Abstract
Seventy-three piglets were weaned at 1 week of age, randomly assigned to 10 groups (A to J), accommodated in stainless steel exposure chambers, and exposed continuously to a controlled environment containing aerosolized ovalbumin. The concentrations of ovalbumin dust were as follows (milligrams per cubic meter): A and F, 16.6; B and G, 8.4; C and H, 4.2; D and I, 2.1; E and J, 0. At weekly intervals, the pigs were bled via venipuncture and anesthetized for nasal lavage and tonsilar biopsies performed for subsequent bacteriologic analysis. At 2 weeks of age, the pigs in groups A to E were challenged with toxigenic Pasteurella multocida (108 CFU pig−1), and at 6 weeks of age, the pigs were euthanatized. At postmortem, the extent of turbinate atrophy was assessed on the snout sections by using a morphometric index. Exposure to aerial ovalbumin resulted in a dose-dependent increase in serum antiovalbumin immunoglobulin G (IgG; P < 0.001) and serum antiovalbumin IgA (P < 0.001). Exposure also caused a significant increase in the numbers of P. multocida organisms isolated from the upper respiratory tract (P < 0.001) and a corresponding increase in turbinate atrophy, as judged by the morphometric index (P < 0.001). Concurrent challenge with P. multocida and ovalbumin resulted in a significant decrease in both the IgG and IgA responses to ovalbumin (P < 0.001). These results show that ovalbumin exposure increases pig susceptibility to P. multocida colonization and that toxigenic P. multocida modifies the serum IgG and IgA responses to ovalbumin in the pig. Both of these effects may enhance the virulence of this respiratory pathogen and so influence the pathogenesis of atrophic rhinitis in pigs.
Atrophic rhinitis is an upper respiratory tract disease of pigs characterized clinically by atrophy and degeneration of the bony and cartilaginous structures of the snout, in particular, the nasal turbinate and septum. In severe cases, this leads to visible shortening or twisting of the snout (12). In addition to these local effects, the disease is also associated with a decreased rate of weight gain, inefficient feed conversion, and increased time to market (6). The etiology and pathogenesis of this disease are multifactorial, involving interactions between primary bacterial pathogens and inhaled environmental pollutants. Toxigenic strains of Bordetella bronchiseptica and Pasteurella multocida types A and D have been established as primary etiological agents by experimental studies (10, 20, 26, 28). Epidemiological studies have also shown that severe turbinate atrophy predisposes young pigs to pneumonia (9, 37).
Experimentally, purified P. multocida toxin induces turbinate atrophy when aerosolized into the nasal cavity or injected into the subcutis, muscle, or peritoneum (6). The more severe, naturally occurring form of the disease is known as progressive atrophic rhinitis and is attributed specifically to colonization of the pig nasal cavity by toxigenic strains of P. multocida (25, 28, 31). However, the failure to reproduce the clinical disease consistently in experimental studies without depriving pigs of passive immunity, i.e., withholding colostrum (24), or pretreating the pigs’ nasal cavities prior to challenge with a chemical irritant such as acetic acid (26) supports the clinical impression that external factors, in particular, atmospheric pollutants, are necessary to the pathogenesis of progressive atrophic rhinitis as seen in commercial pig houses. We have previously established a clear synergistic role for gaseous ammonia in the etiology of P. multocida-induced atrophic rhinitis (18). We have also demonstrated that aerosolized ovalbumin predisposes young pigs to P. multocida colonization, which results in lesions typical of progressive atrophic rhinitis (16, 17). The epidemiological study of Baekbo (2) supports this observation and shows that relatively small aerial dust concentration changes can significantly increase the incidence and severity of atrophic rhinitis.
The seminal observation that infection with microorganisms may decrease the immune response to a different pathogen was made in 1908 by von Pirquet, who showed that the tuberculin reaction, a typical expression of cell-mediated immunity, was depressed in patients with measles (35). This depressed reaction also applies to other clinical infections such as pertussis (4), typhus (38), scarlet fever (22), influenza (3), and brucellosis (1).
There are some data that suggest that toxin derived from P. multocida modulates the humoral immune response to atrophic rhinitis vaccine containing P. multocida toxoid (34). The objective of the present study was to explore the role of organic dusts in the etiology and pathogenesis of progressive atrophic rhinitis by observing (i) the effect of exposing pigs to an organic dust, ovalbumin, on their susceptibility to P. multocida colonization and (ii) whether the humoral immune response to aerial ovalbumin exposure was compromised by P. multocida colonization of the upper respiratory tract.
MATERIALS AND METHODS
Animals.
Seventy-three minimal-disease piglets were derived from Large White sows obtained from the Institute of Animal Health, Compton Laboratory, Newbury, England. The piglets were weaned onto a dry commercial ration (given twice a day) at 1 week of age and randomly assigned to 10 experimental groups (A to J). Before the experiment commenced, all of the pigs were screened for the presence of P. multocida by nasal lavage with 10 ml of phosphate-buffered saline (PBS) as described by Chanter and Rutter (6). The recovered fluid was cultured overnight at 37°C on 5% horse blood agar plates with added antibiotics (29).
Exposure chambers.
All groups of pigs were housed in 1.4-m3 stainless steel Rochester exposure chambers built to the design of Timbrell et al. (32). The chambers were run at −70 kPa, giving an air exchange rate of 60 air changes per hour. Air entered each chamber via a HEPA filter and, after traversing the chamber, was vented via a HEPA filter to prevent the release of biological material. Within the chamber, the air was maintained at a temperature of 25 ± 1.0°C and a relative humidity of 50% ± 5%.
Pigs in groups A to D and F to I were exposed to various concentrations of aerial ovalbumin from 1 to 6 weeks of age. Ovalbumin dust (Sigma) was generated by a Palas RGB-1000 rotating-brush powder dispersion generator (Palas), split and diluted to the required total dust concentrations, and introduced via the inlet pipe to respective chambers. The concentration of aerial ovalbumin (in milligrams per cubic meter) in the chambers was determined weekly by using Institute of Occupational Medicine inspirable personal samples (Negretti Automation Ltd.) and Nuclepore filters (Costar). Twice daily, the ovalbumin levels were monitored with a Rion KC 01A particle counter (Lynjay Services Ltd., Worthing, West Sussex, United Kingdom). This instrument also measured the aerodynamic particle sizes of the dust.
Bacterial inoculum.
P. multocida LFB3, a toxigenic isolate from a clinical case of atrophic rhinitis in a British pig (28), was supplied by the Institute of Animal Health, Compton Laboratory. The isolate was stored at −70°C in brain heart infusion broth containing Robertson’s cooked meat granules and 5% glycerol.
Immediately prior to use, P. multocida LFB3 was cultured overnight under aerobic conditions on 5% horse blood agar at 37°C. A single colony from this plate was used to inoculate 10 ml of brain heart infusion broth, which was incubated overnight aerobically at 37°C. The number of organisms in the broth was determined by a modified method of Miles and Misra (8). This broth was centrifuged at 11,800 × g for 10 min and washed with 37°C PBS three times; the cells were then resuspended in PBS at the required concentration for animal inoculation.
Experimental protocol.
The experiment was designed to challenge pigs with chronic exposure to five different concentrations of inhaled ovalbumin dust with and without inoculation with P. multocida. Pigs were randomly divided into 10 groups. Groups A to E were inoculated at 2 weeks of age with 108 CFU of P. multocida LFB3 in a 0.5-ml dose (suspended in PBS) per nostril. Groups F to J were treated with 0.5 ml of PBS per nostril. Groups were exposed to ovalbumin dust (85% of the particles had an aerodynamic diameter above 5 μm) from weeks 1 to 6 as follows (milligrams per cubic meter ± 10%): A and F, 16.6; B and G, 8.4; C and H, 4.1; D and I, 2.1; E and J, 0. Group sizes were as follows: A to D and F to I, 6 pigs each; E, 7 pigs; J, 18 pigs.
At weekly intervals, all pigs were bled via venipuncture and anesthetized with 20-mg kg−1 intravenous thiopentone (Vet Drug Ltd.) and a tonsilar biopsy was taken by using a Tru-Cut biopsy needle (Baxter Health Care Ltd., Bedford, United Kingdom). While the animal was under anesthesia and in dorsal recumbency, nasal lavage was performed by using a 50-ml catheter-tipped syringe with a 1-ml automatic pipette tip attached. Ten milliliters of PBS was injected into the right nostril, and the wash was collected from the left nostril. At 6 weeks, the pigs were euthanatized by intravenous injection of sodium pentabarbitone and subjected to postmortem examination.
Necropsy.
Postmortem examination was done immediately after death, and any macroscopic signs of disease were noted. The snout was removed by making a transverse cut just in front of the eyes (21) with a band saw and fixed by immersion in 10% neutral buffered formalin for 1 week.
Antibody detection.
The blood samples were left to clot at room temperature for 1 h and then centrifuged at 4,000 × g for 10 min. The serum was decanted and stored at −20°C until the end of the experiment, when the samples were subjected to two enzyme-linked immunosorbent assays (ELISAs) (antiovalbumin immunoglobulin G [IgG] and antiovalbumin IgA). All reagents were diluted in PBS with added 0.05% Tween 20 (PBST) (pH 7.4), apart from the ovalbumin used to coat the plate and the alkaline phosphatase substrate, which were diluted in carbonate-bicarbonate buffer (pH 9.8). The 96-well plates (Greiner Ltd.) used for the ELISAs were thoroughly washed (six times) with PBST between ELISA steps.
The antigen used for both ELISAs was grade V ovalbumin (Sigma), which was used to coat 96-well plastic plates at a concentration of 1 mg ml−1 and left overnight at 4°C. After washing, serum samples were diluted with PBST and pipetted into row A, where they were diluted by tripling dilution columnwise and left overnight at 4°C. For the IgG ELISA, an anti-pig IgG-alkaline phosphatase conjugate (1:10,000) raised in rabbits and affinity purified (Sigma) was used and incubated for 4 h at 4°C, after which the alkaline phosphatase substrate (1 mg ml−1) was added. For the IgA ELISA, anti-pig monoclonal IgA (1:100) (Serotech Ltd.) was incubated overnight at 4°C, followed by an anti-mouse IgG-alkaline phosphatase conjugate (1:8,000) (Sigma) which was incubated again overnight at 4°C. Finally, the alkaline phosphatase substrate (1 mg ml−1) (Sigma) was added. At this stage, all plates were left to incubate for approximately 10 min and read with a Multiskan Bichromatic plate reader (Labsystems Ltd.) at 405 and 495 nm. The data were analyzed by using a computerized ELISA analysis package (ELISANALYSIS, release 5.53; J. H. Peterman, Birmingham, Ala.), and each serum sample was expressed as ELISA units (EU) by reference to a standard.
A positive IgG control and standard was produced in a pig injected at 3 weeks of age with grade V ovalbumin (Freund incomplete adjuvant) and at 5 weeks of age with grade V ovalbumin (Freund complete adjuvant). The serum from the pig was collected at 10 weeks of age. A positive IgA standard was raised by intramammary inoculation of a lactating sow. Milk from the unweaned sow was collected, clarified by ultracentrifugation, and stored at −20°C.
The IgG and IgA responses to ovalbumin exposure were calculated by subtracting the EU value at week 0 from the EU value at week 6 of the experiment.
Bacteriology.
Biopsy tissue samples were weighed and then homogenized in 2 ml of PBS at full speed for 60 s in an Ultra-Turrax T25 Homogenizer (M. J. Patterson Scientific Ltd., Luton, United Kingdom). The homogenates were diluted in a 10-fold dilution series in PBS and plated onto 5% HBA containing neomycin, actidione, and bacitracin (29) by a modified Miles-Misra technique (8). The recovered fluid from the nasal lavage was diluted and plated as described above. After overnight incubation under aerobic conditions at 37°C, colonies of P. multocida were identified by colony morphology and counted. Representative colonies were further tested by Gram staining and indole reactions, and their identity was confirmed by using API 20E test strips (API-bioMerieux UK Ltd.).
Throughout the experiment, random isolates were selected and screened for toxin production by an agar overlay technique (7) using embryonic bovine lung cells provided by the Central Veterinary Laboratory. The data from the tonsilar biopsies and nasal lavages were used to calculate the cumulative number of P. multocida organisms isolated per pig over the course of the experiment.
Snout sections.
After fixation in 10% formalin, the pig snouts were sectioned transversely at 5-mm intervals with a band saw. Snout sections at the level of the second premolar teeth were placed under a dissection microscope (M38; Wild, Heerbrugg, Switzerland) linked to a computerized image analysis system (VIDS III; Analytical Measuring Systems, Oxford, United Kingdom). A morphometric index (MI) of atrophy was calculated based on the ratio of the open area of the nasal cavity to that of the ventral turbinate (12).
Statistics.
Statistical analysis was performed by using a general linear model of variance (GLM) and regression analysis (RA). Both analyses were performed by a statistical computer software package (Minitab, release 10.51; Minitab Inc., State College, Pa.).
RESULTS
Clinical signs.
Throughout the experiments, all animals retained a normal appetite and exhibited behavior consistent with good health. Clinical signs of disease were restricted to sporadic sneezing by some pigs in the groups inoculated with toxigenic P. multocida.
MI and bacteriological findings.
There was a direct linear correlation between the MI and the cumulative number of P. multocida organisms isolated from the tonsil (R2 = 0.968, P < 0.001) [RA]) (Fig. 1). There was no significant difference in the cumulative total number of P. multocida organisms isolated from the nasal cavity between groups B, C, and D and groups E (the group that was inoculated with P. multocida but not exposed to ovalbumin dust) (GLM). However, Fig. 1 does suggest a tendency for both the cumulative numbers of P. multocida organisms and the MI to increase with the concentration of ovalbumin dust (from groups E to A) and fits a quadratic equation (y = 0.010x2 − 1.229x + 37.055 [R2 = 0.921]). This is further illustrated by Fig. 2. The increase in the cumulative numbers of P. multocida organisms isolated from the nasal cavity was significantly greater in group A than in groups B, C, and D (P < 0.001, GLM). Furthermore, a significantly greater cumulative number of P. multocida organisms was isolated in group A than in group E from both the nasal cavity (linear regression; R2 = 0.645 [P < 0.001]) and the tonsil (quadratic regression, y = 0.010x2 − 0.065x + 0.605; R2 = 0.963 [P < 0.001]) compared to the dust concentration. Groups F to J (pigs not challenged with P. multocida) had a mean MI of 48.4% (standard deviation [SD], ±4.43%), which was significantly lower than the MIs of all of the corresponding groups challenged with P. multocida, which ranged from 54.63% (SD, ±2.43%) in group E to 74.23% (SD, ±2.12%) in group A (P < 0.001 [GLM]).
FIG. 1.
Relationship between the cumulative total number of P. multocida organisms isolated from the tonsil (□; linear regression, y = 0.027x + 1.232 [R2 = 0.968]) and nasal cavity (◊; quadratic regression, y = 0.010x2 − 1.229x + 37.055 [R2 = 0.921]) over the course of the experiment plotted in relation to the MI at the end of the experiment.
FIG. 2.
Cumulative total number of P. multocida organisms isolated from the tonsil (□; linear regression, y = 0.024x + 0.393 [R2 = 0.645]) and the nasal cavity (◊; quadratic regression, y = 0.010x2 − 0.065x + 0.605 [R2 = 0.963]) over the course of the experiment plotted in relation to the mean ovalbumin dust concentration in the air.
Serum antibody.
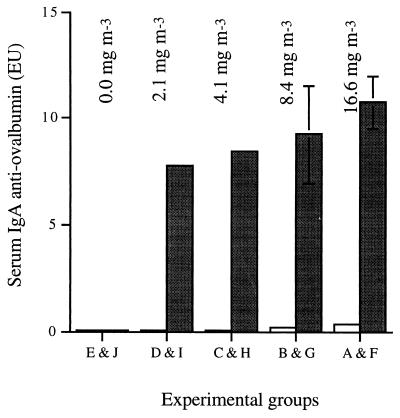
All pigs exposed to ovalbumin dust in the absence of P. multocida showed a significant increase in the level of serum IgG (P < 0.001 [GLM]) and IgA (P < 0.001 [GLM]) to ovalbumin at the end of the experiment (Fig. 3 and 4), and this response was dose dependent (R2 = 0.858, P < 0.001 [RA], and R2 = 0.539, P < 0.001 [RA], respectively). Pigs infected with P. multocida and concurrently exposed to ovalbumin showed significantly lower (effectively no) IgG and IgA responses to ovalbumin (P < 0.001 [GLM]) compared to exposure to ovalbumin alone (Fig. 3 and 4). Levels of antiovalbumin IgG and IgA in pigs exposed to P. multocida plus ovalbumin (groups A to D) were not significantly higher than in pigs exposed to neither (group E; GLM) (Fig. 3 and 4).
FIG. 3.
Mean IgG responses of pigs to exposure to inhaled ovalbumin dust from 1 to 6 weeks of age, with (open bars, groups A to E) and without (closed bars, groups F to J) challenge with P. multocida. Mean concentrations of ovalbumin in air are shown above the respective exposure groups. Mean IgG responses to ovalbumin exposure were calculated by subtracting the EU value at week 0 from the EU value at week 6. Error bars denote the SD (when not shown, the SD is less than 3).
FIG. 4.
Mean IgA responses of pigs to exposure to inhaled ovalbumin dust from 1 to 6 weeks of age, with (open bars, groups A to E) and without (closed bars, groups F to J) challenge with P. multocida. Mean concentrations of ovalbumin in air are shown above the respective exposure groups. Mean IgA responses to ovalbumin exposure were calculated by subtracting the EU value at week 0 from the EU value at week 6. Error bars denote the SD (when not shown, the SD is less than 3).
DISCUSSION
Pigs reared in intensive production systems are continuously exposed to dusts which are principally organic in nature and originate largely from the animals’ feed and integument. Epidemiological studies have demonstrated a link between dust exposure and nasal turbinate scores (2, 27). Ovalbumin is an organic dust, novel to the pigs in this study, which can be milled to a specific range of particle sizes; hence, it has been used in inhalation studies (15). The aerial dust concentrations of 2.1 to 16.6 mg m−3 used in this study cover the upper range of dust concentrations measured in commercial piggeries (5).
In this study, the pigs were presented with the antigen (ovalbumin) in the form of a respirable aerosol which will have come into contact with the upper respiratory tract, conjunctiva, and mucosa of the gastrointestinal tract. The upper respiratory tract has been shown to be an important site for the deposition of organic dust, which has a profound influence on the colonization kinetics of P. multocida (17). Ovalbumin was used in this study to replicate the predominately organic nature of dusts in swine confinement buildings. Furthermore, it is an organic food substance antigenically distinct from pig feed and available as a sterile powder which is easily milled to a size suitable for inhalation. The data presented in this study demonstrate that ovalbumin dust at the maximal exposure concentration (16.6 mg m−3) increased the pigs’ susceptibility to P. multocida colonization of the upper respiratory tract at both the tonsil and nasal cavity (P < 0.001) (Fig. 2). This concentration gave the highest yield of P. multocida from both sites in the upper respiratory tract and resulted in the severest turbinate damage (Fig. 1). This implies increased susceptibility to P. multocida colonization. Potentially, this could be achieved by one or more mechanisms, e.g., (i) modulation of the local immune system, lowering its effectiveness against the bacteria; (ii) disruption of the integrity of the host’s mucosal surfaces; (iii) alteration of the competing commensal flora; or (iv) a direct effect on the viability and/or growth of the pathogen.
The extent of colonization of the tonsil with P. multocida was proportional to the extent of turbinate damage (R2 = 0.968, Fig. 1). Hamilton et al. (18) observed a similar effect when studying interactions between P. multocida and inhaled ammonia. There is a significant increase in the number of P. multocida organisms isolated from the nasal cavity (R2 = 0.921, Fig. 1), which has a quadratic relationship with the MI. However, because the numbers of P. multocida organisms isolated are expressed as CFU per milliliter of recovered fluid from the nasal lavage, it is not possible to calculate the concentration of P. multocida in the recovered mucus alone. Therefore, it is possible that the relationship between the number of P. multocida organisms isolated from the nasal lavage and the MI is influenced by the unmeasured proportion of mucus recovered in the PBS lavage. It is not clear which is the more important site (tonsil or nasal cavity) for toxin absorption, but previous studies suggest that local absorption of toxin may be indirectly mediated through unidentified products of immune cells in the upper respiratory tract mucosa (14, 26). Therefore, both sites may have equal importance in the absorption of the toxin but one site may be more important than the other in the downstream processing of the toxin.
A dose-dependent relationship was found between the concentration of ovalbumin to which the pigs were exposed and the increase in serum IgG and IgA measured at the end of the experiment (R2 = 0.858 and 0.539, respectively) (Fig. 3 and 4). However, at all dust levels, pigs challenged with toxigenic P. multocida showed a much lower immune response to ovalbumin (i.e., increase in serum IgG and IgA) than those not challenged with P. multocida, (P < 0.001, Fig. 3 and 4). No statistically significant difference was observed in the magnitude of the apparent decrease in immunoglobulin levels among groups A to E (pigs challenged with P. multocida).
The strain of P. multocida used in this study produces a dermotoxin which has several biological properties, ranging from nasal turbinate atrophy to pronounced hyperplasia of the transitional epithelium of the urethra and bladder (6). Nakai et al. (23) observed poor immunogenicity of the toxin during infection of pigs, and Williams et al. (36) observed lymphopenia when the toxin was injected parenterally. These findings suggest that the toxin is cytotoxic to lymphocytes, which renders the pig less able to initiate an effective response to foreign antigens, which may include invading bacteria on the mucosa of the respiratory tract.
Modulation of the immune system with P. multocida has also been observed in an epidemiological study which looked at the isolation of B. bronchiseptica and P. multocida from the nasal cavities of piglets (13). This study found that total immunoglobulin concentrations in serum (IgG, IgA, and IgM) decreased in proportion to an increase in the concentration of P. multocida isolated per piglet (CFU per piglet) over a 6-week period. The authors did not comment on this observation. It is, however, consistent with our own observations (Fig. 3 and 4).
Experimental studies on gnotobiotic pigs have shown that crude P. multocida toxin is a poor immunogen but that when the toxin is made inactive (toxoid), it becomes a potent vaccine (6). This implies that active immunity is seen in pigs when the toxin is given in its biologically inactive state (by intranasal vaccination) but not its biologically active state (by natural infection). A recent study (34) found that a commercially available P. multocida vaccine imparted protection against atrophic rhinitis with a subsequent rise in antibodies to P. multocida. However, when animals were vaccinated simultaneously with a P. multocida toxin challenge, no detectable systemic humoral or cellular response to P. multocida toxin was found. Challenge with active P. multocida toxin before vaccination appeared to slow down the immune responses initiated by the toxoid in the vaccine; when vaccination and challenge were simultaneous, the immune response was abolished.
The findings of the current study show that colonization of the upper respiratory tract with toxigenic P. multocida (and consequent exposure to Pasteurella toxin) suppresses the humoral immune response to inhaled and/or ingested ovalbumin. This systemic modulation of the immune system may be an important (and as yet undocumented) virulence factor in this complex multifactorial disease. Indeed, this effect may have more far-reaching implications for other opportunistic bacterial infections in pigs and other farm animals intensively housed in polluted environments. Progressive atrophic rhinitis can severely impair growth rate and food conversion efficiency in commercial pig herds (6, 33). However, it is not clear why such a localized condition can have such a prolonged systemic effect. One possibility is that it exacerbates the effects of concurrent or subsequent infections, such as enzootic pneumonia. Atrophic rhinitis and enzootic pneumonia (mycoplasma-induced respiratory disease complex) are clearly quite distinct conditions, and the majority scientific opinion is that there is no mechanistic or epidemiological relationship between the two (30). However, Cowart et al. (9) found that in individual pigs, an increasing atrophic rhinitis score was related to an increasing pneumonia score at 8 weeks but not at 6 months of age. This finding is consistent with the study by Chanter and Rutter (6) of the colonization kinetics of toxigenic P. multocida following experimental inoculation. This showed that P. multocida colonized the nasal cavity in large numbers at 3 weeks of age but fell to below 10% at 2 months of age. Thus, P. multocida-induced immune modulation may occur early in life and wane when the pig ages. Clinical evidence indicates that enzootic pneumonia can occur as early as 2 to 8 weeks of age (19). This would coincide with the time of maximal immunosuppression due to P. multocida although not with the time the effects of turbinate atrophy are most apparent.
In conclusion, exposure of young pigs to ovalbumin predisposed the nasal cavity and the tonsil to colonization by P. multocida in a dose-dependent manner. Colonization of the upper respiratory tract with P. multocida was directly proportional to the severity of turbinate atrophy, as judged by the MI. Challenge with P. multocida profoundly suppressed the humoral immune response induced by chronic exposure to ovalbumin dust. On the basis of these observations, we propose a new hypothesis which may partly explain the roles of P. multocida and respirable dusts in the etiology and pathogenesis of ill thrift due to endemic respiratory diseases in housed pigs. Organic dusts predispose the upper respiratory tract to colonization with P. multocida. Colonization with P. multocida induces immunomodulation, which may not only increase the severity of atrophic rhinitis but also predispose the pig to enzootic pneumonia and similar, more chronically debilitating infections of the lower respiratory tract.
ACKNOWLEDGMENTS
This investigation was supported by a grant from the Biotechnology and Biological Sciences and Research Council.
We thank Mick Bailey and Karen Haversham for providing the positive antiovalbumin IgA colostra and anti-pig IgA monoclonal antibody, respectively, for the ELISAs. We also thank D. Patel, D. Richards, A. Norris, and A. Rhodes for technical assistance.
REFERENCES
- 1.Alivisators G P, Marketos N, Vava Z. Die Immunitätsparalyse bei Brucellosen. Z Immunitaetsforsch Exp Ther. 1962;122:291–297. [Google Scholar]
- 2.Baekbo P. Proceedings of the 11th Congress of the International Pig Veterinary Society. 1990. Air quality in Danish pig herds; pp. 395–397. [Google Scholar]
- 3.Bloomfield A L, Mateer J G. Changes in skin sensitiveness of tuberculin during epidemic influenza. Am Rev Tuberculosis. 1919;3:166–170. [Google Scholar]
- 4.Caraffa C. Contributo clinico allo studio dell’allergia turberculclinca nelle diverse fasi della pertose. Clin Pediatr. 1932;14:935–940. [Google Scholar]
- 5.Cermack J P, Ross P A. Airborne dust associated with animal housing tasks. Farm Building Progress. 1978;51:11–15. [Google Scholar]
- 6.Chanter N, Rutter J M. Pasteurellosis in swine. In: Adlam C, Rutter J M, editors. Pasteurella and pasteurellosis. Orlando, Fla: Academic Press, Inc.; 1989. pp. 161–195. [Google Scholar]
- 7.Chanter N, Rutter J M, Luther P D. Rapid detection of toxigenic Pasteurella multocida by an agar overlay method. Vet Rec. 1986;119:629–630. [PubMed] [Google Scholar]
- 8.Corry J E L. Quality assessment of culture media by the Miles-Misra method. In: Corry J E L, editor. Quality assurance and quality control of microbiological culture media. Proceedings of a symposium. Darmstadt, Germany: G-I-T Verlag Ernst Giebler; 1982. pp. 21–37. [Google Scholar]
- 9.Cowart R P, Bäckström L, Brim T A. Pasteurella multocida and Bordetella bronchiseptica in atrophic rhinitis and pneumonia in swine. Can J Vet Res. 1989;53:295–300. [PMC free article] [PubMed] [Google Scholar]
- 10.De Jong M F, Oie J L, Testenburg G J. Proceedings of the 6th Congress of the International Pig Veterinary Society. 1980. Atrophic rhinitis pathogenicity tests for Pasteurella multocida isolates; p. 211. [Google Scholar]
- 11.Done J T. Proceedings of the 6th Congress of the International Pig Veterinary Society. 1983. Atrophic rhinitis pathogenicity tests for Pasteurella multocida isolates; p. 211. [Google Scholar]
- 12.Done J T, Upcott D H, Frewin D C, Hebert C N. Atrophic rhinitis; snout morphometry for quantitative assessment of conchal atrophy. Vet Rec. 1984;114:33–35. doi: 10.1136/vr.114.2.33. [DOI] [PubMed] [Google Scholar]
- 13.Eliás B, Krüger M, Gergely P, Voets R, Rafai P. Interaction between immunity to Bordetella bronchiseptica and infection of pig herds by Bordetella bronchiseptica and Pasteurella multocida. J Vet Med Sci. 1996;55:617–622. doi: 10.1292/jvms.55.617. [DOI] [PubMed] [Google Scholar]
- 14.Frymus T, Müller E, Schulte A, Kobatsch B, Ueberschär S, Petzoldt K. Proceedings of the 9th IPVS Congress. 1986. Studies in conventional and gnotobiotic piglets on the pathogenicity and immunogenicity of a toxin from Pasteurella multocida involved in atrophic rhinitis; p. 229. [Google Scholar]
- 15.Gilmour M I. Airborne pollution and respiratory disease in animal houses. Ph.D. thesis. Bristol, England: University of Bristol; 1988. [Google Scholar]
- 16.Hamilton T D C. Airborne pollution and progressive atrophic rhinitis in pigs. Ph.D. thesis. Bristol, England: University of Bristol; 1995. [Google Scholar]
- 17.Hamilton T D C, Roe J M, Taylor F G R, Pearson G, Webster A J F. Proceedings of the Fourth International Symposium of the American Society of Agricultural Engineers. Livestock and Environment IV. 1993. Aerial pollution: an exacerbating factor in atrophic rhinitis; pp. 895–903. [Google Scholar]
- 18.Hamilton T D C, Roe J M, Webster A J F. Synergistic role of gaseous ammonia in etiology of Pasteurella multocida-induced atrophic rhinitis in swine. J Clin Microbiol. 1996;34:2185–2190. doi: 10.1128/jcm.34.9.2185-2190.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmgreen N. Swine enzootic pneumonia: immunologic studies in infected sow-herds. Res Vet Sci. 1974;17:145–153. [PubMed] [Google Scholar]
- 20.Il’ina Z M, Zasukhin M I. Role of Pasteurella toxins in the pathogenesis of infectious atrophic rhinitis. Sb Nauchn Rab Sib Zon Nauchno-Issled Vet Inst. 1975;25:76–81. [Google Scholar]
- 21.Ministry of Agriculture Fisheries and Food. Atrophic rhinitis: a system of snout grading. MAFF booklet LPD 51. London, England: Pinner; 1978. [Google Scholar]
- 22.Mitchell A G, Nelson W E, Le Blanc T J. Studies in immunity. V. Effect of acute diseases on reaction of skin to tuberculin. Am J Dis Child. 1935;49:695–703. [Google Scholar]
- 23.Nakai T, Swata A, Tsuji M, Samejima Y, Kume K. Purification of dermonecrotic toxin from a sonic extract of Pasteurella multocida SP-72 serotype D. Infect Immun. 1984;46:429–434. doi: 10.1128/iai.46.2.429-434.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearce H G, Roe C K. Infectious atrophic rhinitis: a review. Can Vet J. 1966;7:243–256. [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen K B, Barford K. The aetiological significance of Bordetella bronchiseptica and Pasteurella multocida in atrophic rhinitis of swine. Nord Veterinaermed. 1981;33:513–522. [PubMed] [Google Scholar]
- 26.Pedersen K B, Elling F. The pathogenesis of atrophic rhinitis in pigs induced by Pasteurella multocida. J Comp Pathol. 1984;94:203–214. doi: 10.1016/0021-9975(84)90041-0. [DOI] [PubMed] [Google Scholar]
- 27.Robertson J F, Wilson D, Smith W J. Atrophic rhinitis and the aerial environment. Anim Prod. 1990;50:173–182. [Google Scholar]
- 28.Rutter J M, Rojas X. Atrophic rhinitis in gnotobiotic piglets: differences in pathogenicity of Pasteurella multocida in combined infections with Bordetella bronchiseptica. Vet Rec. 1982;110:531–535. [Google Scholar]
- 29.Rutter J M, Taylor R J, Crington W G, Robertson I B, Benson J A. Epidemiological study of Pasteurella multocida and Bordetella bronchiseptica in atrophic rhinitis. Vet Rec. 1984;115:615–619. doi: 10.1136/vr.115.24.615. [DOI] [PubMed] [Google Scholar]
- 30.Straw B E, Leman A D, Robinson R A. Pneumonia and atrophic rhinitis in pigs from a test station—a follow up study. JAMA. 1984;185:1544–1546. [PubMed] [Google Scholar]
- 31.Switzer W P, Farrington D O. Infectious atrophic rhinitis, 687–711. In: Dunne H W, Leman A D, editors. Diseases of swine. 4th ed. Ames: Iowa State University Press; 1975. [Google Scholar]
- 32.Timbrell V, Skidmore J W, Hyett A W, Wagner J C. Exposure chambers for inhalation exposure experiments with standard reference samples of asbestos of the International Union against Cancer (IUCC) Aerosol Sci. 1970;1:215–223. [Google Scholar]
- 33.Turner L W, Wathes C M, Audsley E. Proceedings of the Fourth International Symposium of the American Society of Agricultural Engineers. Livestock and Environment IV. 1993. Dynamic probabilistic modelling of respiratory disease in swine, including production and economic effects; pp. 89–97. [Google Scholar]
- 34.van Diemen P M, de Vries Reilingh G, Parmentier H K. Immune responses of piglets to Pasteurella multocida toxin and toxoid. Vet Immunol Immunopathol. 1994;41:307–321. doi: 10.1016/0165-2427(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 35.von Pirquet C E. Das Verhalten der kutanen Tuberkulinreaction während der Masern. Dtsch Med Wochenschr. 1908;34:1297–1304. [Google Scholar]
- 36.Williams P P, Hall M R, Rimler R B. Proceedings of the 9th Congress of the International Pig Veterinary Society. 1986. Effect of purified Pasteurella multocida turbinate atrophy toxin on porcine peripheral blood lymphocytes in vivo and in vitro; p. 234. [Google Scholar]
- 37.Wilson M R, Takov R, Friendship R M, Martin S W, McMillan I, Hacker R R, Swaminathan S. Prevalence of respiratory diseases and their association with growth rate and space in randomly selected swine herds. Can J Vet Res. 1986;50:209–216. [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C J, Reimann H A. Effect of typhus fever on intracutaneous tuberculin reaction. Natl Med J China. 1931;17:210–216. [Google Scholar]