Keywords: baroreflex sensitivity, blood pressure, endothelin-1, hypoxia, muscle sympathetic nerve activity
Abstract
Repeat exposures to low oxygen (intermittent hypoxia, IH), like that observed in sleep apnea, elicit increases in muscle sympathetic nerve activity (MSNA) and blood pressure (BP) in men. Endothelin (ET) receptor antagonists can attenuate the sympathetic and BP response to IH in rodents; whether these data translate to humans are unclear. We hypothesized that ET-receptor antagonism would ameliorate any rise in MSNA and BP following acute IH in humans. Twelve healthy men (31 ± 1 yr) completed two visits (control, bosentan) separated by at least 1 wk. MSNA, BP, and baroreflex sensitivity (modified Oxford) were assessed during normoxic rest before and following 30 min of IH. The midpoint (T50) for each individual’s baroreflex curve was calculated. Acute IH increased plasma ET-1 (P < 0.01), MSNA burst frequency (P = 0.03), and mean BP (P < 0.01). There was no effect of IH on baroreflex sensitivity (P = 0.46), although an increase in T50 was observed (P < 0.01). MSNA burst frequency was higher (P = 0.04) and mean BP (P < 0.01) was lower following bosentan treatment compared with control. There was no effect of bosentan on baroreflex sensitivity (P = 0.53), although a lower T50 was observed on the bosentan visit (P < 0.01). There was no effect of bosentan on increases in MSNA (P = 0.81) or mean BP (P = 0.12) following acute IH. Acute IH results in an increase in ET-1, MSNA, and BP in healthy young men. The effect of IH on MSNA and BP is not attenuated following ET-receptor inhibition. Present data suggest that acute IH does not increase MSNA or BP through activation of ET-receptors in healthy young men.
NEW & NOTEWORTHY Repeat exposures to low oxygen (intermittent hypoxia, IH) elicit increases in muscle sympathetic nerve activity (MSNA) and blood pressure (BP) in men. Endothelin (ET) receptor antagonists can attenuate the sympathetic and BP response to IH in rodents; whether these data translate to humans were unclear. We show acute IH results in an increase in ET-1, MSNA, and BP in healthy young men; however, the effect of IH on MSNA and BP does not occur through activation of ET-receptors in healthy young men.
INTRODUCTION
Patients with sleep apnea exhibit persistent activation of the sympathetic nervous system and an augmented risk of hypertension and other cardiovascular complications (1–3). Recurrent apneas during sleep, a hallmark of sleep apnea, result in repeated reductions in arterial oxygen saturation (i.e., intermittent hypoxia). These transient exposures to low oxygen during sleep elicit persistent increases in sympathetic nervous system activity (1, 2). Direct relationships between intermittent hypoxia, sympathoexcitation, and hypertension have been established in animal models; however, the contributing mechanisms, particularly in humans, remain unclear.
Endothelin-1 (ET-1) is produced by vascular endothelial cells, neurons within the central nervous system, and Type 1 glomus cells within the carotid bodies (6, 7). Several studies support an increase in plasma ET-1 levels in patients with sleep apnea (8–12) and in healthy men following hypoxia exposure (13). With this, upregulation of ET-1 and ETA receptors (which bind ET-1 and initiate physiological effects) have been linked to increases in hypoxic sensitivity of the carotid body, baroreceptor dysfunction, and the development of hypertension after intermittent hypoxia in rats (2, 14, 15). Perhaps not surprisingly, ETA receptor antagonists were subsequently shown to normalize blood pressure regulatory mechanisms in male rats exposed to intermittent hypoxia (2, 15, 16), due in part to attenuated vascular (16) and neural (15) signaling. Thus, it is reasonable to propose agents that reduce ET-1 and/or block its receptors may ameliorate sympathoexcitation and blood pressure changes induced by intermittent hypoxia in adult men; previously published data in humans support an increase in sympathetic nervous system activity (17–20) and blood pressure (17) following acute (20–30 min) exposure to intermittent hypoxia. However, the contribution of ET-1 to intermittent hypoxia-mediated changes in the sympathetic nervous system activity and blood pressure has not been tested in humans. With this information in mind, we hypothesized that functional ET receptors are necessary to achieve a rise in muscle sympathetic nerve activity (MSNA) and arterial blood pressure following acute intermittent hypoxia in healthy young men.
METHODS
Participants
All experiments and procedures were approved by the Institutional Review Board at the Mayo Clinic (16-004563) and University of Missouri (2007973), were in accordance with institutional guidelines, conformed to the Declaration of Helsinki, and were part of a registered clinical trial (NCT05146089). Data from the control visit, including MSNA and blood pressure responses, were published previously from a subset of participants (21, 22). Data from the bosentan visit testing unrelated hypotheses were published previously from a subset of participants (23). Only healthy, young (<45 yr of age), nonobese (body mass index <30 kg/m2), and nonsmoking men were included in the present investigation. Given risks associated with the study intervention (i.e., bosentan) in women of childbearing age [reduced contraceptive effectiveness and Pregnancy Category X (Actelion Pharmaceuticals (24)], as well as previous data from our group showing women do not exhibit an increase in blood pressure following exposure to intermittent hypoxia (21), this intervention was conducted in men only. Participants with acute and/or chronic disease, as well as those taking any medications were excluded.
Informed consent was obtained from all participants during a screening visit, followed by resting blood pressure (automatic sphygmomanometry), medical history, and fasting blood chemistries to confirm normal blood pressure and liver function before the intervention (per clinical guidelines for the use of bosentan). Participants were given an overnight pulse oximeter (Nonin WristOx2, Model 3150; Nonin Medical Inc; Plymouth, MN) to wear during sleep with oxygen saturation and heart rate monitored continuously. Analysis of the overnight data was conducted (nVISION software, v. 6.5.1; Nonin Medical Inc; Plymouth, MN) and the oxygen desaturation index (ODI) was calculated. A desaturation was defined as a drop in oxygen saturation (SpO2) by at least 4% and results were adjusted for suspected artifacts (i.e., removal of the device to use the restroom). Participants were required to have an adjusted index <10 events/h to participate in the study to avoid the potential effects of undiagnosed sleep apnea.
Instrumentation
Participants were asked to refrain from alcohol, caffeine, and exercise for 24 h before any testing. On the study day, participants arrived in the morning after an overnight fast and rested supine for equipment instrumentation, which included an electrocardiogram (lead II) and finger pulse oximeter. In nine individuals, a 20-gauge, 5-cm catheter was placed in the brachial artery under aseptic conditions after local anesthesia for beat-by-beat arterial blood pressure measurement and periodic blood sampling. In three individuals, arterial blood pressure was assessed noninvasively using finger photoplethysmography (Human NIBP, ADInstruments) calibrated to upper arm sphygmomanometry and periodic blood samples were obtained via an intravenous catheter. All participants had blood pressures within normal ranges on the screening visit. Notably, the use of an arterial catheter on the study day has the potential for underdamping/resonance of the blood pressure tracing, which can cause blood pressure to read artificially high (25, 26). Stroke volume, cardiac output, and total peripheral resistance were estimated from the arterial blood pressure waveform using the Modelflow method (LabChart, ADInstruments), which incorporates age and sex.
Muscle Sympathetic Nerve Activity
MSNA was measured using the technique of microneurography (n = 9: 662 C-3, Bioengineering of University of Iowa; n = 3: Neuro Amp EX, ADInstruments). Multiunit MSNA was recorded with a tungsten microelectrode placed percutaneously into the peroneal nerve, posterior to the fibular head under direct two-dimensional (2-D) ultrasound guidance (29). A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses, and no afferent neural response was evoked by skin stimuli. A reference electrode was positioned subcutaneously ∼4 cm from the recording electrode. The recorded signal was amplified, band-pass filtered (700–2,000 Hz), rectified, and integrated (time constant 0.1 s). Sympathetic neurograms were analyzed according to the study by Hamner and Taylor (30), as implemented by Ensemble (Ensemble-C, Elucimed LTD). An artifact threshold of 0.30 ± 0.01 and burst threshold of 0.045 ± 0.001 was applied. MSNA was expressed as burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats).
Acute Intermittent Hypoxia
Participants were instrumented with a mask connected to a non-rebreathing valve. Breath-by-breath tidal volume (n = 9: Universal Ventilation meter, VacuMed, Ventura, CA; n = 3: Unheated pneumotachograph with differential pressure amplifier, PA-1, Series 1110, Hans Rudolph, Shawnee, KS), respiratory rate, and inspired/expired gases (n = 9: GE Datex-Ohmeda Cardiocap/5, GE Healthcare; n = 3: Gemini 14-10000 Respiratory Monitor, CWE Inc.) were monitored continuously and ventilation was expressed in ambient temperature, pressure saturated (ATPS). Intermittent hypoxia was achieved by alternating between a hypercapnic hypoxic (5% oxygen, 3% carbon dioxide) gas cylinder (30 s) and room air (21% oxygen; 90–120 s) to target approximately 15 hypoxic events over 30 min while avoiding hypocapnia typical of hypoxia-induced hyperventilation. A 50-L meteorological balloon served as a volume reservoir. This technique results in arterial desaturation events similar to that observed in mild-to-moderate sleep apnea (5%–7% desaturation, 15–30 events/h) (31). A hypoxic ventilatory response test was conducted immediately before and following acute intermittent hypoxia. Tests took ∼15 min to complete, at which time variable levels of hypoxemia were achieved using two to six breaths of 5% oxygen/3% carbon dioxide followed by room air (33). Chemosensitivity was assessed as the slope of the regression line for minute ventilation versus SpO2.
Baroreflex Sensitivity
Before and following the hypoxic ventilatory response tests, individuals completed a 5-min quiet resting period. An intravenous bolus of sodium nitroprusside (100 µg) was then administered, followed by an intravenous bolus of phenylephrine (150 µg). This approach (modified Oxford test) is considered the gold standard to examine the sensitivity of the arterial baroreflex (35). Data were available during the modified Oxford test from a subset of individuals (n = 9). During each modified Oxford test, diastolic blood pressure fell 18 ± 1 mmHg (range: 11–29 mmHg) and rose 12 ± 1 mmHg (range: 4–24 mmHg) from baseline levels, consistent with previous work (35, 37, 38). Data from a single modified Oxford test conducted before intermittent hypoxia and one test following intermittent hypoxia were analyzed for measures of cardiac and sympathetic baroreflex sensitivity (Ensemble, Elucimed LTD). For measures of arterial baroreflex sensitivity, diastolic blood pressures were assigned 3-mmHg bins and for each bin, the corresponding MSNA burst incidence (bursts/100 heartbeats) was determined (39). Arterial baroreflex sensitivity was quantified by plotting MSNA burst incidence against mean diastolic pressure for each blood pressure bin. Each data point was weighted according to the number of times the particular value occurred (39). The value of the slope determined via linear regression analysis provided the arterial baroreflex sensitivity for each participant. The midpoint (T50) for each individual’s curve was calculated, representing the diastolic pressure (mmHg) at which there was a 50% likelihood of a burst occurring. For cardiac baroreflex sensitivity, sequences used for systolic blood pressure and R-R interval signals were required to rise or fall in the same direction for at least three consecutive beats (40, 41). Values belonging to the identified sequences were formed into xy-pairs and a regression curve was fitted (R value ≥0.8 acceptance level), with the slope of the curve equaling cardiac baroreflex sensitivity (ms/mmHg). Analysis was performed separately for ascending (up-up) and descending (down-down) sequences, and the results were pooled.
ET Receptor Inhibition
Participants completed two study visits (control, bosentan), separated by a minimum of 1 wk (range: 7–62 days; average = 27 ± 5 days) with visits completed at the same time of day. Visits were not randomized and control visits were completed first; thus study condition was not blinded. For 3 days before the second (bosentan) visit, participants consumed 62.5 mg twice a day orally at home for a total dose of 125 mg/day. A final oral dose (65.2 mg) was taken the morning of the study and experimental sessions were performed 3 h after intake of the final dose. This dosing scheme was based on previous work showing peak plasma concentrations of bosentan are achieved after 3–5 h, the half-life is 5 h, and steady-state concentrations are achieved by 3 days (24, 42). A comparable dose of bosentan has been shown previously to effectively achieve ET-receptor blockade (43, 44) and has thus been applied by other groups asking similar research questions (45–47). Compliance with bosentan was confirmed on the study day via self-report. In addition, plasma ET-1 was assessed using commercially available kits according to the manufacturer’s protocols at the Mayo Clinic (n = 9: QET00B, R&D Systems, Inc.; Minneapolis, MN) and the University of Missouri (n = 3: DET100, R&D Systems, Inc.; Minneapolis, MN).
Data Analysis
Data were recorded at 1,000–10,000 Hz using a computer data acquisition system (PowerLab, ADInstruments) and stored for offline analysis (27). Normoxic resting data were analyzed over an approximate 5-min baseline period before and following acute intermittent hypoxia. Statistical analysis was completed using SigmaPlot 14.0 (Systat Software, Inc.) and P < 0.05 was considered statistically significant. The effect of intermittent hypoxia (pre, post), drug (control, bosentan), and the interaction between intermittent hypoxia and drug on main outcome variables (MSNA, mean blood pressure) was assessed using a two-way repeated-measures analysis of variance (ANOVA). Normality was assessed using the Shapiro–Wilk test. Pairwise comparisons were done with the Holm–Sidak method. Data are reported as means ± SE unless otherwise noted. Based on data from Jouett et al. (48) supporting an attenuation of the MSNA (5 bursts/min) and mean blood pressure (3 mmHg) response to intermittent hypoxia following pharmacotherapy, we determined paired data would be required from 11 individuals to detect a significant effect of bosentan with the power of 0.80 and α of 0.05. Hypoxic ventilatory response and baroreflex sensitivity data were secondary outcomes; thus, the study was not statistically powered to detect differences in these outcomes.
RESULTS
Results from repeat visits in 12 young (31 ± 1 yr), normotensive (screen visit: 114 ± 2/71 ± 2 mmHg), nonobese (26 ± 1 kg/m2) men without sleep apnea (oxygen desaturation index 2.4 ± 0.7 events/h) are presented (Fig. 1). Based on clinical guidelines for the use of bosentan, participants were required to have normal liver function before the intervention [aspartate transaminase: 26.4 ± 1.8 IU/L; alanine transaminase: 27.7 ± 1.8 U/L; alkaline phosphatase: 66.0 ± 4.5 IU/L; bilirubin (total): 0.6 ± 0.1 mg/dL; albumin: 4.6 ± 0.1 g/dL; protein (total): 7.0 ± 0.1 g/dL].
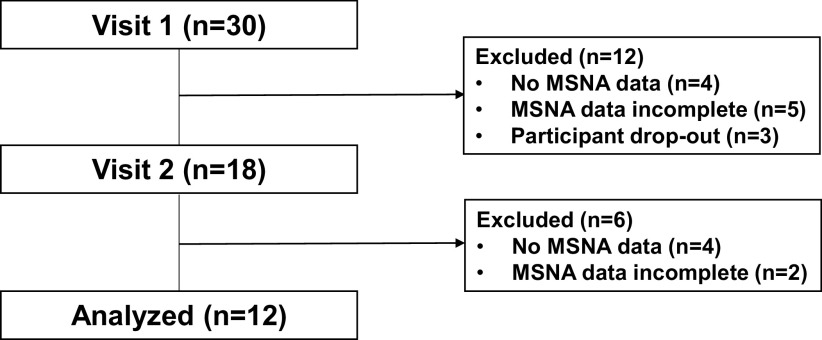
Figure 1.
MSNA data flowchart. MSNA, muscle sympathetic nerve activity.
Representative data are shown in Fig. 2. Acute intermittent hypoxia resulted in repeated reductions in arterial SpO2 (control: baseline 98 ± 1% vs. nadir 92 ± 1%; bosentan: baseline 98 ± 1% vs. nadir 91 ± 1%) and plasma ET-1 was significantly increased following acute intermittent hypoxia (Fig. 3A). The increase in plasma ET-1 did not differ by condition (control Δ: 0.36 ± 0.10, bosentan Δ: 0.17 ± 0.09; P = 0.73).
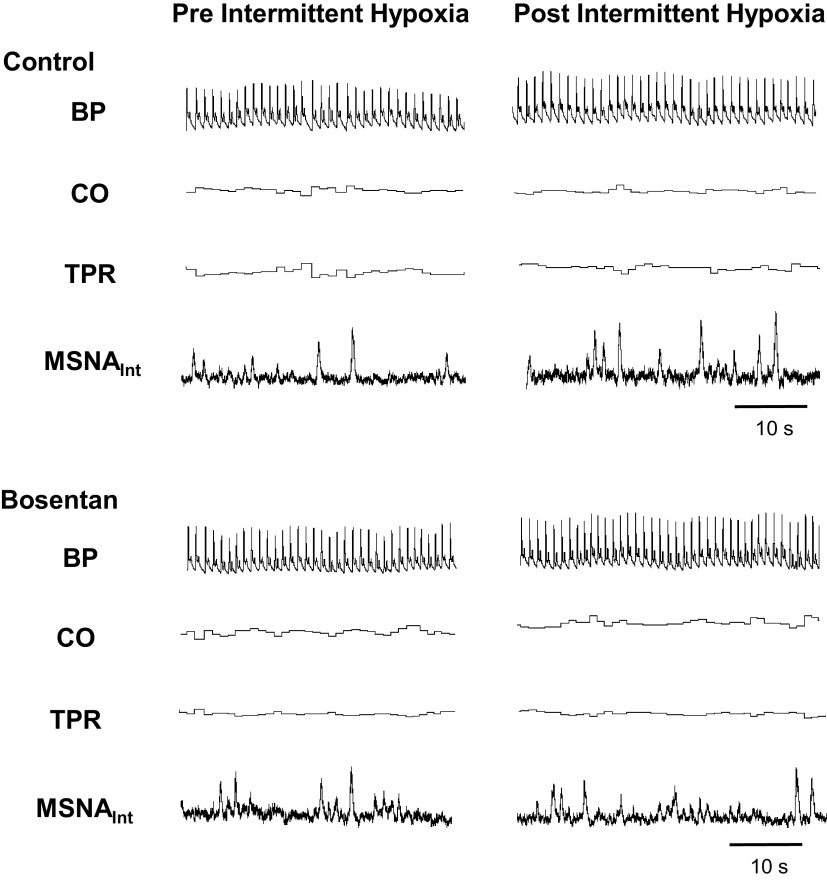
Figure 2.
Steady-state normoxia before (left) and following (right) acute intermittent hypoxia (IH). Representative data from a male participant (26 yr old, BMI: 28 kg/m2). BP, blood pressure; BMI, body mass index, CO, cardiac output; MSNA, muscle sympathetic nerve activity; TPR, total peripheral resistance.
Figure 3.
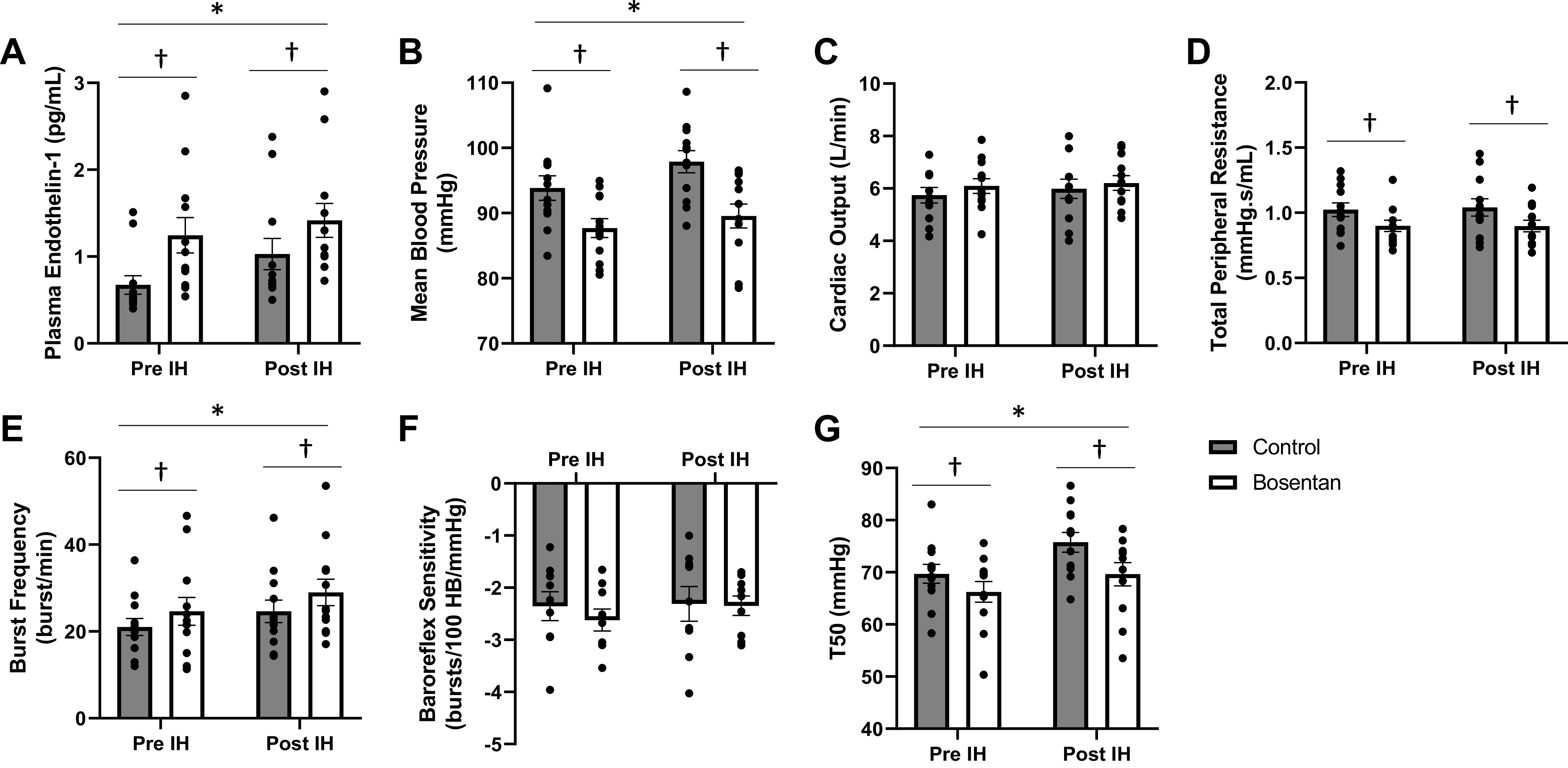
Physiological response to acute intermittent hypoxia and impact of bosentan. Data are from n = 12 men unless noted (baroreflex sensitivity and T50, n = 9). Two-way repeated-measures ANOVA testing the main effect of IH (pre, post) and drug (control, bosentan) and the interaction of IH and drug (normality: Shapiro–Wilk, equal variance: Brown–Forsythe). Pairwise multiple comparison (Holm–Sidak). *P < 0.05 main effect of IH. †P < 0.05 main effect of drug. IH, intermittent hypoxia. A: plasma endothelin-1. B: mean blood pressure. C: cardiac output. D: total peripheral resistance. E: MSNA burst frequency. F: arterial baroreflex sensitivity. G: T50.
Neurocardiovascular Control following Intermittent Hypoxia
As shown previously from this cohort (21, 22), intermittent hypoxia resulted in an increase in diastolic and mean blood pressure (main effect of hypoxia, P < 0.01) with no effect on systolic blood pressure (main effect of hypoxia, P = 0.16) (Table 1, Fig. 3B). There was no observable effect of intermittent hypoxia on heart rate, stroke volume, cardiac output, or total peripheral resistance (main effect of hypoxia, P > 0.05) (Table 1, Fig. 3, C and D). MSNA burst frequency and burst incidence increased following intermittent hypoxia (main effect of hypoxia, P < 0.05) (Table 2, Fig. 3E). There was no effect of intermittent hypoxia on arterial baroreflex sensitivity (main effect of hypoxia, P = 0.46), although an increase in the T50 was observed following the acute exposure to intermittent hypoxia (main effect of hypoxia, P < 0.01) (Table 2, Fig. 3, F and G). There was no effect of intermittent hypoxia on cardiac baroreflex sensitivity (main effect of hypoxia, P = 0.92; Table 2).
Table 1.
Effect of acute intermittent hypoxia on blood pressure regulation and impact of bosentan
| Control |
Bosentan |
P Values |
|||||
|---|---|---|---|---|---|---|---|
| Pre IH | Post IH | Pre IH | Post IH | IH | Drug | Interaction | |
| Systolic blood pressure, mmHg | 139 ± 3 | 143 ± 2 | 137 ± 3 | 138 ± 3 | 0.16 | 0.35 | 0.18 |
| Diastolic blood pressure, mmHg | 73 ± 1 | 76 ± 2 | 68 ± 1 | 69 ± 2 | <0.01 | <0.01 | 0.13 |
| Mean blood pressure, mmHg | 94 ± 2 | 98 ± 2 | 88 ± 2 | 90 ± 2 | <0.01 | <0.01 | 0.12 |
| Heart rate, beats/min | 57 ± 2 | 59 ± 3 | 58 ± 2 | 60 ± 2 | 0.19 | 0.66 | 0.39 |
| Stroke volume, mL/beat | 103 ± 4 | 103 ± 4 | 107 ± 4 | 106 ± 4 | 0.92 | 0.30 | 0.76 |
| Cardiac output, L/min | 5.6 ± 0.4 | 5.7 ± 0.5 | 5.8 ± 0.3 | 6.0 ± 0.3 | 0.20 | 0.27 | 0.45 |
| Total peripheral resistance, mmHg·s/mL | 1.02 ± 0.05 | 1.04 ± 0.07 | 0.90 ± 0.04 | 0.90 ± 0.04 | 0.73 | 0.02 | 0.66 |
Data are reported as means ± SE from n = 12 men. Two-way repeated-measures ANOVA testing the main effect of IH (pre, post) and drug (control, bosentan) and the interaction of IH and drug (normality: Shapiro–Wilk, equal variance: Brown–Forsythe). Pairwise multiple comparison (Holm–Sidak). IH, intermittent hypoxia. Bold type signifies P < 0.05.
Table 2.
Effect of acute intermittent hypoxia on autonomic regulation of cardiovascular function and impact of bosentan
| Control |
Bosentan |
P Values |
|||||
|---|---|---|---|---|---|---|---|
| Pre IH | Post IH | Pre IH | Post IH | IH | Drug | Interaction | |
| MSNA burst frequency, bursts/min | 21.0 ± 1.9 | 24.6 ± 2.6 | 24.6 ± 3.2 | 29.0 ± 3.1 | 0.03 | 0.04 | 0.81 |
| MSNA burst incidence, bursts/100 heart beats | 37.3 ± 2.9 | 42.5 ± 4.3 | 39.5 ± 4.5 | 49.2 ± 4.7 | <0.01 | 0.17 | 0.19 |
| Baroreflex sensitivity, bursts/100 heart beats/mmHg | −2.4 ± 0.3 | −2.3 ± 0.3 | −2.6 ± 0.2 | −2.3 ± 0.2 | 0.46 | 0.53 | 0.39 |
| Baroreflex sensitivity, ms/mmHg | 14.5 ± 9.1 | 13.7 ± 4.8 | 11.2 ± 3.7 | 11.8 ± 4.6 | 0.92 | 0.11 | 0.53 |
| T50, mmHg | 69.7 ± 1.8 | 75.8 ± 1.9 | 66.2 ± 2.0 | 69.6 ± 2.2 | <0.01 | <0.01 | 0.23 |
Data are reported as means ± SE from n = 12 men unless noted (baroreflex sensitivity and T50, n = 9). Two-way repeated-measures ANOVA testing the main effect of IH (pre, post) and drug (control, bosentan) and the interaction of IH and drug (normality: Shapiro–Wilk, equal variance: Brown–Forsythe). Pairwise multiple comparison (Holm–Sidak). IH, intermittent hypoxia; MSNA, muscle sympathetic nerve activity. Bold type signifies P < 0.05.
Role for ET-1 in the Neurocardiovascular Response to Intermittent Hypoxia
Diastolic and mean blood pressures were lower during the bosentan visit compared with control (main effect of bosentan, P < 0.01) (Table 1, Fig. 3B). There was no effect of bosentan on systolic blood pressure, heart rate, stroke volume, or cardiac output (main effect of bosentan, P > 0.05) (Table 1, Fig. 3C). Total peripheral resistance was lower on the bosentan visit when compared with control (main effect of bosentan, P = 0.02) (Table 1, Fig. 3D). MSNA burst frequency was higher on the bosentan visit compared with control (main effect of bosentan, P = 0.04) (Table 2, Fig. 3E). There was no effect of bosentan on arterial (main effect of bosentan, P = 0.53) nor cardiac (main effect of bosentan, P = 0.11) baroreflex sensitivity, although a lower T50 was observed on the bosentan visit (main effect of bosentan, P < 0.01) (Table 2, Fig. 3, F and G). Contrary to our hypothesis, there was no effect of bosentan on the change in main outcome variables (MSNA, blood pressure) following acute intermittent hypoxia (interaction of hypoxia and bosentan, P > 0.05) (Tables 2 and 3).
Table 3.
Effect of acute intermittent hypoxia on ventilation and impact of bosentan
| Control |
Bosentan |
P Values |
|||||
|---|---|---|---|---|---|---|---|
| Pre IH | Post IH | Pre IH | Post IH | IH | Drug | Interaction | |
| Tidal volume, mL/breath | 573 ± 66 | 618 ± 69 | 642 ± 103 | 623 ± 80 | 0.81 | 0.49 | 0.28 |
| Breathing frequency, breaths/min | 11.3 ± 0.7 | 12.3 ± 0.6 | 11.0 ± 0.9 | 11.7 ± 0.9 | 0.03 | 0.43 | 0.61 |
| Minute ventilation, L/min | 6.1 ± 0.4 | 7.4 ± 0.7 | 6.2 ± 0.7 | 6.7 ± 0.7 | 0.12 | 0.64 | 0.12 |
| Hypoxic ventilatory response, L/min/% | −0.81 ± 0.10 | −0.79 ± 0.07 | −0.80 ± 0.19 | −0.71 ± 0.11 | 0.57 | 0.74 | 0.63 |
Data are reported as means ± SE from n = 12. Two-way repeated-measures ANOVA testing the main effect of IH (pre, post) and drug (control, bosentan) and the interaction of IH and drug (normality: Shapiro–Wilk, equal variance: Brown–Forsythe). Pairwise multiple comparison (Holm–Sidak). IH, intermittent hypoxia. Bold type signifies P < 0.05.
Role for ET-1 in the Respiratory Response to Intermittent Hypoxia
Intermittent hypoxia resulted in an increase in breathing frequency (main effect of hypoxia, P = 0.03) with no effect on the tidal volume or minute ventilation (main effect of hypoxia: P = 0.81 and P = 0.12, respectively) (Table 3). There was no effect of bosentan on breathing frequency, tidal volume, or minute ventilation (main effect of bosentan, P > 0.05; interaction of hypoxia and bosentan, P > 0.05). There was no effect of intermittent hypoxia or bosentan on measures of peripheral chemosensitivity (i.e., hypoxic ventilatory response) (main effect of hypoxia, P = 0.57; main effect of bosentan, P = 0.74; interaction of hypoxia and bosentan, P = 0.63).
DISCUSSION
Herein we reiterate previous results from our group (21, 22) and confirm results from others (17, 49–51) showing intermittent hypoxia elicits an increase in MSNA, blood pressure, and plasma ET-1 in healthy young men. We expand these data and show the nonspecific ET-receptor antagonist, bosentan, lowers resting mean arterial blood pressure and total peripheral resistance. Despite the increase in ET-1 following intermittent hypoxia, any effect of intermittent hypoxia on MSNA and blood pressure was unaffected by ET-receptor antagonism with bosentan. Together, these findings suggest that 1) functional ET receptors contribute, at least in part, to the maintenance of resting blood pressure in the healthy young men studied and 2) functional ET receptors do not appear to be necessary to achieve a rise in MSNA and arterial blood pressure following acute intermittent hypoxia in healthy young men.
Intermittent hypoxia in humans elicits increases in MSNA, which persist beyond the period of hypoxia (17–20, 49–51). Confirming previous work (17, 49–51), under control conditions, we observed an increase in MSNA and blood pressure in healthy young men following acute intermittent hypoxia. We further observed an upward shift in the T50 following acute intermittent hypoxia that was independent of a change in arterial baroreflex gain. These data are consistent with baroreflex resetting following acute intermittent hypoxia exposure (52, 53), which has been previously attributed to the development of hypertension in male rodent models of sleep apnea (54).
Nearly a decade ago, Peng et al. (15) in their study showed ET-1 plays an important role in intermittent hypoxia-mediated changes in baroreflex function and a number of studies have linked ET-1 to neural control of blood pressure and the development of hypertension following intermittent hypoxia in rats (14, 15, 55, 56). Accordingly, data have shown ETA receptor antagonists can normalize blood pressure regulatory mechanisms in male rats exposed to intermittent hypoxia (2, 15, 16). In the present investigation, we observed a significant increase in plasma ET-1 following acute hypoxic exposure in healthy young men, which is consistent with data from others (13); however, whether ET-1 is directly related to the rise in MSNA and blood pressure following intermittent hypoxia was previously unknown.
Patients with sleep apnea exhibit greater plasma ET-1 levels compared with control (8–12); this increase in ET-1 is correlated with the severity of nocturnal hypoxia (8, 9) and is attenuated following treatment with continuous positive airway pressure (9, 57). Together, these observations support a role of persistent, recurrent episodes of hypoxemia in elevated circulating ET-1 in humans. Consistent with this, acute hypoxia has been shown previously to increase plasma ET-1 in healthy men (13)—and this finding is supported by present data. Recent pilot work in men and women with untreated sleep apnea suggests the nonspecific ET-1 receptor antagonist, bosentan, may be as effective as continuous positive airway pressure in reducing clinic blood pressure (46). Furthermore, bosentan was able to attenuate the blood pressure response to hypoxia in men with sleep apnea (58). Thus, it is reasonable to propose agents that reduce ET-1 and/or block its receptors may ameliorate sympathoexcitation and blood pressure changes induced by intermittent hypoxia in humans; however, the contribution of ET-1 to intermittent hypoxia-mediated changes in MSNA and blood pressure in humans was previously unknown.
Bosentan is a nonspecific ET-receptor antagonist, and thus has physiological effects associated with both ETA and ETB receptors (59). The neurovascular effects of ET-1 are primarily associated with action at the ETA receptors. However, ETB receptors (in addition to other roles) are important in scavenging ET-1. With this, an elevation in plasma ET-1 following 3 days of oral bosentan administration supports participant compliance with the at-home drug regimen and effective receptor blockade (43, 44). Using doses similar to those presently, data from Gujic et al. (45) found no effect of oral bosentan on baseline blood pressure in healthy young men. Our data disagree with this finding and show 3 days of oral bosentan is sufficient to lower total peripheral resistance, and subsequently blood pressure, in healthy young men. In addition, we observed a reduction in the T50 with no observable change in arterial baroreflex gain under normoxic resting conditions following bosentan treatment. In this context, we also observed greater resting MSNA on the bosentan visit, which was likely a compensatory, baroreflex-mediated response due to lower blood pressures. As noted within the methods and shown in Table 1, the use of an arterial catheter can cause blood pressure to read artificially high (25, 26). This error is systematic and primarily observed in systolic pressures. Importantly, whether blood pressures were collected via automated sphygmomanometry (control: 117 ± 2/66 ± 4 mmHg; bosentan: 113 ± 3/62 ± 1 mmHg) or from beat-to-beat measures (Table 1), conclusions are maintained. Rather, discrepancies between studies may be due to the participants studied, given Gujic et al. (45) report data from a cohort of younger men (average age: 23 yr; range 20–29 yr) than those studied presently. Together, we conclude functional ET receptors contribute, at least in part, to the maintenance of resting blood pressure in the men studied.
Two recent pilot studies in men and women with sleep apnea showed bosentan to be effective at reducing office blood pressure (46), as well as attenuating the blood pressure response to hypoxia in men (58)—although the exact mechanisms are unclear. It is reasonable to speculate this improvement in blood pressure is due to the effects of ET-1 on neurovascular control. Consistent with this, ETA receptor antagonists can lower sympathetic nervous system activity and normalize blood pressure in rats exposed to intermittent hypoxia (2, 15, 16). However, Gujic et al. (45) in their study found no effect of oral bosentan on the sympathetic or blood pressure response to acute steady-state hypoxia in the men studied. Important to this point, mechanisms responsible for the physiological response to acute steady-state (5 min continuous) hypoxia likely differ from those integral to the response to short (30 s), intermittent exposures (60).
Increases in ET-1 in the setting of intermittent hypoxia have been observed and may be due to reactive oxygen species (ROS)-mediated effects on the endothelin-converting enzyme (15). Notably, attenuation of systemic ROS (N-acetylcysteine) can blunt the MSNA response to acute intermittent hypoxia in healthy young men and women (48). With this information in mind, we hypothesized that a nonspecific ET receptor antagonist (bosentan) would similarly attenuate any rise in MSNA and arterial blood pressure following acute intermittent hypoxia. However, it appears antioxidant treatment (48), but not ET-1 receptor antagonism per se, can prevent MSNA-raising effects of intermittent hypoxia in healthy men. Thus, we conclude that ROS-dependent recruitment of ET-1 signaling is not obligatory for the neurovascular response to acute intermittent hypoxia in humans. Other groups have observed an attenuated rise in blood pressure during steady-state hypoxia with bosentan in men with sleep apnea, despite no measurable effect on the increase in MSNA (58). Taken together, any effect of bosentan on blood pressure and total peripheral resistance following intermittent hypoxia is likely the result of other influences that may alter the relationship between MSNA and vasoconstriction (e.g., nitric oxide bioavailability, norepinephrine kinetics). It is also possible the effect of bosentan is most beneficial at the level of the vascular smooth muscle and endothelial cells where ET-1, via the ETA receptor, elicits vasoconstriction.
Experimental Considerations
There are several important limitations that should be acknowledged. First, study visits were not randomly assigned and thus investigators and study participants were not blinded to conditions. This decision was based on the invasiveness of the microneurography procedure (i.e., if MSNA data were not available from the control visit, there was no need to conduct microneurography on the bosentan visit) and the need for study drug to be mailed to participants at home as part of the Bosentan Risk Evaluation and Mitigation Strategy (REMS) program. However, it is possible that this decision could have affected findings in unexpected ways. Second, only one modified Oxford test was conducted before and following intermittent hypoxia, whereas repeated sequential tests are recommended under each condition to minimize potential variability in results (35). Third, the intermittent hypoxia protocol used presently was of a relatively short (∼30 min) duration and resulted in a mild hypoxic exposure (average nadir SpO2 ∼87%). This protocol duration was selected based on its published ability to elicit increases in blood pressure (17) and MSNA (17–20), which are attenuated with both angiotensin II receptor blockade (49) and ROS inhibition (48). With this, it cannot be ruled out that with more severe hypoxia, ET-receptor stimulation may play a role in the increase in MSNA and blood pressure observed with intermittent hypoxia exposure. Furthermore, the dose of bosentan applied was relatively conservative (125 mg/day), and although data support effective receptor blockade (43, 44), other research groups have more recently applied higher doses (61). Finally, the study intervention (bosentan) was conducted on men only and the generalizability of results to women is unlikely (21, 62).
Conclusion
The present study fills a key gap in knowledge and shows lower blood pressure and total peripheral resistance following treatment with bosentan in healthy young men; however, this response is not due to a direct effect on MSNA and instead is more likely the result of peripheral vascular effects following nonspecific ET-1 receptor blockade. Furthermore, bosentan does not appear to alter the neurovascular or cardiorespiratory response to intermittent hypoxia in the men studied. Thus, functional ET receptors may not be necessary to achieve a rise in MSNA and arterial blood pressure following acute intermittent hypoxia in healthy young men.
GRANTS
This work was supported by the National Institutes of Health (NIH) HL130339 (to J.K.L.), NIH U54 AG044170 (to S.E.B.), and Mayo Clinic Center for Biomedical Discovery (to J.K.L.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.L., S.E.B., and C.M.M.-A. conceived and designed research; J.K.L., S.E.B., E.P.O., D.W.J., Z.M.S., J.L.H., and C.M.M.-A. performed experiments; J.K.L., E.P.O., D.W.J., and Z.M.S. analyzed data; J.K.L., S.E.B., D.W.J., Z.M.S., and C.M.M.-A. interpreted results of experiments; J.K.L. and D.W.J. prepared figures; J.K.L. and S.E.B. drafted manuscript; J.K.L., S.E.B., E.P.O., D.W.J., Z.M.S., J.L.H., and C.M.M.-A. edited and revised manuscript; J.K.L., S.E.B., E.P.O., D.W.J., Z.M.S., J.L.H., and C.M.M.-A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Michael J. Joyner, the Human Integrative Physiology Laboratory, and the Clinical Research and Trials Unit at the Mayo Clinic. The authors also thank study participants for the donation of time.
REFERENCES
- 1. Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens 15: 1613–1619, 1997. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- 2. Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 174: 156–161, 2010. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T, American College of Cardiology Foundation. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118: 1080–1111, 2008. [Erratum in Circulation 119: e380, 2009]. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 6. McQueen DS, Dashwood MR, Cobb VJ, Bond SM, Marr CG, Spyer KM. Endothelins and rat carotid body: autoradiographic and functional pharmacological studies. J Auton Nerv Syst 53: 115–125, 1995. doi: 10.1016/0165-1838(94)00179-n. [DOI] [PubMed] [Google Scholar]
- 7. Spyer KM, McQueen DS, Dashwood MR, Sykes RM, Daly MB, Muddle JR. Localization of [125I]endothelin binding sites in the region of the carotid bifurcation and brainstem of the cat: possible baro- and chemoreceptor involvement. J Cardiovasc Pharmacol 17 Suppl 7: S385–389, 1991. doi: 10.1097/00005344-199100177-00108. [DOI] [PubMed] [Google Scholar]
- 8. Gjorup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens 20: 44–52, 2007. doi: 10.1016/j.amjhyper.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 9. Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens 17: 61–66, 1999. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 10. Saarelainen S, Seppala E, Laasonen K, Hasan J. Circulating endothelin-1 in obstructive sleep apnea. Endothelium 5: 115–118, 1997. doi: 10.3109/10623329709079869. [DOI] [PubMed] [Google Scholar]
- 11. Trakada G, Marangos M, Spiropoulos K. Mechanisms of endothelin-1 elevation in chronic obstructive pulmonary disease patients with nocturnal oxyhemoglobin desaturation. Respiration 68: 134–139, 2001. doi: 10.1159/000050482. [DOI] [PubMed] [Google Scholar]
- 12. Zamarron-Sanz C, Ricoy-Galbaldon J, Gude-Sampedro F, Riveiro-Riveiro A. Plasma levels of vascular endothelial markers in obstructive sleep apnea. Arch Med Res 37: 552–555, 2006. doi: 10.1016/j.arcmed.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 13. Cargill RI, Kiely DG, Clark RA, Lipworth BJ. Hypoxaemia and release of endothelin-1. Thorax 50: 1308–1310, 1995. doi: 10.1136/thx.50.12.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 296: R735–R742, 2009. doi: 10.1152/ajpregu.90490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peng YJ, Nanduri J, Zhang X, Wang N, Raghuraman G, Seagard J, Kumar GK, Prabhakar NR. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol (1985) 112: 187–196, 2012. [Erratum in J Appl Physiol 112: 1800, 2012]. doi: 10.1152/japplphysiol.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allahdadi KJ, Cherng TW, Pai H, Silva AQ, Walker BR, Nelin LD, Kanagy NL. Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am J Physiol Heart Circ Physiol 295: H434–H440, 2008. doi: 10.1152/ajpheart.91477.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leuenberger UA, Brubaker D, Quraishi SA, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci 121: 87–93, 2005. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 18. Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol (1985) 96: 754–761, 2004. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- 19. Cutler MJ, Swift NM, Keller DM, Wasmund WL, Burk JR, Smith ML. Periods of intermittent hypoxic apnea can alter chemoreflex control of sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 287: H2054–H2060, 2004. doi: 10.1152/ajpheart.00377.2004. [DOI] [PubMed] [Google Scholar]
- 20. Xie A, Skatrud JB, Crabtree DC, Puleo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent asphyxia in humans. J Appl Physiol (1985) 89: 1333–1339, 2000. doi: 10.1152/jappl.2000.89.4.1333. [DOI] [PubMed] [Google Scholar]
- 21. Jacob DW, Ott EP, Baker SE, Scruggs ZM, Ivie CL, Harper JL, Manrique-Acevedo CM, Limberg JK. Sex differences in integrated neuro-cardiovascular control of blood pressure following acute intermittent hypercapnic-hypoxia. Am J Physiol Regul Integr Comp Physiol 319: R626–R636, 2020. doi: 10.1152/ajpregu.00191.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ott EP, Jacob DW, Baker SE, Holbein WW, Scruggs ZM, Shoemaker JK, Limberg JK. Sympathetic neural recruitment strategies following acute intermittent hypoxia in humans. Am J Physiol Regul Integr Comp Physiol 318: R961–R971, 2020. doi: 10.1152/ajpregu.00004.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Limberg JK, Baker SE, Petersen-Jones H, Guo W, Huang A, Jensen MD, Singh P. Endothelin-1 as a novel target for the prevention of metabolic dysfunction with intermittent hypoxia in male participants. Am J Physiol Regul Integr Comp Physiol 323: R351–R362 2022. doi: 10.1152/ajpregu.00301.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Venitz J, Zack J, Gillies H, Allard M, Regnault J, Dufton C. Clinical pharmacokinetics and drug-drug interactions of endothelin receptor antagonists in pulmonary arterial hypertension. J Clin Pharmacol 52: 1784–1805, 2012. doi: 10.1177/0091270011423662. [DOI] [PubMed] [Google Scholar]
- 25. Romagnoli S, Ricci Z, Quattrone D, Tofani L, Tujjar O, Villa G, Romano SM, De Gaudio AR. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care 18: 644, 2014. doi: 10.1186/s13054-014-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romagnoli S, Romano SM, Bevilacqua S, Lazzeri C, Gensini GF, Pratesi C, Quattrone D, Dini D, De Gaudio AR. Dynamic response of liquid-filled catheter systems for measurement of blood pressure: precision of measurements and reliability of the Pressure Recording Analytical Method with different disposable systems. J Crit Care 26: 415–422, 2011. doi: 10.1016/j.jcrc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 29. Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Auton Neurosci 162: 89–93, 2011. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol (1985) 91: 1199–1206, 2001. doi: 10.1152/jappl.2001.91.3.1199. [DOI] [PubMed] [Google Scholar]
- 31. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD;Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5: 263–276, 2009. [PMC free article] [PubMed] [Google Scholar]
- 33. Chua TP, Ponikowski PP, Harrington D, Chambers J, Coats AJ. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart 76: 483–489, 1996. doi: 10.1136/hrt.76.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 37. Hunt BE, Fahy L, Farquhar WB, Taylor JA. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension 37: 1362–1368, 2001. doi: 10.1161/01.hyp.37.6.1362. [DOI] [PubMed] [Google Scholar]
- 38. Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, Weber DJ, Baric RS. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol 84: 1800–1815, 2010. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 12: 214–222, 1988. doi: 10.1161/01.hyp.12.2.214. [DOI] [PubMed] [Google Scholar]
- 41. Tzeng YC, Sin PY, Lucas SJ, Ainslie PN. Respiratory modulation of cardiovagal baroreflex sensitivity. J Appl Physiol 107: 718–724, 2009. doi: 10.1152/japplphysiol.00548.2009. [DOI] [PubMed] [Google Scholar]
- 42. Weber C, Schmitt R, Birnboeck H, Hopfgartner G, van Marle SP, Peeters PA, Jonkman JH, Jones CR. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther 60: 124–137, 1996. doi: 10.1016/S0009-9236(96)90127-7. [DOI] [PubMed] [Google Scholar]
- 43. Hiramoto Y, Shioyama W, Kuroda T, Masaki M, Sugiyama S, Okamoto K, Hirota H, Fujio Y, Hori M, Yamauchi-Takihara K. Effect of bosentan on plasma endothelin-1 concentration in patients with pulmonary arterial hypertension. Circ J 71: 367–369, 2007. doi: 10.1253/circj.71.367. [DOI] [PubMed] [Google Scholar]
- 44. Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med 338: 784–790, 1998. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 45. Gujic M, Houssiere A, Xhaet O, Argacha JF, Denewet N, Noseda A, Jespers P, Melot C, Naeije R, van de Borne P. Does endothelin play a role in chemoreception during acute hypoxia in normal men? Chest 131: 1467–1472, 2007. doi: 10.1378/chest.06-1775. [DOI] [PubMed] [Google Scholar]
- 46. Joyeux-Faure M, Jullian-Desayes I, Pepin JL, Cracowski JL, Baguet JP, Tamisier R, Levy P, Godin-Ribuot D, Launois SH. Comparison of continuous positive airway pressure and bosentan effect in mildly hypertensive patients with obstructive sleep apnoea: a randomized controlled pilot study. Respirology 21: 546–552, 2016. doi: 10.1111/resp.12713. [DOI] [PubMed] [Google Scholar]
- 47. Modesti PA, Vanni S, Morabito M, Modesti A, Marchetta M, Gamberi T, Sofi F, Savia G, Mancia G, Gensini GF, Parati G. Role of endothelin-1 in exposure to high altitude: Acute Mountain Sickness and Endothelin-1 (ACME-1) study. Circulation 114: 1410–1416, 2006. doi: 10.1161/CIRCULATIONAHA.105.605527. [DOI] [PubMed] [Google Scholar]
- 48. Jouett NP, Moralez G, White DW, Eubank WL, Chen S, Tian J, Smith ML, Zimmerman MC, Raven PB. N-Acetylcysteine reduces hyperacute intermittent hypoxia-induced sympathoexcitation in human subjects. Exp Physiol 101: 387–396, 2016. doi: 10.1113/EP085546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jouett NP, Moralez G, Raven PB, Smith ML. Losartan reduces the immediate and sustained increases in muscle sympathetic nerve activity after hyperacute intermittent hypoxia. J Appl Physiol (1985) 122: 884–892, 2017. doi: 10.1152/japplphysiol.00683.2016. [DOI] [PubMed] [Google Scholar]
- 50. Stuckless TJR, Vermeulen TD, Brown CV, Boulet LM, Shafer BM, Wakeham DJ, Steinback CD, Ayas NT, Floras JS, Foster GE. Acute intermittent hypercapnic hypoxia and sympathetic neurovascular transduction in men. J Physiol 598: 473–487, 2020. doi: 10.1113/JP278941. [DOI] [PubMed] [Google Scholar]
- 51. Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 37: 119–128, 2011. doi: 10.1183/09031936.00204209. [DOI] [PubMed] [Google Scholar]
- 52. Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol 574: 605–613, 2006. doi: 10.1113/jphysiol.2006.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tremblay JC, Boulet LM, Tymko MM, Foster GE. Intermittent hypoxia and arterial blood pressure control in humans: role of the peripheral vasculature and carotid baroreflex. Am J Physiol Heart Circ Physiol 311: H699–H706, 2016. doi: 10.1152/ajpheart.00388.2016. [DOI] [PubMed] [Google Scholar]
- 54. Yamamoto K, Eubank W, Franzke M, Mifflin S. Resetting of the sympathetic baroreflex is associated with the onset of hypertension during chronic intermittent hypoxia. Auton Neurosci 173: 22–27, 2013. doi: 10.1016/j.autneu.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rey S, Del Rio R, Iturriaga R. Role of endothelin-1 on the enhanced carotid body activity induced by chronic intermittent hypoxia. Adv Exp Med Biol 580: 345–350, 2006. doi: 10.1007/0-387-31311-7_53. [DOI] [PubMed] [Google Scholar]
- 56. Rey S, Del Rio R, Iturriaga R. Contribution of endothelin-1 to the enhanced carotid body chemosensory responses induced by chronic intermittent hypoxia. Brain Res 1086: 152–159, 2006. doi: 10.1016/j.brainres.2006.02.082. [DOI] [PubMed] [Google Scholar]
- 57. Jordan W, Reinbacher A, Cohrs S, Grunewald RW, Mayer G, Ruther E, Rodenbeck A. Obstructive sleep apnea: plasma endothelin-1 precursor but not endothelin-1 levels are elevated and decline with nasal continuous positive airway pressure. Peptides 26: 1654–1660, 2005. doi: 10.1016/j.peptides.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 58. Janssen C, Pathak A, Grassi G, van de Borne P. Endothelin contributes to the blood pressure rise triggered by hypoxia in severe obstructive sleep apnea. J Hypertens 35: 118–124, 2017. doi: 10.1097/HJH.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 59. Dingemanse J, van Giersbergen PL. Clinical pharmacology of bosentan, a dual endothelin receptor antagonist. Clin Pharmacokinet 43: 1089–1115, 2004. doi: 10.2165/00003088-200443150-00003. [DOI] [PubMed] [Google Scholar]
- 60. Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol 307: R1181–R1197, 2014. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Derella CC, Blanks AM, Wang X, Tucker MA, Horsager C, Jeong JH, Rodriguez-Miguelez P, Looney J, Thomas J, Pollock DM, Harris RA. Endothelin receptor blockade blunts the pressor response to acute stress in men and women with obesity. J Appl Physiol (1985) 132: 73–83, 2022. doi: 10.1152/japplphysiol.00156.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sebzda KN, Kuczmarski AV, Pohlig RT, Lennon SL, Edwards DG, Wenner MM. Ovarian hormones modulate endothelin-1 receptor responses in young women. Microcirculation 25: e12490, 2018. doi: 10.1111/micc.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]