Abstract
The standardized enzyme-linked immunosorbent assay (ELISA) for measurement of serum immunoglobulin G (IgG) antibody responses to meningococcal C polysaccharide has been modified to employ assay conditions that ensure specificity and favor detection primarily of high-avidity antibodies. The modified and standard assays were used to measure IgG antibody concentrations in sera of toddlers vaccinated with meningococcal polysaccharide vaccine or a meningococcal C conjugate vaccine. The results were compared to the respective complement-mediated bactericidal antibody titers. In sera obtained after one or two doses of vaccine, the correlation coefficients, r, for the results of the standard assay and bactericidal antibody titers were 0.45 and 0.29, compared to 0.85 and 0.87, respectively, for the modified assay. With the standard assay, there were no significant differences between the geometric mean antibody responses of the two vaccine groups. In contrast, with the modified assay, 5- to 20-fold higher postvaccination antibody concentrations were measured in the conjugate than in the polysaccharide group. Importantly, the results of the modified assay, but not the standard ELISA, paralleled the respective geometric mean bactericidal antibody titers. Thus, by employing conditions that favor detection of higher-avidity IgG antibody, the modified ELISA provides results that correlate closely with measurements of antibody functional activity that are thought to be important in protection against meningococcal disease.
Considerable efforts have been made to develop standardized protocols for quantifying serum antibody responses to meningococcal C vaccination (7, 19). The results of a multicenter study demonstrated that one can obtain reproducible measurements of anticapsular antibody concentrations by a standardized enzyme-linked immunosorbent assay (ELISA) (7). However, in some instances, the ability to extrapolate from measurements of anticapsular antibody concentrations in serum to predict bactericidal antibody titers appears to be limited. For example, in infants or young children who receive meningococcal polysaccharide vaccine, one can detect high serum antibody responses to group C polysaccharide by ELISA in the absence of detectable bactericidal antibody (5, 14, 20). The most likely explanation is that administration of the plain polysaccharide vaccine to infants and young children elicits principally low-avidity antibodies. These antibodies are detected by ELISA but appear to be less active in bactericidal function assays than higher-avidity anticapsular antibodies (24). It is likely that the standard meningococcal C ELISA detects both high- and low-avidity antibodies. Hence, for vaccines eliciting primarily low-avidity antibody, there can be high antibody responses measured by this assay but low or absent bactericidal antibody.
Although there are substantial clinical and epidemiologic data indicating that serum bactericidal activity is a good surrogate for protection against meningococcal disease (6, 8), there also are a number of problems in developing reliable and meaningful bactericidal activity assays. These include a large degree of variability in results, depending upon the choice of bacterial strain, bacterial growth conditions, buffer, or complement source (18, 19, 27). In contrast, antibody-binding assays such as ELISAs are easier to standardize. However, to have clinically meaningful results, it is important to choose assay conditions that permit assessment of functionally active antibodies that are likely to confer protection. Toward this goal, we have developed a modified immunoglobulin G (IgG) ELISA to measure concentrations of anticapsular antibody to meningococcal C polysaccharide in serum. As described below, we chose assay conditions that favor detection primarily of high-avidity antibodies (17). Our hypothesis was that by measuring such antibodies selectively, the results of the modified assay would correlate more closely with the results of the bactericidal activity assay than those obtained with the conventional ELISA. The purposes of this report are to describe this modified ELISA procedure and to present the results of studies using this assay to measure IgG anticapsular antibody concentrations in stored serum samples from toddlers immunized with either meningococcal polysaccharide vaccine or an investigational meningococcal C conjugate vaccine. The same sera also were assayed by using the standardized IgG ELISA (7). The results of each of these antibody binding assays were compared to the respective bactericidal antibody titers.
MATERIALS AND METHODS
Standard ELISA.
A detailed description of the standard ELISA procedure for measurement of concentrations of IgG antibody to meningococcal C polysaccharide in serum has been previously given (7). This assay uses a mixture of methylated human serum albumin and meningococcal C polysaccharide as the solid-phase antigen. Alkaline phosphatase-conjugated anti-human IgG mouse monoclonal antibody (clone HP6043) is used to detect bound IgG antibody. The limit of antibody detection in this assay is 0.02 μg/ml.
Modified ELISA procedure. (i) Derivatized polysaccharide.
Meningococcal C polysaccharide (lot 84) was provided by Chiron Vaccines S.p.A. (Siena, Italy). The polysaccharide was derivatized with adipic acid dihydrazide (ADH) by the carbodiimide method (4). Briefly, 5-mg/ml polysaccharide was reacted with 0.5 M ADH (Sigma, St. Louis, Mo.) in the presence of 0.1 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide–HCl (Pierce, Rockford, Ill.) for 15 min at room temperature while the pH was maintained between 6.5 and 7.0. The derivatized polysaccharide was dialyzed against phosphate-buffered saline (PBS) and stored at −20°C. Acid ninhydrin 2 reagent (Sigma) was used to determine the concentration of the polysaccharide in the final product (26), and the extent of ADH incorporation was measured by using the trinitrobenzene sulfonic acid (Sigma) assay (25). The typical antigen lots used had a polysaccharide-to-ADH ratio of 25 ng of ADH per μg of meningococcal C polysaccharide.
(ii) Assay method.
Microtiter plate wells (Immulon II; Dynatech, Chantilly, Va.) were coated with 100 μl of 1-μg/ml test antigen and incubated for 1 h at 37°C in a humidified box. The solution was aspirated, and the wells were washed three times with PBS (SkanWasher300; Skantron Instruments, Inc., Sterling, Va.) and blocked for 1 h at room temperature with 1% bovine serum albumin (BSA; Sigma) in blocking buffer (PBS with 0.02% sodium azide, pH 7.4 [Sigma]). The plates were washed three times with wash buffer (PBS, 0.1% Tween 20 [Sigma], 0.02% sodium azide, pH 7.4). Test and reference sera were prediluted with serum diluting buffer (PBS, 1% BSA, 0.1% Tween 20, 75 mM ammonium thiocyanate [SCN; Sigma], 0.02% sodium azide, pH 7.4). To the first row of wells was added 200 μl of prediluted test sera or samples of an in-house reference serum pool (A289) or quality control (QC) sera (described below). The remaining wells contained 100 μl of serum diluting buffer. Serial twofold dilutions of the serum samples were prepared in the microtiter plate, resulting in a 100-μl final volume in each well. For determination of antibody-binding specificity, all assays were performed with four replicate plates. Serum samples in two of the replicate plates were incubated with serum diluting buffer containing soluble meningococcal C polysaccharide (final concentration, 25 μg/ml) as an inhibitor of antibody binding, whereas serum samples on the other two plates were incubated with serum diluting buffer alone. The microtiter plates were maintained overnight (16 to 18 h) at 4°C in a humidified box. On the following day, wells were aspirated and washed five times with PBS. To each well was added 100 μl of alkaline phosphatase-conjugated murine monoclonal anti-human IgG (clone HP6043, conjugated by American Qualex, La Mirada, Calif.). After 1 h at 37°C, the plates were washed with PBS and 100 μl of 1-mg/ml substrate (Substrate 104; Sigma) prepared in diethanolamine solution (Pierce) was added. After 30 min, the colorimetric reaction was stopped by addition of 25 μl of 3 N NaOH. Absorbance at 405 nm was measured with a 630-nm background filter and a Bio-Tek 312e Microplate Reader (Bio-Tek Instruments Inc., Winooski, Vt.).
(iii) Reference and QC sera.
An in-house reference serum pool, designated A289, was prepared from postvaccination sera of adults immunized with meningococcal polysaccharide vaccine. The concentration of IgG antibody to meningococcal C polysaccharide in this pool was assigned a value of 39 U/ml when assayed by an ELISA (without SCN in the serum diluting buffer). This value was obtained by comparison to the titration curve generated with the Centers for Disease Control and Prevention reference serum (CDC1992, assigned a concentration of 24.1-μg/ml IgG anti-meningococcal C polysaccharide antibody) (11). When the in-house reference pool, A289, was reassayed in replicate in an IgG ELISA performed with and without 75 mM SCN in the serum diluting buffer, approximately 25% less antibody binding was detected in the presence of SCN. Therefore, for use as a reference serum in the modified ELISA, this pool was assigned an IgG value of 30.8 U of antibodies per ml detected in the presence of SCN. Note that in both the ELISA performed with SCN and that done without SCN in the serum diluting buffer, the respective binding curves of the in-house reference serum A289 and the CDC1992 reference serum were parallel (data not shown). For assay of test samples, two QC serum standards were also included on each plate, i.e., a high pool, A288 (mean ± 2 standard deviations, 11.6 ± 4.8 U/ml), and a low pool, A287 (3.4 ± 1.6 U/ml). These were assayed at eight serial twofold dilutions, beginning at 1:50.
(iv) Calculation of antibody concentrations in serum.
Absorbance values for wells containing dilutions of serum incubated with soluble meningococcal C polysaccharide were subtracted as background from the corresponding values for the wells in which sera were diluted with buffer alone (9). Serial twofold dilutions were prepared from in-house reference serum A289, beginning at a dilution of 1:400 and extending to 1:51,200. The best fit for the resulting titration curve was obtained by using a four-parameter logistic equation in the KC-3 software package (Bio Tek Instruments, Inc.). IgG anticapsular antibody concentrations in the test sera were assigned by comparison to the reference curve. To calculate the antibody concentration, multiple data points obtained from dilutions of test sera that yielded optical density (OD) values in the linear portion of the curve (OD, 0.5 to 1.5) were averaged. However, samples with OD values between 0.14 and 0.49 at the lowest dilution tested (1:50) were still considered positive, since an OD of 0.14 is >4 standard deviations above the background value. For these low-titer samples, the assigned values were extrapolated directly from a single point on the standard curve. The average of the IgG concentrations from replicate titration curves of each test serum determined in separate microtiter plates was determined and reported in IgG units per milliliters. The lower limit of antibody detection with this modified ELISA is 0.4 U/ml.
Assay validation.
With a series of dilutions of high-titer serum samples, the results of the modified ELISA were found to be linear within a range of 0.4 to 300 U/ml. There was no significant effect of varying incubation times or incubation temperatures ±10% on the assigned antibody concentration. The coefficient of variation of the modified ELISA performed by different operators on different days was <10%.
Bactericidal antibody procedure.
The method used for measurement of bactericidal antibody titers has been previously described (19). In the present study, the complement source was a normal human serum that lacked detectable intrinsic bactericidal activity. Titers were calculated by the dilution of test serum showing a 50% decrease in the number of CFU after 60 min of incubation compared to the control at time zero.
Serum samples.
Stored serum samples were selected from toddlers, 15 to 23 months of age, who participated in a randomized multicenter safety and immunogenicity study of an investigational meningococcal C conjugate vaccine (Chiron Vaccines) and plain meningococcal polysaccharide vaccine (Menomune; Connaught) (16). Two doses of each of these vaccines were given 2 months apart. For performance comparison of the conventional and modified ELISA methods, samples collected preimmunization, 2 months post-first immunization, and 1 month post-second immunization were selected from toddlers demonstrating a wide range of IgG antibody responses, as determined by the modified ELISA.
Coded sera were sent to the laboratory of George Carlone and Susan Maslanka, where the samples were assayed for IgG anticapsular antibody concentrations by the standard ELISA and for complement-mediated bactericidal antibody by using human complement. The respective antibody results from each laboratory were then exchanged, the vaccine code was broken, and the data were analyzed statistically as described below.
Statistical analysis.
All statistical tests were conducted at the two-sided 5% significance level. For the purpose of analysis, the IgG anti-meningococcal C and bactericidal antibody concentrations in samples collected preimmunization, 2 months post-dose 1, and 1 month post-dose 2 were logarithmically transformed (base 10). Titers below the limit of detection were set to half the limit of detection. Geometric mean concentrations were calculated by vaccine group (conjugate or polysaccharide) for each time point. The two vaccine groups were compared by using a one-way analysis of variance model with a single factor for group.
To assess the relationships between IgG ELISA (standard or modified) and bactericidal titers, scatter plots were drawn on a log scale (base 10) for each time point. Pearson correlation coefficients were computed and regression lines were fitted on the logarithmically transformed values for each vaccine group separately and for both groups combined.
RESULTS
Rationale for ELISA modifications (i) Microtiter plate coating antigen.
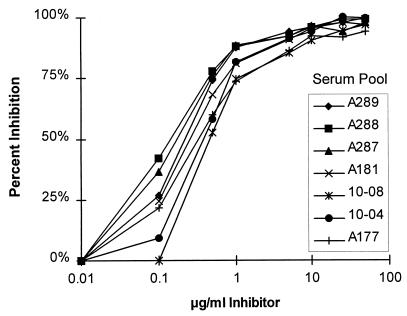
In pilot studies, meningococcal C polysaccharide mixed with methylated human albumin was adsorbed to microtiter wells as described for the standard ELISA (7). With this antigen preparation, high absorbance values were observed when serum samples from some unvaccinated children or adults were assayed. Attempts to determine the specificity of this antibody for polysaccharide binding were inconclusive, since when soluble meningococcal C polysaccharide was added to the serum diluting buffer, <50% inhibition of antibody binding to the solid-phase antigen was observed with some serum samples (data not shown). Therefore, to ensure antibody binding specificity, alternative means for getting meningococcal polysaccharide to adhere to the solid phase were explored. In the process of derivatizing the polysaccharide with biotin by using adipic acid, we observed that the derivatized polysaccharide, in the absence of biotin, bound to untreated polystyrene wells at low optimal coating concentrations (100 μl of a 1.0-μg/ml solution). As shown in Fig. 1, the specificity of meningococcal C antibody binding to this solid-phase antigen appeared to be very high, as determined by dose-response inhibition of antibody binding of a variety of pre- or postvaccination serum pools in the presence of soluble meningococcal C polysaccharide. Therefore, for the modified ELISA, we chose to use the adipic acid-derivitized polysaccharide as the solid-phase antigen.
FIG. 1.
Inhibition of IgG anticapsular antibody by different concentrations of soluble meningococcal C polysaccharide. The serum pools were diluted to an antibody concentration yielding an OD of approximately 1 in the absence of inhibitor. The respective pools had been prepared from preimmune sera or postvaccination sera of adults showing a wide range of anticapsular antibody responses.
(ii) Blocking buffer.
Several blocking buffers were evaluated to determine which formulation would yield the lowest background titers when nonimmune sera were assayed in different microtiter plates. Among those tried, 1% BSA (radioimmunoassay reagent grade) in the absence of a nonionic detergent, such as Brij, was equivalent or superior to other blocking buffers, as determined by the lowest background OD values (data not shown).
(iii) Use of SCN in the serum diluting buffer.
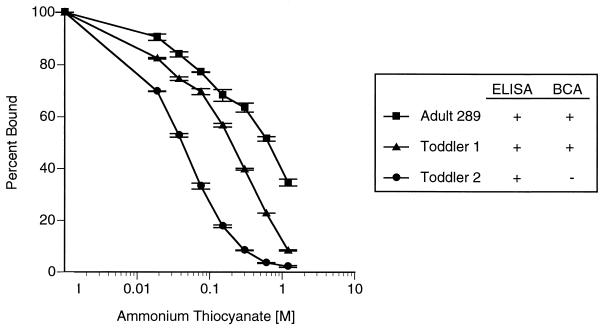
Antibody binding in the presence of increasing SCN concentrations has been reported to correlate directly with antibody avidity (17). Therefore, the chaotropic agent ammonium SCN, which inhibits antigen-antibody interactions in a dose-dependent fashion, was included in the serum dilution buffer in the modified ELISA in an attempt to select for high-avidity antibody binding. Three immune serum pools were prediluted to an antibody concentration that yielded an OD of approximately 1.0 when assayed in the absence of ammonium SCN. Figure 2 shows the results of a representative experiment in which we assessed the effect of increasing concentrations of SCN in the serum diluting buffer on the binding of IgG anti-meningococcal C antibody to the solid-phase antigen. The three pools selected were as follows. Serum pool A289, described further below, is the in-house reference pool prepared from sera of vaccinated adults. The two toddler serum pools were prepared from postvaccination sera from individuals who showed IgG anticapsular antibody responses to vaccination with meningococcal polysaccharide vaccine or meningococcal C conjugate vaccine (concentrations of <0.4 U/ml in serum obtained before vaccination increasing to ≥1.8 U/ml in postvaccination sera, as measured in the absence of SCN in the diluting buffer). Toddler pool 1 (9 subjects) contained postvaccination sera that were selected based on the presence of bactericidal antibody titers of ≥1:8, whereas toddler pool 2 (10 subjects) contained postvaccination sera that were selected based on undetectable bactericidal activity (titers of <1:8). We assumed that toddler pool 1 contained higher-avidity IgG anticapsular antibodies, while toddler pool 2 contained primarily IgG antibodies with lower avidity. A concentration of SCN was sought that would minimally decrease the IgG ELISA binding of toddler pool 1 while significantly decreasing the ELISA titers in toddler pool 2. As shown in Fig. 2, the desired results occurred at a concentration of approximately 75 mM SCN. This concentration of SCN was selected for use in the serum diluting buffer in the modified IgG ELISA.
FIG. 2.
Effect of increasing ammonium SCN concentration in the serum diluting buffer on serum antibody binding to meningococcal C polysaccharide in an ELISA. The serum pools were diluted to an antibody concentration yielding an OD of approximately 1 in the absence of SCN. Pool A289 was prepared from sera of adults vaccinated with meningococcal polysaccharide vaccine. Toddler pools 1 and 2 were prepared from postvaccination sera of toddlers given meningococcal polysaccharide vaccine or meningococcal C conjugate vaccine. All subjects showed greater-than-fourfold increases in concentrations of IgG anticapsular antibody in response to vaccination. Toddler pool 1 included postvaccination sera with bactericidal antibody (BCA) titers of ≥1:8, whereas toddler pool 2 contained postvaccination sera with bactericidal antibody titers of <1:8 (presumed to have lower antibody avidity).
Relationship of IgG antibody concentration to bactericidal antibody titer: comparison of the two ELISA methods. (i) Preimmune sera (naturally induced antibody).
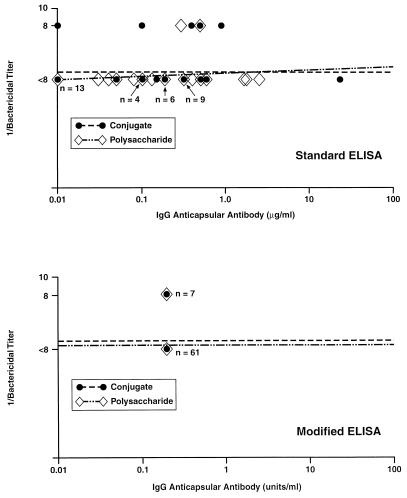
Figure 3 (top panel) shows the relationship between IgG anti-meningococcal C antibody concentrations measured in preimmune sera by the standard ELISA and the respective bactericidal antibody titers measured by using human complement. The IgG antibody concentrations measured with the standard ELISA ranged from 0.02 to 24 μg/ml. Of the 68 samples tested, 7 (10%) had detectable bactericidal antibody titers of 1:8, and none had titers of >1:8. In this collection of preimmune serum samples, there was no discernible relationship between the magnitude of the IgG anticapsular antibody concentrations and the respective bactericidal antibody titers. When these same sera were assayed by the modified ELISA, none had detectable IgG anticapsular antibody (Fig. 3, bottom panel), including the seven samples with bactericidal activity. Note that the lower limit of antibody detection in the modified ELISA is 0.4 U/ml, compared to 0.02 μg/ml with the standard ELISA.
FIG. 3.
Relationship between concentrations of IgG antibody to meningococcal C polysaccharide and bactericidal activity titers measured in prevaccination sera of toddlers 15 to 23 months of age. Except where noted, each point represents the value of a single sample.
(ii) Serum samples obtained 2 months post-dose 1.
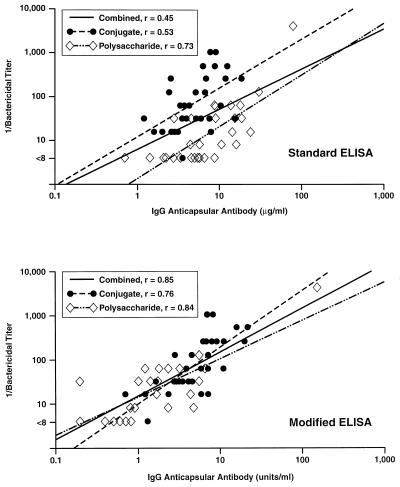
Figure 4 (top panel) shows the relationship between the respective IgG anticapsular antibody concentrations measured with the standard ELISA and the bactericidal antibody titers measured in post-dose 1 toddler sera. The data are stratified by vaccine group (conjugate, plain polysaccharide). For both vaccine groups, there are highly significant (P < 0.001) coefficients of correlation between the respective ELISA and bactericidal results (polysaccharide vaccine, r = 0.73; conjugate vaccine, r = 0.53). However, when the data from the two vaccine groups are combined, the overall correlation coefficient is lower (r = 0.45, P < 0.001). The reason for the lower overall correlation is that the y intercepts of the respective regression lines for the two vaccine groups are separated by nearly a log. The most likely explanation is that the conjugate and polysaccharide vaccines elicit antibody populations that differ in average avidity and are not distinguished by the standard ELISA but affect the biologic functional activity of the antibody measured in the bactericidal activity assay.
FIG. 4.
Relationship between concentrations of IgG antibody to meningococcal C polysaccharide and bactericidal activity titers measured in serum samples obtained 2 months after vaccination of toddlers with meningococcal polysaccharide vaccine or meningococcal C conjugate vaccine.
Figure 4 (bottom panel) shows the results of testing the same sera with the modified IgG ELISA. Although the relationship between the respective IgG antibody concentrations and bactericidal antibody titers is clearly not absolute, with the modified ELISA, the respective regression lines and y intercepts for the samples from the two vaccine groups are similar. The correlation coefficients are as follows: for the polysaccharide group, r = 0.84; for the conjugate group, r = 0.76; for the combined data, r = 0.85 (P < 0.001 for all three coefficients). Thus, by employing conditions that favor detection of higher-avidity antibody, the results of the modified ELISA allow better prediction of the bactericidal antibody titers than does the conventional ELISA.
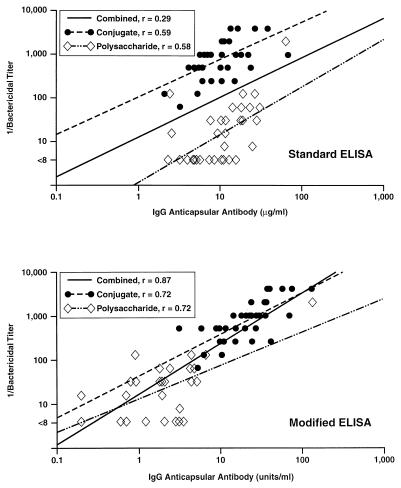
Serum samples obtained 1 month post-dose 2.
Figure 5 (top panel) shows the relationship between IgG anticapsular antibody concentrations measured with the standard ELISA and the bactericidal antibody titers measured in post-dose 2 toddler sera. The correlation coefficient for the polysaccharide group is 0.58, and that for the conjugate group is 0.59 (P < 0.001 for both). However, the y intercepts of the regression lines for the two vaccine groups are even further apart (i.e., nearly 2 logs) than the corresponding data obtained post-dose 1. The most likely explanation is that affinity maturation of the antibody is elicited in response to dose 2 of the conjugate but not in response to the second dose of the plain polysaccharide vaccine. The resulting overall correlation coefficient, r, for the combined data after dose 2 is 0.29 (P < 0.05).
FIG. 5.
Relationship between concentrations of IgG antibody to meningococcal C polysaccharide and bactericidal activity titers measured in serum samples obtained from toddlers 1 month after administration of a second dose of meningococcal polysaccharide vaccine or meningococcal C conjugate vaccine. The second dose of vaccine was administered 2 months after dose 1.
Figure 5 (bottom panel) shows the relationship between IgG antibody concentrations in post-dose 2 sera measured by the modified IgG ELISA and the corresponding bactericidal antibody titers. The correlation coefficient, r, for both the polysaccharide and conjugate groups is 0.72. For the combined data, the correlation coefficient is 0.87 (P < 0.001 for all three r values). Thus, for both post-dose 1 and post-dose 2 sera, the modified ELISA results provide a better prediction of bactericidal antibody titers than do the standard ELISA results.
Geometric mean antibody concentrations as assessed by the different assays.
Table 1 summarizes the geometric mean IgG antibody concentrations and bactericidal antibody titers as measured by the different assays. The discrepant results of the different IgG ELISAs are striking. For example, with the standard ELISA, there is no significant difference in the respective geometric mean IgG antibody concentrations in serum between the conjugate and polysaccharide vaccine groups after dose 1 (5.1 versus 7.0 μg/ml) or after dose 2 (9.3 versus 9.7 μg/ml). In contrast, with the modified ELISA, we observed a nearly fivefold higher IgG response in the conjugate group after dose 1 (4.8 versus 1.0 U/ml, P < 0.001) and a 20-fold higher antibody concentration after dose 2 (21 versus 1.2 U/ml, P < 0.001). As shown in Table 1, the respective geometric mean bactericidal antibody titers paralleled the modified IgG ELISA results and not the standard IgG ELISA results.
TABLE 1.
Antibody concentrations in sera of toddlers vaccinated with meningococcal C conjugate vaccine or meningococcal polysaccharide vaccine, as assessed by different assaysa
| Assay, method, and vaccine | Geometric mean
|
Probabilityb
|
|||
|---|---|---|---|---|---|
| Preimmu- nization | Post- dose 1 | Post- dose 2 | Post- dose 1 | Post- dose 2 | |
| IgG anticapsular antibody | |||||
| Standard ELISAc | |||||
| Polysaccharide vaccine | 0.12 | 7.0 | 9.7 | ||
| 0.11 | 0.81 | ||||
| Conjugate vaccine | 0.18 | 5.1 | 9.3 | ||
| Modified ELISAd | |||||
| Polysaccharide vaccine | 0.22 | 1.0 | 1.2 | ||
| <0.001 | <0.001 | ||||
| Conjugate vaccine | 0.20 | 4.8 | 21.0 | ||
| Bactericidal antibodye | |||||
| Polysaccharide vaccine | 4.2 | 14 | 16 | ||
| <0.001 | <0.001 | ||||
| Conjugate vaccine | 4.4 | 74 | 761 | ||
Serum samples were selected from toddlers showing a wide range of IgG antibody responses to vaccination. There were 35 subjects in the polysaccharide group and 35 in the conjugate group (see Materials and Methods).
Comparing respective geometric mean antibody concentrations or titers elicited by the two vaccines.
In micrograms per milliliter.
In units per milliliter.
Reciprocal of titer.
DISCUSSION
The data of Goldschneider et al. (8) from classic studies performed in the 1960s with military recruits demonstrated the importance of complement-mediated serum bacteriolysis in predicting protection against meningococcal C disease. These and other data (summarized by Frasch in 1995 [6]) provide the basis for using the bactericidal activity assay as a means of assessing protective immunity to meningococcal disease. However, the bactericidal activity assay is very labor-intensive, and even minor variations in the procedure can greatly affect the reproducibility of results (8, 18, 19, 27). For this reason, antibody-binding assays, such as the ELISA, offer a more convenient and reproducible method for quantifying immune responses to vaccination. The question, however, is whether data obtained from an antibody-binding assay can be used as a surrogate to predict bactericidal antibody responses.
The results of previous studies correlating the magnitude of the anticapsular antibody responses to meningococcal C polysaccharide measured by the standard ELISA with bactericidal antibody responses provide conflicting data. In general, higher correlations have been observed when assaying sera from immunized adults (3) or from younger individuals given a single vaccine (12). In contrast, lower correlations have been observed when assaying sera of vaccinated infants or toddlers (13), particularly when the sera are from studies comparing a polysaccharide to a conjugate vaccine (5, 14). The most likely explanation for the high correlations observed in some studies and the low correlations observed in others is the existence of differences in avidity between the antibodies measured in the particular studies. For example, when the analysis is limited to sera from vaccinated adults, the avidity of the antibody populations is likely to be higher and more homogeneous than when sera from infants and children given more than one type of vaccine are assayed. The present analysis is consistent with this explanation. Specifically, with the standard ELISA, higher correlation coefficients were observed when the analysis was limited to one of the two vaccine groups than when coefficients of correlation with bactericidal antibody titers were measured by using combined data from the two vaccine groups. In contrast, higher respective coefficients of correlation with bactericidal antibody titers were observed with the modified ELISA than with the standard ELISA, particularly when the data from the two vaccine groups were combined and analyzed together.
The most likely explanation for the improved performance of the modified ELISA is the use of SCN in the serum diluting buffer, which favors detection of high-avidity over low-avidity antibodies (17). In previous studies of Haemophilus influenzae type b anticapsular antibodies, higher-avidity antibodies were found to be more active than low-avidity antibodies in eliciting complement-mediated bactericidal activity (1, 24), in opsonization (1), or in conferring protection against experimental type b Haemophilus bacteremia (10, 15). Note, however, that two other changes were incorporated into the modified ELISA that could have contributed to the improved performance of the assay, i.e., the use of a novel solid-phase derivatized polysaccharide antigen and the use of replicate microtiter plates in which specificity of antibody binding is assessed by subtracting the respective absorbance values obtained with each serum sample diluted with buffer containing soluble meningococcal polysaccharide inhibitor from the corresponding value obtained from the sample diluted with buffer alone. Of these two modifications, the least important is likely the use of the soluble meningococcal C polysaccharide inhibitor as a control for IgG antibody binding specificity, since inhibition by the soluble polysaccharide with the assay conditions used is >90% for nearly all of the samples tested (data not shown). (Note, however, that this inhibition control is important when using the modified ELISA to measure IgM or total Ig anticapsular antibody concentrations, where nonspecific binding is more of a problem [9 unpublished data].)
The present study did not address the potential importance of lower-avidity anticapsular antibodies in conferring protection against meningococcal disease. Although not apparently as active as high-avidity antibodies in the bactericidal activity assay, production of low-avidity antibodies could confer protection by being present in sufficiently high concentrations to activate complement-mediated bacteriolysis or opsonization. Additional experimental and epidemiological studies are needed to define better the role of low-avidity antibodies in host protection.
Finally, it is important to note that other factors, in addition to antibody avidity, can affect the ability of antibodies to activate complement-mediated bacteriolysis. These include IgM responses (18, 23), IgG subclass distribution (2), and antibody variable region gene expression (15). Given these potential variables, it is perhaps surprising that the simple addition of a chaotropic ion to the dilution buffer to favor detection of high-avidity antibody responses had such a significant influence in improving the correlation between the respective IgG ELISA results and the bactericidal antibody titers.
In summary, the close correspondence between the magnitude of the anticapsular antibody response measured by the modified ELISA and assessment of antibody functional activity, thought to be important in protection against meningococcal disease, suggests that the modified ELISA should be useful in assessing the immunogenicity of new investigational vaccines being developed for prevention of meningococcal C disease. The present data also have implications for interpreting antibody concentrations being measured by ELISA in response to other polysaccharide-based vaccines under development, such as pneumococcal conjugate vaccines. For these vaccines, it will be important to determine whether high- or low-avidity antibody populations elicited by vaccination and detected by antibody-binding assays are active in opsonic assays (21, 22), the principal mechanism of protection for Streptococcus pneumoniae.
ACKNOWLEDGMENTS
We are indebted to the following individuals, who provided important technical or intellectual contributions to this study: Rose Sekulovich, Wai Ping Leong, Marilyn Owens, and Carol Suennen.
REFERENCES
- 1.Amir J, Liang X, Granoff D M. Variability in the functional activity of vaccine-induced antibody to Haemophilus influenzae type b. Pediatr Res. 1990;27:358–364. doi: 10.1203/00006450-199004000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Amir J, Scott M G, Nahm M H, Granoff D M. Bactericidal and opsonic activity of IgG1 and IgG2 anticapsular antibodies to Haemophilus influenzae type b. J Infect Dis. 1990;162:163–171. doi: 10.1093/infdis/162.1.163. [DOI] [PubMed] [Google Scholar]
- 3.Anderson E L, Bowers T, Mink C M, Kennedy D J, Belshe R B, Harakeh H, Pais L, Holder P, Carlone G M. Safety and immunogenicity of meningococcal A and C polysaccharide conjugate vaccine in adults. Infect Immun. 1994;62:3391–3395. doi: 10.1128/iai.62.8.3391-3395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartoloni A, Norelli F, Ceccarini C, Rappuoli R, Costantino P. Immunogenicity of meningococcal B polysaccharide conjugated to tetanus toxoid or CRM197 via adipic acid dihydrazide. Vaccine. 1995;13:463–470. doi: 10.1016/0264-410x(94)00007-a. [DOI] [PubMed] [Google Scholar]
- 5.Campagne G, Garba A, Fabre P, Carlone G, Ryall B, Froeschle J, Schuchat A, Boulanger D, Briantais P, Chippaux J P. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Safety and immunogenicity of three doses of a N. meningitidis A/C diphtheria conjugate vaccine in infants in Niger, abstr. G-1; p. 192. [Google Scholar]
- 6.Frasch C E. Meningococcal vaccines: past, present and future. In: Cartwright K, editor. Meningococcal disease. Chichester, England: John Wiley & Sons Ltd.; 1995. pp. 245–283. [Google Scholar]
- 7.Gheesling L L, Carlone G M, Pais L B, Holder P F, Maslanka S E, Plikaytis B D, Achtman M, Densen P, Frasch C E, Käyhty H, Mays J P, Nencioni L, Peeters C, Phipps D C, Poolman J T, Rosenqvist E, Siber G R, Thiesen B, Tai J, Thompson C M, Vella P P, Wenger J D. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J Clin Microbiol. 1994;32:1475–1482. doi: 10.1128/jcm.32.6.1475-1482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granoff D M, Kelsey S K, Bijlmer H A, Van Alphen L, Dankert J, Mandrell R E, Azmi F H, Scholten R J. Antibody responses to the capsular polysaccharide of Neisseria meningitidis serogroup B in patients with meningococcal disease. Clin Diagn Lab Immunol. 1995;2:574–582. doi: 10.1128/cdli.2.5.574-582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granoff D M, Lucas A H. Laboratory correlates of protection against Haemophilus influenzae type b disease. Importance of assessment of antibody avidity and immunologic memory. Ann N Y Acad Sci. 1995;754:278–288. doi: 10.1111/j.1749-6632.1995.tb44461.x. [DOI] [PubMed] [Google Scholar]
- 11.Holder P K, Maslanka S E, Pais L B, Dykes J, Plikaytis B D, Carlone G M. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin Diagn Lab Immunol. 1995;2:132–137. doi: 10.1128/cdli.2.2.132-137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King W J, MacDonald N E, Wells G, Huang J, Allen U, Chan F, Ferris W, Diaz-Mitoma F, Ashton F. Total and functional antibody response to a quadrivalent meningococcal polysaccharide vaccine among children. J Pediatr. 1996;128:196–202. doi: 10.1016/s0022-3476(96)70389-x. [DOI] [PubMed] [Google Scholar]
- 13.Leach A, Twumasi P A, Kumah S, Banya W S, Jaffar S, Forrest B D, Granoff D M, Butti D E L, Carlone G M, Pais L B, Broome C V, Greenwood B M. Induction of immunological memory in Gambian children by immunization in infancy with a group A plus group C meningococcal polysaccharide and protein-conjugate vaccine. J Infect Dis. 1997;175:200–204. doi: 10.1093/infdis/175.1.200. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman J M, Chiu S S, Wong V K, Partridge S, Chang S J, Chiu C Y, Gheesling L L, Carlone G M, Ward J I. Safety and immunogenicity of a serogroups A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. A randomized controlled trial. JAMA. 1996;275:1499–1503. [PubMed] [Google Scholar]
- 15.Lucas A H, Granoff D M. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide-protein conjugates. J Immunol. 1995;154:4195–4202. [PubMed] [Google Scholar]
- 16.MacDonald N N E, Halperin S, Law B, Forrest B D, Mokatrin A, Raff H V, Costantino P, Ceccarini C, Granoff D M. Biocine meningococcal C (MenC) conjugate vaccine elicits high titers of serum bactericidal activity in toddlers. Pediatr Res. 1996;39:178A. [Google Scholar]
- 17.Macdonald R A, Hosking C S, Jones C L. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods. 1988;106:191–194. doi: 10.1016/0022-1759(88)90196-2. [DOI] [PubMed] [Google Scholar]
- 18.Mandrell R E, Azmi F H, Granoff D M. Complement-mediated bactericidal activity of human antibodies to poly α 2→8 N-acetyl neuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- 19.Maslanka S E, Gheesling L L, Libutti D E, Donaldson K B J, Harakeh H S, Dykes J K, Arhin F F, Devi S J N, Frasch C E, Huang J C, Kriz-Kuzemenska P, Lemmon R D, Lorange M, Peeters C C A M, Quataert S, Tai J Y, Carlone G M The Multilaboratory Study Group. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–167. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maslanka S E, Tappero J W, Plikaytis B D, Brumberg R S, Dykes J K, Gheesling L L, Donaldson K B J, Schuchat A, Pullman J, Jones M, Bushmaker J, Carlone G M. Age-dependent Neisseria meningitidis serogroup C class-specific antibody concentrations and bactericidal titers in sera from young children from Montana immunized with a licensed polysaccharide vaccine. Infect Immun. 1998;66:2453–2459. doi: 10.1128/iai.66.6.2453-2459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahm M H, Olander J V, Magyarlaki M. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae, abstr. G-83; p. 207. [DOI] [PubMed] [Google Scholar]
- 22.Nahm M H, Ward J, Chang S, Yu X H. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Young children may produce antibodies against Streptococcus pneumoniae that are reactive but not opsonophagocytic to cross-reactive serotypes, abstr. G-82; p. 207. [Google Scholar]
- 23.Raff H V, Bradley C, Brady W, Donaldson K, Lipsich L, Maloney G, Shuford W, Walls M, Ward P, Wolff E, et al. Comparison of functional activities between IgG1 and IgM class-switched human monoclonal antibodies reactive with group B streptococci or Escherichia coli K1. J Infect Dis. 1991;163:346–354. doi: 10.1093/infdis/163.2.346. [DOI] [PubMed] [Google Scholar]
- 24.Schlesinger Y, Granoff D M. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA. 1992;267:1489–1494. [PubMed] [Google Scholar]
- 25.Schneerson R, Barrera O, Sutton A, Robbins J B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao K, Ubuka T. Determination of sialic acids by acidic ninhydrin reaction. Acta Med Okayama. 1987;41:237–241. doi: 10.18926/AMO/31741. [DOI] [PubMed] [Google Scholar]
- 27.Zollinger W D, Mandrell R E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]