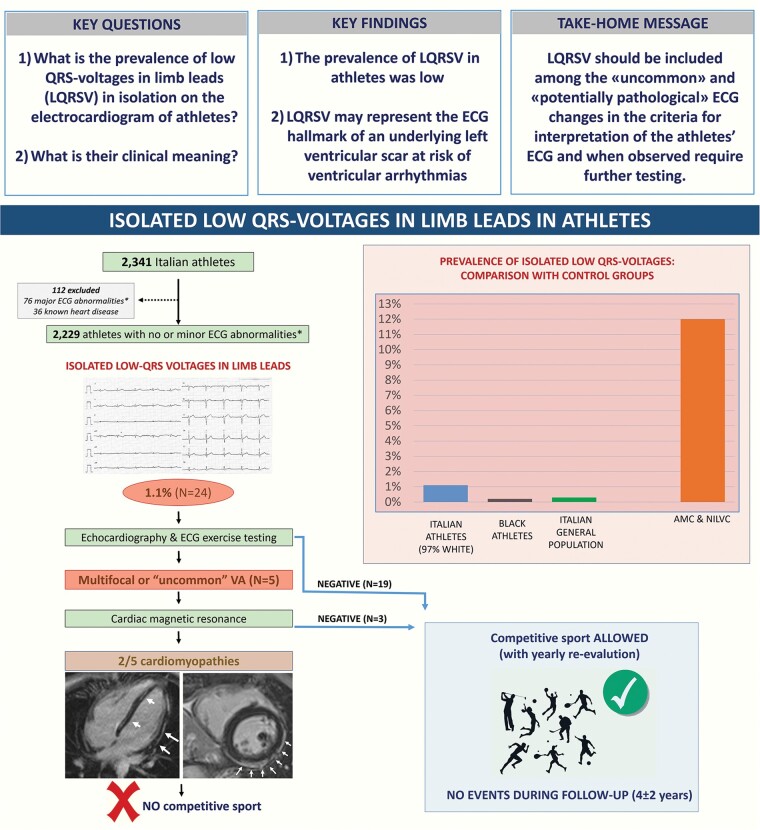
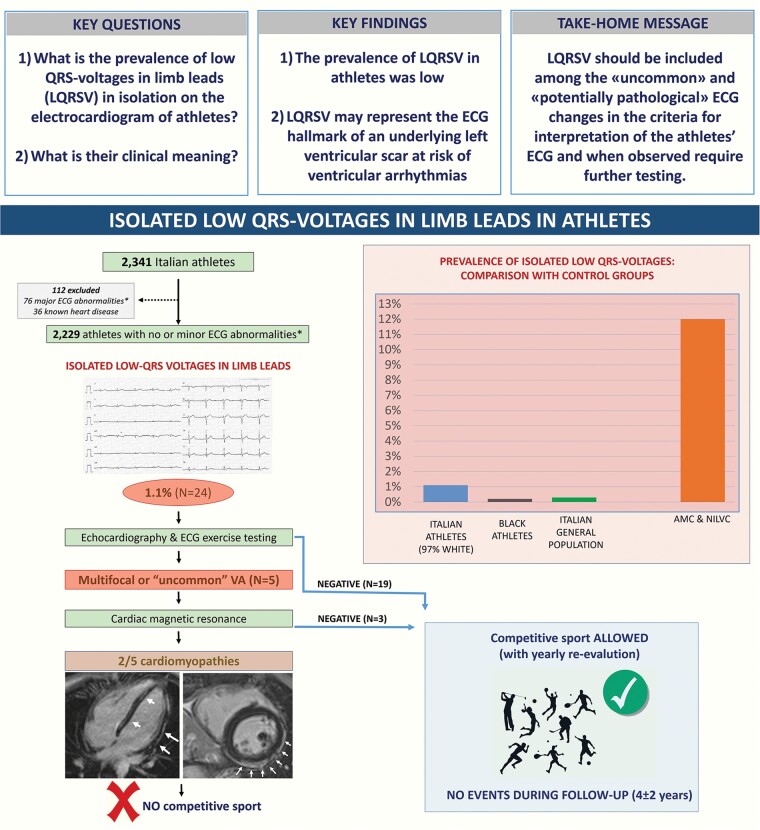
Abstract
Aims
Low QRS voltages (peak to peak <0.5 mV) in limb leads (LQRSV) on the athlete’s electrocardiogram (ECG) may reflect an underlying cardiomyopathy, mostly arrhythmogenic cardiomyopathy (ACM) or non-ischaemic left ventricular scar (NILVS). We studied the prevalence and clinical meaning of isolated LQRSV in a large cohort of competitive athletes.
Methods and results
The index group included 2229 Italian competitive athletes [median age 18 years (16–25), 67% males, 97% Caucasian] without major ECG abnormalities at pre-participation screening. Three control groups included Black athletes (N = 1115), general population (N = 1115), and patients with ACM or NILVS (N = 58). Echocardiogram was performed in all athletes with isolated LQRSV and cardiac magnetic resonance (CMR) in those with ventricular arrhythmias or echocardiographic abnormalities. The isolated LQRSV pattern was found in 1.1% index athletes and was associated with increasing age (median age 28 vs. 18 years; P < 0.001), elite status (71% vs. 34%; P < 0.001), body surface area, and body mass index but not with sex, type of sport, and echocardiographic left ventricular mass. The prevalence of isolated LQRSV was 0.2% in Black athletes and 0.3% in young individuals from the general population. Cardiomyopathy patients had a significantly greater prevalence of isolated LQRSV (12%) than index athletes, Black athletes, and general population. Five index athletes with isolated LQSRV and exercise-induced ventricular arrhythmias underwent CMR showing biventricular ACM in 1 and idiopathic NILVS in 1.
Conclusions
Unlike cardiomyopathy patients, the ECG pattern of isolated LQRSV was rarely observed in athletes. This ECG sign should prompt clinical work-up for exclusion of an underlying cardiomyopathy.
Keywords: Athlete’s heart, Electrocardiogram, Cardiomyopathy, Screening, Sports cardiology
Graphical Abstract
Graphical Abstract.
What’s new?
Low QRS voltages (LQRSV) in the limb leads may be the electrocardiographic (ECG) hallmark of cardiomyopathies at risk of sudden cardiac death but they are not mentioned by the current guidelines for interpretation of the athlete’s ECG.
We found that the ECG pattern of isolated LQRSV was uncommon among both the athletic population and young individuals from the general population while it was relatively common among patients with cardiomyopathies characterized by left ventricular scarring.
In two of five index athletes with isolated LQRSV undergoing cardiac magnetic resonance because of associated effort-induced ventricular arrhythmias, there were morpho-functional and structural abnormalities consistent with heart muscle diseases at risk of sudden cardiac death.
The results of this study suggest that LQRSV in the limb leads should be included in the guidelines for interpretation of the athlete’s ECG among the potentially pathological patterns that should trigger additional investigations.
Introduction
The electrocardiogram (ECG) pattern of low QRS voltages in limb leads (LQRSV) is defined as QRS complexes with a peak-to-peak amplitude <0.5 mV in all peripheral leads. This ECG pattern was found in association with either non-cardiac conditions, such as pulmonary emphysema and obesity or cardiac diseases including pericardial effusion, infiltrative cardiac disease (e.g. amyloidosis) and, more recently, arrhythmogenic cardiomyopathy (ACM) and non-ischaemic left ventricular scar (NILVS).1–7 The common denominator of these conditions underlying LQRSV is the increased electrical impedance between the myocardial mass and the body surface ECG leads and/or the loss of electrically active myocardial mass with replacement inert fibrosis.
The prevalence and clinical meaning of LQRSV in the context of physiologic remodelling of the heart induced by regular exercise is still under investigation. The presence of LQRSV in the athlete’s ECG may raise clinical concern, because intense physical training induces a left ventricular (LV) remodelling with augmentation of ventricular mass and, thus, an expected increase (instead of decrease) in QRS voltages.
The current International recommendations for interpretation of the athlete’s ECG aim to differentiate the electrical changes that reflect the physiologic structural and neuro-autonomic cardiac remodelling induced by exercise conditioning, from training-unrelated abnormalities that are possibly associated with an underlying disease at risk of sudden cardiac death (SCD), based on the relative prevalence of the ECG abnormality in healthy athletes vs. patients with cardiovascular conditions.8,9 However, the current criteria do not mention LQRSV, leaving open whether this ECG pattern deserves attention and requires further investigation.9,10
Therefore, this study was designed to evaluate the prevalence, clinical meaning and underlying pathological substrates of the ECG pattern of LQRSV in a large population of Italian young competitive athletes, either elite or non-elite. In order to assess the inherent clinical relevance of such an ECG pattern in the setting of a sports pre-participation cardiovascular evaluation, our study focused on isolated LQRSV, excluding those athletes with concomitant ECG abnormalities that ‘per se’ represent indication for further cardiovascular investigation for possible underlying cardiovascular disease. The prevalence of isolated LQRSV in this index population of competitive, almost exclusively Caucasian, athletes was compared with that of cardiomyopathy patients with heart muscle diseases characterized by LV scarring, such as ACM and NILVS, and non-cardiomyopathy controls including American athletes of Afro-Caribbean ethnicity and young individuals from the general population who underwent ECG before non-cardiac surgery.
Methods
The prevalence of isolated LQRSV, defined as QRS voltages <0.5 mV in each limb lead and non-associated with major abnormalities according to the 2017 International criteria for interpretation of the athlete's ECG,9 among a group of index athletes was compared with that of cardiomyopathy patients and non-cardiomyopathy controls including American athletes of Afro-Caribbean ethnicity and young individuals from the general population.
Inclusion criteria
Index athletes
The index athletic population included a consecutive series of Italian athletes engaged in competitive sports activity. It included non-elite athletes who underwent annual pre-participation screening at the Sport-Medical centers of Padova, Noale and Treviso (Veneto region, Italy); and elite Olympic athletes evaluated at the sports medical centre of the Italian Olympic Committee in Rome before their participation in the 2016 Rio de Janeiro Olympic Games. The inclusion criteria were the following: (i) age range 14–35 years; (ii) regular competitive sport activity with annual pre-participation cardiovascular evaluation, according to the Italian law;11 (iii) no previously known heart disease; and (iv) no major ECG abnormalities on pre-participation screening, defined according to the 2017 International criteria for interpretation of the ECG in the athlete.9
American athletes of Afro-Caribbean ethnicity
This group included athletes of Afro-Caribbean ethnicity of the same age range (14–35 years old) with no previously known heart disease who underwent ECG pre-participation screening. Athletes with no major ECG abnormalities according to the 2017 International criteria for interpretation of the ECG in the athlete9 were matched 1 to 2 to index athletes for both sex and elite status (National Collegiate elite athletes vs. high school non-elite athletes). According to the screening protocol, athletes with LQRSV but no other major ECG abnormalities did not undergo any additional investigation.
Cardiomyopathy patients
The study included a consecutive series of cardiomyopathy patients with arrhythmogenic cardiomyopathy (ACM) and idiopathic non-ischaemic left ventricular scar (NILVS) who were evaluated at the Inherited Arrhythmogenic Cardiomyopathies Unit of the University of Padova and at the Policlinico Casilino of Roma.
The ACM was diagnosed according to current criteria, as published elsewhere.12,13 Idiopathic NILVS was diagnosed in patients with (i) a subepicardial/midmyocardial stria of late gadolinium enhancement at CMR involving one or more LV segments; (ii) no clinical history of previous acute myocarditis; (iii) the absence of other cardiomyopathic features; and (iv) a negative genotype for gene defect associated with cardiomyopathy.6
The inclusion criteria for cardiomyopathy patients included: (i) age range 14–35 years; (ii) definitive diagnosis of cardiomyopathy based on current criteria; (iii) New York Heart Association (NYHA) functional classes 1–2; (iv) no major ECG abnormalities according to the 2017 International criteria for interpretation of the ECG in the athlete9; and (v) no other known causes of LQRSV.
General population
A consecutive series of ECGs, with a ratio of 1 every 2 athletes for both males and females, from young individuals (age 14–35 years) was also evaluated. The inclusion criteria for the general population group included: (i) age range 14–35 years; (ii) available ECG obtained for non-cardiac preoperative evaluation; (iii) no major ECG abnormalities according to the 2017 International criteria for interpretation of the ECG in the athlete9; and (iv) no other known causes of LQRSV.
Clinical evaluation of index athletes
According to the Italian Law and guidelines, the protocol of pre-participation screening of all study athletes included family history, physical examination, resting 12-lead ECG, and ECG exercise tests for ventricular arrhythmias.11 The protocol of pre-participation cardiovascular evaluation of the subset of elite athletes also included echocardiography. This sample of Olympic athletes was different from that reported in a previous study.10 Athletes with normal findings at first line examination were considered eligible for competition, while those with a positive family history, abnormal physical examination, alterations of basal ECG, or exercise-induced ventricular arrhythmias underwent additional testing to confirm or exclude diagnosis of cardiovascular disease.14,15 All athletes with isolated LQRSV underwent echocardiographic examination, while gadolinium-enhanced cardiac magnetic resonance (CMR) was reserved to those with additional clinical abnormalities, such as a positive family history, the presence of relevant symptoms, the induction of ‘uncommon’ ventricular arrhythmias (i.e. ≥1 multifocal premature ventricular beats with a right-bundle-branch-block pattern suggesting LV origin) by exercise testing, or the evidence of echocardiographic abnormalities.9,15,16
A group of 39 athletes, matched for age range 14–35 years (median 24 years), sex (77% males) and type of sport (51% engaged in mixed disciplines) with normal ECG and no ventricular arrhythmias at exercise testing and 24-h ambulatory ECG monitoring who voluntarily underwent CMR for research purposes was used as control for CMR findings.
Interpretation of the athlete’s ECG
ECGs were acquired in the supine position with the limb leads placed at the wrists and ankles at standard speed (25 mm/s) and amplification (10 mm/mV). Filters were set at 0.05 and 150 Hz. ECG findings were evaluated and classified as ‘major’ or ‘borderline’ according to the 2017 International criteria for ECG interpretation in the athlete.9 Low QRS voltages in the limb leads were defined as an amplitude of the entire QRS complex, from peak to nadir, <0.5 mV in each limb lead, with the sum of all standard leads <3.0 mV.10 We defined ‘isolated’ LQRSV the absence of other major ECG abnormalities. Ventricular arrhythmias in the athletes were classified as either ‘common’ or ‘uncommon’ on the basis of the morphology of the ectopic QRS, the arrhythmia complexity, and the response to exercise, as previously reported.16
The study was planned according to the Helsinki protocol recommendations and was approved by the ethical committee of the University of Padova. All subjects, at the time of their initial evaluation, signed the informed consensus for screening/cardiac diagnostic evaluation. Data were stored in the participant institutions and data analysis was performed with full respect of the privacy of the athletes/patients. The data underlying this article may be shared on reasonable request to the corresponding author.
Statistical analysis
Categorical data were presented as counts (%). Continuous data were presented as mean (±standard deviation) or median (1st–3rd quartiles) according to normal/non-normal distribution. Normality of distribution was assessed with the Shapiro–Wilk test. Categorical data were compared using the χ2 test or the Fisher’s exact test, as appropriate. Continuous data were compared using the Student’s T-test or the rank sum test, as appropriate. Confidence intervals for the prevalence of LQRSV were calculated using the binomial distribution. Because elite athletes were older than non-elite, we performed a binary logistic regression analysis for predictors of LQRSV including elite status and age as co-variates; the low number of athletes with LQRSV did not allow entering other co-variates in the model. Difference in relevant clinical parameters between groups has been estimated using the Wilcoxon or binomial approach respectively for quantitative and categorical variables with confidence intervals. A P-value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS ver. 23 (IBM, USA).
Results
Index athletes
Of 2341 consecutive competitive athletes aged 14–35 years undergoing pre-participation cardiovascular screening, 112 did not fulfil the study inclusion criteria because (i) were diagnosed with a heart disease (N = 36), such as valve disease or repaired congenital heart disease or (ii) had ≥1 major ECG abnormalities (N = 76). Major ECG abnormalities were associated with a LQRSV pattern in 1 of these 76 athletes (1.3%).
The final study population consisted of 2229 young competitive athletes, that were apparently healthy and without major ECG abnormalities [median age 18 years (16–25), 67% males, 96% Caucasian]. The majority of the athletes were engaged in endurance and mixed sports disciplines according to the classification of the European Society of Cardiology (Table 1); 775 of 2229 (35%) were elite athletes. Elite athletes were older than non-elite [23 (20–27) vs. 18 (16–23) years, P < 0.001] but did not differ in terms of sex, body mass index (BMI), body surface area (BSA), and type of sport disciplines.
Table 1.
Characteristics of athletes with and without low QRS voltages in limb leads
| LQRSV (N = 24) | No LQRSV (N = 2205) | P | Abs. diff 95% CI | |
|---|---|---|---|---|
| Male sex | 10 (42%) | 1479 (67%) | 0.36 | −9% (−28%, 10%) |
| Age | 28 (25–31) | 18 (16–25) | <0.001 | −8 (−10, −5) |
| Elite athletes | 17 (71%) | 758 (34%) | <0.001 | 35% (17%, 53%) |
| BSA (m2) | 2.0 ± 0.4 | 1.8 ± 0.3 | <0.001 | −0.1 (−0.2, −0.01) |
| BMI (m2/kg) | 24.6 ± 4.8 | 22.4 ± 4.3 | <0.001 | −0.1 (−0.3, −0.02) |
| Sport discipline | ||||
| Endurance | 8 (33%) | 596 (27%) | 0.49 | |
| Power | 4 (17%) | 280 (13%) | 0.56 | |
| Mixed | 9 (38%) | 1188 (54%) | 0.11 | |
| Skill | 3 (13%) | 141 (6%) | 0.23 | |
| Non-pathologicala ECG changes | ||||
| First-degree AV block, n (%) | 0 0 | 40 (2) | 1.0 | |
| LVH, n (%) | 1 (5) | 286 (13) | 0.35 | |
| RVH, n (%) | 0 0 | 47 (2) | 1.0 | |
| LAE, n (%) | 1 (5) | 15 (1) | 0.16 | |
| RAE, n (%) | 0 0 | 17 (1) | 1.0 | |
| LAD, n (%) | 0 0 | 15 (1) | 1.0 | |
| Early repolarization pattern, n (%) | 6 (27) | 750 (34) | 0.34 |
AV, atrioventricular; BMI, body mass index, BSA, body surface area; LAD, left axis deviation; LAE, left atrial enlargement; LQRSV, low QRS voltages; LVH, left ventricular hypertrophy; RAE, right atrial enlargement; RVH, right ventricular hypertrophy.
According to the 2017 International recommendations for ECG interpretation in the athlete.8
Prevalence
The ECG pattern of isolated LQRSV was found in 24 out of 2229 athletes, accounting for a prevalence of 1.1% (95% CI 0.7–1.6%).
The prevalence of isolated LQRSV was significantly higher among elite (2.2%, 95% CI 1.3–3.5%) compared with non-elite (0.5%, 95% CI 0.2–1.0%, P < 0.001) athletes. Athletes with isolated LQRSV were significantly older than athletes without LQRSV (median age 28 vs 18 years; P < 0.001) and had a higher BSA and BMI, but did not differ significantly with regard to sex, clinical characteristics and, specifically among the elites, cardiac dimensions including LV mass (Tables 1 and 2). At a multivariate model including age and elite status, both variables remained independent predictors of isolated LQRSV with odds ratio of 1.1 (1.1–1.2, P < 0.000) and 2.9 (1.2–7.2, P = 0.02), respectively.
Table 2.
Echocardiographic findings in elite athletes with and without low QRS voltages
| LQRSV (N = 17) | No LQRSV (N = 758) | P | Abs. diff. (95% CI) | |
|---|---|---|---|---|
| LA antero-posterior diameter | 34 ± 5 | 34 ± 4 | 0.58 | 0 (−3, 2) |
| IV septum thickness (mm) | 9 ± 1 | 9 ± 1 | 0.25 | 0 (−1, 1) |
| Posterior wall thickness (mm) | 9 ± 1 | 9 ± 1 | 0.40 | 0 (−1, 1) |
| LV End-diastolic diameter/m2 | 28 ± 2 | 28 ± 2 | 0.74 | 0 (−1, 1) |
| LV End-systolic diameter/m2 | 17 ± 2 | 17 ± 2 | 0.77 | 0 (−1, 1) |
| LV mass/m2 | 88 ± 17 | 92 ± 20 | 0.53 | 4 (−5, 14) |
| RVOT proximal diameter | 27 ± 3 | 27 ± 4 | 0.93 | 0 (−2, 2) |
| RVOT distal diameter | 27 ± 5 | 29 ± 4 | 0.09 | 1 (−1, 4) |
| LV ejection fraction | 65 ± 6 | 66 ± 6 | 0.07 | 1 (−2, 4) |
| E/A ratio | 2 ± 0.4 | 2 ± 0.5 | 0.53 | 0.1 (−0.1, 0.4) |
| E/Ei ratio | 7 ± 1 | 7 ± 1 | 0.48 | −0.1 (−0.7, 0.6) |
LA, left atrium; LV, left ventricle; LQRSV, low QRS voltages; IV, interventricular; RVOT, right ventricular outflow tract.
Clinical meaning
Among the 24 athletes with isolated LQRSV on the basal ECG, none showed a positive family history of SCD/cardiomyopathy or arrhythmic symptoms. The exercise testing showed ventricular arrhythmias in 8 (33%), 6 elite and 2 non-elite athletes. Three athletes showed isolated premature ventricular beats (PVBs) with a ‘common’ pattern (i.e. a left bundle branch block/inferior axis morphology), while five had PVBs with an ‘uncommon’ pattern (i.e. a right bundle branch block morphology in 4 athletes, and multiple morphologies in one). One of 24 (4%) athletes with isolated LQRSV had an abnormal echocardiogram (uncomplicated bicuspid aortic valve, i.e. without significant stenosis, regurgitation, or dilatation of the ascending aorta).
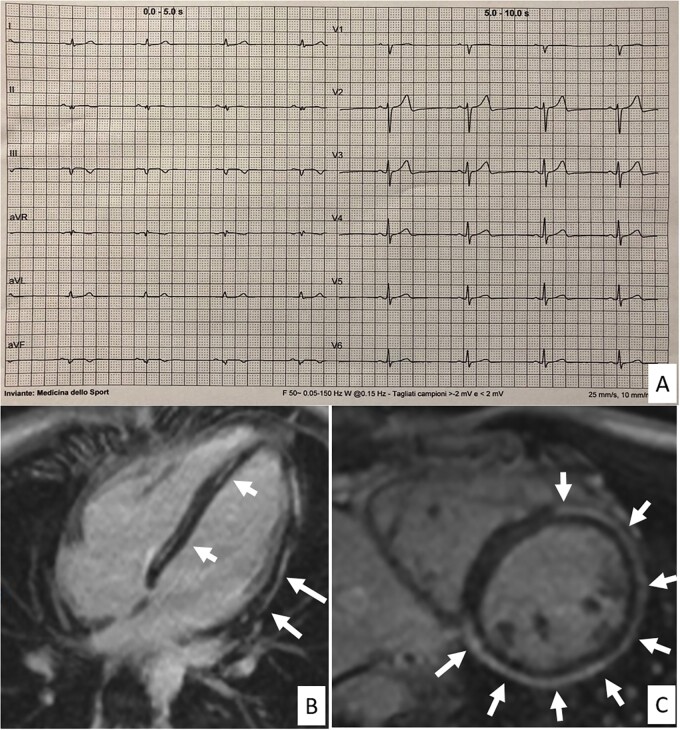
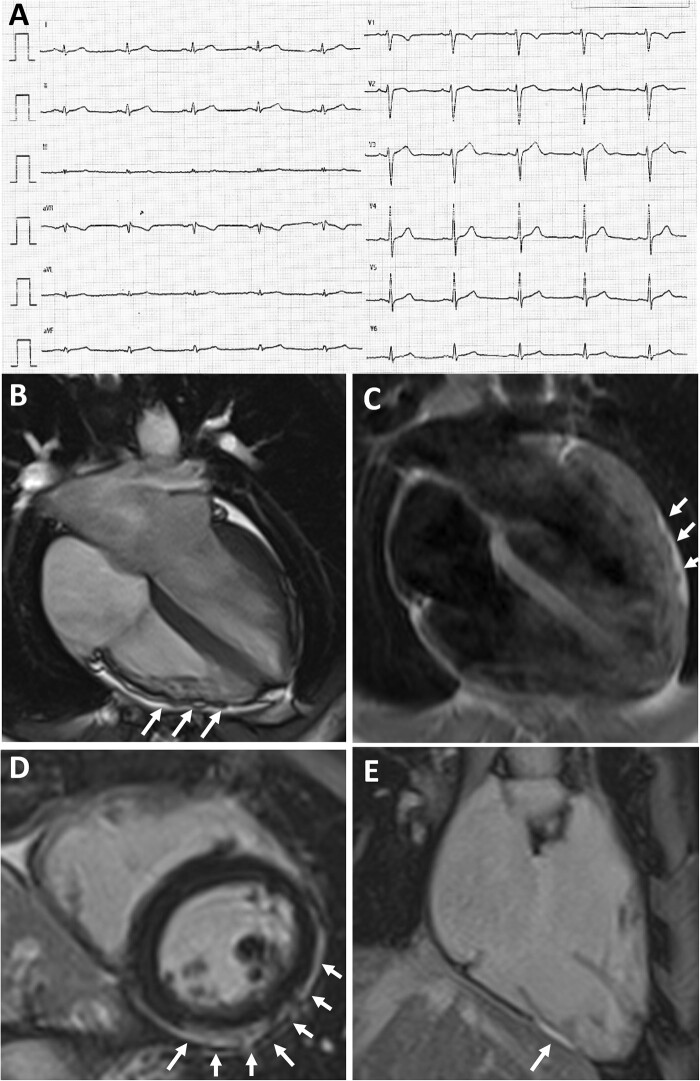
Contrast-enhanced CMR was performed in 5 of 24 athletes with isolated LQRSV because of ‘uncommon’ ventricular arrhythmias. The CMR was abnormal in two of these five athletes, with findings consistent with NILVS (Figure 1) and borderline biventricular ACM (Figure 2). Both athletes were considered non-eligible to competitive sports activity according to the guidelines for management of athletes with heart muscle disease at risk of SCD.15 No young control athlete who underwent CMR for research purposes showed any abnormal findings except for focal junctional LGE that is normal in athletes.
Figure 1.
Representative case of an athlete with low QRS-voltages in the limb leads and underlying isolated (idiopathic) non-ischaemic left ventricular scar. A 25-year-old elite athlete engaged in a mixed discipline with no family history of heart disease and no symptoms showed low QRS voltages in the limb leads in addition to left axis deviation and fragmented QRS in avF and II (A). Isolated premature ventricular beats with a right bundle branch block/superior axis morphology (suggesting origin from the inferior left ventricular wall) were elicited by exercise testing. Echocardiography was normal. Cardiac magnetic resonance, T1-weighted post-contrast sequences, short axis (B) and 2-chamber long axis (C) views showed the presence of subepicardial late gadolinium enhancement with nearly circumferential involvement of the LV wall. The right ventricle showed no wall motion or tissue characterization abnormalities. Genetic testing was negative.
Figure 2.
Representative case of an elite athlete with low QRS-voltages underlying biventricular arrhythmogenic cardiomyopathy. A 28-year-old elite athlete engaged in a skill discipline with no family history of heart disease and no symptoms showed low QRS-voltages in the limb leads (A). Exercise testing showed premature ventricular beats with a right bundle branch block/superior axis configuration, isolated and in couples. Echocardiography was normal. CMR, cine sequences, showed hypokinesis of the mid-apical lateral LV wall and multiple small bulgings (white arrows) of the right ventricular free wall (B). On T1-weighted sequences, epicardial fatty infiltration involving the lateral and inferior LV wall (white arrows, C) and the RV free wall was observed. On post-contrast sequences, subepicardial stria of late gadolinium enhancement involving the antero-lateral, infero-lateral and inferior LV wall (white arrows on D) and the RV was detected (on inferior RV wall, white arrows on E). Genetic testing was negative. Based on RV dilation plus wall motion abnormalities on CMR and non-sustained ventricular tachycardia on 24-hour Holter monitoring a diagnosis of arrhythmogenic cardiomyopathy was made, that was ‘borderline’ according to the 2010 Task Force Criteria and ‘definite’ according to the 2020 International criteria (Padua criteria) that also incorporate tissue characterization findings by CMR.12,13 CMR, cardiac magnetic resonance; LV, left ventricular; RV, right ventricular.
The remaining 22 athletes with isolated LQRSV were considered eligible to competitive sports, with the requirement of periodic re-evaluation on an annual basis. During the subsequent follow-up of 4 ± 2 years, all the 22 athletes with isolated LQRSV who continued to practice competitive sports had an asymptomatic and uneventful outcome with no new diagnoses.
Cardiomyopathy patients
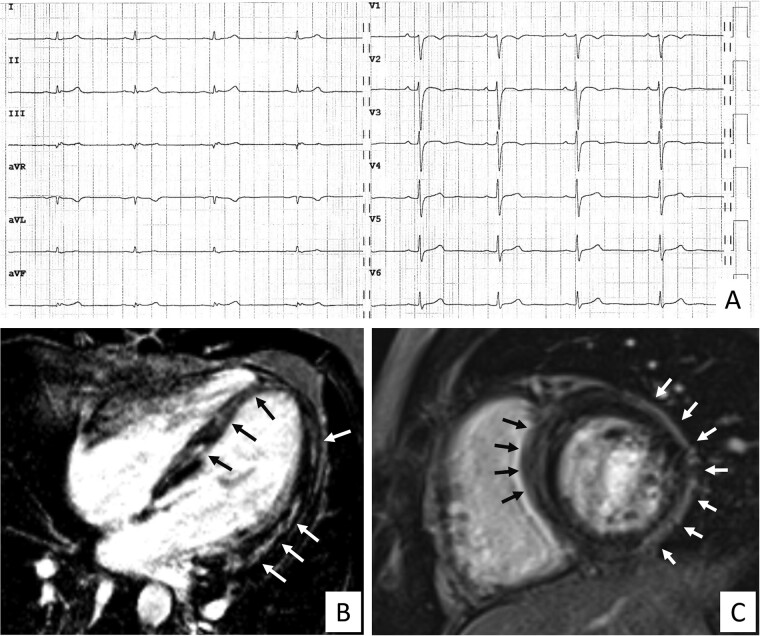
The cardiomyopathy cohort included 58 young patients [69% males, median age 27 (20–34) with either ACM (N = 30) or NILVS (N = 28) and no other major ECG abnormalities]. Isolated LQRSV was found in 7 of 58 patients (12% of all cardiomyopathies, 95% CI 6–23%), and specifically, in 5 of 30 (17%) ACM and 2 of 28 (7%) NILVS patients (Figure 3). The prevalence of isolated LQRSV in cardiomyopathy patients was significantly higher than that of index athletes (1.1%, 95%CI 0.7–1.6%; P < 0.001).
Figure 3.
Representative case of a patient with arrhythmogenic cardiomyopathy with isolated low QRS voltages. The 28-year-old patient has arrhythmogenic cardiomyopathy with predominant left ventricular involvement secondary to a desmoplakin-gene mutation. The electrocardiogram shows low QRS-voltages in the limb leads and increased S-wave duration in V1–V2, but no major abnormalities according to the International recommendations for interpretation of the athlete’s electrocardiogram (A). Cardiac magnetic resonance, T1-weighted post-contrast sequences, 4-chamber long axis (B) and short axis (C) views showed the presence of diffuse subepicardial/midmyocardial late gadolinium enhancement involving both the interventricular septum and the left ventricular wall. No right ventricular late enhancement was evidenced.
Control groups
The prevalence of isolated LQRSV in the control group of the American Afro-Caribbean athletes was 2 of 1115 (0.2%, 95% CI 0.02–0.7%) and in the control group of young people from the general population undergoing ECG before non-cardiac surgery 3 of 1115 (0.3%, 95% CI 0.1–0.8%).
Discussion
This study was designed to assess the prevalence and clinical meaning of the ECG pattern of isolated LQRSV in a large population of young competitive athletes compared with a cohort of cardiomyopathy patients.
The main findings were the following: (i) the ECG pattern of isolated LQRSV was uncommon among the athletic population, with an estimated overall prevalence ranging from 1.1% among the index group of Italian, almost exclusively Caucasian, young competitive athletes to 0.2% among American Afro-Caribbean athletes; (ii) the prevalence of isolated LQRSV was also low in the general population of young individuals undergoing ECG before non-cardiac surgery (0.3%); (iii) the prevalence of isolated LQRSV in patients with cardiomyopathy characterized by LV scarring was significantly higher (12%) than in athletes; (iv) increasing age, elite status, higher BMI/BSA were associated with LQRSV in index athletes; and (v) in 2 of 5 index athletes with isolated LQRSV undergoing CMR because of effort-induced ventricular arrhythmias, there were morpho-functional and structural abnormalities consistent with heart muscle diseases at risk of SCD, specifically biventricular ACM and idiopathic NILVS (Figure 4).
Figure 4.
Summary of the main study findings (see text for details).
Clinical meaning of low QRS voltages
In the clinical setting, the pattern of LQRSV may be observed in patients with diseases that increase the electrical resistance both within the heart (e.g. infiltrative disease) and outside the heart (e.g. pericardial effusion, pulmonary emphysema or obesity), or in patients with conditions that lead to a depletion of electrically active ventricular myocardium, namely, diseases characterized by loss of myocytes with replacement fibrosis, such as ACM, cardiac sarcoidosis, healed myocarditis, or idiopathic NILVS.1–7 In these conditions, the mechanism involved in the reduction of QRS voltages may consist of a decrease in normal LV myocardial mass, which mostly accounts for the generation of the electrical activity, and its substitution with electrically inert fibrous tissue.
The clinical relevance of LQRSV has been established by a previous US population study that assessed the association between LQRSV across the whole 12 ECG leads and the all-cause mortality among 6440 individuals (53% women, mean age 60 years) without a history of cardiovascular disease or major ECG abnormalities. Over a long-term follow-up (median 13.8 years), mortality rate was 61% higher among patients with LQRSV after adjustment for confounding factors.17
Recently, a renewed interest in the ECG pattern of LQRSV was prompted by the availability of CMR that offers the potential to detect ventricular myocardial scar on post-contrast sequences. Studies of correlation between ECG and contrast-enhanced CMR have demonstrated that LQRSV reflects the involvement of the LV by fibrofatty myocardial scar in ACM that is a recognized leading causes of SCD in the athletes.2,4,13,18 Another condition associated with LQRSV is the NILVS, either inflammatory or non-inflammatory, which is characterized by an epicardial/midmyocardial distribution and preferential involvement of the inferolateral regions.4,6,19 Accordingly, the ECG pattern of isolated LQRSV on the pre-participation athlete’s ECG may be of clinical relevance because it may lead to early identification of asymptomatic athletes affected by heart muscle disease at risk of SCD.
Low QRS voltages in athletes
The presence of LQRSV is an unexpected finding in an athlete because the physiologic ventricular remodelling induced by exercise conditioning, with enlargement of LV cavity and increased LV mass, is typically associated with an increase in QRS voltages.8
The results of our study confirm that the ECG pattern of isolated LQRSV in an athletic population of different ethnicities is uncommon, ranging from 0.2% to 1.1%. In index Caucasian athletes, isolated LQRSV were associated with elite status, age and BMI/BSA but unrelated to sex, type of sport and extent of LV remodelling as assessed by echocardiography. Age and elite status were independent predictors at multivariate analysis. Certain hypothesis might be advanced to explain this finding, such as the higher muscular mass in elite athletes, which may increase the electrical impedance between the heart and the recording electrodes. However, type of sport and the extent of cardiac remodelling at echocardiography did not differ between the elite athletes with and without LQRSV, suggesting that this ECG pattern is not closely dependent on training load. We can further speculate that the age-dependent increase of LQRSV prevalence might be explained by the greater probability to detect myocardial fibrosis with the increasing athlete’s age, due to the delayed phenotypic expression of inherited arrhythmogenic cardiomyopathies or later occurrence of myocardial inflammation, as suggested by previous studies on pre-participation screening of adolescents and young adults.11,20,21 In this regard, future studies using CMR T1-mapping sequences, that provide higher sensitivity for interstitial myocardial fibrosis, may offer the potential to diagnose earlier cardiomyopathic changes in association with LQRSV.
It is noteworthy that one third of our athletes with isolated LQRSV had PVBs during exercise testing. Further examination by CMR of five athletes with ventricular arrhythmias showing an ‘uncommon’ morphology of the ectopic QRS16 and/or echocardiographic abnormalities led to identification of a ‘scarring’ heart muscle disease in two cases, accounting for a 8% overall prevalence of pathological substrates associated with the ECG pattern of isolated LQRSV among our athletes.
A previous study in a different cohort of young (aged 25 ± 5 years) Olympic athletes reported a 4% prevalence of LQRSV. At variance with the present study that considered only limb leads voltages, in this previous investigation LQRSV were defined as amplitude of the QRS complex <0.5 mV in limb leads and/or <1.0 mV in precordial leads.10 In keeping with our study results, the previous study reported that athletes with LQRSV were older but did not differ with regard to type of sport and echocardiographic findings. Moreover, among the 23 athletes with LQRSV, CMR was reserved to the 9 with exercise-induced premature ventricular beats (5 with a RBBB morphology). Although CMR did not evidence any significant structural cardiac abnormality, the subset of athletes with LQRSV showed a significantly higher prevalence of ventricular arrhythmias at exercise testing. This finding raises the hypothesis that the appearance of LQRSV on ECG may precede over time the imaging evidence of an arrhythmogenic pathological condition in selected individuals and conveys the recommendation for serial clinical evaluations in apparently healthy athletes presenting with isolated LQRSV on ECG.
Low QRS voltages in cardiomyopathy
Several studies reported that LQRSV in limb leads may reflect the presence of LV scar, which can be evidenced by CMR and is a substrate for re-entrant ventricular tachycardia.2,4,6,13,18,19 In ACM, the LV fibrofatty myocardial replacement typically affects the posterolateral LV regions and, like the right ventricular lesion, progresses from the epicardium to the endocardium, with scar tissue typically confined to subepicardial/midmyocardial layers.22 In our ACM patients the prevalence of isolated LQRSV was 12%, in keeping with the figures reported by previous studies.23 As previously suggested, the presence of LQRSV in ACM patients is highly predictive of LV fibrofatty myocardial involvement2 and our patients with ACM and LQRSV showed a significantly higher prevalence of LV scarring than those with preserved voltages of the QRS complexes.
An isolated NILVS with an epicardial/midmyocardial distribution and preferential involvement of the inferolateral LV regions is usually interpreted as the consequence of previous myocarditis. However, it is a non-specific myocardial lesion that may represent the phenotypic expression of a variety of either inherited or acquired heart muscle diseases leading to non-ischaemic myocardial scarring, including left-dominant ACM, laminopathy, cardiac sarcoidosis, healed myocarditis, and mild dilated cardiomyopathy.6,7,19,22 Most often NILVS is ‘idiopathic’ because it remains of indeterminate aetiology after a deep genetic, clinical and imaging evaluation. Regardless of its aetiology, NLVS has been increasingly reported as a substrate of life-threatening ventricular arrhythmias and SCD in young people including athletes.6,19 In our patients with idiopathic NLVS, the prevalence of isolated LQRSV was 7%. The variable prevalence of LQRSV in our patients with ACM and idiopathic NLVS may be explained by a ‘dose–effect’ relationship between fibrous replacement and reduction in QRS amplitudes in the limb leads.
Implications for the pre-participation cardiovascular evaluation
Recent consensus documents have provided modern criteria for interpretation of the athlete’s ECG, which are based on a better definition of physiological vs. abnormal ECG changes.8,9
The criteria used to classify the ECG abnormalities in the athlete’s ECG relied on their prevalence and relation to training. While common ECG changes are considered secondary to cardiac adaptation to physical exercise and does not require additional investigations, uncommon and non-sports-related ECG abnormalities may potentially reflect an underlying heart disease at increased risk of SCD and requires an appropriate diagnostic work-up. A correct interpretation of the athlete’s ECG is intended to reduce the number of false-positive results but maintain sensitivity, to avoid missing potentially dangerous cardiovascular conditions.
Although the ECG pattern of isolated LQRSV may predict a structural heart disease at risk of SCD, it is not included in the current recommendations for interpretation of the athlete’s ECG.9 The results of our study support updating these ECG criteria to include the isolated LQRSV pattern among the uncommon ECG patterns. Our study showed that isolated LQRSV is much less common in athletes (0.2–1%) compared with patients with cardiomyopathy associated with myocardial scarring (12%). Moreover, 8% of index athletes with isolated LQRSV had evidence of pathologic myocardial fibrosis of the LV in the context of a biventricular ACM or NILVS, which are recognized conditions at risk of SCD during sports. These pathological conditions would have remained undetected if further cardiac evaluation for LQRSV was not conducted.
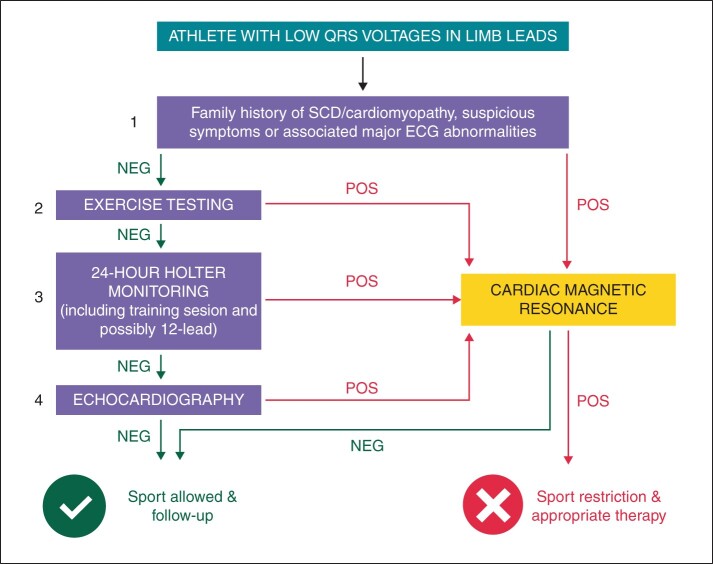
We suggest that the presence of isolated LQRSV should trigger additional clinical work-up of the athlete to exclude an underlying myocardial disease (Figure 5). We propose this evaluation begins with an echocardiogram and an ECG exercise test for ventricular arrhythmias.24 It is worthy to consider that non-ischaemic myocardial scars can be missed by echocardiographic examination because they are often segmental and spare the subendocardial layers of the LV wall6,25: for this reason, normal echocardiographic findings cannot definitely rule out the presence of potentially malignant arrhythmogenic substrate in athletes with LQRSV and, for conclusive diagnosis, investigation by CMR study may be required.
Figure 5.
Proposed diagnostic flow-chart for athletes with isolated LQRSV. The exercise testing is considered positive when one or more premature ventricular beats induced by exercise with ‘uncommon’ morphologies (i.e. multifocal or with a right-bundle-branch-block pattern suggesting left ventricular origin) are observed. LQRSV, low QRS voltages; SCD, sudden cardiac death.
According to the results of previous and this study, CMR is justified in athletes presenting LQRSV in association with positive family history for cardiomyopathy or SCD, additional major ECG abnormalities, such as T-wave inversion in lateral leads, abnormal echocardiographic findings such as a reduced LV ejection fraction or segmental hypokinesis or effort induced ventricular arrhythmias with a right-bundle-branch block pattern (suggesting a LV origin) (Figure 4). Because athletes with isolated LQRSV and no other clinical or ECG abnormalities did not undergo CMR, the results of this study do not provide support to an extended indication of CMR to this athletes subset.
It is noteworthy that the medium-term follow-up of our athletes with isolated LQRSV not undergoing CMR was uneventful despite continuation of sports activity. However, these athletes should remain under close clinical surveillance because a concealed myocardial substrate may become clinically overt thereafter.
Study limitations
Although all athletes with isolated LQRSV underwent echocardiography and exercise testing, CMR study was reserved to the subset of athletes with effort-induced ventricular arrhythmias or echocardiographic abnormalities. As a consequence, the true prevalence of underlying structural heart muscle disease in athletes with such an ECG pattern could be underestimated, which consideration is not in contrast with our study results and likely reinforce our conclusions. Further studies designed to investigate by systematic CMR in all athletes with isolated LQRSV are therefore needed to assess whether and to what extent this ECG pattern may predict a heart muscle disease, regardless of other abnormal cardiac testing and clinical findings. Because the cardiomyopathy patients in this study were non-athletes, the impact of training on the prevalence of isolated LQRSV in ACM and NLVS cannot be evaluated.
Conclusions
The results of this study indicate that isolated LQRSV is an uncommon ECG pattern in young competitive athletes, whereas it is observed in a sizeable proportion of patients with cardiomyopathy. This pattern may represent the only ECG sign of a heart muscle disease at risk of SCD in athletes. These findings support the inclusion of LQRSV isolation among the uncommon ECG patterns of the athlete’s ECG that should prompt comprehensive clinical work-up. Based on the data provided by our study, CMR for the exclusion of underlying cardiomyopathy should be considered when LQRSV are associated with a positive family history, symptoms, additional major ECG abnormalities, abnormal echocardiographic findings, or effort-induced ventricular arrhythmias of LV origin. Although by inference from our study findings a CMR study may be offered to all athletes with isolated LQRSV, the cost-effectiveness of such an expanded indication remains to be determined by further prospective studies.
Contributor Information
Alessandro Zorzi, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani, 2, 35121 Padova, Italy.
Natascia Bettella, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani, 2, 35121 Padova, Italy.
Mario Tatangelo, Institute of Sport Medicine and Science, Rome, Italy.
Alvise Del Monte, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani, 2, 35121 Padova, Italy.
Teresina Vessella, Sports Medicine Unit, ULSS2 Treviso, Treviso, Italy.
Barbara Poscolieri, Sports Medicine Unit, ULSS2 Treviso, Treviso, Italy.
Cinzia Crescenzi, Policlinico Casilino, Rome, Italy.
Davide Pegorin, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani, 2, 35121 Padova, Italy.
Flavio D’Ascenzi, Department of Medical Biotechnologies, Division of Cardiology, University of Siena, Italy.
Valentina Pescatore, Sports Medicine Unit, ULSS3 Venezia, Venezia, Italy.
Franco Giada, Sports Medicine Unit, ULSS3 Venezia, Venezia, Italy.
Patrizio Sarto, Sports Medicine Unit, ULSS2 Treviso, Treviso, Italy.
Leonardo Calò, Policlinico Casilino, Rome, Italy.
Maurizio Schiavon, Sports Medicine Unit, ULSS6 Padova, Padova, Italy.
Dario Gregori, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani, 2, 35121 Padova, Italy.
David M Hadley, Research & Development Department, Cardiac Insight Inc ., Bellevue, WA, USA.
Jonathan A Drezner, Center for Sports Cardiology, University of Washington, Seattle, WA, USA.
Antonio Pelliccia, Institute of Sport Medicine and Science, Rome, Italy.
Domenico Corrado, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani, 2, 35121 Padova, Italy.
Data Availability
Data supporting the article are available from the corresponding author upon reasonable request.
References
- 1. Mussinelli R, Salinaro F, Alogna A, Boldrini M, Raimondi A, Musca Fet al. . Diagnostic and prognostic value of low QRS voltages in cardiac AL amyloidosis. Ann Noninvasive Electrocardiol 2013;18:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Lazzari M, Zorzi A, Cipriani A, Susana A, Mastella G, Rizzo Aet al. . Relationship between electrocardiographic findings and cardiac magnetic resonance phenotypes in arrhythmogenic cardiomyopathy. J Am Heart Assoc 2018;7:e009855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madias JE. Transient attenuation of the amplitude of the QRS complexes in the diagnosis of Takotsubo syndrome. Eur Heart J Acute Cardiovasc Care 2014;3:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valentini F, Anselmi F, Metra M, Cavigli L, Giacomin E, Focardi Met al. . Diagnostic and prognostic value of low QRS voltages in cardiomyopathies: old but gold. Eur J Prev Cardiol 2020; doi: 10.1093/eurjpc/zwaa027. [DOI] [PubMed] [Google Scholar]
- 5. Chandra A, Marhefka GD, DeCaro MV.. Clinical significance of 12 lead ECG changes in patients undergoing pericardiocentesis for cardiac tamponade. Acta Cardiol 2021;76:76–9. [DOI] [PubMed] [Google Scholar]
- 6. Zorzi A, Marra MP, Rigato I, De Lazzari M, Susana A, Niero Aet al. . Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythmia Electrophysiol 2016;9:e004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corrado D, Link MS, Calkins H.. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 8. Zorzi A, Vio R, Bettella N, Corrado D.. Criteria for interpretation of the athlete’s ECG: a critical appraisal. Pacing Clin Electrophysiol 2020;43:882–90. [DOI] [PubMed] [Google Scholar]
- 9. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JMet al. . International recommendations for electrocardiographic interpretation in athletes. Eur Heart J 2018;39:1466–80. [DOI] [PubMed] [Google Scholar]
- 10. Mango F, Caselli S, Luchetti A, Pelliccia A.. Low QRS voltages in Olympic athletes: prevalence and clinical correlates. Eur J Prev Cardiolog 2020;27:1542–8. [DOI] [PubMed] [Google Scholar]
- 11. Vessella T, Zorzi A, Merlo L, Pegoraro C, Giorgiano F, Trevisanato Met al. . The Italian preparticipation evaluation programme: diagnostic yield, rate of disqualification and cost analysis. Br J Sports Med 2020;54:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DAet al. . Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, De Lazzari Met al. . Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–14. [DOI] [PubMed] [Google Scholar]
- 14. Pelliccia A, De Martino L, Borrazzo C, Serdoz A, Lemme E, Zorzi Aet al. . Clinical correlates and outcome of the patterns of premature ventricular beats in Olympic athletes: a long-term follow-up study. Eur J Prev Cardiol 2021;28:1038–47. [DOI] [PubMed] [Google Scholar]
- 15. Biffi A, Delise P, Zeppilli P, Giada F, Pelliccia A, Penco Met al. ; Italian Society of Sports Cardiology and Italian Sports Medicine Federation . Italian cardiological guidelines for sports eligibility in athletes with heart disease: part 1. J Cardiovasc Med (Hagerstown) 2013;14:477–99. [DOI] [PubMed] [Google Scholar]
- 16. Corrado D, Drezner JA, D'Ascenzi F, Zorzi A.. A. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br J Sports Med 2020;54:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Usoro AO, Bradford N, Shah AJ, Soliman EZ.. Risk of mortality in individuals with low QRS voltage and free of cardiovascular disease. Am J Cardiol 2014;113:1514–7. [DOI] [PubMed] [Google Scholar]
- 18. Cipriani A, Bauce B, De Lazzari M, Rigato I, Bariani R, Meneghin Set al. , Arrhythmogenic right ventricular cardiomyopathy: characterization of left ventricular phenotype and differential diagnosis with dilated cardiomyopathy. J Am Heart Assoc 2020;9:e014628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. di Gioia CRT, Giordano C, Cerbelli B, Pisano A, Perli E, De Dominicis Eet al. . Nonischemic left ventricular scar and cardiac sudden death in the young. Hum Pathol 2016;58:78–89. [DOI] [PubMed] [Google Scholar]
- 20. Malhotra A, Dhutia H, Finocchiaro G, Gati S, Beasley I, Clift Pet al. . Outcomes of cardiac screening in adolescent soccer players. N Engl J Med 2018;379:524–34. [DOI] [PubMed] [Google Scholar]
- 21. Pelliccia A, Di Paolo FM, Quattrini FM, Basso C, Culasso F, Popoli Get al. . Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med 2008;358:152–61. [DOI] [PubMed] [Google Scholar]
- 22. Cipriani A, Perazzolo Marra M, Bariani R, Mattesi G, Vio R, Bettella Net al. . Differential diagnosis of arrhythmogenic cardiomyopathy: phenocopies versus disease variants. Minerva Med 2021;112:269–80. [DOI] [PubMed] [Google Scholar]
- 23. Brosnan MJ, Te Riele ASJM, Bosman LP, Hoorntje ET, van den Berg MP, Hauer RNWet al. . Electrocardiographic features differentiating arrhythmogenic right ventricular cardiomyopathy from an athlete’s heart. JACC Clin Electrophysiol 2018;4:1613–25. [DOI] [PubMed] [Google Scholar]
- 24. Zorzi A, Vessella T, De Lazzari M, Cipriani A, Menegon V, Sarto Get al. . Screening young athletes for diseases at risk of sudden cardiac death: role of stress testing for ventricular arrhythmias. Eur J Prev Cardiol 2020;27:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crescenzi C, Zorzi A, Vessella T, Martino A, Panattoni G, Cipriani Aet al. . Predictors of left ventricular scar using cardiac magnetic resonance in athletes with apparently idiopathic ventricular arrhythmias. J Am Heart Assoc 2021;10:e018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the article are available from the corresponding author upon reasonable request.