Abstract
Background and Objectives
Several pathologic processes might contribute to the degeneration of the cholinergic system in aging. We aimed to determine the contribution of amyloid, tau, and cerebrovascular biomarkers toward the degeneration of cholinergic white matter (WM) projections in cognitively unimpaired individuals.
Methods
The contribution of amyloid and tau pathology was assessed through CSF levels of the Aβ42/40 ratio and phosphorylated tau (p-tau). CSF Aβ38 levels were also measured. Cerebrovascular pathology was assessed using automatic segmentations of WM lesions (WMLs) on MRI. Cholinergic WM projections (i.e., cingulum and external capsule pathways) were modeled using tractography based on diffusion tensor imaging data. Sex and APOE ε4 carriership were also included in the analysis as variables of interest.
Results
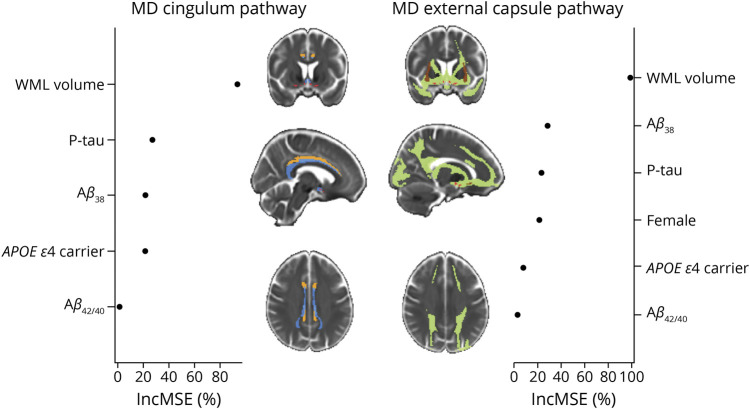
We included 203 cognitively unimpaired individuals from the H70 Gothenburg Birth Cohort Studies (all individuals aged 70 years, 51% female). WM lesion burden was the most important contributor to the degeneration of both cholinergic pathways (increase in mean square error [IncMSE] = 98.8% in the external capsule pathway and IncMSE = 93.3% in the cingulum pathway). Levels of Aβ38 and p-tau also contributed to cholinergic WM degeneration, especially in the external capsule pathway (IncMSE = 28.4% and IncMSE = 23.4%, respectively). The Aβ42/40 ratio did not contribute notably to the models (IncMSE<3.0%). APOE ε4 carriers showed poorer integrity in the cingulum pathway (IncMSE = 21.33%). Women showed poorer integrity of the external capsule pathway (IncMSE = 21.55%), which was independent of amyloid status as reflected by the nonsignificant differences in integrity when comparing amyloid-positive vs amyloid-negative women participants (T201 = −1.55; p = 0.123).
Discussion
In cognitively unimpaired older individuals, WMLs play a central role in the degeneration of cholinergic pathways. Our findings highlight the importance of WM lesion burden in the elderly population, which should be considered in the development of prevention programs for neurodegeneration and cognitive impairment.
The cholinergic neurons located in the nucleus basalis of Meynert (NBM) provide the major cholinergic input to the cerebral cortex and are essential to cognitive functioning.1 Postmortem studies have traced 2 principal cholinergic projection pathways from the NBM to the neocortex: the medial and the lateral pathways.1 The medial pathway advances through the white matter (WM) axons of the rectus gyrus, bends at the rostrum of the corpus callosum, and enters the cingulum bundle, projecting to the paraolfactory, cingulate, and retrosplenial cortices. The lateral pathway advances both through the claustrum and the extreme capsule (i.e., perisylvian division), projecting to the frontoparietal operculum, insula, and superior temporal gyrus, and through the external capsule and uncinate fasciculus (i.e., capsular division), projecting to the remaining parts of the frontal, parietal, and temporal neocortex. Recent diffusion tensor imaging (DTI)-based tractography studies have examined these pathways,2–5 providing the opportunity to study the integrity of the cholinergic system and its potential association with cognitive performance and pathophysiologic processes in vivo.
The strategic location of the NBM and its connective circuitry to the cortex results in increased vulnerability to brain pathology. For example, cholinergic neurons are affected in early stages of Alzheimer disease (AD)-related tauopathy due to their proximity to heavily affected basotemporal regions, which likely also alters their connective circuitry to the cortex.1 Furthermore, other age-related pathologies can also affect the integrity of the cholinergic system. WM lesions (WMLs), which are thought to be a marker of cerebrovascular disease, are commonly found on MRI in the elderly.6 A recent study showed that WMLs are associated with worse integrity of the cholinergic projections in cognitively unimpaired older individuals,4 and cholinergic projections influenced cognitive performance.4 Of interest, despite the association of WMLs with the integrity of the cholinergic projection system, neither WML burden itself nor NBM volume contributed to cognitive performance.4 These findings raised the question of whether other age-associated pathologies apart from WMLs might be affecting the integrity of the cholinergic projections in cognitively unimpaired individuals.
In this study, we investigated the contribution of amyloid and tau pathology in combination with cerebrovascular disease toward the degeneration of cholinergic WM projections in cognitively unimpaired individuals. It is important to address these research questions to assess whether and how other pathologies apart from cerebrovascular disease may affect the integrity of cholinergic projections in cognitively unimpaired individuals.
Methods
Participants
The study sample belongs to the Gothenburg H70 Birth Cohort Studies.7 Every 70-year-old listed in the Swedish Population Registry as a resident in Gothenburg (Sweden) was invited to a comprehensive examination on aging and age-related factors.7 A total of 1,203 individuals born in 1944 (response rate 72.2%; mean age 70.5 years) agreed to participate, of whom 430 consented to a lumbar puncture (response rate 35.8%). Lumbar puncture was considered as contraindicated in participants under anticoagulant therapy, immune-modulated therapy, and cancer therapy. After excluding participants not suitable for a lumbar puncture, the CSF extraction was conducted in 322 (26.8%) individuals. Every participant was also invited to take part in a brain MRI examination, of which 792 individuals (response rate 65.8%) underwent MRI conducted at Aleris in Gothenburg. The MRI examination was conducted within 3 months from the initial study visit. The lumbar puncture was conducted within 2 months from the MRI examination. The general examinations and other procedures have previously been described in detail.7 General cognitive status was measured using the Mini-Mental State Examination (MMSE) and the Clinical Dementia Rating (CDR) scale. For the current study, inclusion criteria were (1) a CDR score of 0; (2) MMSE >24; (3) availability of CSF biomarkers; and (4) availability of MRI data, yielding a final sample of 203 individuals (51% female).
MRI Data Acquisition, Image Processing, and Assessment of WMLs
MRI data were acquired in a 3.0 T Philips Achieva system (Philips Medical Systems), using a 3D T1-weighted turbo field echo sequence (repetition time [RT] = 7.2 ms, echo time [TE] = 3.2 ms, flip angle = 9°, matrix size = 250 × 250 mm, field of view = 256 × 256, and slice thickness = 1.0 mm); a 3D Fluid-attenuated inversion recovery (FLAIR) sequence (RT = 48,000 ms, TE = 280 ms, TI = 1,650 ms, flip angle = 90°, number of slices = 140, matrix size = 250 × 237 mm, and slice thickness = 2.0 mm); a susceptibility-weighted imaging (SWI) sequence (RT = 14.59–17.60 ms, TE = 20.59–24.99 ms, flip angle = 10°, matrix size = 229 × 222 mm, and slice thickness = 1.0 mm); and a DTI sequence encoded with 1 b-value shell: 800 ks/mm2, along with 32 directions and 1 b = 0 image (RT = 7,340 ms, TE = 83 ms, flip angle = 90°, matrix size = 112 × 112 mm, field of view = 224 × 224, and slice thickness = 3.0 mm).7
WMLs were measured as WM hypointensities and WM hyperintensities in T1-weighted and FLAIR sequences, respectively. WML and total intracranial volume (TIV) were automatically segmented using FreeSurfer 6.0.0. FreeSurfer detects hypointense WM signal abnormalities and automatically labels WML volumes for each participant using a probabilistic procedure.8 Hyperintense WMLs were automatically segmented using the open source segmentation toolbox LST 2.0.15.9 It has previously been shown that hypointense and hyperintense WMLs are strongly correlated.6 Previous findings revealed that hypointense WMLs might represent necrotic damage closer to accumulated cerebrovascular pathology,10 whereas hyperintense WMLs might also represent acute damage including peri-inflammatory processes.11 Due to the aim of the current study, we focused on hypointense WMLs, but all the analyses were replicated using hyperintense WMLs and are reported in eFigure 1, links.lww.com/WNL/C220. MRI data management and processing was performed using theHiveDB12 database system. WML volumes in milliliters (mL) were adjusted by TIV to account for variability in head size.13
Previously established ROI masks for the cholinergic WM pathways (i.e., cingulum and external capsule pathways) were used.4 Briefly, the masks were created using probabilistic diffusion-based fiber tracking of the NBM WM projections. These ROI masks of the cholinergic WM pathways were transferred from MNI standard space to each individual DTI image (b0) in native space using the nonlinear SyN registration algorithm14 from advanced normalization tools.15 Native space mean diffusivity (MD) maps were calculated for each subject using the FMRIB Diffusion Toolbox from FSL.16 Microstructural properties of each participant's cholinergic WM tracts were then calculated by averaging the MD values within the back-transformed ROI masks in native space. The MD index was preferred over the fractional anisotropy (FA) index because MD is more robust in the influence of crossing fibers.17
Complementary MRI Markers of Cerebrovascular Disease and Vascular Risk Factors
In addition to the automated measure of WMLs,18 we assessed cerebral microbleeds, lacunes, and superficial siderosis for completeness of information. The presence/absence of cerebral microbleeds was visually assessed on SWI, lacunes (3–15 mm) were assessed on FLAIR images, and superficial siderosis was assessed on SWI. All visual assessments were performed by an experience neuroradiologist blinded to clinical data,19 according to the Standards for Reporting Vascular Changes on Neuroimaging and standard scales and standardized scales.20 We also recorded and described the frequency of vascular risk factors, including hypertension, diabetes, smoking, and ischemia as assessed through a semistructured interview and clinical examination by research nurses or medical doctors.7
CSF Sampling and Biomarker Analysis
Lumbar puncture for CSF sampling and determination of APOE e4 carriership were conducted following standard procedures.7 CSF biomarker levels were determined by a commercially available assay.7 CSF tau phosphorylated at threonine 181 (p-tau) was determined by immunoassay ELISA (INNOTEST PHOSPHO_TAU [181P]). The Aβ42/40 ratio and CSF Aβ38 were determined by the V-PLEX Aβ Peptide Panel 1 (6E10) Kit (Meso Scale Discovery, Rockville, MD). We used p-tau to assess tau neurofibrillary tangle pathology. The CSF Aβ42/40 ratio was used as a marker of amyloidosis.21 For descriptive purposes, each individual was classified as positive (+; i.e., abnormal) or negative (−; i.e., normal) according to CSF biomarkers for Aβ (CSF Aβ42) and p-tau (CSF p-tau) following cohort-specific cutoff values: ≤530 pg/mL for Aβ42 and p-tau >80.22 Aβ38, a shorter isoform of Aβ that can also be found in the CSF, is still poorly understood. A previous study suggested that Aβ38 could be a marker of AD.23 Another study reported a predominant localization of Aβ38 within the vascular vessels in patients with AD.24 In addition, there is also evidence showing the presence of Aβ38 in other non-AD dementias25–27 and patients with chronic neuroinflammation.23 These diverse findings reflect the view that the role of Aβ38 still needs to be elucidated. Hence, we included CSF Aβ38 in this study to determine its association with AD biomarkers and cerebrovascular disease in the general population.
Statistical Analysis
Statistical analyses were conducted using R statistical software.28 A p value <0.05 (2 tailed) was deemed significant in all the analyses.
We used random forest (RF) regression models to assess the differential contributions of the different pathology-specific biomarkers toward the integrity of NBM projections. Two separate RF regression models, treated as the outcome variables, were fitted for the prediction of MD in the cingulum and the external capsule pathway, respectively. MD values were multiplied by a constant (c = 10,000) to facilitate the visualization of the data. WML, CSF Aβ42/40 ratio, Aβ38, and p-tau were included as predictors in all RF models, along with sex (i.e., male/female) and APOE status (i.e., at least 1 ε4 allele to be treated as carrier, otherwise noncarrier). RF is a machine learning method that estimates multiple decision trees via bootstrap aggregation (bagging). Each tree predicts a classification independently and votes for the corresponding class. The majority of the votes decide the overall prediction.29,30 A conditional importance score is computed for each tree in RF analysis. This is performed by measuring the change in the prediction error when the values of a certain variable are permuted within a grid defined by the included covariates. Then, this conditional score is averaged across the entire ensemble. These conditional importance scores are designed to reduce the undesirable effects of collinearity among predictor variables. The final importance of each predictor denotes its contribution to the model. Importance values below or equal to zero denote no contribution. A conditional regression tree is produced as a graphical representation of the model. The RF comprised 5,000 conditional inference trees. R2 was computed to assess the quality of the RF models. Although aging is associated with WM neurodegeneration and greater WML volumes,4,31 age was not included as a covariate in the models because it was controlled from the design (i.e., all participants were aged 70 years). For completeness of information, we also report Pearson correlation coefficients among the predictor variables included in the RF models and independent sample t tests for categorical variables that resulted important in the RF analysis. The randomForest32 and party packages33 were used for these analyses.
Standard Protocol Approvals, Registrations, and Patient Consents
The H70 study was approved by the Regional Ethical Review Board in Gothenburg (Approval Numbers: 869-13, T076-14, T166-14, 976-13, 127-14, T936-15, 006-14, T703-14, 006-14, T201-17, T915-14, 959-15, and T139-15) and by the Radiation Protection Committee (Approval Number: 13-64) in concordance with the 1964 Helsinki Declaration and its later amendment.
Data Availability
The authors state that anonymized data on which the article is based will be shared by request from any qualified investigator.
Results
Demographic, clinical data, vascular risk factors, and MRI markers of cerebrovascular disease are shown in Table 1. In our sample of 203 cognitively unimpaired individuals (all aged 70 years, 51% female), 2% had an AD biomarker profile (i.e., A+ T+), 43% had abnormal CSF levels of β-amyloid only (i.e., A+ T−), and 4.4% had abnormal CSF levels of p-tau only (i.e., A− T+). Results are shown for hypointense WML volume from T1-weighted 3D images. Virtually, the same results were obtained when including hyperintense WMLs instead of hypointense WMLs in the models (eFigure 1, links.lww.com/WNL/C220).
Table 1.
Study Sample Demographic and Clinical Data

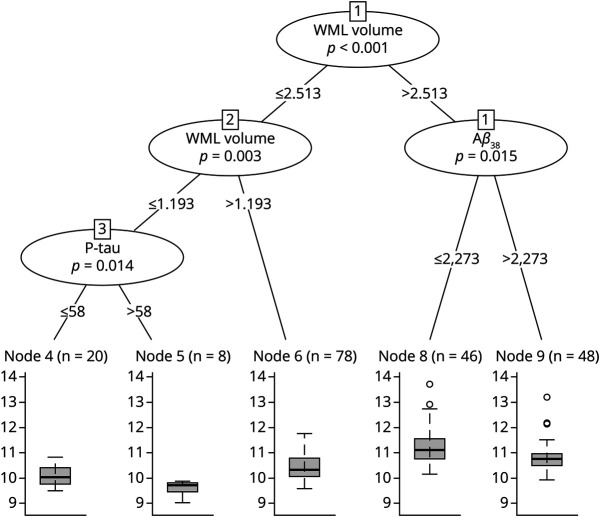
The RF models showed that WML volume was the most important predictor for the average MD of the cingulum pathway (Figure 1). P-tau, Aβ38, and APOE ε4 carriership were also important predictors in the model. The Aβ42/40 ratio received a low importance score. Sex did not contribute to the MD in the cingulum pathway. The RF tree revealed that WML volume was the best predictor splitting individuals according to their MD in the cingulum pathway. Four groups were distinguished (Figure 2). P-tau, Aβ38, Aβ42/40 ratio, sex, and APOE ε4 carriership did not separate any of the groups based on their association with MD in the cingulum pathway.
Figure 1. Contribution of Amyloid, Tau, and Cerebrovascular Biomarkers Toward the Integrity of Cholinergic WM Pathways.
The plot represents the percentage of increase in the prediction error (%IncMSE) when removing each variable from the random forest model. NBM ROI is represented in red. The cingulum pathway is represented in blue (orange for the cingulum mask). The external capsule pathway is represented in green (brown for the external capsule mask). Aβ38 = amyloid β38; Aβ42/40 = amyloid β42/40 ratio; P-tau = phosphorylated tau; WML volume = white matter lesions based on hypointense signal abnormalities of T1-weighted 3D images.
Figure 2. Random Forest Regression Tree for the MD in the Cingulum Pathway.
The figure represents the recursive partitioning for the MD index in the cingulum pathway and the contribution of WML volume to the portioning. Corresponding p values are given for each inner node. Boxplot for MD distribution is shown for each final group. MD = mean diffusivity; WML volume = white matter lesions based on hypointense signal abnormalities of T1-weighted 3D images.
Regarding the prediction of the MD in the external capsule pathway, WML volume was again the most important predictor (Figure 1). Aβ38, p-tau, and sex were also important in the model. Women showed poorer integrity in the external capsule pathway. This finding was independent of amyloid status, as reflected by the nonsignificant differences in integrity when comparing amyloid-positive vs amyloid-negative women participants (T201 = −1.55; p = 0.123). APOE ε4 carriership received a low importance score, and the Aβ42/40 ratio did not contribute to the MD in the external capsule pathway. The RF tree revealed that WML volume, Aβ38, and p-tau were important predictors to split individuals according to their MD in the external capsule. Five groups were distinguished at the end of the tree (Figure 3).
Figure 3. Random Forest Regression Tree for the MD in the External Capsule Pathway.
The figure represents the recursive partitioning for the MD index in the external capsule pathway and the contribution of WML volume, p-tau, and Aβ38 to the portioning. Corresponding p values are given for each inner node. Boxplot for MD distribution is shown for each final group. Aβ38 = amyloid β38; MD = mean diffusivity; P-tau = phosphorylated tau; WML volume = white matter lesions based on hypointense signal abnormalities of T1-weighted 3D images.
Figure 4 shows the correlation matrix for all pairs of continuous predictors in the RF models. Greater WML volumes were associated with lower Aβ38 levels. Higher p-tau levels were associated with lower Aβ42/40 ratio and higher Aβ38 levels.
Figure 4. Correlation Matrix for the Predictors Included in the Random Forest Models.
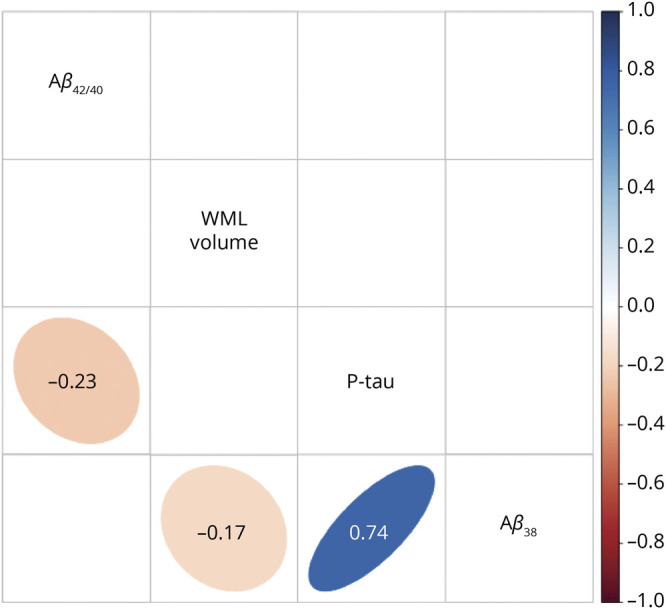
Background of significant correlations (p < 0.05) was colored according to the value of the correlation coefficient and shaped accordingly to the association distribution, otherwise left empty.
Discussion
In our study, we investigated the contribution of cerebrovascular disease compared with amyloid pathology and tau pathology toward the degeneration of cholinergic WM pathways in cognitively unimpaired individuals. We demonstrated the role of WML burden as a central contributor to the degeneration of the cholinergic projections.
The NBM is well known for its key role in cognitive functioning and its deterioration is linked to cognitive impairment in AD.1 It is important to determine the pathologic processes contributing toward degeneration of the cholinergic system as it has previously been demonstrated to be associated with cognitive impairment in advanced aging.4 In this sample of cognitively unimpaired aged individuals, we demonstrated that WMLs were the most important contributor toward the degeneration of the studied cholinergic pathways, followed by CSF Aβ38 and p-tau levels. Conversely, the Aβ42/40 ratio did not show a substantial contribution.
The integrity of the cholinergic system is crucial for proper cognitive functioning.1 The cholinergic hypothesis of cognitive aging postulates that age-related memory decline and other cognitive problems may arise due to declining cholinergic activity.34,35 In a previous study, we demonstrated that the WM integrity of cholinergic projections was closely associated with attention and memory performance in an independent aging cohort of cognitively unimpaired individuals.4 The influence of WML burden on cortical disconnection of the cholinergic system might be associated with subclinical cognitive impairments in the elderly. Longitudinal studies have shown that a high WML burden increases the risk of future cognitive impairment.36 Future studies should determine the disruptive role of WMLs in the association between cholinergic projections and cognitive performance in normal aging and the continuum of AD.
Although WML burden was the most important predictor in our RF models, we found that Aβ38 also contributed to the integrity of the cholinergic system. In contrast, the Aβ42/40 ratio was not an important predictor of neurodegeneration of cholinergic WM projections. The role of CSF Aβ38 and its association with neurodegeneration is still under debate.37,38 CSF Aβ38 levels are lower in frontotemporal dementia25 and dementia with Lewy bodies26,27 than in patients with AD. Furthermore, Aβ38 has previously been linked to increased counts of lacunes and cerebral microbleeds, 2 markers of cerebrovascular disease.39 Deposits of Aβ38 in vascular vessels have also been found in postmortem AD studies.24 Therefore, several studies suggest a potential association of Aβ38 with cerebrovascular pathology. In line with this, we showed that lower CSF Aβ38 levels were associated with a higher WML burden. In our study, both WML burden and CSF Aβ38 were the most important predictors of WM neurodegeneration of the cholinergic system compared with AD biomarkers (CSF Aβ42/40 and p-tau). These findings suggest an association between Aβ38 and cerebrovascular disease in normal aging and their predilection for the cholinergic WM. Recent reports have demonstrated that higher levels of Aβ38 in the CSF may have a protective effect against future cognitive decline and AD dementia in individuals with a positive AD biomarker profile at baseline.40 In support of this, decreased CSF Aβ38 levels have previously been linked to reduced cingulate and insula cortex volumes in our cohort.37 The cingulate cortex receives important cholinergic input from the medial cholinergic pathway and the insula from the lateral cholinergic pathway.1 These areas are well known for their role in emotion regulation, behavior, and executive functioning.41 Future studies should test whether Aβ38, neurodegeneration of the cholinergic system and reduced cingulate and insula gray matter volumes are associated with subclinical changes in emotion regulation and executive functioning in the elderly.
The cholinergic circuitry is highly vulnerable to brain pathology. In our study, we found pathway-dependent associations of WML, Aβ38, and tau (p-tau) pathologic markers with cholinergic WM projections. Our results show that individuals with decreased Aβ38 and high WML burden had the poorest integrity of the external capsule pathway. Of interest, women also showed poorer integrity in the external capsule pathway, independently of amyloid status. In contrast, WML burden was the only predictor of the integrity in the cingulum pathway. These pathway-dependent findings point to a greater vulnerability of the cingulum pathway to vascular pathology, in comparison to amyloid/tau pathologies. Regionally, the cingulum pathway is located in periventricular regions, where the presence of WMLs increases with aging.42 Periventricular WMLs have previously been associated with lower cortical cholinergic activity in normal aging.43 Conversely, the external capsule pathway might be more vulnerable to cerebrovascular disease and pathologies associated with Aβ38.
Regarding tau pathology, our results showed a negative association between p-tau and degeneration of cholinergic WM projections (i.e., a poorer integrity of WM projections was associated with lower levels of CSF p-tau). This counterintuitive finding might be the result of a selection bias in our sample. All our participants were cognitively unimpaired 70-year-olds, and only 6.4% had abnormal CSF p-tau levels. It is important to take into consideration that the combination of abnormal levels of p-tau with other brain pathologies such as WMLs will most probably result in cognitive impairment, and therefore, those individuals may have been excluded from our study. Whether increased CSF p-tau levels are associated with degeneration of cholinergic WM projections needs to be further tested in more diverse populations of older individuals, including patients with cognitive impairment.
The data provided by this study describe the contribution of the CSF Aβ42/40 ratio, Aβ38, and p-tau levels in combination with WML burden toward the degeneration of the cholinergic system in cognitively unimpaired elderly from a population-based cohort.7 However, all individuals included were aged 70 years; therefore, results can only be partially generalized to other age groups. A limitation of the current study, intrinsic to the tractography approach used to generate the cholinergic WM projection masks, is the existence of transverse crossing WM fibers that can lead to distorted information about the WM integrity. We aimed to partly overcome this limitation by using the MD index instead of FA because MD is less affected by crossing fibers.17 The associations between amyloid/tau biomarkers and WMLs might lead to collinearity problems. Using RF regression with conditional inference trees, we were able to handle multicollinearity to some degree. Alternative information about the spatial location of WMLs and cholinergic functional activity profiles based on fMRI could complement the findings of our current study.2 We demonstrated an association between Aβ38 and the degeneration of the cholinergic system. Nevertheless, the literature about the role of Aβ38 in neurodegenerative processes is still limited, and further research is needed. There is currently a discussion ongoing as to whether the validated biomarker cutoffs for dementia diagnosis are clinically relevant for preclinical stages of the disease.44 Subthreshold pathology in individuals exhibiting normal biomarker profiles might already be affecting the brain integrity leading to WM degeneration. Thus, in our study, we used continuous values as the input for the analysis. The integrity of the cholinergic projections across abnormal amyloid/tau profiles in clinical stages of AD needs to be further elucidated. Finally, a previous study demonstrated that WMLs can also be related to AD pathology.45 However, in our study, WMLs were not associated with CSF levels of Aβ42/40 and p-tau, which suggests that our WML measure likely does not reflect AD pathology.20
This study highlights the importance of cerebrovascular pathology relative to amyloid and tau pathology in their contribution to cholinergic neurodegeneration in cognitively unimpaired individuals. WMLs within cholinergic pathways correlate with cognitive impairment46 and executive dysfunction47 in patients with dementia. Given the central role of the cholinergic system in cognition, our study suggests that management of cholinergic WMLs and vascular risk factors should be considered in the development of prevention programs for neurodegeneration and cognitive impairment. As these data are replicated in independent cohorts, it may help in clinical considerations with regard to cerebrovascular and AD biomarkers, cholinergic dysfunction, and cognitive impairment. This knowledge could eventually support therapeutic decisions in the context of acetylcholinesterase inhibitors.
Glossary
- AD
Alzheimer disease
- CDR
Clinical Dementia Rating
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FLAIR
Fluid-attenuated inversion recovery
- MD
mean diffusivity
- MMSE
Mini-Mental State Examination
- NBM
nucleus basalis of Meynert
- RF
random forest
- RT
repetition time
- SWI
susceptibility-weighted imaging
- TE
echo time
- TIV
total intracranial volume
- WM
white matter
- WML
WM lesion
Appendix. Authors

Study Funding
This research was supported by the Swedish Research Council (2012-5041, 2013-8717, 2015-02830, 2016-02282, 2016-02282, 2020-02014, and 2021-01861), the Swedish Research Council for Health, Working Life and Welfare (2013-1202, AGECAP 2013-2300, 2013-2496, and 2013-0475), the Swedish Foundation for Strategic Research (SSF, RB13-0192), the Strategic Research Programme in Neuroscience at Karolinska Institutet (StratNeuro), the Swedish State under the agreement between the Swedish government and the county councils, the ALF agreement (FoUI-954893 and FoUI-962240), the Center for Medical Innovation (CIMED, FoUI-954459 and FoUUI-20200505), the Swedish Alzheimer Foundation (AF-967495, AF-939687, and AF-968032), the Swedish Brain Foundation (FO2020-0150, FO2021-0119, and FO2022-0175), Stonhes Stiftelse, Stiftelsen för Gamla Tjänarinnor, Demensförbundet, Neurofonden, Lindhés Advokatbyrå AB, Eivind och Elsa K: son Sylvans Stiftelse, Stiftelsen Demensfonden, Stiftelsen Hjalmar Svenssons Forskningsfond, Stiftelsen Wilhelm och Martina Lundgrens Vetenskapsfond, Funding for Geriatric diseases at Karolinska Institutet, Research funding at Karolinska Institutet, Fundacion Canaria Dr. Manuel Morales, and the Czech Alzheimer Foundation. MJG is supported by the “Miguel Servet” program [CP19/00031] and a research grant [PI20/00613] of the Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional (ISCIII-FEDER). J.B. Pereira is supported by grants from the Swedish Research Council (2018-02201), the Strategic Research Programme in Neuroscience at Karolinska Institutet (StratNeuro Startup Grant), The Center for Medical Innovation (20200695), Gamla Tjänarinnor (2019-00803), Demensforbundet, Stonhes, and a Senior Researcher faculty position from Karolinska Institutet. H. Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018–02532), the European Research Council (#681712), Swedish State Support for Clinical Research (ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (201809-2016862), the AD Strategic Fund and the Alzheimer's Association (ADSF-21-831376-C, ADSF-21-831381-C, and ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL. S. Kern was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALF GBG-81392 and ALF GBG-771071), the Alzheimerfonden (AF-842471, AF-737641, and AF-939825), and the Swedish Research Council (2019-02075) Stiftelsen Demensfonden, Stiftelsen Hjalmar Svenssons Forskningsfond, Stiftelsen Wilhelm och Martina Lundgrens Vetenskapsfond.
Disclosure
M. Eriksdotter has served as a consultant for Biogen unrelated to the present study. H. Zetterberg has served on scientific advisory boards and/or as a consultant for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, CogRx, and Red Abbey Labs; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). S. Kern has served on a scientific advisory board and/or as a consultant for Geras Solutions and Biogen, unrelated to the present study. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Mesulam M-M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease. J Comp Neurol. 2013;521(18):4124-4144. doi.wiley.com/10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herdick M, Dyrba M, Fritz H-CJ, et al. Multimodal MRI analysis of basal forebrain structure and function across the Alzheimer's disease spectrum. Neuroimage Clin. 2020;28:102495. 10.1016/j.nicl.2020.102495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritz HCJ, Ray N, Dyrba M, Sorg C, Teipel S, Grothe MJ. The corticotopic organization of the human basal forebrain as revealed by regionally selective functional connectivity profiles. Hum Brain Mapp. 2019;40(3):868-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemy M, Cedres N, Grothe MJ, et al. Cholinergic white matter pathways make a stronger contribution to attention and memory in normal aging than cerebrovascular health and nucleus basalis of Meynert. Neuroimage. 2020;211:116607. [DOI] [PubMed] [Google Scholar]
- 5.Teipel SJ, Meindl T, Grinberg L, et al. The cholinergic system in mild cognitive impairment and Alzheimer's disease: an in vivo MRI and DTI study. Hum Brain Mapp. 2011;32(9):1349-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cedres N, Ferreira D, Machado A, et al. Predicting Fazekas scores from automatic segmentations of white matter signal abnormalities. Aging (Albany NY). 2020;12(1):894-901. aging-us.com/article/102662/text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rydberg Sterner T, Ahlner F, Blennow K, et al. The Gothenburg H70 Birth cohort study 2014-16: design, methods and study population. Eur J Epidemiol. 2019;34(2):191-209. link.springer.com/10.1007/s10654-018-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341-355. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774-3783. 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Riphagen JM, Gronenschild EHBM, Salat DH, et al. Shades of white: diffusion properties of T1- and FLAIR-defined white matter signal abnormalities differ in stages from cognitively normal to dementia. Neurobiol Aging. 2018;68:48-58. linkinghub.elsevier.com/retrieve/pii/S0197458018301180. [DOI] [PubMed] [Google Scholar]
- 11.Olsson E, Klasson N, Berge J, et al. White matter lesion assessment in patients with cognitive impairment and healthy controls: reliability comparisons between visual rating, a manual, and an automatic volumetrical MRI method–the gothenburg MCI study. J Aging Res. 2013;2013:198471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muehlboeck J-S, Westman E, Simmons A. TheHiveDB image data management and analysis framework. Front Neuroinform. 2014;7:49-13. journal.frontiersin.org/article/10.3389/fninf.2013.00049/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voevodskaya O. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer's disease. Front Aging Neurosci. 2014;6:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41. ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, Gee JC. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49(3):2457-66. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith S.M, Jenkinson M, Woolrich M.W, Beckmann CF, Behrens T.E.J, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):208-19. [DOI] [PubMed] [Google Scholar]
- 17.Dauguet J, Peled S, Berezovskii V, et al. Comparison of fiber tracts derived from in-vivo DTI tractography with 3D histological neural tract tracer reconstruction on a macaque brain. Neuroimage. 2007;37(2):530-538. sciencedirect.com/science/article/pii/S105381190700328X. [DOI] [PubMed] [Google Scholar]
- 18.Badji A, Pereira JB, Shams S, et al. Cerebrospinal fluid biomarkers, brain structural and cognitive performances between normotensive and hypertensive controlled, uncontrolled and untreated 70-year-old adults. Front Aging Neurosci. 2021;13:777475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rydén L, Sacuiu S, Wetterberg H, et al. Atrial fibrillation, stroke, and silent cerebrovascular disease: a population-based MRI study. Neurology. 2021;97(16):E1608-E1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wardlaw JM, Smith EE, Biessels GJ, et al. , Standards for ReportIng Vascular Changes on Neuroimaging STRIVE v1. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-838. ncbi.nlm.nih.gov/pubmed/23867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's Disease. Alzheimers Res Ther. 2019;11:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kern S, Zetterberg H, Zettergren A, et al. . The prevalence of preclinical Alzheimer's disease in a population study of 70-year-olds. Alzheimers Dement. 2017;13:P848. linkinghub.elsevier.com/retrieve/pii/S1552526017314279. [Google Scholar]
- 23.Wiltfang J, Esselmann H, Bibl M, et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer's disease and in patients with chronic neuroinflammation. J Neurochem. 2002;81(3):481-496. [DOI] [PubMed] [Google Scholar]
- 24.Reinert J, Martens H, Huettenrauch M, et al. Aβ38 in the brains of patients with sporadic and familial Alzheimer's disease and transgenic mouse models. J Alzheimers Dis. 2014;39(4):871-881. [DOI] [PubMed] [Google Scholar]
- 25.Heywood WE, Hallqvist J, Heslegrave AJ, et al. CSF pro-orexin and amyloid-β38 expression in Alzheimer's disease and frontotemporal dementia. Neurobiol Aging. 2018;72:171-176. 10.1016/j.neurobiolaging.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulugeta E, Londos E, Ballard C, et al. CSF amyloid β38 as a novel diagnostic marker for dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2011;82(2):160-164. [DOI] [PubMed] [Google Scholar]
- 27.Van Steenoven I, Van Der Flier WM, Scheltens P, Teunissen CE, Lemstra AW. Amyloid-β peptides in cerebrospinal fluid of patients with dementia with Lewy bodies. Alzheimers Res Ther. 2019;11:8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. https://www.R-project.org/. [Google Scholar]
- 29.Breiman L. Bagging predictions. Mach Learn. 1996;24:123-140. [Google Scholar]
- 30.Breiman L Random forests. Mach Learn.2001;45(1):5-32. [Google Scholar]
- 31.Cedres N, Diaz-Galvan P, Diaz-Flores L, et al. The interplay between gray matter and white matter neurodegeneration in subjective cognitive decline. Aging (Albany NY). 2021;13(16):19963-19977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liaw AL, Wiener M. Classification and regression by randomForest. R News 2. 2003;3:18-22. [Google Scholar]
- 33.Hothorn T, Hornik K, Strobl C, Zeileis A. party: A Laboratory for Recursive Partytioning. R Package Version 09-0. 2015:37. http//CRAN R-project orgparty.r-forge.r-project.org/. [Google Scholar]
- 34.Dumas JA, Kutz AM, Mcdonald BC, et al. . Aged women with cognitive complaints. Neurobiol Aging. 2014;34:1145-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Contestabile A. The history of the cholinergic hypothesis. Behav Brain Res. 2011;221(2):334-340. 10.1016/j.bbr.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 36.Benedictus MR, Van Harten AC, Leeuwis AE, et al. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke. 2015;46(9):2661-2664. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg O, Kern S, Skoog J, et al. . Effects of amyloid pathology and the APOE ε4 allele on the association between cerebrospinal fluid Aβ38 and Aβ40 and brain morphology in cognitively normal 70-years-old. Neurobiol Aging. 2021;101:1-12. [DOI] [PubMed] [Google Scholar]
- 38.Bibl M, Mollenhauer B, Lewczuk P, et al. . Cerebrospinal fluid tau, p-tau 181 and amyloid-β38/40/42 in frontotemporal dementias and primary progressive aphasias, Dement Geriatr Cogn Disord. 2011;31:37-44. karger.com/DOI/10.1159/000322370. [DOI] [PubMed] [Google Scholar]
- 39.Hilal S, Akoudad S, Van Duijn CM, et al. Plasma amyloid-β levels, cerebral small vessel disease, and cognition: The Rotterdam study. J Alzheimers Dis. 2017;60(3):977-987. [DOI] [PubMed] [Google Scholar]
- 40.Cullen N, Janelidze S, Palmqvist S, Stomrud E, Mattsson-Carlgren N, Hansson O, Alzheimer's Disease Neuroimaging Initiative. Association of CSF Aβ38 levels with risk of Alzheimer disease–related decline. Neurology. 2022;98(9):e958-e967. doi: 10.1212/WNL.0000000000013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadland KA, Rushworth MFS, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003;41(8):919-931. [DOI] [PubMed] [Google Scholar]
- 42.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70(1):9-14. ncbi.nlm.nih.gov/pubmed/11118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohnen NI, Müller MLTM, Kuwabara H, Constantine GM, Studenski SA. Age-associated leukoaraiosis and cortical cholinergic deafferentation. Neurology. 2009;72(16):1411-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller A-M, Balasa M, Blennow K, et al. Current approaches and clinician attitudes to the use of cerebrospinal fluid biomarkers in diagnostic evaluation of dementia in europe. J Alzheimers Dis. 2017;60(1):201-210. [DOI] [PubMed] [Google Scholar]
- 45.McAleese KE, Firbank M, Dey M, et al. Cortical tau load is associated with white matter hyperintensities. Acta Neuropathologica Commun. 2015;3:60. 10.1186/s40478-015-0240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SH, Kang HS, Kim HJ, et al. The effect of ischemic cholinergic damage on cognition in patients with subcortical vascular cognitive impairment. J Geriatr Psychiatry Neurol. 2012;25(2):122-127. 10.1177/0891988712445089. [DOI] [PubMed] [Google Scholar]
- 47.Behl P, Bocti C, Swartz RH, et al. Strategic subcortical hyperintensities in cholinergic pathways and executive function decline in treated Alzheimer patients. Arch Neurol. 2007;64(2):266-272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that anonymized data on which the article is based will be shared by request from any qualified investigator.