Abstract
More and more assays for the serodiagnosis of Lyme borreliosis (LB) are based on recombinant antigens. However, so far, there is no consensus as to which are the most specific and sensitive proteins and how they should be used in combination to obtain tests with the best discrimination abilities. The present study was preceded by a detailed analysis of Western blots (WB) using whole-cell lysates of Borrelia burgdorferi sensu stricto strain PKa2, B. afzelii PKo, and B. garinii PBi (U. Hauser, G. Lehnert, R. Lobentanzer, and B. Wilske, J. Clin. Microbiol. 35:1433–1444, 1997). For the present work, the data bank from that study, containing information about the reactivities of 330 sera (from patients at different stages of LB [n = 189]; control group, n = 141), was reused. The specificities and sensitivities of various combinations of proteins from different strains were calculated for different interpretation criteria. For immunoglobulin G (IgG) WB, the recommended combination of antigens available to date as recombinant proteins included p83/100 of PKa2, p83/100 of PKo, p39 of PKo, p39 of PBi, and OspC of PBi (interpretation criterion, at least one reactive band required for a positive WB; specificity, 96.5%; sensitivity, 56.1%). The further addition of p58 of PKo, p17 of PKo, or p14 of PKo was most favorable in terms of both a considerable gain of sensitivity and little loss of specificity. IgG Western blotting with a whole-cell lysate of strain PKo might be improved by the addition of OspC of PBi. For IgG WB, the best combination, out of all bands, was p83/100, p58, p39, p30, and p21 of all three strains and OspC of PBi, p17b of PBi, p56 of PKa2, p43 of PKo, p17 of PKo, and p14 of PKo (interpretation criterion, at least two reactive bands required for a positive WB; specificity, 97.2%; sensitivity, 61.4%). An interpretation criterion of at least two reactive bands is more reliable than one of only one reactive band. For IgM WB, the best combination was OspC of PKo, OspC of PBi, p39 of all three strains, p17 of PKo, and strong reactions with p41 of all three strains (interpretation criterion, at least one reactive band required; specificity, 97.9%; sensitivity, 47.0%).
Lyme borreliosis (LB) is a global disease caused by the tick-borne pathogen Borrelia burgdorferi sensu lato. This disorder develops in stages and with different manifestations involving mainly the skin, the central nervous system, and the joints. In Europe, three species pathogenic for humans (3) and at least eight outer surface protein A (OspA) serotypes of B. burgdorferi sensu lato (40, 43) are known, demonstrating both the inter- and intraspecies heterogeneity. The diagnosis of Lyme disease is based on the recognition of typical clinical signs and is assisted by laboratory tests, especially if the clinical picture is not clear. Since culture is laborious and insensitive and PCRs are still deemed controversial (27), routine testing comprises mostly serological methods.
Several strategies have been developed to increase both the sensitivity and specificity (i.e., the discriminating ability) of these tests. These include preabsorption of cross-reactive antibodies with Treponema phagedenis (48), the use of detergent extracts of B. burgdorferi sensu lato (5), and the use of purified flagella (17) or various recombinant antigens (7, 32, 35, 41, 42).
In the United States, the Centers for Disease Control and Prevention recommend a two-step protocol for the evaluation of sera: an enzyme immunoassay should be used for screening, and positive results should be confirmed by Western blotting (1). However, the interpretation criteria for a positive Western blot (WB) recommended by the Centers for Disease Control and Prevention cannot be used for serodiagnosis in Europe, since in this region the immunoreactivity of the sera is rather restricted, especially in the early stages of the disease, and the heterogeneity of European strains further complicates the situation (2, 9, 18, 30).
The present work was preceded by a study leading to the development of interpretation criteria for WB with three European species (18). However, this method should be used only if sufficient resolution of bands and careful performance and interpretation are ensured. WB prepared by using recombinant antigens are easier to interpret, and as a further advantage, antigens derived from different species can be used (42). However, in our experience, assays using the antigens available to date as recombinant proteins do not reach the same sensitivities as tests using whole-cell preparations of B. burgdorferi sensu lato (unpublished data).
Our preceding analysis of WB of whole-cell lysates (18) included identification of a series of key proteins with monoclonal antibodies and densitometric determination of the molecular masses of all visually distinguishable bands separately for each of the three strains. In that study, a database containing information about the reactivities of 330 sera in both immunoglobulin G (IgG) and IgM WB with three strains each (a total of six assays per serum sample; more than 40 bands per strain were distinguished) was created.
For the present work, this database was reused to analyze the following issues. (i) What are the specificities and sensitivities of the antigens molecularly characterized to date and, thus, presently available as recombinant proteins? (ii) What is the optimal combination of these antigens in WB in terms of both specificity and sensitivity? (iii) Which proteins should be added to increase the discriminating ability further? (iv) Could assays using whole-cell preparations be improved by addition of individual antigens from heterologous strains? (v) What would be the optimal combination of antigens if they were all available as recombinant proteins? All these questions were evaluated for both IgG and IgM assays. Although these analyses are based on data obtained by Western blotting of native antigens and the immunoreactivity of recombinant proteins might be somewhat different, this work should give important implications for further development of improved serological tests.
MATERIALS AND METHODS
For this work, a data bank created during a previous study was reused, and no additional laboratory investigations were performed. Thus, materials, laboratory methods, and identification and designation of bands in WB have already been described in detail previously (18).
Sera.
Sera of the following study groups, comprising patients with LB (n = 189) and controls (n = 141), were investigated. A total of 66 serum specimens were obtained from unselected, untreated patients with erythema migrans. A neuroborreliosis group (n = 83) included 39 patients in which B. burgdorferi sensu lato was isolated from the cerebrospinal fluid (CSF) and 44 other patients with typical signs of acute neuroborreliosis, CSF pleocytosis, and specific CSF/serum IgG ratios of ≥2.0 (39). A late-stage LB group (n = 40) comprised 30 patients with acrodermatitis chronica atrophicans and 10 patients with Lyme arthritis. Possible differential diagnoses had been excluded. The control group consisted of 120 healthy blood donors, 11 patients with stage II or III syphilis, and 10 patients whose sera contained rheumatoid factor at ≥45 IE/ml. The healthy blood donors had no history of frequent tick bites, erythemas, neurological symptoms, or joint disorders.
Preparation of antigens.
Borrelial strains PKa2 (B. burgdorferi sensu stricto, OspA serotype 1), PKo (B. afzelii, OspA serotype 2), and PBi (B. garinii, OspA serotype 4) (43) were used for antigen preparations. Only passages of strains abundantly expressing OspA and OspC were taken for this study.
Western blotting. Cell lysate was electrophoresed (24) on 12.5% polyacrylamide gels of 17 cm in length. Proteins were transferred to nitrocellulose by semidry blotting (23). After blockage of nonspecific binding sites with a solution containing 50 mM Tris-OH–HCl (pH 7.4), 200 mM NaCl, 0.1% Tween 20, and 5% nonfat dry milk for 1 h at 37°C, the membranes were incubated overnight in sera diluted 1:200 (for IgG WB) or 1:100 (for IgM WB), washed, and incubated with horseradish peroxidase-conjugated anti-human IgG or IgM antibodies, respectively (Dakopatts, Copenhagen, Denmark) (dilutions, 1:1,000 for IgG and 1:500 for IgM). Color was developed by adding diaminobenzidine and H2O2.
Analysis of WB and evaluation of various interpretation criteria.
Blots were assessed blindly, always by the same person, after reproducible identification of all bands was established. Band intensities were determined semiquantitatively by visual comparison with WB of defined control sera and defined as very faint (interpreted as negative), weak (interpreted as positive), strong, or very strong. Data (reactivities with all visually distinguishable bands, i.e., more than 40 bands per strain) were imported into a data bank for further analyses. For the present study, interpretation criteria consisting of combinations of reactive bands were evaluated systematically. This was achieved by means of queries which selected subsets of data from the database. Specificities were calculated on the basis of the reactivities of the control group sera (n = 141), and sensitivities were determined from the data of all sera from LB patients (n = 189 for IgG WB, n = 149 for IgM WB; sera from patients with late LB were not tested for IgM). For example, the criterion stating that for a WB to be positive, there must be at least one reactive band of p83/100 of PKa2, p83/100 of PKo, and p39 of PBi resulted in a specificity of 100% and a sensitivity of 41.8%. For a subset of n bands, a total of 2n combinations are possible. Calculations were performed with Microsoft Excel, version 5.0, as well as self-written programs using Borland Turbo Pascal, version 7.0. Putative criteria were sorted according to the resulting overall sensitivities and specificities. From all band combinations resulting in the same specificity, only those with the highest sensitivities were extracted for the tables shown in Results.
Statistics.
When appropriate, results were analyzed by one-sided χ2 tests (loss of specificity and gain of sensitivity [see Tables 4 and 5]).
TABLE 4.
Evaluation of addition of various antigens to IgG WB containing the protein combinationsa shown in Table 2
| Antigen/strain | Loss of specificity (%) | Gain of sensitivity (%) |
|---|---|---|
| p58/PKa2 | 0.7 | 1.6–4.2 |
| p58/PKo | 2.8 | 6.9–9.0 |
| p58/PBi | 0.7 | 1.6–2.6 |
| p30/PKa2 | 3.5 | 1.6–3.2 |
| p30/PKo | 0.7 | 1.1–2.1 |
| p30/PBi | 2.1 | 2.1–3.7 |
| p21/PKa2 | 0.0 | 2.1–3.2 |
| p21/PKo | 0.0 | 1.1–1.6 |
| p21/PBi | 0.0 | 1.6–3.2 |
| p56/PKa2 | 0.0 | 0.5–2.1 |
| p43/PKo | 5.0–6.4 | 9.0–11.1 |
| p17/PKo | 2.8–3.5 | 8.5–11.6 |
| p14/PKo | 0.7 | 5.3–6.9 |
Calculated for combinations with specificities of >95%.
TABLE 5.
Evaluation of addition of various antigens from heterologous strains to IgG WB of whole-cell lysates of different strains
| Basic test: WB with whole-cell lysate of straina: | Additional antigen/strain | Loss of specificity (%) | Gain of sensitivity (%)b |
|---|---|---|---|
| PKa2 | p58/PKo | 1.4 | 6.3 |
| p43/PKo | 6.4 | 8.5 | |
| p39/PKo | 1.4 | 5.3 | |
| p17/PKo | 3.5 | 6.3 | |
| p14/PKo | 0.7 | 3.2 | |
| p39/PBi | 0.0 | 3.2 | |
| OspC/PBi | 2.1 | 4.2 | |
| PKo | OspC/PBi | 2.1c | 6.9c |
| OspC/PKa2 | 2.8c | 2.6c | |
| PBi | p58/PKo | 2.8 | 6.9 |
| p43/PKo | 5.7 | 7.4 | |
| p17/PKo | 3.5 | 7.9 | |
| p14/PKo | 0.7 | 4.8 |
Only additional antigens leading to a gain of sensitivity of at least 3% are included.
Results for criterion of a reactive OspC of strain PBi or at least two reactive bands among p83/100, p58, p43, p39, p30, OspC, p21, p17, and p14 of strain PKo for a positive WB.
RESULTS
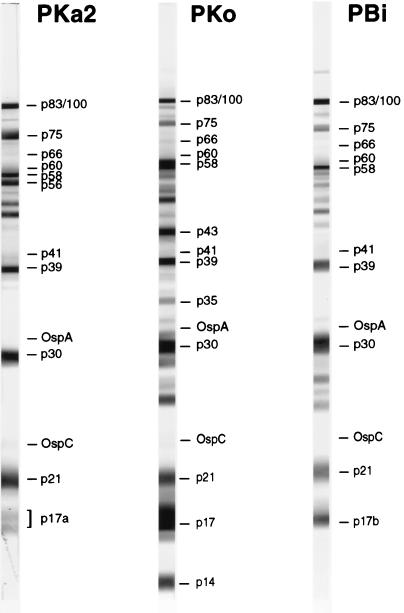
Figure 1 shows representative WB strips of the three strains which were incubated in the reference serum. All bands referred to in the present study are indicated. Homologs of proteins p83/100, p75, p66, p60, p58, p41, p39, OspA, p30, OspC, and p21 were identified in all three strains. p56 and p17a are expressed only by strain PKa2; p43, p35, p17, and p14 are unique for strain PKo; and p17b was identified only in strain PBi.
FIG. 1.
Representative WB strips of the indicated borrelial strains incubated in our broadly reacting reference serum (obtained from a patient with arthritis). All bands referred to in this article are indicated (certain bands were not recognized by this serum). For details of band identification, see reference 18.
Table 1 summarizes the specificities and sensitivities of various individual proteins or combinations of proteins in IgG WB. Bands p75, p66, p60, p41, and OspA were either nonspecific, insensitive, or both. The most sensitive (>35%) of the remaining (individual) antigens were p58 of PKo, p17 of PKo, p43 of PKo, p39 of PKo, p58 of PKa2, and p39 of PBi in order of decreasing sensitivity. No false-positive reactions were obtained with p83/100 of PKa2, p83/100 of PKo, p39 of PBi, any of the homologs of p21, p56 of PKa2, or p17a of PKa2. With the exception of p43 of PKo, all other bands reached specificities of more than 96%.
TABLE 1.
Specificities and sensitivities of various individual proteins and combinations of homolous proteins in IgG WB
| Categorya | Antigen | Strain | Specificity (%)b | Sensitivity (%)c |
|---|---|---|---|---|
| I | p83/100 | PKa2 | 100.0 | 28.0 |
| p83/100 | PKo | 100.0 | 27.5 | |
| p83/100 | PBi | 99.3 | 32.8 | |
| p83/100 | All 3 strainsd | 99.3 | 34.4 | |
| p58 | PKa2 | 99.3 | 37.0 | |
| p58 | PKo | 97.2 | 46.6 | |
| p58 | PBi | 99.3 | 34.4 | |
| p58 | All 3 strainsd | 97.2 | 50.3 | |
| p39 | PKa2 | 97.9 | 16.9 | |
| p39 | PKo | 98.6 | 37.6 | |
| p39 | PBi | 100.0 | 35.4 | |
| p39 | All 3 strainsd | 96.5 | 40.7 | |
| p30 | PKa2 | 96.5 | 27.5 | |
| p30 | PKo | 99.3 | 24.9 | |
| p30 | PBi | 97.9 | 28.6 | |
| p30 | All 3 strainsd | 95.7 | 33.3 | |
| OspC | PKa2 | 97.2 | 14.3 | |
| OspC | PKo | 99.3 | 14.3 | |
| OspC | PBi | 97.9 | 20.6 | |
| OspC | All 3 strainsd | 94.3 | 30.7 | |
| p21 | PKa2 | 100.0 | 25.4 | |
| p21 | PKo | 100.0 | 23.3 | |
| p21 | PBi | 100.0 | 28.6 | |
| p21 | All 3 strainsd | 100.0 | 30.7 | |
| II | p75 | PKa2 | 90.1 | 24.9 |
| p75 | PKo | 92.2 | 19.0 | |
| p75 | PBi | 88.7 | 27.0 | |
| p66 | PKa2 | 100.0 | 11.1 | |
| p66 | PKo | 95.7 | 7.4 | |
| p66 | PBi | 97.9 | 8.5 | |
| p60 | PKa2 | 92.2 | 21.2 | |
| p60 | PKo | 95.7 | 5.8 | |
| p60 | PBi | 90.8 | 23.3 | |
| p41 | PKa2 | 64.5 | 53.4 | |
| p41 | PKo | 78.7 | 45.5 | |
| p41 | PBi | 65.2 | 56.6 | |
| OspA | PKa2 | 99.3 | 0.5 | |
| OspA | PKo | 97.9 | 2.1 | |
| OspA | PBi | 95.7 | 4.8 | |
| III | p56 | PKa2 | 100.0 | 29.6 |
| p43 | PKo | 93.6 | 38.6 | |
| p35 | PKo | 97.2 | 12.7 | |
| p17a | PKa2 | 100.0 | 13.2 | |
| p17 | PKo | 96.5 | 40.7 | |
| p17b | PBi | 99.3 | 15.9 | |
| p14 | PKo | 99.3 | 29.6 |
Category I, highly specific antigens with sensitivities of >14%; homologs of all three strains known. II, well-known antigens with low specificity and/or low sensitivity; homologs of all three strains known. III, antigens identified in only one strain; interesting as additional diagnostic antigens.
Specificities were determined with the data of 141 sera of the control group.
Sensitivities were determined with the data of 189 sera from patients at different stages of LB.
A positive result in the combination means that at least one of the antigens was reactive.
Table 2 summarizes the evaluation of various combinations of proteins available to date as recombinant antigens for use in IgG WB. Only combinations of the highly specific bands p83/100, p39, and OspC were listed in this table. For this evaluation, an interpretation criterion of at least one reactive band for a positive WB was used. The combination of p83/100 of PKa2, p83/100 of PKo, p39 of PKo, p39 of PBi, and OspC of PBi (boxed in the table) seemed to be the most favorable and resulted in a specificity of 96.5% and a sensitivity of 56.1%. The inclusion of other bands (p75, p66, p60, p41, OspA, and p35 of PKo) and the use of an interpretation criterion requiring at least two reactive bands for a positive WB led to much lower sensitivities (data not shown).
TABLE 2.
Optimal combinations of proteins, available as recombinant antigens to date, in IgG WBa
| Combination of homologous protein(s) of strain
|
Specificity (%) | Sensitivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| p83/100
|
p39
|
OspC
|
|||||||
| PKa2 | PKo | PBi | PKo | PBi | PKa2 | PKo | PBi | ||
| + | + | + | 100.0 | 41.8 | |||||
| + | + | + | + | 99.3 | 45.5 | ||||
| + | + | + | + | 98.6 | 46.0 | ||||
| + | + | + | + | 97.9 | 52.9 | ||||
| + | + | + | + | + | 97.2 | 53.4 | |||
| + | + | + | + | + | 97.2 | 53.4 | |||
| + | + | + | + | + | 96.5 | 56.1 | |||
| + | + | + | + | + | + | 95.7 | 56.6 | ||
| + | + | + | + | + | + | 93.6 | 57.1 | ||
| + | + | + | + | + | + | + | 92.9 | 57.7 | |
| + | + | + | + | + | + | + | 92.9 | 57.7 | |
At least one reactive band was required for a positive WB. The recommended combination is boxed.
Table 3 shows the results of the preceding study of WB of whole-cell lysates (18) and was included for comparison purposes only.
TABLE 3.
Specificities and sensitivities of IgG WB of whole-cell lysates of different strains by use of proposed interpretation criteriaa
| Strain | Interpretation criterion | Specificity (%) | Sensitivity (%) |
|---|---|---|---|
| PKa2 | ≥1 reactive band of p83/100, p58, p56, OspC, p21, and p17a | 96.5 | 50.8 |
| PKo | ≥2 reactive bands of p83/100, p58, p43, p39, p30, OspC, p21, p17, and p14 | 97.9 | 56.1 |
| PBi | ≥1 reactive band of p83/100, p39, OspC, p21, and p17b | 97.2 | 56.1 |
Results of preceding study (18).
In Table 4, the theoretical outcomes of the addition of various antigens to the protein combinations listed in Table 2 are shown. The results for IgG WB were calculated using basic combinations (Table 2) with specificities of greater than 95%. The most favorable additional antigens in terms of both specificity and sensitivity were p14, p17, and p58 of strain PKo. Addition of p21 of strain PKa2 or PBi led to an increase in sensitivity of up to 3.2% without a loss of specificity. The addition of p43 of PKo as well as p17 of PKo to the boxed combination in Table 2 resulted in a significant increase in sensitivity (P < 0.05), whereas the loss of specificity resulting from the addition of p43 of PKo was also significant (P < 0.05). The addition of any of the other proteins did not lead to significant changes.
Table 5 shows the theoretical outcome of the addition of various antigens from heterologous strains to IgG WB of (conventional) whole-cell lysates of different strains. If strain PKa2 was used for the basic test, addition of p58 of PKo was most favorable, resulting in a 6.3% gain in sensitivity and a loss of specificity of only 1.4%. A conventional WB with strain PKo would gain 6.9% in sensitivity and lose 2.1% in specificity by the addition of OspC of PBi. (However, these are the results for the interpretation criterion stating that for a WB to be called positive, there must be either a reactive OspC of PBi or at least two reactive bands among p83/100, p58, p43, p39, p30, OspC, p21, p17, and p14 of strain PKo.) Western blotting of strain PBi lysate would improve most by addition of p14, p17, or p58 of strain PKo. None of the additional antigens listed in Table 5 led to a significant gain in sensitivity, whereas p43 of PKo led to a significant loss of specificity (P < 0.05) when added to a WB of strain PKa2 or PBi lysate.
Table 6 summarizes the theoretical optimal combinations of antigens for IgG WB, assuming that they were all available as recombinant proteins. This analysis was based on the requirement of at least two reactive bands for a positive WB. The most favorable combinations, resulting in the highest discrimination abilities, were obtained if only OspC of PBi, but not OspC of the other two strains, was used.
TABLE 6.
Optimal combinations of various antigens from different strains in IgG WBa
| Homologs of all three strains (PKa2, PKo, PBi)b
|
Proteins of one strain only
|
Specificity (%) | Sensitivity (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p83/100 | p58 | p39 | p30 | p21 | OspC | OspC (PBi) | p17b (PBi) | p56 (PKa2) | p43 (PKo) | p17 (PKo) | p14 (PKo) | ||
| + | + | + | + | + | + | 100.0 | 51.3 | ||||||
| + | + | + | + | + | + | + | + | + | 97.9 | 57.7 | |||
| + | + | + | + | + | + | + | 97.2 | 58.2 | |||||
| + | + | + | + | + | + | + | + | + | + | + | 96.5 | 61.4 | |
| + | + | + | + | + | + | + | + | + | 99.3 | 57.7 | |||
| + | + | + | + | + | + | + | 98.6 | 58.2 | |||||
| + | + | + | + | + | + | + | + | + | + | + | 97.2 | 61.4 | |
At least two reactive bands were required for a positive WB. The recommended combination is boxed.
At least one of the homologs of the three strains was reactive.
Table 7 shows the evaluation of various combinations of proteins in IgM WB. OspC of any of the three strains was the most immunodominant protein for IgM detection. OspC of PBi was the most sensitive of the homologs of the three strains (35.6%). However, combination of OspC of PBi with OspC of PKo, p41 (only strong reactions were evaluated) of all three strains, p39 of all three strains, and p17 of PKo increased the sensitivity up to 47.0% without a loss of specificity. Further addition of p35 of PKo led to a considerable decrease in specificity (94.3%) and only a slight increase in sensitivity (49.0%).
TABLE 7.
Evaluation of various combinations of proteins in IgM WBa
| Combination of bands of strains
|
Specificity (%) | Sensitivityc (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OspC
|
Strong p41b
|
p39
|
p17 (PKo) | p35 (PKo) | ||||||||
| PKa2 | PKo | PBi | PKa2 | PKo | PBi | PKa2 | PKo | PBi | ||||
| + | 97.2 | 29.5 | ||||||||||
| + | 98.6 | 30.9 | ||||||||||
| + | 97.9 | 35.6 | ||||||||||
| + | + | 97.2 | 32.2 | |||||||||
| + | + | 96.5 | 36.9 | |||||||||
| + | + | 97.9 | 36.9 | |||||||||
| + | + | + | 96.5 | 37.6 | ||||||||
| + | + | + | 97.9 | 38.3 | ||||||||
| + | + | + | 97.9 | 38.9 | ||||||||
| + | + | + | 97.9 | 38.3 | ||||||||
| + | + | + | + | + | 97.9 | 39.6 | ||||||
| + | + | + | 97.9 | 37.6 | ||||||||
| + | + | + | 97.9 | 39.6 | ||||||||
| + | + | + | 97.9 | 37.6 | ||||||||
| + | + | + | 97.9 | 39.6 | ||||||||
| + | + | + | + | + | + | 97.9 | 43.6 | |||||
| + | + | + | + | + | + | + | + | + | 97.9 | 47.0 | ||
| + | + | + | + | + | + | + | + | + | + | 96.5 | 47.7 | |
| + | + | + | + | + | + | + | + | + | + | 94.3 | 49.0 | |
| + | + | + | + | + | + | + | + | + | + | + | 91.8 | 49.7 |
At least one reactive band was required for a positive WB. The recommended combination is boxed.
Band intensity equal to or greater than that of a strongly reactive control serum sample.
Sensitivities were determined with the data of 149 sera from patients with erythema migrans or acute neuroborreliosis.
DISCUSSION
This study gives an impression of the probable proficiencies of serological tests with various combinations of individual antigens of three strains representing the three species of B. burgdorferi sensu lato that are pathogenic for humans in Europe.
In a first step, the specificities and sensitivities of a series of immunodominant antigens in Ig WB were determined (Table 1). These included molecularly and immunologically well-characterized proteins like p83/100 (21, 25, 31, 33), p66 (6), p60 (a homolog of GroEL of Escherichia coli) (16, 26, 36, 37), p41 (flagellin) (14, 38), p39 (BmpA) (32, 34, 35), OspA (4, 43), and OspC (12, 22, 44, 47). p75 in our WB is presumably identical to the heat shock protein, homologous to DnaK of E. coli, described by Luft et al. (26) and Coleman and Benach (8). As had been recognized for years, p75, p60, and p41 were rather nonspecific (8, 16, 46). OspA was not very sensitive, as was also found earlier, at least in European studies (9, 29, 45, 46). Homologs of p83/100, p39, and OspC of all strains were highly specific (>97%) and, thus, are useful as recombinant antigens for Western blotting or other serological tests. This has already been shown successfully in diagnostic Western blotting (32, 41, 42). In addition to these proteins, a series of specific and sensitive antigens were identified. p43, p35, p17, and p14 were detected only in strain PKo. A 35-kDa protein is one of the diagnostically important bands of the interpretation criteria for IgG WB proposed by Engstrom et al. (11). The gene coding for this protein was characterized in B. burgdorferi sensu stricto strain B31 (15); furthermore, variable expression of this gene could be demonstrated as well (20). p35 of PKo of our WB was shown to be a homolog of this protein, and its expression could also be shown in strain PBi (passages other than the one used for this study) (20a). However, in combination with the other antigens available as recombinant proteins to date, addition of p35 of PKo would not lead to an increase in the overall discrimination ability for both IgG and IgM WB (not included in Table 4 due to a gain in sensitivity of less than 3%). p58, p30, p21, and p56 of PKa2, p17a of PKa2, p17 of PKo, p14 of PKo, and p17b of PBi have not been characterized at the molecular level so far.
Taking into consideration the antigens available as recombinant proteins to date, the combination of p83/100 of PKa2, p83/100 of PKo, p39 of PKo, p39 of PBi, and OspC of PBi (boxed in Table 2) would be the most favorable for IgG WB. The discriminating ability of IgG WB with whole-cell lysate of strain PKo or PBi was only slightly better (Table 3). However, interpretation rules requiring at least two reactive bands for a WB to be positive are more reliable, as discussed later. Our findings with native p39 are very similar to the results obtained with recombinant p39 derived from representatives of these three species (32). Since p39 is highly conserved within the species of B. burgdorferi sensu lato, it might be suggested that in Europe, in general, recombinant p39 of B. afzelii and B. garinii strains would be more sensitive than p39 of B. burgdorferi sensu stricto strains (32). In IgG WB, the use of OspC of PBi alone among the OspC homologs seems to be better than a combination of this protein with the OspCs of the other strains. This finding is consistent with the results of a study by Mathiesen et al., who reported also that OspC of the B. garinii strain utilized by them was as sensitive alone as in combination with the OspCs of the representatives of the two other species (28). However, in an evaluation of IgG WBs with recombinant OspCs derived from the three species, OspC of PKo was the most sensitive and OspC of PBi was the least sensitive (42). The highest sensitivity was obtained with a combination of all three OspCs (42).
The next step of this study was to estimate the theoretical benefit of the addition of other antigens not yet available as recombinant proteins (Table 4). These results might indicate which antigens are worth the effort of future molecular characterization. The most promising proteins were p58 of PKo, p17 of PKo, and p14 of PKo. No false-positive reactions were seen with p21 of any of the strains; however, the gain in sensitivity did not exceed 3.2%. p43 of PKo was the most sensitive of the additional antigens, but due to its rather low specificity, this protein should be used in IgG WB only if an interpretation rule requiring at least two reactive bands for a positive WB is applied. Similar results were obtained when the theoretical addition of various heterologous antigens to WB of whole-cell lysates was evaluated (Table 5). A considerable improvement of Western blotting of strain PKo lysate could result from the addition of OspC of PBi. This approach could be tested right away. For example, a line of recombinant OspC of PBi could be added to the nitrocellulose membrane outside of the gel area. Furthermore, this supplementation could also be tried for enzyme immunoassays. If assays based only on recombinant antigens are developed, the concentration of each protein can be optimized to obtain the best discrimination ability for the whole test. For this study, the intensity of antigen recognition was only critical for the evaluation of IgM WB.
Our analyses were based on data obtained during a very detailed previous WB study including a total of 330 sera (18). Although this is a rather large panel in terms of performance of a study, it is still a small number of samples for an analysis relying so much on statistics. Considering the strategy by which the criteria were defined, the few sera of the control group that react with certain highly specific bands had a strong influence. In other words, one single sample can be crucial for the deletion of a sensitive band of a combination. However, a difference between specificities of 99 and 100%, for example, will never actually be statistically significant unless a control group consisting of at least 500 sera is tested (estimation by one-sided χ2 analysis [P < 0.05]). The probability that a control group sample will recognize two highly specific bands (by chance) will be much lower. Thus, interpretation rules requiring at least two reactive bands for a positive WB will be more reliable. In a final analysis, optimal combinations of various antigens in IgG WB were determined also on the basis of this premise (Table 6). Due to the rather restricted immune response, especially in European patients in the early stages of LB, it is not possible to define interpretation criteria requiring as many bands as the criteria for positivity recommended in the United States (1, 10, 11).
For the evaluation of IgM WB (Table 7), any implementation of a rule requiring more than one reactive band for a positive WB resulted in a significant loss of sensitivity. Detection of specific antibodies is based mainly on the use of OspC. However, sensitivity can be increased considerably by the addition of p39 and p17 of PKo as well as evaluation of strong reactions with p41. Addition of antigens not yet available as recombinant proteins was not shown to be favorable. Another approach might be the use of internal fragments of p41 (7, 19, 41, 42) representing the most heterogeneous region of the amino acid sequence in comparison to flagellin of other species (13, 38). In contrast to our results, Engstrom et al. reported that p39 was the most sensitive antigen for the detection of specific IgM in the United States (11). On the other hand, in the U.S. study of Dressler et al., OspC was the most sensitive protein (10).
In conclusion, in this study, diagnostically valuable proteins that might be worth the effort of further molecular characterization were identified and implications for the optimal combination of antigens were given.
ACKNOWLEDGMENTS
We thank Carolin Feterowski for valuable discussions and Sofia Reinecke for help with the manuscript.
We thank Jürgen Heesemann for generous support of this work.
REFERENCES
- 1.Association of State and Territorial Public Health Laboratory Directors and Centers for Disease Control and Prevention. Proceedings of the Second National Conference on the Serologic Diagnosis of Lyme Disease. Washington, D.C: ASTPHLD; 1995. pp. 1–7. [Google Scholar]
- 2.Assous M V, Postic D, Paul G, Nevot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 3.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J-C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergström S, Sjöstedt A, Dotevall L, Kaijser B, Ekstrand-Hammarström B, Wallberg C, Skogman G, Barbour A G. Diagnosis of Lyme borreliosis by an enzyme immunoassay detecting immunoglobulin G reactive to purified Borrelia burgdorferi cell components. Eur J Clin Microbiol Infect Dis. 1991;10:422–427. doi: 10.1007/BF01968022. [DOI] [PubMed] [Google Scholar]
- 6.Bunikis J, Noppa L, Bergström S. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease borrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- 7.Burkert S, Rössler D, Münchhoff P, Wilske B. Development of enzyme-linked immunosorbent assays using recombinant borrelial antigens for serodiagnosis of Borrelia burgdorferi infection. Med Microbiol Immunol. 1996;185:49–57. doi: 10.1007/s004300050014. [DOI] [PubMed] [Google Scholar]
- 8.Coleman J L, Benach J L. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J Infect Dis. 1992;165:658–666. doi: 10.1093/infdis/165.4.658. [DOI] [PubMed] [Google Scholar]
- 9.Dressler F, Ackermann R, Steere A C. Antibody responses to the three genomic groups of Borrelia burgdorferi in European Lyme borreliosis. J Infect Dis. 1994;169:313–318. doi: 10.1093/infdis/169.2.313. [DOI] [PubMed] [Google Scholar]
- 10.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 11.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992;6:503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 13.Gassmann G S, Jacobs E, Deutzmann R, Göbel U B. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J Bacteriol. 1991;173:1452–1459. doi: 10.1128/jb.173.4.1452-1459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gassmann G S, Kramer M, Göbel U B, Wallich R. Nucleotide sequence of a gene encoding the Borrelia burgdorferi flagellin. Nucleic Acids Res. 1989;17:3590. doi: 10.1093/nar/17.9.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilmore R D, Jr, Kappel K J, Johnson B J B. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J Clin Microbiol. 1997;35:86–91. doi: 10.1128/jcm.35.1.86-91.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen K, Bangsborg J M, Fjordvang H, Pedersen N S, Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988;56:2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen K, Pii K, Lebech A-M. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a μ-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J Clin Microbiol. 1991;29:166–173. doi: 10.1128/jcm.29.1.166-173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser U, Lehnert G, Lobentanzer R, Wilske B. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J Clin Microbiol. 1997;35:1433–1444. doi: 10.1128/jcm.35.6.1433-1444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauser U, Wilske B. Enzyme-linked immunosorbent assays with recombinant internal flagellin fragments derived from different species of Borrelia burgdorferi sensu lato for the serodiagnosis of Lyme neuroborreliosis. Med Microbiol Immunol. 1997;186:145–151. doi: 10.1007/s004300050057. [DOI] [PubMed] [Google Scholar]
- 20.Indest K J, Ramamoorthy R, Solé M, Gilmore R D, Johnson B J B, Philipp M T. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Jauris-Heipke, S. Personal communication.
- 21.Jauris-Heipke S, Fuchs R, Lottspeich F, Preac-Mursic V, Soutschek E, Will G, Wilske B. Molecular characterization of the p100 gene of Borrelia burgdorferi strain PKo. FEMS Microbiol Lett. 1993;114:235–242. doi: 10.1111/j.1574-6968.1993.tb06579.x. [DOI] [PubMed] [Google Scholar]
- 22.Jauris-Heipke S, Liegl G, Preac-Mursic V, Rössler D, Schwab E, Soutschek E, Will G, Wilske B. Molecular analysis of genes encoding outer surface protein C (OspC) of Borrelia burgdorferi sensu lato: relationship to ospA genotype and evidence of lateral gene exchange of ospC. J Clin Microbiol. 1995;33:1860–1866. doi: 10.1128/jcm.33.7.1860-1866.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lefebvre R B, Perng G-C, Johnson R C. The 83-kilodalton antigen of Borrelia burgdorferi which stimulates immunoglobulin M (IgM) and IgG responses in infected hosts is expressed by a chromosomal gene. J Clin Microbiol. 1990;28:1673–1675. doi: 10.1128/jcm.28.7.1673-1675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luft B J, Gorevic P D, Jiang W, Munoz P, Dattwyler R J. Immunologic and structural characterization of the dominant 66- to 73-kDa antigens of Borrelia burgdorferi. J Immunol. 1991;146:2776–2782. [PubMed] [Google Scholar]
- 27.Magnarelli, L. A. 1995. Current status of laboratory diagnosis for Lyme disease. Am. J. Med. 98(Suppl. 4A):10S–14S. [DOI] [PubMed]
- 28.Mathiesen M J, Hansen K, Axelsen N, Halkier-Sorensen L, Theisen M. Analysis of the human antibody response to outer surface protein C (OspC) of Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii. Med Microbiol Immunol. 1996;185:121–129. doi: 10.1007/s004300050021. [DOI] [PubMed] [Google Scholar]
- 29.Moskophidis M, Luther B. Wertigkeit des Immunoblots in der Serodiagnostik der Lyme-Borreliose. Lab Med. 1995;19:231–237. [Google Scholar]
- 30.Norman G L, Antig J M, Bigaignon G, Hogrefe W R. Serodiagnosis of Lyme borreliosis by Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii Western blots (immunoblots) J Clin Microbiol. 1996;34:1732–1738. doi: 10.1128/jcm.34.7.1732-1738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perng G-C, LeFebvre R B, Johnson R C. Further characterization of a potent immunogen and the chromosomal gene encoding it in the Lyme disease agent, Borrelia burgdorferi. Infect Immun. 1991;59:2070–2074. doi: 10.1128/iai.59.6.2070-2074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roessler D, Hauser U, Wilske B. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J Clin Microbiol. 1997;35:2752–2758. doi: 10.1128/jcm.35.11.2752-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rössler D, Eiffert H, Jauris-Heipke S, Lehnert G, Preac-Mursic V, Teepe J, Schlott T, Soutschek E, Wilske B. Molecular and immunological characterization of the p83/100 protein of various Borrelia burgdorferi sensu lato strains. Med Microbiol Immunol. 1995;184:23–32. doi: 10.1007/BF00216786. [DOI] [PubMed] [Google Scholar]
- 34.Simpson W J, Cieplak W, Schrumpf M E, Barbour A G, Schwan T G. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol Lett. 1994;119:381–388. doi: 10.1111/j.1574-6968.1994.tb06917.x. [DOI] [PubMed] [Google Scholar]
- 35.Simpson W J, Schrumpf M E, Schwan T G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990;28:1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamm L V, Gherardini F C, Parrish E A, Moomaw C R. Heat shock response of spirochetes. Infect Immun. 1991;59:1572–1575. doi: 10.1128/iai.59.4.1572-1575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallich R, Helmes C, Schaible U E, Lobet Y, Moter S E, Kramer M D, Simon M M. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992;60:4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallich R, Moter S E, Simon M M, Ebnet K, Heiberger A, Kramer M D. The Borrelia burgdorferi flagellum-associated 41-kilodalton antigen (flagellin): molecular cloning, expression, and amplification of the gene. Infect Immun. 1990;58:1711–1719. doi: 10.1128/iai.58.6.1711-1719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilske B, Bader L, Pfister H W, Preac-Mursic V. Diagnostik der Lyme-Neuroborreliose (Nachweis der intrathekalen Antikörperbildung) Fortschr Med. 1991;109:441–446. [PubMed] [Google Scholar]
- 40.Wilske B, Busch U, Eiffert H, Fingerle V, Pfister H-W, Rössler D, Preac-Mursic V. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferi sensu lato from patients with neuroborreliosis in Germany. Med Microbiol Immunol. 1996;184:195–201. doi: 10.1007/BF02456135. [DOI] [PubMed] [Google Scholar]
- 41.Wilske B, Fingerle V, Herzer P, Hofmann A, Lehnert G, Peters H, Pfister H-W, Preac-Mursic V, Soutschek E, Weber K. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis. Med Microbiol Immunol. 1993;182:255–270. doi: 10.1007/BF00579624. [DOI] [PubMed] [Google Scholar]
- 42.Wilske B, Fingerle V, Preac-Mursic V, Jauris-Heipke S, Hofmann A, Loy H, Pfister H-W, Rössler D, Soutschek E. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferi sensu lato. Med Microbiol Immunol. 1994;183:43–59. doi: 10.1007/BF00193630. [DOI] [PubMed] [Google Scholar]
- 43.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilske B, Preac-Mursic V, Schierz G, Gueye W, Herzer P, Weber K. Immunochemical analysis of the immune response in late manifestations of Lyme borreliosis. Zentbl Bakteriol Microbiol Hyg Ser A. 1988;267:549–558. [PubMed] [Google Scholar]
- 46.Wilske, B., V. Preac-Mursic, G. Schierz, G. Liegl, and W. Gueye. 1989. Detection of IgM and IgG antibodies to Borrelia burgdorferi using different strains as antigen. Zentbl. Bakteriol. 18(Suppl.):299–309.
- 47.Wilske B, Preac-Mursic V, Schierz G, von Busch K. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentbl Bakteriol Microbiol Hyg Ser A. 1986;263:92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- 48.Wilske B, Schierz G, Preac-Mursic V, Weber K, Pfister H W, Einhäupl K. Serological diagnosis of erythema migrans disease and related disorders. Infection. 1984;12:331–337. doi: 10.1007/BF01651147. [DOI] [PubMed] [Google Scholar]