B.K., Pant H.C. TFP5/TP5 peptide provides neuroprotection in the MPTP model of Parkinson’s disease. Neural Regen. Res. 2016;11(5):698–701. doi: 10.4103/1673-5374.182681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binukumar B.K., Shukla V., Amin N.D., Grant P., Bhaskar M., Skuntz S., Pant H.C. Peptide TFP5/TP5 derived from Cdk5 activator P35 provides neuroprotection in the MPTP model of Parkinson’s disease. Mol. Biol. Cell. 2015;26(24):4478–4491. doi: 10.1091/mbc.E15-06-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binukumar B.K., Zheng Y.L., Shukla V., Amin N.D., Grant P., Pant H.C. TFP5, a peptide derived from p35, a Cdk5 neuronal activator, rescues cortical neurons from glucose toxicity. J Alzheimers Dis. 2014;39(4):899–909. doi: 10.3233/JAD-131784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bové J., Perier C. Neurotoxin-based models of Parkinson’s disease. Neuroscience. 2012;211:51–76. doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1002/cbic.200300625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A.I., Chadwick C.A., Gelbard H.A., Cory-Slechta D.A., Federoff H.J. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823(1–2):1–10. doi: 10.1016/S0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Cacabelos R. Parkinson’s disease: from pathogenesis to pharmacogenomics. Int. J. Mol. Sci. 2017;18(3):551. doi: 10.3390/ijms18030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnon L., Braissant O. Role of caspases, calpain and cdk5 in ammonia-induced cell death in developing brain cells. Neurobiol. Dis. 2008;32(2):281–292. doi: 10.1016/j.nbd.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Chase D.L., Koelle M.R. Biogenic amine neurotransmitters in C. elegans. Worm : the online review of C. elegans biology. 2007;1–15 doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis-Oliveira R.J., Remião F., Carmo H., Duarte J.A., Navarro A.S., Bastos M.L., Carvalho F. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology. 2006;27(6):1110–1122. doi: 10.1016/j.neuro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Drolet R.E., Cannon J.R., Montero L., Greenamyre J.T. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol. Dis. 2009;36(1):96–102. doi: 10.1016/j.nbd.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Ellis J.M., Fell M.J. Current approaches to the treatment of Parkinson’s Disease. Bioorg. Med. Chem. Lett. 2017;27(18):4247–4255. doi: 10.1016/j.bmcl.2017.07.075. [DOI] [PubMed] [Google Scholar]
- Fernagut P.O., Hutson C.B., Fleming S.M., Tetreaut N.A., Salcedo J., Masliah E., Chesselet M.F. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of α-synuclein over-expression. Synapse. 2007;61(12):991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Hunt C.P., Leung M.C., Bodhicharla R.K., McKeever M.G., Arrant A.E., Margillo K.M., Ryde I.T., Cyr D.D., Kosmaczewski S.G., Hammarlund M., Meyer J.N. Exposure to mitochondrial genotoxins and dopaminergic neurodegeneration in Caenorhabditis elegans. PloS One. 2014;9(12) doi: 10.1371/journal.pone.0114459. e114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korecka J.A., van Kesteren R.E., Blaas E., Spitzer S.O., Kamstra J.H., Smit A.B., Bossers K. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0063862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston J.W., Forno L.S., Tetrud J., Reeves A.G., Kaplan J.A., Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 1999;46(4):598–605. doi: 10.1002/1531-8249(199910)46:4<598::AID-ANA7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Lopes J.P., Agostinho P. Cdk5: multitasking between physiological and pathological conditions. Prog. Neurobiol. 2011;94(1):49–63. doi: 10.1016/j.pneurobio.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Ma L., Zhao Y., Chen Y., Cheng B., Peng A., Huang K. Caenorhabditis elegans as a model system for target identification and drug screening against neurodegenerative diseases. Eur. J. Pharmacol. 2018;819:169–180. doi: 10.1016/j.ejphar.2017.11.051. [DOI] [PubMed] [Google Scholar]
- Manning-Bog A.B., McCormack A.L., Li J., Uversky V.N., Fink A.L., Di Monte D.A. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J. Biol. Chem. 2002;277(3):1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Maulik M., Mitra S., Bult-Ito A., Taylor B.E., Vayndorf E.M. Behavioral phenotyping and pathological indicators of Parkinson’s disease in C. elegans models. Front. Genet. 2017;8:77. doi: 10.3389/fgene.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki I., Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med. Okayama. 2008;62(3):141–150. doi: 10.18926/AMO/30942. [DOI] [PubMed] [Google Scholar]
- Nandipati S., Litvan I. Environmental exposures and Parkinson’s disease. Int. J. Environ. Res. Publ. Health. 2016;13(9):881. doi: 10.3390/ijerph13090881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R., Blakely R.D. The Caenorhabditis elegans dopaminergic system: opportunities for insights into dopamine transport and neurodegeneration. Annu. Rev. Pharmacol. 2003;43(1):521–544. doi: 10.1146/annurev.pharmtox.43.100901.135934. [DOI] [PubMed] [Google Scholar]
- Peng J., Mao X.O., Stevenson F.F., Hsu M., Andersen J.K. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J. Biol. Chem. 2004;279(31):32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- Richman C., Rashid S., Prashar S., Mishra R., Selvaganapathy P.R., Gupta B.P. C. elegans MANF homolog is necessary for the protection of dopaminergic neurons and ER unfolded protein response. Front. Neurosci. 2018;12:544. doi: 10.3389/fnins.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam S., Ansari A., Amon S., Rezai P., Selvaganapathy P.R., Mishra R.K., Gupta B.P. A microfluidic phenotype analysis system reveals function of sensory and dopaminergic neuron signaling in C. elegans electrostatic swimming behavior. Worm. 2013;2(2) doi: 10.4161/worm.24558. e24558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin E.R., Ranganathan R., Horvitz H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26(3):619–631. doi: 10.1016/S0896-6273(00)81199-X. [DOI] [PubMed] [Google Scholar]
- Shukla V., Zheng Y.L., Mishra S.K., Amin N.D., Steiner J., Grant P., Pant H.C. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. Faseb. J. 2013;27(1):174–186. doi: 10.1096/fj.12-217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.D., Crocker S.J., Jackson-Lewis V., Jordan-Sciutto K.L., Hayley S., Mount M.P., Park D.S. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. Unit. States Am. 2003;100(23):13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.K.B., Minhas H.M., Tong J., Selvaganapathy P.R., Mishra R.K., Gupta B.P. C. elegans electrotaxis behavior is modulated by heat shock response and unfolded protein response signaling pathways. Sci. Rep. 2021;11(3115) doi: 10.1038/s41598-021-82466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkaniec A., Czapski G.A., Adamczyk A. Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: facts and hypotheses. J. Neurochem. 2016;136(2):222–233. doi: 10.1111/jnc.13365. [DOI] [PubMed] [Google Scholar]
- Wu S., Lei L., Song Y., Liu M., Lu S., Lou D., He D. Mutation of hop-1 and pink-1 attenuates vulnerability of neurotoxicity in C. elegans: the role of mitochondria-associated membrane proteins in Parkinsonism. Exp. Neurol. 2018;309:67–78. doi: 10.1016/j.expneurol.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Tiffany-Castiglioni E. The bipyridyl herbicide paraquat produces oxidative stress-mediated toxicity in human neuroblastoma SH-SY5Y cells: relevance to the dopaminergic pathogenesis. J. Toxicol. Environ. Health Part A. 2005;68(22):1939–1961. doi: 10.1080/15287390500226987. [DOI] [PubMed] [Google Scholar]
- Yang W., Tiffany-Castiglioni E. Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: involvement of p53 and mitochondria. J. Toxicol. Environ. Health Part A. 2008;71(4):289–299. doi: 10.1080/15287390701738467. [DOI] [PubMed] [Google Scholar]
- Yin J.A., Liu X.J., Yuan J., Jiang J., Cai S.Q. Longevity manipulations differentially affect serotonin/dopamine level and behavioral deterioration in aging Caenorhabditis elegans. J. Neurosci. 2014;34(11):3947–3958. doi: 10.1523/JNEUROSCI.4013-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.L., Amin N.D., Hu Y.F., Rudrabhatla P., Shukla V., Kanungo J., Pant H.C. A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J. Biol. Chem. 2010;285(44):34202–34212. doi: 10.1074/jbc.M110.134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dose response curve of TP5 in differentiated human neuroblastoma cells. SH-SY5Y cells were treated with increasing doses of TP5 for 12 h then subjected to MTT assays. Cell viability is demonstrated as the percentage of healthy living cells that are normalized to controls. Results are expressed as mean ± SEM with at least five different triplicate wells per condition. Data were analyzed using one-way ANOVA with Dunnett’s post hoc tests (∗∗∗∗p < 0.0001; ∗∗p < 0.01; ∗p < 0.05).
Injection of TP5 induces neuroprotection against PQ in DY328 worms. TP5 (10 μM) was injected into the distal gonads of the worms prior to PQ exposure. DdH20 was used as a negative control to the injections. Quantification of dopaminergic neuronal defects in Day 3 DY328 adults of control, PQ exposed, ddH20 injected then PQ exposure, and TP5 injected then PQ exposure worms. Results are expressed as mean ± SEM and pooled from four different batches, each batch had at least 15 worms per condition. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗∗∗p < 0.0001; ∗∗∗p < 0.001; ∗∗p < 0.01).
Injection of TP5 induces neurorestoration against PQ in DY328 worms. TP5 (1 mM) was injected into the distal gonads of the worm’s prior exposure to PQ. DdH20 was used as a negative control to the injections. Quantification of dopaminergic neuronal defects in Day 5 DY328 adults of control (n = 69), PQ exposed (n = 50), PQ exposure then ddH20 injected (n = 43), and PQ exposure then TP5 injected worms (n = 33). Results are expressed as mean ± standard deviation (SD) and pooled from four different batches. Data were analyzed using one-way ANOVA with Tukey’s post hoc tests (∗∗p < 0.01).