Abstract
Background and Objectives
The timing of neurodegeneration in multiple sclerosis (MS) remains unclear. It is critical to understand the dynamics of neuroaxonal loss if we hope to prevent or forestall permanent disability in MS. We therefore used a deeply phenotyped longitudinal cohort to assess and compare rates of neurodegeneration in retina and brain throughout the MS disease course.
Methods
We analyzed 597 patients with MS who underwent longitudinal optical coherence tomography imaging annually for 4.5 ± 2.4 years and 432 patients who underwent longitudinal MRI scans for 10 ± 3.4 years, quantifying macular ganglion cell-inner plexiform layer (GCIPL) volume and cortical gray matter (CGM) volume. The association between the slope of decline in the anatomical structure and the age of entry in the cohort (categorized by the MRI cohort's age quartiles) was assessed by hierarchical linear models.
Results
The rate of CGM volume loss declined with increasing age of study entry (1.3% per year atrophy for the age of entry in the cohort younger than 35 years; 1.1% for older than 35 years and younger than 41; 0.97% for older than 41 years and younger than 49; 0.9% for older than 49 years) while the rate of GCIPL thinning was highest in patients in the youngest quartile, fell by more than 50% in the following age quartile, and then stabilized (0.7% per year thinning for the age of entry in the cohort younger than 35 years; 0.29% for age older than 35 and younger than 41 years; 0.34% for older than 41 and younger than 49 years; 0.33% for age older than 49 years).
Discussion
An age-dependent reduction in retinal and cortical volume loss rates during relapsing-remitting MS suggests deceleration in neurodegeneration in the earlier period of disease and further indicates that the period of greatest adaptive immune–mediated inflammatory activity is also the period with the greatest neuroaxonal loss.
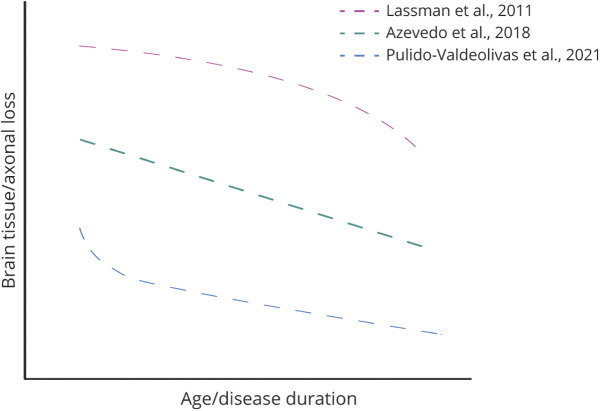
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the CNS associated with neuroaxonal degeneration.1,2 There is unambiguous, substantial loss of neurons and axons by the end of life in MS. This is due to several reasons: Axonal loss can happen immediately as a direct result of adaptive immune–mediated, myelin-targeted inflammatory events but also be a longer-term consequence of factors triggered by these earlier inflammatory events (such as remyelination failure, aberrant glial cell activation, and mitochondrial dysfunction).3 The work that restimulated interest in the neuroaxonal loss in MS principally evaluated acutely demyelinating plaques.4 However, based on a conceptual association of progressive disease with axon loss and as a consequence of the clinical phenotyping of disease, it has been frequently inferred that active neuroaxonal loss occurs most prominently in the progressive phase of the disease.5,6 Recent data have challenged this assumption and point toward early neurodegenerative injury (Figure 1).7-9 Despite this, it remains unclear which component of the neuron is lost first and whether the predominance of loss of each element (dendrite, soma, and axon) occurs during the early adaptive immune–driven phase of disease or later in the disease process. Later in the life of people with MS, processes involved in aging and innate immunity result in further neurodegeneration and clinical progression. It remains unclear how these different age-associated mechanisms affect the rate of neurodegeneration in different CNS structures. Understanding these different dynamics can affect therapeutic decision-making and help with the design of clinical trials that will use measures of neurodegeneration as their outcomes.10
Figure 1. Suggested Models of Brain Tissue Loss in Multiple Sclerosis.
Data from limited autopsy pathology suggest some correlation between axonal loss and the concurrent magnitude of inflammation (quantification of T and B cells, plasma cells, HLA-DR+ cells) in both relapsing and progressive MS.11 However, investigators who have studied this pathologic tissue recognize that—unlike for studying axons where spheroids and axon bulbs can show evidence of degeneration of nerve fibers—pathologic assessment of neuronal cell body loss is difficult in MS, and evaluation of longitudinal change is impossible based on tissue. There has been neuronal cell body loss documented in a limited number of cases within cortical lesions.11-14 It is also recognized that the severity of acute inflammation declines with age in MS to the point where for some patients, it is similar to what is seen in controls.11
Brain MRI imaging studies looking at groups of patients have reported, by contrast, that—at least in some areas of the CNS—neurodegeneration is linear and uniform throughout the course of disease (Figure 1).15 This observation may be unique to particular parts of the CNS and does not necessarily apply to all CNS structures. Optical coherence tomography (OCT) imaging is a tool used to longitudinally quantify retinal neurodegeneration in MS,16-18 and its measures have been shown to correlate with disability,19,20 brain neurodegeneration,21 and cortical lesions.22
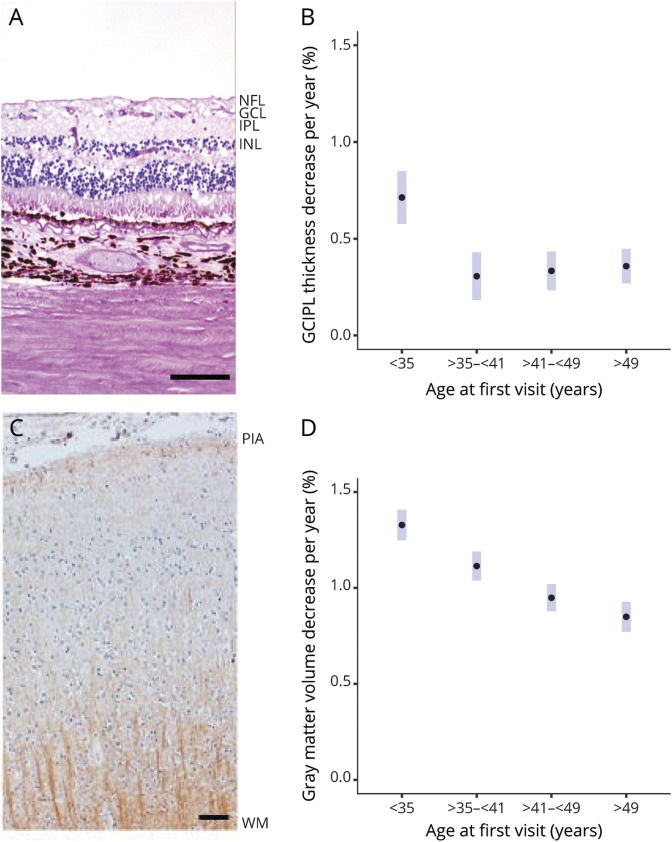
The brain cortex and retina have different cytoarchitectures, with a greater proportion of tissue volume in the retina coming from neurons/axons and greater heterogeneity of neuronal subtypes as well as the presence of oligodendrocytes in the cortex (Figure 2). It is conceivable that the rate of neurodegeneration in these structures could be different.
Figure 2. Rate of Neuronal Volume Loss Declines With Age in MS but Appears to Decline More Rapidly Earlier and Stabilizes in the Retina.
(A, C) Neuropathologic images of retina and brain cortex. (B, D) The association between the rate of atrophy and age at baseline. Scale bars of 200 μm (retina) and 50 μm (brain cortex). The GCIPL is given by retinal ganglion cells nuclei and their synapsis with bipolar cells (GCL + IPL) (A) while the brain cortex is formed by different kinds of neurons, oligodendrocytes, astrocytes, and microglia (C). (B, D) The association between the rate of atrophy and age at baseline. Estimated simple slopes (and 95% CI) for change per year across different age groups in mixed-effects model of GCIPL or cortical gray matter volume with interaction of age and time. GCIPL = ganglion cell-inner plexiform layer; GCL = ganglion cell layer; INL = inner nuclear layer; IPL = inner plexiform layer; NFL = nerve fiber layer; WM = white matter.
This study aimed to explore the differences in age-related retinal and cortical atrophy rates in MS. To that end, we included patients who are part of a large observational cohort curated and phenotyped at the University of California, San Francisco (UCSF EPIC study).23-25 We hypothesized that because of the different impacts of inflammation across the disease course, neurodegeneration is faster in the first stages of MS. We also hypothesized that by evaluating the retina with its unique anatomical organization (ganglion cell-inner plexiform layer [GCIPL] thickness principally reflects retinal ganglion cell volume), to current technical standards, and available high-resolution direct visualization of the retinal layers, OCT can help in measuring inflammation-mediated neuronal cell body loss, earlier than MRI metrics for assessing of neurodegeneration.
Methods
To test our hypothesis, we analyzed 597 patients with MS diagnosed according to 2005 McDonald criteria26 (disease course at the first OCT 90% relapsing-remitting [RRMS] and 10% progressive MS, 69% female patients, median Expanded Disability Status Scale [EDSS] 2.0 [interquartile range (IQR) 1.5–3], with average age and disease duration of 45.3 ± 11 and 11.6 ± 9.7 years, respectively, with macular GCIPL thickness of 79.6 ± 13.0 mm) who underwent longitudinal OCT imaging annually for 4.5 ± 2.4 years. From the 597 patients, longitudinal MRI scans were available from 432 patients (disease course at the first examination 86% RRMS and 14% clinically isolated syndrome, 69% female patients, median EDSS 1.5 [IQR 1–2], average age 41.8 ± 9.6 years, disease duration 8 ± 8.3 years, cortical gray matter [CGM] 657.9 ± 47.0 cm3) who underwent longitudinal MRI for a study period of 10 ± 3.4 years.
The MRI acquisition protocol, as well as the preprocessing and processing procedures, was previously published.27 T1-weighted images were used for cortical volumetric analysis (FreeSurfer). Only the CGM volume was considered for the analysis. The OCT protocol was previously published.16 In brief, individual macular layers were imaged using spectral domain OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany). B scans of the macula were standardized by pattern size (5.9 × 4.4 mm) and scan quantity (19 volume scans/slices). Scans with insufficient quality were excluded in compliance with the OSCAR-IB criteria.28 We followed the APOSTEL guidelines for reporting OCT studies.29,30 For this study, only the macular GCIPL thickness was considered. The GCIPL is a better biomarker of atrophy than peripapillary nerve fiber layer thickness because its loss can be detected much earlier.17,31 Both the reviewers of MRI and OCTs were blinded to the clinical data.
Descriptive statistics for patient characteristics were presented as either median and IQR or mean ± SD. We estimated rates of GCIPL and CGM volume change over time at different age categories with hierarchical linear models. We considered the years since the baseline visit, the age category, and their interaction as the predictor variables, while including eye-specific intercepts and slopes nested within subjects for the GCIPL model and only subject-specific slopes and intercepts for the CGM model. The annualized percent change was modeled by the natural log transformation of the response variable. We assessed pairwise differences of estimated rates of GCIPL or CGM volume change with the Tukey method, adjusting for multiple pairwise comparisons. We fit models using the R package lme4 and assessed model estimates with the R package emmeans.
In a sensitivity analyses, we considered the impact of disease-modifying therapy (DMT), disease duration, and cumulative number of relapses on the estimated GCIPL and CGM volume change.
Three treatment tiers based on their relative effect on relapse rate and measures of neurodegeneration32 were used as time-dependent covariates in a sensitivity analysis: modest (interferons, glatiramer acetate, teriflunomide), moderate (fingolimod, dimethyl fumarate), and high (natalizumab, anti-CD20 monoclonal antibodies, alemtuzumab) efficacy.27 For this analysis, we modeled DMT as a time-dependent variant covariate to account for DMT switches or escalations (eTable 1, links.lww.com/WNL/C282).
Disease duration and number of previous relapses were modeled as variables that interact with age categories. We assessed pairwise differences between age categories in separate models that specified the efficacy of DMT, the disease duration at the first visit, or the number of relapses. Finally, to reduce the influence of recent optic neuritis (ON) episodes on the rates of GCIPL change, we removed eyes with reported ON within 6 months 6 months before the first OCT and during any period during the follow-up, and fit the models considering 2 age categories (18–36 and older than 37), assessing pairwise differences between age categories as described above. We also assessed pairwise differences between age categories considering only eyes with previous ON. We additionally assessed pairwise differences at specified levels of disease duration and the number of relapses.
Data Availability
All data and materials used in the analysis are available to any researcher for purposes of reproducing or extending the analysis.
Results
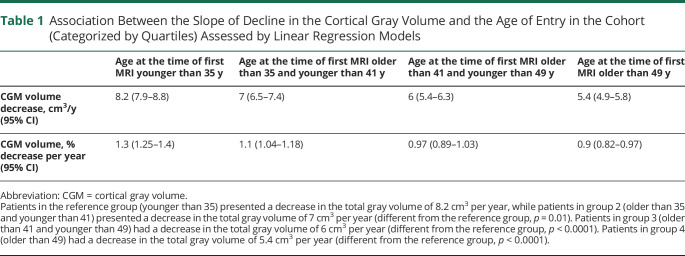
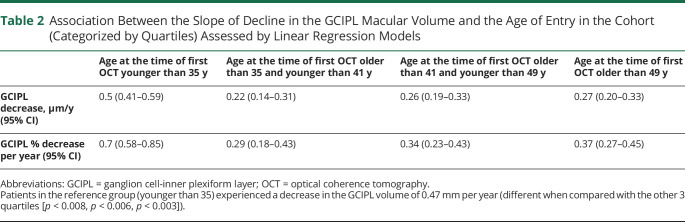
The rate of cortical gray matter atrophy declined with increasing age: 1.3% per year atrophy for the age of entry in the cohort younger than 35 years; 1.1% for age older than 35 and younger than 41 years; 0.97% for age older than 41 and <49 years; 0.9% for age older than 49 years (Table 1). The rates of atrophy were different between the first age category and second (p < 0.05), third (p < 0.001), and fourth (p < 0.001); in addition, second and fourth age categories presented with a statistically significant difference (p = 0.01). However, the rate of GCIPL thinning was at highest in patients in the first age quartile and then almost halved in the following quartile and finally stabilized across ages: 0.7% per year thinning for the age of entry in the cohort younger than 35 years; 0.29% for age older than 35 and younger than 41 years; 0.34% for age older than 41 and younger than 49 years; 0.33% for age older than 49 years (Table 2). GCIPL rates of loss were different between the first age category and second (p < 0.001), third (p < 0.001), and fourth (p < 0.001). In the sensitivity analysis, the rates of atrophy maintained similar slopes within the various age categories for patients on no treatment and for patients treated with modest-efficacy treatments (Figure 3). Interestingly, patients treated with moderate-efficacy and high-efficacy treatments showed a different pattern of neurodegeneration across ages, even if the lower sample size of these 2 groups did not allow to highlight statistically significant differences.
Table 1.
Association Between the Slope of Decline in the Cortical Gray Volume and the Age of Entry in the Cohort (Categorized by Quartiles) Assessed by Linear Regression Models
Table 2.
Association Between the Slope of Decline in the GCIPL Macular Volume and the Age of Entry in the Cohort (Categorized by Quartiles) Assessed by Linear Regression Models
Figure 3. Effect of MS Therapies on the Association of Age and the Estimated Slope of Change in GCIPL Thickness (A) and Cortical Gray Matter Volume (B).
Point estimates with 95% CIs are shown, along with symbols to delineate statistically significative differences in pairwise contrasts between patients who were younger than 35 years at the first visit and other age categories. p Values corrected for multiple comparisons with the Tukey method. GCIPL = ganglion cell-inner plexiform layer; MS = multiple sclerosis.
When we modeled disease duration as a variable that interacted with age categories (Figure 4), we found that the difference between the youngest age category and the other age categories was maintained with disease duration shorter than 10 years. We then decided to analyze the effect of relapses on our models. The differences between age quartiles in the rates of GCIPL and CGM atrophy remained unchanged after accounting for the number of previous clinical relapses (Figure 4).
Figure 4. Estimated Simple Slopes for Change in GCIPL Thickness (A and B) and cGMV (C and D) Given Interactions With Disease Duration or Cumulative Number of Relapses.
Point estimates with 95% CIs are shown, along with symbols to delineate statistically significative differences in pairwise contrasts between patients who were younger than 35 at the first visit and other age categories. p Values corrected for multiple comparisons with the Tukey method. cGMV = cortical gray matter volume; GCIPL = ganglion cell-inner plexiform layer.
Furthermore, to eliminate the possibility that recent ON events may have been driving our observed effect, we performed an additional analysis including only eyes without recent ON (including the 6 months before the first OCT and during any period during the follow-up) (10 eyes from 9 patients were excluded). In this sensitivity analysis, we continued to find a higher rate of atrophy among patients in the lowest age category (0.34 μm per year) when compared with the other 3 age groups (0.25 μm per year, p = 0.04). When analyzing only eyes with ON, we found that the lowest age category shows a higher rate of atrophy when compared with the other age categories (p = 0.0001).
Discussion
In this study, a faster rate of atrophy of the GCIPL and cortical gray matter was observed in the youngest patients with MS that slowed with age. These results provide new insights to understanding neurodegeneration in MS and validating some of the key pathologic concepts.
First, we found that the rate of neurodegeneration in retina and cortical gray matter is faster in the youngest patients. These results are in accordance both with the recent work of Pulido-Valdeolivas et al.9 and with the work of Balk et al.,33 looking at the correlation between retinal neurodegeneration and disease duration. Second, there are differences between the changes in the rate of neurodegeneration in the retina and cortical gray matter. Above the first age quartile, the rate of atrophy in the GCIPL remains relatively constant, but the rate of CGM atrophy progressively declines with age. Normative data for GCIPL34 show lower values of GCIPL in older patients, suggesting that the changes seen here are even more prominent for younger ages. Normative data for gray matter rate of change show constant neurodegeneration across ages in healthy patients.35
Our observations contrast with the recent work of Azevedo et al.15 that reported a linear and constant atrophy in MS, at least for the thalamus. These differences can be due to the different MRI follow-up times (5 vs 11.9 years) and, more likely, to the different structures analyzed (thalami vs CGM).
The reduction of acute inflammatory events, along with reduced proinflammatory lymphocytes, is temporally associated with declining axonal damage during the RRMS disease course.36 This has been already shown from the neuropathologic standpoint36 and it is likely the reason for the progressive slowdown in the rate of neurodegeneration and point to inflammation as the ultimate driver of neurodegeneration. At the same time, the remaining capacity for neuronal self-protection as well as diffuse innate immune activation, and in particular glial response (increased density and size),37 may possibly balance the effect of neuronal loss in the progressive phase. It is indeed known that diffuse microglial activity may persist throughout the course of the disease and may be seen in regions without prominent demyelination.38 Cortical atrophy is also most rapid early in the disease, but the rate of loss never seems to fully stabilize—although the rate of loss decreases, it continues to decrease throughout the period of observation. In addition, different dynamics and regional variation might mean that retinal neurodegeneration measured as macular GCIPL loss is an imperfect proxy of brain neurodegeneration. These results suggest that age is a critical consideration in the design of clinical trials using OCT and MRI as outcome measures. The observed difference between the brain gray matter and retinal neurodegeneration could be due to different tissue susceptibility to inflammation-related with the unique anatomical structure of the retina, devoid of mature oligodendrocytes (Figure 2) and affected only indirectly by acute inflammation, or to differences in the sensitivity and specificity of OCT and MRI in detecting tissue atrophy. Differing rates of injury seen in the retina and cortical gray may have a number of different causes. Plausibly in addition to differences in resolution and reproducibility between OCT and MRI, differences in the antigens found in different regions in the brain and variable susceptibility to damage based on different immunologic targets and neuronal vulnerability may explain the observations and deserve further exploration in the future.
Although the slopes of changes in the GCIPL and CGM thickness in patients on modest-efficacy therapies were similar to those on no treatment, the pattern of changes in GCIPL and CGM was different in patients on moderate-efficacy and high-efficacy therapies. This observation argues that moderate-efficacy and high-efficacy therapies potentially alter the trajectory of neurodegeneration in MS, especially in young patients.
One of the strengths of this study is that we did not exclude eyes with a history of ON. The reason for this choice is that inflammatory acute episodes are likely to be key factors in inducing neurodegeneration, and excluding patients with clinically evident ON from the analysis would have made our analysis incomplete and would have artificially weakened the association between inflammation and neurodegeneration. To directly address concerns about the impact of recent ON on apparent neurodegeneration, we performed an additional analysis excluding eyes with ON 6 months before the first examination and during the follow-up. We found that even analyzing eyes without ON in the 6 months before the first OCT and during the follow-up, the lowest age category shows a higher rate of atrophy when compared with the other 3 age categories combined. This analysis confirms that although ONs may contribute to the magnitude of the observed difference between retinal and brain neurodegeneration, there is still strong evidence that the rate of decline is fastest in the youngest patients on our retinal measures.
A large sample size, an adjustment for different tiers of therapy, a long follow-up, and systematic data collection are other strengths of this study.
This study has limitations. The age of the first OCT was older than the age of the first MRI. This asymmetry is because the great majority of the MRI cohort patients started undergoing MRIs several years before starting to have annual OCTs, resulting in an older cohort of patients when analyzing the OCT of the same MRI cohort. In addition, even if because of the characteristics of our cohort we considered the linear mixed-effects model to be the most appropriate, we cannot exclude nonlinear effects, which deserve to be investigated in future studies with regular sampling over the long term, to determine the optimal model for estimating the rate of neurodegeneration in MS. Furthermore, using DMTs as time-varying covariates did not account for the potential carryover effect and delayed onset of action of medications.
We have shown that the rate of tissue loss on OCT and MRI seem different with retinal degeneration most quickly earlier in the course of the disease. However, the rate of cortical atrophy does not seem to stabilize. Future studies are required to address the causes of the observed difference between the retina and brain cortex, in particular clarifying whether neurodegeneration in the cortex is influenced by the volume of glial cells if the retina has a different tissue susceptibility to inflammation-related neurodegeneration and lastly whether OCT and MRI exhibit differences in their respective sensitivity and specificity in detecting tissue atrophy.
Glossary
- CGM
cortical gray matter
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- GCIPL
ganglion cell-inner plexiform layer
- IQR
interquartile range
- MS
multiple sclerosis
- OCT
optical coherence tomography
- ON
optic neuritis
- RRMS
relapsing-remitting MS
Appendix. Authors
Footnotes
Editorial, page 641
CME Course: NPub.org/cmelist
Study Funding
This work was supported by the Valhalla Foundation and by the National Institute of Neurological Disorders and Stroke grant R35NS111644.
Disclosure
B. Nourbakhsh reports grants from Genentech, outside the submitted work. S.L. Hauser serves on the board of directors for Neurona and on scientific advisory boards for Accure, Alector, Annexon, and Molecular Stethoscope and has received travel reimbursement and writing assistance from F. Hoffmann-La Roche and Novartis for CD20-related meetings and presentations. B.A.C. Cree reports personal fees from Alexion, Atara, Autobahn, Avotres, Biogen, EMD Serono, Horizon, Neuron23, Novartis, Sanofi, TG Therapeutics, and Therini for consulting, outside the submitted work. R.G. Henry reports personal fees from Sanofi/Genzyme, Celgene, Roche/Genentech, Novartis, Boston Pharma, Medday, QIA, grants from Roche/Genentech, Atara, Medday, outside the submitted work. A.J. Green reports other from Bionure, grants, personal fees and other from Inception Sciences, grants from Sherak Foundation, personal fees and other from Pipeline Pharmaceuticals, grants from Hilton Foundation, grants from Adelson Foundation, grants from National MS Society, personal fees from JAMA Neurology, personal fees and other from Mediimmune/Viela, outside the submitted work; in addition, A.J. Green has a patent Small Molecule drug for Remyelination pending and has worked on testing off label compounds for remyelination. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Arnold DL, Matthews PM, Francis G, Antel J. Proton magnetic resonance spectroscopy of human brain in vivo in the evaluation of multiple sclerosis: assessment of the load of disease. Magn Reson Med. 1990;14(1):154-159. doi: 10.1002/mrm.1910140115. [DOI] [PubMed] [Google Scholar]
- 2.Frohman EM, Costello F, Stüve O, et al. Modeling axonal degeneration within the anterior visual system: implications for demonstrating neuroprotection in multiple sclerosis. Arch Neurol. 2008;65(1):26-35. doi: 10.1001/archneurol.2007.10. [DOI] [PubMed] [Google Scholar]
- 3.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183-193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 4.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278-285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 5.Correale J, Gaitán MI, Ysrraelit MC, Fiol MP. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain J Neurol. 2017;140(3):527-546. doi: 10.1093/brain/aww258. [DOI] [PubMed] [Google Scholar]
- 6.Cree BAC, Arnold DL, Chataway J, et al. Secondary progressive multiple sclerosis: new insights. Neurology. 2021;97(8):378-388. doi: 10.1212/WNL.0000000000012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry RG, Shieh M, Okuda DT, Evangelista A, Gorno-Tempini ML, Pelletier D. Regional grey matter atrophy in clinically isolated syndromes at presentation. J Neurol Neurosurg Psychiatry. 2008;79(11):1236-1244. doi: 10.1136/jnnp.2007.134825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Miralles F, Sastre-Garriga J, Tintoré M, et al. Clinical impact of early brain atrophy in clinically isolated syndromes. Mult Scler Houndmills Basingstoke Engl. 2013;19(14):1878-1886. doi: 10.1177/1352458513488231. [DOI] [PubMed] [Google Scholar]
- 9.Pulido-Valdeolivas I, Andorrà M, Gómez-Andrés D, et al. Retinal and brain damage during multiple sclerosis course: inflammatory activity is a key factor in the first 5 years. Sci Rep. 2020;10(1):13333. doi: 10.1038/s41598-020-70255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klistorner A, Barnett M. Remyelination trials: are we expecting the unexpected? Neurol Neuroimmunol Neuroinflammation. 2021;8(6):e1066. doi: 10.1212/NXI.0000000000001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain J Neurol. 2009;132(pt 5):1175-1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50(3):389-400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 13.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain J Neurol. 2007;130(pt 4):1089-1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol Zurich Switz. 2009;19(2):238-253. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azevedo CJ, Cen SY, Khadka S, et al. Thalamic atrophy in multiple sclerosis: a magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol. 2018;83(2):223-234. doi: 10.1002/ana.25150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749-760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasileiou ES, Filippatou AG, Pimentel Maldonado D, et al. Socioeconomic disparity is associated with faster retinal neurodegeneration in multiple sclerosis. Brain. 2021;144(12):3664-3673. doi: 10.1093/brain/awab342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambe J, Risher H, Filippatou AG, et al. Modulation of retinal atrophy with rituximab in multiple sclerosis. Neurology. 2021;96(20):e2525-e2533. doi: 10.1212/WNL.0000000000011933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574-584. doi: 10.1016/S1474-4422(16)00068-5. [DOI] [PubMed] [Google Scholar]
- 20.Lambe J, Fitzgerald KC, Murphy OC, et al. Association of spectral-domain OCT with long-term disability worsening in multiple sclerosis. Neurology. 2021;96(16):e2058-e2069. doi: 10.1212/WNL.0000000000011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study: retinal atrophy reflects brain atrophy in MS. Ann Neurol. 2015;78(5):801-813. doi: 10.1002/ana.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petracca M, Cordano C, Cellerino M, et al. Retinal degeneration in primary-progressive multiple sclerosis: a role for cortical lesions? Mult Scler Houndmills Basingstoke Engl. 2017;23(1):43-50. doi: 10.1177/1352458516637679. [DOI] [PubMed] [Google Scholar]
- 23.University of California, San Francisco MS-EPIC Team, Cree BAC, Hollenbach JA, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653-666. doi: 10.1002/ana.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordano C, Nourbakhsh B, Devereux M, et al. pRNFL as a marker of disability worsening in the medium/long term in patients with MS. Neurol Neuroimmunol Neuroinflammation. 2019;6(2):e533. doi: 10.1212/NXI.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordano C, Yiu HH, Oertel FC, et al. Retinal INL thickness in multiple sclerosis: a mere marker of neurodegeneration? Ann Neurol. 2021;89(1):192-193. doi: 10.1002/ana.25933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol. 2005;58(6):840-846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 27.University of California, San Francisco MS-EPIC Team, Cree BAC, Gourraud PA, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499-510. doi: 10.1002/ana.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. doi: 10.1371/journal.pone.0034823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303-2309. doi: 10.1212/WNL.0000000000002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aytulun A, Cruz-Herranz A, Aktas O, et al. APOSTEL 2.0 recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2021;97(2):68-79. doi: 10.1212/WNL.0000000000012125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77(3):517-528. doi: 10.1002/ana.24351. [DOI] [PubMed] [Google Scholar]
- 32.Mitsikostas DD, Goodin DS. Comparing the efficacy of disease-modifying therapies in multiple sclerosis. Mult Scler Relat Disord. 2017;18:109-116. doi: 10.1016/j.msard.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Balk LJ, Cruz-Herranz A, Albrecht P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263(7):1323-1331. doi: 10.1007/s00415-016-8127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mwanza JC, Durbin MK, Budenz DL, et al. Profile and predictors of normal ganglion cell–inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Investig Opthalmology Vis Sci. 2011;52(11):7872. doi: 10.1167/iovs.11-7896. [DOI] [PubMed] [Google Scholar]
- 35.Crivello F, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Longitudinal assessment of global and regional rate of grey matter atrophy in 1,172 healthy older adults: modulation by sex and age. PLoS One. 2014;9(12):e114478. doi: 10.1371/journal.pone.0114478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhlmann T. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125(10):2202-2212. doi: 10.1093/brain/awf235. [DOI] [PubMed] [Google Scholar]
- 37.Lassmann H. Targets of therapy in progressive MS. Mult Scler Houndmills Basingstoke Engl. 2017;23(12):1593-1599. doi: 10.1177/1352458517729455. [DOI] [PubMed] [Google Scholar]
- 38.Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain. 2017;140(7):1900-1913. doi: 10.1093/brain/awx113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials used in the analysis are available to any researcher for purposes of reproducing or extending the analysis.