Abstract
Purpose
To evaluate the impact of inherited retinal diseases (IRDs) on quality of life (QoL) using multiattributable health utilities derived from primary patient data.
Design
Cross-sectional observational study.
Participants
Seventy adult patients (mean age, 42.7 years) with IRD recruited from state-wide services in Australia.
Methods
Health utility values were calculated from the Assessment of Quality of Life 8-Dimension (AQoL-8D). Linear regressions were used to analyze the relationship between the 25-item and 39-item National Eye Institute Visual Function Questionnaires (NEI-VFQ-25 and NEI-VFQ-39, respectively) and health utilities from the AQoL-8D.
Main Outcome Measures
The AQoL-8D utility values were compared between the IRD cohort and population norms. Regressions were used to determine explanatory power of the NEI-VFQ-25 and NEI-VFQ-39 for health utilities from the AQoL-8D.
Results
Average health-related utility for patients with IRD was 0.58, significantly lower than population norms of 0.80. The IRD patient scores were significantly lower than population norms for all 8 domains of the AQoL-8D. Regressions showed a statistically significant relationship between the NEI-VFQ-39 and AQoL-8D, with the NEI-VFQ-39 and other clinical data explaining up to 73% of the variation in AQoL-8D values and 69% of the variation in the NEI-VFQ-25 values.
Conclusions
Patients with IRD have significantly lower utility values across all dimensions of QoL, with the largest differences in independent living, senses, and relationships. The NEI-VFQ-25 and NEI-VFQ-39 are highly correlated with overall AQoL-8D utilities and, combined with other data, can reasonably estimate QoL utilities required for cost-effectiveness studies.
Keywords: Cost-effectiveness analysis, Inherited retinal diseases, Quality of life, Utilities
Abbreviations and Acronyms: AQoL-8D, Assessment of Quality of Life 8-Dimension; IRD, inherited retinal disease; NEI-VFQ-25, 25-item National Eye Institute Visual Function Questionnaire; NEI-VFQ-39, 39-item National Eye Institute Visual Function Questionnaire; QALY, quality-adjusted life-year; QoL, quality of life
Inherited retinal diseases (IRDs) are a group of heterogeneous conditions causing either progressive or stationary retinal dysfunction resulting in visual loss that can be attributed to variations in more than 250 genes.1,2 Population genomic estimates infer that as many as 1 in 1000 people may be affected by monogenic IRDs,3, 4, 5 and these may occur with associated nonocular conditions.6 Currently, IRDs are the most common cause of blindness certification in the working-age population.7 Blindness is associated with significant lifetime costs as well as lost productivity and quality of life (QoL) for patients and carers.8
The diagnosis of an IRD is a significant stressful event with lifetime impact. Technological advances in genetic testing have the potential to transform the diagnostic and treatment landscape for inherited vision disorders, including IRDs.9 Providing patients with a genetic diagnosis expands treatment from supportive care to the possibility of restoring vision. Gene replacement therapy for RPE65 retinopathy, voretigene neparvovec-rzyl (Luxturna), was recently approved by the United States Food and Drug Administration, the European Medicines Association, the United Kingdom National Institute for Health and Care Excellence, and the Therapeutics Goods Administration (Australia). This new therapy has the potential to halt disease progression, and even to improve the vision of patients who otherwise would lead a lifetime of blindness.10, 11, 12, 13 Other genetic therapies for IRDs including choroideremia, achromatopsia, and x-linked retinitis pigmentosa are being evaluated in clinical trials.14, 15, 16
To obtain public funding to translate treatments such as gene therapy into usual care, robust studies related to the cost of IRDs and cost-effectiveness of genomic testing are urgently needed.17 An important aspect of this analysis is assessment of QoL using a standardized instrument with an associated algorithm to assess utility for use in cost-effectiveness analyses. Utility values from recognized QoL instruments allow comparisons between different health states and can be translated into quality-adjusted life-years (QALYs) to estimate both the cost of an illness and the benefits of interventions.18 However, currently data are very limited on QoL and utility values in patients with IRD, with no studies using primary data for patient-reported health utilities. Zimmerman et al noted that this “leaves uncertainty around comprehensive outcomes such as QALYs, the metric typically used in cost-effectiveness studies.”19
This leads to significant limitations in the use of health utilities in cost-effectiveness studies of voretigene neparvovec-rzyl and other genetic therapies for IRDs. Studies have used simulated data,20 clinical opinion on utility values, or clinicians’ assessments from single-patient vignettes for each visual acuity category (Supplemental Table 1).21,22 A recent review of methodologic challenges in the economic evaluation of gene therapy for RPE65 IRD highlighted the lack of health utility data.23 Halioua-Haubold et al24 also undertook an analysis of a hypothetical gene therapy for choroideremia in 3 hypothetical patients with health outcomes based on assumptions. Together, all current studies of gene therapy related to IRDs rely on proxies for assessment of QoL, and thus have limitations resulting from a lack of patient-reported utility data, use of data not directly relevant for IRD, or both (Supplemental Table 1). (Studies selected for Supplemental Table 1 were based on a literature review of studies examining QoL and IRD. Studies on other vision disorders and diseases were not included.)
Further, it has been reported that reliance on measures of visual function or acuity alone are likely to underestimate the QoL impacts of IRD,25 and thus potentially the benefits of genetic therapies, highlighting the need for a measure that accounts for broader impacts on QoL. The 25-item National Eye Institute Visual Function Questionnaire (NEI-VFQ-25) or 39-item National Eye Institute Visual Function Questionnaire (NEI-VFQ-39) are common measures of vision-related QoL and are widely used measures of QoL in ophthalmic diseases.26 However, they are not among the QoL instruments with an associated utility algorithm to estimate QALYs, limiting their use in cost-effectiveness analyses. An instrument was developed that is related to the NEI-VFQ-25 to generate utility values, the VFQ Utility Index; however, it takes account of only 6 of the 25 questions in the NEI-VFQ-25.27 Some studies have used the EQ-5D, a commonly used QoL instrument with a utility algorithm, to estimate utility among individuals with visual impairment such as glaucoma and age-related macular degeneration. However, they reported a low correlation between the NEI-VFQ-25 score and the EQ-5D,28,29 suggesting that the EQ-5D may predict the utility of those with IRD poorly. We have used the Assessment of Quality of Life 8-Dimension (AQoL-8D),30 another instrument with a utility algorithm in this study, which has 8 dimensions, including senses.
The aim of this study was to estimate the impacts of IRD on QoL using primary IRD patient-reported data and an established measure of health utilities, the AQoL-8D, which includes dimensions related to senses (including vision) and psychosocial factors. This provided an opportunity to use primary IRD patient-reported health utilities data to examine the relationship between IRDs and QoL. We also assessed the association between the NEI-VFQ-25 and NEI-VFQ-39 and a range of patient characteristics with health utilities derived from the AQoL-8D.
Methods
Enrolment
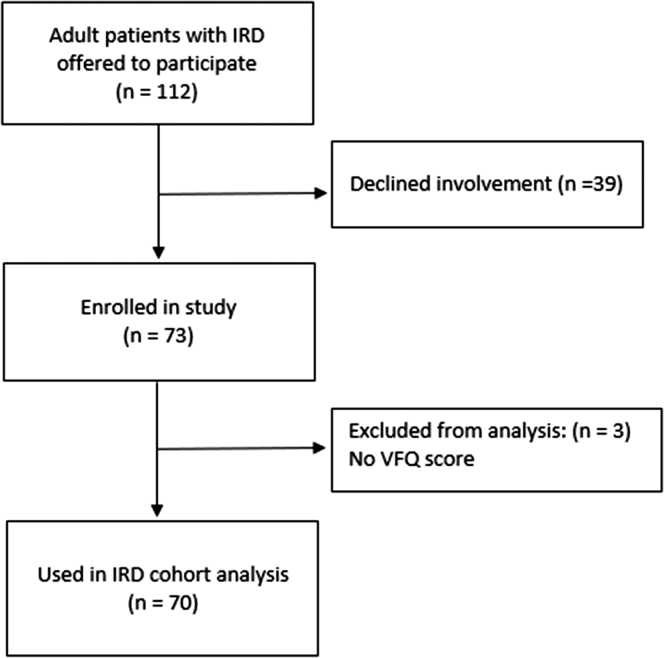
Adults with IRD were recruited from statewide services in New South Wales and were interviewed by clinically trained professionals using a questionnaire that collects primary data regarding visual impairment and its impacts. All patients with a clinical diagnosis of IRD who attend ophthalmic or genetic consultation at the study locations—Sydney Eye Hospital and the Save Sight Institute—were offered participation in the study. Since New South Wales represents approximately 32% of the Australian population, with similar age distributions to Australia,31 patients attending these study locations would broadly represent the general Australian population with IRD. The study was powered to detect a difference of 0.05 units (approximately 30% of the standard deviation of the general Australian population utility score) in AQoL-8D utility between the general Australian population and the patients with IRD. A total of 112 patients were offered inclusion, with a 66% participation rate. Of those participating, the response rate was 96% (Fig 1). As part of the questionnaire, QoL outcomes were collected through the AQoL-8D.30 Visual function was recorded and vision-related QoL was collected through the National Eye Institute Visual Functioning Questionnaire.32,33 This study was approved on September 24, 2018, by the Sydney Children’s Hospitals Network Human Research Ethics Committee (reference, HREC/18/SCHN/292) and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained for all patients.
Figure 1.
Flowchart showing patient enrollment, participation, and response rates. EPIC VE IRD = inherited retinal disease; VFQ = Visual Function Questionnaire.
Data and Measures
Health-Related Quality of Life
Patient QoL was assessed using the AQoL-8D, a reliable and validated instrument for economic evaluation studies.30 The AQoL-8D measures functioning across 8 dimensions: independent living, pain, senses, mental health, happiness, coping, relationships, and self-worth. Utilities are defined for each of the dimensions, which can be translated into QALYs for use in cost-effectiveness analysis. Overall, AQoL-8D utility values range from 0 to 1, with 1 being full or perfect health and 0 being the lowest. The AQoL-8D was chosen because it includes questions of relevance to this cohort including those regarding how well the respondent can see, independent living, social functioning, and relationship and psychosocial impacts.30
Vision and Vision-Related Quality of Life
Vision-related QoL was measured through the NEI-VFQ-39. It is an extended version of the NEI-VFQ-25, consisting of the 25 questions from the NEI-VFQ-25 plus additional questions such as ability to read bills and further questions on general health and difficulty in participating in sport and outdoor activities. It includes questions on visual function for different tasks and activities, along with related questions on life impacts because of vision issues and overall health status.34 The scores range from 0 to 100, with 0 being the lowest and 100 the highest. Participant visual acuity was recorded from patients’ medical records using best-corrected visual acuity.
Socioeconomic and Other Health-Related Data
Socioeconomic and comorbidity data were also collected, including questions on mental health and behavioral disorders, because mental health has been found to be significantly poorer in patients with IRD.35
Statistical Analysis
Descriptive statistics were calculated, including NEI-VFQ-25 and NEI-VFQ-39 scores, visual acuity, each of the AQoL-8D domains and total AQoL-8D utility values, along with other patient characteristics. The AQoL-8D scores from each dimension were converted into the total AQoL-8D utility value using the algorithm provided with the instrument.36 One-sample t tests were performed comparing the total AQoL-8D utility values and each of the domain scores with population norms,37 with statistical significance set at P < 0.05.
For mental health and behavioral disorders, the categorical variable on type of condition was converted into a binary variable on whether the patient had been diagnosed or identified as having any of the following conditions: depression or mood disorders, anxiety, nervous tension or stress, attention deficit disorder or hyperactivity, autism and related disorders, or any other mental health or behavioral disorders. The participant visual acuity was recorded and grouped according to the World Health Organization guidelines,38 in which visual acuities are grouped into visual impairment categories: mild or no visual impairment, moderate visual impairment, severe visual impairment, and 3 separate classifications of blindness. For analysis, patients were grouped further as 20/200 or better or worse than 20/200. This value was chosen because it represents the legal definition for blindness in Australia.
Regressions were estimated to analyze the relationship between a range of explanatory variables and the AQoL-8D, including the NEI-VFQ-39 and visual acuity. Bivariate regression models were used first to analyze the individual explanatory power of each of the variables in explaining the variations in AQoL-8D utility values. Then, a series of multivariate regression models were fitted for the AQoL-8D in a stepwise approach, with the first model containing only the NEI-VFQ-39 score. Model 2 added whether the patient had received a diagnosis of a mental health condition, and model 3 included age, sex, visual acuity, and age at diagnosis. Because the NEI-VFQ-39 is not always collected in clinical trials, we also estimated these models using the NEI-VFQ-25 (models 4, 5, and 6). All analyses were conducted in SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Descriptive statistics of the sample are reported in Table 1. The cohort included 70 individuals with IRD with a mean age of 42.7 years; 65.7% were women. The respondents had an average NEI-VFQ-39 score of 48.5. Fifty-one percent of the sample had a diagnosis of rod-dominated ocular disease, followed by 23% with a diagnosis of macular dystrophy. Futher details on sample are shown in Supplemental Table 2.
Table 1.
Demographics and Descriptive Statistics of Adult Sample of Patients with Inherited Retinal Disease
| Variable | Characteristics (n = 70) |
|---|---|
| Age (yrs) | 42.7 ± 17.4 |
| AQoL-8D utility | 0.58 ± 0.20 |
| NEI-VFQ-39 score | 48.5 ± 17.9 |
| NEI-VFQ-25 score | 47.3 ± 17.7 |
| Gender | |
| Male | 24 |
| Female | 46 |
| IRD diagnosis | |
| Rod-dominated IRD∗ | 36 |
| Macular dystrophy† | 16 |
| Cone-dominated IRD‡ | 12 |
| IRD plus systemic disorder§ | 6 |
| Mental health condition | |
| Yes | 33 |
| No | 37 |
| Age aware of diagnosis (yrs) | |
| ≤18 | 46 |
| 19–30 | 9 |
| 31–45 | 10 |
| ≥45 | 5 |
| Visual acuity | |
| ≥20/200 | 34 |
| <20/200 | 36 |
AQoL-8D = Assessment of Quality of Life 8-Dimension; IRD = inherited retinal disease; NEI-VFQ-25 = 25-item National Eye Institute Visual Function Questionnaire; NEI-VFQ-39 = 39-item National Eye Institute Visual Function Questionnaire.
Data are presented as mean±standard deviation or no. Additional details of variables included in Supplemental Table 2.
Includes rod–cone dystrophy (retinitis pigmentosa), n = 31; choroideremia, n = 2; enhanced S-cone syndrome, n = 2; and Leber congenital amaurosis, n = 1.
Includes Stargardt disease, n = 14; and other, n = 1.
Includes cone–rod dystrophy, n = 6; cone dystrophy, n = 5; and achromatopsia, n = 1.
Includes Usher syndrome, n = 4; and other, n = 2.
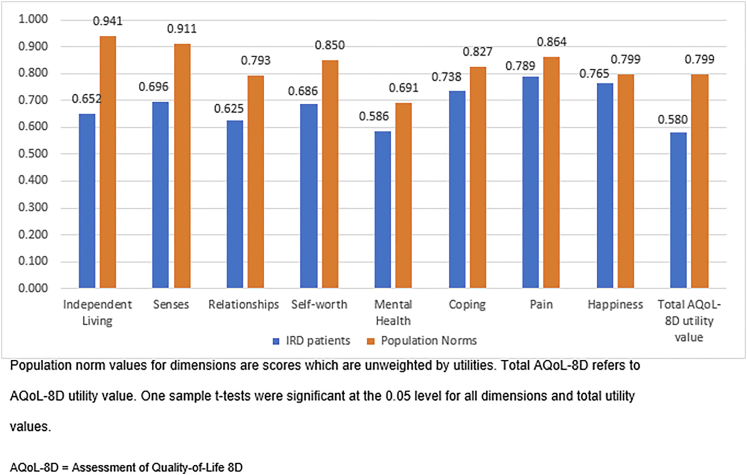
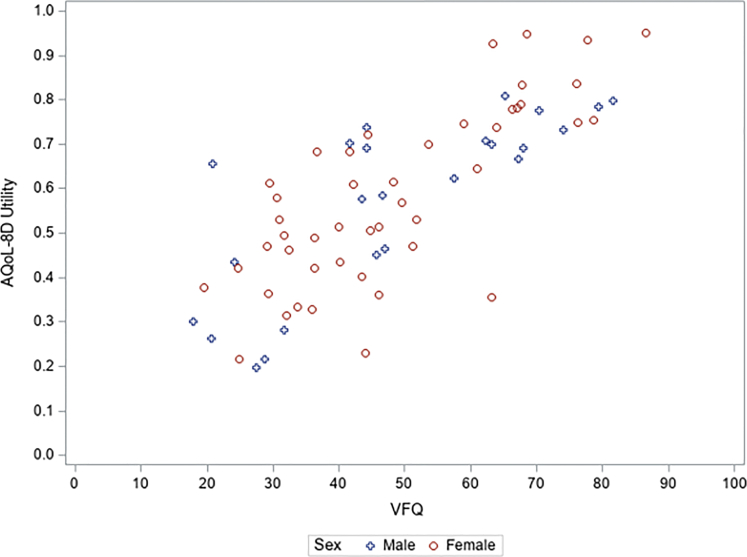
The AQoL-8D scores for each dimension and the total AQoL-8D utility values are shown in Figure 2. The mean AQoL-8D utility value in the sample was 0.58, compared with the population norm of 0.8.37 For total AQoL-8D utility values and for the scores in all the dimensions, t tests were statistically significant. However, as Figure 2 shows, the relative difference varied significantly among the dimensions, with independent living, senses, and relationships showing the greatest impacts for those in the IRD group. Figure 3 shows a positive relationship between the AQoL-8D utility values and NEI-VFQ-39 scores, with AQoL-8D utility values declining as NEI-VFQ-39 scores decline.
Figure 2.
Bar graph showing Assessment of Quality of Life 8-Dimension (AQoL-8D) mean scores and total AQoL-8D utility values of patients with inherited retinal disease (IRD).
Figure 3.
Scatterplot showing positive relationship between Assessment of Quality of Life 8-Dimension (AQoL-8D) utility values and 39-item National Eye Institute Visual Function Questionnaire (NEI-VFQ-39) scores. Pearson correlation coefficient AQoL-8D utility values and NEI-VFQ-39 scores: whole sample, 0.772; male, 0.784; female, 0.768. P < 0.01 for all.
Table 2 shows the bivariate relationships between patient characteristics and AQoL-8D utility values. The R2 value (0.596), indicating goodness of fit, was significantly higher for the NEI-VFQ-39 compared with the other variables such as age of awareness of the IRD or visual acuity alone, showing that it explains much more of the variation in AQoL-8D values than these other variables. Having a mental health condition was estimated to decrease utility by 0.227, whereas profound or severely impaired visual acuity (worse than 20/200) was estimated to decrease utility by 0.151.
Table 2.
Bivariate Regressions with Dependent Variable Assessment of Quality of Life 8-Dimension
| Variable | Coefficient with Assessment of Quality of Life 8-Dimension | 95% Confidence Interval | R2 Value | No. |
|---|---|---|---|---|
| NEI-VFQ-39 | 0.008∗ | 0.007–0.010 | 0.596 | 70 |
| Mental health condition (yes = 1) | –0.227∗ | –0.304 to –0.149 | 0.335 | 70 |
| Age aware of diagnosis | 0.067 | 70 | ||
| ≤18 (reference) | ||||
| 19–30 | 0.110 | –0.032 to 0.251 | ||
| 31–45 | –0.071 | –0.206 to 0.064 | ||
| >45 | –0.644 | –0.247 to 0.118 | ||
| Visual acuity | 0.151 |
0.064–0.239 |
0.150 | 70 |
| Normal/mild/moderate (≥20/200) | ||||
| Profound severe (<20/200; reference) |
NEI-VFQ-39 = 39-item National Eye Institute Visual Function Questionnaire.
Intercept for each bivariate model not included in table.
P < 0.001.
Table 3 shows the multivariate regressions, with mapping equations for the models after the table. Model 1 includes only the NEI-VFQ-39 score (as shown in Fig 2). Model 2 adds whether the patients have a diagnosis of a mental health condition. Model 3 adds age, sex, visual acuity, and age at diagnosis. The R2 value rose from 0.6 in the base model to 0.73 in the extended model (model 3). All models showed strong explanatory power. In the extended model (model 3), a 1-unit increase in the NEI-VFQ-39 score (scale, 0–100) was associated with a 0.007 increase in AQoL-8D utility values (scale, 0–1). Having a diagnosis of a mental health condition is negatively associated with AQoL-8D and increases the R2 value (model 2). Although model 3 has a slightly higher R2 value, visual acuity and other variables in the extended model were not statistically significant. Models 4, 5, and 6 produced very similar results, although with a slightly lower R2 value: 0.55 when including NEI-VFQ-25 alone (model 4), 0.682 when mental health is added (model 5), and 0.688 when sex, age, age at diagnosis, and visual acuity are added (model 6). This shows that the additional questions in the NEI-VFQ-39 slightly increase its explanatory power for AQoL-8D health utilities over the NEI-VFQ-25.
Table 3.
Multivariate Regression Analyses for Assessment of Quality of Life 8-Dimension Utility Values
| Variable | Base Model (Model 1) |
Extended Model (Model 2), Mental Health Conditions |
Extended Model (Model 3), Mental Health Conditions, Age, Gender, Diagnosis, Age at Diagnosis |
Base Model (Model 4) 25-Item National Eye Institute Visual Function Questionnaire |
Extended Model (Model 5), 25-Item National Eye Institute Visual Function Questionnaire Mental Health Conditions |
Extended Model (Model 6), 25-Item National Eye Institute Visual Function Questionnaire Mental Health Conditions, Age, Gender, Diagnosis, Age at Diagnosis |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% Confidence Interval | Coefficient | 95% Confidence Interval | Coefficient | 95% Confidence Interval | Coefficient | 95% Confidence Interval | Coefficient | 95% Confidence Interval | Coefficient | 95% Confidence Interval | |
| NEI-VFQ-39 score | 0.0084∗ | 0.007–0.010 | 0.007∗ | 0.006–0.009 | 0.007∗ | 0.005–0.009 | ||||||
| NEI-VFQ-25 score | 0.0082∗ | 0.006–0.010 | 0.0069∗ | 0.005–0.009 | 0.0064∗ | 0.004–0.009 | ||||||
| Mental health condition (yes =1) | –0.144∗ | –0.197 to –0.090 | –0.145∗ | –0.204 to –0.086 | –0.150∗ | –0.206 to –0.093 | –0.154∗ | –0.216 to –0.092 | ||||
| Gender (male = 1) | –0.005 | –0.062 to 0.052 | –0.016 | –0.074 to 0.043 | ||||||||
| Age | 0.001 | –0.001 to 0.003 | 0.001 | –0.002 to 0.003 | ||||||||
| Age at awareness of diagnosis (yrs) | ||||||||||||
| 19–30 | 0.001 | –0.084 to 0.087 | –0.0002 | –0.091 to 0.091 | ||||||||
| 31–45 | –0.028 | –0.118 to 0.062 | –0.023 | –0.117 to 0.072 | ||||||||
| >45 | –0.028 | –0.148 to 0.091 | –0.009 | –0.136 to 0.118 | ||||||||
| Visual acuity | 0.026 | –0.048 to 0.099 | 0.048 | –0.026 to 0.123 | ||||||||
| Normal/mild/moderate (≥20/200) | ||||||||||||
| Profound severe (≤20/200), reference | ||||||||||||
| Intercept | 0.168∗ | 0.081–0.256 | 0.308∗ | 0.218–0.398 | 0.298∗ | 0.168 – 0.427 | 0.189∗ | 0.098–0.280 | 0.332 | 0.238–0.427 | 0.321∗ | 0.184–0.457 |
| No. | 70 | 70 | 70 | 70 | 70 | 70 | ||||||
| R2 value | 0.596 | 0.717 | 0.730 | 0.550 | 0.682 | 0.688 | ||||||
NEI-VFQ-25 = 25-item National Eye Institute Visual Functioning Questionnaire; NEI-VFQ-39 = 39-item National Eye Institute Visual Functioning Questionnaire.
Dependent variable AQoL-8D utility values are presented.
P < 0.001.
The results from the models can also be expressed as equations found in Figure 4 for use in mapping health utilities where AQoL-8D data were not collected. Mapping the NEI-VFQ-25 (a commonly used subset of the NEI-VFQ-39) produced similar results, although with slightly lower R2 values.
Figure 4.
Mapping equations for 39-item National Eye Institute Visual Function Questionnaire (NEI-VFQ-39), 25-item National Eye Institute Visual Function Questionnaire (NEI-VFQ-25), and Assessment of Quality of Life 8-Dimension (AQoL-8D) utility values.
Discussion
In this study, we estimated the patient-reported impact of IRDs on QoL using established measures of health utilities. Using patient-reported primary data, we found that patients with IRD have a significantly lower average health utility value of 0.58 compared with the population norm of 0.8. Scores were lower across all dimensions of the AQoL-8D, showing that IRDs have profound impacts across many different aspects of life. The largest relative differences were in the independent living, senses, and relationships dimensions of the AQoL-8D.
Regressions showed a strong positive relationship between the NEI-VFQ-39 with health utility measured through the AQoL-8D. The NEI-VFQ-39 scores alone explained about 60% of the variation in AQoL-8D utility values. This increased to 72% with the addition of mental health metrics and to 73% when, age, sex, age at diagnosis, and visual acuity were also included. The models produced similar results when using the NEI-VFQ-25. Previous studies found that mental health conditions were commonly reported in this population35 and emphasized the need for attention to mental health in these disorders. Visual acuity was not statistically significant in the multivariate model, consistent with previous literature, suggesting that measures of visual acuity alone are unlikely to capture the QoL impacts resulting from IRD.25
Use of Utility Values in Inherited Retinal Disease Treatment Evaluations
Clinical genetic and molecular assessments have advanced significantly in recent years and have the potential to transform the diagnostic and treatment landscape for inherited vision disorders.39, 40, 41 Genomic medicine can avoid unnecessary additional tests, can reduce prognostic uncertainty, and can determine eligibility for emerging therapies and clinical trials and access to reproductive technologies.42 With the cost of genomic testing falling and the development of gene therapies, it is crucial to have robust cost-effectiveness analyses, which will require QoL and patient-reported related utility data for patients with IRD.
The successful translation of potential therapies into clinical practice requires not only disease-specific outcome improvements, but also improvements in QoL. However, the lack of the collection of patient-reported data for utilities has meant that these have been estimated and retrofitted onto the clinical studies, a significant limitation in studies of gene therapies.23
In the absence of utility values for patients with IRD, studies have used values associated with visual acuity.19 The lack of data relevant to economic analysis is likely to underestimate health utility gains because of the use of data for other vision conditions, some of which have a later onset than RPE65 pathogenic variants.43 In its evaluation, United Kingdom National Institute for Health and Care Excellence noted, “The committee was disappointed that no direct measure of health-related quality of life (HRQoL) had been used in the clinical trials and considered that the lack of patient-reported outcomes was a key limitation.”12
In the absence of patient-reported data, authors relied on the use of clinician’s assessment of utility based on proxy vignettes associated with visual acuity to estimate utility values.10,44 The results of one of those studies suggested a significant association between utility estimated from the EQ5D-5L.10,44 However, our bivariate analysis suggests the explanatory power for AQoL-8D of visual acuity alone is much lower than the NEI-VFQ-39 score. We believe the reason for the difference in the findings between our study incorporating AQoL-8D score, visual acuity, and NEI-VFQ-39 score and the previous study may be the result of single patient vignettes for each visual acuity category that assumed that all patients share the same experience (for example, depression). In practice, in individual patient-reported data, experiences vary, sometimes markedly. This emphasizes the importance of patient-reported data, as in our study.
The use of assumptions and reliance on data related to other diseases has meant the reported cost-effectiveness of the example case of voretigene neparvovec-rzyl are contradictory.19 These vastly different results occur because economic models are sensitive to key inputs, including how QoL and health utilities are measured,23 but given that data specific to IRD are unavailable, little alternative has been available to using assumptions or data related to other diseases that may not accurately reflect the circumstances of patients with IRD.10,24 Given these factors, we considered a range of utility tools to elicit patient-reported values including the EQ-5D, which has been criticized for ophthalmic populations because of a lack of relevance of the questions to vision-relation QoL, particularly related to earlier stages of vision loss.45 In our study, we chose the AQoL-8D because it includes questions on vision, independent living, social functioning, relationships, and psychosocial impacts,30 all factors know to be relevant in eye diseases.46
Our modeling showed that the NEI-VFQ-39 and other clinical data have strong explanatory power for AQoL-8D health utilities. The results were also very similar when using the NEI-VFQ-25. This supports the potential use of other instruments such as the NEI-VFQ-39 and NEI-VFQ-25 to derive utility measures where these have not been collected. Similarly, previous studies have reported utility scores being mapped from other measures.28 The algorithms we identified incorporating the NEI-VFQ-25, NEI-VFQ-39, and additional clinical data can be used in this way to estimate health utilities when QoL for IRD with an associated utility algorithm is not collected. We recommend the use of model 3 where possible because it has the highest R2 value; however, the other models can be used if data are insufficient to use model 3.
Strengths and Limitations of Study
A strength of this study is that it used health-related utility of patients with IRD directly from patient-reported data from a validated instrument. This revealed significant differences in overall health utilities between those with IRD and the general population. We also demonstrated significant differences within specific QoL dimensions, providing a more complete picture of the multiple impacts of IRD. Importantly, health utilities from the AQoL-8D allow comparisons between different health states and can be translated into QALYs. This is crucial for cost-effectiveness studies, including for gene therapies to improve outcomes for patients with IRD.
A limitation of the study is that some of the conditions used in the models were patient reported, such as the mental health conditions. However, many of the other measures, such as visual acuity, were assessed clinically. Our broader group of IRDs included many different IRD diagnoses. The associations (or lack of associations) found in the multivariate models may only apply to the broader categories that were used, and it is possible that the overall findings may differ for specific types of IRD. However, because of the small number of patients with less common types of IRD, we have used the broader grouping of all IRDs. Further, a small number of records had missing data (Fig 1).
In conclusion, patient-reported health utility data confirmed significantly lower utility across all dimensions of the AQoL-8D for those with IRD compared with population norms. The NEI-VFQ-25 and NEI-VFQ-39 showed a strong correlation with the AQoL-8D, with the capacity to explain between 60% of the variation in AQoL-8D-related utility values, rising to 73% when including mental health measures, age, sex, age at diagnosis, and visual acuity, providing a useful method for estimating health utilities in cost-effectiveness studies.
Acknowledgments
The authors thank Laura Wedd for confirming patient participation information.
Manuscript no. D-21-00143
Footnotes
Supplemental material available atwww.ophthalmologyscience.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): J.G.: Consultant and Lecturer – Novartis Australia; Financial support – Retina Australia
R.V.J.: Consultant and Lecturer – Novartis Australia; Financial support – Cure Blindness Australia
The authors may be contacted regarding data used in this study at mqbs-gi-admin@mq.edu.au.
Supported by the National Health and Medical Research Council (NHMRC) Centre for Research Excellence, Canberra, Austrlia, (grant no.: APP 1116360); and Luminesce Alliance, Westmead, NSW Australia, which is a not-for-profit cooperative joint venture across the Sydney Children’s Hospitals Network, Children’s Medical Research Institute, and Children’s Cancer Institute, established with the support of the New South Wales Government. Luminesce Alliance is also affiliated with the University of Sydney and the University of New South Wales. The sponsor or funding organization had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Sydney Children’s Hospitals approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Schofield, Kraindler, Tan, Shrestha, West, Ma, Grigg, Jamieson
Analysis and interpretation: Schofield, Kraindler, Tan, Shrestha
Data collection: Schofield, Tan, Shrestha, West, Ma, Grigg, Jamieson
Obtained funding: N/A; Study was performed as part of the authors' regular employment duties. No additional funding was provided.
Overall responsibility: Schofield, Kraindler, West, Ma, Grigg, Jamieson
Supplementary Data
References
- 1.Sahel J.A., Dalkara D. Gene therapy for retinal dystrophy. Nat Med. 2019;25(2):198–199. doi: 10.1038/s41591-019-0346-1. [DOI] [PubMed] [Google Scholar]
- 2.Grigg J, Jamieson R, Chen F, et al. Guidelines for the assessment and management of patients with inherited retinal diseases (IRD): the Royal Australia and New Zealand College of Ophthalmologists. https://ranzco.edu/wp-content/uploads/2020/05/RANZCO-Guidelines-for-the-assessment-and-management-of-patients-with-inherited-retinal-diseases-IRD.pdf. Accessed June 20, 2021
- 3.Liew G., Michaelides M., Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanany M., Rivolta C., Sharon D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc Natl Acad Sci U S A. 2020;117(5):2710–2716. doi: 10.1073/pnas.1913179117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birtel J., Eisenberger T., Gliem M., et al. Clinical and genetic characteristics of 251 consecutive patients with macular and cone/cone-rod dystrophy. Sci Rep. 2018;8(1):4824. doi: 10.1038/s41598-018-22096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahim A.T., Daiger S.P., Weleber R.G. Nonsyndromic retinitis pigmentosa overview. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle. GeneReviews. 20002017:1993–2022. https://www.ncbi.nlm.nih.gov/books/NBK1417 [Google Scholar]
- 7.Deloitte Access Economics Socioeconomic cost of macular degeneration in New Zealand. 2016. https://www2.deloitte.com/au/en/pages/economics/articles/socioeconomic-cost-macular-degeneration-in-nz.html Available at:
- 8.Chakravarthy U., Biundo E., Saka R.O., et al. The economic impact of blindness in Europe. Ophthal Epidemiol. 2017;24(4):239–247. doi: 10.1080/09286586.2017.1281426. [DOI] [PubMed] [Google Scholar]
- 9.Lee K., Garg S. Navigating the current landscape of clinical genetic testing for inherited retinal dystrophies. Genet Med. 2015;17:245–252. doi: 10.1038/gim.2015.15. [DOI] [PubMed] [Google Scholar]
- 10.Johnson S., Buessing M., O’Connell T., et al. Cost-effectiveness of voretigene neparvovec-rzyl vs standard care for RPE65-mediated inherited retinal disease. JAMA Ophthalmol. 2019;137(10):1115–1123. doi: 10.1001/jamaophthalmol.2019.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sergouniotis P.I. Inherited retinal disorders: using evidence as a driver for implementation. Ophthalmologica. 2019;242:187–194. doi: 10.1159/000500574. [DOI] [PubMed] [Google Scholar]
- 12.United Kingdom National Institute for Health and Care Excellence VN for treating inherited retinal dystrophies caused by RPE65 gene mutations. 9 October 2019. 9 October 2019. www.nice.org.uk/guidance/hst11 Available at: Accessed 27.1.21.
- 13.Garafalo A.V., Cideciyan A.V., Héon E., et al. Progress in treating inherited retinal diseases: early subretinal gene therapy clinical trials and candidates for future initiatives. Prog Retin Eye Res. 2020;77:100827. doi: 10.1016/j.preteyeres.2019.100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer M.D., Michalakis S., Wilhelm B., et al. Safety and vision outcomes of subretinal gene therapy targeting cone photoreceptors in achromatopsia: a nonrandomized controlled trial. JAMA Ophthalmol. 2020;138(6):643–651. doi: 10.1001/jamaophthalmol.2020.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue K., Jolly J.K., Barnard A.R., et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat Med. 2018;24:1507–1512. doi: 10.1038/s41591-018-0185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cehajic-Kapetanovic J., Xue K., Martinez-Fernandez de la Camara C., et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med. 2020;26:354–359. doi: 10.1038/s41591-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Academies of Sciences, Engineering, and Medicine . The National Academies Press; Washington, DC: 2017. An Evidence Framework for Genetic Testing. [PubMed] [Google Scholar]
- 18.Whitehead S.J., Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. doi: 10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann M., Lubinga S.J., Banken R., et al. Cost utility of voretigene neparvovec for biallelic RPE65-mediated inherited retinal disease. Value Health. 2019;22(2):161–167. doi: 10.1016/j.jval.2018.09.2841. [DOI] [PubMed] [Google Scholar]
- 20.Uhrmann M.F., Lorenz B., Gissel C. Cost effectiveness of voretigene neparvovec for RPE65-mediated inherited retinal degeneration in Germany. Trans Vis Sci Tech. 2020;9(9):17. doi: 10.1167/tvst.9.9.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viriato D., Bennett N., Sidhu R., et al. An economic evaluation of voretigene neparvovec for the treatment of biallelic RPE65-mediated inherited retinal dystrophies in the UK. Adv Ther. 2020;37(3):1233–1247. doi: 10.1007/s12325-020-01243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ascenção R., Paquete A.T., Santos L.C., et al. PSS11 costs and quality of life of RPE65-mediated inherited retinal dystrophies in Portugal. Value Health. 2020;23(2):S735. [Google Scholar]
- 23.Huygens S.A., Versteegh M.M., Vegter S., et al. Methodological challenges in the economic evaluation of a gene therapy for RPE65-mediated inherited retinal disease: the value of vision. Pharmacoeconomics. 2021;39(4):383–397. doi: 10.1007/s40273-021-01003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halioua-Haubold C.L., Jolly J.K., Smith J.A., et al. Potential lifetime quality of life benefits of choroideremia gene therapy: projections from a clinically informed decision model. Eye (Lond) 2019;33(8):1215–1223. doi: 10.1038/s41433-019-0492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assi L., Rosman L., Chamseddine F., et al. Eye health and quality of life: an umbrella review protocol. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noble J., Forooghian F., Sproule M., et al. Utility of the National Eye Institute VFQ-25 questionnaire in a heterogeneous group of multiple sclerosis patients. Am J Ophthalmol. 2006;142(3):464–468. doi: 10.1016/j.ajo.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 27.Rentz A.M., Kowalski J.W., Walt J.G., et al. Development of a preference-based index from the National Eye Institute Visual Function Questionnaire-25. JAMA Ophthalmol. 2014;132(3):310–318. doi: 10.1001/jamaophthalmol.2013.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browne C., Brazier J., Carlton J., et al. Estimating quality-adjusted life years from patient-reported visual functioning. Eye (Lond) 2012;26(10):1295–1301. doi: 10.1038/eye.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payakachat N., Summers K.H., Pleil A.M., et al. Predicting EQ-5D utility scores from the 25-item National Eye Institute Vision Function Questionnaire (NEI-VFQ 25) in patients with age-related macular degeneration. Qual Life Res. 2009;18(7):801–813. doi: 10.1007/s11136-009-9499-6. [DOI] [PubMed] [Google Scholar]
- 30.Richardson J., Iezzi A., Khan M.A., Maxwell A. Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient. 2014;7(1):85–96. doi: 10.1007/s40271-013-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Australian Bureau of Statistics Australian demographic statistics, June 2019. June 2019. https://www.abs.gov.au/ausstats/abs@.nsf/0/1cd2b1952afc5e7aca257298000f2e76 Available at:
- 32.Mangione C.M., Lee P.P., Gutierrez P.R., et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 33.Mangione C.M., Lee P.P., Pitts J., et al. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116(11):1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 34.Rand Corporation Visual Function Questionnaire. https://www.rand.org/health-care/surveys_tools/vfq.html Available at:
- 35.Sainohira M., Yamashita T., Terasaki H., et al. Quantitative analyses of factors related to anxiety and depression in patients with retinitis pigmentosa. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assessment of Quality of Life. Scoring: psychometric (unweighted) or utility (weighted)? https://www.aqol.com.au/index.php/scoring-algorithms Available at: Accessed 14.12.20.
- 37.Maxwell A., Özmen M., Iezzi A., Richardson J. Centre for Health Economics, Monash University; Monash Business School, Monash University, Clayton, Victoria: 2016. Norms for the AQoL-6D and AQoL-8D multi attribute utility instruments. Research paper 2016 (94) [Google Scholar]
- 38.World Health Organization Blindness and vision impairment. https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment Available at:
- 39.Prokudin I., Li D., He S., et al. Value of whole exome sequencing for syndromic retinal dystrophy diagnosis in young patients. Clin Exp Ophthalmol. 2015;43(2):132–138. doi: 10.1111/ceo.12391. [DOI] [PubMed] [Google Scholar]
- 40.Stone E.M., Andorf J.L., Whitmore S.S., et al. Clinically focused molecular investigation of 1000 consecutive families with inherited retinal disease. Ophthalmology. 2017;124:1314–1331. doi: 10.1016/j.ophtha.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hafler B.P. Clinical progress in inherited retinal degenerations: gene therapy clinical trials and advances in genetic sequencing. Retina. 2017;37:417–423. doi: 10.1097/IAE.0000000000001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenassi E., Clayton-Smith J., Douzgou S., et al. Clinical utility of genetic testing in 201 preschool children with inherited eye disorders. Genet Med. 2020;22(4):745–751. doi: 10.1038/s41436-019-0722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buessing M., O’Connell T., Johnson S., et al. Important considerations in modeling the cost-effectiveness for the first Food and Drug Administration–approved gene therapy and implications for future one-time therapies. Value Health. 2019;22(8):P970–P971. doi: 10.1016/j.jval.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 44.Lloyd A., Piglowska N., Ciulla T., et al. Estimation of impact of RPE65-mediated inherited retinal disease on quality of life and the potential benefits of gene therapy. Br J Ophthalmol. 2019;103(11):1610–1614. doi: 10.1136/bjophthalmol-2018-313089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bozzani F.M., Alavi Y., Jofre-Bonet M., Kuper H. A comparison of the sensitivity of EQ-5D, SF-6D and TTO utility values to changes in vision and perceived visual function in patients with primary open-angle glaucoma. BMC Ophthalmol. 2012;12:43. doi: 10.1186/1471-2415-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Amanda C.S., Nolen R., Huryn L.A., Turriff A. Psychosocial impacts of Mendelian eye conditions: a systematic literature review. Surv Ophthalmol. 2020;65(5):562–580. doi: 10.1016/j.survophthal.2020.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.