Abstract
Purpose
To determine whether peripheral blood leukocyte numbers and serum markers of inflammation can be used to predict which patients with primary uveal melanoma will develop metastasis.
Design
Retrospective study.
Participants
Medical records of patients with uveal melanoma (UM) who received treatment for primary UM between February 1992 and December 2020 at the Erasmus University Medical Center (Rotterdam, The Netherlands) and the Rotterdam Eye Hospital (Rotterdam, The Netherlands) were reviewed.
Methods
Inclusion criteria were the presence of a melanoma of the choroid or ciliary body and the availability of data from peripheral blood samples taken before treatment of the melanoma. Data including patient demographics, C-reactive protein (CRP) levels; erythrocyte sedimentation rate (ESR); number of leukocytes, neutrophils, monocytes, and lymphocytes; and histopathologic findings were obtained from medical records. Neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) were calculated.
Main Outcome Measures
Metastasis-free survival.
Results
Of the 807 patients with UM, serum and leukocyte data were available for 183 of them at the time of primary tumor treatment. In the total group, no correlation was found between ESR before treatment; the number of leukocytes; percentages of neutrophils, monocytes, and lymphocytes; or NLR or LMR values and any of the clinical characteristics or metastasis-free survival. Among patients who underwent enucleation, those with negative BAP1 findings showed significantly lower numbers of leukocytes (P < 0.05). In the entire cohort, a significant association was found between high CRP levels and longer metastasis-free survival (MFS; P = 0.049).
Conclusions
The total blood leukocyte number was related to loss of BAP1 staining in patients who underwent enucleation, with lower leukocyte counts correlating with absent BAP1 staining. Higher CRP levels were associated with a longer MFS in the entire cohort. Neither the NLR nor the LMR is a good predictor for metastasis developing in patients with UM.
Keywords: Leukocytes, Markers of inflammation, Metastasis, Neutrophil-to-lymphocyte ratio, Uveal melanoma
Abbreviations and Acronyms: CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GNA11, guanine nucleotide-binding protein, subunit alpha-11; GNAQ, guanine nucleotide-binding protein, q polypeptide; HR, hazard ratio; LMR, lymphocyte-to-monocyte ratio; MFS, metastasis-free survival; NLR, neutrophil-to-lymphocyte ratio; UM, uveal melanoma
Although uveal melanoma (UM) is a rare type of melanoma, it constitutes the most common primary intraocular malignancy in adults. Uveal melanoma arises from melanocytes and is located mainly in the choroid (>90%).1 The incidence of UM in the United States and Northern Europe is approximately 6 to 7 per million.2,3
Secondary somatic driver mutations and chromosomal abnormalities play a role in the development of metastasis: somatic mutations in the SF3B1 and EIF1AX genes are associated with normal copies of chromosome 3 and carry a relatively favorable prognosis.4, 5, 6 Monosomy 3 is often seen in combination with a somatic mutation in the BAP1 gene and carries a high chance of metastasis, leading to a poor prognosis.7,8
Inflammation is a known major driver for the development and progression of cancer. Immune cells can have either a protumoral or an antitumoral role, which is regulated by cytokines released in the tumor microenvironment. The presence of monosomy 3 and loss of BAP1 expression in UM is associated with an inflammatory phenotype, which, in contrast with many other tumors, is associated with a bad prognosis.9,10 This inflammatory phenotype is characterized by a high density of infiltrating macrophages and T cells.10, 11, 12
Neutrophils are associated with tumor progression and poor prognosis. It seems that neutrophils generate a niche for seeding, indicated by the large number of neutrophils accumulating at metastatic sites.13, 14, 15 Neutrophils are important players in the metastatic process of several malignancies: they play a protumorigenic role in the early stages of cancer biology in cutaneous melanoma and colorectal, lung, and breast cancer.16,17 Neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) in the peripheral blood have a robust prognostic value associated with worse overall survival in many of these malignancies. Both are inexpensive markers of systemic inflammation.15,18, 19, 20 In UM tissue, neutrophils are quite rare; although the role of inflammation and tumor-infiltrating macrophages in UM has been extensively reviewed, it is yet unknown whether peripheral blood neutrophils or monocytes have any adverse function in UM, especially with regard to outgrowth of metastasis.
We hypothesized that systemic inflammation may also play a role in the prognosis of UM. Well-known markers of systemic inflammation are the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), NLR, LMR, and high numbers of peripheral blood leukocytes. The objectives of this study were to determine if patients with UM who have an increased risk of metastasis developing already have aberrant markers of inflammation at the time of treatment of the primary UM. We analyzed several of these markers under the hypothesis that an aberrant ESR, CRP, leukocyte count, LMR, or NLR could be an indication of systemic inflammation and specifically would occur in those who later demonstrate metastasis. To our knowledge, this is the first study to analyze systemic inflammation markers in patients with UM. This may provide insight into the systemic changes at the time that metastases have not yet become clinically detectable.
Methods
Design and Participants
This is a retrospective study of medical records of patients with UM who received treatment for primary UM. Data were collected between February 1992 and December 2020 at the Erasmus University Medical Center (Rotterdam, The Netherlands) and the Rotterdam Eye Hospital (Rotterdam, The Netherlands). A total of 807 patients received treatment for UM. The research followed the tenets of the Declaration of Helsinki. The local ethics committee waived the need for its approval. Participants provided informed consent at the Erasmus University Medical Center.
Histopathologic analysis included tumor largest basal diameter, tumor thickness, cell type, ciliary body involvement, extraocular extension, presence of epithelial cells and necrosis, and immunohistochemical staining for BAP1.21 Inclusion criteria were: having a melanoma of the choroid or ciliary body and data from peripheral blood samples taken before any treatment of the UM, including any surgical therapy. Clinicopathologic characteristics, including patient demographics; CRP levels; ESR; and total number of leukocytes with percentages of lymphocytes monocytes, and neutrophils were obtained from the medical records. Patients with elevated levels of leukocytes (>11 × 109/l) were excluded because this could be the result of other factors, such as an active infection or autoimmune disease.
Main Outcomes and Measures
The NLRatio and LMR were obtained by dividing the total neutrophil fraction by the total lymphocyte fraction and the total lymphocyte fraction by the total monocyte fraction, respectively. The NLR and LMR were graded as either high or low, using the median as a cutoff point. Metastasis-free survival was the main outcome measure of this study. Secondary outcome measures were CRP levels, ESR, and leukocyte counts and the relationship with secondary oncogenic driver mutations, chromosomal abnormalities, and histopathologic findings of the tumor. The Rotterdam Ocular Melanoma Study cohort provided information on clinical and pathologic characteristics, and all patients provided informed consent.6
Peripheral Blood Markers
C-reactive protein levels; ESR; total leukocyte numbers; and percentages of neutrophils, basophils, eosinophils, lymphocytes, and monocytes in peripheral blood were analyzed with automated blood fluid module and matching reagents. Before 2003, the Advia (Siemens Healthcare Diagnostics, Ltd) was used to measure peripheral blood markers; from 2003 through 2013, the Sysmex XE (Sysmex Corporation) was used; and from 2013 onward, the Sysmex XN 9000 (Sysmex Corporation) was used.
Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation and associations among the groups were evaluated using the independent-samples t test. Categorical variables were expressed as absolute frequencies and percentages and were compared between groups with the chi-square test. Metastasis-free survival (MFS) was calculated as the interval between the date of diagnosis and the detection of metastasis or the date of death or last follow-up for surviving patients. Patients who were alive at the last visit or who were lost to follow-up were censored in the analysis. Kaplan-Meier analysis using log-rank testing estimated the difference in survival between patients with high and low ESR, CRP levels, leukocyte counts, neutrophil counts, basophil counts, eosinophil counts, lymphocyte counts, monocyte counts, NLR, and LMR in the complete cohort, with the cutoff for high and low values defined as the median. A P value of < 0.05 (2-sided) was considered to reflect a significant difference. SPSS software version 25 (SPSS IBM) was used to perform the analyses.
Results
Study Population
Information on inflammatory markers before treatment was available for 195 patients. Of those 195 patients, 12 patients had leukocyte counts of more than 11 × 109/l, suggesting an infectious condition, and these were excluded. Mean age at diagnosis of the included 183 patients was 65 years, and 49% of the participants were men. Tumors were treated with enucleation (45%), stereotactic radiotherapy (42%), brachytherapy (6%), transpupillary thermotherapy (1%), photodynamic therapy (1%), or proton beam therapy (4%). Two patients (1%) already showed dissemination when UM was diagnosed and did not undergo UM treatment. We therefore excluded them from the analysis. The CRP level of the 2 patients with disseminated disease at diagnosis was 1.0 mg/l and 11.0 mg/l. Of 183 patients, 62 (34%) demonstrated metastasis. For 91 patients, tumor tissue was available for pathologic assessment. Baseline demographic data for all included patients and patients treated with enucleation are presented in Tables 1 and 2.
Table 1.
Demographic and Clinical Characteristics of Patients with Uveal Melanoma Analyzed for Neutrophil-to-Lymphocyte Ratio (n = 183)
| Characteristic | Data |
|---|---|
| Sex | |
| Male | 89 (49) |
| Female | 94 (51) |
| Age at diagnosis (yrs), mean (range) | 65 (19–89) |
| Follow-up time (mos), median/mean (range) | 21/42 (0–1437) |
| Primary treatment | |
| Enucleation | 82 (45) |
| Stereotactic radiotherapy | 77 (42) |
| Brachytherapy | 10 (6) |
| Transpupillary thermotherapy | 2 (1) |
| Photodynamic therapy | 2 (1) |
| Proton beam therapy | 8 (4) |
| No therapy | 2 (1) |
| Metastasis | |
| No | 121 (66) |
| Yes | 62 (34) |
Data are presented as no. (%), unless otherwise indicated.
Table 2.
Pathologic Characteristics of Patients Treated Who Underwent Enucleation (n = 82)
| Characteristic | Data |
|---|---|
| Sex | |
| Male | 45 (55) |
| Female | 37 (45) |
| Age at diagnosis (yrs), mean (range) | 62 (28–88) |
| Follow-up time (mos), median/mean (range) | 23/49 (0–272) |
| Metastasis | |
| No | 43 (52) |
| Yes | 39 (48) |
| Tumor diameter (mm), mean (range) | 13.4 (3–23) |
| Cell type | |
| Spindle cell | 27 (33) |
| Epithelioid | 13 (16) |
| Mixed | 42 (51) |
| Primary tumor location | |
| Choroid | 66 (81) |
| Ciliary body | 16 (19) |
| BAP1 staining results | |
| Negative | 40 (49) |
| Positive | 28 (34) |
| Not determined | 14 (17) |
| AJCC T classification | |
| T1 | 11 (13) |
| T2 | 24 (29) |
| T3 | 38 (47) |
| T4 | 9 (11) |
| Pretreatment NLR, median (SD) | 2.72 (1.47) |
| Pretreatment LMR, median (SD) | 3.28 (1.38) |
AJCC = American Joint Committee on Cancer; LMR = lymphocyte-to-monocyte ratio; NLR = neutrophil-to-lymphocyte ratio; SD = standard deviation.
Data are presented as no. (%), unless otherwise indicated.
For the entire cohort of 183 patients, the mean NLR before treatment was 3.0 (median, 2.7; range, 0.9–7.7) and the mean LMR before treatment was 3.4 (median, 3.1; range, 0.9–9.3). As for patients who underwent enucleation, tissue was available for analyses; therefore, this group was also evaluated separately. Of these patients, 55% were men; the mean age at diagnosis was 62 years. Almost half of these patients (48%) demonstrated metastasis (Table 2).
Peripheral Blood Markers in Full Cohort of 183 Patients in Relationship to Clinical Characteristics
We analyzed whether clinical and pathologic characteristics were related to blood values using an independent-samples t test. No significant differences in ESR, CRP levels, or total leukocyte numbers were found in the entire cohort in relationship to primary tumor location or the American Joint Committee on Cancer T classification (Table 3). Similarly, no significant difference in the percentages of blood neutrophils, monocytes, or lymphocytes; NLR; or LMR were observed (Table 4).
Table 3.
Erythrocyte Sedimentation Rate, C-Reactive Protein Levels, and Total Leukocyte Number in Relationship to Clinical and Histopathologic Findings in the Total Cohort of 183 Patients and the 82 Patients Who Underwent Enucleation for Uveal Melanoma Analyzed with an Independent Samples t Test
| Clinical and Pathologic Findings | Erythrocyte Sedimentation Rate |
C-Reactive Protein Level |
Leukocytes (109/l) |
|||
|---|---|---|---|---|---|---|
| Mean ± Standard Deviation | P Value | Mean ± Standard Deviation | P Value | Mean ± Standard Deviation | P Value | |
| All patients | ||||||
| Tumor location | ||||||
| Choroid (n = 151) | 11.6 ± 13.4 (n = 91) | 0.71 | 4.6 ± 11.8 (n = 95) | 0.82 | 7.6 ± 1.6 (n = 150) | 0.12 |
| Ciliary body (n = 20) | 13.2 ± 12.8 (n = 12) | 3.8 ± 4.3 (n = 12) | 6.8 ± 1.9 (n = 19) | |||
| AJCC T classification | ||||||
| T1 (n = 26) | 9.0 ± 8.1 (n = 18) | 0.40 | 2.8 ± 2.6 (n = 16) | 0.25 | 7.8 ± 1.4 (n = 26) | 0.51 |
| T2 (n = 49) | 10.7 ± 15.3 (n = 32) | 5.1 ± 14.0 (n = 33) | 7.4 ± 1.7 (n = 48) | |||
| T3 (n = 73) | 12.4 ± 12.1 (n = 41) | 3.2 ± 3.4 (n = 45) | 7.5 ± 1.7 (n = 72) | |||
| T4 (n = 21) | 17.4 ± 18.1 (n = 11) | 10.1 ± 23.0 (n = 12) | 7.1 ± 1.7 (n = 21) | |||
| Patients who underwent enucleation | ||||||
| Ciliary body involvement | ||||||
| No (n = 52) | 9.3 ± 7.1 (n = 22) | 0.50 | 7.7 ± 16.3 (n = 24) | 0.24 | 7.7 ± 1.6 (n = 52) | 0.24 |
| Yes (n = 28) | 11.6 ± 10.2 (n = 12) | 2.6 ± 3.3 (n = 15) | 7.2 ± 1.8 (n = 26) | |||
| Presence of epithelioid cells | ||||||
| No (n = 25) | 10.2 ± 7.5 (n = 9) | 0.94 | 6.6 ± 5.3 (n = 13) | 0.74 | 7.8 ± 1.9 (n = 23) | 0.24 |
| Yes (n = 56) | 10.0 ± 8.5 (n = 26) | 5.2 ± 15.1 (n = 28) | 7.4 ± 1.6 (n = 56) | |||
| Necrosis | ||||||
| No (n = 47) | 9.1 ± 7.7 (n = 27) | 0.63 | 3.0 ± 3.0 (n = 30) | 0.55 | 7.5 ± 1.8 (n = 47) | 0.71 |
| Yes (n = 16) | 11.0 ± 9.0 (n = 5) | 2.4 ± 2.7 (n = 9) | 7.3 ± 1.6 (n = 15) | |||
| Extraocular extension | ||||||
| No (n = 54) | 9.0 ± 7.8 (n = 26) | 0.23 | 3.2 ± 2.5 (n = 27) | 0.57 | 7.4 ± 1.8 (n = 52) | 0.80 |
| Yes (n = 13) | 13.1 ± 8.9 (n = 7) | 2.5 ± 2.9 (n = 8) | 7.6 ± 1.5 (n = 13) | |||
| BAP1 staining | ||||||
| Positive (n = 28) | 9.1 ± 7.0 (n = 16) | 0.79 | 4.1 ± 4.8 (n = 19) | 0.42 | 8.0 ± 1.7 (n = 27) | 0.034 |
| Negative (n = 40) | 9.8 ± 8.7 (n = 16) | 2.9 ± 3.6 (n = 17) | 7.1 ± 1.6 (n = 39) | |||
AJCC = American Joint Committee on Cancer.
Boldface indicates statistical significance.
Table 4.
Neutrophil, Monocyte, and Lymphocyte Fractions, Neutrophil-to-Lymphocyte Ratio, and Lymphocyte-to-Monocyte Ratio in Relationship to Clinical and Histopathologic Findings in the Total Cohort of 183 Patients and in the 82 Patients Who Underwent Enucleation for Uveal Melanoma Analyzed with an Independent-Samples t Test
| Clinical and Pathologic Findings | Neutrophils (%) |
Monocytes (%) |
Lymphocytes (%) |
Neutrophil-to-Lymphocyte Ratio |
Lymphocyte-to-Monocyte Ratio |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± Standard Deviation | P Value | Mean ± Standard Deviation | P Value | Mean ± Standard Deviation | P Value | Mean ± Standard Deviation | P Value | Mean ± Standard Deviation | P Value | |
| All patients | ||||||||||
| Primary tumor location | ||||||||||
| Choroid (n = 151) | 65.2 ± 7.9 | 0.95 | 7.9 ± 2.2 | 0.23 | 24.3 ± 6.7 | 0.74 | 3.0 ± 1.3 | 0.59 | 3.3 ± 1.4 | 0.21 |
| Ciliary body (n = 20) | 65.3 ± 10.0 | 7.3 ± 2.4 | 24.9 ± 8.6 | 3.2 ± 1.8 | 3.7 ± 1.5 | |||||
| AJCC T classification | ||||||||||
| T1 (n = 26) | 63.6 ± 9.6 | 0.76 | 7.8 ± 2.4 | 0.90 | 25.6 ± 8.5 | 0.79 | 2.9 ± 1.6 | 0.96 | 3.5 ± 1.5 | 0.72 |
| T2 (n = 49) | 65.6 ± 8.1 | 7.7 ± 2.5 | 24.3 ± 6.4 | 3.0 ± 1.3 | 3.4 ± 1.5 | |||||
| T3 (n = 73) | 64.5 ± 8.1 | 7.8 ± 2.2 | 24.0 ± 6.9 | 3.1 ± 1.3 | 3.3 ± 1.4 | |||||
| T4 (n = 21) | 65.1 ± 7.1 | 8.1 ± 1.4 | 24.4 ± 6.7 | 3.0 ± 1.2 | 3.1 ± 1.1 | |||||
| Patients who underwent enucleation | ||||||||||
| Ciliary body involvement | ||||||||||
| No (n = 52) | 64.7 ± 9.2 | 0.11 | 7.8 ± 2.2 | 0.12 | 24.9 ± 7.3 | 0.26 | 3.0 ± 1.4 | 0.30 | 3.4 ± 1.2 | 0.38 |
| Yes (n = 28) | 68.1 ± 8.6 | 7.0 ± 2.3 | 23.0 ± 7.6 | 3.4 ± 1.6 | 3.7 ± 1.6 | |||||
| Presence of epithelioid cells | ||||||||||
| No (n = 25) | 65.9 ± 7.7 | 0.84 | 7.4 ± 1.7 | 0.90 | 24.4 ± 6.3 | 0.89 | 3.0 ± 1.3 | 0.75 | 3.4 ± 1.1 | 0.66 |
| Yes (n = 56) | 65.4 ± 9.7 | 7.5 ± 2.4 | 24.6 ± 8.0 | 3.1 ± 1.6 | 3.6 ± 1.5 | |||||
| Necrosis | ||||||||||
| No (n = 47) | 64.5 ± 9.5 | 0.17 | 7.5 ± 2.3 | 0.69 | 25.6 ± 7.6 | 0.052 | 2.9 ± 1.4 | 0.12 | 3.6 ± 1.2 | 0.07 |
| Yes (n = 16) | 68.1 ± 7.1 | 7.8 ± 2.1 | 21.4 ± 6.4 | 3.5 ± 1.4 | 3.0 ± 1.4 | |||||
| Extraocular extension | ||||||||||
| No (n = 54) | 4.7 ± 8.8 | 0.22 | 7.4 ± 2.2 | 0.29 | 25.3 ± 7.3 | 0.13 | 2.9 ± 1.3 | 0.11 | 3.7 ± 1.5 | 0.09 |
| Yes (n = 13) | 68.1 ± 8.4 | 8.2 ± 2.7 | 21.8 ± 7.3 | 3.6 ± 1.7 | 2.9 ± 1.3 | |||||
| BAP1 staining | ||||||||||
| Positive (n = 28) | 64.8 ± 8.2 | 0.72 | 7.1 ± 1.9 | 0.17 | 25.2 ± 6.7 | 0.53 | 2.9 ± 1.6 | 0.31 | 3.7 ± 1.3 | 0.09 |
| Negative (n = 40) | 65.8 ± 9.9 | 7.9 ± 2.3 | 24.0 ± 8.1 | 3.2 ± 1.6 | 3.2 ± 1.2 | |||||
AJCC = American Joint Committee on Cancer.
Peripheral Blood Markers versus Clinical and Histopathologic Characteristics in Patients with Uveal Melanoma Treated with Enucleation
Because histopathologic data were available for a group of 82 patients who underwent enucleation (Table 2), we subsequently compared blood values with histologic parameters. We observed a significant difference in leukocytes based on BAP1 staining: patients with tumor tissue that stained positive for BAP1 (a good prognostic sign) showed higher total leukocyte numbers compared with those with negative staining results, 8.0 × 109/l versus 7.1 × 109/l (P < 0.05). No correlation was found among ESR; CRP levels; total leukocyte numbers (Table 3); the number of neutrophils, monocytes, and lymphocytes; NLR; and LMR values before treatment (Table 4) and the following parameters: ciliary body involvement, the presence of epithelioid cells, necrosis, extraocular extension, BAP1 staining, tumor size as defined by the American Joint Committee on Cancer T classification, or the development of metastasis.
Blood Values and Metastasis-Free Survival
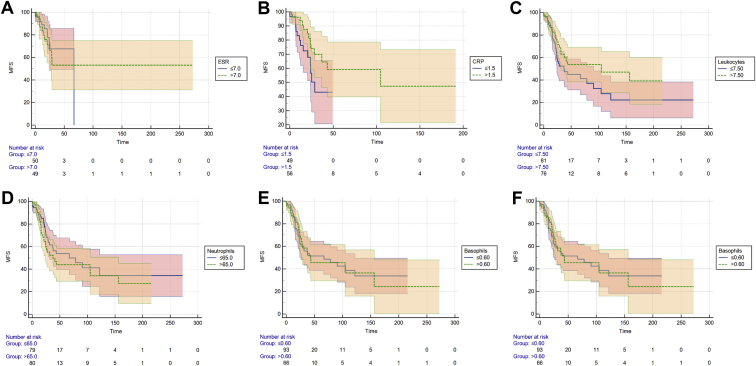
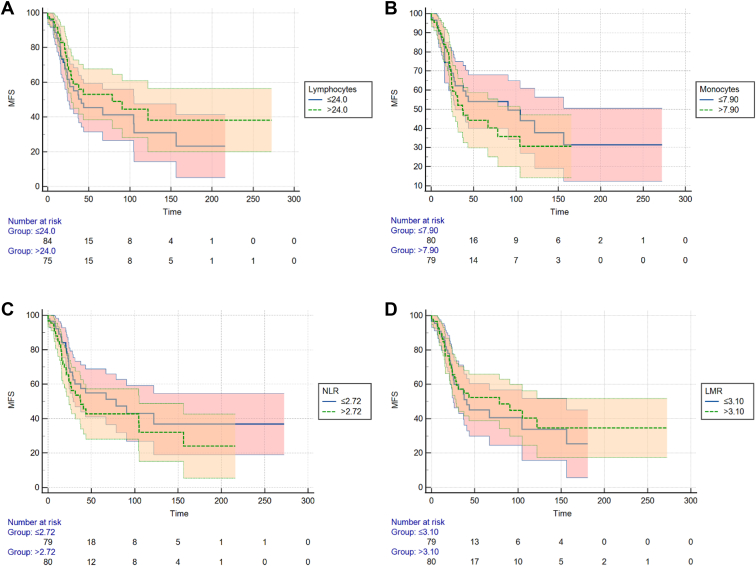
Kaplan-Meier analyses were created for the total cohort of 183 patients for ESR; CRP levels; the total number of leukocytes; percentages of neutrophils, basophils, eosinophils, lymphocytes, and monocytes; the NLR; and the LMR (Figs 1 and 2). A significant difference in MFS was observed in which patients with a longer MFS showed high CRP values (P = 0.049). The other parameters showed no significant differences in MFS between patients with high and low values.
Figure 1.
Kaplan-Meier curves for disease-free survival in the total cohort of 183 cases. A, Erythrocyte sedimentation rate (ESR), separated into low and high, based on the median value (P = 0.56, log-rank test). B, C-reactive protein (CRP) levels, separated into low and high, based on the median value (P = 0.049, log-rank test). C, Total number of leukocytes, separated into low and high, based on the median value (P = 0.13, log-rank test). D, Percentage of neutrophils, separated into low and high, based on the median value (P = 0.38, log-rank test). E, Percentage of basophils, separated into low and high, based on the median value (P = 0.85, log-rank test). F, Percentages of eosinophils, separated into low and high, based on the median value (P = 0.63, log-rank test). MFS = metastasis-free survival (%).
Figure 2.
Kaplan-Meier curves for disease-free survival in the total cohort of 183 cases. A, Percentage of lymphocytes, separated into low and high, based on the median value (P = 0.28, log-rank test). B, Percentage of monocytes, separated into low and high, based on the median value (P = 0.50, log-rank test). C, Neutrophil-to-lymphocyte ratio (NLR), separated into low and high, based on the median value (P = 0.21, log-rank test). D, Lymphocyte-to-monocyte ratio (LMR), separated into low and high, based on the median value (P = 0.68, log-rank test). MFS = metastasis-free survival (%).
Discussion
Because hematological tests are noninvasive and cost-effective, the NLR and LMR can act as simple and convenient parameters of a systemic inflammatory response. An increased NLR as calculated from peripheral blood samples has been shown to be an independent predictive marker for different malignancies,16,17 and several studies have demonstrated that a higher NLR or LMR is often associated with worse outcomes and advanced disease. The possible underlying mechanism is the presence of a chronic inflammatory reaction, which has been reported to be involved in tumor growth, invasion, and metastasis and has been reported as one of the hallmarks for cancer.22, 23, 24 An environment high in neutrophils is favorable for tumor development and progression.13, 14, 15 In many malignancies, a higher NLR is therefore associated with aggressive tumor behavior and negative treatment outcomes.25
We analyzed the NLR, LMR, and leukocyte numbers of patients with UM at the time of treatment of the primary tumor as well as some specific serum markers associated with systemic inflammation. To our knowledge, this study is the first to report on the prognostic implication of NLR and LMR in patients with UM. However, we did not find a correlation between the NLR or LMR and the development of metastasis in these patients.
When comparing other white blood cell counts, a difference in total leukocyte count between the patients with positive and negative BAP1 staining results was observed in the group treated with enucleation. Patients with negative BAP1 staining results showed lower leukocyte counts at the time of enucleation. Looking at the total cohort of 183 patients, the leukocyte counts in patients who demonstrated metastases showed a similar trend (P = 0.057). It is known that negative BAP1 staining results are associated with an increased number of tumor-associated lymphocytes and macrophages in tumor tissue and a high chance of metastasis. Therefore, it seems that high blood leukocyte counts may be associated with a favorable outcome for patients with UM opposed to the unfavorable association between the presence of tumor-associated leukocytes and survival in patients with UM.7,8
When we looked at the serum markers we noted an association between high CRP levels and long MFS in the entire cohort. C-reactive protein level has been suggested as a prognostic marker and an independent predictor in cutaneous melanoma, in which a markedly elevated CRP level identifies a subgroup of patients at high risk of disease recurrence and death.26 Again, we found a contradiction between cutaneous melanoma and UM: in cutaneous melanoma, an elevated CRP level was associated with a higher risk at recurrence, whereas in UM, elevated CRP levels are associated with a longer MFS. For many types of cancer, blood differential leukocyte parameters have well-established prognostic value, where an increased count in circulating neutrophils and monocytes is associated with adverse outcomes.13, 14, 15, 16 We did not find this, nor did we observe any other differences in peripheral blood cell markers between the patients who did and did not demonstrate metastasis.
This study confirms that UM differs from other types of cancer, and also when comparing it with cutaneous melanoma. The immunologic difference between UM and cutaneous melanoma might be one of the reasons most drugs used to treat metastatic cutaneous melanoma are largely ineffective in patients with UM.27,28 Only recently was tebentafusp found to result in longer overall survival among previously untreated patients with metastatic UM.29,30 An important difference between UM and other tumors is that UM cells benefit from the immune privilege in the eye and may adopt several mechanisms involved in this privilege for tumor escape that act even after leaving the niche.31
Some limitations of the present study should be considered when interpreting the data, such as the relatively small sample group and the study’s retrospective character, inherent to the rare type of malignancy studied. Patients with very elevated leukocyte counts were excluded because these usually are the result of other medical conditions, such as an infection or nonmalignant inflammatory disease. Another limitation may be lack of long follow-up. Approximately 40% of the patients with UM demonstrate metastasis, with a peak within 4 years after initial treatment.6 This raises the possibility that some of the patients included in the nonmetastasized group still could demonstrate metastasis at a later stage. This may have influenced the results of this study. The major limitation is that this was not a large series. It is important to repeat the study in a large series.
In conclusion, this study demonstrated that lower levels of peripheral blood leukocytes are associated with negative tissue BAP1 staining, which carries a bad prognosis. In contrast with cutaneous melanoma, high CRP levels in patients with UM are associated with a longer MFS. Neither NLR nor LMR seems to be a good predictor of metastasis developing in patients with UM. Further studies are needed to clarify the importance of these peripheral blood markers and ratios as biomarkers and to evaluate the exact clinical significance for patients with UM.
Manuscript no. XOPS-D-21-00159.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Dr. Martine J. Jager, an editorial board member of this journal, was recused from the peer-review process of this article and had no access to information regarding its peer-review.
HUMAN SUBJECTS: Human subjects were not included in this study. This is a retrospective study of medical records. Data was collected at the Erasmus University Medical Center and the Rotterdam Eye Hospital. The research followed the tenets of the Declaration of Helsinki. The local ethics committee waived the need for approval. Participants provided informed consent at the Erasmus University Medical Center.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Meijer, Jager, Kiliç
Analysis and interpretation: Meijer, Berendschot, Jager, Kiliç
Data collection: Meijer, de Bruyn, de Klein, Paridaens, Verdijk, Brosens, Jager, Kiliç
Obtained funding: N/A; Study was performed as part of the authors' regular employment duties. No additional funding was provided.
Overall responsibility: Meijer, Jager, Kiliç
References
- 1.Grisanti S., Tura A. In: Noncutaneous Melanoma. Scott J.F., Gerstenblith M.R., editors. Codon Publications; Brisbane: 2018. Uveal melanoma; pp. 1–17. [Google Scholar]
- 2.Singh A.D., Turell M.E., Topham A.K. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118(9):1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Virgili G., Gatta G., Ciccolallo L., et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114(12):2309–2315. doi: 10.1016/j.ophtha.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 4.Furney S.J., Pedersen M., Gentien D., et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013;3(10):1122–1129. doi: 10.1158/2159-8290.CD-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin M., Masshofer L., Temming P., et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat Genet. 2013;45(8):933–936. doi: 10.1038/ng.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yavuzyigitoglu S., Koopmans A.E., Verdijk R.M., et al. Uveal melanomas with SF3B1 mutations: a distinct subclass associated with late-onset metastases. Ophthalmology. 2016;123(5):1118–1128. doi: 10.1016/j.ophtha.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 7.van Essen T.H., van Pelt S.I., Versluis M., et al. Prognostic parameters in uveal melanoma and their association with BAP1 expression. Br J Ophthalmol. 2014;98(12):1738–1743. doi: 10.1136/bjophthalmol-2014-305047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewens K.G., Kanetsky P.A., Richards-Yutz J., et al. Chromosome 3 status combined with BAP1 and EIF1AX mutation profiles are associated with metastasis in uveal melanoma. Invest Ophthalmol Vis Sci. 2014;55(8):5160–5167. doi: 10.1167/iovs.14-14550. [DOI] [PubMed] [Google Scholar]
- 9.Souri Z., Wierenga A.P.A., van Weeghel C., et al. Loss of BAP1 is associated with upregulation of the NFkB pathway and increased HLA class I expression in uveal melanoma. Cancers (Basel) 2019;11(8):1102. doi: 10.3390/cancers11081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gezgin G., Dogrusoz M., van Essen T.H., et al. Genetic evolution of uveal melanoma guides the development of an inflammatory microenvironment. Cancer Immunol Immunother. 2017;66(7):903–912. doi: 10.1007/s00262-017-1991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maat W., Ly L.V., Jordanova E.S., et al. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49(2):505–510. doi: 10.1167/iovs.07-0786. [DOI] [PubMed] [Google Scholar]
- 12.Bronkhorst I.H., Vu T.H., Jordanova E.S., et al. Different subsets of tumor-infiltrating lymphocytes correlate with macrophage influx and monosomy 3 in uveal melanoma. Invest Ophthalmol Vis Sci. 2012;53(9):5370–5378. doi: 10.1167/iovs.11-9280. [DOI] [PubMed] [Google Scholar]
- 13.Wculek S.K., Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thio Q., Goudriaan W.A., Janssen S.J., et al. Prognostic role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with bone metastases. Br J Cancer. 2018;119(6):737–743. doi: 10.1038/s41416-018-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahat M.A., Coffelt S.B., Granot Z., Muthana M., Amedei A. Macrophages and neutrophils: regulation of the inflammatory microenvironment in autoimmunity and cancer. Mediators Inflamm. 2016;2016:5894347. doi: 10.1155/2016/5894347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Templeton A.J., McNamara M.G., Seruga B., et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 17.Kenyon A., Gavriouchkina D., Zorman J., et al. Generation of a double binary transgenic zebrafish model to study myeloid gene regulation in response to oncogene activation in melanocytes. Dis Model Mech. 2018;11(4) doi: 10.1242/dmm.030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffelt S.B., Kersten K., Doornebal C.W., et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishijima T.F., Deal A.M., Lund J.L., et al. Inflammatory markers and overall survival in older adults with cancer. J Geriatr Oncol. 2019;10(2):279–284. doi: 10.1016/j.jgo.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Bi H., Yan Y., Wang D., et al. Predictive value of preoperative lymphocyte-to-monocyte ratio on survival outcomes in bladder cancer patients after radical cystectomy. J Cancer. 2021;12(2):305–315. doi: 10.7150/jca.50603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koopmans A.E., Verdijk R.M., Brouwer R.W., et al. Clinical significance of immunohistochemistry for detection of BAP1 mutations in uveal melanoma. Mod Pathol. 2014;27(10):1321–1330. doi: 10.1038/modpathol.2014.43. [DOI] [PubMed] [Google Scholar]
- 22.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 23.Moore M.M., Chua W., Charles K.A., Clarke S.J. Inflammation and cancer: causes and consequences. Clin Pharmacol Ther. 2010;87(4):504–508. doi: 10.1038/clpt.2009.254. [DOI] [PubMed] [Google Scholar]
- 24.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guthrie G.J., Charles K.A., Roxburgh C.S., et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Fang S., Wang Y., Sui D., et al. C-reactive protein as a marker of melanoma progression. J Clin Oncol. 2015;33(12):1389–1396. doi: 10.1200/JCO.2014.58.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin Y., Petaccia de Macedo M., Reuben A., et al. Parallel profiling of immune infiltrate subsets in uveal melanoma versus cutaneous melanoma unveils similarities and differences: a pilot study. Oncoimmunology. 2017;6(6) doi: 10.1080/2162402X.2017.1321187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jager M.J., Dogrusoz M., Woodman S.E. Uveal melanoma: identifying immunological and chemotherapeutic targets to treat metastases. Asia Pac J Ophthalmol (Phila) 2017;6(2):179–185. doi: 10.22608/APO.201782. [DOI] [PubMed] [Google Scholar]
- 29.Damato B.E., Dukes J., Goodall H., Carvajal R.D. Tebentafusp: T cell redirection for the treatment of metastatic uveal melanoma. Cancers (Basel) 2019;11(7):971. doi: 10.3390/cancers11070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan P., Hassel J.C., Rutkowski P., et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196–1206. doi: 10.1056/NEJMoa2103485. [DOI] [PubMed] [Google Scholar]
- 31.Niederkorn J.Y. Ocular immune privilege and ocular melanoma: parallel universes or immunological plagiarism? Front Immunol. 2012;3:148. doi: 10.3389/fimmu.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]