Abstract
Purpose
To describe the prevalence, risk factors, and associations of vitreoretinal interface (VRI) abnormalities in a population-based study of older adults.
Design
Cross-sectional analysis of cohort study participants.
Participants
Of the 1149 participants (mean age, 76.1 ± 6.9 years) in the 15-year Blue Mountains Eye Study follow-up examination from 2007 through 2009, 905 (1791 eyes) had gradable time-domain or spectral-domain OCT scans of the macula from at least 1 eye.
Methods
OCT scans were graded according to the International Vitreomacular Traction Study Group classification system of VRI abnormalities. Best-corrected visual acuity (BCVA) was recorded.
Main Outcome Measures
Prevalence of VRIs.
Results
Overall, 451 participants showed any VRI abnormality (49.8%). Prevalence of VRI abnormality by person was: vitreomacular adhesion (VMA), 33.6%; vitreomacular traction (VMT), 1.6%; epiretinal membrane (ERM), 21.4%; full-thickness macular hole (FTMH), 0.7%; and lamellar macular hole (LMH), 0.7%. Twenty-two percent of VMAs were focal, and 78% were broad based; 76% of VMTs were focal, and 24% were broad based. All FTMHs observed were large (>400 μm), with mean aperture size of 573 μm (range, 459–771 μm). Increased age was associated with higher ERM and lower VMA prevalence (P < 0.001 for both). Pseudophakia and myopia were associated with ERM (age- and sex-adjusted odds ratios [ORs], 1.48 [95% confidence interval (CI), 1.01–2.17] and 1.72 [95% CI, 1.05–2.81], respectively). Moderate or severe ERM and FTMH were associated with worse BCVA of 9.2 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (95% CI, 3.4–15.0 ETDRS letters; P = 0.008) and 26.0 ETDRS letters (95% CI, 10.9–41.1 ETDRS letters; P = 0.001), respectively.
Conclusions
The prevalence of VRI abnormalities is high in older individuals. Epiretinal membrane was associated with increasing age, pseudophakia, and myopia. Epiretinal membrane and FTMH may account for significant visual loss in the affected eye. This study provided useful population-based data on the prevalence of VRI abnormalities in older individuals.
Keywords: Epiretinal membrane, Vitreomacular traction, population
Abbreviations and Acronyms: BCVA, best-corrected visual acuity; BMES, Blue Mountains Eye Study; CI, confidence interval; D, diopter; ERM, epiretinal membrane; ETDRS, Early Treatment Diabetic Retinopathy Study; FTMH, full-thickness macular hole; LMH, lamellar macular hole; NICOLA, Northern Ireland Cohort for the Longitudinal Study of Ageing; OR, odds ratio; PVD, posterior vitreous detachment; SD, spectral-domain; TD, time-domain; VMA, vitreomacular adhesion; VMT, vitreomacular traction; VRI, vitreoretinal interface
Vitreoretinal interface (VRI) disorders, such as epiretinal membrane (ERM), vitreomacular traction (VMT), vitreomacular adhesion (VMA), full-thickness macular hole (FTMH), and lamellar macular hole (LMH), are commonly reported abnormalities at the vitreomacular interface, but their prevalence is unclear. Before the invention of OCT, these disorders were diagnosed from slit-lamp biomicroscopy and stereoscopic retinal photographs, which often missed milder cases of VRI abnormalities.1, 2, 3 With OCT imaging, the vitreoretinal interface can be imaged directly, and the presence and severity of VRI abnormalities can be quantified objectively.2,3
A recent meta-analysis of 13 population-based studies1 reported an ERM prevalence of 9.1% (95% confidence interval [CI], 6.0%–12.2%) based on photographic grading. This is a useful estimate, but the true prevalence is likely to be higher. To date, only a small number of population-based studies have reported VRI prevalence based on OCT imaging,1, 2, 3, 4, 5 and even fewer on ocular and systemic associations.4,5 Such data are needed to determine the public health impact of VRI abnormalities on visual function. This is particularly relevant given the availability of surgical and, more recently, nonoperative interventions such as pneumatic vitreolysis to treat VRI abnormalities.6
Therefore, we aimed to report the prevalence of VRI abnormalities from a well-characterized population-based study of older adults, the Blue Mountains Eye Study (BMES) from Australia.7, 8, 9 We also report ocular and systemic associations of VRI abnormalities and their effect on vision in the affected eye.
Methods
Study Population
The BMES is a large population-based cohort study focusing on vision and eye diseases in an older White population.7, 8, 9 At baseline in 1992, all permanent, noninstitutionalized persons 49 years of age and older were included, with no upper age limit. Of the 4433 eligible residents, 3654 (81.4%) participated in the BMES I baseline examination. Participants were followed up at 5-year intervals for 3 subsequent examinations, with details provided elsewhere.10 This report focuses on the 1149 participants (56.1% of survivors) who were re-examined 15 years later (2007–2009) for the BMES IV.11 This was the first visit at which time-domain (TD) OCT was available. Spectral-domain (SD) OCT became available midway through the study.
All 4 BMES examinations were approved by the Western Sydney Area Health Service’s Human Research Ethics Committees and the University of Sydney. All participants provided written, informed consent at each visit. The study adhered to the recommendations of the Declaration of Helsinki.
Definitions of Vitreoretinal Interface Disorders
Vitreoretinal interface abnormalities were defined according to the OCT-based anatomic classification system recommended by the International Vitreomacular Traction Study Group published in 2013.12 We classified VMA, VMT, ERM, FTMH, and LMH.
Participants’ pupils were dilated with 0.5% tropicamide and underwent retinal photography and OCT scanning at each visit. Thirty-degree stereoscopic color retinal photographs of the macula, optic disc, and other retinal fields of both eyes were taken obtained a Zeiss FF3 fundus camera (Carl Zeiss, Oberkochen, Germany). Details of photographic grading for diabetic retinopathy, age-related macular degeneration, retinal vein occlusion, and other retinal lesions performed in the BMES have been reported previously.10 Two OCT machines were used in the BMES IV: the Carl Zeiss Stratus TD OCT device and the Carl Zeiss Cirrus SD OCT device. Most eyes (77.6%) were scanned with the Stratus OCT. The Cirrus OCT was used later in the study when it became commercially available for participants who had not yet undergone OCT examination (22.4% of scanned eyes). For Stratus OCT imaging, the macular thickness map scan protocol was used. This protocol consists of 6 linear scans in a spoke pattern centered at the fovea. The line scans were 6 mm in the transverse direction, had a 2-mm axial depth, and comprised 512 axial scans each. For Cirrus OCT imaging, the macular cube 200 × 200 combo protocol was used. This protocol consists of a cube scan centered at the fovea of 6 × 6 mm with a 2-mm axial depth and comprising 200 × 200 axial scans. A single trained grader (H.N.) masked to patient details retrieved OCT scans and graded for VRI abnormalities. Intragrader reliability was excellent, with κ values for VMA, VMT, ERM, FTMH, and LMH of 0.85, 0.94, 0.92, 0.93, and 0.93, respectively.
Measurement of Other Variables
At each visit, participants underwent interviewer-administered and take-home questionnaires, followed by a general health, vision, and eye examination. Data on eye-specific conditions, including late and early aged-related macular degeneration, diabetic retinopathy, branch and central retinal vein occlusion, cataracts, and previous cataract surgery, were collected from history and examination.10,11 Autorefraction followed by subjective refraction were performed and recorded in diopters (D) of spherical equivalent. Best-corrected visual acuity (BCVA) in Early Treatment Diabetic Retinopathy Study Scale (ETDRS) letters from each eye was recorded. Myopia was defined in 3 groups as no myopia (>–1 D), low myopia (–1 to –3 D), and moderate to high myopia (≤–3 D). Hyperopia was defined as more than +1 D, and emmetropia was defined as between –1 and +1 D inclusive.
Clinical measurements included resting blood pressure (systolic and diastolic) using a mercury sphygmomanometer with appropriate cuff size. Self-reported smoking status was recorded into 3 different smoking statuses: never, past, or current (within last 12 months). Presence of diabetes was defined as concurrent use of hypoglycemic medication or insulin or a fasting blood plasma of 126 mg/dl (7.0 mmol/l) or more.
Statistical Analyses
Participant characteristics were analyzed as follows: for categorical variables, using Pearson chi-square tests for 2 × 2 tables and Fisher exact tests for 3 × 2 tables, and for continuous variables, independent samples t tests. For disorders with low observed counts, Fisher exact tests were used instead of Pearson chi-square tests to investigate person-specific distribution and frequencies.
Associations between person-specific factors and VRI disorders were analyzed using generalized logistic regression models to account for correlation between eyes. Because of low numbers, low and moderate to high myopia were combined. We adjusted for a maximum of 2 covariates (age and sex) to avoid overfitting and spurious associations.13 Associations were expressed as age- and sex-adjusted odds ratios (ORs) and 95% confidence intervals (CIs). SAS software version 9.4 (SAS Institute) was used for all analyses.
Results
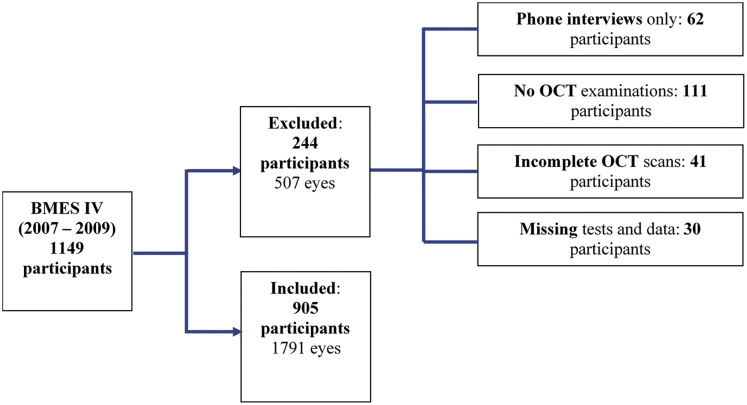
Of the 1149 BMES IV participants, a total of 244 participants were excluded from this study, as shown in Figure 1. The 244 excluded participants comprised 62 participants who were available only for a phone interview and had no images taken, 111 participants who declined OCT examinations, 41 participants who had incomplete OCT scans, and 30 participants who had missing systemic or ocular data. This resulted in a total of 905 BMES IV participants (79%) who were included for cross-sectional analyses and 1791 eyes. Of the 905 participants, 19 eyes were omitted because of incomplete (n = 8) or missing (n = 11) OCT scans. This did not decrease the number of included participants because 1 eye from each participant was still available.
Figure 1.
Flowchart showing the included and excluded participants in the Blue Mountains Eye Study fourth follow-up (BMES IV).
Table 1 shows the characteristics of included and excluded participants. Participants who were excluded from this analysis were similar to those included in terms of age, sex, weight, and diabetes status. Those excluded had slightly lower systolic blood pressure (144.1 mmHg vs. 148.1 mmHg) and were more likely to be past smokers. The prevalence of eye conditions among those included and excluded was similar for diabetic retinopathy, retinal vein occlusion, age-related macular degeneration, and cataract surgery. Included participants were slightly more likely to have moderate to high myopia than those excluded.
Table 1.
Characteristics of Participants Included and Excluded from Analysis
| Characteristics | Included (n = 905 Participants) | Excluded (n = 244 Participants) | P Value |
|---|---|---|---|
| Age (yrs) | 76.1 (6.9) | 76.5 (6.7) | 0.42 |
| Male sex | 41.9 | 42.6 | 0.83 |
| Blood pressure (mmHg) | |||
| Systolic | 148.1 (22.9) | 144.1 (22.3) | 0.04 |
| Diastolic | 79.3 (14.3) | 79.5 (14.8) | 0.89 |
| Weight (kg) | 75.1 (15.1) | 76.8 (15.7) | 0.21 |
| Diabetes | 15.9 | 14.6 | 0.62 |
| Smoking | |||
| Never | 55.9 | 47.9 | 0.008 |
| Past | 37.8 | 48.3 | |
| Current | 6.3 | 3.8 | |
| Best-corrected visual acuity | |||
| Better eye | 51.8 (9.4) | 51.3 (10.1) | 0.57 |
| Worse eye | 45.4 (18.1) | 43.7 (21.1) | 0.32 |
| Myopia | |||
| No | 86.7 | 81.7 | 0.02 |
| Low | 10.2 | 10.5 | |
| Moderate to high | 8.6 | 7.8 | |
| Diabetic retinopathy | 22.5 | 33.3 | 0.25 |
| Retinal vein occlusion | 2.7 | 1.2 | 0.41 |
| Age-related macular degeneration | |||
| Early | 36.2 | 38.5 | 0.59 |
| Late | 5.7 | 7.1 | 0.46 |
| Cataract surgery | 31 | 35.7 | 0.20∗ |
Data are presented as mean (standard deviation) or percentage, unless otherwise indicated. Boldface values indicate significance.
Pearson chi-square test.
Overall, 451 participants had any VRI disorder (49.8%). The overall prevalence of VRI disorders was as follows: VMA, 33.6%; VMT, 1.6%; ERM, 21.4%; FTMH, 0.7%; and LMH, 0.7% (Table 2). The prevalence, when considered by eye, was lower at 24.8%, 0.9%, 13.6%, 0.4%, and 0.3%, respectively, for the same VRI abnormalities. The prevalence of VRI abnormalities was similar for both OCT machines used (Table 2).
Table 2.
Prevalence of Vitreoretinal Interface Disorders by Eye
| Vitreomacular Adhesion | Vitreomacular Traction | Epiretinal Membrane | Full-Thickness Macular Hole | Lamellar Macular Hole | |
|---|---|---|---|---|---|
| By person (n = 905) | 33.6 | 1.6 | 21.4 | 0.7 | 0.7 |
| All participant eyes (n = 1791) | 24.8 | 0.9 | 13.6 | 0.4 | 0.3 |
| By OCT type | |||||
| Stratus OCT (n = 1390) | 24.2 | 0.9 | 13.5 | 0.4 | 0.4 |
| Cirrus OCT (n = 401) | 26.9 | 1.0 | 14.0 | 0.3 | 0.3 |
| P value | 0.35 | 0.93 | 0.80 | 0.57 | 0.71 |
| By age (yrs) | |||||
| < 75 | 34.0 | 0.9 | 9.8 | 0.4 | 0.4 |
| 75–84 | 20.3 | 1.2 | 16.5 | 0.3 | 0.3 |
| > 85 | 9.0 | 0.4 | 16.7 | 0.9 | 0.4 |
| P value | <0.001 | 0.49 | <0.001 | 0.62 | 0.88 |
| By sex | |||||
| Male | 29.6 | 0.9 | 14.9 | 0.3 | 0.3 |
| Female | 21.4 | 1.0 | 12.6 | 0.5 | 0.4 |
| P value | 0.002 | 0.96 | 0.22 | 0.50 | 0.66 |
| By laterality | |||||
| Bilateral | 15.6 | 0.3 | 5.4 | 0.1 | 0.0 |
| Best-corrected visual acuity (ETDRS letters) | |||||
| Present | 50.0 (48.8–51.2) | 47.4 (41.9–53.0) | 47.2 (45.5–49.0) | 23.1 (7.2–39.0) | 37.1 (24.6–49.5) |
| Absent | 48.9 (47.7–50.2) | 49.0 (48.2–49.8) | 49.3 (48.4–50.1) | 49.1 (48.3–49.9) | 49.0 (48.2–49.8) |
| P value | 0.06 | 0.59 | 0.02 | 0.001 | 0.06 |
ETDRS = Early Treatment Diabetic Retinopathy Study.
Data are presented as % or % (95% confidence interval), unless otherwise indicated.
Of eyes with VMA, 98 (22%) had focal VMA and 347 (78%) had broad-based VMA. Of eyes with VMT, 13 (76%) had focal VMT and 4 had broad-based VMT (24%). The mean vertical height of VMT (from retinal pigment epithelium to internal limiting membrane) was 270 μm (range, 186–430 μm). All 7 FTMHs were large (> 400 μm). The mean aperture size of FTMH was 573 μm (range, 459–771 μm).
A strong relationship was found between VRI abnormalities and age (Table 2). The prevalence of VMA was highest in eyes of patients younger than 75 years (34.0%) and lowest in those older than 85 years (9.0%; P < 0.001 for trend). Conversely, the prevalence of ERM was lowest in patients younger than 75 years (9.8%), increased to 16.5% in those 75 to 84 years of age, and stayed similar in those older than 85 years (16.7%; P < 0.001 for trend). The prevalence of other VRI abnormalities (VMT, FTMH, and LMH) was similar across the age groups. Vitreomacular adhesion was more common in men (29.6%) than women (21.4%; P = 0.002), whereas other VRI abnormalities did not differ by sex. Bilateral VMA, VMT, ERM, and FTMH were present in 15.6%, 0.3%, 5.4%, and 0.1% of eyes, respectively. Dual pathologic features were present in some participants. Among participants with ERM in one eye, the fellow eye sometimes showed a dual pathologic feature of VMA (11.9%), VMT (1.0%), or ERM (25.3%). No participants showed an ERM in one eye and FTMH or LMH in the other eye. Vitreomacular adhesion, VMT, and LMH were not associated significantly with BCVA (Table 2). However, presence of ERM and FTMH was associated with worse BCVA of 2.1 ETDRS letters (95% CI, 1.1–2.9 ETDRS letters; P = 0.02) and 26.0 ETDRS letters (95% CI, 10.9–41.1 ETDRS letters; P = 0.001), respectively, compared with eyes without these disorders.
The relationship between BCVA and ERM is examined further in Table 3. Mild ERM was not associated with worse vision, but moderate to severe ERM was associated with a 9.2-ETDRS letter (95% CI, 3.4–15.0 ETDRS letters; P = 0.008) reduction in BCVA compared with eyes with no ERM. The prevalence of mild to moderate ERM was 3.1% in all participants and 2.0% in all participant eyes.
Table 3.
Vision and Severity of Epiretinal Membrane Analyzed by Eye
| Epiretinal Membrane Severity | No. (%) | Mean Best-Corrected Visual Acuity (95% Confidence Interval)∗ | P Value (vs. None) |
|---|---|---|---|
| None | 1558 | 48.9 (48.0–49.8) | Reference |
| Mild | 207 | 48.1 (46.5–49.6) | 0.35 |
| Moderate and severe | 35 | 39.7 (33.0–46.4) | 0.008 |
Early Treatment Diabetic Retinopathy Study letters.
We further examined the association of ocular and systemic risk factors with prevalent VRI abnormalities, adjusting for age and sex (Table 4). In addition to age and sex, presence of pseudophakia was associated with higher odds of ERM (OR, 1.48; 95% CI, 1.01–2.17), but not with VMA. Low myopia was associated with higher odds of ERM (OR, 1.72; 95% CI, 1.05–2.81), but lower likelihood of VMA (OR, 0.51; 95% CI, 0.31–0.81). Blood pressure, smoking, diabetes, diabetic retinopathy, retinal vein occlusion, and age-related macular degeneration were not associated with either ERM or VMA in this study (Table 4). The mean age, sex, prevalence of diabetes, myopia, and pseudophakia did not differ between those with TD OCT and SD OCT imaging.
Table 4.
Risk Factors Associated with Epiretinal Membrane and Vitreomacular Adhesion, Eye-Specific Analyses
| Age- and Sex-Adjusted Odds Ratio (95% Confidence Interval) |
||
|---|---|---|
| Epiretinal Membrane | Vitreomacular Adhesion | |
| Age | 1.03 (1.01–1.05) | 0.92 (0.90–0.94) |
| Male sex | 1.22 (0.90–1.67) | 1.54 (1.18–2.02) |
| Blood pressure (mmHg) | ||
| Systolic | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) |
| Diastolic | 1.01 (0.99–1.02) | 1.01 (0.99–1.02) |
| Smoking | ||
| Past | 1.08 (0.77–1.51) | 1.11 (0.83–1.48) |
| Current | 1.41 (0.73–2.71) | 1.07 (0.59–1.94) |
| Diabetes | 0.68 (0.43–1.08) | 0.80 (0.55–1.17) |
| Diabetic retinopathy | 1.58 (0.75–3.34) | 0.60 (0.25–1.45) |
| Retinal vein occlusion | 1.04 (0.42–2.60) | 1.40 (0.65–3.04) |
| Pseudophakic | 1.48 (1.01–2.17) | 0.75 (0.52–1.07) |
| Age-related macular degeneration | ||
| Early | 0.96 (0.67–1.37) | 0.98 (0.73–1.36) |
| Late | 0.96 (0.42–2.20) | 1.46 (0.74–2.90) |
| Myopia | ||
| Low | 1.72 (1.05–2.81) | 0.51 (0.31–0.81) |
| Moderate to high | 0.86 (0.35–2.13) | 0.46 (0.20–1.03) |
Discussion
This study reports the prevalence of OCT-diagnosed VRI abnormalities in a population of older adults. A high prevalence of VRI abnormalities was found, with VMA present in 33.6% of participants, VMT in 1.6%, ERM in 21.4%, FTMH in 0.7%, and LMH in 0.7%. Only a small number of population studies have reported OCT-defined prevalence of VRI abnormalities. The VRI disorder prevalences reported here from the BMES are most comparable with those reported from the Beaver Dam Eye Study (n = 1540),3 which had a similar age profile (mean age, 74.1 years compared with 76.1 years in the BMES) and methodology. The Beaver Dam Eye Study3 found a similar prevalence of VMT (1.6%) and FTMHs (0.4%), a somewhat lower prevalence of VMA (26.0%), and a higher prevalence of ERM (34.1%) and LMH (3.6%). The Northern Ireland Cohort for the Longitudinal Study of Ageing (NICOLA) Study5 (n = 3351; subsample, n = 1481) with a younger participant group (mean age, 62 years) reported a lower prevalence of VMA (22.6%), VMT (0.5%), ERM (7.6%), and macular holes (FTMH, LMH, and pseudomacular holes together; 0.3%). The Alienor Study14 (n = 610) in France reported an ERM prevalence of 52.8% in an older population (mean age, 79.4 years), whereas the Gutenberg Health Study4 (n = 1890) in Germany (mean age, 58.8 years) reported a much lower ERM prevalence of 4.7%, FTMH prevalence of 0.1%, and LMH prevalence of 0.6%. In China, the Handan Eye Study15 (n = 6565; mean age, 51.2 years), using a combination of OCT and color fundus photographs, reported a low ERM prevalence of 3.4%, whereas the Jiangning Eye Study16 (n = 2005; mean age not published, but in participants were older than 50 years), also using a combination of OCT and color fundus photographs, reported an ERM prevalence of 8.4%. The Beijing Eye Study17 (n = 3468; mean age, 64.3 years) found a comparable VMT prevalence of 2.4%. Differences in age and ethnicity distribution likely account for much of the different VRI disorder prevalence rates reported. A number of studies have reported VRI disorder prevalence based on photographic grading,7,18, 19, 20, 21, 22, 23, 24 including the BMES, which reported a prevalence of 7%.7 Photographic estimates of ERM prevalence range from 2.8% to 28.6%, with a recent meta-analysis reporting a pooled prevalence of 9.1%.1 Our results and others show that photographic grading may underestimate ERM prevalence by 2- to 3-fold compared with OCT grading.
In addition to VRI prevalence, we recorded the size of VMA, VMT, and FTMH according to guidelines published by the International Vitreomacular Traction Study Group.12 Most VMAs (78%) were broad based (>1500 μm), whereas most VMTs (76%) were focal (≤ 1500 μm). The only other study to report size of VRI abnormalities was the Gutenberg study,4 which reported similar distribution of focal versus broad-based VRI abnormalities. A novel finding in our study is that mean VMT height from the retinal pigment epithelium to internal limiting membrane was 270 μm. All 7 FTMHs in our study were large (>400 μm), with a mean aperture size of 573 μm. The NICOLA study5 likewise reported that all 2 FTMHs found in that study were large. These findings suggest that large FTMHs are considerably more common than small or medium macular holes in the general population.
Epiretinal membrane was associated strongly with increasing age, a relationship that also has been reported in most other studies of ERM.1 This is likely related to the onset of posterior vitreous detachment (PVD), a trigger for ERM formation.25,26 In this context, it is interesting that our study observed that prevalence of ERM plateaued at 74 to 85 years of age, with a stable prevalence in those older than 85 years. This suggests that PVD evolution is essentially complete by 85 years of age, an observation supported by the Beijing Eye Study, which also found a plateau in PVD prevalence by age 80 years and older.17 The low prevalence of VMA (signifying incomplete PVD) in those 85 years of age and older in our study also supports this proposition. Pseudophakia and myopia were associated with presence of ERM, as has been reported in the NICOLA Study,5 Alienor Study,14 Beaver Dam Eye Study,3 Gutenberg Study,4 and other studies.7,19,21 This is consistent with the role of PVD in ERM formation, because both pseudophakia and myopia are associated with PVD, which then leads to ERM.27 Similarly, myopia was associated with lower prevalence of VMA, likely because of higher rates of PVD with myopia.
Male sex was associated with VMA. A similar result was reported in the NICOLA Study,5 Alienor Study,28 and Beijing Eye Study.17 The reasons for this are unclear, but may be related to lower levels of hyaluronic acid in women, leading to earlier PVD.29 Vitreomacular adhesion also has been hypothesized to be associated with age-related macular degeneration30,31 through possible direct traction or effects on diffusion of oxygen and growth factors. More recent studies,28,32 including the current study, found no evidence to support this hypothesis.
Our results highlight the impact of VRI abnormalities on visual function. Although VMA, VMT, and LMH were not associated with BCVA, ERM and FTMH were associated significantly with poorer BCVA. Mild ERM did not affect BCVA significantly, but moderate to severe ERM was associated with 9.2 ETDRS letters (2 lines) worse vision. Full-thickness macular hole showed the most impact on vision and reduced BCVA by 26 ETDRS letters (5 lines). These results show that VRI abnormalities significantly affect vision, and at a prevalence of 3.1% for moderate to severe ERM and 0.7% for FTMH, VRI abnormalities represent an important contributor to vision impairment among older adults. This is particularly relevant because surgical vitrectomy may be able to restore vision in many patients with these conditions.
This study has several strengths including a moderately large sample size from a well-established and well-characterized cohort and relatively high participation rate of 79%. Excluded and included participants were similar, improving the generalizability of the findings. To the best of our knowledge, this study is one of the first to perform a complete evaluation and documentation of VRI disorder parameters according to International Vitreomacular Traction Study Group criteria12 and to include impact on vision. Some limitations should be considered. First, 2 OCT machines were used in the study. Time-domain OCT has lower resolution than SD OCT, but accuracy for detecting all VRI abnormalities is reported to be similar for both machines, except for ERM, which is underestimated by TD OCT.33 The possibility exists that use of TD OCT may have underestimated the prevalence of ERM, but we believe this is unlikely, because the prevalences of all VRI abnormalities including ERM were similar across both machines, and therefore, results were combined to obtain the reported estimates. These estimates are similar to those of other studies using SD OCT as described earlier. Second, the study is a cross-sectional survey of a cohort. Survival bias also may have affected the findings; for example, if patients who smoked and harbored VRI abnormalities showed higher mortality from smoking and hence were not surveyed, this could account for the lack of association of smoking with VRI abnormalities. This is unlikely to have occurred because other surveys in younger participants have not reported an association of smoking with VRI disorder prevalence.1,5
In conclusion, this report found a high prevalence of VRI abnormalities diagnosed from OCT. Increasing age was associated strongly and positively with ERM and was associated inversely with VMA. Most VMAs were broad based, whereas most VMTs were focal. All FTMHs detected in this study were large. Presence of ERM and FTMH were associated significantly with worse visual acuity. These findings are useful for estimating the prevalence of VRI abnormalities and their effects on visual function in older adults.
Acknowledgments
The authors thank the participants of the Blue Mountains Eye Study for their contributions.
Manuscript no. D-21-00042.
Footnotes
Disclosure(s): All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supported by the Australian National Health and Medical Research Council (grant nos.: 153948, 211069, and 302068).
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at Western Sydney Area Health Service and the University of Sydney approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Liew, Nguyen, Mitchell
Analysis and interpretation: Liew, Nguyen, Ho, White, Burlutsky, Gopinath, Mitchell
Data collection: Liew, Nguyen, Mitchell
Obtained funding: N/A; Study was performed as part of regular employment duties at Westmead Institute for Medical Research. No additional funding was provided.
Overall responsibility: Liew, Nguyen, Ho, White, Burlutsky, Gopinath, Mitchell
References
- 1.Xiao W., Chen X., Yan W., et al. Prevalence and risk factors of epiretinal membranes: a systematic review and meta-analysis of population-based studies. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2016-014644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zapata M.A., Figueroa M.S., Esteban González E., et al. Prevalence of vitreoretinal interface abnormalities on spectral-domain OCT in healthy participants over 45 years of age. Ophthalmol Retina. 2017;1(3):249–254. doi: 10.1016/j.oret.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Meuer S.M., Myers C.E., Klein B.E., et al. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: the Beaver Dam Eye Study. Ophthalmology. 2015;122(4):787–795. doi: 10.1016/j.ophtha.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuster A.K., Kluck A.K., Korb C.A., et al. Characteristics and pathologies of the vitreo-macular interface-results from the Gutenberg Health Study. Acta Ophthalmol. 2020;98(3):e273–e281. doi: 10.1111/aos.14285. [DOI] [PubMed] [Google Scholar]
- 5.Quinn N.B., Steel D.H., Chakravarthy U., et al. Assessment of the vitreomacular interface using high-resolution OCT in a population-based cohort study of older adults. Ophthalmol Retina. 2020;4(8):801–813. doi: 10.1016/j.oret.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Flynn H.W., Jr., Relhan N. The Charles Schepens Lecture. Management options for vitreomacular traction: use an individualized approach. Ophthalmol Retina. 2017;1(1):3–7. doi: 10.1016/j.oret.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell P., Smith W., Chey T., et al. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104(6):1033–1040. doi: 10.1016/s0161-6420(97)30190-0. [DOI] [PubMed] [Google Scholar]
- 8.Liew G., Joachim N., Mitchell P., et al. Validating the AREDS simplified severity scale of age-related macular degeneration with 5- and 10-year incident data in a population-based sample. Ophthalmology. 2016;123(9):1874–1878. doi: 10.1016/j.ophtha.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Wang S.B., Mitchell P., Plant A.J., et al. Prevalence and risk factors of epiretinal membrane in a cohort with cardiovascular disease risk, compared with the Blue Mountains Eye Study. Br J Ophthalmol. 2015;99(12):1601–1605. doi: 10.1136/bjophthalmol-2015-306776. [DOI] [PubMed] [Google Scholar]
- 10.Joachim N., Colijn J.M., Kifley A., et al. Five-year progression of unilateral age-related macular degeneration to bilateral involvement: the Three Continent AMD Consortium report. Br J Ophthalmol. 2017;101(9):1185–1192. doi: 10.1136/bjophthalmol-2016-309729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopinath B., Liew G., Kifley A., et al. Dietary flavonoids and the prevalence and 15-y incidence of age-related macular degeneration. Am J Clin Nutr. 2018;108(2):381–387. doi: 10.1093/ajcn/nqy114. [DOI] [PubMed] [Google Scholar]
- 12.Duker J.S., Kaiser P.K., Binder S., et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 13.Babyak M.A. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66(3):411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 14.Delyfer M.N., Legout P., Le Goff M., et al. Prevalence of epiretinal membranes in the ageing population using retinal colour images and SD-OCT: the Alienor Study. Acta Ophthalmol. 2020;98(7):e830–e838. doi: 10.1111/aos.14422. [DOI] [PubMed] [Google Scholar]
- 15.Duan X.R., Liang Y.B., Friedman D.S., et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2009;50(5):2018–2023. doi: 10.1167/iovs.08-2624. [DOI] [PubMed] [Google Scholar]
- 16.Ye H., Zhang Q., Liu X., et al. Prevalence and associations of epiretinal membrane in an elderly urban Chinese population in China: the Jiangning Eye Study. Br J Ophthalmol. 2015;99(12):1594–1597. doi: 10.1136/bjophthalmol-2015-307050. [DOI] [PubMed] [Google Scholar]
- 17.Shao L., Xu L., You Q.S., et al. Prevalence and associations of incomplete posterior vitreous detachment in adult Chinese: the Beijing Eye Study. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You Q., Xu L., Jonas J.B. Prevalence and associations of epiretinal membranes in adult Chinese: the Beijing Eye Study. Eye (Lond) 2008;22(7):874–879. doi: 10.1038/sj.eye.6702786. [DOI] [PubMed] [Google Scholar]
- 19.Koh V., Cheung C.Y., Wong W.L., et al. Prevalence and risk factors of epiretinal membrane in Asian Indians. Invest Ophthalmol Vis Sci. 2012;53(2):1018–1022. doi: 10.1167/iovs.11-8557. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki R., Wang J.J., Sato H., et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata Study. Eye (Lond) 2009;23(5):1045–1051. doi: 10.1038/eye.2008.238. [DOI] [PubMed] [Google Scholar]
- 21.Fraser-Bell S., Ying-Lai M., Klein R., Varma R. Prevalence and associations of epiretinal membranes in Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2004;45(6):1732–1736. doi: 10.1167/iovs.03-1295. [DOI] [PubMed] [Google Scholar]
- 22.Cheung N., Tan S.P., Lee S.Y., et al. Prevalence and risk factors for epiretinal membrane: the Singapore Epidemiology of Eye Disease Study. Br J Ophthalmol. 2017;101(3):371–376. doi: 10.1136/bjophthalmol-2016-308563. [DOI] [PubMed] [Google Scholar]
- 23.Aung K.Z., Makeyeva G., Adams M.K., et al. The prevalence and risk factors of epiretinal membranes: the Melbourne Collaborative Cohort Study. Retina. 2013;33(5):1026–1034. doi: 10.1097/IAE.0b013e3182733f25. [DOI] [PubMed] [Google Scholar]
- 24.Ng C.H., Cheung N., Wang J.J., et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118(4):694–699. doi: 10.1016/j.ophtha.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steel D.H., Lotery A.J. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye (Lond) 2013;27(Suppl 1):S1–S21. doi: 10.1038/eye.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bu S.C., Kuijer R., Li X.R., et al. Idiopathic epiretinal membrane. Retina. 2014;34(12):2317–2335. doi: 10.1097/IAE.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 27.Johnson M.W. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371–382.e1. doi: 10.1016/j.ajo.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Gattoussi S., Cougnard-Grégoire A., Delyfer M.N., et al. Vitreomacular adhesion and its association with age-related macular degeneration in a population-based setting: the Alienor Study. Invest Ophthalmol Vis Sci. 2017;58(4):2180–2186. doi: 10.1167/iovs.16-20741. [DOI] [PubMed] [Google Scholar]
- 29.Palacio A.C., Gupta A., Nesmith B.L., et al. Vitreomacular adhesion evolution with age in healthy human eyes. Retina. 2017;37(1):118–123. doi: 10.1097/IAE.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 30.Simpson A.R., Petrarca R., Jackson T.L. Vitreomacular adhesion and neovascular age-related macular degeneration. Surv Ophthalmol. 2012;57(6):498–509. doi: 10.1016/j.survophthal.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.J., Lee C.S., Koh H.J. Posterior vitreomacular adhesion and risk of exudative age-related macular degeneration: paired eye study. Am J Ophthalmol. 2009;147(4):621–626.e1. doi: 10.1016/j.ajo.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Maggio E., Polito A., Guerriero M., et al. Vitreomacular adhesion and the risk of neovascular age-related macular degeneration. Ophthalmology. 2017;124(5):657–666. doi: 10.1016/j.ophtha.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Folgar F.A., Toth C.A., DeCroos F.C., et al. Assessment of retinal morphology with spectral and time domain OCT in the phase III trials of enzymatic vitreolysis. Invest Ophthalmol Vis Sci. 2012;53(11):7395–7401. doi: 10.1167/iovs.12-10379. [DOI] [PubMed] [Google Scholar]