Abstract
Purpose
To investigate the 2-year effectiveness of reduced-fluence photodynamic therapy (rf-PDT) for chronic central serous chorioretinopathy (cCSC).
Design
Retrospective cohort study.
Participants
A total of 223 consecutive patients with newly diagnosed cCSC with active serous retinal detachment (SRD) were included from May 2007 to June 2017 and followed up for at least 2 years. Patients who underwent ocular treatment other than cataract surgery before the beginning of recruitment and those who had macular neovascularization at baseline were excluded.
Methods
All patients underwent a comprehensive ophthalmic evaluation, including measurements of best-corrected visual acuity (BCVA), slit-lamp examination, dilated fundus examination, color fundus photography, fundus autofluorescence, fluorescein angiography, indocyanine green angiography, and spectral-domain OCT. An inverse probability of treatment weighting (IPTW) methodology was applied to balance 18 baseline characteristics between patients who received rf-PDT (rf-PDT group) and those who did not receive treatment (controls). Inverse probability of treatment weighting survival analysis and regression were performed.
Main Outcome Measures
The proportion of patients whose BCVA at 24 months was the same or improved compared with the baseline visual acuity (VA) (VA maintenance rate).
Results
A total of 155 eyes (rf-PDT group: 74; controls: 81) were analyzed. The patients’ backgrounds were well balanced after IPTW with standardized differences of < 0.10. An IPTW regression analysis revealed that the VA maintenance rate was significantly higher in the rf-PDT group than in the controls (93.6% vs. 70.9%, P < 0.001, 12 months; 85.7% vs. 69.8%, P = 0.019, 24 months). The rf-PDT group tended to show better VA improvement, but was not statistically significant (–0.06 vs. –0.008, P = 0.07, 12 months; –0.06 vs. –0.03, P = 0.32, 24 months). An IPTW Cox regression showed a significantly higher rate of complete SRD remission in the rf-PDT group (hazard ratio, 5.05; 95% confidence interval, 3.24–7.89; P < 0.001).
Conclusions
The study suggests the beneficial effect of rf-PDT for cCSC for both VA maintenance and higher proportion of complete SRD remission in the clinical setting.
Abbreviations and Acronyms: AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; cCSC, chronic central serous chorioretinopathy; CSC, central serous chorioretinopathy; FA/ICGA, fluorescein angiography/indocyanine green angiography; hd-/hf-PDT, half-dose/half-fluence photodynamic therapy; IPTW, inverse probability of treatment weighting; logMAR, logarithm of the minimum angle of resolution; MNV, macular neovascularization; PDT, photodynamic therapy; PS, propensity score; RCT, randomized clinical trial; rf-PDT, reduced-fluence photodynamic therapy; RPE, retinal pigment epithelium; SRD, serous retinal detachment; VA, visual acuity
Keywords: Central serous chorioretinopathy, Propensity score, Reduced-fluence photodynamic therapy, Serous retinal detachment
Central serous chorioretinopathy (CSC) is a chorioretinal disease that can cause idiopathic serous detachment of the retina, commonly observed in middle-aged men.1 Patients often present with decreased vision, metamorphopsia, or central scotoma, and 58% of the cases are unilateral.2 Although the choroid is suggested to play a role in causing the disease, there are few reports on the epidemiology of the choroid.3 The latest nationwide population-based incidence in Japan is reportedly 54.2 (per 100 000 person-years) in men and 15.7 (per 100 000 person-years) in women, which suggests higher incidence of CSC in Asians compared with Whites.4
Historically, CSC has been recognized as a self-limiting benign condition, and the visual prognosis has been considered favorable. However, it is known that there is a clinically distinct entity characterized by retinal pigment epithelium (RPE) damage with a worse visual prognosis, namely, chronic CSC (cCSC). Mrejen et al5 recently reported the long-term visual prognosis of patients with cCSC and found that 12.8% of patients are socially blind in both eyes during a mean follow-up of 11.3 years. We have also reported that a known susceptibility gene for age-related macular degeneration (AMD), TNFRSF10A, showed a stronger association with CSC than with AMD using 1546 CSC samples and 13 029 controls. This indicated the possibility that in Asian individuals with AMD, there may be a large number of cases of macular neovascularization (MNV) secondary to CSC (recently named as “pachychoroid neovasculopathy”).6, 7, 8, 9 This finding also suggests the possibility of poor visual prognosis of cCSC. Because the majority of patients with newly diagnosed CSC are men of working age (peak of 41–45 years old),1 the social impact of CSC is currently being reevaluated.
Photodynamic therapy (PDT) has been recognized as an effective treatment for CSC. Moreover, half-dose/half-fluence PDT (hd-/hf-PDT) has been reported as a treatment option with similar efficacy and low risk of adverse events compared with full-dose/full-fluence PDT.10, 11, 12 Nevertheless, the number of studies comparing PDT with untreated controls is limited, including only 1 randomized clinical trial (RCT). Even this RCT had limitations because the study included a relatively small number of participants and targeted patients with acute CSC alone, that is, patients with CSC whose symptoms lasted < 3 months.13 In other words, there is no high-level evidence evaluating the effectiveness of reduced-fluence PDT (rf-PDT) for cCSC.
Therefore, this study aimed to evaluate the long-term effectiveness and safety of rf-PDT in patients with cCSC by comparing them with untreated controls. We investigated the real-world data of 223 consecutive patients with cCSC with a 2-year follow-up period and used propensity score (PS) analysis for minimizing the bias of confounding by indication.
Methods
This study was approved by the Institutional Review Board of Kyoto University Graduate School of Medicine (R0532-4). Informed consent was obtained from all patients before the start of the study. The procedures of our study followed the tenets of the Declaration of Helsinki.
Study Participants and Ophthalmic Examinations
From the Kyoto CSC cohort of 548 patients with CSC as of September 2020, cCSC patients who had serous retinal detachment (SRD) during the first diagnosis and could be followed up for at least 2 years were selected. The eligibility period was from May 2007 to June 2017. Overall, 223 patients with cCSC were eligible for this study. Patients with the following conditions were excluded: (1) Patients who underwent treatments other than rf-PDT (i.e., photocoagulation, anti-VEGF treatment); (2) MNV existence at the first visit examined using OCT and fluorescein angiography/indocyanine green angiography (FA/ICGA); (3) history of CSC treatment or ocular surgery other than cataract surgery; and (4) cataract surgery during the observation period of 2 years.
All patients underwent a comprehensive ophthalmic evaluation, including measurements of best-corrected visual acuity (BCVA), slit-lamp examination, dilated fundus examination, color fundus photography, fundus autofluorescence, FA/ICGA, and spectral-domain OCT. The details of each examination are summarized in the Supplementary Note (available at www.ophthalmologyscience.org).
Diagnosis of cCSC
Central serous chorioretinopathy was diagnosed independently by 2 of the 3 retina specialists (Y.H., M.M., and K.Y.) based on the comprehensive ophthalmic examination data. Patients with SRD with other systemic diseases that could mimic CSC, such as eclampsia, diabetes, or uveitis, were carefully excluded. If the independent specialists did not agree, the other retina specialist made the final judgment. The presence of MNV was also diagnosed in the same manner.
Chronic central serous chorioretinopathy was diagnosed by multimodal imaging with reference to the PLACE trial.14 Concretely, in addition to SRD that affected the fovea on spectral-domain OCT, ≥ 1 ill-defined hyperfluorescent leakage areas (focal or diffuse) on FA with RPE window defect(s), hyperfluorescent changes typical of cCSC on ICGA, and RPE abnormality on fundus autofluorescence were considered. Patients with focal leakage with no RPE window defects were categorized as acute CSC and thus were excluded from the current study. The decision was made after agreement between 2 retina specialists (N.A. and M.M.). Representative cCSC cases in both the rf-PDT group and control group are shown in Figures 1 and 2, respectively.
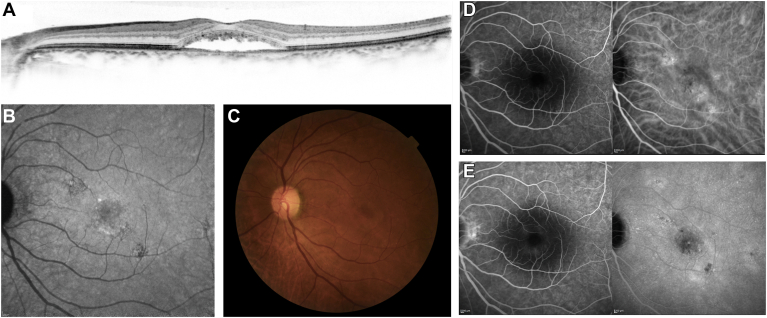
Figure 1.
A 65-year-old man visited the macular clinic at Kyoto University Hospital with chief symptoms of color anomaly and visual loss in the left eye. This was his third episode, with the former 2 episodes resolving after observation. His baseline visual acuity was 20/25. He underwent reduced-fluence photodynamic therapy with a diagnosis of chronic central serous chorioretinopathy. Spectral-domain OCT (SD-OCT) (A), fundus autofluorescence (B), color fundus photography (C), early-phase fluorescein and indocyanine green angiography (FA/ICGA), and late-phase FA/ICGA (E) images are shown. Multimodal imaging showed serous retinal detachment involving the fovea on SD-OCT (A), retinal pigment epithelium alteration (B−E), and diffuse leakage on FA (E), accompanied by choroidal vascular hyperpermeability on ICGA (D, E).
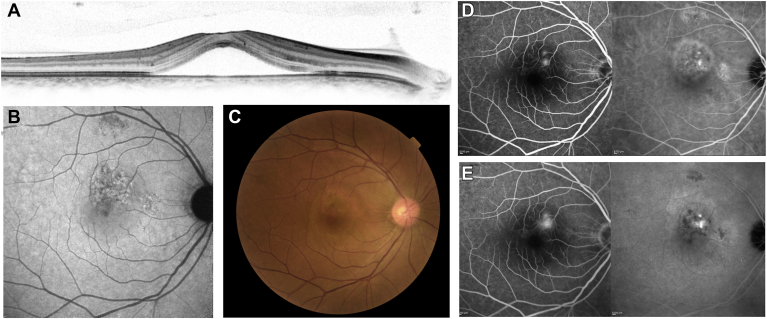
Figure 2.
A 46-year-old man visited the macular clinic at Kyoto University Hospital with a chief symptom of metamorphopsia in the right eye that lasted for 6 months. His baseline visual acuity was 20/16. Despite the recommendation for reduced-fluence photodynamic therapy (rf-PDT) for the existing chronic central serous chorioretinopathy, he did not receive rf-PDT because he did not want any treatment. Spectral-domain (SD)-OCT (A), fundus autofluorescence (B), color fundus photography (C), early-phase fluorescein angiography/indocyanine green angiography (FA/ICGA), and late-phase FA/ICGA (E) images are shown. Multimodal imaging showed serous retinal detachment involving the fovea on SD-OCT (A), retinal pigment epithelium alteration (B−E), and multifocal leakage on FA (E), accompanied by choroidal vascular hyperpermeability on ICGA (D, E).
Phenotyping
In our study, a total of 18 baseline characteristics were phenotyped for the use of balancing the background. All 18 characteristics are listed in Table 1 and explained in detail next. These baseline data were collected within 1 month before the treatment date for the rf-PDT group and at the first visit date for the control group.
Table 1.
Characteristics of the Reduced-fluence Photodynamic Therapy Group and Control Group
| Unweighted |
IPTW Adjusted |
|||||
|---|---|---|---|---|---|---|
| rf-PDT (n = 74) | Control (n = 81) | P Value | rf-PDT | Control | Standardized Difference | |
| Male∗ | 56 (75.7) | 59 (72.8) | 0.83 | (78.0) | (74.8) | –0.032 |
| Age (yrs)∗ | 60.1 ± 10.9 | 55.1 ± 11.5 | 0.006 | 57.3 ± 10.4 | 56.4 ± 11.1 | 0.083 |
| Smoking status∗ | ||||||
| Current | 17 (23.0) | 17 (21.0) | 0.72 | (18.9) | (20.6) | –0.017 |
| Former | 22 (29.7) | 29 (35.8) | (37.0) | (34.4) | 0.026 | |
| Never | 35 (47.3) | 35 (43.2) | (44.1) | (44.9) | –0.009 | |
| Brinkman index∗ | 323 ± 461 | 274 ± 380 | 0.46 | 310 ± 402 | 288 ± 412 | 0.050 |
| Steroid intake∗ | 5 (6.8) | 3 (3.7) | 0.62 | (3.8) | (2.9) | 0.009 |
| Phakic eyes∗ | 70 (94.6) | 76 (93.8) | 1.0 | (96.0) | (95.3) | 0.007 |
| No. of previous episodes∗ | 0.53 ± 0.87 | 0.36 ± 1.25 | 0.33 | 0.40 ± 0.80 | 0.39 ± 1.38 | 0.020 |
| Symptom duration of the current episode (mo)∗ | 13.0 ± 31.3 | 8.5 ± 32.5 | 0.40 | 9.95 ± 23.0 | 9.39 ± 37.4 | 0.017 |
| Initial BCVA (logMAR)∗ | 0.16 ± 0.27 | 0.01 ± 0.17 | <0.001 | 0.08 ± 0.23 | 0.06 ± 0.22 | 0.051 |
| FA findings | ||||||
| Diffuse leakage∗ | 70 (94.6) | 67 (87.0) | 0.19 | (88.9) | (89.1) | 0.020 |
| Number of focal leakages∗ | 0.89 ± 0.84 | 0.69 ± 0.80 | 0.13 | 0.77 ± 0.76 | 0.72 ± 0.87 | 0.060 |
| ICGA findings | ||||||
| CVH in affected eye∗ | 40 (54.8) | 42 (52.5) | 0.90 | (55.0) | (54.9) | 0.001 |
| CVH in fellow eye∗ | 33 (45.2) | 37 (46.2) | 1.0 | (48.0) | (49.3) | –0.012 |
| OCT findings | ||||||
| Foveal neurosensory retinal thickness (μm)∗ | 129 ± 31 | 145 ± 37 | 0.003 | 141 ± 39 | 138 ± 36 | 0.090 |
| Height of SRD (μm)∗ | 184 ± 104 | 163 ± 113 | 0.22 | 163 ± 103 | 165 ± 112 | –0.014 |
| Cystoid macular edema∗ | 0 (0.0) | 4 (4.9) | 0.15 | (0.0) | (2.9) | –0.029 |
| Pigment epithelium detachment∗ | 9 (12.2) | 10 (12.3) | 1.0 | (8.9) | (8.3) | 0.006 |
| RPE alteration grading∗ | ||||||
| <1 optic disc area | 41 (55.4) | 52 (67.5) | 0.27 | (58.5) | (63.2) | –0.046 |
| 1−5 optic disc area | 26 (35.1) | 21 (27.3) | (35.6) | (32.1) | 0.035 | |
| > 5 optic disc area | 7 (9.5) | 4 (5.2) | (5.9) | (4.8) | 0.012 | |
Presented by mean ± standard deviation or count (%).
BCVA = best-corrected visual acuity; CVH = choroidal vascular hyperpermeability; FA = fluorescein angiography; ICGA = indocyanine green angiography; IPTW = inverse probability of treatment weighting; logMAR = logarithm of the minimum angle of resolution; rf-PDT = reduced-fluence photodynamic therapy; RPE = retinal pigment epithelium; SRD = serous retinal detachment.
Eighteen characteristics used for creating the IPTW model.
Smoking status was evaluated using the Brinkman index ([number of cigarettes per day] × [number of years smoking]).15 Continuous steroid intake was recorded at the initial visit (yes or no). We measured the neurosensory retinal thickness defined as the distance between the internal limiting membrane and the ellipsoid zone; SRD height was defined as the distance between the ellipsoid zone and the inner portion of the RPE using spectral-domain OCT images. The presence of cystoid macular edema and pigment epithelium detachment was evaluated as a binary trait (i.e., present or not). Further details of the OCT measurements are summarized in the Supplementary Note (available at www.ophthalmologyscience.org).
Fluorescein angiography and ICGA results were reviewed to evaluate the number of focal leakages, presence of diffuse leakages, and choroidal vascular hyperpermeability according to previous studies.8,16, 17, 18 Briefly, focal leakage and diffuse leakage are FA findings defined as hot spots of leakage and a larger area of widespread leakage, respectively. A hot spot of leakage was defined as an increase in hyperfluorescence area between early- and late-phase FA, whereas diffuse leakage was defined as a larger area of hyperfluorescent leakage that could not be linked with a single spot of leakage on the FA image at 3 minutes after the infusion of fluorescein. If there were multiple focal leakage points within 1 papillary diameter, they were counted as a single leakage. Choroidal vascular hyperpermeability was evaluated using the sequential images of ICGA obtained until approximately 15 minutes after dye injection. Choroidal vascular hyperpermeability was defined as multifocal areas of hyperfluorescence with blurred margins within the choroid, followed by minimal extension of the focal hyperfluorescent area.8,17,18 This status was evaluated independently by 2 retina specialists (M.M. and Y.H.) who were blinded to all other medical information. If there were discrepancies, a third retina specialist (A.T.) made the final decision.
Retinal pigment epithelium alteration was diagnosed by multimodal imaging, including FA/ICGA, fundus autofluorescence, fundus photography, and OCT. The extent of the RPE alteration area was categorized into 3 groups based on their cumulative surface: <1 optic disc area, between 1 and 5 optic disc areas, or >5 optic disc areas.16
Outcomes
The BCVA was collected at baseline and at 1, 3, 6, 9, 12, and 24 months from the baseline. If the BCVA was not available at the 12-month or 24-month visit, the last observation carried forward method was used for imputing the missing values. The visual acuity (VA) maintenance rate was calculated using the rate of patients with the logarithm of the minimum angle of resolution (logMAR) change of ≤0, which indicates the constancy or improvement in BCVA from baseline. The complete SRD remission date was defined as the middle date between the last visit with SRD existence and the first visit with complete SRD disappearance as confirmed using OCT.
Photodynamic Therapy
All patients in the rf-PDT group received rf-PDT using a standard dose of verteporfin (6 mg/m2) infused over 10 minutes, followed by laser treatment at 15 minutes after the start of drug infusion. The total light energy was reduced to 25 J/cm2 by adjusting the lens magnification setting and was irradiated for 83 seconds. The laser was delivered to cover both the areas with the leak point based on FA images and the choroidal vascular hyperpermeability based on the ICGA images19 that were obtained before treatment. After treatment, patients were instructed to avoid sunlight for 48 hours.
Basically, we considered all cCSC cases as an indication for rf-PDT. However, in the clinical setting, some patients cannot undergo treatment or refuse treatment for various reasons. Therefore, for each individual case, the final judgement was made on the basis of discussions by doctors at the macular clinic, after considering patient factors such as BCVA, symptom duration, recurrence number, ophthalmic status, multimodal imaging data, and consent for treatment, among others.
Statistical Analyses
Statistical comparisons were made using an unpaired t test and Fisher exact test for continuous data (shown as mean and standard deviation) and categorical data (shown as count), respectively.
In a clinical setting, physicians determine whether to perform PDT based on the clinical manifestation, such as RPE alteration, duration of symptoms, and BCVA, among others. To account for the confounding by indication, a PS was developed.20, 21, 22 The PS was derived to reflect the probability of receiving the treatment, which is calculated by a set of characteristics observed at the time of treatment decision. As a result, all the baseline characteristics can be replaced with PS. Moreover, this method can account for a higher number of potential confounders than conventional regression methods.23, 24, 25
Among the several PS methodologies, the inverse probability of treatment weighting (IPTW) was used to balance the baseline characteristics between the rf-PDT group and controls.23,26 The characteristics that can influence the selection of treatment and outcomes were selected. All 18 of the baseline characteristics presented in Table 1 were used for creating the IPTW model. Group differences were assessed by calculating weighted means and standardized mean differences. The following were reported: the IPTW VA maintenance rate at 12 and 24 months, IPTW BCVA change from baseline, IPTW hazard ratios for SRD remission, and IPTW Kaplan–Meier curve. For a sensitivity analysis, we conducted the same analysis excluding patients with steroid intake. We used an intention-to-treat principle.
All statistical analyses were conducted using Software R (R version 4.0.1, Foundation for Statistical Computing). The R packages “survival,” “Cobalt,” and “WeightIt” were used. P values of ≤ 0.05 were considered statistically significant.
Results
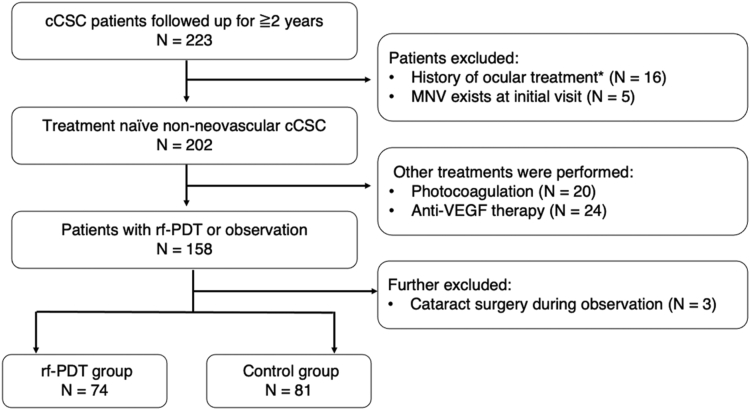
The consort diagram is presented in Figure 3. Among the 223 cCSC patients, 202 with treatment-naive non-neovascular cCSC were identified. Sixteen patients with a history of ocular treatment except for cataract surgery and 5 patients with MNV at baseline were excluded. After excluding patients who underwent non-PDT treatment such as photocoagulation and anti-VEGF therapy (n = 44) and those who had cataract surgery during observation (n = 3), a total of 74 eyes of 74 patients who received rf-PDT and 81 eyes of 81 control patients were finally analyzed.
Figure 3.
CONSORT diagram. Among the 223 patients, 202 cases of treatment-naive non-neovascular chronic central serous chorioretinopathy (cCSC) were identified by excluding 16 patients with a treatment history and 5 patients with macular neovascularization (MNV). After excluding patients who underwent other treatments such as photocoagulation and anti-VEGF therapy (n = 44), and those who underwent cataract surgery during observation (n = 3), 74 eyes of 74 patients who underwent reduced-fluence photodynamic therapy (rf-PDT) and 81 eyes of 81 control patients were analyzed. ∗History of ocular treatment: history of photocoagulation, anti-VEGF therapy, photodynamic therapy, or any other ocular surgery except for cataract surgery.
The unweighted and IPTW clinical characteristics of the 2 groups are summarized in Table 1. Before IPTW, patients who received rf-PDT were significantly older (60.1 ± 10.9 years vs. 55.1 ± 11.5 years, P = 0.006), had significantly worse BCVA (logMAR) (0.16 ± 0.27 vs. 0.01 ± 0.17, P < 0.001), and had significantly thinner neurosensory retinal thickness (129 ± 31 μm vs. 145 ± 37 μm, P = 0.003) at baseline than the control group. The model to develop a PS, which included 18 covariates, yielded a c-statistic of 0.80, indicating a strong ability to determine whether patients underwent rf-PDT. The IPTW resulted in adequate between-group balance on baseline characteristics, as the standardized difference for every parameter was < 0.1 (Table 1).
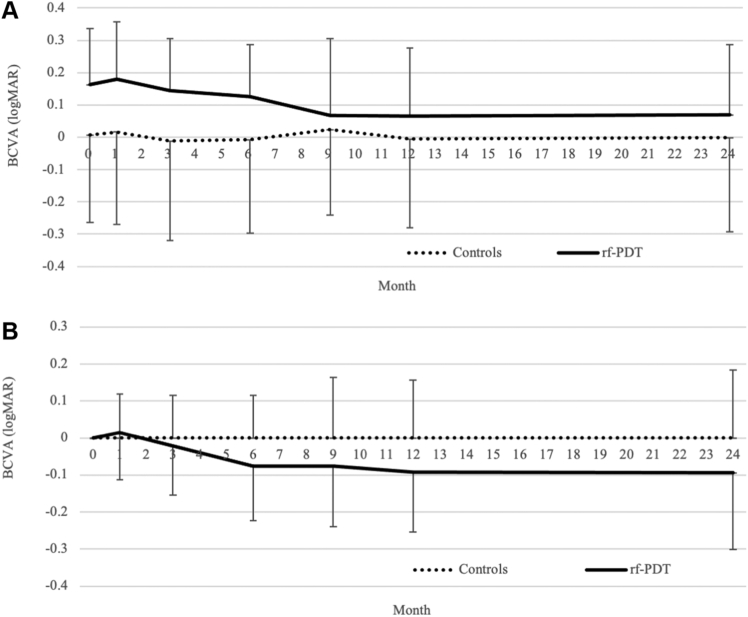
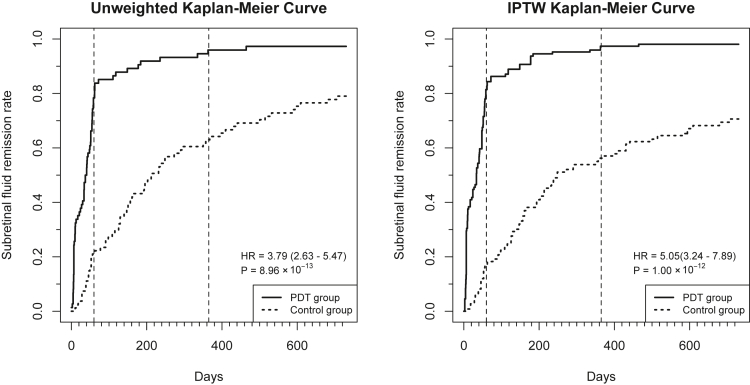
The trajectories of the mean BCVA and mean BCVA change from baseline, before IPTW, are presented in Figure 4A and B, respectively. Although the rf-PDT group nominally showed relatively better BCVA improvement (Fig 4B) and the IPTW regression analysis showed the same trend of relatively better BCVA improvement in the rf-PDT group, it did not reach statistical significance (12-month BCVA change, −0.06 ± 0.02 vs. −0.008 ± 0.02, P = 0.07; 24-month BCVA change, −0.06 ± 0.03 vs. −0.03 ± 0.03, P = 0.32, respectively). However, the BCVA maintenance rate showed a statistically significant difference between the 2 groups (12-month BCVA maintenance rate, 93.6% vs. 70.9%, P < 0.001; 24-month BCVA maintenance rate was 85.7% vs. 69.8%, P = 0.019, respectively) (Table 2). The sensitivity analysis that excluded patients with steroid intake showed almost similar results (Supplementary Table S1, available at www.ophthalmologyscience.org). The unweighted and IPTW Kaplan−Meier curves for SRD remission are presented in Figure 5. More than 80% of patients in the rf-PDT group reached complete SRD remission at 60 days from treatment in contrast to only 20% in the control group. At 1 year from treatment, > 90% of patients in the rf-PDT group achieved complete SRD remission in contrast to only 50% in the control group. The IPTW Cox regression analysis showed a significantly higher rate of complete SRD remission in the rf-PDT group than in the control group (hazard ratio, 5.05; 95% confidence interval, 3.24–7.89; P < 0.001).
Figure 4.
The trajectory of best-corrected visual acuity (BCVA) and its change from the baseline before inverse probability of treatment weighting. A, The trajectory of the BCVA from baseline to 24 months after treatment. B, The trajectory of the BCVA change from the baseline to 24 months after treatment. ∗The BCVA is shown in the logarithm of the minimum angle of resolution (logMAR) scale. The error bars show the standard deviation of each value. rf-PDT = reduced-fluence photodynamic therapy.
Table 2.
Visual Prognosis for Reduced-fluence Photodynamic Therapy Group and Control Group after Inverse Probability of Treatment Weighting
| PDT Group | Control Group | P Value | |
|---|---|---|---|
| VA maintenance rate (%) | |||
| At 12 mos | 93.6 | 70.9 | <0.001 |
| At 24 mos | 85.7 | 69.8 | 0.019 |
| VA change (mean ± SE)∗ | |||
| At 12 mos | –0.06 ± 0.02 | –0.008 ± 0.02 | 0.07 |
| At 24 mos | –0.06 ± 0.02 | –0.03 ± 0.03 | 0.32 |
PDT = photodynamic therapy; SE = standard error; VA = visual acuity.
The VA is presented by logMAR. Weighted mean (weighted standard error) is shown.
Figure 5.
Unweighted and inverse probability of treatment weighting (IPTW) Kaplan–Meier curves for serous retinal detachment remission. Left: Kaplan–Meier curve of the 2 unweighted groups. Right: Kaplan–Meier curve of the 2 IPTW balanced groups. HR = hazard ratio; PDT = photodynamic therapy.
Regarding the safety profile, we have listed the potential adverse events of rf-PDT in Supplementary Table S2 (available at www.ophthalmologyscience.org).14 In the rf-PDT group, 1 patient reported a headache after treatment that disappeared spontaneously. No other systemic or ocular events were encountered.
Discussion
This study revealed that rf-PDT was associated with a significantly higher BCVA maintenance rate, higher proportion of complete SRD remission, and a trend toward better visual improvement in patients with cCSC. To the best of our knowledge, this is the only study with a 2-year follow-up evaluating the effectiveness of rf-PDT for cCSC compared with untreated controls. Although this is a cohort study, the application of PS analysis successfully eliminated the imbalance between cases and controls, leading to a fair comparison. The current results support the use of rf-PDT for patients with cCSC.
A recent review article suggested that hd-/hf-PDT should be the first-line treatment for CSC;27 however, the evidence supporting this recommendation is limited.28 Except for one RCT reported in 2008,13 there is no high-level evidence; other existing research studies are single-arm observational studies and comparative observational studies with a small sample size, which could not adequately control for confounders. Additionally, RCTs that performed hd-/hf-PDT for CSC evaluated the efficacy of various other interventions (i.e., subthreshold micropulse laser, anti-VEGF therapy, and 30% dose PDT) by comparing with hd-/hf-PDT as a control and did not evaluate the efficacy of rf-PDT by comparing with placebo.29, 30, 31, 32 Furthermore, the only RCT that evaluated the efficacy of hd-PDT by comparing with placebo targeted acute CSC cases but not cCSC cases.13 Thus, there is no reliable evidence for cCSC. Propensity score analysis, including IPTW, was developed to overcome the limitations of observational data. Previous literature has suggested that a well-conducted PS analysis could be comparable to an RCT.33, 34, 35 In the current situation where rf-PDT is recommended as the first-line treatment for CSC despite the absence of reliable evidence, a comprehensive factual data analysis should be the best alternative for RCT. Our study has provided important evidence for the clinical effectiveness of rf-PDT for cCSC.
We summarized the effect of reduced-intensity PDT obtained in previous RCTs with a relatively large sample size (Table 3). The logMAR was converted to ETDRS letters and vice versa using a conversion table reported by Beck et al.36 Although our study analyzed data in a clinical setting, the VA maintenance and complete SRD remission rates were comparable to those reported in previous RCTs. Interestingly, although the initial BCVAs of the 4 studies were comparable, BCVA improvement was smaller in studies targeting cCSC, which is likely due to the RPE damage observed in cCSC. Because the current study also targeted cCSC cases, the results might have been underpowered by this limited improvement in BCVA. This is likely the reason why the current study failed to detect a statistically significant improvement in mean BCVA change. By increasing the sample size, the mean BCVA change is expected to reach statistical significance, in addition to the significantly higher rate of VA maintenance.
Table 3.
Summary of the Performance of Reduced-Intensity Photodynamic Therapy in Current Study and Previous Randomized Controlled Trials
| The current study (unweighted) | Chan et al, Ophthalmology 2008 | Zhao et al, JAMA Ophthalmol 2015 | van Dijik et al, Ophthalmology 2018 | |
|---|---|---|---|---|
| Design | PS analysis using data in clinical setting | Randomized controlled trial | Randomized controlled trial | Randomized controlled trial |
| Comparison | rf-PDT vs. no treatment | hd-PDT vs. placebo PDT | hd-PDT vs. 30% dose PDT | hd-PDT vs. HSML |
| Sample size for half-intensity PDT (analyzed) | 74 | 39 | 56 | 67 |
| Classification | Chronic | Acute | Acute | Chronic |
| VA (logMAR) | ||||
| Initial | 0.16 ± 0.27 | 0.16 ± 0.19 | 0.19 ± 0.20 | 0.16 ± 0.17 |
| 6−8 mos | – | 0.03 ± 0.11∗ | –0.01 ± 0.19 | 0.03 ± 0.33 |
| 1 yr | 0.07 ± 0.27 | –0.05 ± 0.09 | 0.01 ± 0.18 | – |
| Change | –0.09 ± 0.16 | –0.21‡ | –0.18 ± 0.14 | –0.14 ± 0.17 |
| VA (ETDRS letters) | ||||
| Initial | 77.0 ± 13.5 | 77.0 ± 9.5 | 75.3 ± 9.8 | 76.9 ± 8.32 |
| 6−8 mos | – | 83.5 ± 5.4 | 85.0 ± 9.3 | 83.7† |
| 1 yr | 81.5 ± 13.5 | 87.5 ± 4.5 | 84.7 ± 9.2 | – |
| Change | 4.5 ± 8.0 | 10.5‡ | 9.0 ± 6.9 | 6.8 ± 8.5 |
| VA maintenance rate (%) | ||||
| 1 yr | 93.6% | 100% | – | – |
| Subretinal fluid remission rate (%) | ||||
| 6−8 mos | 90.7% | 92.3% | 92.9% | 67.2% |
| 1 yr | 97.4% | 94.9% | 94.6% | – |
hd-PDT = half-dose photodynamic therapy; HSML = high-density subthreshold micropulse laser; logMAR = logarithm of the minimum angle of resolution; PDT = photodynamic therapy; PS = propensity score; rf-PDT = reduced-fluence photodynamic therapy; VA = visual acuity.
Calculated from presented Figure.
Calculated by adding VA change to initial VA.
Calculated by subtracting initial VA from final VA.
This study has certain strengths. First, we included a relatively large sample size and reported long-term results (2-year outcomes) in contrast to most of the previous studies evaluating 1-year outcomes. Second, the study had higher generalizability than RCTs because we used data in a clinical setting. As the effectiveness in a clinical setting can provide complementary information to efficacy trials such as RCTs,37 the results could be more widely applicable to clinicians. Last, the appropriate application of PSs was one of the strengths.
Study Limitations
The study has some potential limitations. First, the study only evaluated VA and complete SRD remission as outcomes. Because there are many other symptoms of CSC besides VA change, evaluating various outcomes such as metamorphopsia, micropsia, and hyperopic change may be useful. Additionally, the occurrence of choroidal neovascularization is an important event in patients with CSC, and these outcomes need to be evaluated in future studies. Second, although the current study included a relatively large number of patients with cCSC, the sample size remains a limitation. An increase in sample size would enable a more robust estimation and detailed analysis, such as stratification analysis; a multicenter clinical research study or meta-analysis is needed. Third, of the patients in the cCSC group, 44 were excluded because they had received non-PDT treatments such as focal laser or anti-VEGF injection. Although IPTW can eliminate background imbalance, the exclusion of a relatively large number of patients is a limitation. Fourth, the time of baseline data collection differed between the rf-PDT group (within 1 month before the treatment) and the control group (the first visit). There is a possibility of bias in the evaluation of the actual effect of the treatment. However, because the duration of symptoms and other clinical manifestations at baseline were included in the PS model, we believe we minimized any such potential bias. Fifth, the lack of considerations of clinical characteristics of cCSC (i.e., subfoveal choroidal thickness, pigment epithelial detachment subtypes) in the analysis is also a limitation. These characteristics may play an important role in treatment response to rf-PDT and should be considered in future studies. Nevertheless, despite the difficulty in including subfoveal choroidal thickness or pigment epithelial detachment subtypes in our PS model, the C-statistic was 0.80, implying that the current model adequately fulfills the “assumption of strongly ignorable treatment assignment,” an assumption that unobserved parameters did not affect the treatment decision. Therefore, we speculate that other factors used in the analysis accounted for the influence of these characteristics on the decision for rf-PDT. Last, because the study population included patients referred to the tertiary referral center, our results cannot be generalized to all care settings. Specifically, those with milder symptoms or quick spontaneous resolution may be underrepresented in this study. However, because such patients are not considered for treatment, this selection strategy does not decrease the importance of our study.
Conclusions
The long-term effectiveness of rf-PDT was evaluated using factual data and PS methodologies. We revealed that the VA maintenance rate and complete SRD remission rate were significantly higher in the rf-PDT group and could be generalized to patients with cCSC. Further studies, such as RCT and large-scale factual analysis, are expected to validate our results.
Manuscript no. XOPS-D-21-00170
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): M. Miyake: Grants – Novartis Pharma; Receipt of equipment, drugs, medical writing, gifts or other services – Bayer Yakuhin, Kowa Pharmaceutical, Alcon Japan, HOYA, Novartis Pharma, AMO Japan, Santen Pharmaceutical, Senju Pharmaceutical, Johnson & Johnson K.K., Japan Ophthalmic Instruments Association
M. Miyata: Grants – Alcon Japan, Novartis Pharma; Receipt of equipment, drugs, medical writing, gifts or other services: HOYA, Santen Pharmaceuticals
Y. Mori: Receipt of equipment, drugs, medical writing, gifts or other services – Santen Pharmaceutical
Y. Muraoka: Grants – Bayer Yakuhin, Novartis Pharma; Receipt of equipment, drugs, medical writing, gifts or other services – Canon, Santen Pharmaceutical, Senju Pharmaceutical, Bayer Yakuhin, Novartis Pharma
A. Oishi: Grants – Alcon Japan, Tokai Optical Co, Ltd; Receipt of equipment, materials, drugs, medical writing, gifts or other services – Canon, Bayer Yakuhin, Novartis Pharma, Santen Pharmaceutical, Senju Pharmaceutical, Kowa Pharmaceutical, HOYA
S. Ooto: Receipt of equipment, materials, drugs, medical writing, gifts or other services – Bayer Yakuhin, Kowa Pharmaceutical, Janssen Pharmaceutical, Novartis Pharma, AMO Japan, Santen Pharmaceutical, Alcon Japan, Senju Pharmaceutical, Japan Focus Company, Ltd.
A. Takahashi: Receipt of equipment, materials, drugs, medical writing, gifts or other services – Bayer Yakuhin, Novartis Pharma, Santen Pharmaceutical, MSD
H. Tamura: Grants – Findex; Receipt of equipment, materials, drugs, medical writing, gifts or other services – Bayer Yakuhin, Novartis Pharma, Santen Pharmaceutical, Suntory
A. Tsujikawa: Payments made to institution – Canon, Findex, Santen Pharmaceutical, Kowa Pharmaceutical, Pfizer, AMO Japan, Senju Pharmaceutical, Wakamoto Pharmaceutical, Alcon Japan, Novartis Pharma, Otsuka Pharmaceutical, Bayer Yakuhin, Nitten Pharmaceutical; Consulting fees – Senju Pharmaceutical, Bayer Yakuhin, Novartis Pharma, HOYA, Ellex, MSD, Allergan Japan, Eisai, Daiich-Sankyo, Chugai Pharmaceutical, Kyowa Hakko Bio co, Ltd; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events – Bayer Yakuhin, Senju Pharmaceutical, Novartis Pharma, Santen, Alcon Pharma Pharmaceutical, Alcon Japan, AbbVie GK, AMO Japan, Kowa Pharmaceutical, Canon, Otsuka Pharmaceutical, Wakamoto Pharmaceutical, NIDEK Co, Ltd.
K. Yamashiro: Receipt of equipment, materials, drugs, medical writing, gifts or other services – Novartis Pharma, Bayer Yakuhin, Alcon Pharma, Senju Pharmaceutical, Santen Pharmaceutical, Kowa Pharmaceutical, Canon, Chugai Pharmaceutical
Supported by JSPS KAKENHI (grant no. 20H03841) and AMED (grant no. JP20lk1403038). The sponsor or funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. Institutional Review Board of Kyoto University Graduate School of Medicine (R0532-4) approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Aisu, Miyake, Hosoda
Data collection: Aisu, Hosoda
Analysis and interpretation: Aisu, Miyake, Hosoda, Mori, Takahashi, Muraoka, Ueda-Arakawa, Miyata, Oishi, Tamura, Ooto, Yamashiro, Tsujikawa
Obtained funding: N/A; Study was performed as part of regular employment duties at Kyoto University Hospital. No additional funding was provided.
Overall responsibility: Aisu, Miyake
Supplementary Data
References
- 1.Liew G., Quin G., Gillies M., Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41:201–214. doi: 10.1111/j.1442-9071.2012.02848.x. [DOI] [PubMed] [Google Scholar]
- 2.How A.C., Koh A.H. Angiographic characteristics of acute central serous chorioretinopathy in an Asian population. Ann Acad Med Singap. 2006;35:77–79. [PubMed] [Google Scholar]
- 3.Mori Y., Miyake M., Hosoda Y., et al. Distribution of choroidal thickness and choroidal vessel dilation in healthy Japanese individuals. Ophthalmol Sci. 2021;1 doi: 10.1016/j.xops.2021.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kido A., Miyake M., Tamura H., et al. Incidence of central serous chorioretinopathy (2011-2018): a nationwide population-based cohort study of Japan. Br J Ophthalmol. 2021 Jul 14 doi: 10.1136/bjophthalmol-2021-319403. bjophthalmol-2021-319403. doi: 10.1136/bjophthalmol-2021-319403. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mrejen S., Balaratnasingam C., Kaden T.R., et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126:576–588. doi: 10.1016/j.ophtha.2018.12.048. [DOI] [PubMed] [Google Scholar]
- 6.Hosoda Y., Miyake M., Schellevis R.L., et al. Genome-wide association analyses identify two susceptibility loci for pachychoroid disease central serous chorioretinopathy. Commun Biol. 2019;2:468. doi: 10.1038/s42003-019-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosoda Y., Yamashiro K., Miyake M., et al. Predictive genes for the prognosis of central serous chorioretinopathy. Ophthalmol Retina. 2019;3:985–992. doi: 10.1016/j.oret.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Miyake M., Ooto S., Yamashiro K., et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep. 2015;5 doi: 10.1038/srep16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosoda Y., Miyake M., Yamashiro K., et al. Deep phenotype unsupervised machine learning revealed the significance of pachychoroid features in etiology and visual prognosis of age-related macular degeneration. Sci Rep. 2020;10:18423. doi: 10.1038/s41598-020-75451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng C.K., Chang C.K., Peng C.H. Comparison of photodynamic therapy using half-dose of verteporfin or half-fluence of laser light for the treatment of chronic central serous chorioretinopathy. Retina. 2017;37:325–333. doi: 10.1097/IAE.0000000000001138. [DOI] [PubMed] [Google Scholar]
- 11.Nicolo M., Eandi C.M., Alovisi C., et al. Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2014;157:1033–1037. doi: 10.1016/j.ajo.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Shin J.Y., Woo S.J., Yu H.G., Park K.H. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2011;31:119–126. doi: 10.1097/IAE.0b013e3181e378f2. [DOI] [PubMed] [Google Scholar]
- 13.Chan W.M., Lai T.Y., Lai R.Y., et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008;115:1756–1765. doi: 10.1016/j.ophtha.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Breukink M.B., Downes S.M., Querques G., et al. Comparing half-dose photodynamic therapy with high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (the PLACE trial): study protocol for a randomized controlled trial. Trials. 2015;16:419. doi: 10.1186/s13063-015-0939-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinkman G.L., Coates E.O., Jr. The effect of bronchitis, smoking, and occupation on ventilation. Am Rev Respir Dis. 1963;87:684–693. doi: 10.1164/arrd.1963.87.5.684. [DOI] [PubMed] [Google Scholar]
- 16.van Rijssen T.J., van Dijk E.H.C., Scholz P., et al. Patient characteristics of untreated chronic central serous chorioretinopathy patients with focal versus diffuse leakage. Graefes Arch Clin Exp Ophthalmol. 2019;257:1419–1425. doi: 10.1007/s00417-019-04333-y. [DOI] [PubMed] [Google Scholar]
- 17.Guyer D.R., Puliafito C.A., Monés J.M., et al. Digital indocyanine-green angilography in chorioretinal disorders. Ophthalmology. 1992;99:287–291. doi: 10.1016/s0161-6420(92)31981-5. [DOI] [PubMed] [Google Scholar]
- 18.Miyake M., Tsujikawa A., Yamashiro K., et al. Choroidal neovascularization in eyes with choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014;55:3223–3230. doi: 10.1167/iovs.14-14059. [DOI] [PubMed] [Google Scholar]
- 19.Fujita K., Imamura Y., Shinoda K., et al. One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology. 2015;122:555–561. doi: 10.1016/j.ophtha.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 20.Haukoos J.S., Lewis R.J. The propensity score. JAMA. 2015;314:1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurth T., Walker A.M., Glynn R.J., et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 23.Brookhart M.A., Schneeweiss S., Rothman K.J., et al. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 25.Williamson E.J., Forbes A. Introduction to propensity scores. Respirology. 2014;19:625–635. doi: 10.1111/resp.12312. [DOI] [PubMed] [Google Scholar]
- 26.Bergstra S.A., Sepriano A., Ramiro S., Landewe R. Three handy tips and a practical guide to improve your propensity score models. RMD Open. 2019;5 doi: 10.1136/rmdopen-2019-000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rijssen T.J., van Dijk E.H.C., Yzer S., et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019;73 doi: 10.1016/j.preteyeres.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Salehi M., Wenick A.S., Law H.A., et al. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;(12) doi: 10.1002/14651858.CD011841.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dijk E.H.C., Fauser S., Breukink M.B., et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: The PLACE Trial. Ophthalmology. 2018;125:1547–1555. doi: 10.1016/j.ophtha.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Bae S.H., Heo J., Kim C., et al. Low-fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one-year results of a randomized trial. Ophthalmology. 2014;121:558–565. doi: 10.1016/j.ophtha.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Semeraro F., Romano M.R., Danzi P., et al. Intravitreal bevacizumab versus low-fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Jpn J Ophthalmol. 2012;56:608–612. doi: 10.1007/s10384-012-0162-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhao M., Zhang F., Chen Y., et al. A 50% vs. 30% dose of verteporfin (photodynamic therapy) for acute central serous chorioretinopathy: one-year results of a randomized clinical trial. JAMA Ophthalmol. 2015;133:333–340. doi: 10.1001/jamaophthalmol.2014.5312. [DOI] [PubMed] [Google Scholar]
- 33.Iwagami M., Yasunaga H., Doi K., et al. Postoperative polymyxin B hemoperfusion and mortality in patients with abdominal septic shock: a propensity-matched analysis. Crit Care Med. 2014;42:1187–1193. doi: 10.1097/CCM.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 34.Payen D.M., Guilhot J., Launey Y., et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975–984. doi: 10.1007/s00134-015-3751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dellinger R.P., Bagshaw S.M., Antonelli M., et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES Randomized Clinical Trial. JAMA. 2018;320:1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck R.W., Moke P.S., Turpin A.H., et al. A computerized method of visual acuity testing. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 37.Singal A.G., Higgins P.D., Waljee A.K. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5:e45. doi: 10.1038/ctg.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.