Abstract
Purpose
To identify systemic health factors associated with a thinner choroid, which has been hypothesized as a cause of poor visual outcomes in low–birth weight infants.
Design
The prospective, observational Study of Eye Imaging in Preterm Infants (BabySTEPS) enrolled infants recommended for retinopathy of prematurity screening based on the American Association of Pediatrics guidelines.
Participants
Infants who underwent imaging with investigational handheld OCT at 36 ± 1 weeks’ postmenstrual age (PMA) as part of BabySTEPS.
Methods
Average choroidal thickness was measured across the central subfoveal 1 mm. We concurrently collected maternal and infant clinical health data. Univariate and multivariate linear regression analyses were performed to evaluate factors associated with choroidal thickness. The left and right eyes showed similar thicknesses, so their average was used for analysis.
Main Outcomes Measures
Association between infant health factors and subfoveal choroidal thickness.
Results
Subfoveal choroidal thickness was measurable in 82 of 85 infants and 94% of eyes. Mean choroidal thickness was 231 ± 78 μm. In the univariate analysis, a thinner choroid was associated with decreased growth velocity (P < 0.001), lower birth weight (P < 0.001), smaller head circumference (P < 0.001), younger gestational age (P = 0.01), the presence of patent ductus arteriosus (P = 0.05), sepsis or necrotizing enterocolitis (P = 0.03), bronchopulmonary dysplasia (P = 0.03), pulmonary interstitial emphysema (P = 0.002), more days on oxygen support (P < 0.001), and being on oxygen support at 36 weeks (P < 0.001) and at the time of imaging (P < 0.001). In the multivariate analysis, growth velocity (P = 0.002) and oxygen support at the time of OCT imaging (P = 0.004) remained associated with a thinner choroid.
Conclusions
A thinner choroid is associated independently with growth velocity and receiving oxygen support at 36 ± 1 weeks PMA. This suggests that choroidal development in preterm infants may be related to growth rate in the first weeks of life and the prolonged use of supplemental oxygen. Longitudinal studies are needed to assess differences in choroidal thickness before 36 weeks PMA and to assess their impact on visual outcomes.
Keywords: Choroid, Infant, OCT, Oxygen, Weight gain
Abbreviations and Acronyms: BabySTEPS, Study of Eye Imaging in Preterm Infants; BPD, bronchopulmonary dysplasia; EPO, erythropoietin administration; ICH, intracranial hemorrhage; ICN, intensive care nursery; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PIE, pulmonary interstitial emphysema; PMA, postmenstrual age; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; RBC, transfusion of packed red blood cells; RPE, retinal pigment epithelium; VEGF, vascular endothelial growth factor
Preterm and low–birth weight infants are at high risk for visual impairment, with nearly 3% of preterm infants and more than 50% of very preterm infants experiencing some degree of visual dysfunction.1 Sometimes the cause of visual impairment can be attributed to retinopathy of prematurity (ROP), cortical visual impairment, myopia, strabismus, or amblyopia, all more commonly found in these populations. In many cases, however, no known cause exists for the poor visual outcomes. Recently, it has been hypothesized that early changes in the choroid may contribute. The metabolic demands of the developing retina are among the highest in the human body, and the choroid serves as the primary blood supply for the photoreceptors. Poor choroidal blood flow can result in photoreceptor death or dysfunction.2
Prior studies have investigated associations between systemic health factors and retinal disorders, such as ROP and retinal nerve fiber layer development. Rothman et al3 showed that retinal nerve fiber layer thinning is associated with brain abnormalities on magnetic resonance imaging and lower neurodevelopmental scores. Studies of ROP have found associations between ROP and younger gestational age, lower birth weight, slower postnatal weight gain, sepsis, oxygen therapy and mechanical ventilation, pulmonary disease, and patent ductus arteriosus.4, 5, 6, 7, 8
As imaging with OCT has improved, it has allowed us to visualize the choroid better and to develop an appreciation for its role in many diseases, including high myopia, age-related macular degeneration, and diabetic retinopathy.9, 10, 11 Data about choroidal development remain limited, likely because of a paucity of reliable techniques for measuring choroidal thickness in preterm infants. It was shown only recently that handheld OCT can be used to image the choroid in preterm and term infants.12,13 Two retrospective studies of former-preterm infants investigated associations between choroidal health and birth weight, body length at birth, and gestational age using OCT and found that those born at a lower birth weight harbored a thinner choroid during adolescence.14,15 A recent report from this study group also found that the presence of plus disease is associated with a thinner choroid.16
In this study, we used OCT to analyze the impact of systemic health factors on choroidal thickness at 36 weeks. We hypothesized that the choroid is particularly susceptible to systemic insults given its highly vascularized nature. This prospective study provided us the unique opportunity to study systemic influences in the controlled setting of the intensive care nursery (ICN), where all health data are recorded meticulously.
Methods
Between August 2016 and November 2019, 118 infants were enrolled as part of the longitudinal, prospective National Institutes of Health-funded Study of Eye Imaging in Preterm Infants (BabySTEPS; ClinicalTrials.gov identifier, NCT02887157). The data were obtained with approval from the institutional review board at Duke University, and the described research adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from the parent or legal guardian of all participants. Both eyes of infants were imaged with a bedside investigational swept-source OCT system at multiple time points during their stay in the ICN. All eyes were dilated before imaging. For this cross-sectional analysis, we chose infants who had undergone an OCT imaging session at 36 ± 1 weeks’ postmenstrual age (PMA) because this was the latest age at which most infants had undergone imaging and had not yet been discharged or transferred out of the ICN. Detailed demographic data, imaging, and image processing techniques used for each of the BabySTEPS study participants at 36 weeks’ PMA were reported previously.16
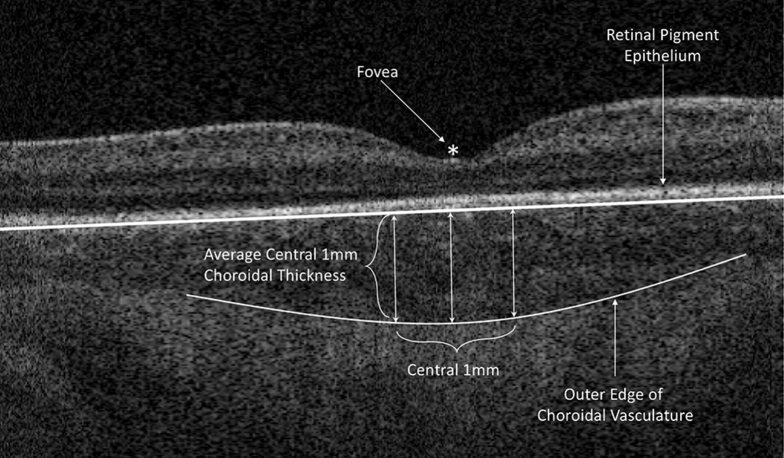
The Duke OCT Retinal Analysis Program Marking Code Baby version 2.017 custom segmentation software was used to delineate the central 1-mm subfoveal choroidal thickness on OCT images for each eye of each patient at each time point (Fig 1). The central 1 mm centered on the fovea was selected as the primary outcome because the contour of the outer border of the choroid varied across the outer choroidal vessels, making an average of the central 1 mm more accurate than a single subfoveal point. Errors in segmentation were corrected manually by a single trainer grader (K.P.W. or S.M.), because good intragrader and intergrader reproducibility in the BabySTEPS cohort was shown previously.16 Similarly, choroidal thickness in the BabySTEPS study cohort previously was found to be similar between left and right eyes, so their average was used for analysis. All graders were masked to study participants.
Figure 1.
OCT image showing the method for measuring the central 1-mm subfoveal choroidal thickness. The fovea, outer border of the retinal pigment epithelium, and outer edge of the choroidal vasculature (choroidal–scleral junction) were identified using the Duke Optical Coherence Tomography Retinal Analysis Program Marking Code Baby version 2.0 and were confirmed by trained graders. Choroidal thickness was measured across the central 1 mm centered at the fovea and averaged.
Infants’ birth histories and clinical health courses as well as maternal health data were extracted from the medical record consistent with data collected for the Generic Database, a registry of clinical information of very low–birth weight infants born alive in Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network centers (ClinicalTrials.gov identifier, NCT00063063) and stored securely in Research Electronic Data Capture. All data entry was audited independently by members of the BabySTEPS study group. The study group selected 20 infant and 8 maternal health metrics for analysis based on previous literature (Tables 1 and 2, respectively). Only systemic health factors experienced by at least 10 infants were included in the analysis. Necrotizing enterocolitis and early-onset and late-onset sepsis were combined as in prior neonatology studies to represent the hyperinflammatory response.18 To calculate growth velocity (grams per kilogram per day of weight gain) for each infant, we used the 2-point average method: (W2 – W1) / [W2 + W1) / 2] / 1000/number of days, where W2 is the infant’s weight on the day of OCT imaging and W1 is the infant’s weight on day 7 of life.19,20 Systemic health factors were grouped in Table 1 into commonly used categories by the World Health Organization to represent average choroidal thickness trends.21,22 The 5-minute Apgar score was grouped in Table 1 into reassuring (7–10) versus abnormal (0–6) as defined by the American Academy of Pediatrics and the American College of Obstetrics and Gynecology.23 We tracked both the weight at 36 weeks, which corresponded to one of the time points at which clinical health data are recorded as part of the Generic Database registry, and the weight on the specific date the OCT imaging was obtained (“weight at OCT imaging” in Table 1) for each infant.
Table 1.
Mean Choroidal Thickness by Infant Systemic Factors Grouped by Demographics and Organ System
| Systemic Health Factor | No. | Choroidal Thickness (Mean ± Standard Deviation) | P Value |
|---|---|---|---|
| Patient demographics | |||
| Birth weight (g) | |||
| <1000 (ELBW) | 43 | 202.23 ± 71.48 | |
| 1000–1500 (VLBW) | 37 | 259.91 ± 72.28 | |
| >1500–2500 (LBW) | 2 | 318.89 ± 45.55 | <0.001 |
| Body length at birth (cm) | |||
| Mean ± SD, 34.80 ± 3.93 | 81 | 229.87 ± 77.18 | 0.42 |
| Gestational age (wks) | |||
| <28 (extremely preterm) | 38 | 206.05 ± 70.00 | |
| 28–32 (very preterm) | 40 | 253.83 ± 78.79 | |
| >32–37 (preterm) | 4 | 241.93 ± 78.13 | 0.01 |
| Growth velocity (g/kg/day) | |||
| Mean ± SD, 12.50 ± 4.18 | 80 | 231.64 ± 78.35 | <0.001 |
| Head circumference at birth (cm) | |||
| Mean ± SD, 24.58 ± 2.38 | 82 | 229.87 ± 77.18 | <0.001 |
| Sex | |||
| Female | 39 | 227.95 ± 84.38 | |
| Male | 43 | 233.97 ± 71.61 | 0.73 |
| Weight at 36 wks (g) | |||
| <1000 | 12 | 151.33 ± 56.21 | |
| 1000–1500 | 22 | 198.20 ± 54.30 | |
| >1500 | 47 | 266.88 ± 70.66 | 0.15 |
| Cardiac | |||
| Patent ductus arteriosus | |||
| No | 44 | 246.97 ± 84.52 | |
| Yes | 38 | 212.73 ± 64.86 | 0.05 |
| Treated patent ductus arteriosus | |||
| No | 57 | 234.46 ± 84.82 | |
| Yes | 25 | 223.45 ± 58.34 | 0.56 |
| Hematologic | |||
| Received erythropoietin | |||
| No | 63 | 231.06 ± 79.60 | |
| Yes | 19 | 231.25 ± 72.22 | 0.99 |
| Transfused packed red blood cells | |||
| No | 21 | 260.49 ± 85.88 | |
| Yes | 62 | 221.63 ± 72.85 | 0.05 |
| Hyperinflammatory response | |||
| Sepsis or necrotizing enterocolitis | |||
| No | 67 | 239.70 ± 77.62 | |
| Yes | 15 | 192.73 ± 66.56 | 0.03 |
| Neurologic | |||
| Apgar score at 5 min | |||
| 0–6 (abnormal) | 32 | 235.01 ± 80.45 | |
| 7–10 (reassuring) | 50 | 228.61 ± 76.30 | 0.33 |
| Intracranial hemorrhage, periventricular leukomalacia, or ventriculomegaly | |||
| No | 29 | 232.27 ± 72.86 | |
| Yes | 53 | 230.47 ± 80.62 | 0.92 |
| Pulmonary | |||
| Bronchopulmonary dysplasia | |||
| No | 71 | 238.55 ± 79.61 | |
| Yes | 11 | 183.05 ± 37.02 | 0.03 |
| Days receiving oxygen support | |||
| 0–7 | 46 | 250.86 ± 80.58 | |
| 8–28 | 20 | 228.92 ± 62.14 | |
| >28 | 16 | 177.04 ± 61.26 | <0.001 |
| Receiving oxygen support at time of OCT imaging | |||
| No | 62 | 251.15 ± 73.12 | |
| Yes | 20 | 168.99 ± 55.49 | <0.001 |
| Pulmonary interstitial emphysema | |||
| No | 68 | 242.72 ± 75.02 | |
| Yes | 14 | 174.69 ± 65.66 | 0.002 |
| Receiving oxygen support at 36 wks’ PMA | |||
| No | 63 | 248.13 ± 75.63 | |
| Yes | 19 | 174.67 ± 54.40 | <0.001 |
| Surfactant administration | |||
| No | 23 | 250.15 ± 66.29 | |
| Yes | 59 | 223.68 ± 80.78 | 0.17 |
ELBW = extremely low birth weight; LBW = low birth weight; PMA = postmenstrual age; SD = standard deviation; VLBW = very low birth weight.
Averages for select continuous variables are presented in bins according to commonly used categories by the World Health Organization to represent average choroidal thickness trends.21, 22 All P values were generated using a univariate linear regression model.
Statistically significant P values are bolded.
Table 2.
Mean Choroidal Thickness by Maternal Systemic Factors Grouped by Demographics and Health Factors
| Maternal Factors | No. | Choroidal Thickness (Mean ± Standard Deviation) | P Value |
|---|---|---|---|
| Demographics | |||
| Age at delivery (yrs) | |||
| <35 | 61 | 220.95 ± 69.36 | |
| 35+ | 21 | 260.62 ± 93.14 | 0.25 |
| Mother’s race | |||
| Black | 36 | 231.94 ± 69.05 | |
| White | 38 | 238.31 ± 84.04 | |
| Other | 8 | 193.12 ± 80.16 | 0.33 |
| Health factors | |||
| Antepartum hemorrhage | |||
| No | 70 | 231.98 ± 75.65 | |
| Yes | 11 | 216.40 ± 89.09 | 0.36 |
| Clinical chorioamnionitis | |||
| No | 63 | 226.76 ± 81.09 | |
| Yes | 15 | 239.46 ± 63.81 | 0.53 |
| Histologic chorioamnionitis | |||
| No | 51 | 239.03 ± 80.80 | |
| Yes | 24 | 210.54 ± 63.11 | 0.30 |
| Hypertension | |||
| No | 44 | 231.26 ± 74.40 | |
| Yes | 37 | 228.22 ± 81.36 | 0.43 |
| Multiple births | |||
| No | 62 | 223.93 ± 79.03 | |
| Yes | 20 | 253.36 ± 69.81 | 0.14 |
| Smoking during pregnancy | |||
| No | 66 | 229.69 ± 79.69 | |
| Yes | 16 | 236.96 ± 69.89 | 0.74 |
All P values were generated using a univariate linear regression model.
Literature Search
A literature search was performed on December 30, 2020, using PubMed. No restrictions on year published were imposed. Search terms included the following: choroid, infant, and optical coherence tomography. We reviewed all citations for reports of choroidal thickness in articles identified by the literature search. Foreign literature for which an abstract was available in English were reviewed.
Statistical Analysis
All statistical analysis was performed using R software version 3.6.2 (R Foundation for Statistical Computing). P values were obtained using linear regression for the univariate analysis and a forward variable selection was used for the multivariate analysis. Continuous data were used for statistical analysis of all variables, even if variables were displayed as categorical variables in Tables 1 or 2. Statistical significance was defined as P < 0.05. Variables that were associated significantly with a thinner choroid in the univariate model were included in the multivariate model for forward variable selection. All means are reported with a standard deviation.
Results
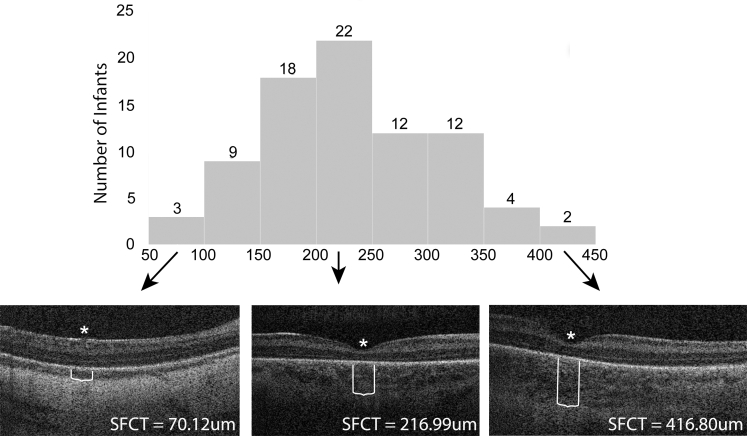
OCT images were obtained at 36 ± 1 weeks’ PMA in 85 infants and 170 eyes, and subfoveal choroidal thickness was measurable in 82 infants and 159 eyes (94%). Mean birth weight was 968 ± 271 g, and mean gestational age was 28 ± 2 weeks. Mean growth velocity from 7 days after birth to 36 weeks’ PMA was 13 ± 4 g/kg per day and ranged from 4 to 25 g/kg per day. Mean choroidal thickness was 231.1 ± 77.5 μm and ranged from 70.1 to 419.9 μm (Fig 2).
Figure 2.
Histogram showing distribution of choroidal thicknesses for all infants with illustrative OCT images for the thinnest (left), average (center), and thickest (right) choroids in the data set. The asterisks denote the fovea and the white lines denote the choroidal thickness. SFCT = average central 1-mm subfoveal choroidal thickness.
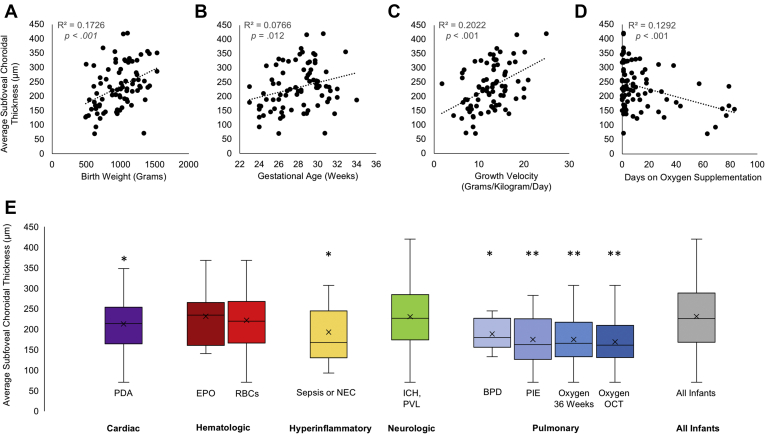
In the univariate analysis, a thinner choroid was associated significantly with decreased growth velocity, lower birth weight, younger gestational age, smaller head circumference at birth, the presence of bronchopulmonary dysplasia or pulmonary interstitial emphysema, oxygen support at 36 weeks’ PMA, oxygen support at the time of OCT imaging, increased total days on oxygen, patent ductus arteriosus, and hyperinflammatory response (either necrotizing enterocolitis, early-onset sepsis, or late-onset sepsis; Table 1; Fig 3). None of the maternal factors investigated were found to be associated significantly with a thinner choroid (Table 2).
Figure 3.
A–D, Scatterplots representing the relationship between continuous variables (birth weight, gestational age, growth velocity, number of days receiving supplemental oxygen) and average 1-mm subfoveal choroidal thickness. E, Box-and-whisker plots illustrating the relationship between the presence of systemic categorical variables and average 1-mm subfoveal choroidal thickness in infants with each condition. ∗P < 0.05 in the univariate analysis. ∗∗P < 0.005 in the univariate analysis. BPD = bronchopulmonary dysplasia; EPO = erythropoietin administration; ICH,PVL = intracranial hemorrhage, periventricular leukomalacia, or ventriculomegaly; NEC = necrotizing enterocolitis; Oxygen OCT = required oxygen supplementation at the time of OCT imaging; Oxygen 36 Weeks = required oxygen supplementation at 36 weeks’ postmenstrual age; PDA = patent ductus arteriosus; PIE = pulmonary interstitial emphysema; RBC = transfusion of packed red blood cells.
In the multivariate analysis, a thinner choroidal was associated significantly with decreased growth velocity (P = 0.002) and receiving oxygen support at the time of OCT imaging (P = 0.004; Table 3). A total of 20 infants were receiving supplemental oxygen at the time of OCT imaging. We collected data on the type of supplemental oxygen; however, each of the individual groups were too small to draw conclusions (n < 10). Four infants were receiving continuous positive airway pressure, 9 were administered high-flow nasal cannula treatment, 5 were receiving nasal cannula treatment, 2 were receiving synchronized intermittent mandatory ventilation, and 62 were not receiving any supplemental oxygen.
Table 3.
Results of Multivariate Regression Analysis for Factors Associated with a Thinner Choroid
| Systemic Factor | Adjusted Estimate (μm) | Standard Error | t Value | P Value |
|---|---|---|---|---|
| Receiving oxygen support at time of OCT imaging | 30.11 | 9.48 | 3.18 | 0.002 |
| Growth velocity (per g/kg/day increase) | 5.80 | 1.98 | 2.93 | 0.004 |
All factors with P < 0.05 in the univariate analysis (see Table 1) were included in the multivariate model through forward variable selection.
Statistically significant P values are bolded.
Discussion
In this prospective, cross-sectional study using handheld OCT images for 82 preterm infants at 36 ± 1 weeks’ PMA, we found that a thinner choroid is associated independently with slower postnatal growth velocity and the use of supplemental oxygen. We also showed that in univariate analysis, a thinner choroid is associated with several other systemic health conditions, including baseline health metrics (low birth weight, early gestational age, small head circumference), cardiac abnormalities (patent ductus arteriosus), hyperinflammatory response (sepsis or necrotizing enterocolitis), and pulmonary abnormalities (bronchopulmonary dysplasia [BPD], patent ductus arteriosus, oxygen use). One prior study of 80 premature infants with an average gestational age of 30.7 weeks showed a positive association between subfoveal choroidal thickness and higher birth weight in preterm infants using spectral-domain OCT between 36 and 42 weeks.13 Our study suggests that a thinner choroid in premature infants is associated with several other systemic health factors and conditions that infants experience in the newborn nursery.
In our study, the most common systemic factors associated with a thinner choroid were pulmonary and either directly or indirectly related to the use of supplemental oxygen (e.g., requiring oxygen at 36 weeks of age or BPD and pulmonary interstitial emphysema, conditions closely related to the use of supplemental oxygen).24,25 Many preterm infants require oxygen supplementation, and debate exists among neonatologists regarding how to find the ideal oxygen saturation target for each neonate that both avoids hypoxia (having too little oxygen in the blood) and hyperoxia (having an excess of oxygen).26,27 The effects of hyperoxia on vasculature development have been well studied in the context of BPD and ROP. Hyperoxia induces oxidative stress and the formation of reactive oxygen species, impairing vascular development and resulting in a reduced density of the pulmonary vascular bed in BPD and arrest and pruning of the retinal vasculature in ROP.28,29 Additionally, it has been shown that hyperoxia during development results in downregulation of vascular endothelial growth factor (VEGF), a key mechanism in angiogenesis.30,31 In premature infants receiving supplemental oxygen, we postulate that increased oxidative stress and downregulation of VEGF also may arrest the vascular development of the choroid, resulting in a thinner choroid in these infants. In support of this hypothesis, studies in mouse models of hyperoxia during development have shown choroidal involution.32 In ROP, the initial hyperoxia resulting in arrest and regression of retinal vasculature often leads to subsequent neovascularization resulting from retinal ischemia. However, choroidal neovascularization resulting from hyperoxia has not been described, and studies using the oxygen-induced retinopathy model in rats have shown the choroid lacks the ability to revascularize after involution caused by oxygen-induced retinopathy.29,32 This may explain why some studies have shown that a thin choroid persists in former preterm infants.14,15,33
Given the apparent inability of the choroid to revascularize, it is likely that the thin choroids associated with oxygen supplementation at 36 weeks’ PMA and at the time of OCT imaging persist into adulthood, but we cannot rule out the possibility that the thinning is transient. Studies in adults and mice have found that supplemental oxygen results in temporary vasoconstriction, allowing tissues to autoregulate while they are receiving higher levels of oxygen.34,35 Particularly because the strongest correlation was between infants receiving oxygen at the time of OCT imaging, it is possible that the choroidal thinning is a result of transient vasoconstriction, rather than permanent vessel attenuation and loss. Future studies of choroidal thickness and choroidal blood flow in infants receiving supplemental oxygen will be important to clarify these findings.
Slower growth velocity in the neonatal intensive care unit has become highlighted increasingly as an important factor in the development of ROP,7,36,37 so we were not surprised to find a significant, independent association between growth velocity and a thin choroid in our study. The WINROP (Weight, Insulin-like growth factor-1 Neonatal, ROP) algorithm, which takes into account weekly weight measurements and serum insulin-like growth factor 1 levels to evaluate an infant’s individual risk of ROP developing, was published first in 2006 by Löfqvist et al.38 A modified version of the algorithm, using only weekly weights from birth to 36 weeks’ PMA, gestational age, and PMA, also was found to be an excellent screening tool for identifying infants at high risk for ROP development.39 The exact mechanism by which poor weight gain contributes to a thinner choroid has yet to be elucidated.
Birth weight and gestational age were significant in the univariate, but not multivariate, analysis, likely because of the adjustments of decreased growth velocity and oxygen supplementation that also are associated strongly with birth weight and gestation age. Several previous studies also found associations between choroidal thickness and birth weight, gestational age, or both. One prior study using OCT in 80 preterm infants between 36 and 42 weeks’ PMA showed that infants with lower birth weight harbored thinner choroids.13 A study of 388 former preterm and full-term infants found that infants who were small for their gestational age harbored a thinner peripapillary choroid compared with infants who were of an appropriate gestational age.15 Another study of 1406 children 11 to 12 years of age, including 51 former lower–birth weight infants, also found that thinner choroids were associated with being small for gestational age and lower birth weights.14 These associations between a thinner choroid and lower birth weight, earlier gestational age, or both have been hypothesized to occur either secondary to global developmental delay or to delayed development of the retinal pigment epithelium (RPE).14,15 Preterm and low–birth weight infants develop at a slower rate than they would have in utero, and they have been shown to have higher rates of subnormal height and neurosensory impairment in adulthood.40 The global developmental delay hypothesis also could explain the association between a smaller head circumference at birth and a thinner choroid at 36 weeks. Another hypothesis is that the RPE is not developed fully at preterm birth. The RPE expresses VEGF, which is essential to development of the choroidal vasculature, and a decrease in the expression of VEGF from an immature RPE may lead to impairments in choroidal development.41
Patent ductus arteriosus and the hyperinflammatory response (i.e., necrotizing enterocolitis or sepsis) were found to be significant in the univariate, but not multivariate, analysis. Patent ductus arteriosus is defined as failure of the ductus arteriosus to close within 72 hours of birth, and it is correlated very closely with birth weight and gestational age.42 This may explain why patent ductus arteriosus was significant in the univariate analysis, but not in the multivariate analysis, because it may be more related to gestational age than choroidal thickness. Necrotizing enterocolitis and sepsis are grouped together frequently in the neonatology literature as morbidities that cause a hyperinflammatory response, and previously they were found to be associated with the development of BPD and ROP.43 It is possible that inflammation associated with these characteristics affects choroidal vascular growth by a similar mechanism as it affects the pulmonary and retinal vascular development, but our study was underpowered to detect this association.
In this study, we also investigated several maternal health factors that we suspected may be associated with a thin choroid. Birth weight previously was shown as a significant factor associated with thinner choroids in preterm infants,13 making it seem plausible that intrauterine processes may affect choroidal development. Maternal hypertension has been shown to affect infant vascular development, resulting in an increased risk of BPD and ROP.44, 45, 46, 47 Studies in mice have shown that maternal hypertension leads to reduced microvascular densities of fetal vasculature in the placenta.48 Similarly, maternal smoking also has been shown in some studies to increase the risk of BPD, although its impact on ROP has been less consistent, with some studies showing an increased risk of ROP and others showing a decreased risk or no impact.49, 50, 51, 52 In our study, we did not find any significant associations between the 8 maternal factors we investigated and a thinner choroid (Table 2).
This study has several limitations. One is the inability to pinpoint the time in development at which choroidal growth is impacted in relationship to the infant’s systemic health conditions. Second, we do not yet have long-term follow-up data in these patients showing that these thin choroids persist or whether they correlate with poor visual outcomes. Because BabySTEPS is a longitudinal study, these children will be followed up to school age, and longitudinal findings will publish at that time. Failure to find associations with particular variables also may be limited by our relatively small sample size in this study of 82 infants. In addition, we did not evaluate the association of uncommon systemic conditions (n < 10) with choroidal thickness. Another limitation comes from difficulties inherent to imaging awake neonates with handheld OCT at bedside. Although OCT technology developed in house by our research team has been improving rapidly and it is now possible to measure the choroidal thickness in most infants, the current capture resolution and processing of the scans do not allow for distinguishing individual choroidal layers or a choroidal vascularity index in most infants. Future directions include investigating which layer of choroid is thinned in premature infants receiving prolonged supplemental oxygen (e.g., is the choriocapillaris primarily affected, similar to the capillary network in BPD, or are the larger vessels affected?) and investigating development of the choroid over the infants’ entire nursery course, including earlier age groups.
In conclusion, we found that a thinner choroid at 36 weeks’ PMA in preterm infants is associated independently with slower postnatal growth velocity and the use of supplemental oxygen. To our knowledge, this is the first study in humans to show that decreased growth velocity in the ICN and the prolonged use of supplemental oxygen may play a significant role in choroidal development. More studies are needed to elucidate the pathophysiologic features of choroidal growth and development and to determine the impact of these choroidal changes on long-term visual outcomes.
Manuscript no. D-21-00034.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): L.L.S.: Financial support – Boehringer Ingelheim
C.A.T.: Royalties (through institution) – Alcon; Patent (unlicensed and pending) – the investigational device and imaging in this study; Equity owner – Theia Imaging, LLC
L.V.: Consultant – AERI, Alcon, Alimera Sciences, Allergan, AGTC, Bausch and Lomb, BVI, DORC, Genentech, Guidepoint, Janssen Pharmaceutical, Orbit Biomedical, Second Sight; Financial support – Heidelberg Engineering, Inc, Oculus Surgical, Orbit Biomedical, Inc, Novartis, Second Sight, Inc, Regenxbio, Roche/Genentech, Evolve Medical Education
Supported by the National Eye Institute, National Institutes of Health, Bethesda, Maryland (grant nos.: R01 EY025009, P30 EY005722, and K23 EY02827); and the Vitreoretinal Surgery Foundation. The contents in this manuscript are solely the responsibility of the authors and do not represent the official view of National Eye Institute, National Institutes of Health, or the Vitreoretinal Surgery Foundation. The sponsor or funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Duke University approved the study. All research adhered to the tenets of the Declaration of Helsinki. The parent or legal guardian of all participants provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Michalak, Mangalesh, Shen, McGeehan, Winter, Sarin, Finkle, Cotten, Ying, Toth, Vajzovic
Analysis and interpretation: Michalak, Mangalesh, Shen, McGeehan, Sarin, Cotten, Ying, Toth, Vajzovic
Data collection: Michalak, Mangalesh, Shen, McGeehan, Winter, Sarin, Finkle, Cotten, Ying, Toth, Vajzovic
Obtained funding: Michalak, Toth, Vajzovic; Study was performed as part of the authors' regular employment duties. No additional funding was provided.
Overall responsibility: Michalak, Toth, Vajzovic
Supplementary Data
References
- 1.Burgess P., Johnson A. Ocular defects in infants of extremely low birth weight and low gestational age. Br J Ophthalmol. 1991;75(2):84–87. doi: 10.1136/bjo.75.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao J., McLeod S., Merges C.A., Lutty G.A. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998;116(5):589–597. doi: 10.1001/archopht.116.5.589. [DOI] [PubMed] [Google Scholar]
- 3.Rothman A.L., Sevilla M.B., Mangalesh S., et al. Thinner retinal nerve fiber layer in very preterm versus term infants and relationship to brain anatomy and neurodevelopment. Am J Ophthalmol. 2015;160(6):1296–1308 e1292. doi: 10.1016/j.ajo.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn Y.J., Hong K.E., Yum H.R., et al. Characteristic clinical features associated with aggressive posterior retinopathy of prematurity. Eye (Lond) 2017;31(6):924–930. doi: 10.1038/eye.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourla D.H., Gonzales C.R., Valijan S., et al. Association of systemic risk factors with the progression of laser-treated retinopathy of prematurity to retinal detachment. Retina. 2008;28(3 Suppl):S58–S64. doi: 10.1097/IAE.0b013e31815075b0. [DOI] [PubMed] [Google Scholar]
- 6.Chen M., Citil A., McCabe F., et al. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology. 2011;99(2):125–132. doi: 10.1159/000312821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellström A., Ley D., Hansen-Pupp I., et al. New insights into the development of retinopathy of prematurity—importance of early weight gain. Acta Paediatr. 2010;99(4):502–508. doi: 10.1111/j.1651-2227.2009.01568.x. [DOI] [PubMed] [Google Scholar]
- 8.VanderVeen D.K., Martin C.R., Mehendale R., et al. Early nutrition and weight gain in preterm newborns and the risk of retinopathy of prematurity. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye J., Shen M., Huang S., et al. Visual acuity in pathological myopia is correlated with the photoreceptor myoid and ellipsoid zone thickness and affected by choroid thickness. Invest Ophthalmol Vis Sci. 2019;60(5):1714–1723. doi: 10.1167/iovs.18-26086. [DOI] [PubMed] [Google Scholar]
- 10.Govetto A., Sarraf D., Figueroa M.S., et al. Choroidal thickness in non-neovascular versus neovascular age-related macular degeneration: a fellow eye comparative study. Br J Ophthalmol. 2017;101(6):764–769. doi: 10.1136/bjophthalmol-2016-309281. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Tao Y. Choroidal structural changes correlate with severity of diabetic retinopathy in diabetes mellitus. BMC Ophthalmol. 2019;19(1):186. doi: 10.1186/s12886-019-1189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno T.A., O’Connell R.V., Chiu S.J., et al. Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci. 2013;54(6):4140–4147. doi: 10.1167/iovs.12-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erol M.K., Coban D.T., Ozdemir O., et al. Choroidal thickness in infants with retinopathy of prematurity. Retina. 2016;36(6):1191–1198. doi: 10.1097/IAE.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 14.Li X.Q., Munkholm A., Larsen M., Munch I.C. Choroidal thickness in relation to birth parameters in 11- to 12-year-old children: the Copenhagen Child Cohort 2000 Eye Study. Invest Ophthalmol Vis Sci. 2014;56(1):617–624. doi: 10.1167/iovs.14-15016. [DOI] [PubMed] [Google Scholar]
- 15.Fiess A., Christian L., Kolb-Keerl R., et al. Peripapillary choroidal thickness in former preterm and full-term infants aged from 4 to 10 years. Invest Ophthalmol Vis Sci. 2016;57(15):6548–6553. doi: 10.1167/iovs.16-20128. [DOI] [PubMed] [Google Scholar]
- 16.Mangalesh S., McGeehan B., Tai V., et al. Macular OCT characteristics at 36 weeks’ postmenstrual age in infants examined for retinopathy of prematurity. Ophthalmol Retina. 2021;5(6):580–592. doi: 10.1016/j.oret.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu S.J., Li X.T., Nicholas P., et al. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18(18):19413–19428. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng P.C., Ang I.L., Chiu R.W.K., et al. Host-response biomarkers for diagnosis of late-onset septicemia and necrotizing enterocolitis in preterm infants. J Clin Invest. 2010;120(8):2989–3000. doi: 10.1172/JCI40196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton T.R., Anderson D., Groh-Wargo S., et al. An attempt to standardize the calculation of growth velocity of preterm infants-evaluation of practical bedside methods. J Pediatr. 2018;196:77–83. doi: 10.1016/j.jpeds.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Fenton T.R., Griffin I.J., Hoyos A., et al. Accuracy of preterm infant weight gain velocity calculations vary depending on method used and infant age at time of measurement. Pediatr Res. 2019;85(5):650–654. doi: 10.1038/s41390-019-0313-z. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . 2nd ed. World Health Organization; Geneva: 2004. International Statistical Classification of Diseases and Related Health Problems. Tenth Revision. [PubMed] [Google Scholar]
- 22.World Health Organization . World Health Organization; Geneva: 2015. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. [PubMed] [Google Scholar]
- 23.American College of Obstetricians and Gynecology TFoNEAAoP . 2nd ed. American College of Obstetricians and Gynecologists; Washington, DC: 2014. Neonatal Encephalopathy and Neurologic Outcome. [Google Scholar]
- 24.Nuñez-Ramiro A., Aguar M., Cernada M., et al. Oxygen needs during resuscitation and surfactant to achieve stabilisation were independent risks factors for pulmonary interstitial emphysema in preterm infants. Acta Paediatr. 2018;107(1):28–32. doi: 10.1111/apa.14048. [DOI] [PubMed] [Google Scholar]
- 25.Buczynski B.W., Maduekwe E.T., O’Reilly M.A. The role of hyperoxia in the pathogenesis of experimental BPD. Semin Perinatol. 2013;37(2):69–78. doi: 10.1053/j.semperi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sola A., Golombek S.G., Montes Bueno M.T., et al. Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014;103(10):1009–1018. doi: 10.1111/apa.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Askie L.M. Meta-analysis of oxygenation saturation targeting trials: do infant subgroups matter? Clin Perinatol. 2019;46(3):579–591. doi: 10.1016/j.clp.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Pabelick C.M., Thompson M.A., Britt R.D., Jr. Effects of hyperoxia on the developing airway and pulmonary vasculature. Adv Exp Med Biol. 2017;967:179–194. doi: 10.1007/978-3-319-63245-2_11. [DOI] [PubMed] [Google Scholar]
- 29.Zhou T.E., Zhu T., Rivera J.C., et al. The inability of the choroid to revascularize in oxygen-induced retinopathy results from increased p53/miR-Let-7b activity. Am J Pathol. 2019;189(11):2340–2356. doi: 10.1016/j.ajpath.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Hosford G.E., Olson D.M. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L161–L168. doi: 10.1152/ajplung.00285.2002. [DOI] [PubMed] [Google Scholar]
- 31.Fujinaga H., Baker C.D., Ryan S.L., et al. Hyperoxia disrupts vascular endothelial growth factor-nitric oxide signaling and decreases growth of endothelial colony-forming cells from preterm infants. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1160–L1169. doi: 10.1152/ajplung.00234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao Z., Dorfman A.L., Seshadri S., et al. Choroidal involution is a key component of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2011;52(9):6238–6248. doi: 10.1167/iovs.10-6742. [DOI] [PubMed] [Google Scholar]
- 33.Park K.A., Oh S.Y. Analysis of spectral-domain optical coherence tomography in preterm children: retinal layer thickness and choroidal thickness profiles. Invest Ophthalmol Vis Sci. 2012;53(11):7201–7207. doi: 10.1167/iovs.12-10599. [DOI] [PubMed] [Google Scholar]
- 34.van der Bel R., Çalişkan M., van Hulst R.A., et al. Blood pressure increase during oxygen supplementation in chronic kidney disease patients is mediated by vasoconstriction independent of baroreflex function. Front Physiol. 2017;8:186. doi: 10.3389/fphys.2017.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajima Y., Takuwa H., Nishino A., et al. Cerebral hemodynamic response to acute hyperoxia in awake mice. Brain Res. 2014;1557:155–163. doi: 10.1016/j.brainres.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 36.Wu C., Löfqvist C., Smith L.E., et al. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2012;130(8):992–999. doi: 10.1001/archophthalmol.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binenbaum G. Algorithms for the prediction of retinopathy of prematurity based on postnatal weight gain. Clin Perinatol. 2013;40(2):261–270. doi: 10.1016/j.clp.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Löfqvist C., Andersson E., Sigurdsson J., et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124(12):1711–1718. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 39.Hellström A., Hård A.L., Engström E., et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123(4):e638–e645. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- 40.Hack M., Flannery D.J., Schluchter M., et al. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346(3):149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 41.Marneros A.G., Fan J., Yokoyama Y., et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167(5):1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dice J.E., Bhatia J. Patent ductus arteriosus: an overview. J Pediatr Pharmacol Ther. 2007;12(3):138–146. doi: 10.5863/1551-6776-12.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoll B.J., Hansen N.I., Adams-Chapman I., et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 44.Gagliardi L., Rusconi F., Bellù R., Zanini R. Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics. 2014;134(1):e154–e161. doi: 10.1542/peds.2013-3898. [DOI] [PubMed] [Google Scholar]
- 45.Zayed M.A., Uppal A., Hartnett M.E. New-onset maternal gestational hypertension and risk of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2010;51(10):4983–4988. doi: 10.1167/iovs.10-5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozkan H., Cetinkaya M., Koksal N., et al. Maternal preeclampsia is associated with an increased risk of retinopathy of prematurity. J Perinat Med. 2011;39(5):523–527. doi: 10.1515/jpm.2011.071. [DOI] [PubMed] [Google Scholar]
- 47.Shulman J.P., Weng C., Wilkes J., et al. Association of maternal preeclampsia with infant risk of premature birth and retinopathy of prematurity. JAMA Ophthalmol. 2017;135(9):947–953. doi: 10.1001/jamaophthalmol.2017.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furuya M., Ishida J., Inaba S., et al. Impaired placental neovascularization in mice with pregnancy-associated hypertension. Lab Invest. 2008;88(4):416–429. doi: 10.1038/labinvest.2008.7. [DOI] [PubMed] [Google Scholar]
- 49.Morrow L.A., Wagner B.D., Ingram D.A., et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am J Respir Crit Care Med. 2017;196(3):364–374. doi: 10.1164/rccm.201612-2414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isayama T., Shah P.S., Ye X.Y., et al. Adverse impact of maternal cigarette smoking on preterm infants: a population-based cohort study. Am J Perinatol. 2015;32(12):1105–1111. doi: 10.1055/s-0035-1548728. [DOI] [PubMed] [Google Scholar]
- 51.Hirabayashi H., Honda S., Morioka I., et al. Inhibitory effects of maternal smoking on the development of severe retinopathy of prematurity. Eye (Lond) 2010;24(6):1024–1027. doi: 10.1038/eye.2009.263. [DOI] [PubMed] [Google Scholar]
- 52.Hudalla H., Bruckner T., Pöschl J., et al. Maternal smoking as an independent risk factor for the development of severe retinopathy of prematurity in very preterm infants. Eye (Lond) 2021;35(3):799–804. doi: 10.1038/s41433-020-0963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.