Abstract
Introduction
The optimal surgical treatment for giant pituitary neuroendocrine tumors(GPitNETs) is debated.
Research question
The aim of this paper is to optimize the surgical management of these patients and to provide a consensus statement on behalf of the EANS Skull Base Section.
Material and methods
We constituted a task force belonging to the EANS skull base committee to define some principles for the management of GPitNETs. A systematic review was performed according to PRISMA guidelines to perform a meta-analysis on surgical series of GPitNETs. Weighted summary rates were obtained for the pooled extent of resection and according to the surgical technique. These data were discussed to obtain recommendations after evaluation of the selected articles and discussion among the experts.
Results
20articles were included in our meta-analysis, for a total of 1263 patients. The endoscopic endonasal technique was used in 40.3% of cases, the microscopic endonasal approach in 34% of cases, transcranial approaches in 18.7% and combined approaches in 7% of cases. No difference in terms of gross total resection (GTR) rate was observed among the different techniques. Pooled GTR rate was 36.6%, while a near total resection (NTR) was possible in 45.2% of cases. Cavernous sinus invasion was associated with a lower GTR rate (OR: 0.061). After surgery, 35% of patients had endocrinological improvement and 75.6% had visual improvement. Recurrent tumors were reported in 10% of cases
Discussion and conclusion
After formal discussion in the working group, we recommend the treatment of G-PitNETs tumors with a more complex and multilobular structure in tertiary care centers. The endoscopic endonasal approach is the first option of treatment and extended approaches should be planned according to extension, morphology and consistency of the lesion. Transcranial approaches play a role in selected cases, with a multicompartmental morphology, subarachnoid invasion and extension lateral to the internal carotid artery and in the management of residual tumor apoplexy.
Keywords: Giant pituitary adenoma, Giant PitNET, Surgery, Endoscopy, Transcranial approach, Apoplexy
1. Introduction
Giant pituitary neuroendocrine tumors (G-PitNETs) constitute 6–10% of pituitary tumors (Goel et al., 2004; Iglesias et al., 2021) and continue to represent a therapeutic challenge because of their size, invasiveness and extrasellar extension (Iglesias et al., 2018). Different definitions were proposed to define G-PitNET and we adopted the most common definition, where G-PitNETs are considered tumors with a largest diameter ≥40 mm (Iglesias et al., 2018).
While the endonasal transsphendoidal approach is generally considered as the gold standard for the treatment of PitNET, surgery for G-PitNET presents some important differences from surgery for smaller tumors. A gross total resection in G-PitNET after a single surgical procedure can be achieved in less than half the cases, even in specialized tertiary-care centers, while the operative morbidity and mortality rates remain high (Sinha and Sharma, 2010; Mortini et al., 2007).
In this paper, to optimize and standardize the surgical management of G-PitNETs, we performed a systematic literature review and meta-analysis on the subject. A task force composed of members of the EANS skull base committee along with some international experts in the field, enabled the compilation of a consensus statement and recommendations on the surgical management of these difficult tumors.
2. Methods
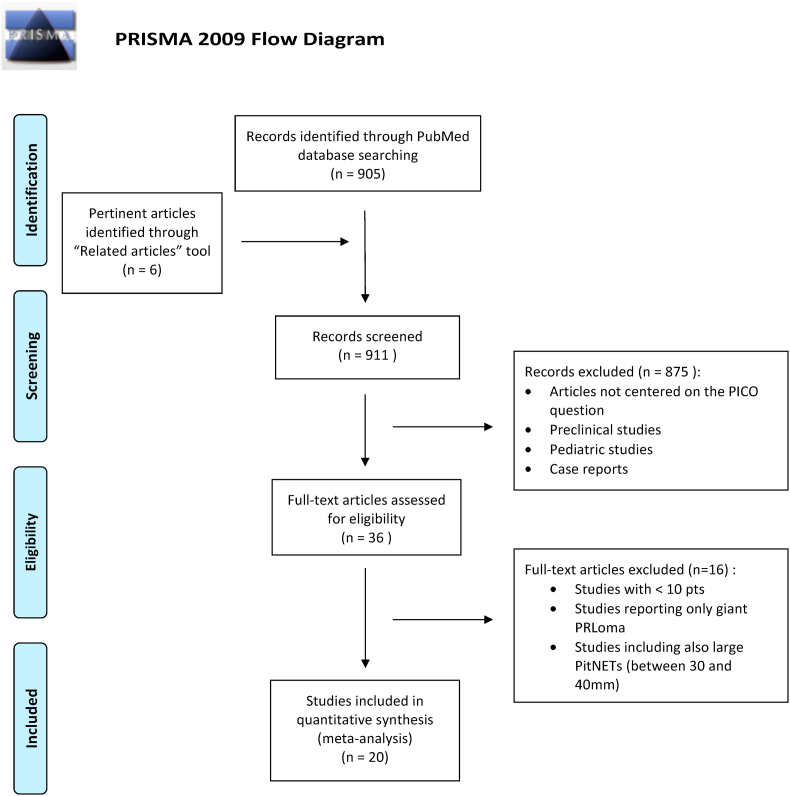
A systematic review was conducted according to the PRISMA criteria (Liberati et al., 2009). A literature search was performed using PubMed database, including articles published between January 2000 and January 2020. The search was conducted using the medical subject headings (MeSH) and free text terms: “giant pituitary adenoma” combined with “surgery”, “endoscopy”, “microscopy”, “resection”, “recurrence”, “survival” and “outcome”. Our search was limited to studies conducted in adults. Additional relevant studies were manually searched in the reference list of identified studies and through the use of the “related articles” tool in PubMed. Duplicate studies were eliminated. Two authors (GC and MM) independently reviewed abstracts, full-text articles and citations to select pertinent studies. A PICO question was formulated to identify pertinent studies: the population was defined as adult patients with G-PitNETs defined as tumors ≥40 mm in diameter, the intervention was any type of surgery performed and outcomes included endocrinological, visual and clinical outcomes, the extent of resection, recurrence rate and overall survival, early and long-term morbidity and quality of life. No language restrictions were used. Studies with less than 10 patients were excluded, as well as those including PitNETs of all sizes without a subgroup analysis for giant lesions or studies with an inconsistent follow-up. Studies reporting only giant prolactinomas, case reports, preclinical studies and pediatric trials were also excluded. The selection process was summarized in Fig. 1.
Fig. 1.
The selection process of the pertinent articles included in the meta-analysis is detailed according to the PRISMA statement.
Data from the individual studies were combined and compared. ANOVA test with the Tukey's HSD was used to compare the means of the different samples. Weighted summary rates were determined using meta-analysis models. Pooled estimates using meta-analytical techniques were obtained for the pooled rate of gross total resection (GTR) and near total resections (NTR) and according to the surgical technique used. The authors used the term GTR to define a macroscopically complete resection, with no residual tumor visible at the 3 months postoperative MRI, while a resection >95% was defined as NTR. When a residual tumor was present, the term subtotal resection (STR) was used.
The methodological quality of selected articles was evaluated using the GRADE system (Atkins et al., 2004) without masking the authorship of the article. The results of the systematic review and meta-analysis were discussed within the task force, to elaborate a consensus and evidence-based recommendations on the preferred surgical strategies. If randomized blinded trials or prospective matched pair cohort studies were identified, the recommendations were Level A or B. For controlled non-randomized trials or uncontrolled studies the recommendations were Level C or “expert opinion”, respectively. If unanimous responses were recorded, we used the phrase: “we recommend”. Divergent opinions were discussed until a consensus was reached and we used the terms: “we suggest”.
3. Results
Twenty articles were included in our meta-analysis, for a total of 1263 patients with G-PitNETs (Goel et al., 2004; Iglesias et al., 2021; Sinha and Sharma, 2010; Mortini et al., 2007; Yang et al., 2019; Elshazly et al., 2018; Nishioka et al., 2017; Han et al., 2017; Yano et al., 2017; Kuo et al., 2016; Shimon et al., 2015; Landeiro et al., 2015; Wang et al., 2014; de Paiva Neto et al., 2010; Koutourousiou et al., 2013; Gondim et al., 2014; Nakao and Itakura, 2011; D'Ambrosio et al., 2009; Xue-Fei et al., 2008; Guo et al., 2012) (Table 1). Three studies were multicentric (Iglesias et al., 2021; Shimon et al., 2015; Landeiro et al., 2015) while the others were monocentric (Goel et al., 2004; Sinha and Sharma, 2010; Mortini et al., 2007; Yang et al., 2019; Elshazly et al., 2018; Nishioka et al., 2017; Han et al., 2017; Yano et al., 2017; Kuo et al., 2016; Wang et al., 2014; de Paiva Neto et al., 2010; Koutourousiou et al., 2013; Gondim et al., 2014; Nakao and Itakura, 2011; D'Ambrosio et al., 2009; Xue-Fei et al., 2008; Guo et al., 2012). The mean age of the population included was 48.3±5.5 years. 64.1% of patients were male (810 patients) and the mean tumor diameter was 5.0±0.5 cm. In 613 cases (613/1214; 50.5% of cases) the tumor was Knosp grade 3 or 4 or cavernous sinus invasion was confirmed intraoperatively.
Table 1.
The main findings of the pertinent articles included in our meta-analysis are here summarized.
| Authors | N° patients | Mean age | % of males | Median tumor diameter (cm) | % cavernous sinus invasion | % of non functioning tumors | % EEA | % microscopic TSA | % TCA | % GTR | % STR | Median FU (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iglesias P. 2020 | 40 | 54 | 60% | 4.60 | 27.5% | 100% | 77,5% | 10% | 12.5% | 25% | 36 | |
| Yang C. 2019 | 60 | 51 | 68.3% | 5.26 | 58% | 88% | 100% | 0% | 0% | 46.7% | 41.7% | 42.5 |
| Elshazly K. 2018 | 55 | 55.5 | 64% | 5.1 | 69% | 92.7% | 96.4% | 0% | 0% | 44% | 47% | 41 |
| Han S. 2017 | 62 | 47.5 | 66.1% | 4.7 | 42% | 87% | 69.3% | 0% | 6.5% | 35.5% | 75.8% | 46.9 |
| Nishioka H. 2017 | 128 | 48.1 | 55.5% | 4.8 | 54% | 100% | 46.9% | 38.2% | 0% | 29.7% | 68.7% | 62.2 |
| Yano S. 2017 | 34 | 54.5 | 58.8% | 4.55 | 82.40% | 100% | 0% | 0% | 47% | 76.1 | ||
| Kuo C. 2016 | 38 | 50.8 | 63.1% | 71% | 100% | 0% | 0% | 21.1% | 72.9 | |||
| Landeiro JA 2015 | 35 | 48.2 | 54.2% | 20% | 100% | 85.7% | 0% | 0% | 68.6% | 49 | ||
| Shimon I. 2015 | 34 | 34.9 | 44.1% | 4.94 | 88.2% | 0% | 3% | 106.8 | ||||
| Gondim JA 2014 | 50 | 48.2 | 66% | 5.4 | 32% | 84% | 100% | 0% | 0% | 38% | 18% | 60 |
| Wang S. 2014 | 36 | 44 | 61.1% | 5.38 | 38.9% | 72.20% | 0% | 77.8% | 22.2% | 22.2% | 15.5 | |
| Koutourousiou M. 2013 | 54 | 52.9 | 85.1% | 5.0 | 94.4% | 76% | 100% | 0% | 0% | 20.4% | 66.7% | 37.9 |
| Guo F. 2012 | 15 | 50 | 53.3% | 66.70% | 0% | 0% | 100% | 67% | 40 | |||
| Nakao N. 2011 | 43 | 55 | 53.5% | 4.7 | 9.3% | 100% | 97.7% | 0% | 0% | 47% | ||
| de Paiva Neto MA 2010 | 51 | 48 | 63% | 4.5 | 60.8% | 76.50% | 0% | 100% | 0% | 41.2% | 20% | 30 |
| Sinha S. 2010 | 250 | 36.8 | 75.2% | 5.4 | 41.2% | 54.30% | 0% | 38.4% | 58% | 74% | 29.6 | |
| D'ambrosio AL 2009 | 11 | 48 | 72.7% | 27.2% | 82% | 0% | 0% | 0% | 55% | 9% | 51.6 | |
| Xue-Fei S. 2008 | 54 | 51 | 53.7% | 6.3 | 25.9% | 77% | 0% | 29.6% | 70.4% | 33.3% | 51.9% | 42.9 |
| Mortini P. 2007 | 95 | 48.4 | 69.5% | 4.65 | 75.8% | 73.30% | 0% | 72.7% | 12.6% | 14.7% | 56.9 | |
| Goel A. 2004 | 118 | 43.6 | 55% | 5.1 | 52.5% | 100% | 0% | 89% | 2.5% | 29.7% | 40.6% | 31 |
Abbreviations: cm: centimeters; EEA: endoscopic endonasal approach; GTR: gross total resection; STR: subtotal resection; TSA: transsphenoidal approach; TCA: transcranial approach.
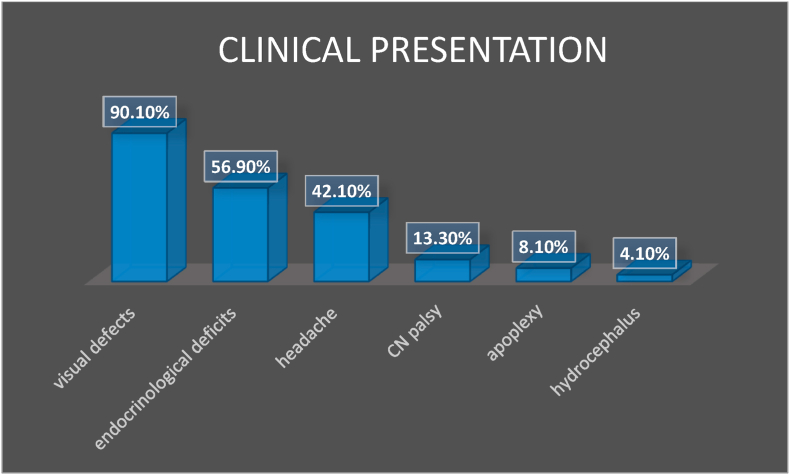
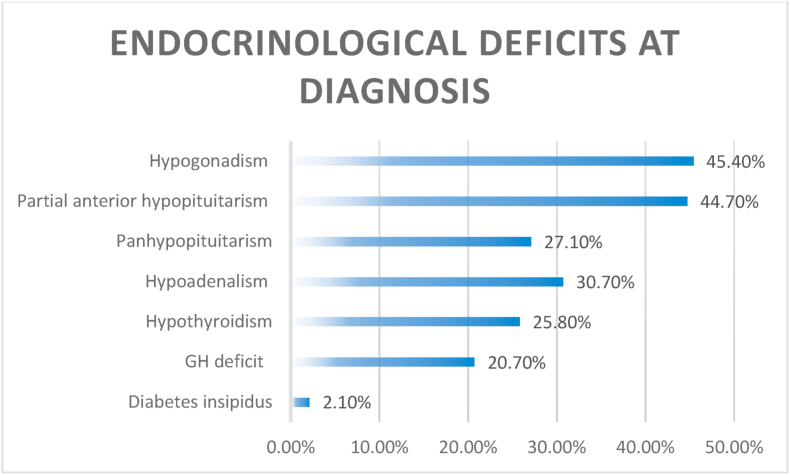
Visual problems represented the most common clinical presentation (90.1%), followed by endocrinological deficits in 57% of cases (Fig. 2, Fig. 3). Almost one third of patients (27%) had a complete anterior panhypopituitarism at diagnosis, while diabetes insipidus was extremely rare (2%) (Fig. 3). 260 patients (260/1222, 21%) of these surgical series had a functioning tumor, while in 79% of cases a non-functioning tumor was detected. Some studies included only non-functioning GPit-NETs (Goel et al., 2004; Iglesias et al., 2021; Nishioka et al., 2017; Landeiro et al., 2015; Nakao and Itakura, 2011) while one included only functioning tumors (Shimon et al., 2015). Recurrent tumors represented 5.8% (CI: 3.5–8.1%) of the surgical series.
Fig. 2.
The most common symptoms at diagnosis are resumed. The most common clinical presentation was represented by visual deficits, followed by symptoms of anterior hypopituitarism and headaches.
Fig. 3.
The endocrinological deficits at diagnosis are detailed. The most common finding was hypogonadism, while panhypopituitarism was found in almost a third of patients. Diabetes insipidus was extremely rare at diagnosis.
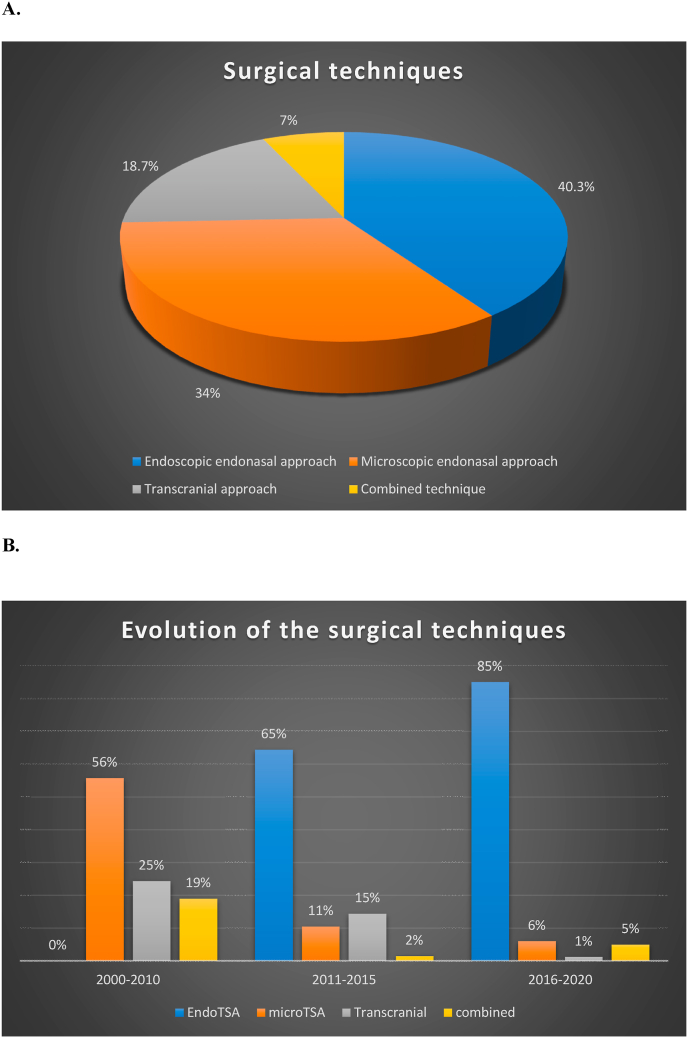
We analyzed the different surgical techniques used in each study, that were classified as: endoscopic endonasal approaches (used in 40.3% of cases), microscopic endonasal approaches (34% of cases), transcranial approaches (18.7% of cases) and combined approaches (endonasal and transcranial, used in 7% of cases). (Fig. 4). Their timeline evolution is shown in Fig. 4.
Fig. 4.
Fig. 4A: The different surgical techniques used across the papers are here summarized. In 495 out of 1229 patients (40.3%) an endoscopic endonasal approach (EEA) was used, in 418 a microscopic transsphenoidal approach (34%), in 230 patients a transcranial approach (18.7%) and in 86 patients a combined approach (endonasal and transcranial, 7%).
Fig. 4B: The timeline evolution of the different techniques across the different periods is summarized. For the years 2011–2015 and 2016–2020 the surgical technique was not specified in 7% and 3% of cases respectively.
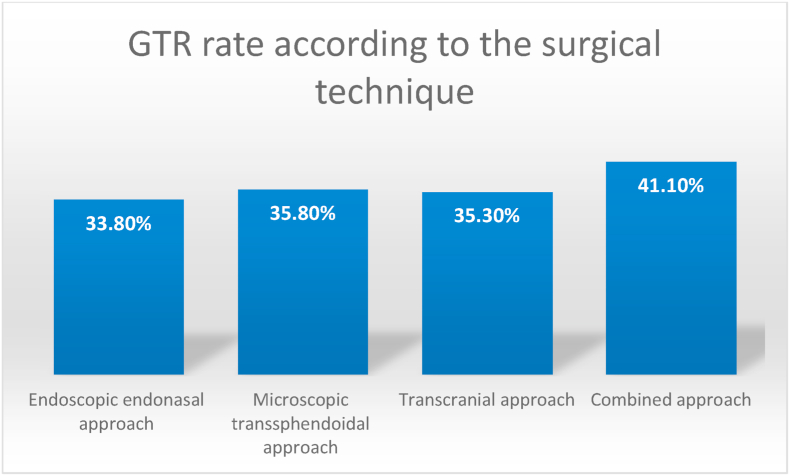
A comparative analysis of the extent of resection related to each surgical technique showed that only 8 studies reported the details after endoscopic endonasal (Yang et al., 2019; Elshazly et al., 2018; Nishioka et al., 2017; Han et al., 2017; Kuo et al., 2016; Koutourousiou et al., 2013; Gondim et al., 2014; Nakao and Itakura, 2011), 4 after microscopic transsphenoidal techniques (Mortini et al., 2007; Nishioka et al., 2017; de Paiva Neto et al., 2010; Xue-Fei et al., 2008), 4 after transcranial approaches (Mortini et al., 2007; Han et al., 2017; Xue-Fei et al., 2008; Guo et al., 2012) and 3 after combined techniques (Nishioka et al., 2017; Han et al., 2017; D'Ambrosio et al., 2009) Of the 403 patients where an EEA was performed, meta-analytic techniques showed that GTR was achieved in 33.8% (±3.9%) while GTR was achieved in 35.8% of 185 patients with microscopic transsphenoidal approaches (±7.9%). Transcranial approaches in 54 patients provided a GTR in 35.3% of cases (±14.9%) and combined approaches in 45 patients provided a GTR in 41.1% of cases (±12.1%). These differences were not statistically significant. (Fig. 5).
Fig. 5.
The rate of gross total resection (GTR) is detailed according to the surgical technique used. The results were pooled according to the meta-analysis technique (not shown). These differences were not statistically significant.
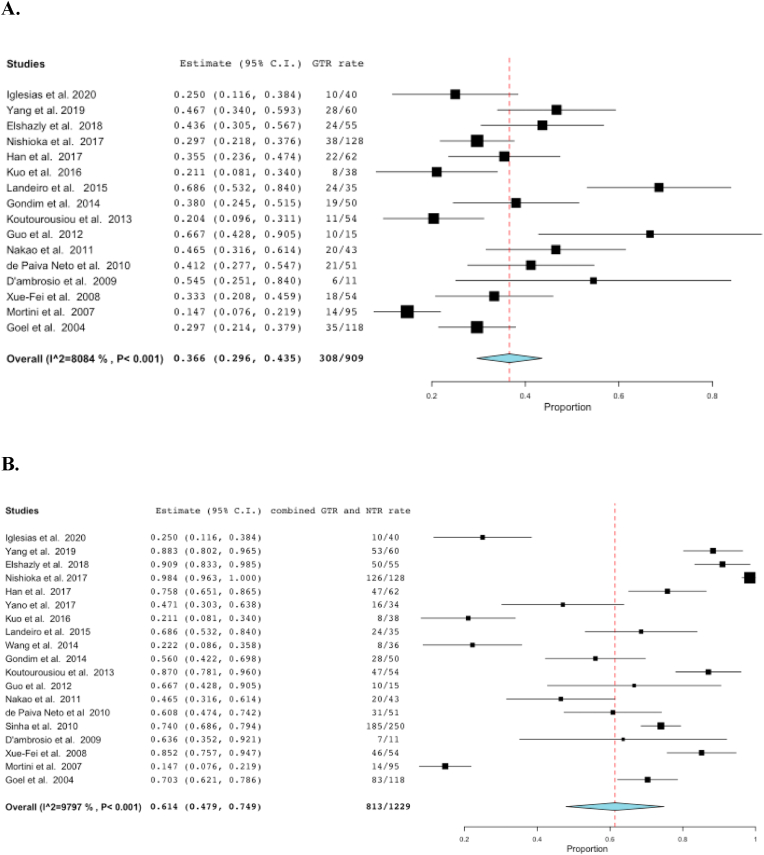
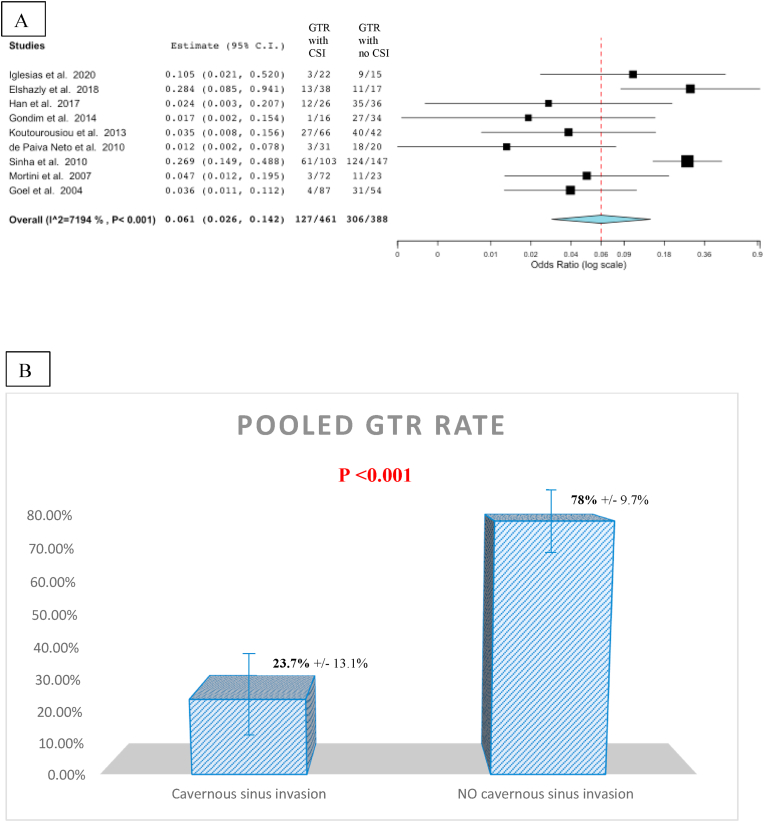
If we consider all the techniques together, the pooled GTR rate was 36.6%, while a NTR was possible in 45.2% of cases (pooled GTR + NTR: 61.4%) (Fig. 6). The comparison in terms of NTR and GTR + NTR among the different surgical techniques was not possible because in most of papers the specific technique used was not detailed. As expected, invasion of the cavernous sinus limited the rate of GTR to 23.7% and this difference was statistically significant when compared to GTR in patients with no cavernous sinus invasion (OR: 0.061; CI: 0.026–0.142) (Fig. 7).
Fig. 6.
Forest plots showing the pooled rate of gross total resection (GTR) rate (Figure 6A) across the different studies and the merged results of GTR and near total resection (NTR) (Figure 6B). The meta-analyzed measure is represented as a diamond. The pooled GTR rate was 36.6% and the pooled GTR and NTR rates were 61.4%.
Fig. 7.
Forest plot (A) representing the pooled gross total resection (GTR) rate when the invasion of the cavernous sinus was present (first column) and when it was absent (second column). When the invasion of the cavernous sinus was present, the pooled GTR rate was 23.7% (±13.1%) and it increased to 78% (±9.7%) when no cavernous sinus invasion was reported (B). A statistically significant difference was found (p < 0.001).
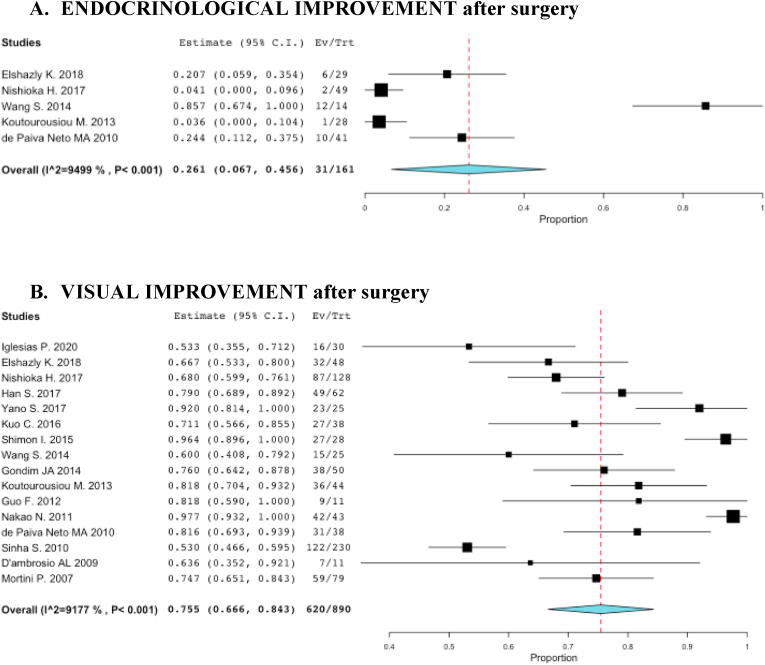
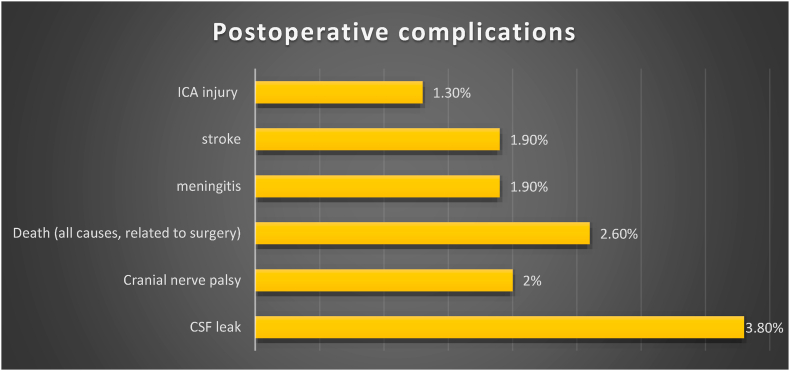
The pooled rate for endocrinological improvement (in terms of recuperation of at least one hormonal axis or resolution of stalk compression hyperprolactinaemia in the postoperative period) was 26.1% according to the analysis of six studies specifying these data (Elshazly et al., 2018; Nishioka et al., 2017; Shimon et al., 2015; Wang et al., 2014; de Paiva Neto et al., 2010; Koutourousiou et al., 2013) and no correlation was found with a specific surgical technique. A new partial anterior pituitary deficit was observed in 21% of patients, while a new anterior panhypopituitarism in 2.2%. New permanent diabetes insipidus was observed in 4.9% of cases. Visual improvement after surgery was reported in 75.5% of patients (in terms of visual acuity and visual field recovery), while 3.1% of patients experienced a visual worsening (Fig. 8). The median follow-up was of 42.9 months (range 15.5–106.8 months). The pooled rates for the most common surgical complications are summarized in Fig. 9.
Fig. 8.
Forest plots summarizing the studies that reported the rate of postoperative endocrinological improvement (A) and of visual improvement (B). The pooled rate is reported as a diamond: 26.1% of patients had a recuperation of at least one hormonal axis or resolution of stalk compression hyperprolactinaemia after surgery (A), while visual improvement was reported in 75.5% of patients (in terms of visual acuity and visual field recovery) (B).
Fig. 9.
The most common postoperative complications are summarized. Data from single studies were pooled according to the meta-analytic method (not shown).
The most common complication was CSF leak that remained rare and was reported in less than 4% of cases.
Early surgery for a large residual tumor was performed in 12.6% of cases (CI: 7.1%–18.2%). The surgical technique used for the second procedure varied according to the study considered: Kuo (Kuo et al., 2016) and Gondim(Gondim et al., 2014) repeated an EEA while Landeiro (Landeiro et al., 2015) and Mortini (Mortini et al., 2007) used a second endonasal approach or combined it with transcranial approaches.
Adjuvant radiotherapy was delivered in 33% of cases for growing residual or recurrent tumors and an adjuvant medical treatment was used in 58% of cases to obtain a biological remission in functional tumors. Recurrent tumors at last follow up were reported in 10.4% of cases (CI: 5.9%–14.8%) and a second surgery was performed in 6% of cases (3%–9%).
Concerning the procedures performed for recurrent tumors, the technique used was specified in five studies: Gondim (Gondim et al., 2014) and Koutourousiou (Koutourousiou et al., 2013) used a second endoscopic approach, D'Ambrosio used a second transcranial approach (D'Ambrosio et al., 2009), while Shimon et al. used a combination of the two techniques (Shimon et al., 2015). Yano et al. used both techniques (Yano et al., 2017). The surgical results after these redo surgeries were not specified.
4. Discussion
4.1. Definition of GPitNETs
There exists some ambiguity and debate around the definition of GPitNET. In 1969, Hardy et al. defined giant tumors as those having a suprasellar extension ≥30mm calculated from the tuberculum sellae (Hardy, 1969). Ten years later Symon et al. defined these tumors with the same measure extending ≥40 mm from the tuberculum sellae (Symon et al., 1979). These two definitions were developed before the introduction of the cerebral magnetic resonance imaging (MRI), when computed tomography was the main imaging modality in the diagnosis of these tumors.
Measurements from the tuberculum sellae are no longer performed after the advent of MRI. In the modern era, some authors still define GPitNETs as tumors with a maximal diameter ≥30 mm (Cusimano et al., 2012; Garibi et al., 2002; Alleyne et al., 2002), but the majority of the authors refer to tumors with a diameter ≥40 mm (Goel et al., 2004; Iglesias et al., 2021; Sinha and Sharma, 2010; Mortini et al., 2007; Yang et al., 2019; Elshazly et al., 2018; Nishioka et al., 2017; Han et al., 2017; Yano et al., 2017; Kuo et al., 2016; Shimon et al., 2015; Wang et al., 2014; de Paiva Neto et al., 2010; Koutourousiou et al., 2013; Gondim et al., 2014; Nakao and Itakura, 2011; D'Ambrosio et al., 2009; Xue-Fei et al., 2008; Guo et al., 2012) Tumors with a diameter between 30 and 40 mm should be defined as large PitNETs.
An important point to discuss is that inside this category of G-PitNETs, we include tumors larger than 40mm presenting with a regular round shape, without invasion of the cavernous sinus, that could extend mainly into the sphenoid sinus and/or into the suprasellar space. On the other side, we also include tumors with a more complex and multilobular structure, with invasion of the subarachnoid space and encasement of vasculo-nervous structures and large subfrontal or temporal fossa extensions, which are more challenging to treat. The definition of a cut-off for the size is important, as in the differentiation between micro- and macroadenomas but the size is not synonymous of prognosis, even if it helps in the categorization and in the analysis of case series.
-
•
We recommend defining G-PitNETs as tumors with a maximal diameter ≥ 40 mm on cerebral MRI or on high-definition contrasted CT when the patient present a contraindication to perform an MRI. (Level C)
-
•
We recommend the treatment of G-PitNETs tumors with a more complex and multilobular structure extending in several directions in tertiary care centers as they are surgically challenging compared to the rounded suprasellar G-PiNETs. (Level C)
4.2. Epidemiological and clinical characteristics of GPitNETs
GPitNETs are most commonly diagnosed between the 4th and the 5th decade and present a slight male predominance. In surgical series, clinically non-functioning giant pituitary adenomas are more frequently diagnosed (>70% of cases according to our analysis and to literature analysis)(Goel et al., 2004) and they are in general discovered in the context of pituitary hormone deficiency or mass effect on surrounding structures. Headaches and visual symptoms (essentially a chiasmatic syndrome) are the most common clinical manifestations (Iglesias et al., 2018, 2021). Syndromes associated with hormonal hypersecretion may be present in a minority of cases. Partial hypopituitarism is recorded in >40% of cases with GPitNETs and panhypopituitarism was reported in about one third of cases. Diabetes insipidus at diagnosis is rare.
-
•
We recommend preoperative endocrine and ophthalmological evaluations to establish the hormonal and the visual status of patients before surgery. (Level C)
4.3. Surgical approach
Most GPitNETs, with the exception of giant prolactinomas, require surgical treatment to decompress neural structures. They are classically associated with a limited rate of GTR and thus higher recurrence rates, as well as an increased postoperative rate of morbidity and mortality with an overall poor long-term prognosis (Marigil Sanchez et al., 2019). The aim of surgery for non-functioning GPitNETs should be to perform a maximal tumor resection to decompress the optic structures and the pituitary gland and stalk(Gondim et al., 2014; Cappabianca et al., 2015), while maintaining the quality of life of the patient with limited endocrinological and neurological morbidity. For functioning GPitNETs, the purpose is, in addition to the decompression of the optic apparatus, to normalize the hormonal hypersecretion and, if possible, to restore a physiological pituitary function. Surgical approaches should be tailored according to the size and extension of the GPitNET, its configuration, the need for a hormonal cure and the patient-specific goal of treatment.
To achieve those objectives some questions should be addressed before surgery, such as:
-
1.
Is the endonasal approach the first choice when dealing with GPitNET?
-
2.
What are the indications for transcranial approaches?
-
3.
How should postoperative pituitary apoplexy in GPitNETs be managed?
4.3.1. Is the endonasal approach the first choice when dealing with G-PitNET?
The endonasal approach is the most common approach used with G-PitNETs. In our meta-analysis including articles published during the last 20 years, an endonasal approach was performed in 74.3% of patients; the endoscopic approach in 40.3% and a microscopic transsphenoidal approach in 34% of cases. No statistically-significant differences were detected in terms of resection rates between these two techniques. However, during the last few decades, several studies have compared the endoscopic and microscopic transsphenoidal techniques in the management of PitNETs. The endoscopic technique was associated with better results in terms of resection rates (Gao et al., 2014; Almutairi et al., 2018; Dhandapani et al., 2016; Messerer et al., 2011), visual and endocrinological outcomes, essentially for tumors with an important suprasellar and parasellar extensions (Yu et al., 2018; Guo et al., 2021; Esquenazi et al., 2017). In 2012 a meta-analysis performed by Komotar et al., including 478 patients with tumors >30 mm, reported higher rates of GTR in the endoscopic cohort when compared with the microscopic cohort (P < 0.008) (Komotar et al., 2012). However, this analysis included also large tumors (with a maximal diameter <40 mm) and some surgical cohorts included patients treated with different surgical techniques, rendering the comparisons of the surgical outcomes for each surgical technique difficult.
No statistically significant differences were found in terms of resection rates between the endonasal and transcranial approaches. It is likely that the choice of a transsphenoidal versus a transcranial approach, reported in various series, was based on the specific characteristics of the individual tumors to achieve the best resection rate. Apart from approaches and surgical adjuncts, the factors that will influence the surgical outcomes are the shape of the tumor and the diaphragmatic opening, a multicompartmental extension, a lateral intradural extension beyond the ICA, the invasion of the subarachnoid space with vascular encasement, the invasion of the cavernous sinus and the consistency of the tumors (Elshazly et al., 2018; Koutourousiou et al., 2013; Cappabianca et al., 2015; Messerer et al., 2019). Rounded, dumbbell shaped and multilobulated adenomas are respectively associated with a decreased GTR rate (Koutourousiou et al., 2013). The presence of multiple compartments is an important factor in limiting the extent of resection (Elshazly et al., 2018; Koutourousiou et al., 2013) and is often associated with an extension into the subarachnoid space with arterial encasement.
Aside from the classic transsphenoidal approach, extended endoscopic endonasal approaches such as transtuberculum or transplanum approaches could be used when the tumor presents a significant suprasellar extension with a small sella turcica and to cut the diaphragm for a larger access in dumbbell-shaped adenomas. Furthermore, extended approaches may be considered when the tumor presents with subfrontal extension.
Cavernous sinus invasion is a well-known limiting factor in the achievement of GTR in PitNETs, in particular when there is extension to the lateral portion of the cavernous sinus or an encasement of the carotid artery (Koutourousiou et al., 2013, 2017; Messerer et al., 2011; Cossu et al., 2019; Starnoni et al., 2016; Fernandez-Miranda et al., 2018; Nishioka et al., 2014). This consideration was also confirmed by our literature analysis in giant lesions. The intracavernous portion of the tumor may be addressed by experienced surgeons through an endoscopic transcavernous approach, in particular with functional tumors (Koutourousiou et al., 2013, 2017; Fernandez-Miranda et al., 2018; Nishioka et al., 2014; Zoli et al., 2016; Cohen-Cohen et al., 2018), even if the efficiency of an aggressive resection of a large intracavernous extension remains controversial in terms of cure.
As regards tumor consistency, Cappabianca et al. reported that a soft consistency would, understandably, increase the possibility of achieving GTR for giant adenomas (Cappabianca et al., 2015) and an hemorrhagic component of the tumor could potentially help in the resection of the tumor (Koutourousiou et al., 2013; Azab et al., 2019; Juraschka et al., 2014). On T2-weighted MRI sequences, a soft consistency is generally associated with hyperintensity, while a fibrous tumor is generally hypointense on this sequence. For firm tumors, a more extended approach should be planned with the option of performing an extracapsular resection.
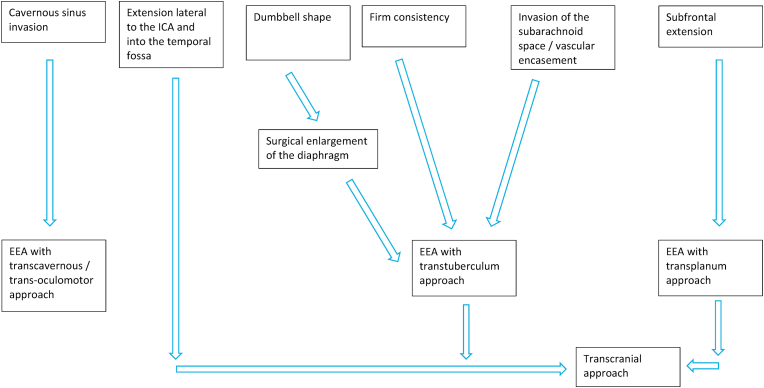
All these factors should be considered together in a preoperative algorithm (Fig. 10) and the main goal is to perform the safest maximal resection to avoid postoperative complications, especially apoplexy of the residual tumor.
-
•
We recommend the endoscopic endonasal approach as the first option when dealing with GPitNETs. Extended approaches can be performed according to the extension, morphology and expected consistency of the lesion. (Level C)
Fig. 10.
An algorithm for the surgical management of G-PitNETs is here proposed. The factors to consider to choose the most appropriate surgical approach are.
4.3.2. What are the indications for transcranial approaches in GPitNETs?
Since the introduction of endoscopy, increased experience and improvement in techniques has resulted in a more widespread application of extended endoscopic endonasal approaches, even for 3rd ventricular and subfrontal extensions of pituitary tumors, limiting the use of transcranial approaches to specific cases. In some distinct and well-selected cases however, transcranial techniques remain valid and they may be used as isolated approaches or in combination with transsphenoidal approaches (Han et al., 2017; Graillon et al., 2020). Flexibility in the choice of the best surgical approach is fundamental and the morphology and extension of the tumor, its invasiveness and its consistency should be considered, as well as the possibility of achieving a surgical cure (Goel et al., 2004; Mortini et al., 2007; de Paiva Neto et al., 2010).
Transcranial approaches at present represent an option in 0.5–4% of cases of pituitary adenomas (Graillon et al., 2020; Youssef et al., 2005).
After a consensus among members of the task force, the indications for transcranial approaches for the treatment of GPitNETs are:
-
-
Tumors with multicompartimental extensions, possibly with invasion of the subarachnoid space and encircling of the arteries of the polygon of Willis
-
-
Tumors encircling the optic nerve or the oculomotor nerve in its cisternal portion
-
-
Tumors extending laterally to the supraclinoid ICA and invading the temporal fossa
-
-
Tumors with a large subfrontal extension
Tumors extending lateral to the ICA may be addressed with an EEA in some cases in experienced hands if the tumor has expanded the oculomotor triangle which is the common pathway for these tumors to achieve this lateral extension (Ferrareze Nunes et al., 2018).
A combined endonasal and transcranial approach may be proposed in well-selected cases to combine the strengths of the two approaches. We advise an endonasal approach to address the majority of the lesion, depending on the consistency of the lesion, while the transcranial approach would address the portion of the tumor not accessible through an extended endoscopic endonasal route. Tumor extension lateral to the cavernous ICA and into the basal cisterns with encasement of neuro-vascular structures can be removed through transcranial skull base approaches (trans-cavernous if needed) that should be tailored according to the extension of the tumor.
The discussion remains open about performing the combined approach during the same sitting or in a staged fashion. Some surgeons prefer a two-staged strategy, where the second procedure is performed weeks or months after the first stage to reduce operative time (Mortini et al., 2007). Others prefer to perform the two stages during one single surgery (D'Ambrosio et al., 2009; Alleyne et al., 2002; Leung et al., 2011; Leung et al., 2012) to reduce the risk of postoperative apoplexy between the two stages, thereby avoiding visual deterioration and/or acute hydrocephalus (Sinha and Sharma, 2010; Mortini et al., 2007; Alleyne et al., 2002; Leung et al., 2011, 2012; Zada et al., 2011). However, a simultaneous approach is associated with a longer procedure and possibly a higher infection rate. In general, care should be taken to minimize significant residual in contact with the optic apparatus, as the risk of symptomatic apoplexy in this setting is greatest.
-
•
We recommend the use of transcranial approaches in combination with endonasal approaches in selected cases, namely with GPitNETs with a multicompartmental morphology, subarachnoid invasion with arterial and/or nervous encasement and extension lateral to the ICA into the temporal fossa. (Level C)
4.3.3. How should postoperative pituitary apoplexy in GPitNETs be managed?
The rate of symptomatic postoperative apoplexy after a first incomplete resection remains low even in series of giant lesions and according to our pooled analysis it was reported in <4% of cases. After a transsphenoidal approach, if the surgeon suspects a large residual tumor, an early clinical evaluation with visual assessment is fundamental for a proper management. If a new visual deficit is suspected, urgent pituitary MRI or a high-definition contrasted CT (if the MRI is not available) should be performed to exclude an apoplexy of the residual tumor.
When this rare eventuality occurs, different therapeutic options can be proposed. The most important factor to consider is the timing of the second surgery as the visual deterioration may be sudden and severe and an emergency surgery for decompression of the visual pathways can be crucial for recovery.
Transcranial approaches can be performed to remove the residual tumor and perform a decompression of the visual pathway. Firm tumor consistency encountered at the first endonasal surgery precluding a complete removal represents a good indication for transcranial surgery (Mortini et al., 2007; Elshazly et al., 2018; Han et al., 2017).
A second standard transsphenoidal approach after a first unsuccessful attempt because of unfavorable conditions, might not be fruitful. In such cases, an extended endoscopic endonasal approaches associated with an extracapsular resection of the residual tumor is more likely to give satisfying results.
-
•
We suggest the use of transcranial techniques in the management of postoperative residual tumor apoplexy for giant tumors after endonasal approaches in the aforementioned craniotomy preferred locations, especially for tumors with a firm consistency. In tertiary referral centers with an endoscopic expertise, an extended endoscopic approach may be performed with an extracapsular resection. (Level C)
5. Summary of recommendations
-
•
We recommend defining GPitNETs as tumors with a maximal diameter > 40 mm on cerebral MRI or on high-definition contrasted CT when the patient present a contraindication to perform an MRI. (Level C)
-
•
We recommend the treatment of G-PitNETs tumors with a more complex and multilobular structure extending in several directions in tertiary care centers as they are surgically challenging compared to the rounded suprasellar G-PiNETs. (Level C)
-
•
We recommend preoperative endocrine and ophthalmological evaluations to establish the hormonal and the visual status of patients before surgery. (Level C)
-
•
We recommend the endoscopic endonasal approach as the first option when dealing with GPitNETs. Extended approaches can be performed according to the extension, morphology and expected consistency of the lesion. (Level C)
-
•
We recommend the use of transcranial approaches in combination with endonasal approaches in selected cases, namely with GPitNETs with a multicompartmental morphology, subarachnoid invasion with arterial and/or nervous encasement and extension lateral to the ICA into the temporal fossa.
-
•
We suggest the use of transcranial techniques in the management of postoperative residual tumor apoplexy for giant tumors after endonasal approaches in the aforementioned craniotomy preferred locations, especially for tumors with a firm consistency. In tertiary referral centers with an endoscopic expertise, an extended endoscopic approach may be performed with an extracapsular resection.
The main findings of the pertinent articles included in our meta-analysis are here summarized.
-
-
the shape of the tumor and the opening of the diaphragm
-
-
the extension of the tumor into the cavernous sinus or subfrontally
-
-
the invasion of the subarachnoid space with encasement of neurovascular structures
-
-
the consistency, as predicted on the preoperative MRI.
Standard and extended endoscopic endonasal approaches may be used with different nuances to address the different compartments of the tumor and also transcranial approaches keep a role in selected cases.
Conflict of interest
None.
References
- Alleyne C.H., Jr., Barrow D.L., Oyesiku N.M. Combined transsphenoidal and pterional craniotomy approach to giant pituitary tumors. Surg. Neurol. 2002;57(6):380–390. doi: 10.1016/s0090-3019(02)00705-x. discussion 390. [DOI] [PubMed] [Google Scholar]
- Almutairi R.D., Muskens I.S., Cote D.J., Dijkman M.D., Kavouridis V.K., Crocker E., Ghazawi K., Broekman M.L.D., Smith T.R., Mekary R.A., et al. Gross total resection of pituitary adenomas after endoscopic vs. microscopic transsphenoidal surgery: a meta-analysis. Acta Neurochir (Wien) 2018;160(5):1005–1021. doi: 10.1007/s00701-017-3438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins D., Best D., Briss P.A., Eccles M., Falck-Ytter Y., Flottorp S., Guyatt G.H., Harbour R.T., Haugh M.C., Henry D., et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab W.A., Nasim K., Abdelnabi E.A., Yousef W., Najibullah M., Khan T., Zaidan S.N., Bokeris A.A., Mostafa K.H., Geng D. Endoscopic endonasal excision of large and giant pituitary adenomas: radiological and intraoperative correlates of the extent of resection. World Neurosurg. 2019;126:e793–e802. doi: 10.1016/j.wneu.2019.02.151. [DOI] [PubMed] [Google Scholar]
- Cappabianca P., Cavallo L.M., de Divitiis O., de Angelis M., Chiaramonte C., Solari D. Endoscopic endonasal extended approaches for the management of large pituitary adenomas. Neurosurg. Clin. 2015;26(3):323–331. doi: 10.1016/j.nec.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Cohen-Cohen S., Gardner P.A., Alves-Belo J.T., Truong H.Q., Snyderman C.H., Wang E.W., Fernandez-Miranda J.C. The medial wall of the cavernous sinus. Part 2: selective medial wall resection in 50 pituitary adenoma patients. J. Neurosurg. 2018;131(1):131–140. doi: 10.3171/2018.5.JNS18595. [DOI] [PubMed] [Google Scholar]
- Cossu G., Daniel R.T., Pierzchala K., Berhouma M., Pitteloud N., Lamine F., Colao A., Messerer M. Thyrotropin-secreting pituitary adenomas: a systematic review and meta-analysis of postoperative outcomes and management. Pituitary. 2019;22(1):79–88. doi: 10.1007/s11102-018-0921-3. [DOI] [PubMed] [Google Scholar]
- Cusimano M.D., Kan P., Nassiri F., Anderson J., Goguen J., Vanek I., Smyth H.S., Fenton R., Muller P.J., Kovacs K. Outcomes of surgically treated giant pituitary tumours. Can. J. Neurol. Sci. 2012;39(4):446–457. doi: 10.1017/s0317167100013950. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio A.L., Syed O.N., Grobelny B.T., Freda P.U., Wardlaw S., Bruce J.N. Simultaneous above and below approach to giant pituitary adenomas: surgical strategies and long-term follow-up. Pituitary. 2009;12(3):217–225. doi: 10.1007/s11102-009-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva Neto M.A., Vandergrift A., Fatemi N., Gorgulho A.A., Desalles A.A., Cohan P., Wang C., Swerdloff R., Kelly D.F. Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clin. Endocrinol. 2010;72(4):512–519. doi: 10.1111/j.1365-2265.2009.03665.x. [DOI] [PubMed] [Google Scholar]
- Dhandapani S., Singh H., Negm H.M., Cohen S., Anand V.K., Schwartz T.H. Cavernous sinus invasion in pituitary adenomas: systematic review and pooled data meta-analysis of radiologic criteria and comparison of endoscopic and microscopic surgery. World Neurosurg. 2016;96:36–46. doi: 10.1016/j.wneu.2016.08.088. [DOI] [PubMed] [Google Scholar]
- Elshazly K., Kshettry V.R., Farrell C.J., Nyquist G., Rosen M., Evans J.J. Clinical outcomes after endoscopic endonasal resection of giant pituitary adenomas. World Neurosurg. 2018;114:e447–e456. doi: 10.1016/j.wneu.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Esquenazi Y., Essayed W.I., Singh H., Mauer E., Ahmed M., Christos P.J., Schwartz T.H. Endoscopic endonasal versus microscopic transsphenoidal surgery for recurrent and/or residual pituitary adenomas. World Neurosurg. 2017;101:186–195. doi: 10.1016/j.wneu.2017.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Miranda J.C., Zwagerman N.T., Abhinav K., Lieber S., Wang E.W., Snyderman C.H., Gardner P.A. Cavernous sinus compartments from the endoscopic endonasal approach: anatomical considerations and surgical relevance to adenoma surgery. J. Neurosurg. 2018;129(2):430–441. doi: 10.3171/2017.2.JNS162214. [DOI] [PubMed] [Google Scholar]
- Ferrareze Nunes C., Lieber S., Truong H.Q., Zenonos G., Wang E.W., Snyderman C.H., Gardner P.A., Fernandez-Miranda J.C. Endoscopic endonasal transoculomotor triangle approach for adenomas invading the parapeduncular space: surgical anatomy, technical nuances, and case series. J. Neurosurg. 2018:1–11. doi: 10.3171/2017.10.JNS17779. [DOI] [PubMed] [Google Scholar]
- Gao Y., Zhong C., Wang Y., Xu S., Guo Y., Dai C., Zheng Y., Wang Y., Luo Q., Jiang J. Endoscopic versus microscopic transsphenoidal pituitary adenoma surgery: a meta-analysis. World J. Surg. Oncol. 2014;12:94. doi: 10.1186/1477-7819-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibi J., Pomposo I., Villar G., Gaztambide S. Giant pituitary adenomas: clinical characteristics and surgical results. Br. J. Neurosurg. 2002;16(2):133–139. doi: 10.1080/02688690220131723. [DOI] [PubMed] [Google Scholar]
- Goel A., Nadkarni T., Muzumdar D., Desai K., Phalke U., Sharma P. Giant pituitary tumors: a study based on surgical treatment of 118 cases. Surg. Neurol. 2004;61(5):436–445. doi: 10.1016/j.surneu.2003.08.036. ; discussion 445-436. [DOI] [PubMed] [Google Scholar]
- Gondim J.A., Almeida J.P., Albuquerque L.A., Gomes E.F., Schops M. Giant pituitary adenomas: surgical outcomes of 50 cases operated on by the endonasal endoscopic approach. World Neurosurg. 2014;82(1–2):e281–290. doi: 10.1016/j.wneu.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Graillon T., Castinetti F., Fuentes S., Gras R., Brue T., Dufour H. Transcranial approach in giant pituitary adenomas: results and outcome in a modern series. J. Neurosurg. Sci. 2020;64(1):25–36. doi: 10.23736/S0390-5616.16.03889-3. [DOI] [PubMed] [Google Scholar]
- Guo F., Song L., Bai J., Zhao P., Sun H., Liu X., Yang B., Wang S. Successful treatment for giant pituitary adenomas through diverse transcranial approaches in a series of 15 consecutive patients. Clin. Neurol. Neurosurg. 2012;114(7):885–890. doi: 10.1016/j.clineuro.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Guo S., Wang Z., Kang X., Xin W., Li X. A meta-analysis of endoscopic vs. Microscopic transsphenoidal surgery for non-functioning and functioning pituitary adenomas: comparisons of efficacy and safety. Front. Neurol. 2021;12:614382. doi: 10.3389/fneur.2021.614382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Gao W., Jing Z., Wang Y., Wu A. How to deal with giant pituitary adenomas: transsphenoidal or transcranial, simultaneous or two-staged? J. Neuro Oncol. 2017;132(2):313–321. doi: 10.1007/s11060-017-2371-6. [DOI] [PubMed] [Google Scholar]
- Hardy J. Transphenoidal microsurgery of the normal and pathological pituitary. Clin. Neurosurg. 1969;16:185–217. doi: 10.1093/neurosurgery/16.cn_suppl_1.185. [DOI] [PubMed] [Google Scholar]
- Iglesias P., Rodriguez Berrocal V., Diez J.J. Giant pituitary adenoma: histological types, clinical features and therapeutic approaches. Endocrine. 2018;61(3):407–421. doi: 10.1007/s12020-018-1645-x. [DOI] [PubMed] [Google Scholar]
- Iglesias P., Arcano K., Trivino V., Guerrero-Perez F., Rodriguez Berrocal V., Vior C., Cordido F., Villabona C., Diez J.J. Giant non-functioning pituitary adenoma: clinical characteristics and therapeutic outcomes. Exp. Clin. Endocrinol. Diabetes. 2021;129(4):309–313. doi: 10.1055/a-1017-3288. [DOI] [PubMed] [Google Scholar]
- Juraschka K., Khan O.H., Godoy B.L., Monsalves E., Kilian A., Krischek B., Ghare A., Vescan A., Gentili F., Zadeh G. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: institutional experience and predictors of extent of resection. J. Neurosurg. 2014;121(1):75–83. doi: 10.3171/2014.3.JNS131679. [DOI] [PubMed] [Google Scholar]
- Komotar R.J., Starke R.M., Raper D.M., Anand V.K., Schwartz T.H. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary. 2012;15(2):150–159. doi: 10.1007/s11102-011-0359-3. [DOI] [PubMed] [Google Scholar]
- Koutourousiou M., Gardner P.A., Fernandez-Miranda J.C., Paluzzi A., Wang E.W., Snyderman C.H. Endoscopic endonasal surgery for giant pituitary adenomas: advantages and limitations. J. Neurosurg. 2013;118(3):621–631. doi: 10.3171/2012.11.JNS121190. [DOI] [PubMed] [Google Scholar]
- Koutourousiou M., Vaz Guimaraes Filho F., Fernandez-Miranda J.C., Wang E.W., Stefko S.T., Snyderman C.H., Gardner P.A. Endoscopic endonasal surgery for tumors of the cavernous sinus: a series of 234 patients. World Neurosurg. 2017;103:713–732. doi: 10.1016/j.wneu.2017.04.096. [DOI] [PubMed] [Google Scholar]
- Kuo C.H., Yen Y.S., Wu J.C., Chang P.Y., Chang H.K., Tu T.H., Huang W.C., Cheng H. Primary endoscopic transnasal transsphenoidal surgery for giant pituitary adenoma. World Neurosurg. 2016;91:121–128. doi: 10.1016/j.wneu.2016.03.092. [DOI] [PubMed] [Google Scholar]
- Landeiro J.A., Fonseca E.O., Monnerat A.L., Taboada G.F., Cabral G.A., Antunes F. Nonfunctioning giant pituitary adenomas: invasiveness and recurrence. Surg. Neurol. Int. 2015;6:179. doi: 10.4103/2152-7806.170536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G.K., Law H.Y., Hung K.N., Fan Y.W., Lui W.M. Combined simultaneous transcranial and transsphenoidal resection of large-to-giant pituitary adenomas. Acta Neurochir (Wien) 2011;153(7):1401–1408. doi: 10.1007/s00701-011-1029-y. ; discussion 1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung G.K., Yuen M.M., Chow W.S., Tse P.Y., Lui W.M. An endoscopic modification of the simultaneous 'above and below' approach to large pituitary adenomas. Pituitary. 2012;15(2):237–241. doi: 10.1007/s11102-011-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigil Sanchez M., Karekezi C., Almeida J.P., Kalyvas A., Castro V., Velasquez C., Gentili F. Management of giant pituitary adenomas: role and outcome of the endoscopic endonasal surgical approach. Neurosurg. Clin. 2019;30(4):433–444. doi: 10.1016/j.nec.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Messerer M., De Battista J.C., Raverot G., Kassis S., Dubourg J., Lapras V., Trouillas J., Perrin G., Jouanneau E. Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg. Focus. 2011;30(4):E11. doi: 10.3171/2011.1.FOCUS10308. [DOI] [PubMed] [Google Scholar]
- Messerer M., Daniel R.T., Cossu G. No doubt: the invasion of the cavernous sinus is the limiting factor for complete resection in pituitary adenomas. Acta Neurochir (Wien) 2019;161(4):717–718. doi: 10.1007/s00701-018-03784-2. [DOI] [PubMed] [Google Scholar]
- Mortini P., Barzaghi R., Losa M., Boari N., Giovanelli M. Surgical treatment of giant pituitary adenomas: strategies and results in a series of 95 consecutive patients. Neurosurgery. 2007;60(6):993–1002. doi: 10.1227/01.NEU.0000255459.14764.BA. ; discussion 1003-1004. [DOI] [PubMed] [Google Scholar]
- Nakao N., Itakura T. Surgical outcome of the endoscopic endonasal approach for non-functioning giant pituitary adenoma. J. Clin. Neurosci. 2011;18(1):71–75. doi: 10.1016/j.jocn.2010.04.049. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Fukuhara N., Horiguchi K., Yamada S. Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: predictive factors, strategies, and outcomes. J. Neurosurg. 2014;121(3):505–510. doi: 10.3171/2014.3.JNS132214. [DOI] [PubMed] [Google Scholar]
- Nishioka H., Hara T., Nagata Y., Fukuhara N., Yamaguchi-Okada M., Yamada S. Inherent tumor characteristics that limit effective and safe resection of giant nonfunctioning pituitary adenomas. World Neurosurg. 2017;106:645–652. doi: 10.1016/j.wneu.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Shimon I., Jallad R.S., Fleseriu M., Yedinak C.G., Greenman Y., Bronstein M.D. Giant GH-secreting pituitary adenomas: management of rare and aggressive pituitary tumors. Eur. J. Endocrinol. 2015;172(6):707–713. doi: 10.1530/EJE-14-1117. [DOI] [PubMed] [Google Scholar]
- Sinha S., Sharma B.S. Giant pituitary adenomas--an enigma revisited. Microsurgical treatment strategies and outcome in a series of 250 patients. Br. J. Neurosurg. 2010;24(1):31–39. doi: 10.3109/02688690903370305. [DOI] [PubMed] [Google Scholar]
- Starnoni D., Daniel R.T., Marino L., Pitteloud N., Levivier M., Messerer M. Surgical treatment of acromegaly according to the 2010 remission criteria: systematic review and meta-analysis. Acta Neurochir (Wien) 2016;158(11):2109–2121. doi: 10.1007/s00701-016-2903-4. [DOI] [PubMed] [Google Scholar]
- Symon L., Jakubowski J., Kendall B. Surgical treatment of giant pituitary adenomas. J. Neurol. Neurosurg. Psychiatry. 1979;42(11):973–982. doi: 10.1136/jnnp.42.11.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Lin S., Wei L., Zhao L., Huang Y. Analysis of operative efficacy for giant pituitary adenoma. BMC Surg. 2014;14:59. doi: 10.1186/1471-2482-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue-Fei S., Yong-Fei W., Shi-Qi L., Jing-Song W., Yao Z., Ying M., Liang-Fu Z. Microsurgical treatment for giant and irregular pituitary adenomas in a series of 54 consecutive patients. Br. J. Neurosurg. 2008;22(5):636–648. doi: 10.1080/02688690802346083. [DOI] [PubMed] [Google Scholar]
- Yang C., Zhang J., Li J., Wu N., Jia D. The role of multimodal navigation in endoscopic endonasal surgery for giant pituitary adenomas. Gland Surg. 2019;8(6):663–673. doi: 10.21037/gs.2019.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S., Hide T., Shinojima N. Efficacy and complications of endoscopic skull base surgery for giant pituitary adenomas. World Neurosurg. 2017;99:533–542. doi: 10.1016/j.wneu.2016.12.068. [DOI] [PubMed] [Google Scholar]
- Youssef A.S., Agazzi S., van Loveren H.R. Transcranial surgery for pituitary adenomas. Neurosurgery. 2005;57(1 Suppl):168–175. doi: 10.1227/01.neu.0000163602.05663.86. ; discussion 168-175. [DOI] [PubMed] [Google Scholar]
- Yu S.Y., Du Q., Yao S.Y., Zhang K.N., Wang J., Zhu Z., Jiang X.B. Outcomes of endoscopic and microscopic transsphenoidal surgery on non-functioning pituitary adenomas: a systematic review and meta-analysis. J. Cell Mol. Med. 2018;22(3):2023–2027. doi: 10.1111/jcmm.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zada G., Woodmansee W.W., Ramkissoon S., Amadio J., Nose V., Laws E.R., Jr. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J. Neurosurg. 2011;114(2):336–344. doi: 10.3171/2010.8.JNS10290. [DOI] [PubMed] [Google Scholar]
- Zoli M., Milanese L., Bonfatti R., Sturiale C., Pasquini E., Frank G., Mazzatenta D. Cavernous sinus invasion by pituitary adenomas: role of endoscopic endonasal surgery. J. Neurosurg. Sci. 2016;60(4):485–494. [PubMed] [Google Scholar]