Abstract
Introduction
Neurocognitive problems associated with posttraumatic stress disorder (PTSD) can interact with impairment resulting from traumatic brain injury (TBI).
Research question
We aimed to identify neurocognitive problems associated with probable PTSD following TBI in a civilian sample.
Material and methods
The study is part of the CENTER-TBI project (Collaborative European Neurotrauma Effectiveness Research) that aims to better characterize TBI. For this cross-sectional study, we included patients of all severities aged over 15, and a Glasgow Outcome Score Extended (GOSE) above 3. Participants were assessed at six months post-injury on the PTSD Checklist-5 (PCL-5), the Trail Making Test (TMT), the Rey Auditory Verbal Learning Test (RAVLT) and the Cambridge Neuropsychological Test Automated Battery (CANTAB). Primary analysis was a complete case analysis. Regression analyses were performed to investigate the association between the PCL-5 and cognition.
Results
Of the 1134 participants included in the complete case analysis, 13.5% screened positive for PTSD. Probable PTSD was significantly associated with higher TMT-(B-A) (OR = 1.35, 95% CI: 1.14–1.60, p < .001) and lower RAVLT-delayed recall scores (OR = 0.74, 95% CI: 0.61–0.91, p = .004) after controlling for age, sex, psychiatric history, baseline Glasgow Coma Scale and education.
Discussion and conclusion
Poorer performance on cognitive tests assessing task switching and, to a lesser extent, delayed verbal recall is associated with probable PTSD in civilians who have suffered TBI.
Keywords: Cognition, Head injury, Neuropsychology, Posttraumatic stress disorder, Stress
Highlights
-
•
Six months after traumatic brain injury 13.5% of people screen positive for PTSD.
-
•
Task switching performance and verbal memory are related to probable PTSD.
-
•
PTSD severity is related to processing speed and task switching performance.
1. Introduction
Each year, more than 50 million people worldwide suffer a traumatic brain injury (TBI) (Maas et al., 2017) often resulting in a wide range of cognitive, emotional and physical problems in survivors (Filley and Kelly, 2018). Typical deficits include impaired memory, attention and executive functioning, slowed information processing, behavioural difficulties and psychological distress (Azouvi et al., 2017; Pavlovic et al., 2019; Wang and Li, 2016; Yeates et al., 2017). Individual consequences are the result of many factors including the severity of the TBI, its location, and injury-specific recovery mechanisms (Cristofori and Levin, 2015). In addition to psychological and cognitive symptoms as a consequence of TBI, PTSD may be a contributing factor, and is also associated to cognitive impairment. TBI is an established risk factor for posttraumatic stress disorder (PTSD): PTSD is diagnosed in 14% of TBI cases in the civilian setting within the first year after injury and in 7% after one year (Scholten et al., 2016; Van Praag et al., 2019). According to the Diagnostic and Statistical Manual of Mental Disorders–fifth edition (DSM-5), PTSD is a trauma-stressor-related disorder that can develop following exposure to a traumatic event (Diagnostic and Statistica, 2013). For a PTSD diagnosis, patients need to manifest symptoms in four clusters: intrusion, avoidance, negative alterations in cognitions and mood, and alterations in arousal and reactivity. However, particularly in the cognitive, mood and arousal domains, the symptoms of PTSD show overlap with those characteristic of TBI (Tanev et al., 2014).
People diagnosed with PTSD may suffer from long-term cognitive deficits (Hayes et al., 2012). A meta-analysis identified associations between PTSD and neurocognitive impairment in verbal learning, speed of information processing, attention/working memory and verbal memory with medium effect sizes (Scott et al., 2015). Individuals with PTSD appear to have difficulty in remembering specific details and contextual information but show enhanced memory functioning for threat-related information. A key issue, here, is that they may have difficulty in disengaging attention from negative stimuli (Hayes et al., 2012). In their review, Qureshi et al. (2011) suggest that after trauma, attentional impairment can account for the observed memory problems (Qureshi et al., 2011). Moreover, neurocognitive deficits in individuals with PTSD can be the consequence of the PTSD, but a pretrauma cognitive vulnerability can also be a risk factor for developing PTSD (Scott et al., 2015).
Symptoms typical of PTSD may coincide with or be related to neurocognitive deficits resulting from the TBI as the latter may not only cause neurocognitive impairment (Barman et al., 2016) but also constitutes a risk factor for the development of PTSD (Pavlovic et al., 2019). Despite the overlap in the neurocognitive symptoms observed in PTSD and TBI, differences have been reported. As noted above, the cognitive impairment following mTBI generally resolves within three months after incurring the injury, whilst the cognitive problems associated with PTSD do not (Jacob et al., 2019; Karr et al., 2014). Various studies mainly focusing on mTBI reported greater attentional distraction and less proficient verbal memory, executive functioning (task switching) and verbal fluency in persons with PTSD compared to those with mTBI (Pineau et al., 2014; Shandera-Ochsner et al., 2013). A study of veterans showed a clear association between less severe PTSD symptoms and more proficient visual memory, irrespective of TBI (Vasterling et al., 2018). However, most studies of PTSD following TBI either had relatively small cohorts or assessed veterans retrospectively (Pineau et al., 2014; Shandera-Ochsner et al., 2013; Vasterling et al., 2018). As the nature of trauma sustained in conflict settings is generally not comparable to that incurred in civilian events, a particular need exists for more prospective research on PTSD in civilian TBI populations (Buckley et al., 2000).
The present study aims to delineate neurocognitive correlates of probable PTSD following mild, moderate and severe non-combat-related TBI. As the only cognitive function that was consistently found to be associated with PTSD/TBI in previous studies, we hypothesised that verbal memory performance would be associated with probable PTSD after civilian TBI (Pineau et al., 2014; Shandera-Ochsner et al., 2013). We further explored associations between cognitive functioning and the symptom burden for each of the four PTSD clusters (intrusion, avoidance, cognition and mood, and arousal). In general, we expect that strong cognitive functioning is associated with low PTSD symptoms for each of the clusters (Scott et al., 2015). Based on neurocognitive theories and previous work, we expect associations between intrusion symptoms and attention (Vasterling et al., 1998), avoidance symptoms and verbal learning and memory (Foa and Kozak, 1986) and/or intrusion symptoms and working memory (Brewin et al., 1996).
2. Method
2.1. Study design and participants
The data for the present study was collected within the context of the European CENTER-TBI Core study (Collaborative European Neurotrauma Effectiveness Research: www.center-tbi.eu), a prospective, observational trial that aims to better characterize TBI and identify the most effective clinical TBI management interventions (clinicaltrials.gov NCT02210221), (Maas et al., 2015). Between December 2014 and December 2017, 4509 children and adults with a TBI were recruited from 65 hospitals across 19 countries. To be eligible, candidates had to have a clinical diagnosis of TBI defined by the treating physician, an indication for a CT-scan and needed to have been seen in an affiliated study centre within 24 h of the injury. For the current study, we selected participants aged over 15 years. To exclude individuals unlikely to be able to complete the cognitive assessment, we only included candidates with a 6-month post-TBI score above 3 on the Glasgow Outcome Score Extended (GOSE) (Wilson et al., 1998). Excluded were candidates with a severe pre-existing neurological disorder that would confound test outcomes.
2.2. Procedure
Demographic variables, pre-TBI history and TBI-related data were collected at the time of recruitment. Six months post-injury, candidates completed all self-report questionnaires and cognitive assessments under the supervision of a trained research nurse or neuropsychologist, who were instructed to record test validity issues using test completion codes (Bagiella et al., 2010), and results flagged as invalid were removed. When a visit to the research centre was not possible or candidates declined to take the neuropsychological tests, the self-report questionnaires were sent by post. All efforts were made to obtain responses. The data collected was entered into an electronic case report form, de-identified and stored in a secure database.
2.3. Ethical approval
The CENTER-TBI study (EC grant 602150) has been conducted in accordance with all relevant EU laws if directly applicable or of direct effect, and all relevant laws of the country where the recruiting sites are located, including but not limited to, the relevant privacy and data protection laws and regulations (the “Privacy Law”), the relevant laws and regulations on the use of human materials, and all relevant guidance relating to clinical studies from time to time in force including, but not limited to, the ICH Harmonised Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95) (“IH GCP”) and the World Medical Association Declaration of Helsinki entitled “Ethical Principles for Medical Research Involving Human Subjects”. Informed consent was obtained from all patients recruited in the Core Dataset of CENTER-TBI and documented in the electronic case report form. Ethical approval was obtained for each recruiting site. The list of sites, Ethics Committees, approval numbers and approval dates can be found on the following website: https://www.center-tbi.eu/project/ethical-approval.
2.4. Measures
2.4.1. Posttraumatic stress disorder checklist for DSM-5 (PCL-5)
The PCL-5 is a self-report measure to screen for PTSD, determine PTSD symptom severity, monitor symptom change after treatment or make a provisional diagnosis of PTSD. Although a formal diagnosis requires a more thorough evaluation (Weathers et al., 2013), the checklist includes 20 items reflecting the DSM-5 diagnostic criteria for PTSD. Patients are asked to indicate how much they have been bothered by each problem over the past month on a 5-point Likert scale ranging from 0 to 4. The sum score can range from 0 to 80, with higher scores indicating more pronounced symptoms. We used the four DSM-5 symptom cluster scores to arrive at a probable PTSD diagnosis to ensure that all PTSD symptoms and not just symptoms of depression (cognition and mood) or arousal (arousal cluster) were present. Items with a score of 2 or higher are considered clinically relevant. For a probable diagnosis of PTSD, this needs to apply to at least one item in the intrusion and one item in the avoidance clusters, two or more negative alterations in cognition and mood, and two or more arousal symptoms. The symptom cluster method is a well-established measure with sensitivity scores up from 0.39 to 1.00 and specificity scores from 0.79 to 0.97 (Hoge et al., 2014; McDonald and Calhoun, 2010).
2.4.2. Other outcome instruments
Overall functional outcome was assessed by the Glasgow Outcome Scale-Extended (GOS-E) (Wilson et al., 1998). The GOSE has 8 categories: death, vegetative state, severe disability (lower and upper), moderate disability (lower and upper), and good recovery (lower and upper). A GOSE of less than 8 indicates that recovery is incomplete.
Symptoms of anxiety and depression were measured with respectively the Generalised Anxiety Disorder-7 (GAD-7) (Kroenke et al., 2007) and the Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al., 2001). The GAD-7 consists of 7 symptoms of anxiety that are rated on a four-point scale. Higher scores indicate more emotional distress. The clinical cut-off is a score of 8 or more. The PHQ-9 includes 9 symptoms of depression that are rated on a four-point scale. Higher scores indicate greater emotional distress. The clinical cut-off is a score of 10 or more.
Postconcussion symptoms were assessed with the Rivermead Post-concussion symptom Questionnaire (RPQ) (King et al., 1995). The RPQ consists of 16 symptoms typically reported after concussion that are rated on a five-point scale. Higher scores indicate more severe symptoms. Scores equal to or greater than 16 were considered indicative of significant post-concussion symptoms (Thompson et al., 2016).
2.5.3. Cognitive assessment battery
The test battery comprised the Trail Making Test (TMT) (Periáñez et al., 2007; Reitan, 1992), the Rey Auditory Verbal Learning Test (RAVLT) (Callahan and Johnstone, 1994; Rey, 1994; Schmidt, 1996), and the Cambridge Neuropsychological Test Automated Battery (CANTAB) (CANTAB Cambridge Cognition, 2014; Schulz-Heik et al., 2020; Stenberg et al., 2020). The TMT is a two-part test that assesses information processing, attentional functioning and task switching/cognitive flexibility and the RAVLT assesses verbal learning and memory. The CANTAB is a computerised neuropsychological battery examining a range of domains including attention, memory and executive functioning. Using mainly nonverbal stimuli, the test is language- and culture-independent. We included the following subtests: the reaction time task (RTI), the attention switching task (AST), the spatial working memory task (SWM), the paired associate learning task (PAL), the rapid visual information processing task (RVP) and the stockings of Cambridge task (SOC). Appendix A provides an overview of the cognitive outcomes, which neuropsychological functions they reflect and short descriptions of the tests.
2.5. Statistical analysis
We used SPSS version 27 for our analyses (IBM Corporation Released, 2017). The CENTER-TBI data (version 2.1) was accessed using the bespoke data management tool Neurobot (https://neurobot.incf.org/).
To identify the neurocognitive test outcomes most strongly related to PTSD following TBI, we used multiple logistic regression, with the probable PTSD diagnosis as the binary dependent variable. Age, sex, educational level, history of psychiatric disorders and baseline Glasgow coma Scale (GCS) score (Teasdale and Jennett, 1974) were entered as demographic and TBI-related covariates. Multiple imputation with chained equations was used to address missing data for these covariates, assuming the data was missing at random (educational level: n = 92, GCS: n = 30 and psychiatric history: n = 8). Covariates were selected from the following tests; TMT-A, TMT-(B-A), RAVLT-immediate recall, RAVLT-interference recall, RAVLT-delayed recall, CANTAB RTI, AST, PAL, SOC, SWM and RVP. For comparability across tests, outcomes were converted to z-scores based on the sample descriptive statistics. We explored interaction effects of TBI severity and cognitive test scores on the PCL-5 diagnosis of PTSD. The primary analysis was a complete case analysis for the outcome of interest. As a sensitivity analysis, we repeated our main analysis with the PCL-5 total score as the dependent variable in a linear regression.
Linear regression models were used to study the association between cognitive test outcomes and the four PCL-5 clusters (symptoms indicative of intrusion and/or avoidance, negative alterations in cognition and mood, and alterations in arousal).
For all linear and logistic models, model selection was based on covariate significance (p < .2) and adjusted R2. The selection procedure only included the cognitive variables after controlling for demographic and injury-related variables. Multicollinearity was checked by means of the variance inflation factor (VIF). Models with an issue of multicollinearity were not considered (VIF >4), (Miles and Shevlin, 2001). In general, the RAVLT outcome variables were highly correlated and could not be entered simultaneously. Significance was set at p < .01.
3. Results
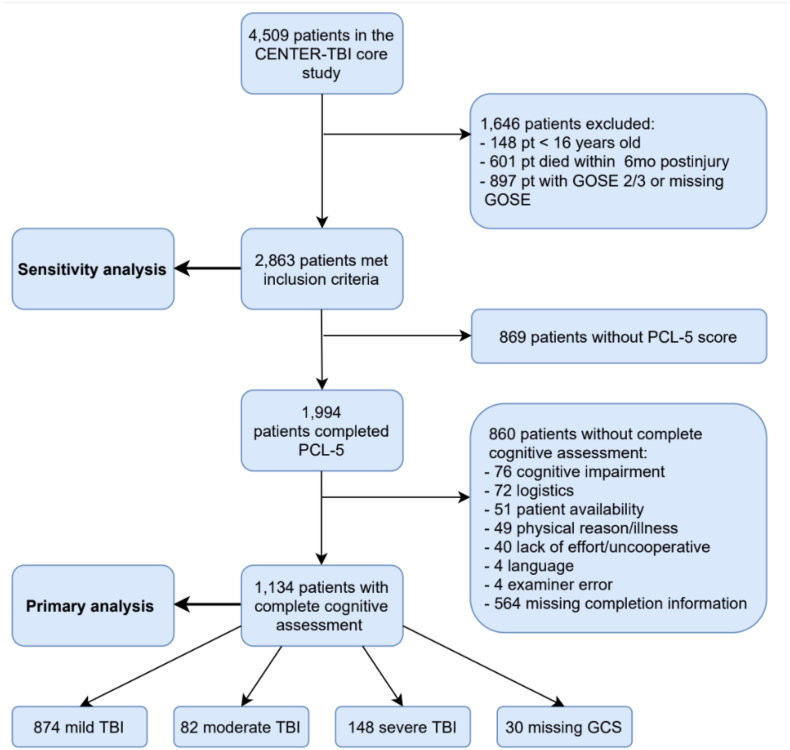
Of the 4509 participants in the CENTER-TBI study, 2863 met the inclusion criteria for the present study. Of these, 1134 (39.6%) completed the PCL-5 and all cognitive tests, and were included in the complete case analysis (Figure 1) (Steyerberg et al., 2019). Most had suffered mild TBI (77.1%), with 7.2% and 13.1% having sustained moderate and severe TBI, respectively, 2.6% had a missing GCS.
Fig. 1.
Flowchart of patient inclusion and exclusion.
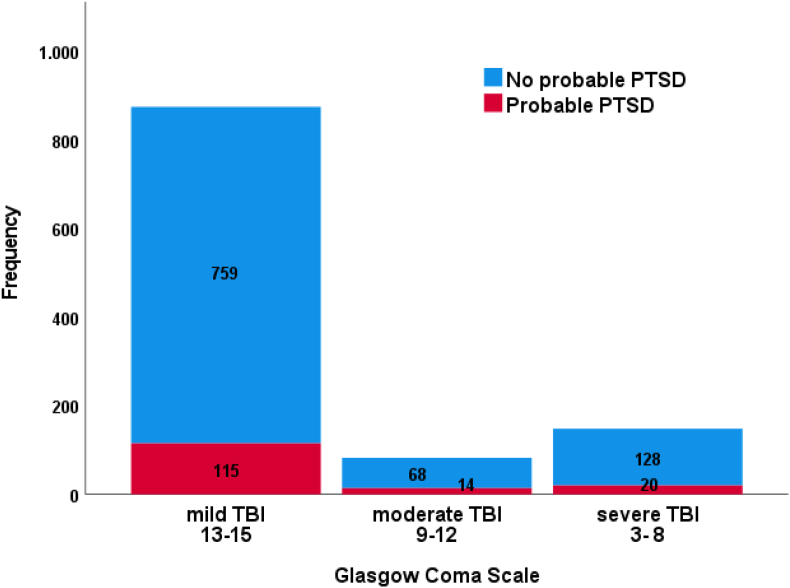
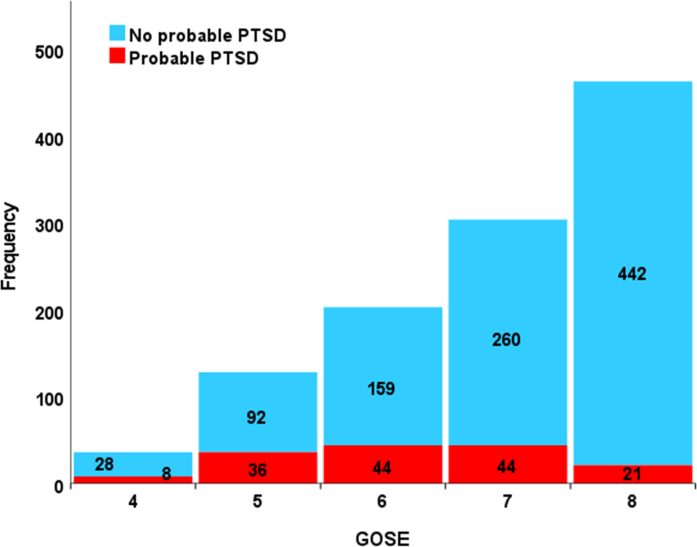
A total of 153 participants screened positive for PTSD (13.5%) on the PCL-5. Table 1 summarises the descriptive statistics of the study cohort differentiated for probable PTSD. The occurrence of PTSD differentiated for initial severity as defined by the GCS is presented in Appendix B, Figure 1. Probable PTSD occurred more frequently in patients with moderate TBI (17.1%) compared to those with mild (13.2%) or severe TBI (13.5%). Overall, the participants with suspected PTSD were younger, had lower levels of education, more frequently reported a history of psychiatric disorders and had more often been injured in road traffic accidents or by violence. Six months post-TBI, two thirds of the study cohort had a GOSE score of 7 or 8, with 14.5% and 4.5%, respectively screening positive for PTSD. The participants with GOSE scores of 4, 5 and 6, were more likely to have PTSD (28.6%, 28.1% and 21.7%, respectively) (see Appendix B, Figure 2).
Table 1.
Participant characteristics.
|
Demographic characteristics at baselineb |
Probable PTSDa N = 153 |
No probable PTSD N = 981 |
p-value |
|---|---|---|---|
| Age in years, Median [IQR]c | 43 [28–55] | 49 [30–61] | .009 |
| Male, n (%) | 102 (66.7) | 673 (68.6) | .63 |
| Highest educational level, n (%) | .019 | ||
| Primary school or less | 22 (15.6) | 100 (11.1) | |
| Secondary school/High school | 56 (39.7) | 273 (30.3) | |
| Post-high school training | 25 (17.7) | 191 (21.2) | |
| College/University | 38 (27.0) | 337 (37.4) | |
| Missing | 12 | 80 | |
| Marital status, n (%) | .17 | ||
| Never been married | 49 (32.7) | 304 (32.5) | |
| Married/Living together/common law | 75 (50.0) | 519 (55.4) | |
| Divorced/Separated/Widowed/Other | 26 (17.3) | 113 (12.1) | |
| Missing |
3 |
45 |
|
|
TBI-related characteristics at baselineb | |||
| Glasgow Coma Scale, n (%) | .61 | ||
| Mild TBI | 115 (77.2) | 759 (79.5) | |
| Moderate TBI | 14 (9.4) | 68 (7.1) | |
| Severe TBI | 20 (13.4) | 128 (13.4) | |
| Missing | 4 | 26 | |
| Cause of injury, n (%) | .003 | ||
| Road traffic incident | 74 (49.3) | 435 (45.1) | |
| Incidental fall | 45 (30.0) | 397 (41.1) | |
| Violence/Assault/Act of mass violence | 14 (9.3) | 34 (3.5) | |
| Suicide attempt | 3 (2.0) | 9 (0.9) | |
| Other | 14 (9.3) | 90 (9.3) | |
| Missing | 3 | 16 | |
| Care pathway, n (%) | .058 | ||
| Emergency Room | 25 (16.3) | 244 (24.9) | |
| Admitted to hospital | 60 (39.2) | 366 (37.3) | |
| Intensive care unit | 68 (44.4) | 371 (37.8) | |
| Psychiatric historyb,d | |||
| Psychiatric disorders, n (%) | .001 | ||
| Yes | 29 (19.1) | 95 (9.8) | |
| No | 123 (80.9) | 879 (90.2) | |
| Missing | 1 | 7 | |
| Type of psychiatric disorder, n (%) | |||
| Anxiety | 7 (4.6) | 27 (2.8) | .65 |
| Depression | 17 (11.1) | 51 (5.2) | .64 |
| Substance abuse | 3 (2.0) | 11 (1.1) | .85 |
| Sleep disorder | 3 (2.0) | 15 (1.5) | .47 |
| Schizophrenia | 2 (1.3) | 2 (0.2) | .20 |
| Other |
7 (4.6) |
14 (1.4) |
.24 |
|
Characteristics 6 months post-TBIe | |||
| RPQ total score, Median [IQR] PHQ-9 total score, Median [IQR] |
23.0 [14.0–34.5] 10.5 [6.0–16.8] |
4.0 [0–13.0] 2.0 [1.0–5.0] |
< .001 < .001 |
| GAD-7 total score Median [IQR] | 8.0 [5.0–14.0] | 1.0 [0–4.0] | < .001 |
| Medication, n (%) | .001 | ||
| Yes | 43 (30.9) | 168 (18.7) | |
| No | 96 (69.1) | 732 (81.3) | |
| Missing | 14 | 81 | |
| Type of medication, n (%) | |||
| Psychostimulants | 0 (0.0) | 3 (0.3) | .38 |
| Antidepressants | 13 (8.5) | 40 (4.1) | .39 |
| Antipsychotic agents | 3 (2.0) | 9 (0.9) | .68 |
| Anxiolytics | 9 (5.9) | 17 (1.7) | .054 |
Note.
Diagnosis based on the PCL-5 self-report questionnaire.
At study entry/TBI evaluation upon admission.
The Mann-Whitney Test was conducted for age and Pearson's Chi2 tests for the other variables.
Information about the psychiatric history and type of psychiatric disorder(s) was obtained by interview from the patient and/or carer upon admission.
The Rivermead Postconcussion Questionnaire (RPQ, sum scores from 0 to 64 with higher scores reflecting more severe postconcussive symptoms), the Patient Health Questionnaire-9 (PHQ-9, sum scores from 0 to 27 with higher scores reflecting more severe depressive symptoms) and the Generalised Anxiety Disorder-7 (GAD-7, sum scores from 0 to 21 with higher scores reflecting higher levels of anxiety symptoms).
Compared to the participants with TBI only, participants with probable PTSD scored significantly worse on the TMT-A, TMT-(B-A), RAVLT immediate recall, interference recall, and delayed recall, and the SWM, RVP and RTI subtests from the CANTAB. The cognitive outcome scores (raw and z-scores) for participants with and without probable PTSD are shown in Appendix C.
3.1. Neuropsychological correlates of probable PTSD following TBI
The regression model associating probable PTSD with the results of cognitive tests is shown in Table 2 (Nagelkerke R2 = 0.081). After selection, only TMT-(B-A) and RAVLT-delayed recall were included in the final model in addition to the following fixed covariates: age, sex, educational level, psychiatric history and GCS. Adding other (sub)test scores did not improve the model. None of the interaction effects of GCS and subtest scores were significant. Higher TMT-(B-A) scores and lower RAVLT-delayed recall scores were significantly associated with the PCL-5-based diagnosis of PTSD, as were the fixed covariates age and psychiatric history. Associations with sex, educational level and GCS were not significant in the multivariable analysis.
Table 2.
Logistic regression: covariates associated with probable PTSD - primary analysis.
| Covariate | B (SE(B)) | Odds ratio (95% CI) | p-valueb | VIF (ranges)c |
|---|---|---|---|---|
| Age | -.026 (.006) | .97 (.91–.99) | < .001 | 1.30 |
| Sex (male) | .30 (.20) | 1.34 (.91–1.98) | .14 | 1.07–1.08 |
| Educational levela | 1.16–1.19 | |||
| Primary school or less | .13 (.31) | 1.13 (.62–2.08) | .69 | |
| Secondary school/high school | .23 (.24) | 1.25 (.78–2.00) | .34 | |
| Post-high school training | -.012 (.27) | .99 (.58–1.69) | .97 | |
| Psychiatric historyd | .79 (.24) | 2.20 (1.37–3.53) | .001 | 1.01 |
| GCS | .030 (.026) | 1.03 (.98–1.09) | .25 | 1.09 |
| TMT-(B-A) | .30 (.085) | 1.35 (1.14–1.60) | < .001 | 1.22–1.25 |
| RAVLT-delayed recall | -.30 (.10) | .74 (.61–.91) | .004 | 1.36–1.41 |
Note.
Reference category: college/university.
Significance level p < .01.
VIF = variance inflation factor (range) of the original and 5 imputed datasets.
Information about the psychiatric history and type of psychiatric disorder(s) was obtained by interview from the patient and/or carer upon admission.
Sensitivity analysis with the PCL-5 total score as the dependent variable is shown in Table 3 (Nagelkerke R2 = 0.058). Similar to our main results in Table 2, higher TMT-(B-A) scores and lower RAVLT-delayed recall scores were significantly related to PTSD symptoms. In addition to age and psychiatric history, sex and GCS were also significantly related to PTSD symptoms. The association for educational level was not significant.
Table 3.
Continuous analysis (linear regression) of covariates associated with PTSD symptoms – sensitivity analysis.
| Covariate | B | SE(B) | p-valueb | VIF (ranges)c |
|---|---|---|---|---|
| Age | -.13 | .025 | .003 | 1.30 |
| Sex (male) | 1.99 | .87 | .001 | 1.07–1.08 |
| Educational levela | 1.16–1.19 | |||
| Primary school or less | 1.30 | 1.41 | .022 | |
| Secondary school/high school | 2.01 | 1.02 | .060 | |
| Post-high school training | 1.08 | 1.10 | .026 | |
| Psychiatric historyd | 6.04 | 1.25 | .006 | 1.01 |
| GCS | .019 | .12 | .004 | 1.09 |
| TMT-(B-A) | 2.08 | .43 | .003 | 1.22–1.25 |
| RAVLT-delayed recall | -.69 | .46 | .002 | 1.36–1.41 |
Note.
Reference category: college/university.
Significance level p < .01.
VIF = variance inflation factor (range) of the original and 5 imputed datasets.
Information about the psychiatric history and type of psychiatric disorder(s) was obtained by interview from the patient and/or carer upon admission.
3.2. Neuropsychological correlates of PTSD clusters following TBI
Table 4 lists the results of the linear regression models predicting symptoms of intrusion, avoidance, negative alterations in cognition and mood, and alterations in arousal. The outcomes on the TMT-(B-A), the CANTAB RTI and the CANTAB SWM were significantly associated with the intrusion cluster, while only the TMT-(B-A) scores also showed significant associations with the avoidance cluster. Both the TMT-(B-A) and the RTI were related to both the cognition and mood cluster and the arousal cluster. Correlations between clusters are given in Appendix D.
Table 4.
Linear regression models: cognitive tests associated with the four PTSD symptom clusters – primary analysis.
| Covariates | Intrusion cluster |
Avoidance cluster |
Cognition/Mood cluster |
Arousal cluster |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B(SE) | p-valueb | B(SE) | p-valueb | VIF (range)c | B(SE) | p-valueb | B(SE) | p-valueb | VIF (range)c | |
| Age | -.037 (.007) | < .001 | -.019 (.003) | < .001 | 1.33–1.34 | -.048 (.009) | < .001 | -.036 (.008) | < .001 | 1.15–1.16 |
| Sex (Male) | .21 (.23) | .36 | .10 (.12) | .38 | 1.05 | .42 (.34) | .22 | .78 (.29) | .007 | 1.03–1.04 |
| Educational levela | 1.13–1.15 | 1.11–1.13 | ||||||||
| Primary school or less | .77 (.39) | .049 | .023 (.19) | .90 | -.061 (.55) | .91 | .53 (.47) | .26 | ||
| Secondary school/high school | .71 (.29) | .016 | .22 (.13) | .11 | .39 (.40) | .33 | .59 (.34) | .084 | ||
| Post-high school training | .33 (.30) | .26 | .085 (.15) | .57 | .19 (.44) | .67 | .67 (.38) | .082 | ||
| Psychiatric historyd | 1.19 (.34) | .001 | .53 (.17) | .002 | 1.01 | 2.39 (.49) | < .001 | 1.88 (.42) | < .001 | 1.01 |
| GCS | .081 (.031) | .010 | .026 (.016) | .092 | 1.09–1.10 | -.073 (.045) | .10 | .032 (.039) | .41 | 1.08 |
| TMT-(B-A) | .41 (.12) | .001 | .21 (.061) | .001 | 1.35–1.36 | .46 (.18) | .009 | .53 (.15) | < .001 | 1.28–1.29 |
| CANTAB RTI | .35 (.12) | .002 | .15 (.057) | .010 | 1.19 | .67 (.17) | < .001 | .52 (.14) | <.001 | 1.16–1.17 |
| CANTAB SWM | .38 (.13) | .003 | .15 (.063) | .020 | 1.44–1.47 | |||||
| Nagelkerke R2 | .075 | .057 | .061 | .059 | ||||||
Note.
Reference category: college/university.
Significance level p < .01.
VIF = variance inflation factor (range) of the original and 5 imputed datasets.
Information about the psychiatric history and type of psychiatric disorder(s) was obtained by interview from the patient and/or carer upon admission.
3.3. Sensitivity analysis with imputed data
Of the 2863 participants that met inclusion criteria, 1994 (69.6%) had completed the full PCL-5. The response rate for the cognitive measures ranged from 46.5 to 61.8% (Fig. 1). Appendix E shows the characteristics for the participants in our main analysis with complete sets of cognitive scores (n = 1134) and those with missing scores (n = 1729). Sensitivity analysis of all 2863 study participants were performed with multiple imputation for missing data on demographics, TBI-related features, PCL-5 and cognitive test outcomes. The results show a similar pattern to those of the complete case analysis, with the TMT-(B-A) again being significantly associated with probable PTSD, however the association between the RAVLT-delayed recall was no longer significant (Appendix F Table 1). Similar to the main analysis, the CANTAB-RTI is significantly associated to the intrusion, cognition and mood, and arousal symptoms. For the imputed data, TMT-(B-A) is only significantly related to intrusion symptoms, and the associations between TMT-(B-A) and avoidance, cognition and mood, and arousal, and the association between intrusive symptoms and SWM could not be confirmed (Appendix F Table 2).
4. Discussion
Exploring the neurocognitive correlates of probable PTSD following civilian TBI, we found task switching performance and, to a lesser extent, delayed verbal recall to be associated with probable PTSD after controlling for age, sex, educational level, history of psychiatric disorders and TBI severity. For each of the PTSD clusters, the severity of PTSD symptoms was associated with poorer task switching/cognitive flexibility and lower processing speed. To our knowledge, we are the first to study PTSD in a large cohort of individuals having sustained TBI in the civilian setting while considering head trauma of all severities. The percentage of probable PTSD we obtained, i.e. 13.5%, is consistent with the overall prevalence rate reported in a recent meta-analysis of civilian TBI (15.6%, 95% CI:12.9–18.4), (Van Praag et al., 2019). Rates were higher in patients with upper severe or moderate disability (24.0%) compared to those with a GOSE of 7 (14.5%) or 8 (4.5%).
4.1. Neuropsychological correlates of probable PTSD after TBI
We included the TMT-(B-A) as a measure of task switching/cognitive flexibility and the RAVLT-delayed recall as component of long-term verbal memory since previous studies have shown the importance of executive functioning and verbal memory in differentiating between patients with co-occurring mild TBI and PTSD and patients with mild TBI only (Pineau et al., 2014; Shandera-Ochsner et al., 2013). Pineau et al. (2014) found more pronounced attentional distraction in patients with PTSD than they did in those with mTBI only (Pineau et al., 2014), with additional problems in long-term verbal memory in patients with both TBI and PTSD. A longitudinal study in a military population reported similar results, with impairments in verbal memory coinciding with increasing PTSD severity. Follow-up results showed an additional association between reduced proficiency in visual learning and memory, and PTSD severity (Vasterling et al., 2018). Another study of veterans observed significant differences in executive functioning (cognitive flexibility), verbal fluency and verbal memory between individuals with mTBI and PTSD and those with PTSD without mTBI, compared to veterans with mTBI only and a control group without either condition (Shandera-Ochsner et al., 2013). Extending previous findings to civilians and all TBI severities, the outcomes we obtained with the RAVLT-delayed recall confirm that this component of long-term verbal memory is associated with probable PTSD following TBI irrespective of the severity of the head trauma. The second correlate, cognitive flexibility as assessed with the TMT-(B-A), was even more strongly associated with probable PTSD/TBI compared to TBI only, which is also consistent with previous literature. However, cognitive functioning appears not to be specific for PTSD/mTBI as it was also observed in PTSD-only groups in previous studies (Pineau et al., 2014; Shandera-Ochsner et al., 2013). In addition, we found lower age and history of psychiatric illness to be significantly related to probable PTSD after TBI. A pre-injury history of mental illness may thus point to a vulnerability for PTSD, which is a risk factor that clinicians need to take into account when treating patients having suffered a TBI.
4.2. Neuropsychological correlates of PTSD clusters following TBI
Examining the four PTSD symptom clusters (intrusion, avoidance, cognition and mood, and arousal) we found associations for processing speed and cognitive flexibility, in which higher levels of intrusion correlated with reduced processing speed and cognitive flexibility. Re-experiencing symptoms (e.g. recurring nightmares of the trauma or reliving the trauma) may be an expression of difficulties with directing attentional focus away from trauma-related cues (Vasterling et al., 1998). Our complete case analysis revealed an additional association between visual working memory and the intrusion cluster. This finding is consistent with the idea that memory encoding and consolidation issues play a role in intrusive symptoms (Brewin et al., 1996). However, the sensitivity analysis with imputed data did not confirm the relationship between the CANTAB SWM task and intrusion. Reduced processing speed was highly associated with intrusive, cognition and mood, and arousal symptoms, but not to probable PTSD, a finding which was confirmed by the sensitivity analysis with imputed data. The finding that processing speed relates to PTSD symptoms (Vasterling et al., 2018; Jurick et al., 2021), but not to probable PTSD (Pineau et al., 2014; Shandera-Ochsner et al., 2013), is in line with previous studies. We also found that the cognitive correlates for the PTSD-specific (intrusion, avoidance) and non-specific symptoms (cognition and mood, arousal) are the same (TMT-B-A and RTI), with the strongest relation between speed and non-specific PTSD symptoms. That the same cognitive variables (TMT-B-A and CANTAB-RTI) were associated with the symptom burden in each of the four clusters, may be attributed to the fact that the PTSD cluster scores are highly correlated.
4.3. Limitations
As this is a cross-sectional study, we cannot draw any conclusions about causality. Moreover, although the 20-item PCL-5 self-report questionnaire can be used to screen for PTSD, it is insufficient for a formal diagnosis. However, to include all PTSD symptoms and not just symptoms of depression (cognition and mood cluster) or arousal (arousal cluster), we used the symptom cluster method to ensure that symptoms were present relating to all four DSM-5 cluster criteria, i.e. intrusion, avoidance, cognition and mood, and arousal (Diagnostic and Statistica, 2013).
Since model selection may increase the risk of type-I errors, we used the more stringent significance level of p < .01. Although the correlates we identified were significant, both for the complete case analysis and the sensitivity analysis of the imputed dataset of the full cohort, we recognize that the Nagelkerke R2 was low, indicating that discriminatory performance was limited. The associations between cognitive functions and probable PTSD/PTSD symptoms are significant but effect sizes are small.
Limited information on premorbid functioning precluded us from controlling for potential pretrauma cognitive deficits or for cognitive abilities that may have buffered the effects of traumatic stress (e.g. cognitive control, emotion regulation, adaptive re-appraisal of trauma-related cognitions). In people dealing with PTSD following TBI, we need to be aware of possible response bias due to a lack of effort (Wisdom et al., 2014). Although we did not include a formal performance validity test, the examiners did record apparent low effort and test scores labelled as such were removed from the database. Additionally, rather than entire cognitive profiles, we compared cognitive functions separately while the development of PTSD will depend on the sum of protecting and obstructive cognitive functions. Further, we acknowledge that cognitive tests do not measure single, isolated functions. Cognitive concepts overlap, where an adequate attentional focus, for instance, is a condition for cognitive flexibility. We further recognize that patients screening positive for probable PTSD also had more postconcussion symptoms and symptoms of anxiety and depression. There is overlap between PTSD and postconcussion symptoms (e.g. sleep disturbance, poor memory, irritability), as well with symptoms of depression and anxiety, making accurate attribution complex. We decided not to enter these symptom scales into our regression models to prevent overcontrolling for these symptoms as they are part of the PTSD diagnosis (cluster mood/cognition and arousal). Instead, we performed PTSD cluster analysis which gave more insight in the PTSD-specific symptoms (intrusion, avoidance) and the non-specific symptoms (mood/cognition and arousal) and their relation with cognitive test scores. Finally, we did not control for cognitive-behavioural or psychopharmacological treatments in our analyses.
4.4. Conclusion and future directions
Our study showed that approximately one out of seven adults with TBI screens positive for probable PTSD six months after sustaining the head injury. Performance on tests of cognitive flexibility and, to a lesser extent, delayed verbal recall, are associated with probable PTSD following TBI, regardless of the severity of the injury.
Future research should investigate the impact of cognitive functioning after TBI on the natural course of PTSD symptoms, explore which cognitive strengths or weaknesses influence its course, and investigate the effects of PTSD treatment on attention, cognitive flexibility and verbal memory.
Irrespective of the need for future research, our findings have implications for clinical practice: All clinicians treating patients after TBI should be aware of the relatively high occurrence of PTSD after TBI. Structured follow-up of patients, especially after mild TBI, is often deficient and needs to be improved (Foks et al., 2017; Seabury et al., 2018). Our data suggest that all patients who do not attain full good recovery (GOSE = 8) should be screened for PTSD. The PCL-5, which has now been linguistically validated in many languages (von Steinbuechel et al., 2021), provides a simple and efficient screening tool. Patients screening positive for probable PTSD should be referred for psychiatric or neuropsychological evaluation for diagnostic confirmation, cognitive evaluation and treatment.
Disclosure of interest
The authors report no conflict of interest.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The data used in preparation of this manuscript was obtained in the context of CENTER-TBI, a large collaborative research project funded by the European Union 7th Framework program (EC grant 602150).
Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA) and from Integra LifeSciences Corporation (USA).
Contributor Information
Dominique L.G. Van Praag, Email: Dominique.vanpraag@uza.be.
Kristien Wouters, Email: Kristien.wouters@uza.be.
Filip Van Den Eede, Email: Filip.vandeneede@uza.be.
Lindsay Wilson, Email: L.wilson@stir.ac.uk.
Andrew I.R. Maas, Email: Andrew.maas@uza.be.
the CENTER-TBI investigators and participants:
Cecilia Åkerlund, Krisztina Amrein, Nada Andelic, Lasse Andreassen, Audny Anke, Anna Antoni, Gérard Audibert, Philippe Azouvi, Maria Luisa Azzolini, Ronald Bartels, Pál Barzó, Romuald Beauvais, Ronny Beer, Bo-Michael Bellander, Antonio Belli, Habib Benali, Maurizio Berardino, Luigi Beretta, Morten Blaabjerg, Peter Bragge, Alexandra Brazinova, Vibeke Brinck, Joanne Brooker, Camilla Brorsson, Andras Buki, Monika Bullinger, Manuel Cabeleira, Alessio Caccioppola, Emiliana Calappi, Maria Rosa Calvi, Peter Cameron, Guillermo Carbayo Lozano, Marco Carbonara, Simona Cavallo, Giorgio Chevallard, Arturo Chieregato, Giuseppe Citerio, Iris Ceyisakar, Hans Clusmann, Mark Coburn, Jonathan Coles, Jamie D. Cooper, Marta Correia, Amra Čović, Nicola Curry, Endre Czeiter, Marek Czosnyka, Claire Dahyot-Fizelier, Paul Dark, Helen Dawes, Véronique De Keyser, Vincent Degos, Francesco Della Corte, Hugo den Boogert, Bart Depreitere, Đula Đilvesi, Abhishek Dixit, Emma Donoghue, Jens Dreier, Guy-Loup Dulière, Ari Ercole, Patrick Esser, Erzsébet Ezer, Martin Fabricius, Valery L. Feigin, Kelly Foks, Shirin Frisvold, Alex Furmanov, Pablo Gagliardo, Damien Galanaud, Dashiell Gantner, Guoyi Gao, Pradeep George, Alexandre Ghuysen, Lelde Giga, Ben Glocker, Jagoš Golubovic, Pedro A. Gomez, Johannes Gratz, Benjamin Gravesteijn, Francesca Grossi, Russell L. Gruen, Deepak Gupta, Juanita A. Haagsma, Iain Haitsma, Raimund Helbok, Eirik Helseth, Lindsay Horton, Jilske Huijben, Peter J. Hutchinson, Bram Jacobs, Stefan Jankowski, Mike Jarrett, Ji-yao Jiang, Faye Johnson, Kelly Jones, Mladen Karan, Angelos G. Kolias, Erwin Kompanje, Daniel Kondziella, Evgenios Koraropoulos, Lars-Owe Koskinen, Noémi Kovács, Ana Kowark, Alfonso Lagares, Linda Lanyon, Steven Laureys, Fiona Lecky, Didier Ledoux, Rolf Lefering, Valerie Legrand, Aurelie Lejeune, Leon Levi, Roger Lightfoot, Hester Lingsma, Andrew I.R. Maas, Ana M. Castaño-León, Marc Maegele, Marek Majdan, Alex Manara, Geoffrey Manley, Costanza Martino, Hugues Maréchal, Julia Mattern, Catherine McMahon, Béla Melegh, David Menon, Tomas Menovsky, Ana Mikolic, Benoit Misset, Visakh Muraleedharan, Lynnette Murray, Ancuta Negru, David Nelson, Virginia Newcombe, Daan Nieboer, József Nyirádi, Otesile Olubukola, Matej Oresic, Fabrizio Ortolano, Aarno Palotie, Paul M. Parizel, Jean-François Payen, Natascha Perera, Vincent Perlbarg, Paolo Persona, Wilco Peul, Anna Piippo-Karjalainen, Matti Pirinen, Horia Ples, Suzanne Polinder, Inigo Pomposo, Jussi P. Posti, Louis Puybasset, Andreea Radoi, Arminas Ragauskas, Rahul Raj, Malinka Rambadagalla, Jonathan Rhodes, Sylvia Richardson, Sophie Richter, Samuli Ripatti, Saulius Rocka, Cecilie Roe, Olav Roise, Jonathan Rosand, Jeffrey V. Rosenfeld, Christina Rosenlund, Guy Rosenthal, Rolf Rossaint, Sandra Rossi, Daniel Rueckert, Martin Rusnák, Juan Sahuquillo, Oliver Sakowitz, Renan Sanchez-Porras, Janos Sandor, Nadine Schäfer, Silke Schmidt, Herbert Schoechl, Guus Schoonman, Rico Frederik Schou, Elisabeth Schwendenwein, Charlie Sewalt, Toril Skandsen, Peter Smielewski, Abayomi Sorinola, Emmanuel Stamatakis, Simon Stanworth, Robert Stevens, William Stewart, Ewout W. Steyerberg, Nino Stocchetti, Nina Sundström, Anneliese Synnot, Riikka Takala, Viktória Tamás, Tomas Tamosuitis, Mark Steven Taylor, Braden Te Ao, Olli Tenovuo, Alice Theadom, Matt Thomas, Dick Tibboel, Marjolein Timmers, Christos Tolias, Tony Trapani, Cristina Maria Tudora, Andreas Unterberg, Peter Vajkoczy, Shirley Vallance, Egils Valeinis, Zoltán Vámos, Mathieu van der Jagt, Gregory Van der Steen, Joukje van der Naalt, Jeroen T.J.M. van Dijck, Thomas A. van Essen, Wim Van Hecke, Caroline van Heugten, Dominique Van Praag, Thijs Vande Vyvere, Roel P.J. van Wijk, Alessia Vargiolu, Emmanuel Vega, Kimberley Velt, Jan Verheyden, Paul M. Vespa, Anne Vik, Rimantas Vilcinis, Victor Volovici, Nicole von Steinbüchel, Daphne Voormolen, Petar Vulekovic, Kevin K.W. Wang, Eveline Wiegers, Guy Williams, Lindsay Wilson, Stefan Winzeck, Stefan Wolf, Zhihui Yang, Peter Ylén, Alexander Younsi, Frederick A. Zeiler, Veronika Zelinkova, Agate Ziverte, and Tommaso Zoerle
Appendices.
Appendix A. Cognitive Covariates
| Test | Cognitive domain | Variable |
|---|---|---|
| Trail Making Test (TMT) | ||
| TMT A | Attention and processing speed | TMT A: Connect numbers sequentially as fast as possible |
| TMT B-A | Task switching/Cognitive flexibility | TMT B: Connect numbers and letters alternately as fast as possible (TMT B minus A was calculated for analysis) |
| Rey Auditory Verbal Learning Test (RAVLT) | Repeat as many words as possible of a list of 15 unrelated words read by the assessor | |
| RAVLT Immediate recall | Verbal short-term memory | Sum of the first 5 trials |
| RAVLT Interference recall | Interference | Trial 6 after an interference list |
| RAVLT Delayed recall | Verbal long-term memory | Trial 7 after 20 min |
| Cambridge Neuropsychological Test Automated Battery (CANTAB) | ||
| CANTAB SWM: Spatial working memory | Spatial working memory | Find hidden tokens in displayed boxes. Outcome is the number of times a box is selected in which a token was already presented |
| CANTAB PAL: Paired associate learning | Visual learning and memory | Number of errors, adjusted for the estimated number of errors they would have made on any problems, attempts and unfinished items |
| CANTAB RVP: Rapid visual information processing task | Sustained attention and concentration | Detect specific sequences by pushing a button |
| CANTAB SOC: Stockings of Cambridge task | Spatial planning and problem solving | Number of occasions upon which the participant successfully completed a test problem in the minimum possible number of moves |
| CANTAB RTI: Choice Reaction Time | Processing speed | Median duration between the onset of the stimulus and the time at which button is released |
| CANTAB AST: Attention Switching Task | Attention, task switching | Difference between the median latency of responses between assessments in the block in which the rule was switched vs those in the block in which the rule remained constant. Close to zero indicates less variation in latencies across non-switch and switch trials. |
Appendix B. Probable PTSD Diagnosis Differentiated for GCS and GOSE Rating
Fig. 1.
Probable PTSD diagnosis differentiated for GCS rating.
Fig. 2.
Probable PTSD diagnosis differentiated for GOSE rating
Note: GOSE 4: Upper Severe Disability – needs full assistance in activities of daily living, GOSE 5: Lower Moderate Disability – independent, but cannot resume work/school or all previous social activities, GOSE 6: Upper Moderate Disability – Some disability exists, but can partly resume work or previous activities, GOSE 7: Lower Good Recovery – Minor physical or mental deficits that affects daily life, GOSE 8: Upper Good Recovery – Full recovery or minor symptoms that do not affect daily life
Appendix C. Cognitive outcomes for probable or no PTSD
| Raw Scores |
Z-scores |
||||||
|---|---|---|---|---|---|---|---|
| Probable PTSD (n = 153) |
No probable PTSD (n = 981) |
Probable PTSD (n = 153) |
No probable PTSD (n = 981) |
||||
| Mean (SD) | Min-Max | Mean (SD) | Min-Max | Mean (SD) | Mean (SD) | p-value | |
| TMT-A | 38.45 (18.70) | 13–101 | 34.65 (16.93) | 8–101 | .19 (1.09) | -.03 (.98) | .011 |
| TMT-(B-A) | 62.17 (46.86) | 12–248 | 49.34 (36.95) | -68-241 | -.29 (1.21) | -.04 (.96) | .001 |
| RAVLT Immediate | 42.78 (11.33) | 12–66 | 45.33 (11.31) | 13–72 | -.20 (1.01) | .03 (.99) | .009 |
| RAVLT Interference | 8.75 (3.47) | 1–15 | 9.40 (3.37) | 0–15 | -.17 (1.02) | .03 (.99) | .027 |
| RAVLT Delayed | 8.38 (3.70) | 1–15 | 9.18 (3.55) | 0–15 | -.19 (1.03) | .03 (.99) | .010 |
| CANTAB SWM | 30.90 (21.89) | 0–118 | 27.15 (20.21) | 0–88 | .16 (1.07) | -.02 (.99) | .035 |
| CANTAB PAL | 25.31 (30.26) | 0–134 | 22.98 (29.18) | 0–156 | .07 (1.03) | -.01 (1.00) | .36 |
| CANTAB RVP | .88 (.06) | .66–1.00 | .89 (.06) | .35–1.00 | -.20 (1.00) | .03 (1.00) | .008 |
| CANTAB SOC | 8.01 (2.04) | 3–12 | 8.25 (2.00) | 2–12 | -.10 (1.02) | .02 (1.00) | .18 |
| CANTAB RTI | 407.47 (146.59) | 228.5–1162.5 | 376.03 (92.57) | 218.0–1168.0 | .27 (1.44) | -.04 (.91) | .011 |
| CANTAB AST | 165.21 (166.48) | -99.5-633.5 | 164.02 (172.88) | -270.0-890.0 | .01 (.97) | -.01 (1.01) | .94 |
Participants with complete outcome data (i.e. PCL-5 and all cognitive tests). Independent samples t-tests were conducted to compare outcomes for patients with and without probable PTSD.
Appendix D. Pearson correlations between symptom clusters
| Intrusion | Avoidance | Cognition/mood | Arousal | |
|---|---|---|---|---|
| Intrusion | .74 | .66 | .69 | |
| Avoidance | .62 | .60 | ||
| Cognition/mood | .75 | |||
| Arousal |
Significance level of each of the correlations: p < .001.
Appendix E. Comparison of Patients Characteristics – for Patients with Outcome Data and with Missing Outcome Data (n = 2863)
| Patients with outcome data (complete cases) n = 1134 |
Patients with missing outcome data (added for sensitivity analysis) n = 1729 | p-value | Missing (%) | |
|---|---|---|---|---|
| Age in years, Median (IQR) | 47 [29–60] | 51 [31–66] | < .001 | 0 |
| Male, n (%) | 775 (68.3) | 1109 (64.1) | .020 | 0 |
| Highest educational level, n (%) | 122 (11.7) | 213 (14.5) | < .001 | 12.4 |
| Primary school or less Secondary school/High school | 329 (31.6) | 549 (37.5) | ||
| Post-high school training | 216 (20.7) | 316 (21.6) | ||
| College/University | 375 (36.0) | 387 (26.4) | ||
| GCS, n (%) | 0.78 | 2.9 | ||
| Mild TBI | 874 (79.2) | 1309 (78.1) | ||
| Moderate TBI | 82 (7.4) | 126 (7.5) | ||
| Severe TBI | 148 (13.4) | 240 (14.3) | ||
| Care pathway, n (%) | 0.62 | 0 | ||
| Emergency Room | 269 (23.7) | 398 (23.0) | ||
| Admitted to hospital | 426 (37.6) | 681 (39.4) | ||
| Intensive Care Unit | 439 (38.7) | 650 (37.6) | ||
| History of psychiatric disorders, n (%) | 124 (11.0) | 240 (14.2) | .013 | 1.6 |
Patients with complete outcome data (incl. PCL-5 and all cognitive tests). Mann-Whitney Test for age and Pearson's Chi2 test for other variables were conducted.
Appendix F. Sensitivity analysis of imputed data (n = 2863)
Table 1.
Logistic regression: covariates associated with probable PTSD 6 months post-TBI - sensitivity analysis of imputed data (full cohort).
| Covariate | B (SE(B)) | Odds ratio (95% CI) | p-valueb | VIF (range)c |
|---|---|---|---|---|
| Age | -.026 (.004) | .97 (.96–.99) | < .001 | 1.30 |
| Sex (male) | .26 (.16) | 1.29 (.96–1.94) | .13 | 1.07–1.08 |
| Educational levela | 1.16–1.19 | |||
| Primary school or less | .32 (.43) | 1.38 (.85–2.41) | .48 | |
| Secondary school/high school | .29 (.26) | 1.33 (.83–1.99) | .29 | |
| Post-high school training | .26 (.30) | 1.30 (.71–2.32) | .41 | |
| Psychiatric historyd | .71 (.29) | 2.03 (1.52–2.93) | .041 | 1.01 |
| GCS | .038 (.021) | 1.04 (.99–1.07) | .083 | 1.09 |
| TMT-(B-A) | .25 (.065) | 1.28 (1.13–1.50) | <.001 | 1.22–1.25 |
| RAVLT Delayed recall | -.22 (.085) | .80 (.65–.99) | .013 | 1.36–1.41 |
Note:a Reference category: College/University, b Significance level p < .01, c VIF = variance inflation factor (range) of the original and 5 imputed datasets, d Information about the psychiatric history and type of psychiatric disorder(s) was obtained by interview from the patient and/or carer upon admission. Nagelkerke R2 = 0.074.
Table 2Linear regression models: cognitive tests associated with the four PTSD symptom clusters – sensitivity analysis of imputed data
| Covariates | Intrusion cluster |
Avoidance cluster |
Cognition/Mood cluster |
Arousal cluster |
|||||
|---|---|---|---|---|---|---|---|---|---|
| B(SE) | p-valueb | B(SE) | p-valueb | B(SE) | p-valueb | B(SE) | p-valueb | VIF (range)c | |
| Age | -.026 (.005) | < .001 | -.014 (.002) | < .001 | -.050 (.007) | < .001 | -.033 (.005) | < .001 | 1.17–1.22 |
| Sex (Male) | .17 (.15) | .26 | .068 (.083) | .42 | .12 (.24) | .62 | .42 (.21) | .043 | 1.03–1.04 |
| Educational levela | 1.12–1.13 | ||||||||
| Primary school or less | .55 (.29) | .063 | .15 (.13) | .27 | .17 (.38) | .66 | .47 (.34) | .17 | |
| Secondary school/high school | .47 (.18) | .008 | .16 (.090) | .071 | .13 (.30) | .68 | .15 (.23) | .53 | |
| Post-high school training | .39 (.20) | .053 | .17 (.10) | .11 | .38 (.33) | .25 | .76 (.28) | .009 | |
| Psychiatric historyd | 1.36 (.32) | .001 | .52 (.17) | .013 | 2.16 (.51) | .002 | 1.80 (.30) | < .001 | 1.01–1.02 |
| GCS | .038 (.025) | .13 | .022 (.011) | .052 | -.091 (.039) | .032 | .015 (.031) | .64 | 1.08–1.10 |
| TMT-(B-A) | .33 (.088) | < .001 | .13 (.054) | .028 | .23 (.16) | .15 | .32 (.14) | .045 | 1.30–1.35 |
| CANTAB RTI | .34 (.10) | .005 | .13 (.053) | .035 | .61 (.11) | < .001 | .50 (.10) | < .001 | 1.16–1.21 |
| Nagelkerke R2 | .064 | .051 | .070 | .061 | |||||
Note:a Reference category: College/University, b Significance level p < .01, c VIF = variance inflation factor (range) of the original and 5 imputed datasets, d Information about the psychiatric history and type of psychiatric disorder(s) was obtained by interview from the patient and/or carer upon admission.
References
- Azouvi P., Arnould A., Dromer E., Vallat-Azouvi C. Neuropsychology of traumatic brain injury: an expert overview. Rev. Neurol. (Paris) 2017;173(7–8):461–472. doi: 10.1016/j.neurol.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Bagiella E., Novack T.A., Ansel B., Diaz-Arrastia R., Dikmen S., Hart T., Temkin N. Measuring outcome in traumatic brain injury treatment trials: recommendations from the traumatic brain injury clinical trials network. J. Head Trauma Rehabil. 2010;25(5):375–382. doi: 10.1097/HTR.0b013e3181d27fe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman A., Chatterjee A., Bhide R. Cognitive impairment and rehabilitation strategies after traumatic brain injury. Indian J. Psychol. Med. 2016;38(3):172–181. doi: 10.4103/0253-7176.183086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin C.R., Dalgleish T., Joseph S. A dual representation theory of posttraumatic stress disorder. Psychol. Rev. 1996;103:670–686. doi: 10.1037/0033-295x.103.4.670. [DOI] [PubMed] [Google Scholar]
- Buckley T.C., Blanchard E.B., Neill W.T. Information processing and PTSD: a review of the empirical literature. Clin. Psychol. Rev. 2000;20(8):1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Callahan C.D., Johnstone B. The clinical utility of the Rey Auditory-Verbal Learning Test in medical rehabilitation. J. Clin. Psychol. Med. Settings. 1994;1(3):261–268. doi: 10.1007/BF01989627. [DOI] [PubMed] [Google Scholar]
- CANTAB Cambridge Cognition . Cambridge Cognition Ltd; Cambridge, UK: 2014. CANTAB Research Suite 6: Test Administration Guide. [Google Scholar]
- Cristofori I., Levin H.S. Traumatic brain injury and cognition. Handb. Clin. Neurol. 2015;128:579–611. doi: 10.1016/B978-0-444-63521-1.00037-6. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders: DSM-5. fifth ed. American Psychiatric Association; 2013. DSM-V, doi-org.db29.linccweb.org/10.1176/appi. [Google Scholar]
- Filley C.M., Kelly J.P. White matter and cognition in traumatic brain injury. J. Alzheimers Dis. 2018;65(2):345–362. doi: 10.3233/JAD-180287. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Kozak M.J. Emotional processing of fear: exposure to corrective information. Psychol. Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- Foks K.A., Cnossen M.C., Dippel D.W.J., Maas A.I.R., Menon D., van der Naalt J., Steyerberg E.W., Lingsma H.F., Polinder S. The center-TBI investigators and participants. Management of mild traumatic brain injury at the emergency department and hospital admission in Europe: a survey of 71 Neurotrauma centers participating in the CENTER-TBI study. J. Neurotrauma. 2017 Sep 1;34(17) doi: 10.1089/neu.2016.4919. 2529-2523. [DOI] [PubMed] [Google Scholar]
- Hayes J.P., Vanelzakker M.B., Shin L.M. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front. Integr. Neurosci. 2012;6:89. doi: 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge C.W., Riviere L.A., Wilk J.E., Herrell R.K., Weathers F.W. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD Checklist. Lancet Psychiatr. 2014;1:269–277. doi: 10.1016/s2215-0366(14)70235-4. [DOI] [PubMed] [Google Scholar]
- IBM Corporation Released . IBM Corp; Armonk, NY: 2017. IBM SPSS Statistics for Windows, Version 25.0. [Google Scholar]
- Jacob S.N., Dodge C.P., Vasterling J.J. Posttraumatic stress disorder and neurocognition: a bidirectional relationship? Clin. Psychol. Rev. 2019;72:101747. doi: 10.1016/j.cpr.2019.101747. [DOI] [PubMed] [Google Scholar]
- Jurick S.M., Crocker L.D., Merritt V.C., Sanderson-Cimino M.E., Keller A.V., Glassman L.H., Twamley E.W., Rodgers C.S., Schiehser D.M., Aupperle R.L., et al. Independent and synergistic associations between TBI characteristics and PTSD symptom clusters on cognitive performance and postconcussive symptoms in Iraq and Afghanistan veterans. J. Neuropsychiatry Clin. Neurosci. 2021;33(2):98–108. doi: 10.1176/appi.neuropsych.20050128. [DOI] [PubMed] [Google Scholar]
- Karr J.E., Areshenkoff C.N., Garcia-Barrera M.A. The neuropsychological outcomes of concussion: a systematic review of the meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology. 2014;28:321–336. doi: 10.1037/neu0000037. [DOI] [PubMed] [Google Scholar]
- King N.S., Crawford S., Wenden F.J., Moss N.E.G., Wade D.T. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head-injury and its reliability. J. Neurol. 1995;242(9):587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B., Monahan P.O., Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann. Intern. Med. 2007;146(5):317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- Maas A.I., Menon D.K., Steyerberg E.W., Citerio G., Lecky F., Manley G.T., Hill S., Legrand V. Sorgner A and CENTER-TBI participants and investigators. Collaborative European NeuroTrauma Effectiveness research in traumatic brain injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76(1):67–80. doi: 10.1227/NEU.0000000000000575. [DOI] [PubMed] [Google Scholar]
- Maas A.I., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Buki A., Chesnut R.M., et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- McDonald S.D., Calhoun P.S. The diagnostic accuracy of the PTSD checklist: a critical review. Clin. Psychol. Rev. 2010 Dec;30(8):976–987. doi: 10.1016/j.cpr.2010.06.012. Epub 2010 Jul 6. PMID: 20705376. [DOI] [PubMed] [Google Scholar]
- Miles J., Shevlin M. Sage Publishers; Londen, VK: 2001. Applying Regression & Correlation. A Guide for Students and Researchers. [Google Scholar]
- Pavlovic D., Pekic S., Stojanovic M., Popovic V. Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary. 2019;22:270–282. doi: 10.1007/s11102-019-00957-9. [DOI] [PubMed] [Google Scholar]
- Periáñez J.A., Ríos-Lago M., Rodríguez-Sánchez J.M., Adrover-Roig D., Sánchez-Cubillo I., Crespo-Facorro B., Quemada J.I., Barceló F. Trail Making Test in traumatic brain injury, schizophrenia, and normal ageing: sample comparisons and normative data. Arch. Clin. Neuropsychol. 2007;22(4):433–447. doi: 10.1016/j.acn.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Pineau H., Marchand A., Guay S. Objective neuropsychological deficits in posttraumatic stress disorder and mild traumatic brain injury: what remains beyond symptom similarity? Behav. Sci. 2014;4:471–486. doi: 10.3390/bs4040471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi S.U., Long M.E., Bradshaw M.R., Pyne J.M., Magruder K.M., Kimbrell T., Hudson T.J., Jawaid A., Schulz P.E., Kunik M.E. Does PTSD impair cognition beyond the effect of trauma? J. Neuropsychiatry Clin. Neurosci. 2011;23(1):16–28. doi: 10.1176/jnp.23.1.jnp16. [DOI] [PubMed] [Google Scholar]
- Reitan R.M. Reitan Neuropsychology Laboratory; Tucson, AZ: 1992. Trail Making Test: Manual for Administration and Scoring. [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch. Psychol. 1994;28:21. [Google Scholar]
- Schmidt M. Western Psychological Services; Los Angeles, CA: 1996. Rey Auditory Verbal Learning Test: A Handbook. [Google Scholar]
- Scholten A.C., Haagsma J.A., Cnossen M.C., Olff M., van Beeck E.F., Polinder S. Prevalence of and risk factors for anxiety and depressive disorders after traumatic brain injury: a systematic review. J. Neurotrauma. 2016;33(22):1969–1994. doi: 10.1089/neu.2015.4252. [DOI] [PubMed] [Google Scholar]
- Schulz-Heik R.J., Fahimi A., Durazzo T.C., Friedman M., Bayley P.J. Evaluation of adding the CANTAB computerized neuropsychological assessment battery to a traditional battery in a tertiary care center for veterans. Appl. Neuropsychol. Adult. 2020;27(3):256–266. doi: 10.1080/23279095.2018.1534735. [DOI] [PubMed] [Google Scholar]
- Scott J.C., Matt G.E., Wrocklage K.M., Crnich C., Jordan J., Southwick S.M., Krystal J.H., Schweinsburg B.C. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull. 2015;141(1):105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabury S.A., Gaudette É., Goldman D.P., Markowitz A.J., Brooks J., McCrea M.A., Okonkwo D.O., Manley G.T., TRACK-TBI Investigators, Adeoye O., et al. Assessment of follow-up care after emergency department presentation for mild traumatic brain injury and concussion: results from the TRACK-TBI study. JAMA Netw. Open. 2018 May 18;1(1) doi: 10.1001/jamanetworkopen.2018.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandera-Ochsner A.L., Berry D.T., Harp J.P., Edmundson M., Graue L.O., Roach A., High W.M., Jr. Neuropsychological effects of self-reported deployment-related mild TBI and current PTSD in OIF/OEF veterans. Clin. Neuropsychol. 2013;27(6):881–907. doi: 10.1080/13854046.2013.802017. [DOI] [PubMed] [Google Scholar]
- Stenberg J., Karr J.E., Terry D.P., Saksvik S.B., Vik A., Skandsen T., Silverberg N.D., Iverson G.L. Developing cognition endpoints for the CENTER-TBI neuropsychological test battery. Front. Neurol. 2020;11:670. doi: 10.3389/fneur.2020.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg E.W., Wiegers E., Sewalt C., Buki A., Citerio G., De Keyser V., Ercole A., Kunzmann K., Lanyon L., Lecky F., et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicenter, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–934. doi: 10.1016/S1474-4422(19)30232-7. [DOI] [PubMed] [Google Scholar]
- Tanev K.S., Pentel K.Z., Kredlow M.A., Charney M.E. PTSD and TBI co-morbidity: scope, clinical presentation and treatment options. Brain Inj. 2014;28(3):261–270. doi: 10.3109/02699052.2013.873821. [DOI] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974 Jul 13;304(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thompson C., Davies P., Herrmann L., Summers M., Potter S. Approaches to establishing validated cut-off scores on the Rivermead post concussion symptoms questionnaire (RPQ) Brain Inj. 2016;30(5–6):770. [Google Scholar]
- Van Praag D.L.G., Cnossen M.C., Polinder S., Wilson L., Maas A.I.R. Posttraumatic stress disorder after civilian traumatic brain injury: a Systematic review and meta-analysis of prevalence rates. J. Neurotrauma. 2019;36(23):3220–3232. doi: 10.1089/neu.2018.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Constans J.I., Sutker P.B. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Aslan M., Lee L.O., Proctor S.P., Ko J., Jacob S., Concato J. Longitudinal associations among posttraumatic stress disorder, traumatic brain injury, and neurocognitive functioning in Army soldiers deployed to the Iraq War. J. Int. Neuropsychol. Soc. 2018;24:311–323. doi: 10.1017/S1355617717001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Steinbuechel N., Rauen K., Krenz U., Wu Y.-J., Covic A., Plass A.M., Cunitz K., Mueller I., Bockhop F., Polinder S., et al. The linguistic validation group of CENTER-TBI. Translation and linguistic validation of outcome instruments for traumatic brain injury research and clinical practice: a step-by-step approach within the observational CENTER-TBI study. J. Clin. Med. 2021;10(13):2863. doi: 10.3390/jcm10132863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.L., Li W.B. Cognitive impairment after traumatic brain injury: the role of MRI and possible pathological basis. J. Neurol. Sci. 2016;370:244–250. doi: 10.1016/j.jns.2016.09.049. [DOI] [PubMed] [Google Scholar]
- Weathers F.L., Litz B.T., Keane T.M., Palmieri P.A., Marx B.P., Schnurr P.P. 2013. The PTSD Checklist for DSM-5 (PCL-5)www.ptsd.va.gov Scale available from the National Center for PTSD at. [Google Scholar]
- Wilson J.T.L., Pettigrew L.E.L., Teasdale G.M. Structured interviews for the Glasgow outcome scale and extended Glasgow outcome scale: guidelines for their use. J. Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Wisdom N.M., Pastorek N.J., Miller B.I., Booth J.E., Romesser J.M., Linck J.F., Sim A.H. PTSD and cognitive functioning: importance of including performance validity testing. Clin. Neuropsychol. 2014;28(1):128–145. doi: 10.1080/13854046.2013.863977. [DOI] [PubMed] [Google Scholar]
- Yeates K.O., Levin H.S., Ponsford J. The neuropsychology of traumatic brain injury: looking back, peering ahead. J. Int. Neuropsychol. Soc. 2017;23:806–817. doi: 10.1017/S1355617717000686. [DOI] [PubMed] [Google Scholar]