Abstract
Purpose
To establish the safety, tolerability, pharmacokinetics, and pharmacodynamics of an intravitreal injection of recombinant human complement factor H (CFH), GEM103, in individuals with genetically defined age-related macular degeneration (AMD) and geographic atrophy (GA).
Design
Phase I single ascending-dose, open-label clinical trial (ClinicalTrials.gov identifier, NCT04246866).
Participants
Twelve individuals 50 years of age or older with a confirmed diagnosis of foveal GA in the study eye.
Methods
Participants were assigned to the increasing dose cohorts and received 1 50-μl intravitreal injection of GEM103 at doses of 50 μg/eye, 100 μg/eye, 250 μg/eye, or 500 μg/eye; dose escalation was dependent on the occurrence of dose-limiting toxicities.
Main Outcome Measures
Safety assessments included ocular and systemic adverse events (AEs), ocular examinations, clinical laboratory and vital signs, and serum antidrug antibody levels. Biomarkers, measured in the aqueous humor (AH), included CFH and complement activation biomarkers factor Ba and complement component 3a.
Results
No dose-limiting toxicities were reported, enabling escalation to the maximum study dose. No anti-GEM103 antidrug antibodies were detected during the study. Four participants experienced AEs; these were nonserious, mild or moderate in severity, and unrelated to GEM103. The AEs in 2 of these participants were related to the intravitreal injection procedure. No clinically significant ophthalmic changes and no ocular inflammation were observed. Visual acuity was maintained and stable throughout the 8-week follow-up period. No choroidal neovascularization occurred. CFH levels increased in a dose-dependent manner after GEM103 administration with supraphysiological levels observed at week 1; levels were more than baseline for 8 weeks or more in all participants receiving single doses of 100 μg or more. Complement activation biomarkers were reduced 7 days after dose administration.
Conclusions
A single intravitreal administration of GEM103 (up to 500 μg/eye) was well tolerated in individuals with GA. Of the few mild or moderate AEs reported, none were determined to be related to GEM103. No intraocular inflammation or choroidal neovascularization developed. CFH levels in AH were increased and stable for 8 weeks, with pharmacodynamic data suggesting that GEM103 restored complement regulation. These results support further development in a repeat-dose trial in patients with GA with AMD.
Keywords: Age-related macular degeneration, Complement factor H, Complement regulation, GEM103, Geographic atrophy
Abbreviations and Acronyms: AE, adverse event; AH, aqueous humor; AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; CFB, complement factor B; CFH, complement factor H; CFP, color fundus photography; C3(a/b), complement component 3(a/b); FA, fluorescein angiography; FAF, fundus autofluorescence; GA, geographic atrophy; IRC, image reading center; LLVA, low-luminance visual acuity; nAMD, neovascular age-related macular degeneration; NI, near infrared; OCTA, optical coherence tomography angiography; RPE, retinal pigment epithelium; SD, standard deviation
Age-related macular degeneration (AMD) is a progressive retinal disease affecting older adults and is the leading cause of irreversible blindness.1,2 It was estimated that approximately 196 million people worldwide were affected by AMD in 2020, representing a substantial global disease burden.1 Geographic atrophy (GA), the most advanced form of dry AMD, is a primary cause of vision loss, affecting 20% of patients with AMD worldwide.3 Currently, no curative treatment is available for early, intermediate, or advanced dry AMD (including GA),4 although vitamin supplements have been found to reduce the risk of progression to advanced AMD.5
Clinical risk factors that predispose individuals to AMD include advanced age, cigarette smoking, previous cataract surgery, obesity, and cardiovascular risk factors.6, 7, 8, 9 Genetic factors also enhance the risk of disease onset and play a role in the cause of AMD, explaining up to approximately 70% of the variation in overall disease severity.6,9 The key genetic factors that contribute to AMD progression include mutations in the complement factor H (CFH) gene and the age-related maculopathy susceptibility 2 gene.10 Other rare genetic variants in the complement pathway have been implicated in the development of AMD, including complement component 2 and component 3, complement factor B (CFB; cleaved to Ba and Bb), and complement factor I.11,12 Substantial evidence supports the role of CFH dysfunction in the pathogenesis of AMD,9,13, 14, 15, 16 and the addition of several risk factors could mean that approximately 83% of patients progress to advanced AMD.12 In particular, the CFH polymorphism Y402H has been found to confer up to a six-fold increased risk of AMD developing and is estimated to play a role in almost 60% of AMD cases overall.17
Complement factor H is the major regulatory protein of the alternative pathway of complement activation18 and plays a role in irreversible decay of complement component 3 (C3)-convertase and preventing formation of complement component 5-convertase.19 Therefore, when bound to cells or free in circulation, CFH inhibits the formation of C3b (by C3-convertase)19 and also can inactivate existing C3b, downregulating the accelerating inflammatory signals produced by the complement system. Complement factor H binds to the glycosaminoglycans and sialic acids on mammalian self-cell surfaces (but not pathogen surfaces), which protects from deposition of activated C3b on surfaces of self-cells,20,21 such as retinal pigment epithelium (RPE) cells. Therefore, when functioning normally, CFH helps to ensure that only pathogens are targeted for clearance through C3b deposition and provides noncanonical effects that protect RPE cells against oxidative stress.22 Dysfunction of CFH can lead to inappropriate immune cell activation and cell lysis and to reduced clearance of lipid oxidation products and cellular debris.23, 24, 25 Beyond inhibition of the alternative pathway, CFH also is involved in lipid metabolism, and dysfunction of CFH is implicated in the formation of lipid-rich deposits in several conditions, including AMD.26 Therefore, CFH has emerged as a therapeutic target for the treatment of dry AMD and GA.
GEM103 is a novel recombinant human CFH in clinical development for the treatment of dry AMD and GA. GEM103 is designed to restore appropriate regulation of the complement system in patients with dry AMD and is administered via intravitreal injection. Herein, we report results of a phase I clinical trial aimed at establishing the safety, tolerability, pharmacokinetics, and pharmacodynamics of GEM103 at several dose levels in patients with dry AMD and GA to enable the accurate design of subsequent clinical trials.
Methods
This study was conducted in accordance with the tenets of the Declaration of Helsinki and all other local regulations. Institutional review board (Advarra IRB) or ethics committee approval was obtained, and the study was registered at ClinicalTrials.gov (identifier: NCT04246866). The clinical study protocol, the investigator’s brochure, a sample informed consent form, and other study-related documents were reviewed and approved by the local or central institutional review boards of all study sites. All individuals provided written informed consent to participate. Gemini Therapeutics was the study sponsor, responsible for the design and oversight of the study, and provided the study drug.
Study Design
This was a phase I, open-label single ascending-dose clinical trial in patients with AMD and GA. The primary objectives were to evaluate the safety and tolerability of GEM103 and to establish the single intravitreal maximum tolerated dose of GEM103. The secondary objectives were to evaluate the concentration of GEM103 in aqueous humor (AH), to evaluate the endogenous CFH concentration in plasma before and after GEM103 administration, and to evaluate the immunogenicity of GEM103 in serum.
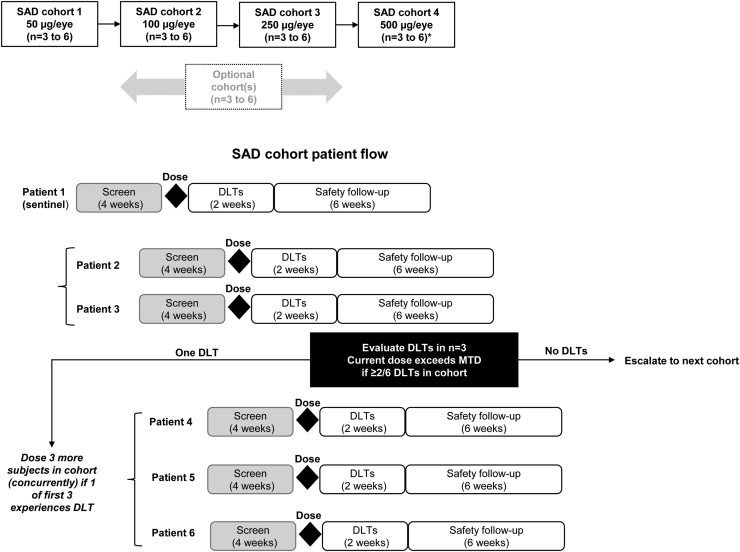
Participants were enrolled from 9 study sites in the United States from December 2019 through October 2020, and the study was conducted at 11 sites in the United States. The safety review committee comprised 3 independent members who were not affiliated with the sponsor and was responsible for reviewing safety data and deciding whether to proceed with dose escalation to subsequent cohorts. A summary of the study design is shown in Figure 1. The sample size of 12 participants was based on clinical assessment and not statistical considerations (no formal statistical hypothesis tests were planned). This sample size was considered sufficient to provide safety, biomarker, pharmacokinetic, and pharmacodynamic response data to inform dose and regimen selection for additional clinical studies.
Figure 1.
Flowchart showing study design (single ascending dose). ∗To determine the duration of elevated levels of complement factor H in aqueous humor after dosing with GEM103, participants in the 500-μg/eye cohort were followed up for up to 4 months after receiving GEM103. DLT = dose-limiting toxicity; MTD = maximum tolerated dose; SAD = single ascending dose.
The study included 4 cohorts, with participants assigned to receive 1 dose of the study drug at escalating doses of 50 μg/eye, 100 μg/eye, 250 μg/eye, or 500 μg/eye. Dose escalation to the next cohort was based on the safety data after dosing in each patient in the cohort (Fig 1). The minimum planned duration of each patient’s participation was approximately 3 months: 1 month for screening and 2 months for follow-up after the day of dosing. To determine the duration of elevated levels of CFH in AH after dosing with GEM103, participants in the 500-μg/eye cohort were followed up for up to 4 months after receiving GEM103.
Study Population
Patients eligible for inclusion in the study were at least 50 years of age at the time of the signed informed consent provision and had sufficiently clear ocular media, adequate pupillary dilation, fixation to permit quality fundus imaging, and a confirmed diagnosis of foveal-involving GA in the study eye. Geographic atrophy could have been multifocal, and cumulative GA lesions must have resided completely within the fundus autofluorescence (FAF) imaging field (field 2, 30° image centered on the fovea), as confirmed by the image reading center (IRC). Geographic atrophy must have been central, defined as GA that affects the foveal center point (diagnosis of GA and location relative to the foveal center point was determined by an IRC eligibility read, based on multimodal imaging with color fundus photography [CFP], fluorescein angiography [FA], OCT, and near infrared [NI] imaging). Total size of all GA lesions in the study eye must have been within 0.5 to 15.0 disc areas. Best-corrected visual acuity (BCVA) in the study eye using an ETDRS chart visual acuity score of 5 to 45 letters (Snellen equivalent, approximately 20/800 to 20/125) also was required. Exclusion criteria for the study eye included exudative AMD or choroidal neovascularization (see Supplemental Appendix 1 for a detailed list of study inclusion and exclusion criteria).
After providing informed consent, participants provided saliva and blood samples for genetic testing. The samples were sent for targeted sequencing using the sponsor’s genetic testing assay. Although the initial protocol included criteria to limit enrollment to participants with functionally adverse variants in the CFH gene, it was amended to accommodate enrollment during the global coronavirus disease 2019 pandemic, with feedback and recommendations received from the independent safety review committee. This amendment removed the genetic inclusion and exclusion criteria. Genetic results were tracked and used for analysis but were not used for eligibility assessment, providing a more complete understanding of the pharmacokinetic and pharmacodynamic biomarker profile in all participants with GA secondary to dry AMD. Data were collected on genetics, demographics, medical and ocular history, family history of AMD, and concomitant medications at this time; pregnancy testing also was performed.
Dose and Administration of Study Intervention
The study drug was manufactured centrally under current Good Manufacturing Practices standards; dilutions were prepared by investigators and study personnel at each individual trial site at the time of administration. GEM103 (manufactured by Catalent Pharma Solutions and Berkshire Sterile Manufacturing), a recombinant human CFH, was supplied in 10-mg/ml vials. A pharmacy manual provided study drug preparation instructions for dilution and dosing. Doses of 50 μg/eye, 100 μg/eye, 250 μg/eye, and 500 μg/eye were prepared and administered by intravitreal injection to the study eye only.
Safety Assessments
Safety assessments included ocular and systemic adverse events (AEs), ocular examinations, clinical laboratory and vital signs, and serum antidrug antibody levels.
Visual Acuity, Retinal Architecture, and Pathologic Assessments
Exploratory efficacy end points included AH biomarker evaluation, BCVA, and low-luminance visual acuity (LLVA) scores as assessed by the ETDRS and area of GA (in square millimeters) as assessed by CFP, FAF, NI imaging, and FA. Ocular imaging included CFP, FAF, OCT, NI imaging, and FA, as well as OCT angiography (OCTA) when available at the study site. Drusen volume and OCT assessment of total retinal and choroidal thickness, photoreceptor layer thickness, features of nascent GA, RPE thickening, and integrity of the RPE layer also were assessed.
Imaging and visual function assessments were performed on both eyes. Color fundus photography, FAF, FA, OCT, OCTA, and NI imaging were performed at screening and 8 weeks after dosing. Color fundus photography, FAF, OCT, OCTA, and NI imaging took place at baseline before dosing; optional OCT and OCTA took place at 1 week after dosing; and FAF, OCT, OCTA, and NI imaging took place 2 weeks after dosing. Color fundus photography, FAF, OCT, OCTA, and NI imaging were performed at 4 weeks after dosing. Images were read by an IRC (Duke Reading Center, Chapel Hill, NC).
Biomarker Assessments
Assessment of biomarkers included AH and plasma concentrations of GEM103, CFH, complement activation biomarkers (Ba and C3a), and other complement factors or components (C3 and CFB). Aqueous humor sampling of the study eye was conducted by anterior chamber paracentesis using a 27- to 30-gauge needle attached to a 1-ml syringe. Samples of up to 100 μl were obtained at baseline and 1, 4, and 8 weeks. Blood sampling for plasma CFH detection was conducted at visit 1 and visit 2 (2 hours ± 5 minutes after dose administration), in addition to the remaining time points (24 hours after dose administration and 1, 2, 4, and 8 weeks); the blood samples collected at baseline and weeks 1, 2, 4, and 8 were tested for complement activation biomarkers. Samples from participants in the 500-μg/eye cohort were assessed during the extended follow-up if the CFH levels at the preceding visits were approximately twice the baseline value.
For quantification of CFH in AH, a double-capture multiplex bead-based immunoassay conjugated to antifactor H capture antibodies was used (Luminex; MilliporeSigma, Inc). Complement factor B and C3 also were analyzed in this assay with specific anti-CFB and anti-C3 antibodies. Quantitative measurement of AH C3a and Ba, as well as plasma CFH, was carried out using a direct capture immunoassay (MicroVue; Qidel Corporation).
Statistical Analysis
Descriptive statistics (mean ± standard deviation [SD], median, and range) were calculated for all participant characteristics and biomarker, visual acuity, and pathologic measurements. No formal statistical hypothesis tests were planned because of the sample size. All analyses were performed using Statistical Analysis System software, version 9.4 or higher (SAS Institute, Inc).
Results
Study Population
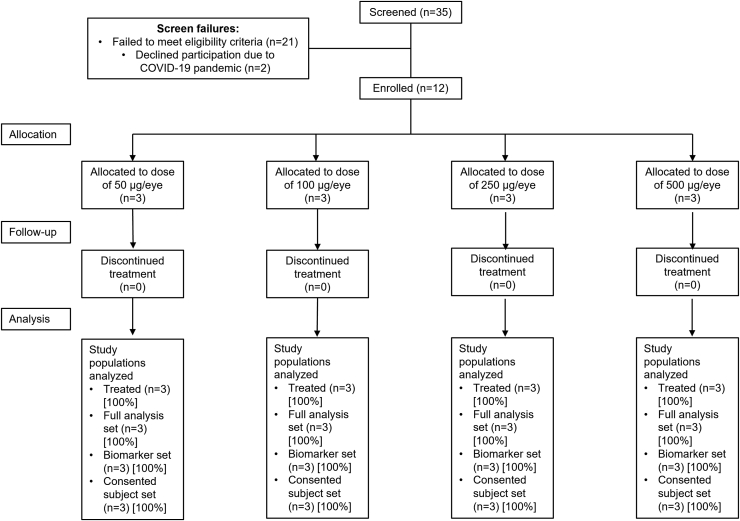
Thirty-five patients were screened initially, with 23 patients excluded primarily because of failure to meet eligibility criteria (Fig 2): 11 patients were excluded before introduction of the protocol amendment that removed the genetic requirements. Twelve patients (9 women and 3 men) who met eligibility criteria were enrolled, and all completed 8 weeks of follow-up. One participant in the 500-μg cohort continued to a 3-month follow-up visit. No participants discontinued from the study. Demographics and baseline characteristics were similar between groups (Table 1). No participant had a history of neovascular AMD (nAMD) in the study eye, but 3 participants had a history of nAMD in the fellow eye (n = 1 in the 50-μg, 250-μg, and 500-μg dose groups). Half of the patients were homozygous for the Y402H CFH variant; 2 participants were heterozygous, and 4 did not show the variant.
Figure 2.
Flowchart showing participant disposition. COVID-19 = coronavirus disease 2019.
Table 1.
Demographics and Baseline Characteristics
| Characteristic | All Participants (n = 12) | Dose Group |
|||
|---|---|---|---|---|---|
| 50 μg (n = 3) | 100 μg (n = 3) | 250 μg (n = 3) | 500 μg (n = 3) | ||
| Age (yrs) | |||||
| Mean ± SD | 81.5 ± 6.2 | 84.7 ± 8.3 | 79.7 ± 2.1 | 77.0 ± 7.0 | 84.7 ± 5.0 |
| Range | 70–94 | 78–94 | 78–84 | 70–84 | 80–90 |
| Female sex, no. (%) | 9 (75.0) | 2 (66.7) | 3 (100.0) | 3 (100.0) | 1 (33.3) |
| Tobacco amount (pack-years), no. (%) | |||||
| Former | 7 (58.3) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 2 (66.7) |
| Never | 5 (41.7) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 1 (33.3) |
| Genetics, no. (%) | |||||
| Y402H/Y402H | 6 (50) | 2 (67) | 2 (67) | 2 (67) | 0 (0) |
| Y402H/Y402Y | 2 (17) | 0 (0) | 1 (33) | 1 (33) | 0 (0) |
| Y402Y/Y402Y | 4 (33) | 1 (33) | 0 (0) | 0 (0) | 3 (100) |
| GA lesion size (mm2), study eye (fellow eye)∗ | |||||
| Mean ± SD | 9.5 ± 11.8 (7.1 ± 9.3) | 6.7 ± 4.3 (5.8 ± 8.0) | 14.3 ± 18.2 (12.3 ± 15.6) | 13.2 ± 17.2 (5.0 ± 3.5) | 3.7 ± 0.9 (2.8 ± 1.7) |
| Range | 1.7–35.3 (0.2–29.9) | 2.2–10.8 (0.2–11.4) | 2.6–35.3 (0.2–29.9) | 1.7–33.0 (2.5–7.5) | 2.9–4.7 (1.6–4.0) |
| BCVA (ETDRS letters), study eye (fellow eye) | |||||
| Mean ± SD | 39.7 ± 6.0 (54.5 ± 23.1) | 44.0 ± 6.1 (53.0 ± 29.6) | 35.7 ± 9.3 (52.0 ± 25.2) | 40.3 ± 3.1 (58.7 ± 32.6) | 38.7 ± 3.5 (54.3 ± 17.9) |
| Range | 28–51 (21–84) | 40–51 (25–84) | 28–46 (35–81) | 37–43 (21–78) | 35–42 (43–75) |
| LLVA (ETDRS), study eye (fellow eye) | |||||
| Mean ± SD | 29.3 ± 7.7 (38.2 ± 16.4) | 34.0 ± 4.6 (37.0 ± 14.1) | 32.0 ± 12.1 (34.3 ± 18.9) | 27.7 ± 2.5 (43.3 ± 26.6) | 23.3 ± 7.0 (38.0–12.1) |
| Range | 16–43 (14–66) | 30–39 (22–50) | 19–43 (18–55) | 25–30 (14–66) | 16–30 (27–51) |
| AH CFH (ng/ml)† | |||||
| Mean ± SD | 134.3 ± 97.0 | 102.1 ± 24.8 | 161.0 ± 100.4 | 89.4 ± 61.7 | 210.1 ± 205.7 |
| Range | 50–356 | 76–126 | 56–256 | 50–161 | 65–356 |
| Plasma CFH (ng/ml) | |||||
| Mean ± SD | 284 252.6 ± 53 681.5 | 304 275.0 ± 51 570.0 | 228 260.2 ± 23 392.9 | 318 468.1 ± 27 152.3 | 286 007.3 ± 69 574.9 |
| Range | 204 793–363 540 | 269 622–363 540 | 204 793–251 578 | 290 996–345 289 | 208 507–343 087 |
| AH Ba (ng/ml)‡ | |||||
| Mean ± SD | 21.6 ± 10.6 | 26.5 ± 10.7 | 25.4 ± 13.8 | 17.8 ± 9.7 | 12.2 ± NA |
| Range | 8–41 | 19–34 | 16–41 | 8–28 | 12 |
| AH C3a (ng/ml)† | |||||
| Mean ± SD | 5.4 ± 2.4 | 4.6 ± 2.2 | 5.2 ± 1.3 | 5.2 ± 3.5 | 7.6 ± 2.9 |
| Range | 1.5–9.6 | 2.2–6.6 | 4.4–6.9 | 1.5–8.6 | 5.6–9.6 |
| AH CFB (ng/ml)† | |||||
| Mean ± SD | 545.7 ± 305.4 | 447.2 ± 311.9 | 558.7 ± 223.1 | 500.1 ± 405.1 | 742.4 ± 443.8 |
| Range | 166–1056 | 169–785 | 301–694 | 166–951 | 429–1056 |
| AH C3 (ng/ml)† | |||||
| Mean ± SD | 598.8 ± 414.3 | 926.9 ± 272.6 | 611.4 ± 510.7 | 216.3 ± 76.0 | 661.6 ± 513.9 |
| Range | 131–1181 | 617–1129 | 196–1181 | 131–277 | 298–1025 |
AH = aqueous humor; BCVA = best-corrected visual acuity; CFB = complement factor B; CFH = complement factor H; C3(a) = complement component 3(a); GA = geographic atrophy; LLVA = low-luminance visual acuity; NA = not applicable; SD = standard deviation.
All participants were White; 8% were Hispanic/Latino. All AH biomarker data are in relationship to the study eye. Data are in relationship to all participants unless otherwise stated. Demographic information and patient characteristics at baseline show a total of 12 participants, 3 in each of the following dose groups: 50 μg, 100 μg, 250 μg, or 500 μg. Characteristics include age, sex, tobacco history, genetic information, GA lesion size, visual acuity, and biomarker measurements at baseline.
Data were not available for 1 participant each in the 50-μg, 250-μg, and 500-μg dose groups.
Data were not available for 1 participant in the 500-μg dose group.
Data were not available for 1 participant in the 50-μg dose group and 2 participants in the 500-μg dose group.
GEM103 Was Well Tolerated in Patients with Dry AMD and GA
A summary of safety events by treatment group is shown in Table 2. No participants reported incidences of dose-limiting toxicities, including patients in the maximum dose cohort (500 μg). Only 4 patients experienced 12 AEs, and all were nonserious, mild, or moderate in severity and were determined to be unrelated to GEM103 by investigators. Nine ocular AEs occurred in the study eye in 4 participants; all were mild in severity and were assessed by the investigator not to be related to GEM103. In the 50-μg dose group, 1 participant demonstrated mild retinal hemorrhage in the study eye observed at study day 15 that was not considered related to GEM103 or the injection procedure, and another participant demonstrated mild ocular hyperemia in the study eye, considered by the investigator probably to be related to the intravitreal injection procedure. The investigator did not record any additional information as to a potential cause of the mild, intraretinal retinal hemorrhage, noted as not associated with tortuosity, vascular dilation, or retinal vein or artery occlusion. Improvement began by the day 28 visit and was marked as trace retinal hemorrhage at day 57 but did not resolve during the study period and required no treatment. Fluorescein angiography and CFP were performed at the visit of discovery and ruled out the possibility of vasculitis. Two participants in the 250-μg group experienced mild eye irritation. For 1 of these participants, 2 reports of ocular irritation in the study eye as well as epiretinal membrane (Medical Dictionary for Regulatory Activities preferred term, macular fibrosis) in the study eye were made, and 2 reports of posterior capsular opacification in the study eye were made. This participant also demonstrated focal RPE atrophy in the fellow eye. The other participant in the 250-μg group showed ocular irritation in both eyes and pain in the study eye. This participant also experienced a fall with bruising that was moderate in severity and did not require hospitalization. The mild ocular hyperemia experienced in the participant who received 50 μg and the mild eye pain in the participant who received 250 μg were determined by the investigators to be unrelated to the study drug but related to intravitreal administration. No participants experienced serious AEs or AEs leading to study discontinuation. No deaths or other significant AEs occurred during the study.
Table 2.
Number of Participants with Reported Safety Events by Treatment Group
| Assessment | All Participants (n = 12) | GEM103 Dose Group |
|||
|---|---|---|---|---|---|
| 50 μg (n = 3) | 100 μg (n = 3) | 250 μg (n = 3) | 500 μg (n = 3) | ||
| Any TEAEs | 4 (33.3) | 2 (66.7) | 0 (0) | 2 (66.7) | 0 (0) |
| DLTs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| SAEs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| GEM103-related AEs | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| GEM103-unrelated AEs | 4 (33.3) | 2 (66.7) | 0 (0) | 2 (66.7) | 0 (0) |
| IVT injection-related AEs | 2 (16.7) | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) |
| Ocular inflammation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Serum antibodies | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TEAEs by system organ class preferred term | |||||
| Eye disorders | 4 (33.3) | 2 (66.7) | 0 (0) | 2 (66.7) | 0 (0) |
| Eye irritation | 2 (16.7) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) |
| Eye pain | 1 (8.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) |
| Macular fibrosis | 1 (8.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) |
| Ocular hyperemia | 1 (8.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Posterior capsule opacification | 1 (8.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) |
| Retinal depigmentation | 1 (8.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) |
| Retinal hemorrhage | 1 (8.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Injury, poisoning, and procedural complications | 1 (8.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) |
| Contusion | 1 (8.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) |
| Fall | 1 (8.3) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) |
AE = adverse event; DLT = dose-limiting toxicity; IVT = intravitreal; SAE = serious adverse event; TEAE = treatment-emergent adverse event.
Data are presented as no. (%) within each group. At each level of participant summarization, a participant is counted once if the participant reported 1 or more events. Treatment-emergent adverse events are shown for the total of 12 participants, 3 in each of the following dose groups: 50 μg, 100 μg, 250 μg, or 500 μg. Adverse events related to the study drug and AEs related to the IVT injection are included, as are TEAEs by system organ class and preferred term.
No anti-GEM103 antibodies were detected in the plasma of any participants at any dose or treatment time point. No clinically significant changes were observed on ophthalmic examinations (including intraocular pressure and slit-lamp biomicroscopy) during the course of the study, and no ocular inflammation was observed; no incidences of endophthalmitis, iritis, vitreitis, vasculitis, or vascular occlusive events were reported. No choroidal neovascularization was reported or noted on examination or imaging. Systemically, no clinically significant abnormalities were observed for hematologic analysis, clinical chemistry or urinalysis (which included liver and kidney function), or vital signs. In addition, no treatment-emergent abnormalities in physical examinations were observed.
Visual Acuity Was Maintained after GEM103 Administration, Which Was Accompanied by Stable Clinical Parameters
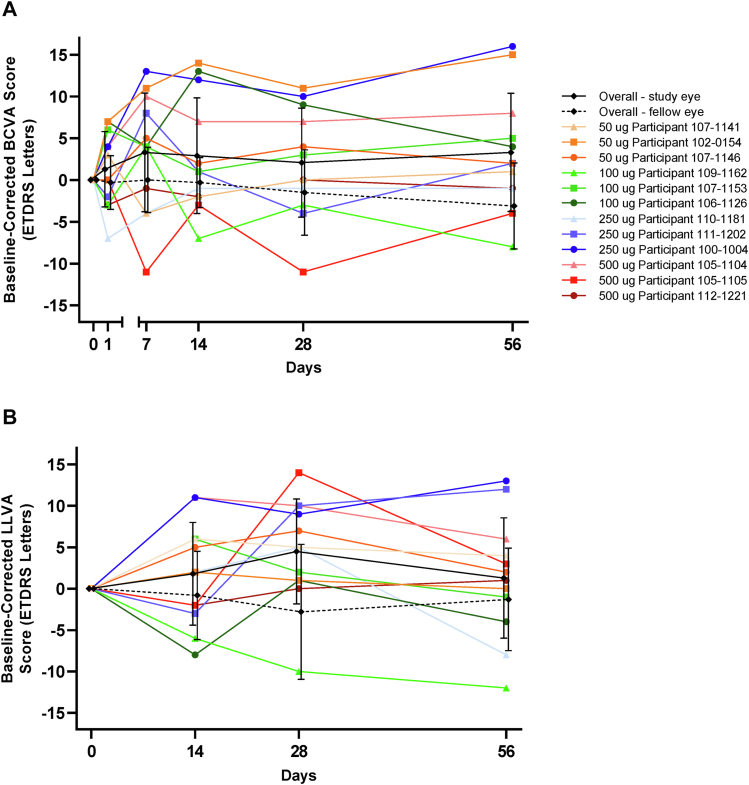
Individual patient visual acuity scores for the study eye and mean scores of the study and fellow eyes are provided in Figure 3. Median and mean BCVA and LLVA for the study eye remained within 5 ETDRS letters of baseline throughout the study; at week 8, median BCVA pooled across all dose groups was +2.0 ETDRS letters compared with baseline, and the mean ± SD was +3.3 ± 7.07 ETDRS letters. In the study eye, the lowest individual BCVA score at week 8 compared with baseline was −8 ETDRS letters, recorded in a participant in the 100-μg/eye group, and the maximum was +16 ETDRS letters in the 250-μg/eye group. Median LLVA at week 8 pooled across all dose groups in the study eye was +1.5 ETDRS letters compared with baseline, and the mean ± SD was +1.3 ± 7.28 ETDRS letters. The lowest individual LLVA score in the study eye at week 8 compared with baseline was −12 ETDRS letters, recorded in a participant in the 100-μg/eye group, and the maximum was +13 ETDRS letters in the 250-μg/eye group.
Figure 3.
Line graphs showing that visual acuity was maintained during the course of follow-up: mean ± standard deviation overall (study eye and fellow eye) and individual participant (study eye) baseline-corrected visual acuity scores (ETDRS letters) for (A) best-corrected visual acuity (BCVA) and (B) low-luminance visual acuity (LLVA).
Baseline GA lesions in the study eye in the 12 participants ranged in size from 1.7 to 35.3 mm2. Overall mean ± SD change from baseline in GA area within the study eye at week 8 in the 10 participants for whom these data were available was +0.2 ± 0.4 mm2 (Supplemental Fig 1). Data in relationship to drusen volume and total retinal and choroidal thickness, photoreceptor layer thickness, features of nascent GA, RPE thickening, and integrity of RPE layer as assessed by OCT showed substantial variation and did not provide any additional insights.
Complement Factor H Levels in AH Increased in a Dose-Dependent Manner after GEM103 Administration, Which Was Accompanied by Reductions in Complement Activation Biomarkers
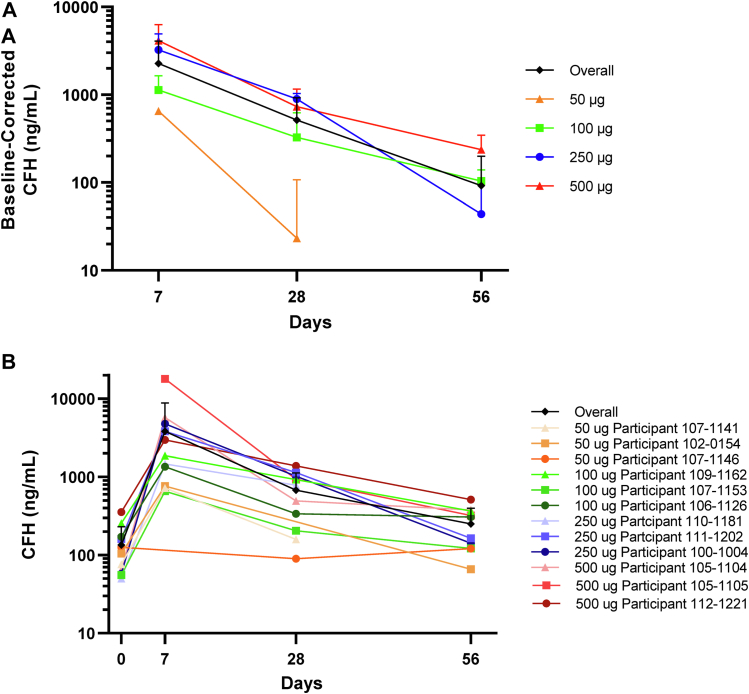
Aqueous humor samples from 11 participants were available for assessment of baseline CFH levels; inadequate AH sample volume and sample mishandling prevented assessment in all participants. The range of baseline AH CFH levels measured was 50 to 356 ng/ml, and the mean ± SD was 134.3 ± 97.0 ng/ml. All participants showed a week 1 AH CFH level of more than both the maximum observed and the mean baseline level, indicating achievement of more than physiologic levels of CFH and the presence of GEM103. Mean CFH levels at week 1 showed a dose-dependent increase when comparing the actual values between dose groups (Fig 4). Aqueous humor CFH levels decreased over time. Within each of the cohorts, the median AH CFH level remained greater than baseline at weeks 4 and 8 after intravitreal GEM103 injection. Plasma levels of CFH were highly variable throughout the course of the study; no dose effect was evident (Supplemental Fig 2).
Figure 4.
Line graphs showing that GEM103 increased and maintained levels of complement factor H (CFH) to more than baseline in aqueous humor: (A) mean ± standard deviation baseline-corrected levels of CFH (study eye) by treatment group and (B) absolute mean ± standard deviation overall and individual participant CFH levels (study eye). Note that data were not available from all dose groups, nor at all time points. Negative data points or error bars are not included because of the logarithmic scale.
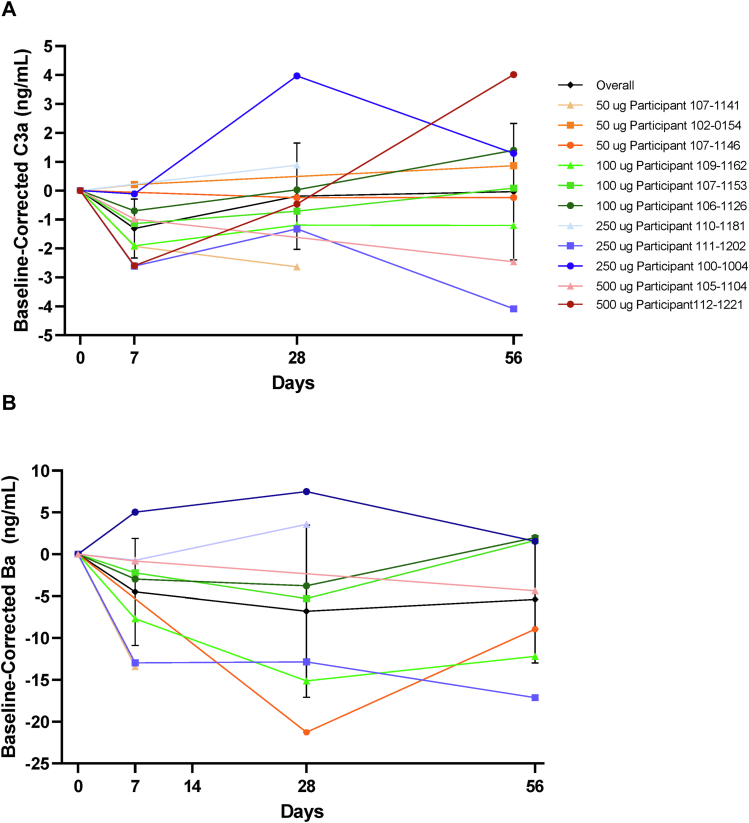
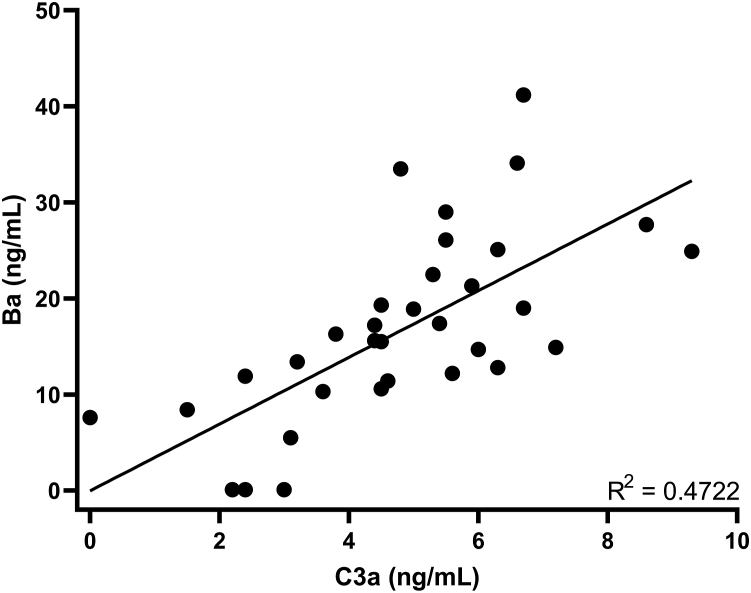
Overall mean decreases in biomarkers of complement activation (C3a and Ba) were observed from baseline after administration of GEM103, showing a 24.0% reduction in C3a at day 7 after GEM103 dose administration (Fig 5A). The overall reduction in Ba was 20.8% on day 7 (Fig 5B). Individualized data show that most participants demonstrated a reduction in C3a and Ba in AH at week 1 compared with baseline. Linear regression showed a positive correlation between concentration of Ba and C3a in AH (Fig 6). Other complement biomarkers in AH (CFB and C3) were stable during the course of the study (Supplemental Fig 3).
Figure 5.
Line graphs showing that GEM103 affected levels of complement activation biomarkers in aqueous humor: mean ± standard deviation overall and individual participant (study eye) baseline-corrected levels of (A) complement component C3a (C3a) and (B) complement component Ba (Ba). Note that data were not available from all dose groups or at all time points.
Figure 6.
Scatterplot showing the correlation between concentrations of complement components complement component C3a (C3a) and Ba (individual participant data) in aqueous humor.
Discussion
This phase I ascending-dose trial demonstrated that a novel recombinant human CFH (GEM103) is well tolerated in patients with GA at a dose of up to 500 μg. A single intravitreal injection of GEM103 was well tolerated at each of the doses tested, and no safety signals were evident with doses of up to 500 μg. This supports further evaluation of doses in the range of 500 μg on efficacy end points in higher-powered clinical trials. Only 4 participants experienced AEs; all were nonserious, mild, or moderate in severity, and none were determined by investigators to be related to GEM103. Mild ocular hyperemia and mild eye pain were determined by the investigator to be related to intravitreal injection. No clinically significant changes were observed from ophthalmic examinations during the study, and no ocular inflammation was observed. No evidence of neovascularization in the study eye was observed on retinal ophthalmic examination by investigators or on imaging as assessed by the independent IRC. Best-corrected visual acuity and LLVA scores remained stable compared with baseline through week 8, further supporting the safety of GEM103.
Secondary biomarker evaluation provides key evidence of biologic activity. Dose-dependent increases in CFH in AH were observed after intravitreal GEM103 infusion across the cohorts, with supraphysiologic levels achieved for at least 7 days. The endogenous CFH levels measured in this study are consistent with the AH CFH levels reported in age-matched control participants and patients with AMD.27 Because GEM103 is a recombinant human CFH, measured CFH levels after GEM103 administration are a combined level of endogenous CFH and GEM103. At week 1, CFH levels in AH exceeded those previously reported in age-matched control participants without AMD27 with sustained CFH levels up to week 8.
The increase in CFH was accompanied by simultaneous reductions in complement activation biomarkers. Although research has linked AMD to biomarkers of local complement activation, particularly C3a and Ba,27,28 data demonstrating biologic activity among intraocularly administered complement methods to decrease these biomarkers in the human eye are limited. At week 1, a decrease in biomarkers from baseline was observed in most dose cohorts. Evidence of continued activity through reductions in biomarkers was observed later in the study, although variability was found in measured levels.
Currently, no curative treatment is approved for patients with dry AMD, representing a significant unmet need. The status of CFH dysfunction as a well-established risk factor for the development of dry AMD and GA8 has led to focus on modulation of the alternative complement pathway as a therapeutic approach. Intravitreal therapies currently in phase II/III development are targeting complement factors downstream of CFH (i.e., C3a and C5 inhibitors).29,30 Results of these trials support targeting the alternative pathway as a therapeutic approach, with reduction in GA area observed.
In contrast to those therapies targeting downstream factors with exogenous inhibition, GEM103 is designed to restore balance to the complement system through its own endogenous regulator, CFH. The present study supports this concept, with downregulation of downstream complement factors observed as levels of functional CFH increased. Beyond the alternative pathway, evidence of the role of CFH in modulating inflammation and maintaining extracellular retinal homeostasis also exists.22 In vitro data (data not published) suggest that GEM103 localizes and binds to RPE, blocks damage by complement, protects RPE from lipid-mediated oxidative stress, and decreases macrophage infiltration and inflammation while not affecting normal RPE phagocytic function. The lack of macular or choroidal neovascularization associated with nAMD further supports the safety of GEM103. Although the incidence rate of such neovascularization in patients with GA is low, research shows that repeat dosing of intravitreal complement inhibitors can increase evidence of nAMD by > 17 times over 18 months compared with a sham control.31 The length and size of the present study limit comparison between GEM103 and other therapies with respect to neovascular risk. Nevertheless, aspects supporting the potentially lower neovascular risk conferred by GEM103 are its absence of pegylated molecules, which have been shown to be potentially immunogenic32 and have been linked to neovascularization in animal models,33,34 and the possible protective effect of CFH against neovascularization.35 A multiple-dose phase II trial is underway to understand the safety of GEM103.
The present trial was not powered to assess effects in relationship to the exploratory efficacy end points; therefore, it was expected that effects on GA area similar to those observed in trials of C3a and C5 inhibitors would not be observed. Best-corrected visual acuity and LLVA remained stable throughout the study; considering known variability in vision of patients with advanced AMD,36 no concerning losses in BCVA or LLVA were observed. Median and mean BCVA and LLVA scores pooled across all dose groups did not decrease at any time point compared with baseline and increased by 5.0 ETDRS letters or fewer. The present trial was underpowered to evaluate this clinical outcome fully, and future studies will clarify this potential further, particularly considering CFH’s role in protecting retinal cells.
Some limitations of the study should be acknowledged. The small sample size was deemed sufficient for investigating safety and pharmacokinetic, pharmacodynamic, and biomarker end points only. Although sampling of the AH provides useful information about intraocular effects of GEM103, it may not capture the complete picture because it likely underrepresents pharmacokinetics local to the RPE and retina and may be inadequate at detecting measurable levels of certain biomarkers such as C3b. Open-label clinical trials have inherent limitations, primarily the risk of investigator bias37; however, because no formal statistical comparisons were planned and because most of the end points were not subjective, that is, the exploratory efficacy and biomarker end points, an open-label design was deemed sufficient within the context of the aims of the study. Nevertheless, further well-controlled clinical studies will be required to confirm safety outcomes and to explore further the clinical and biomarker end points. In addition, based on the nature of GEM103 as a recombinant version of a normally occurring functional endogenous human protein, the minimal anticipated systemic exposure based on nonhuman primate evaluation and the associated bioanalytical challenges of differentiating endogenous CFH from GEM103, bioanalysis of GEM103 systemic exposure was limited. Endogenous plasma CFH levels before and after intravitreal dosing of GEM103 were assessed, and no clinically relevant changes were noted. In addition, the variability in effect of GEM103 on biomarker levels at least in part can be attributed to the low number of participants in this study and the fact that participants demonstrated a variable genetic background.
In conclusion, GEM103 was well tolerated in individuals with dry AMD and GA, with the limited number of mild or moderate AEs observed determined not to be related to the compound. GEM103 administration led to supraphysiologic levels being achieved for at least 56 days in higher-dose cohorts, with biomarker data supporting that GEM103 restores complement regulation. GEM103 may represent a well-tolerated and potentially effective therapeutic option for the treatment of dry AMD, and results of this study support further development in a repeat-dose trial to evaluate safety and pharmacokinetic and effects on complement activation biomarkers further.
Manuscript no. XOPS-D-22-00030.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org
See Commentary (XOPS 100155) in this issue
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): A.M.K.: Financial support – Gemini Therapeutics, Genentech, Apellis, Iveric Bio, Gyroscope
R.K.M.: Consultant – Neurotech, Oxurion, Aiviva, ForwardVue, Allegenesys, Eli Lilly, DORC International BV; Financial support – Allegro Ophthalmics LLC, Allergan/Abbvie, Samsung Bioepis, Oxurion NV, Boehringer Ingelheim Pharma GmbH & Co. KG, Santen Pharmaceutical Co. Ltd, Roche/Genentech, Gyroscope Therapeutics, GlaxoSmithKline, KalVista Pharmaceuticals, Graybug, Aerpio; Data Safety Monitoring Board or Advisory Board – Forward Vue, Aiviva; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid – DRCR Retina
N.B.: Financial support – Gemini Therapeutics; Consultant – Genentech, Alimera, Oxurion, Alcon; Lecturer – Alcon
D.S.B.: Financial support – 4D Molecular Therapeutics (4DMT), Achillion Pharma, Acucela, Adverum Biotech, Aerie, Aerpio, AiViva Biopharma, Alcon, Aldeyra Therapeutics, Alkahest, Allegro, Allergan, Allgenesis, Alzheon, Amgen, Amydis, Annexon Biosciences, Apellis Pharma, AsclepiX Therapeutics, Aviceda Therapeutics, Bausch & Lomb, Bayer, Biogen Inc, BioMotiv, Bionic Vision Technologies, Biovisics Medical, Boehringer-Ingelheim Pharma, Chengdu Kanghong Biotech, Ciana Therapeutics, Clearside Biomedical, Delsitech, DTx Pharma, Duet Pharma, Eloxx Pharma, EyePoint Pharma, Galimedix Therapeutics, Gemini Therapeutics, Genentech, GenSight Biologics, Glaukos, GrayBug Vision, Gyroscope Therapeutics, Horizon Therapeutics, jCyte, Kala Pharma, Iconic Therapeutics, Interface Biologics, Ionis Pharma, Isarna Therapeutics, Iveric Bio, Lineage Cell, Lumithera, Inc, MantraBio, NGMB Biopharma, Notal Vision, Novartis Ophthalmics, Ocular Therapeutix, Ocugen, Oculis SA, Ocuphire Pharma, OcuTerra Therapeutics, Opthea, OptoVue, Ora, Oxurion NV, Palatin Technologies, Quark Pharma, Ray Therapeutics, Regeneron, Regenxbio, Regulus Therapeutics, RetinAi Medical AG, Ripple Therapeutics, Roche, Santen, Shenyang XingQi Pharma, StealthBio Therapeutics, Surrozen, Inc., Thea Laboratories, Unity Biotech, Verseon Corp, Viewpoint Therapeutics, Vinci Pharma, Vitranu, Inc.
S.S.C.: Consultant – Genentech
D.S.D.: Financial support – Genentech, Regeneron, Novartis, Apellis, Allergan, Alimera Sciences, Eyepoint, Bayer
N.M.H.: Consultant – Adverum, Allergan, Annexon, Apellis, Bayer, Lineage Cell Therapeutics, Clearside Biosciences, Gemini Therapeutics, Genentech, Gyroscope, Katalyst Surgical, Nacuity, Notal Vision, Novartis, Polyactiva, Regeneron, Stealth Biosciences; Lecturer – Allergan, Genentech, Novartis, Regeneron, Spark; Data Safety Monitoring Board or Advisory Board – Editas phase 1 clinical trial; Equity owner – Katalyst Surgical, Apellis, Gemini Therapeutics
D.M.M.: Financial support – Gemini Therapeutics, Genentech Roche, Regenxbio, vial.com, Allergan, Aiviva, Amgen, Boehringer Inglheim, Alcon, Aerpio, Kalvista, Ionis, Xbrane Xplore, Mylan, Regeneron, Samsung, Novartis, Opthea, Chenghdu, Clearside, Astella, Allegro, Alimera, Iveric, Outlook, Gemini, Thrombogenics, Tyrogenex, Graybug, Topcon, Optos, Gyroscope, Stealth, Aerie, Apellis, Ohr, Kodiak, Zeiss, Annexon, Alexion, Oculis, Mylan
D.P.: Consultant – Genentech, IVERIC, Regenx, NGM, Regeneron, Adverum, Gemini Therapeutics; Financial support – Genentech, Regeneron, Gemini Therapeutics, Kodiac, Regenx, Stealth, Novarits, NGM, Apellis, IVERIC, Adverum, Unity, IONIS; Data Safety Monitoring Board or Advisory Board – Genentech, Regeneron; Equity owner – Gemini Therapeutics
A.A.A.: Consultant – Gemini Therapeutics
K.C.P.: Equity owner – Gemini Therapeutics
Supported by Gemini Therapeutics, Cambridge, Massachusetts. The sponsor participated in the design of the study; conducting the study; data collection; data management; data analysis; interpretation of the data; and preparation, review, or approval of the manuscript.
The data that support the findings of this study are available from Gemini Therapeutics, Inc, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Presented at: American Academy of Ophthalmology Annual Meeting, November 2020.
HUMAN SUBJECTS: Human subjects were included in this study. Local or central IRBs (Advarra IRB) of all study sites approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Patki
Analysis and interpretation: Khanani, Patki
Data collection: Khanani, Maturi, Bagheri, Bakall, Boyer, Couvillion, Dhoot, Holekamp, Jamal, Marcus, Pieramici, Aziz, Bridges
Obtained funding: N/A
Overall responsibility: Khanani, Maturi, Bagheri, Bakall, Boyer, Couvillion, Dhoot, Holekamp, Jamal, Marcus, Pieramici, Aziz, Patki, Bridges
Supplementary Data
References
- 1.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Bressler N.M. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz-Valckenberg S., Sahel J.A., Danis R., et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study) Ophthalmology. 2016;123:361–368. doi: 10.1016/j.ophtha.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Bowes Rickman C., Farsiu S., Toth C.A., Klingeborn M. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013;54:ORSF68–ORSF 80. doi: 10.1167/iovs.13-12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seddon J.M., Cote J., Page W.F., et al. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123:321–327. doi: 10.1001/archopht.123.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarthy U., Wong T.Y., Fletcher A., et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. doi: 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jager R.D., Mieler W.F., Miller J.W. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 9.Seddon J.M. Macular degeneration epidemiology: nature-nurture, lifestyle factors, genetic risk, and gene-environment interactions—The Weisenfeld Award Lecture. Invest Ophthalmol Vis Sci. 2017;58:6513–6528. doi: 10.1167/iovs.17-23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black J.R., Clark S.J. Age-related macular degeneration: genome-wide association studies to translation. Genet Med. 2016;18:283–289. doi: 10.1038/gim.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geerlings M.J., de Jong E.K., den Hollander A.I. The complement system in age-related macular degeneration: a review of rare genetic variants and implications for personalized treatment. Mol Immunol. 2017;84:65–76. doi: 10.1016/j.molimm.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seddon J.M., Reynolds R., Maller J., et al. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis Sci. 2009;50:2044–2053. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calippe B., Guillonneau X., Sennlaub F. Complement factor H and related proteins in age-related macular degeneration. C R Biol. 2014;337:178–184. doi: 10.1016/j.crvi.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Sobrin L., Seddon J.M. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40:1–15. doi: 10.1016/j.preteyeres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toomey C.B., Kelly U., Saban D.R., Bowes Rickman C. Regulation of age-related macular degeneration-like pathology by complement factor H. Proc Natl Acad Sci U S A. 2015;112:E3040–E3049. doi: 10.1073/pnas.1424391112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triebwasser M.P., Roberson E.D., Yu Y., et al. Rare variants in the functional domains of complement factor H are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:6873–6878. doi: 10.1167/iovs.15-17432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakkinstian A., Han P., McEvoy M., et al. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet. 2006;15:2784–2790. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- 18.Pickering M.C., Cook H.T. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol. 2008;151:210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hourcade D.E., Mitchell L.M., Medof M.E. Decay acceleration of the complement alternative pathway C3 convertase. Immunopharmacology. 1999;42:167–173. doi: 10.1016/s0162-3109(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 20.Blaum B.S., Hannan J.P., Herbert A.P., et al. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol. 2015;11:77–82. doi: 10.1038/nchembio.1696. [DOI] [PubMed] [Google Scholar]
- 21.Morgan H.P., Schmidt C.Q., Guariento M., et al. Structural basis for engagement by complement factor H of C3b on a self surface. Nat Struct Mol Biol. 2011;18:463–470. doi: 10.1038/nsmb.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armento A., Honisch S., Panagiotakopoulou V., et al. Loss of complement factor H impairs antioxidant capacity and energy metabolism of human RPE cells. Sci Rep. 2020;10:10320. doi: 10.1038/s41598-020-67292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calippe B., Augustin S., Beguier F., et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity. 2017;46:261–272. doi: 10.1016/j.immuni.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Mattapallil M.J., Caspi R.R. Compliments of factor H: what’s in it for AMD? Immunity. 2017;46:167–169. doi: 10.1016/j.immuni.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu C., Roubeix C., Sennlaub F., Saban D.R. Microglia versus monocytes: distinct roles in degenerative diseases of the retina. Trends Neurosci. 2020;43:433–449. doi: 10.1016/j.tins.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meri S., Haapasalo K. Function and dysfunction of complement factor H during formation of lipid-rich deposits. Front Immunol. 2020;11:611830. doi: 10.3389/fimmu.2020.611830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altay L., Sitnilska V., Schick T., et al. Early local activation of complement in aqueous humour of patients with age-related macular degeneration. Eye (Lond) 2019;33:1859–1864. doi: 10.1038/s41433-019-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schick T., Steinhauer M., Aslanidis A., et al. Local complement activation in aqueous humor in patients with age-related macular degeneration. Eye (Lond) 2017;31:810–813. doi: 10.1038/eye.2016.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao D.S., Grossi F.V., El Mehdi D., et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127:186–195. doi: 10.1016/j.ophtha.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Jaffe G.J., Westby K., Csaky K.G., et al. C5 Inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128:576–586. doi: 10.1016/j.ophtha.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Wykoff C.C., Rosenfeld P.J., Waheed N.K., et al. Characterizing new-onset exudation in the randomized phase 2 FILLY trial of complement inhibitor pegcetacoplan for geographic atrophy. Ophthalmology. 2021;128(9):1325–1336. doi: 10.1016/j.ophtha.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Shiraishi K., Yokoyama M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: a review. Sci Technol Adv Mater. 2019;20:324–336. doi: 10.1080/14686996.2019.1590126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyzogubov V.V., Tytarenko R.G., Liu J., et al. Polyethylene glycol (PEG)-induced mouse model of choroidal neovascularization. J Biol Chem. 2011;286:16229–16237. doi: 10.1074/jbc.M110.204701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudolf X.V., Lyzogubov V.V., Bora N.S., Bora P.S. Understanding the polyethylene glycol-induced mouse model of retinal degeneration and choroidal neovascularization. J Cell Mol Pharmacol. 2018;1:1–5. [Google Scholar]
- 35.Borras C., Delaunay K., Slaoui Y., et al. Mechanisms of FH protection against neovascular AMD. Front Immunol. 2020;11:443. doi: 10.3389/fimmu.2020.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel P.J., Chen F.K., Rubin G.S., Tufail A. Intersession repeatability of visual acuity scores in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:4347–4352. doi: 10.1167/iovs.08-1935. [DOI] [PubMed] [Google Scholar]
- 37.Naci H., Davis C., Savovic J., et al. Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014–16: cross sectional analysis. BMJ. 2019;366:15221. doi: 10.1136/bmj.l5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.