Abstract
Serologic testing for the presence of antibodies to Bartonella henselae is a widely accepted diagnostic procedure for laboratory confirmation of the diagnosis of cat scratch disease (CSD). In this study a commercially available indirect immunofluorescence assay (IFA) based on B. henselae-infected human larynx carcinoma cells (test A) was evaluated. Sera from 42 patients with CSD (20 confirmed by PCR) and 270 sera from healthy controls (consisting of 63 cat owners, 65 individuals whose last close contact with cats was >6 months previously, and 142 persons who had never been exposed to cats) were investigated for antibodies to B. henselae. All patients with CSD had titers of immunoglobulin G (IgG) to B. henselae of 128 or higher (test A; sensitivity, 100%). Of the 270 controls 189 (70%) were seronegative (titer, <64), 38 (14.1%) had titers of 64, 30 (11.1%) had titers of 128, 9 (3.3%) had titers of 256, and 4 (1.5%) had high titers, 512 (test A; specificity, 70%). Of the cat owners and individuals who had never had close contact with cats, 71.4 and 71.12%, respectively, were seronegative, and titers of 64, 128, 256, and 512 were found in 14.3 and 16.2%, 1.6 and 10.5%, 9.5 and 0.7%, and 3.2 and 1.4%, respectively. The sera from the patients and from the first 100 healthy adults without a history of close contact with cats were additionally tested with a second commercially available IFA, based on Vero cells infected with B. henselae and Bartonella quintana (test B). The sensitivity and specificity of test B were 93 and 73%, respectively. For patients with CSD the cross-reactivity between B. henselae and B. quintana in this test was 95%. Both systems are highly sensitive but less specific for detection of IgG antibodies to B. henselae in samples from patients with clinically apparent CSD. For detection of IgM antibodies, test A seems to be more sensitive (88%) and more specific (95%) than test B (sensitivity and specificity of 64 and 86%, respectively). The data show that the seroprevalence of antibodies to B. henselae in German individuals is high (30%). Low antibody levels are not sufficient evidence of active or prior infection.
Bartonella henselae is now accepted as the causative agent of cat scratch disease (CSD). Until recently the diagnosis of CSD was by exclusion. The diagnosis required the presence of at least three of the following: a history of contact with a cat and the presence of a scratch or a primary lesion, a positive cat scratch skin test reaction, regional lymphadenopathy with negative results for other causes of lymphadenopathy, and characteristic histopathologic features in a lymph node biopsy specimen (2). CSD occurs mainly in children and young adults, and uncomplicated CSD-mediated lymphadenopathy usually resolves spontaneously within 2 to 6 months (4). Moreover, the histopathological findings are typical, but not specific, for CSD, and obtaining them requires the surgical removal of a lymph node or a biopsy. In the last few years, serological tests to detect antibodies to B. henselae have been developed (7, 8) and serologic testing for antibodies to B. henselae was proposed as an adequate alternative to skin testing (5). To evaluate the reliability of serological tests, we studied the specific antibody responses to B. henselae in 270 healthy German students and 42 patients with CSD. Two commercially available indirect immunofluorescence assays (IFAs) were compared.
MATERIALS AND METHODS
A total of 42 patients with CSD ranging in age from 3 to 53 years (mean, 19.2 years) were enrolled in this study. CSD was suspected clinically in 22 patients with regional lymphadenopathy together with a history of close contact with cats, in most of them associated with cat scratches and subsequent skin lesions appearing within 3 months before the onset of illness. In the other 20 patients CSD was diagnosed histologically. For each of these 20 patients a lymph node biopsy showing characteristic histologic findings consistent with CSD was available. DNA was extracted from all lymph nodes with a commercial kit (Qiagen GmbH, Hilden, Germany). The extracted DNA was used as a template in the PCR assay. The primers p24E and p12B, designed by Relman et al. (9), were used to amplify a 241-bp fragment from the Bartonella 16S rRNA gene fragment as described elsewhere (10). The control group consisted of 270 clinically healthy students (age range, 21 to 30 years; mean, 23 years) without symptoms of CSD. The controls were classified into three groups according to their contact with cats: group A, cat owners (n = 63); group B, those whose last close contact with cats was more than 6 months previously (n = 65); and group C, those who never had had close contact with cats (n = 142). Additionally, sera from patients with serologically confirmed infections by Epstein-Barr virus (EBV; n = 9), cytomegalovirus (CMV; n = 16), Toxoplasma gondii (n = 15), and Chlamydia pneumoniae (n = 15) were assessed for cross-reactive antibodies to B. henselae.
All sera were examined with a Bartonella IFA (BION Enterprises, Park Ridge Chicago, Ill.; distributed in Germany by BIOS GmbH Labordiagnostik, Munich) (test A) for immunoglobulin G (IgG) antibodies. The patients’ sera and the first 100 sera from group C volunteers were also tested for IgM antibodies by test A, and these sera were additionally examined for the presence of IgG and IgM antibodies with a second commercially available test (MRL Diagnostics, Cypress, Calif.; distributed in Germany by Genzyme Virotech GmbH, Rüsselsheim) (test B). Serum testing for IgG and IgM antibodies to Bartonella species and interpretation of the results were performed as recommended by the manufacturers. Each test was read independently by two persons who were blinded regarding the clinical data. Interobserver differences never exceeded one dilution step. Titers are reported as the reciprocals of serum dilutions.
For both IgG and IgM, test A incorporates human larynx carcinoma cells which have been infected with B. henselae Houston (ATCC 19882). Titers of ≥64 were defined as positive for both Ig classes by the manufacturer.
For IgG antibodies test B incorporates Vero cells which have been infected with either B. henselae or Bartonella quintana, the individual substrates permitting the qualitative detection and quantification of human serum IgG antibodies to Bartonella. For this test as well titers of ≥64 are considered positive. For IgM antibodies test B includes purified B. henselae and B. quintana cells diluted in yolk sac fluid, which permits the qualitative detection and quantification of human serum IgM antibodies to Bartonella. Titers of ≥20 were regarded as positive. In contrast to all the other tests, the serum dilution recommended for IgM detection in test B starts at 1:20.
RESULTS
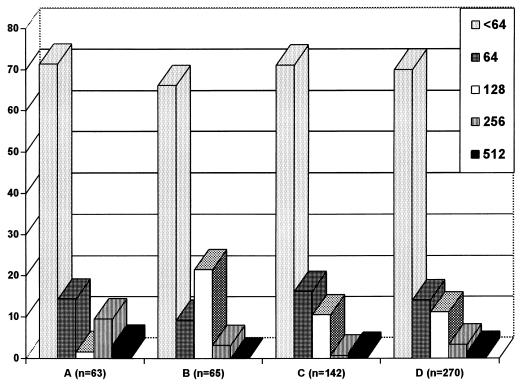
The results of test A for the students’ sera are shown in Fig. 1. Of the 270 control sera 189 (70%) were seronegative (titer, <64); 84% of all students had titers of ≤64, and 95% had titers of ≤128. Four (1.5%) individuals had titers of 512, and none of the control sera showed titers above 512. The data show a relatively high seroprevalence (30%) of antibodies to B. henselae at low titers in German individuals. It remains to be clarified if the high seroprevalence in healthy individuals is evidence of prior or active infection or merely of cross-reactivity to other agents. There were no differences between the cat owners and the other controls. Of the 55 sera from patients with other lymphadenopathy diseases (due to CMV, EBV, T. gondii, or C. pneumoniae) only 5 (9%) had a titer of 64 (2 from patients infected with EBV and 3 from patients infected with C. pneumoniae). These results suggest that there is apparently no substantial serological cross-reactivity between B. henselae and these infectious agents.
FIG. 1.
Prevalence (expressed as percentages) of IgG antibodies to B. henselae (test A) in healthy young adults. A, group A; B, group B; C, group C; D, groups A, B, and C.
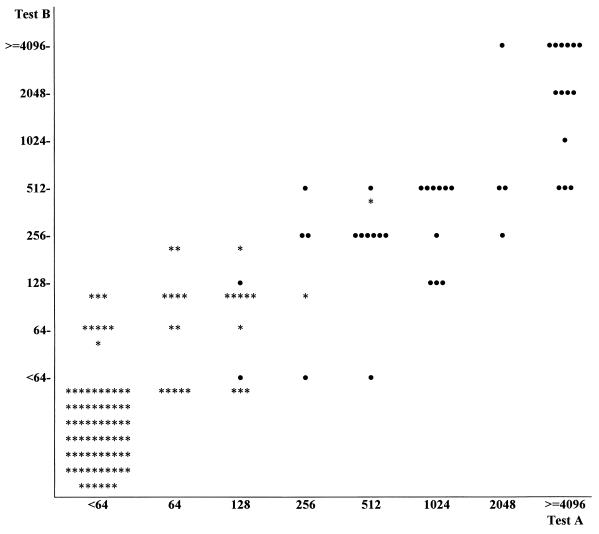
Comparison of the two tests (A and B) for IgG antibodies (Fig. 2) showed concordant results for 83% of the 100 control sera (both negative for 66% and both positive for 17%); 8 and 9 sera were positive only in test A and only in test B, respectively. The results for the patient group showed an overall concordance, with 39 sera (93%) found to be positive in both tests; 3 sera were positive only in test A. However, of the concordant sera only 10 had the same titers in both tests; 16 and 14 were one dilution step higher and two or more steps higher in test A, respectively, and only 2 sera had higher titers in test B.
FIG. 2.
Comparison of B. henselae IgG detection by the two IFAs. •, patients (n = 42); ∗, controls (the first 100 healthy adults who reported never having had close contact with cats).
Comparison of the IgM test results (Fig. 3) is possible only if the different dilutions steps (multiples of 1:64 in test A and of 1:20 in test B) are accepted as equal. For 83% of sera from the control group the two test results were concordant (82 negative and 1 positive). Thirteen percent were negative in test A but positive in test B, while only 4 sera negative in test B were positive in test A. Of the 42 sera from patients with CSD 30 (71.4%) showed concordant results, either positive (26 sera) or negative (4 sera). Eleven sera (26.2%) were positive in test A but negative in test B, and only 1 serum was negative in test A but positive in test B. A comparison of titers is not feasible because of the differences in dilution. In general test A seems to result in higher titers than test B. The sensitivities and specificities of the two B. henselae IFAs are shown in Table 1.
FIG. 3.
Comparison of B. henselae IgM detection by the two IFAs. •, patients (n = 42); ∗, controls (the first 100 healthy adults who reported never having had close contact with cats).
TABLE 1.
Sensitivities and specificities of B. henselae assaysa
| IFA | Test groupb | n | Positive (n) | Negative (n) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| IgG (test A) | PCR, H | 20 | 20 | 0 | 100 | |
| Clinical | 22 | 22 | 0 | 100 | ||
| Control (total) | 270 | 81 | 189 | 70 | ||
| Controlc | 100 | 25 | 75 | 75 | ||
| LA | 55 | 5 | 50 | 91 | ||
| IgM (test A) | PCR, H | 20 | 16 | 4 | 80 | |
| Clinical | 22 | 21 | 1 | 95 | ||
| Controlc | 100 | 5 | 95 | 95 | ||
| IgG (test B) | PCR, H | 20 | 17 | 3 | 85 | |
| Clinical | 22 | 22 | 0 | 100 | ||
| Controlc | 100 | 27 | 73 | 73 | ||
| IgM (test B) | PCR, H | 20 | 10 | 10 | 50 | |
| Clinical | 22 | 17 | 5 | 77 | ||
| Controlc | 100 | 14 | 86 | 86 |
Sensitivity was determined by dividing the number of positive cases by the total number of cases and multiplying the quotient by 100; specificity was determined by dividing the number of negative controls by the total number of controls and multiplying the quotient by 100.
PCR, H, patients for whom PCR and histologic analysis results were consistent with CSD; clinical, patients clinically suspected of having CSD; LA, patients with lymphadenopathy caused by infectious agents other than Bartonella spp.
Sera from the first 100 healthy adults in group C.
A high percentage of the sera tested (5% of the control sera and 95% of the patients’ sera) showed antibodies to B. quintana, at titers ranging from 64 to 32,000 (test B). Among the 38 patients with CSD with titers of antibodies to both B. henselae and B. quintana of ≥64, 6 had higher titers of antibody to B. henselae, 14 had higher titers of antibody to B. quintana, and 18 had equal titers of antibodies to both species. Of the 100 sera from group C tested by both assays only 5 had antibodies to both B. henselae and B. quintana, 22 had antibodies only to B. henselae, and none had antibodies only to B. quintana.
DISCUSSION
Since B. henselae was identified as the etiologic agent of CSD several diagnostic laboratory procedures have been developed. Isolation of Bartonella is time-consuming and often not successful. PCR is a rapid and specific method of detecting the organism in clinical samples but requires appropriate laboratory facilities and equipment. For histopathologic or immunohistochemical examination lymph node excision or biopsy is required. Therefore, for many clinical laboratories the most practical diagnostic method of confirming clinically suspected CSD is serologic testing. Specific serological tests have been evaluated recently and are available.
In 1992, Regnery et al. (8) developed a Bartonella IFA at the Centers for Disease Control and Prevention. Eighty-eight percent (36 of 41) of patients with suspected CSD were seropositive for B. henselae, with titers of ≥64, and 94% (101 of 107) of the sera of healthy controls were negative. Antibodies to B. quintana were not detected in sera from the control group. Using the same Bartonella IFA, Zangwill et al. (12) confirmed a sensitivity of 84% (38 of 45 patients with CSD were found to be seropositive) and a specificity of 96% (108 of 112 control sera were negative) for this test. However, they found that a high percentage (18%) of asymptomatic family members had elevated B. henselae antibody titers. In a study performed in Switzerland, 20 of 20 (100%) children with CSD had IFA titers of antibody to B. henselae of ≥512, and 26 to 60% of controls living in various urban and rural regions were seropositive (titer, ≥64) (6). In contrast, only 3% (11 of 332) of the controls had titers of ≥256, and a cutoff level of 256 was proposed for this assay (6). In another study over 3,000 serum samples submitted for Bartonella serology to the Centers for Disease Control and Prevention were tested by IFA (3). Of 91 patients with profiles meeting a strict clinical definition of CSD, 86 (91%) had titers of antibody to either B. henselae or B. quintana of ≥64 (3). The majority of the first 600 patients’ sera were positive for both B. henselae and B. quintana (94%), indicating a high cross-reactivity between these two Bartonella species (3). The concordance for these two antigens in our study was 95% in the patient group but only 18% in the controls. Cross-reactivity between B. quintana and B. henselae was less frequently found in controls than in the patient group. This difference is probably caused by the high prevalence of low titers of antibody to Bartonella spp. in the control group. As the results indicate a considerable cross-reaction between the two species at higher titers, the IFA does not seem useful for etiological differentiation in cases which could be caused by either Bartonella species.
Barka et al. (1) described an enzyme immunoassay (EIA) for detection of B. henselae-reactive IgG, IgM, and IgA antibodies and suggested that the EIA was highly specific and more sensitive (95%) than the IFA. In contrast, serological investigations performed by Szelc-Kelly et al. (11) showed that the IFA measuring IgG antibodies was the most sensitive (83 to 93%) and the most specific (98%) serologic test and that the IgG EIA is not sensitive enough (sensitivity, 16 to 35%) for use in the clinical diagnosis of CSD.
We found a high seroprevalence of antibodies to B. henselae among the healthy young adults. Of the 270 individuals tested, 81 (30%), 43 (16%), 13 (5%), and 4 (1.5%) had titers of serum antibody to B. henselae of ≥64, ≥128, ≥256, and 512, respectively. Similar results were also found in Switzerland (6), and it seems possible that the seroprevalence in Europe is higher than that in the United States (6, 8, 11). Surprisingly, no differences in seroprevalence were found among the control subgroups. Of cat owners and individuals who reported never having had close contact with cats, 71.4 and 71.12%, respectively, were seronegative for B. henselae, and titers of 64, 128, 256, and 512 were found in 14.3 and 16.2%, 1.6 and 10.5%, 9.5 and 0.7%, and 3.2 and 1.4%, respectively. Our data suggest that cat owners are not more often infected by B. henselae. It remains to be clarified if there are additional vectors for transmission of B. henselae. Moreover, an influence from cross-reactions, especially with antibodies to B. quintana or other agents, cannot be excluded.
EBV, CMV, and T. gondii infections are important differential diagnoses of lymphadenitis, especially in children. No significant serological cross-reactivity was found between B. henselae and these infectious agents in the sera we tested. The two commercially available tests are both highly sensitive but not as highly specific for detection of B. henselae IgG antibodies (Fig. 2). Based on our results, titers of 256 or higher in both tests together with symptoms of CSD strongly indicate an active disease (predictive values, 95% for test A and 90% for test B). This supports the results of Nadal and Zbinden (6). Concerning the detection of IgM antibodies, there was a marked difference between the two IFA systems, with sensitivity and specificity of 88 and 95%, respectively, for the BION IgM IFA and 64 and 86%, respectively, for the MRL IgM IFA. The positive predictive value for test A, with titers of ≥128, (100%) is sufficiently high. Test B, with titers of ≥40, is less reliable (positive predictive value, 70%).
In both test systems, IgM antibodies were less often detected in patients with histologically diagnosed CSD than in the patients with clinically diagnosed CSD. Usually lymph node biopsies are performed at a later stage of lymphadenopathy, and for these patients serological investigations for CSD are probably done later in the course of the illness than for the patients with early clinically diagnosed CSD.
The application of these serologic tests is very useful in the diagnosis of CSD, avoiding invasive surgical diagnostic procedures and the CSD skin test. Serologic titers in symptomatic patients with CSD, when positive, were usually high (references 3 and 6 and our results). Low antibody levels could indicate the onset of CSD or prior contact with B. henselae. If in the case of clinically suspected CSD the first titer is low, a titer increase in a serum specimen obtained later in disease should confirm the diagnosis.
REFERENCES
- 1.Barka N E, Hadfield T, Patnaik M, Schwartzman W A, Peter J B. EIA for detection of Rochalimaea henselae-reactive IgG, IgM and IgA antibodies in patients with suspected cat-scratch disease. J Infect Dis. 1993;167:1503–1504. doi: 10.1093/infdis/167.6.1503. [DOI] [PubMed] [Google Scholar]
- 2.Carithers H A, Carithers C M, Edwards R O. Cat-scratch disease, its natural history. JAMA. 1969;207:312–316. [PubMed] [Google Scholar]
- 3.Dalton M J, Robinson L E, Cooper J, Regnery R L, Olson J G, Childs J E. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national referral center. Arch Intern Med. 1995;155:1670–1676. [PubMed] [Google Scholar]
- 4.Margileth A M. Antibiotic therapy for cat-scratch disease: clinical study of therapeutic outcome in 268 patients and a review of the literature. Pediatr Infect Dis J. 1992;11:474–478. [PubMed] [Google Scholar]
- 5.Margileth A M. Sorting out the causes of lymphadenopathy. Contemp Pediatr. 1995;12:23–40. [Google Scholar]
- 6.Nadal D, Zbinden R. Serology to Bartonella (Rochalimaea) henselae may replace traditional diagnostic criteria for cat-scratch disease. Eur J Pediatr. 1995;154:906–908. doi: 10.1007/BF01957503. [DOI] [PubMed] [Google Scholar]
- 7.Raoult D, Tissot Dupont H, Enea-Mutillod M. Positive predictive value of Rochalimaea henselae antibodies in the diagnosis of cat-scratch disease. Clin Infect Dis. 1994;19:355. doi: 10.1093/clinids/19.2.355. [DOI] [PubMed] [Google Scholar]
- 8.Regnery R L, Olson J G, Perkins B A, Bibb W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet. 1992;339:1443–1445. doi: 10.1016/0140-6736(92)92032-b. [DOI] [PubMed] [Google Scholar]
- 9.Relman D A, Loutit J S, Schmidt T M, Falkow S, Tompkins L S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 10.Sander A, Bühler C, Pelz K, von Cramm E, Bredt W. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J Clin Microbiol. 1997;35:584–587. doi: 10.1128/jcm.35.3.584-587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szelc-Kelly C M, Goral S, Perez-Perez G I, Perkins B A, Regnery R L, Edwards K M. Serologic response to Bartonella and Afipia antigens in patients with cat-scratch disease. Pediatrics. 1995;96:1137–1142. [PubMed] [Google Scholar]
- 12.Zangwill K M, Hamilton D H, Perkins B A, Regnery R L, Plikaytis B D, Hadler J L, Cartter M L, Wenger J D. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993;329:8–13. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]