Highlights
-
•
Gross total removal has a pivotal role in surgical treatment of intradural spinal tumors.
-
•
Sodium fluorescein prevents vascular injuries also preserving pial vessels in posterior myelotomy.
-
•
Fluorescence before the durotomy helps to distinguishing tumor from healthy tissue in intradural lesions.
-
•
Intraoperative fluorescence is safe and effective, also preserving functional anatomy in tumor removal.
Abbreviations: GTR, gross total removal; iUS, intraoperative ultrasound; MR, magnetic resonance; IONM, intraoperative neuromonitoring
1. Introduction
Spinal intradural tumors are rare lesions accounting for less than 15% of central nervous system neoplasms. They are categorized as intramedullary and extramedullary according to their location (Kumar et al., 2020). They are often associated with significant morbidity mainly related to motor impairment and sphincter disorders. The differential diagnosis of spinal tumors considers the localization, age, sex, and the onset symptoms (Hongo et al., 2019).
Despite several literature controversies, a gross total removal (GTR) retains its pivotal role in the surgical treatment of spinal tumors.
In recent years the use of technological innovations such as intraoperative neuromonitoring (IONM), magnetic resonance (MR) tractography, and intraoperative ultrasound (iUS) have become increasing to reach a safe and maximum surgical resection (Setzer et al., 2010; Ghadirpour et al., 2019). Furthermore, surgery under the guidance of fluorescence (Sodium fluorescein) has already become a well-known practice in the treatment of brain tumors. This tool provides an advantage in cases where the blood-brain barrier is disrupted, so a staining with the pathologic tissue is possible (Schebesch et al., 2013). Also, in spinal intradural tumors the blood-brain barrier is disrupted, so the use of fluorescence is feasible (Olguner et al., 2021). Often in intramedullary spinal tumor is not always possible to find a clear cleavage and this may affect the extent of resection. Indeed, a crucial factor determining the GTR is the perioperative tumor cleavage plan also in spine tumor surgery.
To our knowledge, only three studies evaluated spinal intradural tumors under yellow filter microscope in the presence of sodium fluorescein (Olguner et al., 2021; Falco et al., 2019; Acerbi et al., 2017).
The aim of our study is to examine the staining pattern of sodium fluorescein in intradural spinal tumors and demonstrate how this is feasible for the purpose of a maximal safe resection.
2. Material and methods
2.1. Patient selection
The Institutional Review Board of our hospital approved this study, and a written informed consent was obtained from all patients for off-label use of Sodium fluorescein in spinal procedures.
In our study we retrospectively examined the database of Neurosurgery Department, University of Messina and we performed a retrospective analysis of all the patients who underwent a microsurgical removal of intra- and extramedullary intradural spinal lesion between January 2019 and May 2020. Among 21 patients, 14 patients fulfilled the selection criteria. 6 patients were excluded from our selection because of the contraindication in the administration of sodium fluorescein (Table 1).
Table 1.
Contraindication in the use of sodium fluorescein.
| Contraindication in the use of sodic fluorescein |
|---|
| Chronic renal failure |
| Creatinine value > 1.5 mg/dl |
| Known hypersensitivity to fluorescein sodium |
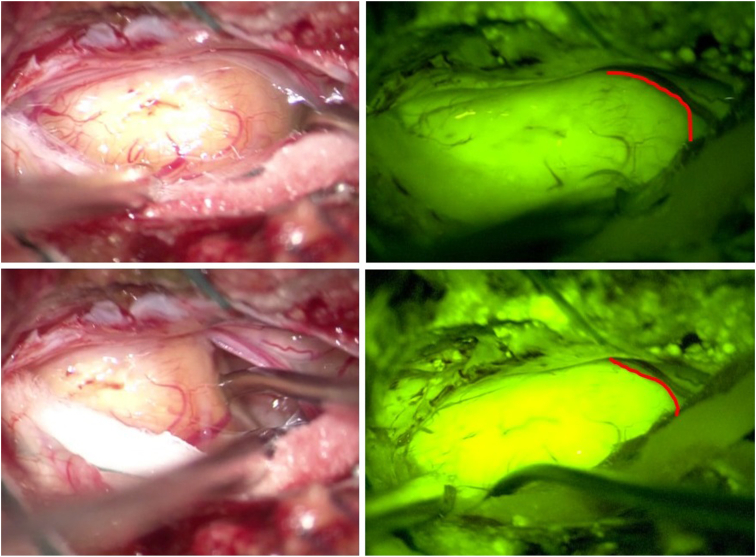
14 patients were selected for our study: 9 women (64%; age range 23–67 years) and 5 men (36%; age range 18–70 years):1 hemangioblastoma, 2 anaplastic ependymomas (WHO Grade 3), 1 primitive neuroectodermal tumor (PNET), 2 ependymomas (WHO Grade 1), 3 meningiomas (WHO Grade 1), 1 capillary hemangioma, 1 plexiform schwannoma (Fig. 1), 2 sarcomas, 1 plasmacytoma.
Fig. 1.
Plexiform schwannoma arising from cauda. Microsurgical dissection with microscope Pentero 900 Zeiss (Carl Zeiss Meditec, Oberkochen, Germany) under white light (left) and with a special filter for intraoperative visualization of the YELLOW560 fluorescence (right), highlighting tumor resection boundaries with spinal nerve roots. (Red line). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
These lesions were in the cervical spine (21.4%), cervico-thoracic junction (14.3%), thoracic spine (28.6%), lumbar spine (35.7%).
All patients underwent a pre-operative spinal MRI with Gadolinium, also evaluating the eventual lesion contrast-enhancement, and early post-operative (within 48 h) MRI. In only 1 case it was not possible to perform the postoperative imaging study within 48 h, but after 1 month-follow-up the patient performed spine MRI at another institution, showing a GTR of the lesion (meningioma WHO grade 1).
Extent of resection (EOR) was evaluated by comparing pre-operative and post-operative images. Volumetric assessments were performed with Horos software for MAC (https://horosproject.org/).
We retrospectively analyzed the video recorded and extracted.TIF images. Directly at dural opening, regions of interest were set on the most illuminating part of the tumor and the most illuminating area of the spinal cord in case of intramedullary lesions. Red, green, blue (RGB) values of each ROI were evaluated using ImageJ (Version 1.52t).
2.2. Surgical protocol
The operative protocol includes the intravenous administration of sodium Fluorescein (Monico SpA, Venice, Italy) at a dosage of 5 mg/kg at the time of general anesthesia induction.
All patients underwent a pre-operative neurophysiological study the day before surgery according to standard protocol. Surgical treatment was subsequently performed with standard microsurgical technique. The operative microscope Pentero 900 Zeiss (Carl Zeiss Meditec, Oberkochen, Germany) equipped with a special filter for intraoperative visualization of the YELLOW560 fluorescence was used for all surgical procedures. In all cases, intraoperative neurophysiological monitoring was used with evaluation of SSEP, MEP and the D wave that was used in 7 cases (50%) for lesions located above T9.
3. Results
An intense contrast-enhancement on the MRI study was found for all lesions except for the WHO I ependymomas and PNET.
Image analysis extracted from intraoperative videos confirmed the intraoperative findings of increased fluorescence of the tumor in contrast with spinal cord or dura mater. Even before the opening of the dura, surgeons had the impression of stronger fluorescent stain of the lesion in comparison with the non-affected structures. Considering that yellow is mainly a mixture of red and green, values of these colors were significantly increased in tumors compared with normal tissues.
A homogeneous and high fluorescein involvement was found in 13 out of 14 treated lesions (92.8%). No fluorescence was found in PNET. No patient underwent adjuvant or neoadjuvant chemo/radiotherapy treatment either for previous pathologies or for the current spinal tumors.
In all cases it was possible to associate the intense contrast-enhancement on the pre-operative MRI with the intraoperative lesional fluorescence. Especially in extramedullary intradural lesions (6 out of 14–42%) the fluorescence allowed to visualize the lesions extradurally, leading to shorter operative times and related complications.
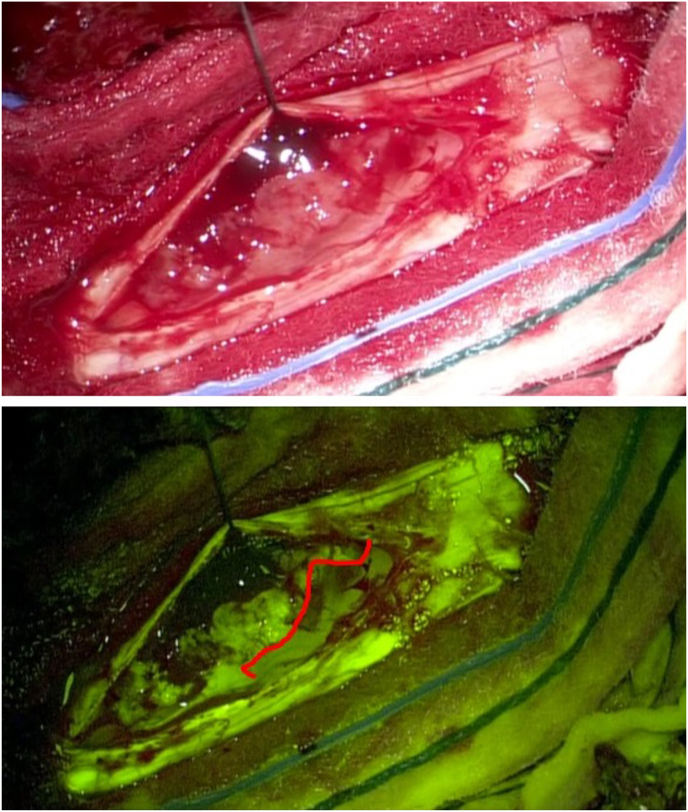
GTR was achieved in 11 out of 14 patients’ cohort (78%). In cases where it was not possible to obtain GTR, there were alterations in the neurophysiological monitoring. Nevertheless, in the 3 cases (22%) in which it was not possible to reach a GTR, a subtotal resection (STR) > 75% was performed (Fig. 2).
Fig. 2.
Thoracic anaplastic ependymoma. Microsurgical resection after durotomy, under white light (up) and YELLOW560 filter (down) that is effective in detect a tumor cleavage plan (Red line), thus preserving surgical and functional anatomy. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In our patients’ cohort, no adverse events related to sodium fluorescein administration were reported.
4. Discussion
Several intraoperative tools, such as neuronavigation, fluorescence, intraoperative CT, MRI and iUS, are routinely used to detect and distinguish tumors from normal brain tissue, thus improving a maximal safe resection. However, those technologies are not yet largely used in the resection of spinal intradural tumors. Sodium fluorescein is an intraoperative fluorescent agent that can accumulate in the extracellular space through an altered blood brain barrier (Diaz et al., 2015).
Most of spinal intradural tumors showed a various degree of contrast-enhancement at preoperative MRI.
There are many studies that compare spinal cord intradural tumors resection with 5-aminolevulinic acid (5-ALA) but there is scant literature on sodium fluorescein (Wainwright et al., 2019; Krause Molle et al., 2018; Kamp et al., 2018). 5-ALA guided surgery was proven to be useful technique in spinal glioma resection (Kamp et al., 2018).
The scenario of using sodium fluorescein as an in vivo tracer for spinal lesions, like in brain tumors, offers a wide range of scenarios in the treatment of spinal tumors in which the gold standard is the maximal safe resection and in which chemo and radiotherapy play a role mainly in recurrence or in case of growing tumor remnant.
We think that sodium fluorescein is useful in preventing vascular injuries in intramedullary lesions, preserving pial vessels in posterior myelotomy. Also, fluorescence visualization even before the durotomy helps to distinguishing between tumor and healthy tissue in intradural extramedullary tumors with dense vascularity.
The decades of experience of neurosurgeons with fluorescent tracers and the increasingly widespread availability of operating microscopes equipped with special intraoperative fluorescence filters could give way to a new standard tool for the treatment of spinal tumors (Sun et al., 2020). Indeed, in our patients’ cohort, fluorescence was found in 13 out of 14 cases (92.8%). These histotypes allowed us to understand how the cohort of spinal intradural tumors capable of fluorescence tracking reflects about 80% of all spinal tumors. This could make the use of fluorescein a standardized practice in the surgical treatment of spinal tumors that assume significant contrast-enhancement in pre-operative T1-weighted MRI sequences. For this reason, we stated that surgery under a microscope with yellow filter in the presence of sodium fluorescein is safe and effective in determining the tumor cleavage plan, thus preserving surgical and functional anatomy.
4.1. Study limitations
This study presents some limitations due to its retrospective design and the limited number of cases in some of the anatomopathological diagnoses. Also, for some patients there is not a significant long-term follow-up, not allowing a possible correlation between the use of sodium fluorescein, extent of resection, progression free survival and overall survival. Future prospective studies could better address these limitations by stratifying the cohorts according to tumor histology and the identification of a plane of dissection.
5. Conclusion
Intradural spinal tumors surgery under microscope guidance that uses yellow fluorescence filter, lead to a safe and effective maximal safe resection, determining the tumor cleavage plan, thus preserving surgical and functional anatomy. After an appropriate learning curve, sodium fluorescein may represent a new, promising tool also in intramedullary lesions, preventing vascular injuries, preserving pial vessels in posterior myelotomy, thus reducing the recurrence rate and increasing GTR rate.
Ethics approval
There is no ethical issue in this paper.
Funding support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Patients gave their consent for off-label Sodium Fluorescein administration and for images or other clinical information reported in the paper.
Declaration of competing interest
There are no conflicts of interest.
Acknowledgement
none.
References
- Acerbi F., Cavallo C., Schebesch K.M., Akçakaya M.O., de Laurentis C., Hamamcioglu M.K., Broggi M., Brawanski A., Falco J., Cordella R., Ferroli P., Kiris T., Höhne J. Fluorescein-guided resection of intramedullary spinal cord tumors: results from a preliminary, multicentric, retrospective study. World Neurosurg. 2017;108:603–609. doi: 10.1016/j.wneu.2017.09.061. [DOI] [PubMed] [Google Scholar]
- Diaz R.J., Dios R.R., Hattab E.M., Burrell K., Rakopoulos P., Sabha N., Hawkins C., Zadeh G., Rutka J.T., Cohen-Gadol A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015;122:1360–1369. doi: 10.3171/2015.2.JNS132507. [DOI] [PubMed] [Google Scholar]
- Falco J., Cavallo C., Vetrano I.G., et al. Fluorescein application in cranial and spinal tumors enhancing at preoperative MRI and operated with a dedicated filter on the surgical microscope: preliminary results in 279 patients enrolled in the FLUOCERTUM prospective study. Front Surg. 2019;6:49. doi: 10.3389/fsurg.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadirpour R., Nasi D., Iaccarino C., Romano A., Motti L., Sabadini R., et al. Intraoperative neurophysiological monitoring for intradural extramedullary spinal tumors: predictive value and relevance of D-wave amplitude on surgical outcome during a 10-year experience. J. Neurosurg. Spine. 2019;30:259–267. doi: 10.3171/2018.7.SPINE18278. [DOI] [PubMed] [Google Scholar]
- Hongo H., Takai K., Komori T., Taniguchi M. Intramedullary spinal cord ependymoma and astrocytoma: intraoperative frozen-section diagnosis, extent of resection, and outcomes. J. Neurosurg. Spine. 2019;30:133–139. doi: 10.3171/2018.7.SPINE18230. [DOI] [PubMed] [Google Scholar]
- Kamp M.A., Krause Molle Z., Munoz-Bendix C., Rapp M., Sabel M., Steiger H.J., et al. Various shades of red—a systematic analysis of qualitative estimation of ALA-derived fluorescence in neurosurgery. Neurosurg. Rev. 2018;41:3–18. doi: 10.1007/s10143-016-0745-4. [DOI] [PubMed] [Google Scholar]
- Krause Molle Z., Gierga K., Turowski B., Steiger H.J., Cornelius J.F., Rapp M., et al. 5-ALA-Induced fluorescence in leptomeningeal dissemination of spinal malignant glioma. World Neurosurg. 2018;110:345–348. doi: 10.1016/j.wneu.2017.10.069. [DOI] [PubMed] [Google Scholar]
- Kumar N., Tan W.L.B., Wei W., Vellayappan B.A. An overview of the tumors affecting the spine-inside to out. Neurooncol. Pract. 2020;7:i10–i17. doi: 10.1093/nop/npaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguner S.K., Arslan A., Açık V., İstemen İ., Can M., Gezercan Y., Ökten A.İ. Sodium fluorescein for spinal intradural tumors. Front. Oncol. 2021;10 doi: 10.3389/fonc.2020.618579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebesch K.M., Proescholdt M., Höhne J., Hohenberger C., Hansen E., Riemenschneider M.J., et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery - a feasibility study. Acta Neurochir. 2013;155:693–699. doi: 10.1007/s00701-013-1643-y. [DOI] [PubMed] [Google Scholar]
- Setzer M., Murtagh R.D., Murtagh F.R., Eleraky M., Jain S., Marquardt G., et al. Diffusion tensor imaging tractography in patients with intramedullary tumors: comparison with intraoperative findings and value for prediction of tumor resectability: presented at the 2009 Joint Spine Section Meeting. J. Neurosurg. Spine. 2010;13:371–380. doi: 10.3171/2010.3.SPINE09399. [DOI] [PubMed] [Google Scholar]
- Sun Z., Jing L., Fan Y., Zhang H., Chen L., Wang G., Sharma H.S., Wang J. Fluorescein-guided surgery for spinal gliomas: analysis of 220 consecutive cases. Int. Rev. Neurobiol. 2020;151:139–154. doi: 10.1016/bs.irn.2020.03.004. [DOI] [PubMed] [Google Scholar]
- Wainwright J.V., Endo T., Cooper J.B., Tominaga T., Schmidt M.H. The role of 5-aminolevulinic acid in spinal tumor surgery: a review. J. Neuro Oncol. 2019;141:575–584. doi: 10.1007/s11060-018-03080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]