Abstract
Purpose
To investigate the clinical and morphologic factors related to asymmetric dilated vortex veins in central serous chorioretinopathy (CSC).
Design
Retrospective, comparative study.
Participants
One hundred fifty-eight eyes of 158 patients with CSC.
Methods
All patients with CSC underwent ophthalmic examination and multimodal imaging, including measurements of axial length (AL), fluorescein angiography, indocyanine green angiography, swept-source OCT, and anterior segment OCT. Using en face OCT images at the level of the outer choroid, the eyes were divided into 2 groups: eyes with symmetric vortex veins (symmetry group) and those with asymmetric vortex veins (asymmetry group).
Main Outcome Measures
Clinical and morphologic factors related to asymmetric vortex veins in CSC.
Results
Of the 158 eyes, 120 eyes (75.9%) were classified into the asymmetry group and 38 eyes (24.1%) were classified into the symmetry group. The asymmetry group showed significantly greater spherical equivalent (–0.32 ± 1.78 diopters [D] vs. –1.35 ± 2.64 D; P = 0.033), shorter AL (23.52 ± 0.86 mm vs. 24.10 ± 1.06 mm; P = 0.005), and greater subfoveal choroidal thickness (414.6 ± 105.3 μm vs. 360.4 ± 91.8 μm; P = 0.005) than the symmetry group. No significant differences existed between the 2 groups regarding age, sex, or all scleral thicknesses at the superior, temporal, inferior, and nasal points. In the multivariate analyses, shorter AL (odds ratio, 0.56; 95% confidence interval, 0.36–0.88; P = 0.011) was found to be significantly associated with the presence of asymmetric vortex veins.
Conclusions
The asymmetric dilated vortex vein is a common finding in patients with CSC. Our results suggest that certain biometric factors, such as short AL, may be associated with asymmetric dilated vortex veins developing in patients with CSC.
Keywords: Asymmetric dilated vortex vein, Axial length, Central serous chorioretinopathy, Choroidal thickness, Choroidal vascular hyperpermeability, Pachychoroid, Scleral thickness
Abbreviations and Acronyms: AL, axial length; AS, anterior-segment; CSC, central serous chorioretinopathy; D, diopter; ICGA, indocyanine green angiography; SCT, subfoveal choroidal thickness; SE, spherical equivalent; SRD, serous retinal detachment; SS, swept-source
Central serous chorioretinopathy (CSC) is a common disease characterized by the accumulation of serous retinal detachment (SRD) with or without pigment epithelium detachment in the posterior pole of the eye.1,2 Usually, CSC develops in middle-aged men.3 In most cases of CSC, SRD is absorbed spontaneously, and the visual prognosis is relatively good. However, some patients with CSC may demonstrate recurrent or prolonged SRD and may sustain damage to the retinal pigment epithelium, resulting in irreversible vision loss.4 From previous studies, it is well known that male gender,5 type A personality,6 psychological stress,7 exposure to steroids,5 pregnancy,8 short axial length (AL),9,10 scleral thickening,11 and genetic factors12, 13, 14, 15, 16, 17 are associated with CSC. However, the exact pathologic mechanism of CSC is not fully understood.
Choroidal abnormalities are a well-known cause of CSC pathologic features. Using indocyanine green angiography (ICGA), researchers have observed signs of filling delays, dilated choroidal vessels, and choroidal vascular hyperpermeability.18,19 Owing to significant advances in OCT technology, such as enhanced depth imaging20 and high-penetration swept-source (SS) OCT,21 subfoveal choroidal thickness (SCT) in CSC eyes was found to be significantly greater than that in normal eyes.22 After further research, a thick choroid was observed as a result of dilated choroidal vessels in the outer choroid.23
Recently, Pang et al24 observed dilated outer choroidal vessels of the macular area associated with congested vortex vein ampullas before exiting the sclera using ultra-widefield ICGA. Hiroe and Kishi25 showed that the patterns of outer choroidal vessels in the posterior pole were divided into asymmetric or symmetric vortex veins using en face OCT, and asymmetric dilated vortex veins were observed in all CSC eyes. Moreover, Kishi et al26 reported that the asymmetric dilated vortex vein regions and filling delay areas on ICGA showed substantial overlap in acute CSC. Based on these findings, asymmetric dilated vortex veins in the outer choroid are expected to be related closely to CSC pathologic features.
However, the mechanism underlying asymmetric dilated vortex vein development in CSC remains unclear. We previously studied the biometric aspects in CSC eyes and demonstrated that short AL,10 hyperopic refractive error,10 and thickened sclera11 were associated with the pathogenesis of CSC. In contrast, patients with CSC accompanied by high myopia are seldom encountered in a clinical setting.27
This study investigated the relationship between the asymmetric dilated vortex vein and the clinical and morphologic factors. We conducted a comparative study in CSC eyes with symmetric or asymmetric vortex veins, paying particular attention to some biometric factors such as AL and scleral thickness.
Methods
Participants
This retrospective study was conducted in a single institution. The study was approved by the institutional review board of the University of the Ryukyus Hospital (approval no., 1503) and was conducted following the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants before their participation in this study.
We retrospectively reviewed the charts and imaging data of 158 eyes of 158 patients (28 women and 130 men; mean age, 52.0 years; standard deviation, 11.8 years) with CSC who visited the Macula Service of the University of the Ryukyus Hospital between October 2018 and November 2020. The right eye was used in patients with bilateral CSC.
Central serous chorioretinopathy was diagnosed by fundus ophthalmoscopy and multimodal imaging, including fluorescein angiography, ICGA, fundus autofluorescence photography, SS OCT, and OCT angiography. The clinical findings of CSC included the presence of SRD involving the macula on SS OCT and 1 or more leakage points at the level of the retinal pigment epithelium on fluorescein angiography and multifocal choroidal vascular hyperpermeability in mid- or late-phase ICGA. Fundus autofluorescence photography revealed at least 1 area of retinal pigment epithelium alteration. OCT angiography was also used to confirm the absence of choroidal neovascularization. Eyes with a history of any other retinal diseases, including choroidal neovascularization, a history of intraocular surgery, a history of photodynamic therapy, or a history of related systemic diseases associated with CSC, were excluded. Moreover, we also excluded patients with a history of taking systemic corticosteroids, considering the different causes of steroid-induced CSC.28, 29, 30
Multimodal Imaging and Imaging Analysis
At the initial visit, all patients underwent complete ocular examinations with autorefraction (ARK-1a; NIDEK), best-corrected visual acuity (BCVA) testing with Landolt C charts (SC-1000; TOMEY), measurements of the AL (IOL Master 700; Carl Zeiss Meditec), slit-lamp biomicroscopy with a noncontact fundus lens, color fundus photography and fundus autofluorescence photography (TRC-50DX; Topcon), fluorescein angiography and ICGA (Spectralis HRA + OCT; Heidelberg Engineering), SS OCT (DRI-OCT Triton; Topcon), OCT angiography (between October 2018 and March 2019: DRI-OCT Triton [Topcon]; between April 2019 and November 2020: Zeiss Plex Elite 9000 [Carl Zeiss Meditec]), and anterior-segment (AS) OCT (CASIA 2; TOMEY). The spherical equivalent (SE) was defined as adding half of the cylindrical power to the principal spherical power. We measured SCT using B-scan images of the 12-mm horizontal line scans at the foveal center by measuring the distance from the hyperreflective line corresponding to Bruch’s membrane beneath the retinal pigment epithelium to the inner surface of the sclera using the calliper measurement tool of the SS OCT. To evaluate the symmetry of vortex veins in the outer choroid, we referred to the methods that used en face OCT described in a previous study.25 First, the volume scan data were obtained using a raster scan protocol of 512 (horizontal) × 128 (vertical) B-scans, which covered a 12 × 9-mm area centered on the middle point of the foveal center and the optic disc. Then, en face OCT images were obtained as coronal slices from 3-dimensional OCT images that were flattened relative to Bruch’s membrane using a software tool (EnView software; Topcon). The definition of a symmetric vortex vein was based on en face OCT images in the outer choroid showing a symmetric pattern between the superior and inferior vortex veins and the watershed horizontally passing through the macular region. The symmetry group (Fig 1) typically comprised patients with CSC with no difference in running pattern and vascular diameter between the superior and inferior vortex veins. The patients with CSC without symmetric vortex veins were categorized as having asymmetric vortex veins. The asymmetry group (Fig 2) typically showed dilated vortex veins extending over the horizontal line through the fovea. The symmetry or asymmetry groups were determined by 2 retinal specialists (N.T. and N.I.). Although the 2 examiners were blinded to the clinical findings, a third retinal specialist (S.S.) made the final decision in controversial cases. Interrater agreement was assessed by calculating Cohen’s κ coefficient.
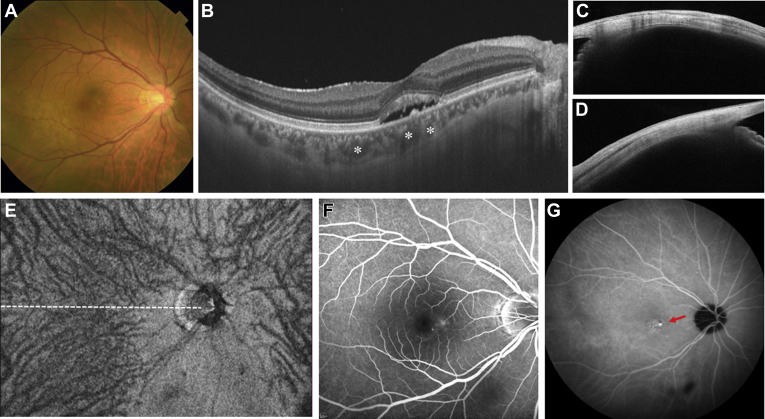
Figure 1.
Images of the right eye of a 55-year-old man with central serous chorioretinopathy Axial length is 26.44 mm, and spherical equivalent is –5.375 diopters. A, Color fundus photograph revealing serous retinal detachment (SRD) involving the fovea. B, Horizontal B-scan OCT image through the fovea showing SRD with dilated choroidal vessels (asterisks). The subfoveal choroidal thickness is 227 μm. C, Cross-sectional image of the sclera at the superior point showed with anterior-segment (AS) OCT. The scleral thickness is 363 μm. D, Cross-sectional image of the sclera at the inferior point showed with AS OCT. The scleral thickness is 415 μm. The mean value of scleral thicknesses at 4 directions is 390 μm. E, En face OCT image of 12 × 9 mm showing vortex veins in the outer choroid. The superior and inferior vortex veins demonstrate symmetric patterns, and the horizontal watershed zone passes through the fovea (white dashed line). This eye was classified in the symmetry group. F, Fluorescein angiogram within a 30 × 30 range revealing diffuse leakage in the macular region. G, Indocyanine green angiogram within a 55 × 55 range in the late phase revealing typical choroidal vascular hyperpermeability (red arrow).
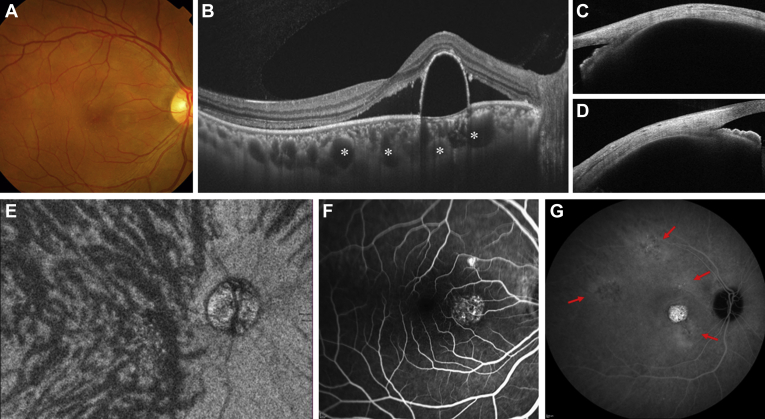
Figure 2.
Images of the right eye of a 37-year-old man with central serous chorioretinopathy. Axial length is 21.33 mm, and spherical equivalent is +1.000 diopter. A, Color fundus photograph revealing serous retinal detachment (SRD) with pigment epithelium detachment in the macular region. B, Horizontal B-scan OCT image through the fovea showing SRD with dome-shaped pigment epithelium detachment and significantly dilated choroidal vessels (asterisks). The subfoveal choroidal thickness is 452 μm. C, Cross-sectional images of the sclera at the superior point showed with anterior segment (AS) OCT. The scleral thickness is 432 μm. D, Cross-sectional images of the sclera at the inferior point showed with AS OCT. The scleral thickness is 470 μm. The mean value of scleral thicknesses at 4 directions is 450 μm. E, En face OCT image of 12 × 9 mm showing dilated vortex veins in the outer choroid. The horizontal watershed zone has disappeared because of the superior dilated vortex veins evident in more than the upper half of the posterior pole. This eye was classified in the asymmetry group. F, Fluorescein angiogram within a 30° × 30° range revealing typical leakages in the macular area. G, Indocyanine green angiogram within a 55° × 55° range in the late phase, revealing multifocal areas of choroidal vascular hyperpermeability (red arrows).
Using AS OCT, the scleral thickness was measured manually in 4 directions (superior, temporal, inferior, and nasal). In brief, we identified the upper scleral line based on the low brightness of each rectus muscle and delineated the lower scleral line from the difference in brightness. Scleral thickness was calculated vertically as the distance between the upper scleral line and the lower scleral line 6-mm posterior to the scleral spur using the calliper tool of the AS OCT. The detailed methodology for measuring scleral thickness has been described previously.11
Outcomes
The symmetry and asymmetry groups were compared in terms of age, sex, SE, AL, SCT, and scleral thickness. Further, we investigated the possible factors related to the asymmetric vortex veins using multivariate logistic regression analyses.
Statistical Analysis
All data were analyzed using Statistical Analysis System software version 9.4 (SAS, Inc). Statistical significance was set at P < 0.05. Unless otherwise stated, the results were expressed as mean and standard deviation, and categorical data were assessed using the Fisher exact test. The Mann–Whitney U test was used to compare the quantitative variables between the 2 independent groups. The significantly associated factors in the univariate analysis were assessed further for their associations with the presence of asymmetric vortex veins using multivariate logistic regression analyses adjusting for possible confounders. The associations among the variables and the presence of asymmetric vortex veins in CSC eyes were assessed using odds ratios and their 95% confidence intervals by multivariate logistic regression analyses.
Results
Of the 158 eyes, 120 eyes (75.9%) were classified into the asymmetry group and 38 eyes (24.1%) were classified into the symmetry group (κ statistic, 0.92; 95% confidence interval, 0.85–0.99; P < 0.001). The demographic and clinical characteristics of the 2 groups are summarized in Table 1. The asymmetry group showed significantly greater SE (–0.32 ± 1.78 diopters [D] vs. –1.35 ± 2.64 D; P = 0.033), shorter AL (23.52 ± 0.86 mm vs. 24.10 ± 1.06 mm; P = 0.005), and greater SCT (414.6 ± 105.3 μm vs. 360.4 ± 91.8 μm; P = 0.005) than the symmetry group. No significant differences existed between the 2 groups regarding age, sex, and all scleral thicknesses at the superior, temporal, inferior, and nasal points. Multivariate logistic regression analyses of the factors associated with asymmetric vortex veins are summarized in Table 2. We included age and sex, which are clinically important confounders, in the final multivariate models, although they were not factors that were significantly associated in the univariate analysis. Because AL and SE were highly correlated (rs = −0.50; P < 0.001, Spearman rank correlation test), we needed to exclude 1 of these 2 variables from the multivariate logistic regression analyses. Therefore, 2 multivariate logistic regression models, model 1 including AL and model 2 including SE, were used for further analyses. In model 1, multivariate analysis revealed that AL (odds ratio, 0.56; 95% confidence interval, 0.36–0.88; P = 0.011) was the sole variable significantly associated with asymmetric vortex veins in CSC. Age, sex, and SCT were not significantly associated with asymmetric vortex veins in CSC. In model 2, we did not find statistically significant associations among age, sex, SE, SCT, and asymmetric vortex veins in CSC. Table 3 shows the distribution of the symmetry or asymmetry groups with the AL subgroup. With a shorter AL, the prevalence of the asymmetry group was significantly higher than that of the symmetry group in the AL subgroup dataset (P < 0.001).
Table 1.
Demographic and Clinical Characteristics of Patients with Asymmetric and Symmetric Vortex Veins in Central Serous Chorioretinopathy
| Characteristic | Asymmetry Group (n = 120) | Symmetry Group (n = 38) | P Value |
|---|---|---|---|
| Age (yrs) | 52.8 ± 12.1 | 49.7 ± 10.6 | 0.300∗ |
| Female sex | 23 (19.2) | 5 (13.2) | 0.473† |
| SE (D) | –0.32 ± 1.78 | –1.35 ± 2.64 | 0.033∗ |
| AL (mm) | 23.52 ± 0.86 | 24.10 ± 1.06 | 0.005∗ |
| SCT (μm) | 414.6 ± 105.3 | 360.4 ± 91.8 | 0.005∗ |
| Scleral thickness (μm) | |||
| Superior | 417.5 ± 60.8 | 405.2 ± 60.7 | 0.220∗ |
| Temporal | 437.0 ± 55.9 | 425.3 ± 51.0 | 0.226∗ |
| Inferior | 450.3 ± 58.0 | 440.0 ± 64.2 | 0.194∗ |
| Nasal | 434.6 ± 61.0 | 430.7 ± 56.7 | 0.580∗ |
AL = axial length; D = diopter; SCT = subfoveal choroidal thickness; SE = spherical equivalent.
Data are presented as mean ± standard deviation or no. (%), unless otherwise indicated.
Mann–Whitney U test.
Fisher exact test.
Table 2.
Multivariable Logistic Regression Analysis of the Factors Associated with Asymmetric Vortex Vein in Central Serous Chorioretinopathy
| Variable | Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | P Value | Odds Ratio | 95% Confidence Interval | P Value | |
| Age (yrs) | 1.03 | 0.99–1.07 | 0.147 | 1.03 | 0.99–1.07 | 0.187 |
| Male (vs. female) | 1.46 | 0.48–4.46 | 0.503 | 1.75 | 0.58–5.24 | 0.321 |
| AL (mm) | 0.56 | 0.36–0.88 | 0.011 | — | — | — |
| SE (D) | — | — | — | 1.20 | 0.99–1.47 | 0.071 |
| SCT (μm) | 1.00 | 1.00–1.01 | 0.051 | 1.00 | 1.00–1.01 | 0.055 |
D = diopter; AL = axial length; SCT = subfoveal choroidal thickness; SE = spherical equivalent; — = analysis not carried out.
Table 3.
Distribution of Asymmetry or Symmetry Groups with the Axial Length Subgroup
| Variable | Asymmetry Group (n = 120) | Symmetry Group (n = 38) | P Value |
|---|---|---|---|
| AL subgroup (mm), no. (%) | < 0.001∗ | ||
| 21–< 22 | 5 (4.2) | 0 (0) | |
| 22–< 23 | 24 (20.0) | 6 (15.8) | |
| 23–< 24 | 55 (45.8) | 15 (39.5) | |
| 24–< 25 | 30 (25.0) | 10 (26.3) | |
| 25–< 26 | 6 (5.0) | 4 (10.5) | |
| 26–< 27 | 0 (0) | 3 (7.9) |
AL = axial length.
Fisher exact test.
Discussion
An asymmetric dilated vortex vein in the outer choroid is a newly identified morphologic feature associated with pachychoroid-related diseases.25 The current study investigated the relationship between asymmetric vortex veins and the clinical and morphologic features of CSC. We revealed the following: (1) the asymmetric vortex vein was seen in 75.9% of CSC eyes; (2) the asymmetry group showed greater SE, shorter AL, and greater SCT than the symmetry group in the univariate analysis; and (3) shorter AL was the sole factor associated with asymmetric vortex veins in CSC in the multivariate regression analyses. These findings suggest that a shorter AL may be associated with the development of asymmetric vortex veins and the pathogenesis of CSC.
Choroidal circulation is formed by short posterior ciliary arteries, long posterior ciliary arteries, and anterior ciliary arteries as inflow vessels and vortex veins as outflow vessels. The vortex veins were located in each of the 4 quadrants. These vortex veins form an ampulla and penetrate the sclera at the equator. The boundary of each vortex vein region is called a watershed zone. It is observed as a border separating each vortex vein region, usually passing horizontally through the disc area and vertically through the papillomacular area.31 However, various choroidal venous vascular patterns exist, such as the asymmetric vortex vein.32 In the current study, of the 158 CSC eyes, 120 eyes (75.9%) were in the asymmetry group and 38 eyes (24.1%) were in the symmetry group. Hiroe and Kishi25 reported the running patterns of the vortex veins in 38 eyes with CSC using en face OCT and ICGA images, and asymmetric vortex veins were observed in all CSC eyes. Although the observed prevalence of asymmetric vortex veins in CSC eyes was higher than that detected in a large cohort in our study, our results are seemingly much higher than those in normal participants.25 Shiihara et al33 also analyzed the running pattern of the choroidal vessels in en face OCT images determined by a machine learning-based quantitative method and showed that CSC eyes harbored dilated and asymmetric vessels running in the outer choroid. The pathogenesis of CSC is presumably involved in venous congestion and increased venous pressure associated with these morphologic features, such as asymmetric dilated vortex veins.
In this study, shorter AL seemed to be the sole factor associated with the presence of asymmetric vortex veins in CSC eyes using multivariate regression analyses. We recently proposed that the thick sclera may play a role in the pathogenesis of CSC by showing significantly greater scleral thickness in CSC eyes than in normal control eyes.11 Therefore, we postulated that the thick sclera was considered to disturb the choroidal venous outflow. Unexpectedly, the scleral thickness was not significantly associated with the presence of an asymmetric vortex vein in the current study. Furthermore, previous reports have shown that asymmetric vortex veins also are present in healthy participants.25,33 These facts imply that the asymmetric vortex vein may be a congenital morphologic factor and may be related to the misalignment of the watershed associated with the shorter AL. In addition to the presence of asymmetric vortex veins, thick sclera may stagnate the choroidal circulation further or may enlarge the choroidal vessels, leading to the development of CSC.
Central serous chorioretinopathy is a multifactorial disease, typically caused by many physiologic and psychological factors,34,35 and is also influenced by genetic factors.12, 13, 14, 15, 16, 17 Notably, Hosoda et al13 reported significant associations between CFH and VIPR2 genes and choroidal thickness. They proposed that CFH and VIPR2 contribute to the pathogenesis of CSC via their effect on choroidal thickness. These genetic factors and anatomic features, such as short AL, may play an essential role in asymmetric vortex vein development.
This study has several limitations. First, only eyes with CSC were analyzed in this study. It is necessary to consider whether the results apply to both CSC eyes and healthy eyes. Second, because this study was hospital-based, the enrolled CSC eyes consisted of mainly severe cases referred to our hospital for treatment, and few mild CSC cases were found. Third, the en face images used in this study covered 12 × 9 mm of the posterior pole. We observed asymmetric dilated vortex veins in the posterior pole. Still, we were unable to observe the full features of the vortex veins from the macular region to the ampulla before entering the sclera. Fourth, we measured the scleral thickness 6 mm posterior to the scleral spur. Therefore, the morphologic features of the sclera surrounding the vortex veins remain unclear. Finally, the diagnosis of symmetric or asymmetric vortex veins was made subjectively based on the previous literature.25 More objective and quantitative methods are needed for future analyses.
In conclusion, this study analyzed the relationship between asymmetric vortex veins and the clinical and morphologic features of CSC. We showed that a shorter AL is associated with solely asymmetric vortex veins in CSC. Therefore, certain biometric factors, such as shorter AL, may be associated with asymmetric dilated vortex veins developing in eyes with CSC. To better understand the morphologic features of the vortex veins, it is necessary to further investigate the features of asymmetric vortex veins from their posterior pole to the ampulla using widefield images.
Acknowledgment
The authors thank Kengo Yoshii, Department of Mathematics and Statistics in Medical Sciences, Kyoto Prefectural University of Medicine, Kyoto, Japan, for statistical expertise.
Manuscript no. D-21-00091
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): N.T.: Lecturer – Topcon, Novartis, AbbVie, Kowa, Bayer
N.I.: Lecturer – HOYA, Senju
S.W.: Lecturer – Senju
S.S.: Lecturer – Novartis, Senju
T.T.: Lecturer – Novartis, Alcon, Senju
Y.Y.: Lecturer – Senju, Alcon, HOYA
H.K.: Financial support – Novartis, Bayer, Alcon, HOYA, Senju, Otsuka, AMO, Kowa, Star Japan, Santen; Lecturer – Novartis, Bayer, Alcon, HOYA, Topcon, Senju, Nidek, AbbVie, Otsuka, Pfizer, AMO, JFC, Kowa, Bausch & Lomb, Allergan, Canon, TOMEY, Sumitomo Dainippon Pharma, SANOFI, Santen
Supported by Japan Society for the Promotion of Science KAKENHI (grant no.: JP20K18349), Tokyo, Japan.
HUMAN SUBJECTS: Human subjects were included in this study. This retrospective study was conducted in a single institution. The study was approved by the institutional review board of the University of the Ryukyus Hospital and was conducted following the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants before their participation in this study.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Terao, Koizumi
Analysis and interpretation: Terao, Imanaga, Koizumi
Data collection: Terao, Imanaga, Wakugawa, Sawaguchi, Tamashiro, Yamauchi
Obtained funding: Imanaga, Koizumi
Overall responsibility: Terao, Imanaga, Wakugawa, Sawaguchi, Tamashiro, Yamauchi, Koizumi
References
- 1.Daruich A., Matet A., Dirani A., et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Cheung C.M.G., Lee W.K., Koizumi H., et al. Pachychoroid disease. Eye (Lond) 2019;33(1):14–33. doi: 10.1038/s41433-018-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitzmann A.S., Pulido J.S., Diehl N.N., et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. 2008;115(1):169–173. doi: 10.1016/j.ophtha.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 4.Mrejen S., Balaratnasingam C., Kaden T.R., et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126(4):576–588. doi: 10.1016/j.ophtha.2018.12.048. [DOI] [PubMed] [Google Scholar]
- 5.Tittl M.K., Spaide R.F., Wong D., et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128(1):63–68. doi: 10.1016/s0002-9394(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 6.Yannuzzi L.A. Type-A behavior and central serous chorioretinopathy. Retina. 1987;7(2):111–131. doi: 10.1097/00006982-198700720-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gelber G.S., Schatz H. Loss of vision due to central serous chorioretinopathy following psychological stress. Am J Psychiatry. 1987;144(1):46–50. doi: 10.1176/ajp.144.1.46. [DOI] [PubMed] [Google Scholar]
- 8.Quillen D.A., Gass D.M., Brod R.D., et al. Central serous chorioretinopathy in women. Ophthalmology. 1996;103(1):72–79. doi: 10.1016/s0161-6420(96)30730-6. [DOI] [PubMed] [Google Scholar]
- 9.Oh J.H., Oh J., Togloom A., et al. Biometric characteristics of eyes with central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1502–1508. doi: 10.1167/iovs.13-13183. [DOI] [PubMed] [Google Scholar]
- 10.Terao N., Koizumi H., Kojima K., et al. Short axial length and hyperopic refractive error are risk factors of central serous chorioretinopathy. Br J Ophthalmol. 2020;104(9):1260–1265. doi: 10.1136/bjophthalmol-2019-315236. [DOI] [PubMed] [Google Scholar]
- 11.Imanaga N., Terao N., Nakamine S., et al. Scleral thickness in central serous chorioretinopathy. Ophthalmol Retina. 2021;5(3):285–291. doi: 10.1016/j.oret.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Miki A., Kondo N., Yanagisawa S., et al. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology. 2014;121(5):1067–1072. doi: 10.1016/j.ophtha.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Hosoda Y., Yoshikawa M., Miyake M., et al. CFH and VIPR2 as susceptibility loci in choroidal thickness and pachychoroid disease central serous chorioretinopathy. Proc Natl Acad Sci U S A. 2018;115(24):6261–6266. doi: 10.1073/pnas.1802212115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schellevis R.L., van Dijk E.H.C., Breukink M.B., et al. Role of the complement system in chronic central serous chorioretinopathy: a genome-wide association study. JAMA Ophthalmol. 2018;136(10):1128–1136. doi: 10.1001/jamaophthalmol.2018.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong E.K., Breukink M.B., Schellevis R.L., et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology. 2015;122(3):562–570. doi: 10.1016/j.ophtha.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 16.Schubert C., Pryds A., Zeng S., et al. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat. 2014;35(7):859–867. doi: 10.1002/humu.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosoda Y., Miyake M., Schellevis R.L., et al. Genome-wide association analyses identify two susceptibility loci for pachychoroid disease central serous chorioretinopathy. Commun Biol. 2019;2:468. doi: 10.1038/s42003-019-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spaide R.F., Hall L., Haas A., et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16(3):203–213. doi: 10.1097/00006982-199616030-00004. [DOI] [PubMed] [Google Scholar]
- 19.Iida T., Kishi S., Hagimura N., Shimizu K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999;19(6):508–512. doi: 10.1097/00006982-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Spaide R.F., Koizumi H., Pozzoni M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Yasuno Y., Hong Y., Makita S., et al. In vivo high-contrast imaging of deep posterior eye by 1-microm swept source optical coherence tomography and scattering optical coherence angiography. Opt Express. 2007;15(10):6121–6139. doi: 10.1364/oe.15.006121. [DOI] [PubMed] [Google Scholar]
- 22.Imamura Y., Fujiwara T., Margolis R., Spaide R.F. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda S., Ikuno Y., Yasuno Y., et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013;33(2):302–308. doi: 10.1097/IAE.0b013e318263d11f. [DOI] [PubMed] [Google Scholar]
- 24.Pang C.E., Shah V.P., Sarraf D., Freund K.B. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol. 2014;158(2):362–371 e2. doi: 10.1016/j.ajo.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Hiroe T., Kishi S. Dilatation of asymmetric vortex vein in central serous chorioretinopathy. Ophthalmol Retina. 2018;2(2):152–161. doi: 10.1016/j.oret.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Kishi S., Matsumoto H., Sonoda S., et al. Geographic filling delay of the choriocapillaris in the region of dilated asymmetric vortex veins in central serous chorioretinopathy. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0206646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manayath G.J., Arora S., Parikh H., et al. Is myopia a protective factor against central serous chorioretinopathy? Int J Ophthalmol. 2016;9(2):266–270. doi: 10.18240/ijo.2016.02.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi T., Koizumi H., Takahashi Y., et al. Differences in choroidal structures between idiopathic and steroid-induced central serous chorioretinopathy. J Vitreoretin Dis. 2018;3(1):10–15. [Google Scholar]
- 29.Araki T., Ishikawa H., Iwahashi C., et al. Central serous chorioretinopathy with and without steroids: a multicenter survey. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda S., Miki A., Kusuhara S., et al. Choroidal thickness of central serous chorioretinopathy secondary to corticosteroid use. Retina. 2017;37(8):1562–1567. doi: 10.1097/IAE.0000000000001380. [DOI] [PubMed] [Google Scholar]
- 31.Hayreh S.S. In vivo choroidal circulation and its watershed zones. Eye (Lond) 1990;4(Pt 2):273–289. doi: 10.1038/eye.1990.39. [DOI] [PubMed] [Google Scholar]
- 32.Mori K., Gehlbach P.L., Yoneya S., Shimizu K. Asymmetry of choroidal venous vascular patterns in the human eye. Ophthalmology. 2004;111(3):507–512. doi: 10.1016/j.ophtha.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Shiihara H., Sakamoto T., Terasaki H., et al. Running pattern of choroidal vessel in en face OCT images determined by machine learning-based quantitative method. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1879–1887. doi: 10.1007/s00417-019-04399-8. [DOI] [PubMed] [Google Scholar]
- 34.Liu B., Deng T., Zhang J. Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis. Retina. 2016;36(1):9–19. doi: 10.1097/IAE.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 35.Kaye R., Chandra S., Sheth J., et al. Central serous chorioretinopathy: an update on risk factors, pathophysiology and imaging modalities. Prog Retin Eye Res. 2020;79:100865. doi: 10.1016/j.preteyeres.2020.100865. [DOI] [PubMed] [Google Scholar]