Abstract
Introduction
Postoperative residual tumor can occur for intentional or unintentional reasons. Decision-making regarding second-look surgery has to weigh molecular biology, probability of total resection and prognostic relevance against potential additional morbidity. In interdisciplinary tumor boards the neurosurgeon has to estimate risk and efficacy of second-look surgery in individual cases, based on precise data.
Research question
Aim of this study was to provide such data by analyzing morbidity and volumetric efficacy of second-look surgery at a designated pediatric neuro-oncology unit.
Material and methods
Children who received second-look surgery in 2007–2018 after incomplete resections were analyzed retrospectively. Measurements were performed on early postoperative magnetic resonance imaging, comparing axial diameter-based measurement as well as computer-assisted volumetric analysis.
Results
59 patients (37% of the overall cohort; 21 female; mean age: 8 ± 5 years) received a subtotal (n = 35) or near total (n = 24) resection. After interdisciplinary case review, 12 of these patients received second-look surgery mainly for residual ependymoma. This led to further tumor volume reduction in all cases (new degrees of resection: subtotal = 2, near total = 6, gross total = 4). No new permanent morbidity or perioperative mortality was observed.
Discussion and conclusion
Second-look surgery did not increase mortality and permanent morbidity, had an 8% rate of transient morbidity and achieved tumor volume reduction above 95% in 75% of selected cases, with 4 additional gross total resections. Second-look surgery is safe and effective with regard to volumetric outcome parameters even in cases with good initial resections, although the role of second-look surgery regarding oncological outcome has to be further investigated in times of personalized molecular medicine.
Keywords: Children, Neuro-oncology, Residual tumor, Volumetric analysis
Highlights
-
•
Second-look surgery after incomplete initial resection of a pediatric brain tumor does not increase mortality and permanent surgical morbidity.
-
•
It achieves a reduction of tumor volume above 95% in 75% of selected cases, with 4 additional gross total resections per 12 patients undergoing second-look surgery.
-
•
Irrespective of two-dimensional or three-dimensional measurement methodology, criteria for near total resection correspond well and consistently showed an extent of resection above 95%.
-
•
In the era of molecular and personalized medicine, children with specific tumors and molecular biology (e.g. PF-EPN-A ependymoma or group 4 medulloblastoma) might be candidates for second-look surgery after interdisciplinary review. This study gives the neurosurgeon accessible information to precisely characterize the neurosurgical implications of second-look surgery in such tumor board discussions.
Abbreviations
- DICOM =
digital imaging and communications in medicine
- GT =
gross total
- IOM =
intraoperative neuromonitoring
- MRI =
magnetic resonance imaging
- NT =
near total
- SHH =
sonic hedgehog
- SIOP =
International Society of Pediatric Oncology
- ST =
subtotal
- VPS =
ventriculoperitoneal shunt
- WHO =
World Health Organization
- WNT =
wingless-related integration site
1. Introduction
Primary brain tumors are the most common solid tumors in childhood and a leading cause of morbidity and mortality (Udaka and Packer, 2018). Surgical resection plays a major role in the treatment regime: Several studies highlighted the impact of gross total (GT) or near total (NT) resection prior to adjuvant therapy for several pediatric tumor entities (Albright et al., 1996; Snider et al., 2018; Packer et al., 1999; Lam et al., 2018). However, in some cases an incomplete resection occurs, with reasons varying from intentional termination of surgery (to preserve eloquent structures and avoid deficits) to unintentional tumor remnants. Especially the second category of tumor remnants can lead to a challenging situation: Risks and benefits of a second-look resection have to be weighed against each other. As published data and guideline recommendations for this decision-making are limited, these cases are usually subject to interdisciplinary discussion and are decided on an individual basis. However, methods of measurements of tumor remnants and classification parameters for the grade of resection differed in previous studies, making comparison and pooling of data difficult. Albright et al. found an improved 5-year progression-free-survival with less than 1,5 cm2 of residual tumor volume in medulloblastoma patients and further publications adopted this value for distinguishing near-total (NT, <1,5 cm2) from subtotal (ST, >1,5 cm2) resections (Albright et al., 1996; Packer et al., 1999; Thompson et al., 2016; Zeltzer et al., 1999). Other publications used a 5 mm tumor margin in the greatest dimension of postoperative MRI or performed a volumetric analysis and set the cut-off level to 1,5 cm3 to delineate NT and ST resections (Merchant et al., 2009; VanPoppel et al., 2011). Gupta et al. defined GT resection as > 90% and ST as <90% resection of the initial tumor mass (Gupta et al., 2017). Defined cut-off values for inclusion of patients in clinical trials or study protocols, as for example the definition of residual tumor with a diameter below 5 mm in any direction as used in the recruiting SIOP Ependymoma II trial (EudraCT No. 2013-002766-39), may also be adopted to guide decision-making and compensate for the lack of evidence-based recommendations in some situations.
Within the past decades, microneurosurgery has been refined and, combined with technological advances (such as modern neuromonitoring), this has led to improved surgical outcomes across several studies and age groups. Similar to the above-mentioned prognostic importance of neurosurgical resections in pediatric neuro-oncology, such results were confirmed in adult brain tumor entities as well (Stummer et al., 2008). There is one caveat though: The survival benefit of a GT resection appears to be diminished if the patient suffers a permanent neurological deficit (Rahman et al., 2017). A careful balance between extensive resection and risk of neurological damage is therefore one of the most important considerations before surgery; especially when a good degree of resection has already been achieved and the consideration is made for a second-look surgery of small remnants. Having encountered such scenarios in our interdisciplinary pediatric tumor board, we aimed to systematically analyze technical feasibility, safety and further tumor size reduction achieved by second-look surgery after ST or NT initial resections in a contemporary series at a designated pediatric neuro-oncology unit.
2. Materials and methods
2.1. Study design
This retrospective study was approved by the Ethics Committee of the Medical Faculty of Heinrich-Heine-University Düsseldorf (Study no. 2018-83-RetroDEuA). The medical records and radiological images of all pediatric patients who received a neurosurgical resection of a primary brain tumor between January 2007 and March 2018 at our institution were reviewed. Inclusion criteria were 1) age under 18 years at time of initial surgery, 2) histologically confirmed primary brain tumor WHO grade I to IV and 3) pre- and postoperative (i.e. obtained within 72 h after surgery) MRI images available for analysis.
We further specified if 1) subtotal (ST; >1,5 cm2 remnant), near-total (NT; <1,5 cm2 remnant) or gross-total (GT; no remnant) resection of the tumor was achieved and if 2) patients with ST or NT resection received second-look surgery. At our institution the decision to offer second-look surgery is made after individual case discussion in a multidisciplinary pediatric tumor board (comprising pediatric oncology, pediatric radiology, radiotherapy and pediatric neurosurgery).
2.2. Surgery
Neurosurgical resection was performed in a standardized setting. All patients received intraoperative neurophysiological monitoring (IOM). Surgery was performed in microsurgical technique and intraoperative neuronavigation (Brainlab Cranial Navigation, Brainlab AG, Munich, Germany) and/or intraoperative ultrasound (ProSound Alpha 6, Hitachi Aloka Medical Ltd, Tokyo, Japan) were used. No patient in this cohort received a fluorescence-guided resection.
2.3. Radiological imaging
Standard MRI imaging was performed at 1.5 to 3 T and comprised T1-weighted sequences before and after administration of a gadolinium-based contrast agent as well as T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences. Slice thickness varied between 1 and 1.5 mm.
2.4. Analysis of tumor and tumor remnants
Analysis of tumor size and tumor remnant size was performed on pre- and postoperative MRI scans, respectively. Data was anonymized and processed in Digital Imaging and Communications in Medicine (DICOM) format. Advanced measurements were performed with a dedicated DICOM processing software (OsiriX, Version 9.5.2; Pixmeo SARL, Berne, Switzerland). T1-weighted sequences after administration of a gadolinium-based contrast agent were used in contrast-enhancing tumors, whereas T2-weighted sequences were primarily used in non-contrast-enhancing lesions. Early postoperative MRI scans were defined as baseline scans to classify tumor remnants. This baseline scan was also used as reference for comparison of any further MRI scans obtained within the interval before second-look surgery; in case of radiological progression before second-look surgery the patient did not qualify for the study. Tumor margins were outlined at each slice manually and the size of the outlined area was measured automatically. Highest value of cm2 on postoperative scans was used to classify ST or NT resection grade. Additionally, volumetric analysis was performed. All slices with regions of interest (ROIs) containing outlined areas were selected and rendered as three-dimensional figures. Volume was measured automatically using the software’s algorithm.
2.5. Data collection and statistical analysis
Epidemiological data, morbidity and mortality, data regarding tumor location and histology as well as pre-, postoperative and follow-up images were collected from the files and electronic records. Descriptive statistics were calculated for all continuous variables (GraphPad Prism for Windows, GraphPad Software, La Jolla, USA).
3. Results
3.1. Patients
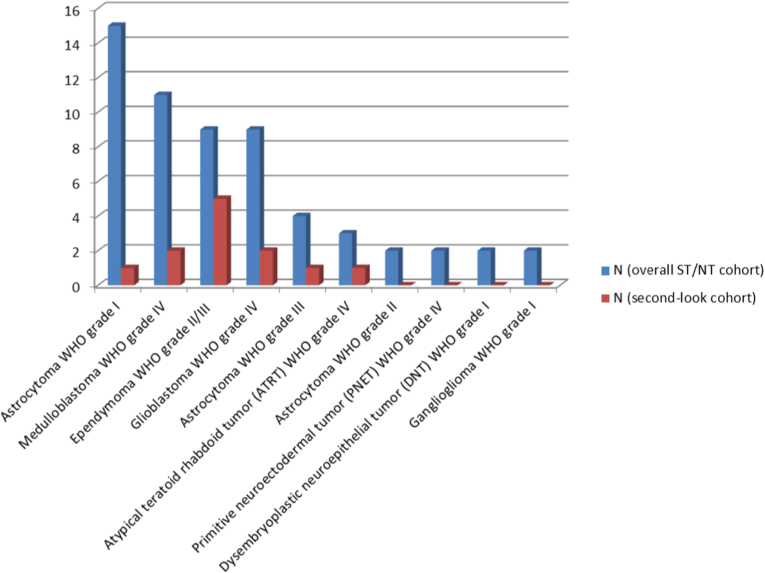
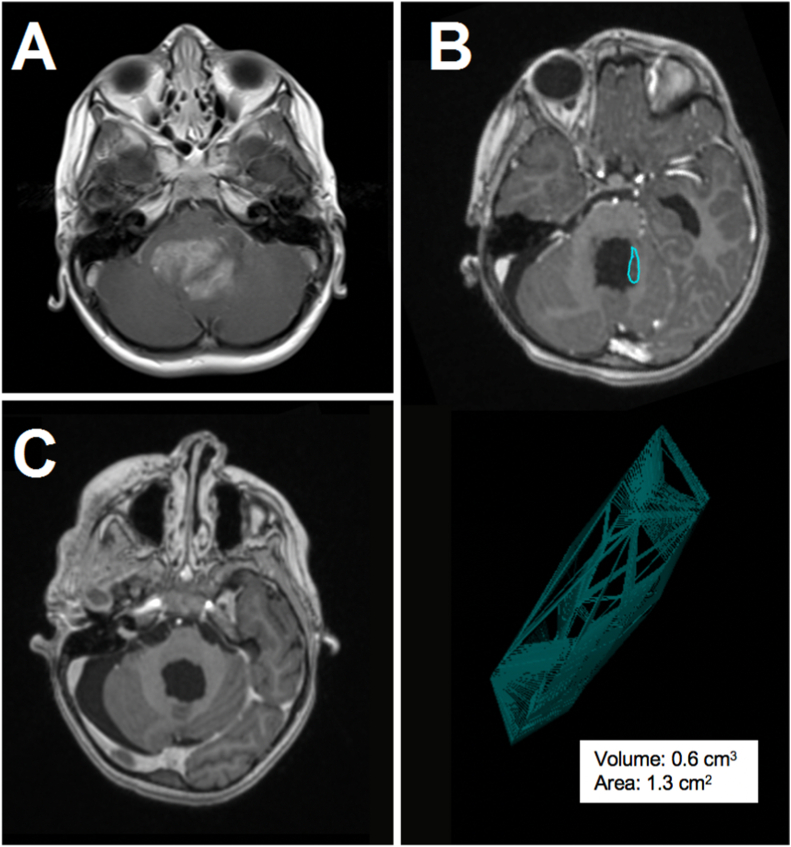
One hundred sixty-one pediatric patients fulfilling the criteria for this study were identified. Of this overall cohort, 59 patients received a NT (n = 24) or ST (n = 35) resection (37%; 21 female and 38 male; mean age: 8 ± 5 years). Histopathological findings obtained after the initial operations are summarized in Fig. 1. Second-look surgery was performed in 12 of these patients after case discussion in the interdisciplinary pediatric tumor board, with individual decisions mainly based on oncological relevance given the histology, volume of tumor remnant as well as neurosurgical accessibility and risk assessment. Histopathological confirmation of a tumor remnant was positive in all second-look cases, with ependymoma being overrepresented in this subgroup (Fig. 1). Clinical data for the second-look surgery cohort is summarized in Table 1. The tumor was located in the posterior fossa in six, the right hemisphere in three and the left hemisphere in three cases. The medium interval between first and second-look surgery was 15 days (range 2–244 days). As per definition, no radiologically dynamic disease burden was observed within the interval between first and second-look surgery. Two patients were treated with radiotherapy and chemotherapy after first surgery. All other patients did not receive adjuvant treatment between primary and second-look resections. A second repeat surgery was performed in only one patient. A typical example of a tumor residual after initial surgery with volumetric assessment and consecutive second-look surgery is presented in Fig. 2.
Fig. 1.
Overview of histological results for the cohort with ST or NT initial resections and the subgroup receiving second-look surgery.
Table 1.
Summary of patient data, VPS = ventriculoperitoneal shunt.
| Patient No. | Gender | Age [y] | Histological diagnosis | WHO grade | Tumor loca-lization | Interval (d) before 2nd surgery | New neuro-logical deficit | VPS |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 13 | Ependymoma | II | central right | 6 | N | N |
| 2 | M | 13 | Ependymoma | II | posterior fossa | 12 | Y | Y |
| 3 | M | 2 | Ependymoma | III | posterior fossa | 87 | N | Y |
| 4 | M | 6 | Ependymoma | III | posterior fossa | 44 | N | N |
| 5 | M | 11 | Ependymoma | III | central left | 4 | N | N |
| 6 | F | 1 | Astrocytoma | I | posterior fossa | 83 | N | Y |
| 7 | M | 16 | Astrocytoma | III | parietal right | 237 | N | N |
| 8 | F | 16 | Glioblastoma | IV | temporal left | 13 | N | N |
| 9 | M | 16 | Glioblastoma | IV | parietal left | 3 | N | N |
| 10 | M | 7 | Medulloblastoma | IV | posterior fossa | 244 | N | N |
| 11 | F | 4 | Medulloblastoma | IV | posterior fossa | 17 | N | N |
| 12 | M | 1 | ATRT | IV | posterior fossa | 2 | N | Y |
Fig. 2.
Exemplary case of a 6-year-old boy with posterior fossa ependymoma WHO grade III. Preoperative contrast-enhanced T1-weighted MRI demonstrates a large fourth ventricular tumor (A). Postoperative MRI reveals unintended residual tumor along the left lateral wall of the fourth ventricle with a volume of 0.6 cm3 (B). Considering the histology and the easily accessible and low-risk location of the residual tumor, a second-look operation was performed and ultimately a GT was achieved (C).
No intraoperative or postoperative mortality occurred. None of the patients showed a new permanent neurological deficit after second-look surgery. In one case, a transient postoperative dysphagia occurred after second-look surgery for residual tumor of a posterior fossa ependymoma, but the deficit was completely resolved after four weeks of logopedic therapy. Reviewing the operation notes, we identified IOM warning criteria in four cases and a vascular conflict in one case as the reasons to stop second-look resections. Within a median follow-up of 546 ± 407 days, four patients developed hydrocephalus and received a ventriculoperitoneal shunt.
3.2. Analysis of tumor and tumor remnants
Measurements obtained in the second-look surgery cohort are summarized in Table 2. Mean tumor volume before initial resection was 55.26 cm3 (range 11.0–127.0 cm3). NT resection was achieved in three and ST resection in nine cases after initial surgery. After second-look surgery MRI confirmed a GT resection in four patients, including the three patients where a NT resection had been performed before. In the remaining nine cases, a tumor volume reduction could be achieved ranging from 73.7% to 99.7%. After second-look surgery, 9 of 12 patients now demonstrated a tumor volume reduction above 95% of the initial tumor mass, corresponding to less than 1.5 cm2 or 1.5 cm3, respectively.
Table 2.
Summary of volumetric analyses and classification of degree of resection, comparing initial tumor, initial resection and second-look resection.
| Initial tumor |
After initial resection |
After second-look resection |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Tumor size [cm3] | Tumor size [cm2] | Tumor residual size [cm3] | Tumor residual size [cm2] | Degree of re-section | Tumor volume reduc-tion [%] | Tumor residual size [cm3] | Tumor residual size [cm2] | Degree of re-section | Tumor volume reduc-tion [%] |
| 1 | 48.7 | 12.0 | 1.5 | 1.5 | NT | 96.9 | 0 | 0 | GT | 100 |
| 2 | 37.0 | 10.0 | 4.6 | 3.0 | ST | 87.6 | 0.9 | 1.0 | NT | 97.6 |
| 3 | 33.8 | 10.8 | 1.6 | 1.6 | ST | 95.3 | 0.1 | 0.7 | NT | 99.7 |
| 4 | 49.3 | 13.4 | 0.6 | 1.3 | NT | 98.8 | 0 | 0 | GT | 100 |
| 5 | 82.4 | 12.9 | 10.3 | 2.2 | ST | 87.5 | 1.2 | 0.9 | NT | 98.5 |
| 6 | 127.0 | 33.7 | 19.4 | 4.6 | ST | 84.7 | 12.1 | 4.9 | ST | 90.4 |
| 7 | 113.1 | 28.0 | 34.7 | 14.2 | ST | 69.3 | 0 | 0 | GT | 100 |
| 8 | 86.9 | 23.7 | 39.0 | 12.0 | ST | 55.1 | 22.8 | 9.6 | ST | 73.7 |
| 9 | 33.5 | 14.1 | 3.0 | 1.7 | ST | 91.0 | 1.1 | 1.1 | NT | 96.7 |
| 10 | 11.0 | 6.2 | 1.9 | 1.7 | ST | 82.7 | 0.1 | 0.7 | NT | 99.1 |
| 11 | 24.9 | 9.3 | 0.4 | 1.0 | NT | 98.4 | 0 | 0 | GT | 100 |
| 12 | 15.5 | 2.4 | 2.0 | 0.6 | ST | 87.1 | 0.4 | 0.6 | NT | 97.4 |
4. Discussion
Although many studies suggested an outcome benefit associated with GT resections of pediatric brain tumors, the risk-benefit assessment and thus the indication for second-look surgery remains controversial in a subgroup of patients with residual tumors, certainly influenced by histology and extent of resection (e.g. additional benefit of GT vs. NT resection) (Albright et al., 1996; Snider et al., 2018; Lam et al., 2018). Molecular features have to be taken into account as well: Thompson et al. could not confirm a benefit with regard to progression-free survival or overall survival for GT resection of medulloblastoma compared to NT resection in general or ST resection in three distinct molecular subgroups (WNT, SHH, group 3) (Thompson et al., 2016). In contrast, GT resection seemed to be superior to ST resection with regard to progression-free survival of group 4 medulloblastoma in this analysis, highlighting the relevance of molecular subtype as a prognostic factor. In contrast, ependymoma patients with GT have better outcome independent of molecular subgroup (Ramaswamy et al., 2016).
Only few publications explicitly addressed the topic of second-look surgery in pediatric brain tumor cohorts so far. Massimino et al. analyzed a group of ependymoma patients (n = 29) undergoing second-look surgery (Massimino et al., 2011). While no additional severe morbidity occurred, rates of local tumor control appeared similar when comparing GT resections achieved by initial versus second-look operations. Moiyadi et al., Kahn et al. and Patel et al. conclude that second-look surgery by experienced pediatric neurosurgeons has an acceptable morbidity rate (Khan et al., 2001; Patel et al., 2019; Moiyadi and Shetty, 2012). Millward et al. expressed the need for a surgical review panel in pediatric posterior fossa ependymoma treatment and highlighted that based on imaging criteria 68% of patients with tumor remnants would have been offered a re-resection (Millward et al., 2016).
In our study, less than 40% of children had residual tumor, similar to the published rates (Massimino et al., 2011; Tomasello et al., 2015; Winkler et al., 2016). Among the patients with incomplete initial resections, a ST resection (64%) was more common than a NT resection (36%). This proportion is also reflected for the 12 patients who received second-look surgery. Higher WHO grade, ependymoma histology and posterior fossa location appeared to be overrepresented in this subgroup. We performed second-look surgery at a median interval of 15 days after initial surgery, similar to previously published data (Patel et al., 2019). In our series no mortality occurred in association with second-look surgery. Perioperative transient morbidity was observed in one case, which corresponds to the lower end of the reported spectrum (Khan et al., 2001; Patel et al., 2019). Reviewing our operation notes, the main reasons for aborting second-look operations appeared to be IOM warning criteria (especially related to cranial nerve monitoring) and vascular conflicts, i.e. adherence of tumor to eloquent structures. In addition, Patel et al. described unintentional reasons for unexpected tumor remnants, such as camouflage of tumor tissue by blood, altered cerebellar parenchyma or hemostatic agents as well as anatomical regions with difficult visual angles for the surgeon, such as the roof and lateral recess of the fourth ventricle (Patel et al., 2019). Such unintentional tumor residuals might be avoided with the use of intraoperative ultrasound, endoscopy assistance, intraoperative MRI and neuronavigation (Sweeney et al., 2018; Tejada et al., 2018).
Analyzing tumor size, a further substantial tumor volume reduction was achieved in all second-look cases. A GT resection was finally achieved in almost half of patients. In the majority of the remaining patients the degree of resection now was between 97% and 99%. Irrespective of two-dimensional or three-dimensional measurement methodology, criteria for NT correspond well and all cases meeting both 2D and 3D criteria for classification as NT consistently showed an extent of resection above 95%. Although the results of different measurement methods did not diverge in our study, estimation of tumor size should nevertheless be standardized to allow consistent, reproducible and comparable findings. There is evidence that volumetric assessment of tumor burden is more accurate (Dempsey et al., 2005; Galanis et al., 2006; Warren et al., 2001). Availability of volumetric analyzing tools and technical advances regarding automatic measurements via auto-segmentation will potentially facilitate routine volumetric assessments in the future.
As we present results of a retrospective single center study, we acknowledge several limitations. Our results are influenced by typical limitations inherent to a retrospective study design. Due to limited case numbers, potential confounders, bias and missing data we cannot comment on oncological outcomes, such as overall or progression free survival. The limited cohort size makes statistically meaningful comparison of different tumor entities or even molecular subgroups impossible and thus only descriptive statistics could be performed. The relatively long study period might introduce some bias due to continuous refinement of surgical techniques and evolving oncological concepts, such as molecular subgroups, hampering comparison and pooling of patients. However, on the other hand this reflects data from a clinical routine setting. To overcome the limitations of single center series, especially regarding limited case numbers, we would like to emphasize the importance of multicenter prospective registries. Such registries provide high quality datasets through structured data collection and can thus answer open questions, such as the impact of molecular subsets and variables such as age and tumor location within the cerebellum with regard to risk-benefit assessment of second look surgery, and ultimately its prognostic relevance.
5. Conclusions
In this retrospective study of a contemporary cohort at a dedicated pediatric neurosurgery unit, second-look surgery after incomplete initial resection of a pediatric brain tumor did not increase mortality and permanent surgical morbidity, had an 8% rate of transient morbidity and achieved reduction of tumor volume above 95% in 75% of selected cases, with 4 additional GT resections. In our experience second-look surgery thus appears to be feasible, safe and effective with regard to volumetric outcome parameters. Nevertheless, the role of repeat surgery regarding oncological outcomes and its meaning within a multimodal therapeutic concept have to be further investigated using adequately powered methodology. Additional factors, such as delay of adjuvant therapy by pursuing second-look surgery, as well as refined subgroups, for example based on molecular information, might be relevant in the risk-benefit assessment of second-look surgery. Conversely improved degree of resection might allow for adapted adjuvant therapies and thus influence long-term risks. Such important treatment decisions should be made in interdisciplinary pediatric tumor boards at specialized centers. Randomized controlled trials or large prospective registries with comparable and transparent measurement and outcome parameters are needed to evaluate the role of second-look surgery in an era of the concepts of evidence-based medicine, molecular medicine and personalized medicine.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interestCOI
None.
References
- Albright a L., Wisoff J.H., Zeltzer P.M., Boyett J.M., Rorke L.B., Stanley P. Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurg. 1996;38(2):265–271. doi: 10.1097/00006123-199602000-00007. http://www.ncbi.nlm.nih.gov/pubmed/8869053 [DOI] [PubMed] [Google Scholar]
- Dempsey M.F., Condon B.R., Hadley D.M. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D? AJNR (Am. J. Neuroradiol.) 2005;26(4):770–776. http://www.ncbi.nlm.nih.gov/pubmed/15814919 [PMC free article] [PubMed] [Google Scholar]
- Galanis E., Buckner J.C., Maurer M.J., et al. Validation of neuroradiologic response assessment in gliomas: measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol. 2006;8(2):156–165. doi: 10.1215/15228517-2005-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Mallick S., Benson R., Haresh K.P., Julka P.K., Rath G.K. Extent of surgical resection and adjuvant temozolomide improves survival in pediatric GBM: a single center experience. Child’s Nerv.Syst. 2017;33(6):951–956. doi: 10.1007/s00381-017-3381-6. [DOI] [PubMed] [Google Scholar]
- Khan R.B., Sanford R.A., Kun L.E., Thompson S.J. Morbidity of second-look surgery in pediatric central nervous system tumors. Pediatr. Neurosurg. 2001;35(5):225–229. doi: 10.1159/000050426. [DOI] [PubMed] [Google Scholar]
- Lam S., Lin Y., Zinn P., Su J., Pan I.-W. Patient and treatment factors associated with survival among pediatric glioblastoma patients: a Surveillance, Epidemiology, and End Results study. J. Clin. Neurosci. 2018;47:285–293. doi: 10.1016/j.jocn.2017.10.041. [DOI] [PubMed] [Google Scholar]
- Massimino M., Solero C.L., Garrè M.L., et al. Second-look surgery for ependymoma: the Italian experience. J. Neurosurg. Pediatr. 2011;8(3):246–250. doi: 10.3171/2011.6.PEDS1142. [DOI] [PubMed] [Google Scholar]
- Merchant T.E., Li C., Xiong X., Kun L.E., Boop F.A., Sanford R.A. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. doi: 10.1016/S1470-2045(08)70342-5. http://www.ncbi.nlm.nih.gov/pubmed/19274783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward C.P., Mallucci C., Jaspan T., et al. Assessing “second-look” tumour resectability in childhood posterior fossa ependymoma-a centralised review panel and staging tool for future studies. Child’s Nerv.Syst. 2016;32(11):2189–2196. doi: 10.1007/s00381-016-3225-9. [DOI] [PubMed] [Google Scholar]
- Moiyadi A.V., Shetty P. Feasibility of repeat surgery for pediatric brain tumors: an objective assessment of perioperative outcomes. J. Neurosurg. Pediatr. 2012;10(5):411–417. doi: 10.3171/2012.8.PEDS12133. [DOI] [PubMed] [Google Scholar]
- Packer R.J., Goldwein J., Nicholson H.S., et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group Study. J. Clin. Oncol. 1999;17(7):2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- Patel P., Wallace D., Boop F.A., et al. Reoperation for medulloblastoma prior to adjuvant therapy. Neurosurg. 2019;84(5):1050–1058. doi: 10.1093/neuros/nyy095. [DOI] [PubMed] [Google Scholar]
- Rahman M., Abbatematteo J., De Leo E.K., et al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J. Neurosurg. 2017;127(1):123–131. doi: 10.3171/2016.7.JNS16396. [DOI] [PubMed] [Google Scholar]
- Ramaswamy V., Hielscher T., Mack S.C., et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: a retrospective multicohort analysis. J. Clin. Oncol. 2016;34(21):2468–2477. doi: 10.1200/JCO.2015.65.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider C.A., Yang K., Mack S.C., et al. Impact of radiation therapy and extent of resection for ependymoma in young children: a population-based study. Pediatr. Blood Cancer. 2018;65(3) doi: 10.1002/pbc.26880. [DOI] [PubMed] [Google Scholar]
- Stummer W., Reulen H.-J., Meinel T., et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurg. 2008;62(3):564–576. doi: 10.1227/01.neu.0000317304.31579.17. 76. [DOI] [PubMed] [Google Scholar]
- Sweeney J.F., Smith H., Taplin A., Perloff E., Adamo M.A. Efficacy of intraoperative ultrasonography in neurosurgical tumor resection. J. Neurosurg. Pediatr. 2018;21(5):504–510. doi: 10.3171/2017.11.PEDS17473. [DOI] [PubMed] [Google Scholar]
- Tejada S., Avula S., Pettorini B., Henningan D., Abernethy L., Mallucci C. The impact of intraoperative magnetic resonance in routine pediatric neurosurgical practice-a 6-year appraisal. Child’s Nerv.Syst. 2018;34(4):617–626. doi: 10.1007/s00381-018-3751-8. [DOI] [PubMed] [Google Scholar]
- Thompson E.M., Hielscher T., Bouffet E., et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17(4):484–495. doi: 10.1016/S1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello F., Conti A., Cardali S., La Torre D., Angileri F.F. Telovelar approach to fourth ventricle tumors: highlights and limitations. World Neurosurg. 2015;83(6):1141–1147. doi: 10.1016/j.wneu.2015.01.039. [DOI] [PubMed] [Google Scholar]
- Udaka Y.T., Packer R.J. Pediatric brain tumors. Neurol. Clin. 2018;36(3):533–556. doi: 10.1016/j.ncl.2018.04.009. [DOI] [PubMed] [Google Scholar]
- VanPoppel M., Klimo P., Dewire M., et al. Resection of infantile brain tumors after neoadjuvant chemotherapy: the St. Jude experience. Clinical article. J. Neurosurg. Pediatr. 2011;8(3):251–256. doi: 10.3171/2011.6.PEDS11158. [DOI] [PubMed] [Google Scholar]
- Warren K.E., Patronas N., Aikin A.A., Albert P.S., Balis F.M. Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J. Natl. Cancer Inst. 2001;93(18):1401–1405. doi: 10.1093/jnci/93.18.1401. [DOI] [PubMed] [Google Scholar]
- Winkler E.A., Birk H., Safaee M., et al. Surgical resection of fourth ventricular ependymomas: case series and technical nuances. J. Neuro Oncol. 2016;130(2):341–349. doi: 10.1007/s11060-016-2198-6. [DOI] [PubMed] [Google Scholar]
- Zeltzer P.M., Boyett J.M., Finlay J.L., et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J. Clin. Oncol. 1999;17(3):832–845. doi: 10.1200/JCO.1999.17.3.832. http://www.ncbi.nlm.nih.gov/pubmed/10071274 [DOI] [PubMed] [Google Scholar]