Abstract
Purpose
Subretinal injections (SRis) are commonly used in retinal gene therapy procedures to deliver adeno-associated virus (AAV) to photoreceptors and retinal pigment epithelial cells. We present an optimized surgical protocol to minimize off-target application of AAV in the vitreous, which in turn reduces the risk of extensive biodistribution and inflammation, ultimately leading to enhanced safety of the therapy.
Design
Experimental animal research study.
Participants
Eight cynomolgus monkeys (Macaca fascicularis).
Methods
Subretinal injections with an AAV2/8 vector were performed. The animals were allocated to 2 different vector dose groups (1×10ˆ11 and 5×10ˆ11 viral genomes [vg]). Samples of intravitreal fluid were taken at the end of the SRi procedure and again after a 3-minute lavage (wash-out) with balanced salt solution (BSS).
Main Outcome Measures
Intravitreal vector genome copies were analyzed with quantitative polymerase chain reaction and compared between groups.
Results
Even uneventful SRi leads to dissemination of millions of AAV particles (0.1–0.7% of viral vector loading dose) into the vitreous cavity. Three minutes of lavage led to a substantial decrease (on average 96%) of intravitreal vector load.
Conclusions
Multiple studies have shown that the intravitreal space is not as immune privileged as the subretinal space. Intravitreal AAV particles disseminate into the bloodstream, lead to increased biodistribution into lymphatic tissue, and help to stage an immune response with implications for both safety and efficacy. Therefore, minimizing off-target vector application after reflux of vector from the subretinal space is of significant interest. We show that a simple lavage of intravitreal fluid efficiently decreases the intravitreal vector load. Such a step should be considered when performing subretinal gene therapy.
Keywords: AAV, Gene therapy, Retina, Subretinal surgery, Viral vector
Abbreviations and Acronyms: AAV, adeno-associated virus; BSS, balanced salt solution; IRD, inherited retinal disease; SRi, subretinal injection; TIVL, total intravitreal vector load; vg, viral genome
Adeno-associated virus (AAV)-mediated gene therapies provide treatment options for a range of inherited retinal diseases (IRDs). In recent years, the number of disease targets has expanded rapidly and now include monogenic IRD and multifactorial diseases such as age-related macular degeneration.1 For many of these diseases, gene therapy products are already in the stage of clinical trials. With voretigene-neparvovec (Luxturna), ocular gene therapy has been approved in multiple countries, including the United States and Europe, for the treatment of patients with visual impairment due to confirmed biallelic RPE65 mutation–associated inherited retinal dystrophy in the presence of viable retinal cells.2
As with Luxturna, most of these new gene therapies will be applied by subretinal injection (SRi). This route of application provides the best dose-efficacy relationship when outer retinal cells (e.g., retinal pigment epithelium or photoreceptors) are being targeted. Therefore, SRi is the route of choice for most retinal gene therapy approaches targeting the outer retina. In addition, the subretinal space is especially immune privileged.3 This is ideal when a potentially immunogenic viral vector such as AAV is used. Although AAV is generally considered to be well tolerated, it has become clear that AAV is recognized by the immune system and does lead to a dose-dependent inflammation in the eye, termed “gene therapy–associated uveitis.”4 Gene therapy–associated uveitis has been observed in preclinical and clinical trials, and is a potential threat to the visual function of the eye.5, 6, 7
Comparisons between intravitreal and subretinal application of AAV gene therapies suggest that the subretinal space might be more immune privileged than the intravitreal space.8, 9, 10 Intravitreal AAV, but not subretinal AAV, has the potential to elicit capsid-directed humoral immune responses that can inhibit effective readministration.8 A reason for this could be the enhanced and prolonged biodistribution of the vector after intravitreal injection.11 Consequently, inadvertent placement of the vector into the vitreous (e.g., through reflux from the retinotomy site) has potential implications for treatment outcome.
The aim of this study was to (1) measure the amount of AAV escaping into the vitreous after uneventful subretinal delivery and to (2) investigate whether a lavage can help to further limit intravitreal AAV load after SRi. These findings may help to understand off-target effects after subretinal AAV gene therapy and enhance safety and efficacy of subretinal gene therapies worldwide.
Methods
Cynomolgus monkeys (Macaca fascicularis, 4 male and 4 female) were kept in pair or group housing in a climate-controlled room and fed twice daily with a certified laboratory diet (LabDiet 5048; PMI Nutritional International, Inc.) supplemented with fresh fruits and vegetables. Housing included stainless steel mirrors, wooden chips, colored plastic tools, and balls. All procedures involving animals were performed in adherence to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, in compliance with Good Laboratory Practice regulations and with approval from the relevant local regulatory authority (Regierungspräsidium, Düsseldorf, Germany) at Covance, Münster.
Subretinal injections with good manufacturing practice–grade vector (recombinant AAV serotype 8, rAAV8) were administered to 8 monkeys. Animals were fasted overnight before general anesthesia with isoflurane. Before the surgery, the periorbital region was thoroughly cleaned with povidone-iodine and sterile surgical drapes were applied. After applying a pediatric lid speculum to the left eye, pars plana location was confirmed by transillumination and 3 sclerotomies were made approximately 1 to 2 mm posterior to the limbus using valved 23G trocars (Retilock; FCI S.A.S.). Where necessary, a temporal canthotomy was applied to facilitate access. All surgeries were performed by the same experienced vitreoretinal surgeon (M.D.F.) using the PentaSys vitrectomy machine (Ruck GmbH) for pars plana vitrectomy. After vitrectomy, a localized retinal detachment was induced with balanced salt solution (BSS Plus; Alcon Pharma GmbH) using an extendible 41-gauge cannula (DORC 1270.EXT; Dutch Ophthalmic Research Center [International] B.V.). In a second step, an SRi of rAAV8 vector solution of up to 0.17 ml into the induced bleb (same retinotomy) was administered using 1 to 2 psi positive pressure on the silicon oil infusion program of the PentaSys system. After completing the SRi, a small sample of vitreous fluid was taken before and after 3 minutes of fluid/fluid exchange with BSS via the vitrectomy probe and the 23G trocar system. The vitreous sample was aspirated from the center of the vitreous cavity. In our 23-gauge vitrectomy system, 50 ml of BSS is running through the infusion and is aspirated through the vitrectomy probe during the 3 minutes of intravitreal lavage.
For quantitative polymerase chain reaction analysis, 50 μl of the vitreous fluid samples were used for DNA extraction. Of the 110 μl DNA elute, 5 μl were used for quantitative polymerase chain reaction analysis. When calculating the virus load per eye based on the reported number of vector genome copies, we assumed a 2 ml total volume of the vitreous cavity in the cynomolgus monkey. Thus, total intravitreal vector load (TIVL) is presented as the amount of vector genomes measured in the 50 μl sample multiplied by the factor 40 to account for the total volume of the vitreous of 2 ml. Statistical analysis was performed using JMP, Version 15 (SAS Institute Inc, 1989–2019). Differences were tested for statistical significance by a 2-sided, paired-sample t test and with the Wilcoxon-signed rank test. A P < 0.05 was considered as the level of significance.
Results
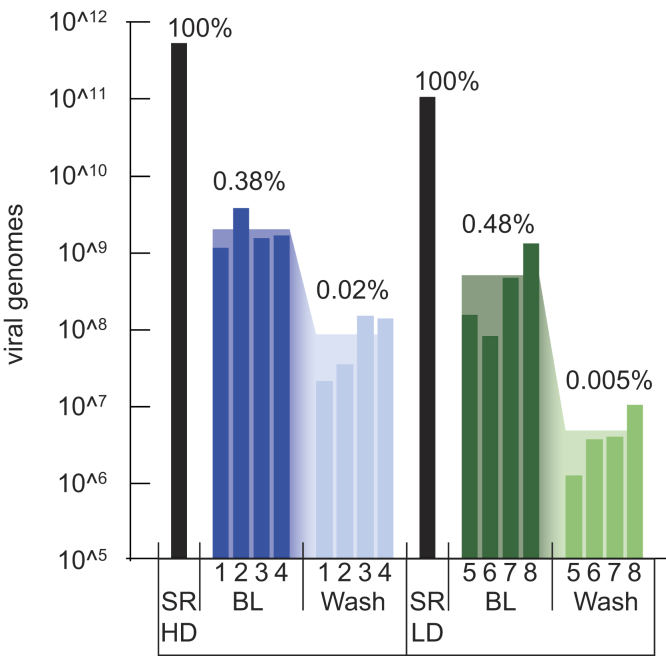
Total intravitreal vector load ranged from 7.8×10ˆ7 viral genomes (vg) to 1.3×10ˆ9 vg in the low-dose group and between 1.1×10ˆ9 vg and 3.6×10ˆ9 vg in the high-dose group (Fig 1). Mean TIVL was 4.8×10ˆ8 vg in the low-dose group and 1.9×10ˆ9 vg in the high-dose group. After a 3-minute lavage, mean TIVL in the low-dose group was reduced to 4.6×10ˆ6 vg and 8.2×10ˆ7 vg in the high-dose group. This reduction of approximately 2 log units was significant in the paired-sample t test and the Wilcoxon signed-rank test (parametric P < 0.021, nonparametric P < 0.008). Total intravitreal vector load after lavage varied from 1.2×10ˆ6 vg and 9.8×10ˆ6 vg in the low-dose group and between 2.0×10ˆ7 vg and 1.3×10ˆ8 vg in the high-dose group. When calculated as a percentage of the subretinal injected vector dose of 1.0×10ˆ11 vg (low dose) and 5.10ˆ11 vg (high dose), 0.08% to 1.26% of vector in the low-dose group and 0.22% to 0.31% of vector in the high-dose group was found in vitreous. Intravitreal lavage reduced the virus load in the vitreous to 0.001% to 0.01% of subretinally injected dose in the low-dose group and 0.004% to 0.03% in the high-dose group. This equates to an average reduction of 95% (high dose)/98% (low dose) of virus load in the vitreous by lavage for 3 minutes (Fig 2).
Figure 1.
Intravitreal viral genomes (vg) measured after subretinal injection (SRi) of high dose = 5×10ˆ11 (SR HD) or low dose = 10ˆ11 (SR LD). Subretinal dose is marked as black bar. Blue indicates intravitreal vg after high-dose injection, and green indicates intravitreal vg after low-dose injection. Narrow bars with numbers below mark each animal, and broad bars in background show mean value. BL = baseline vg measured in vitreous after SRi. Wash = vg after 3 minutes of fluid-fluid exchange. Percentage values indicate mean intravitreal vg dose as percentage of subretinal dose.
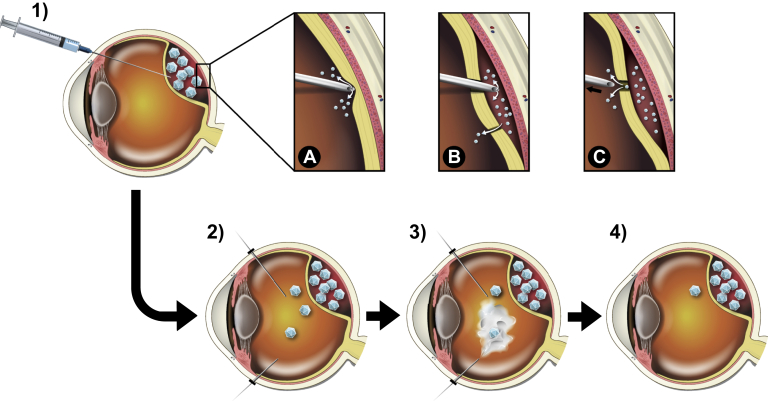
Figure 2.
1–4, Subretinal injection followed by intravitreal lavage with balanced salt solution (BSS). A–C, Possible ways for vector to escape into the intravitreal space during the procedure: (A) inadvertent injection of viral vector solution before full penetration of neurosensory retina; (B) reflux of viral vector solution from a previous retinotomy, macular hole, or retinal tear; (C) reflux of viral vector solution through the retinotomy after retraction of injection needle.
Discussion
Subretinal injections are a highly effective way to treat the cells of the outer retina with gene therapy. Although an intravitreal injection would be favorable in terms of being the far less-invasive technique, retinal barriers limit the transduction efficacy when cells of the outer retina are being targeted. In addition, comparative studies, where the vector was delivered either to the intravitreal space or to the subretinal space have shown that the subretinal space has the added benefit of limiting the systemic biodistribution and providing greater immune privilege.9,10 We investigated how much of the vector solution applied in the subretinal space can be found in the vitreous through reflux from the retinotomy or inadvertent placement in the wrong compartment.
We show that after an uneventful SRi (i.e., without obvious reflux, no second retinotomy, and no retinal tear/macular hole formation), less than 1% of the viral vector load injected into the subretinal space can be found in the vitreous. However, given the high absolute number of viral particles injected, that still equates to millions or even billions of virus particles with potential implications for gene therapy–associated uveitis. Of note, the amount of virus in the vitreous after uneventful SRi varied between animals. This may be due to variations during surgery, such as the amount of inadvertently placed vector solution in vitreous or reflux, or due to variability associated with the sampling or quantification of vg in the samples. All surgeries were performed by the same experienced surgeon (M.D.F.) in the same setting in one session to aim for greatest reproducibility. Sampling was also done by the same team consistently and according to protocol. Molecular analysis has been done using Good Laboratory Practices according to a protocol validated for regulatory submission as part of a formal toxicology and biodistribution study. We conclude that the observed variance reflects how difficult it is to standardize the injection procedure. When performing an SRi, a retinotomy just central to the superior temporal arcade is most likely to deliver vector solution in the foveal region.12 This placement of the retinotomy is also recommended in the protocol for use of voretigene neparvovec. Use of a foot-pedal–controlled injection system and intraoperative OCT can further aid a standardized delivery of subretinal gene therapy.12,13 However, viral vector can escape into the vitreous at different critical time points during the procedure (Fig 2). First, correct positioning of the cannula tip for subretinal delivery is not confirmed through haptic feedback, but usually is confirmed only post hoc by injecting and observing a bleb formation. If there is no bleb formation, the needle tip might be too far advanced and retinal and subretinal tissue are compressed to a point, where they occlude the tip of the needle, or the needle tip might not be sufficiently advanced and fluid directly escapes into the vitreous. Second, if a second retinotomy is performed and the resulting bleb has or gains direct access to the first retinotomy, vector solution injected through one retinotomy can reflux through the other. Third, at the end of the injection procedure, a positive pressure difference between the subretinal space and the vitreous can lead to reflux of vector through the retinotomy.
Potentially, viral vector could also escape into the vitreous in the hours after the surgery is performed. In this study, no samples of the vitreous were taken at later timepoints, but future studies could overcome this limitation to provide insight of egress over time.
It is important to note that the absolute number of virus particles in the vitreous is still very high even if this only constitutes 1% of the total dose given. For example, a dose of 1×10ˆ11 delivered subretinally may lead to 1×10ˆ9 viral vector particles in the vitreous cavity, a dose that has been shown to be sufficient to elicit immunological reactions such as intraocular inflammation and antibody formation.14 To reduce intravitreal vector load, a lavage of 3 minutes duration proved to be effective in reducing intravitreal vector concentrations to a minimum. Our study was limited in that only 1 surgical protocol (2-step procedure, no fluid-air exchange) was assessed in nonhuman primates, which feature smaller eyes and stronger adhesion between vitreous and retina compared with humans. It is conceivable that different surgical protocols yield different levels of viral biodistribution, and a fluid-air exchange, as an alternative, may be another means of accomplishing a similar washout effect.
Another limitation of the study is the different adherence of the posterior hyaloid between nonhuman primates (NHP) and IRDs, in whom a posterior vitreous detachment is usually performed. It may well be that the posterior vitreous detachment, which was not done in the current study, has an impact on vector distribution.
Although the recommended approach for voretigene neparvovec is a direct injection into the subretinal space, many clinical trials work with a 2-step approach and use BSS to preform the subretinal space to avoid inadvertent injection into the vitreous during the initiation of the bleb. Such a 2-step approach, however, necessitates reentry of an injection needle through the same retinotomy and risks enlarging the same or creating a second retinotomy.15 We have only tested the 2-step approach because the study was part of an Investigational New Drug–enabling program toward the first-in-man application of gene therapy for PDE6A-associated retinitis pigmentosa. Taken together, there are a variety of intraoperative factors that can potentially influence the amount of vector that is unintentionally left in the vitreous. We show that when a 2-step technique is performed and only 1 bleb with 1 retinotomy is raised, up to 1.3% of the subretinal dose can be detected in the vitreous. We speculate that multiple blebs, multiple retinotomies, and a 1-step technique potentially lead to even higher levels of intravitreal virus load, and further studies would need to assess this.
However, the exact number of intravitreal vector genomes that lead to a clinically relevant inflammation is unknown. Intravitreal vector load has not been routinely measured after SRi and therefore has not been compared with inflammatory outcomes. It can only be hypothesized that in some cases of gene therapy–associated uveitis, excessive intravitreal spill of vector may play a role. Future studies could measure intravitreal spill of vector and correlate this with clinical signs of inflammation. This could be useful to compare different surgical techniques and would enable threshold values to be established for clinical significance.
In conclusion, we have shown that a small but potentially relevant percentage of the subretinal dose is unintentionally delivered to the intravitreal space. A simple lavage of 3 minutes can effectively reduce intravitreal vector load by approximately 96%. This information is highly relevant for vitreoretinal surgeons performing subretinal delivery of AAV and will help to enhance the safety and efficacy of ocular gene therapy in general.
Manuscript no. D-21-00027.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): M.D.F.: Grant – Kerstan Stiftung.
HUMAN SUBJECTS: Human subjects were not included in this study. All procedures involving animals were performed in adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, in compliance with good laboratory practice regulations and with approval from the relevant local regulatory authority (Regierungspräsidium, Düsseldorf, Germany) at Covance, Münster. All research adhered to the tenets of the Declaration of Helsinki.
Animal subjects were used in this study.
Author Contributions:
Conception and design: Bartz-Schmidt, Peters, Fischer
Data collection: Reichel, Wozar, Seitz, Ochakovski, Peters, Fischer
Analysis and interpretation: Reichel, Wozar, Seitz, Ochakovski, Fischer
Obtained funding: N/A; Study was performed as part of the authors' regular employment duties. No additional funding was provided.
Overall responsibility: Reichel, Fischer
Appendix: RD-CURE Consortium
| Coordination: | Project Coordinator: Prof. Dr. Bernd Wissinger Molecular Genetics Laboratory, Institute for Ophthalmic Research, Centre for Ophthalmology, Project Co-Coordinator: Prof. Dr. Martin Biel Department of Pharmacy – Center for Drug Research, Ludwig-Maximilians-Universität München Scientific Representative of the Tistou and Charlotte Kerstan Foundation: Prof. Dr.med. Dr.h.c.mult. Eberhart Zrenner Institute for Ophthalmic Research, Centre for Ophthalmology, University of Tübingen |
| Further Project Partners: | University Eye Hospital, Centre for Ophthalmology, University of Tübingen Prof. Karl Ulrich Bartz-Schmidt Prof. Dr. Dominik Fischer Institute for Ophthalmic Research, Centre for Ophthalmology, University of Tübingen: Dr. Susanne Kohl Dr. Laura Kühlewein Prof. Dr. Francois Paquet-Durand Prof. Dr.med. Dipl.Ing. Mathias Seeliger Prof. Dr. Marius Ueffing Dr. Nicole Weisschuh PD Dr. Ditta Zobor STZ eyetrial, Centre for Ophthalmology, University of Tübingen Dr. Nadine Kahle Dr. Tobias Peters Prof. med. Barbara Wilhelm Department of Pharmacy – Center for Drug Research, Ludwig-Maximilians-Universität München: PD Dr. Stylianos Michalakis |
| Reviewers and Advisory Board: | Prof. Jean Bennett (F.M. Kirby Center for Molecular Ophthalmology, University of Pennsylvania, Philadelphia, USA) Dr. Olav Hagemann (Achromatopsie Selbsthilfe e.V., Dorsten, Germany) Prof. Peter Humphries (Smurfit Institute of Genetics, Trinity College, Dublin, Ireland) Prof. Robert Molday (Dept. of Biochemistry and Molecular Biology, University of British Columbia, Vancouver, Canada) Prof. Jan Wijnholds (Netherlands Institute for Neuroscience, Royal Netherlands Academy of Arts & Sciences, Amsterdam, The Netherlands) |
| Funding Agency: | Tistou & Charlotte Kerstan Stiftung |
| Website | www.rd-cure.de |
References
- 1.Bordet T., Behar-Cohen F. Ocular gene therapies in clinical practice: viral vectors and nonviral alternatives. Drug Discov Today. 2019;24:1685–1693. doi: 10.1016/j.drudis.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Russell S., Bennett J., Wellman J.A., et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand V., Duffy B., Yang Z., et al. A deviant immune response to viral proteins and transgene product is generated on subretinal administration of adenovirus and adeno-associated virus. Mol Ther. 2002;5:125–132. doi: 10.1006/mthe.2002.0525. [DOI] [PubMed] [Google Scholar]
- 4.Reichel F.F., Dauletbekov D.L., Klein R., et al. AAV8 can induce innate and adaptive immune response in the primate eye. Mol Ther. 2017;25:2648–2660. doi: 10.1016/j.ymthe.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bainbridge J.W.B., Mehat M.S., Sundaram V., et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimopoulos I.S., Hoang S.C., Radziwon A., et al. Two-year results after AAV2-mediated gene therapy for choroideremia: The Alberta Experience. Am J Ophthalmol. 2018;193:130–142. doi: 10.1016/j.ajo.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Timmers A.M., Newmark J.A., Turunen H.T., et al. Ocular inflammatory response to intravitreal injection of adeno-associated virus vector: relative contribution of genome and capsid. Hum Gene Ther. 2020;31:80–89. doi: 10.1089/hum.2019.144. [DOI] [PubMed] [Google Scholar]
- 8.Kotterman M.A., Yin L., Strazzeri J.M., et al. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22:116–126. doi: 10.1038/gt.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q., Miller R., Han P.Y., et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- 10.Reichel F.F., Peters T., Wilhelm B., et al. Humoral immune response after intravitreal but not after subretinal AAV8 in primates and patients. Invest Ophthalmol Vis Sci. 2018;59:1910–1915. doi: 10.1167/iovs.17-22494. [DOI] [PubMed] [Google Scholar]
- 11.Seitz I.P., Michalakis S., Wilhelm B., et al. Superior retinal gene transfer and biodistribution profile of subretinal versus intravitreal delivery of AAV8 in nonhuman primates. Invest Ophthalmol Vis Sci. 2017;58:5792–5801. doi: 10.1167/iovs.17-22473. [DOI] [PubMed] [Google Scholar]
- 12.Xue K., Groppe M., Salvetti A.P., MacLaren R.E. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye. 2017;31:1308–1316. doi: 10.1038/eye.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasconcelos H.M., Lujan B.J., Pennesi M.E., et al. Intraoperative optical coherence tomographic findings in patients undergoing subretinal gene therapy surgery. Int J Retina Vitreous. 2020;6:13. doi: 10.1186/s40942-020-00216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cukras C., Wiley H.E., Jeffrey B.G., et al. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol Ther. 2018;26:2282–2294. doi: 10.1016/j.ymthe.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis J.L., Gregori N.Z., MacLaren R.E., Lam B.L. Surgical technique for subretinal gene therapy in humans with inherited retinal degeneration. Retina. 2019;39(Suppl 1):S2–S8. doi: 10.1097/IAE.0000000000002609. [DOI] [PubMed] [Google Scholar]