Abstract
Introduction
Incidence of Chronic Subdural Hematoma (cSDH) is rising worldwide, partly due to an aging population, but also due to increased use of antithrombotic medication. Many recent studies have emerged to address current cSDH management strategies.
Research question
What is the state of the art of cSDH management.
Material and methods
Review.
Results
Head trauma, antithrombotic use and craniocerebral disproportion increase the risk of cSDH development. Most patients present with disorientation, GCS 13–15, and symptoms arising from cortical irritation and increased intracranial pressure. cSDH occurs bilaterally in 9–22%. CT allows assessment of cerebral compression (herniation, hematoma thickness, ventricle collapse, midline shift), hematoma age and presence of membranes, factors that ultimately determine treatment urgency and surgical approach. Recurrence remains the principle complication (9–33%), occurring more commonly with older age and bilateral cSDHs.
Discussion and conclusion
While incompletely understood, it is generally believed that injury in the dural cell layer results in bleeding from bridging veins, resulting in a hematoma formation, with or without a preceding hygroma, in a potential space approximating the junction between the dura and arachnoid. Neovascularization and leaking from the outer membrane are thought to propagate this process. Evidence that MMA embolization may reduce recurrence rates is a potentially exciting new treatment option, but also supports the theory that the MMA is implicated in the cSDH pathophysiology. The use of steroids remains a controversial topic without clear treatment guidelines. cSDH represents a common neurosurgical problem with burr-hole treatment remaining the gold standard, often in conjunction with subgaleal drains. MMA embolization to stop recurrence may represent an important evolution in understanding the pathophysiology of cSDH and improving treatment.
Keywords: Surgery, Head trauma, Trepanation, Burr hole, Injury, Elderly
Highlights
-
•
Incidence of cSDH is rising, partly due to the aging population and increased antithrombotic use.
-
•
cSDH occurs bilaterally in 9–22% of cases.
-
•
Recurrence remains the principle complication and has been estimated at 9–33%.
-
•
Risk factors for recurrence include old age and bilateral cSDHs.
-
•
MMA embolization may reduce recurrence, but its efficacy and target population remain unclear.
1. Introduction
Chronic Subdural Hematomas (cSDH) are believed to result from slow venous bleeding, typically from bridging veins on the cerebral surface, but the pathophysiological process is likely more complex and remains incompletely understood. Although they can occur anywhere along the neuroaxis, they classically present on the convexity of the cerebral hemispheres, unilaterally or bilaterally. The hematomas contain liquefied older blood, with a “motor oil” appearance, and are characteristically contained by an outer membrane (Macdonald and Winn, 2017). Their manifestation was first detailed in a case-report in 1695 by Johan J. Wepfer (Sahyouni et al., 2017a). However, the surgical solution of trepanation is broadly described earlier in several different cultures, including: Mesolithic era as far back as 10,000 before Christ (BC); Hippocratic schools from the 4th century BC; the Roman period by Galen (3rd century) (Missios, 2007). The question remains: how many of these patients had a cSDH? Why was the trepanation performed?
While cSDH can have multiple etiologies, they classically manifest after a minor head trauma, with subsequent augmentation of the hematoma due to slow bleeding over a few weeks, until the signs and symptoms become apparent. Patients typically present with confusion, headache and a reduced state of consciousness. A small subset may present with seizures. However, the constellation of symptoms can range widely amongst patients.
cSDH is one of the most common pathologies in neurosurgery and typically presents in the elderly population. Their importance in neurosurgery has been growing due to their increasing incidence, likely attributable to the increasing aging population and use of antithrombotic medication (Aspegren et al., 2013; Adhiyaman et al., 2017; Rauhala et al., 2019). The management of cSDH involves either careful observation or surgical treatment. While surgical treatment is fairly simple by neurosurgical standards, the post-operative course can be complicated by a number of factors given the old age of patients, including the presence of multiple comorbidities and the frequent use of antithrombotics, that renders these patients susceptible to recurrent bleeding.
While cSDH is a common disease in neurosurgical practice, there remains ongoing controversies, differences in management approaches, and many uncertainties prinicipally due to an incomplete understanding of the pathophysiology. It is therefore the objective of the present paper to comprehensively review the literature, with a particular emphasis on these topics, and to provide an overview of the current state of the art.
2. Epidemiology
Despite being one of the most common pathologies in neurosurgery, the epidemiology of cSDH has not been well-studied. However, in the last few years, a number of papers have provided insight into its incidence in specific regions. Men are more commonly affected than women, representing a minimum of two-thirds of patients (Rauhala et al., 2019; Toi et al., 2017; Kudo et al., 1992; Nayil et al., 2012; MORI and MAEDA, 2001). Furthermore, the incidence and median age of cSDH has been steadily increasing worldwide (Adhiyaman et al., 2017; Rauhala et al., 2019). The average age of onset reported from multiple different countries ranges between 60 in India to 81 years of age in North Wales (Adhiyaman et al., 2017; Nayil et al., 2012). In Finland, it was reported that the incidence more than doubled over a 25-year period from 8.2 to 17.6/100,000, and that the incidence in patients 80 years or older specifically, nearly tripled from 46.9 to 129.5 per 100,000 a year (Rauhala et al., 2019). These findings are supported by similar increases noted in North Wales, where the incidence has increased from 8.2 (reported in 2002) to 48/100,000 people (reported in 2017) over a 15 year period (Adhiyaman et al., 2017; Asghar et al., 2002). In Japan, patients 80 years or older represented close to half of all patients in a cohort of 63,358 patients (Toi et al., 2017). Factors that drive these trends include the increased use of antithrombotics and the ageing population.
3. Pathophysiology
Our understanding of the pathophysiology of cSDH has evolved considerably over the decades, and continued research in the field may eventually modify current theories. Presently, it is generally well accepted that the initial hemorrhage in cSDH derives from bleeding of bridging veins as they traverse the dural cell layer of the dural border. This can occur due to traumatic events, anatomical changes that result in cranio-cerebral disproportion (i.e. brain-shrinkage with age), intracranial hypotension, or through anatomical manipulation (cranial surgery) (Macdonald and Winn, 2017). These bridging veins are susceptible to bleeding as their vessel walls are thin, and are believed to be thinnest in the dural cell border layer, being enclosed by as little as a single endothelial cell layer (Macdonald and Winn, 2017; Yamashima and Friede, 1984). The subsequent hematoma-formation from the shearing of the veins is believed to create a potential space within the dural cell border layer as it is composed of flattened, elongated cells, with comparatively weaker junctions that provide a natural cleavage plane of membrane separation (Macdonald and Winn, 2017; Holl et al., 2018). As this collection forms in a potential space, and given that the bleeding derives from low-pressure venous blood, the hematoma takes time to develop, explaining why many patients become symptomatic weeks after the initial injury. An inflammatory response is triggered and drives clot fibrinolysis, production of granulation tissue, and release of angiogenic factors, finally leading to “neo-membrane” formation (Stanisic et al., 2012; Edlmann et al., 2017). This perpetuates inflammation and it is thought that its granulation tissue becomes rich in immature, fragile, and leaky blood vessels prone to microbleedings. Altogether, chronic inflammatory response and the neo-membrane, potentiate the enlargement of the hematoma and impede proper clotting (Gandhoke et al., 2013).
The neo-membranes of cSDH have been previously classified into 4 types and associated with membrane thickness and radiodensity on imaging (Table 1): Type I (non-inflammatory membrane), Type II (inflammatory membrane), Type III (hemorrhagic inflammatory), Type IV (scar inflammatory membrane) (Gandhoke et al., 2013; Nagahori et al., 1993). Interestingly, Gandhoke et al. noted that patients with a GCS<12 exclusively presented with Type II neo-membranes (Gandhoke et al., 2013).
Table 1.
Histopathological description of the various neo-membranes as described by Nagahori T, Nishijima M, Takaku A: Histological study of the outer membrane of chronic subdural hematoma: possible mechanism for expansion of hematoma cavity. No shinkei geka. Neurological surgery 21:697-701, 1993.
| Type | Original Description |
|---|---|
| I, Non-inflammatory membrane. | Contains immature fibroblasts and collagen fibers, was associated with very slight or sparse cell infiltration and neo-capillaries. |
| II, Inflammatory membrane | Consisting of one layer of immature connective tissue, was associated with marked cell infiltration and vascularization throughout the entire thickness. |
| III, Hemorrhagic-inflammatory membrane. | Presents with structure of 2 or 3 layers, and was associated with capillaries with a large lumen on the side of the dura mater and marked cell infiltration and many thin new vessels on the side of the hematoma cavity. Some patients showed a layer consisting of only collagen fibers and fibroblasts between two such layers. In addition, hemorrhage into the membrane was often observed. |
| IV, Scar-inflammatory membrane. | Inflammatory cell infiltration, neovascularization and hemorrhage in the outer membrane of cicatricial tissue. |
Another pathophysiological mechanism that has been proposed is that cSDH develops after formation of hygromas (Lee, 1998). This mechanism has been suggested due to the observation that cSDH often appears after subdural hygromas, and that both may manifest after cranial trauma (Park et al., 2008; Ahn et al., 2016). The incidence of cSDH following hygromas ranges widely in the literature and has been reported in up to 58% (Lee et al., 2000). Bilateral hygromas and male sex have been found to be predictors of this cSDH conversion (Ahn et al., 2016). While a number of mechanisms have been proposed, this pathophysiological process remains incompletely understood. It is generally believed, however, that the CSF leakage into the dural cell layer creates a potential space and that the stretching of this layer results in the leaking of the aforementioned thin bridging veins (Kristof et al., 2008). It has been shown that beta-trace protein is present in a relatively high degree compared with serum levels in both cSDH and hygromas, confirming not only that CSF is present in the fluid, but also that hygromas are composed of CSF and do not simply represent serum or effusions (Kristof et al., 2008).
It seems that regardless of whether a hygroma forms, cSDH appears to manifest in the same dural layer region and therefore suggests that a traumatic injury to this area remains a common step in this process. Expansion of this area appears to occur via slow venous bleeding and CSF appears to precipitate this event, suggesting that multiple patho-mechanisms exist for cSDH development.
In babies with non-accidental head injury and the classical triad (encephalopathy, retinal hemorrhage and subdural hematoma), the typical pattern of subdural hemorrhage includes a bilateral thin film of SDH over the cerebral convexities and in the posterior interhemispheric fissure (Squier, 2011).
4. Risk factors
There are multiple factors that have been shown to predispose to cSDH formation; however, a combination of these factors is frequently present. They can be broken into general themes including trauma, medical and physiological, craniocerebral disproportion, and iatrogenic:
4.1. Trauma
For the majority of cSDH patients, there is a history of some form of cranial trauma that has occurred weeks before the onset of symptoms. The trauma is believed to be the initiating event that causes a dural border cell layer disruption. While in some circumstances a clear history is not attainable from the patient, a preceding traumatic event has been identified in a range of 50–77% of cases. (Rauhala et al., 2019; MORI and MAEDA, 2001; Oishi et al., 2001; Nakaguchi et al., 2001; Ramachandran and Hegde, 2007; Baechli et al., 2004; Gelabert-González et al., 2005). In a Brazilian study of 778 patients with cSDH, traumatic causes included falls (36.2%), traffic accidents (9.5%), aggression (4.5%), or other accidents (10.1%), together representing 60.4% of cases attributed to cSDH development (Sousa et al., 2013). It is noteworthy, that it is not uncommon for trauma to be precipitated secondary to accidents related to alcohol overconsumption.
4.2. Medical and physiological
A number of medical and physiological factors can also play a role in the development of cSDH. Common factors include male gender, dural leaks, environmental factors, antithrombotic medication and medication or conditions that increase the risk of falls. However, many of these factors have not been well explored. Particularly male gender, traditionally pointed to as a major risk factor by epidemiological studies (Kanat et al., 2010), is not further explained. It was a common belief that male cSDH bias was related to increased frequencies of trauma and/or alcohol abuse, but this assumption was antagonized by Marshman et al. who demonstrated that this relationship is not supported by literature, and that paradoxically, other known risk factors such as anticoagulation were in fact more commonly present in females (Marshman et al., 2015). New research to enlighten the true explanation for this male bias is needed.
Another example includes the potential role of high altitude; only case reports of high altitude-induced cSDH have been reported in the literature, and it has been proposed that this may be partially due to increased venous pressure (Ganau et al., 2012; Lu et al., 2015). Better evidence exists for antithrombotic medication. It has been reported that at least 4–6 out 10 patients with cSDH take antithrombotic medication (Aspegren et al., 2013; Baechli et al., 2004; Rust et al., 2006; Gaist et al., 2017; Castellani et al., 2017). In a recent study from Gaist et al. (2017) of 10,010 patients, use of low-dose aspirin, clopidogrel, a direct oral anticoagulant, or a vitamin K-antagonist were associated with an increased risk of cSDH and of cSDH-associated mortality at 30 days. Vitamin K-antagonists exposed patients to the highest risk (OR 3.63, CI = 3.31–3.98), with patients using this medication between 1 and 3 months having the highest risk (Gaist et al., 2017). Other authors have also shown the increased risk of cSDH due to warfarin use (Rust et al., 2006). While not a direct focus of the study, Gaist et al. also showed that patients on hypnotics/sedatives had a higher risk of cSDH, likely due to their increased risk of falls (Gaist et al., 2017).
The occurrence rate of subdural hematoma in long-term dialysis patients is 10 times higher than that of the general population, which may be related to increased use of anticoagulants in long-term hemodialysis patients (Sood et al., 2007).
4.3. Craniocerebral disproportion
Craniocerebral disproportion refers to a mismatch between brain size relative to cranial size. This disproportion can be the result of natural phenomena such as age-related brain atrophy, or can be secondary to severe dehydration or chronic alcohol consumption (Alderazi and Brett, 2007). It is thought that craniocerebral disproportion increases the chance of cleavage of the dural cell layer, and provides space for the growth of subdural hematoma as there is less opposing pressure from the brain parenchyma. It also increases the stretching on the bridging veins (Yamashima and Friede, 1984). However, literature on this subject is limited.
4.4. Iatrogenic
Dural leaks are a known factor which can result in cSDH. These leaks most commonly occur due to iatrogenic causes involving intentional or unintentional trauma to the dura mater, as a consequence of surgery or over-drainage of CSF during lumbar punctures or CSF drainage systems. This process can lead to intracranial hypotension. It has also been suggested that the mechanism of cSDH formation in such instances involves inferior displacement of the brain due to low intracranial pressure and the lack of CSF-buffering, thereby stretching cortical bridging veins and arachnoidian microvasculature (Kerr et al., 2014), similar to the mechanism of craniocerebral disproportion. However, the incidence of cSDH occurrence attributable to dural leaks is relatively low. In terms of surgery, extended endonasal endoscopic approaches in particular have been noted to predispose to cSDH due to the risk of postoperative CSF-leakage (Kerr et al., 2014). In terms of CSF-drainage, one large study on patients with normal pressure hydrocephalus showed that over-drainage resulted in a 5% incidence of cSDH requiring surgical evacuation (Khan et al., 2013). These authors showed that all patients with this complication had an opening pressure >160 mmH2O on lumbar puncture. The occurrence of cSDH from lumbar punctures is rare: a study on spinal anesthesia in Sweden identified only 5 reported cases in the country over a decade (Moen et al., 2004), while another study on 1,089 patients who underwent lumbar punctures did not report a single case (Zetterberg et al., 2010).
5. Clinical presentation
The clinical presentation of cSDH can vary widely, ranging from asymptomatic to severely reduced Glasgow Coma Scale (GCS) scores. The severity of neurological symptoms is partially a function of the hematoma volume, the speed of hematoma volume expansion, the hematoma location, and whether there is a mass effect (signified by signs of herniation, loss of gyri visibility on imaging, reduction of ventricular size). The vast majority of patients with cSDH present with a GCS between 13-15,6,16,36 with an estimate that only about 8% present with a GCS between 9 and 12, and 3% with a GCS between 3 and 8 (Rauhala et al., 2019).
The clinical signs and symptoms most often observed are shown in Table 2. It has been suggested that the clinical evolution can be broken down into 3 phases (Yang and Huang, 2017): (1) An initial period, highlighted by a potential subclinical traumatic episode initiating the pathophysiological disruption of the dural cell layer and potentially a hematoma formation; (2) Followed by a period of slow hematoma maturation and augmentation, and neo-membrane formation lasting weeks to months; (3) And finally, a period wherein progressive decompensation of intracranial capacity occurs due to hematoma growth and patients begin to manifest signs and symptoms of cerebral irritation and increased intracranial pressure. Patients with cSDH can be categorized according to a grading system proposed by Markwalder et al. (1981) to identify the clinical severity from 0 to 4 based on their clinical presentation (Table 3).
Table 2.
Clinical and Radiological Presentation of cSDH.
| Clinical Findings | Radiological Findings |
|---|---|
Symptoms
Pronator drift (Barré test) Mingazzini test Romberg test Assessment of orientation (time, space, self) Pupil exam (to assess intracranial pressure) |
Crescent shaped hematoma typically present at the cerebral convexity unliteral or bilaterally, with varying radiodensities helping to differentiate age:
Herniation Collapse of ventricular space Hematoma membrane formation Midline shift “Thickening” of cortex |
Table 3.
Markwalder grading of cSDH severity, adapted from Markwalder, T.M., Steinsiepe, K.F., Rohner, M., Reichenbach, W. and Markwalder, H., 1981. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. Journal of neurosurgery, 55(3), pp.390-396.
| Grade | Description |
|---|---|
| 0 | Neurologically normal |
| 1 | Alert and oriented; mild symptoms such as headache; absent or mild neurological deficit, such as reflex asymmetry |
| 2 | Drowsy or disoriented with variable neurological deficit, such as hemiparesis |
| 3 | Stuporous but responding appropriately to noxious stimuli; severe focal signs, such as hemiplegia |
| 4 | Patient comatose with absent motor responses to painful stimuli; decerebrate or decorticate posturing. |
The symptoms and natural history of cSDH due to dural leaks may be slightly different, and depending on the size of the leak, clinical symptoms may not present immediately. These patients typically present with symptoms typical of intracranial hypotension. A systematic review of cases with neuro-axial anesthesia leading to intracranial subdural hematoma showed that these patients most commonly present with non-postural (81%) and postural headaches (77%) (Cuypers et al., 2016), and less commonly present symptoms which occur in cSDH due to other cases, such as trauma. In addition, patients with intracranial hypotension can also present with tinnitus (Rettenmaier et al., 2017) which is relatively uncommon in cSDH from other etiologies.
6. Imaging and classification of cSDH types
The imaging modality typically used to identify and follow patients with cSDH is computed tomography (CT). cSDH typically presents on the cerebral convexities either unilaterally or bilaterally. Bilateral hematomas represent between 9 and 22% of cases (Rauhala et al., 2019; Nayil et al., 2012; MORI and MAEDA, 2001; Gelabert-González et al., 2005; Sousa et al., 2013; Castellani et al., 2017; MacFarlane et al., 2009). Of note, in children the presentation is most frequently bilateral (Tzioumi and Oates, 1998). Interestingly, the literature has shown a slight predominance of left-sided hematomas (Rauhala et al., 2019; MORI and MAEDA, 2001; Gelabert-González et al., 2005; Sousa et al., 2013; Castellani et al., 2017). It has been hypothesized that this is because the left side is dominant in 95% of patients and may therefore manifest with more clinically important symptoms (MacFarlane et al., 2009). It is also possible that this is related to transverse sinus drainage dominance; however, to our knowledge, no study has assessed this relationship.
Some imaging classifications of cSDH have been previously proposed; however, none are commonly reported in the literature. Typically, CT scans of cSDH are analyzed with respect to hematoma location (typically on the convexity), if they are unilateral or bilateral, hematoma age, hematoma thickness, and for the presence of membranes. Additional CT findings which may be described include obliteration of sulci, herniation, collapse of ventricular spaces, midline shift, and “thickening” of the cortex (hematoma isointensity with cortex), (Wilms et al., 1992; Altaf et al., 2018) (Table 2, Table 4).
Table 4.
The Oslo Chronic Subdural Hematoma Grading System for Prediction of Postoperative Recurrence Requiring Reoperation (Stanisic M et al. A Reliable Grading System for Prediction of Chronic Subdural Hematoma Recurrence Requiring Reoperation After Initial Burr-Hole Surgery. Neurosurgery 2017; 81:752–760).
| CT scan imaging appearance based on density changes | ||||
|---|---|---|---|---|
| Isodense or hyperdense subtypes and Laminar or separated types | 2 | |||
| Hypodense or gradation subtypes and trabecular type | 0 | |||
| Preoperative volume (mL) | ||||
| >130 | 1 | |||
| ≤130 | 0 | |||
| Postoperative residual cavity volume (mL) | ||||
| >200 | 2 | |||
| 80-200 | 1 | |||
| <80 | 0 | |||
| Total score |
0–5 |
|||
| Total score points |
Non-recurrence (n) |
Recurrence requiring reoperation (n) |
Rate of recurrence requiring reoperation (95%CI) |
P-value |
| 0 | 18 | 0 | 0% (0%–18%) | <.001 |
| 1–2 | 48 | 3 | 6% (1%–16%) | |
| 3–4 | 21 | 9 | 30% (15%–49%) | |
| 5 | 3 | 5 | 63% (25%–92%) | |
There has been growing interest to investigate whether follow-up imaging is necessary in patients operated for cSDH. Multiple recent studies have shown that routine imaging seems unlikely to make a significant difference (Frechon et al., 2020; Pedersen et al., 2017; Schucht et al., 2019). One study showed that for the few patients with recurrent hematomas needing surgical treatment, the onset of symptoms preceded planned CT imaging at 6-week follow-up (Pedersen et al., 2017). Another study showed a recurrence in 21 of 423 operated patients (5%), 14 of whom were symptomatic while the remaining 7 patients were reoperated despite being asymptomatic (Frechon et al., 2020).
7. Treatment
The management of cSDH is determined by the presence and severity of symptoms (mild headache vs epileptic seizures) related to the hematoma as well as its size and mass effect. If symptomatic, or thicker than 10 mm or with more than 7 mm of midline shift, surgery is offered (Holl et al., 2018; Torihashi et al., 2008; Mehta et al., 2018; Ivamoto et al., 2016). Evidence of radiographic progression during conservative follow-up is also a reasonable indication for surgical treatment (Holl et al., 2018; Mehta et al., 2018). Surgery remains the definitive treatment, while MMA embolization is potentially useful, but requires further investigation. Furthermore, MMA embolization is potentially useful for stopping further bleeding but will not provide direct help in reducing the mass effect of blood accumulation on the brain.
7.1. Surgical evacuation
A wide variety of surgical techniques have been proposed and motivated several reviews and clinical trials: twist-drill craniostomy and spontaneous hematoma efflux, twist-drill craniostomy and catheter drainage, small craniotomy and endoscopic removal, subduro-peritoneal shunt as an alternative for infants and elderly patients, larger craniotomy, hematoma removal and membranectomy, or burr-hole evacuation without or with continuous closed-system drainage (Gelabert-González et al., 2005; Mehta et al., 2018; Sahyouni et al., 2017b; Abecassis and Kim, 2017; Ding et al., 2020; Soleman et al., 2019). This last option has been the most commonly applied all over the world, despite the fact that comparative outcomes are still debated. Regardless of several single-center reports and reviews promoting other surgical possibilities (Almenawer et al., 2014), the current discussion seems to focus mostly on the use of drainage, while burr-hole evacuation has increasingly become the standard (Mehta et al., 2018). However, recent level I evidence shows lower rates of recurrence, drain misplacements, parenchymal injuries, as well as overall morbidity for subperiosteal/subgaleal drains but similar outcome and mortality when compared with subdural drains (Greuter et al., 2020).
A Monte Carlo simulation on meta-analysis data, despite having limitations, supports burr-hole craniostomy as the most efficient choice for surgical drainage of uncomplicated cSDH, as it shows the best balance between low recurrence rate and low incidence of complications resulting in morbidity (Lega et al., 2010). The utility-rating of burr hole craniostomy (0.9608) was superior to that of twist-drill (0.9202; P = 0.001) or craniotomy (0.9169; P = 0.006) (Lega et al., 2010).
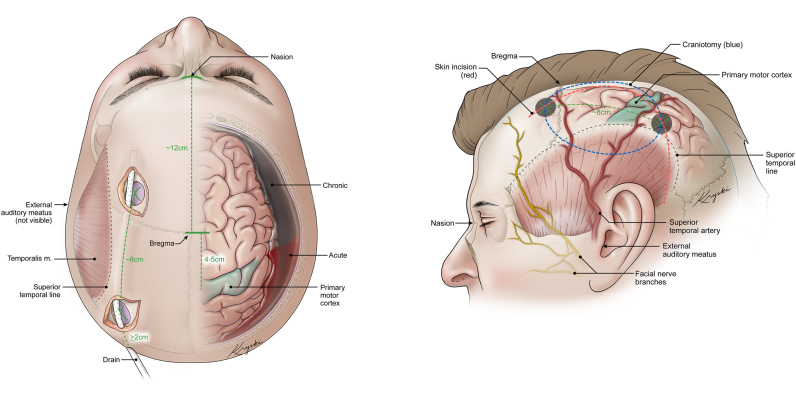
Burr-hole evacuation in the operating room can be performed under conscious sedation or general anesthesia as per surgeon preference or patient tolerance, compliance, and comorbidities. The technical aspects of this procedure are subject to several variants and preferences. In our hands, for a large convexity cSDH, two burr-holes are typically placed, (1) anterior, just in front of the coronal suture, and (2) posterior, on the tuber parietalis, approximately 8 cm posterior to the first one. Both are placed cranial to the superior temporal line, as this normally coincides with the cranio-caudal center of the hematoma and avoids the risk of injuring eloquent brain areas (operculum or primary motor cortex) (Fig. 1a). If placed more medially and superiorly, the risk of injuring a bridging vein is higher and the gravity-induced passive hematoma efflux is lower. After opening the dura in a cruciate fashion, an outer membrane may be present and should be coagulated. However, the outer membrane should not be cut beyond the edges of the burr-holes (MORI and MAEDA, 2001) and evidence does not support opening the inner membrane (Unterhofer et al., 2016). The vascularized membrane can bleed and such bleeding may be difficult to control if remote from the surgical site (Sahyouni et al., 2017b; Abecassis and Kim, 2017).
Fig. 1.
Illustrations of a superior and lateral view of important anatomical landmarks to consider during surgical treatment of cSDH. A) The superior view highlights the placements of the burr-holes, with consideration of the motor cortex. Shown here is the subcutaneous/subgaleal drain placement which is preferred by the authors. B) The lateral view demonstrated the same Burr-hole placements but also demonstrated the skin incision for a conversion to a craniotomy. Anatomical landmarks are highlighted to demonstrated why this skin incision is preferred.
If hemorrhage cannot be controlled despite continued irrigation, consideration must be given to converting the burr-holes into a craniotomy (Fig. 1). Also, an upfront craniotomy for cSDH might be indicated for hematoma recurrence and presence of multiple cavities and membranes (Abecassis and Kim, 2017). When converting the two burr-holes into a craniotomy, the skin incision that we propose negotiates different concerns: (1) preservation of subcutaneous vascularization and superior temporal artery branches; (2) preservation of the frontal branch of the facial nerve; (3) reaching the upper part of the hematoma and cavity, between the superior temporal line and the superior sagittal sinus. Putting together these criteria, we propose a skin incision for such scenarios that we believe is safe and easy to replicate (Fig. 1b).
A last technical detail that frequently goes unnoticed is the long-term cosmetic outcome resulting from the scalp depression secondary to the burr-hole defect. Im et al. showed a good reconstruction with excellent cosmetic and functional outcomes and without significant complications after covering with titanium plates (Im et al., 2014).
7.2. Middle meningeal artery embolization
Inflammatory responses of the dural layer have been implicated in the pathophysiology of cSDH by promoting neovascularization and formation of bleeding granulation tissue (neo-membranes) (Edlmann et al., 2017). It is believed that this neovascularization is supplied by branches of the middle meningeal artery (MMA), wherefore MMA-embolization has gathered a growing interest, particularly in the treatment of recurrent cSDH (Ban et al., 2018). This procedure is an anti-angiogenic modulation that is believed to stop the rebleeding and inflammation cascade.
Given the small size of most studies on this topic, with a level of evidence lower than II in reported series, the best information derives from a systematic review that includes almost 200 patients and 15 studies (Waqas et al., 2019). The results indicate a recurrence rate after MMA-embolization of only 3.6% with no notable complications. Several embolization materials were used (polyvinyl alcohol, N-butyl-2-cyanoacrylate, Onyx, coils and gelatin), with no differences in outcome.
It is still unclear which population would benefit most and would be ideal candidates for MMA-embolization. Accordingly, proposals for randomized controlled trials (RCTs) should probably target a minimally symptomatic cohort with mass effect but without major deficits or radiographic progression of disease, as those patients could potentially tolerate the delay between a potential need for surgical treatment and the expected progressive reduction of the hematoma.
7.3. Medical treatment
Non-surgical treatment of cSDH can only be considered if there are no signs of intracranial hypertension or cortical irritation, such as epileptic seizures. There is limited evidence with respect to non-surgical treatment of CSDH and case series and single-center reports focus mostly on antiangiogenic and anti-inflammatory drugs (Fernandes de Oliveira, 2019). However, higher evidence studies are emerging.
Among the drugs studied, corticosteroids remain a major target, as they are powerful anti-inflammatory substances that may inhibit formation of new blood vessels. The drawbacks of steroids, including dexamethasone, are well-known side-effects such as diabetes, infections, and cognitive and sleep disturbances. A review and meta-analysis by Holl et al. (2019) assembles quite well the current evidence on this matter; in their review of 7 studies, the results indicated a higher incidence of re-interventions (4–58%) in patients treated with steroids only when compared to those treated surgically (7–26%) or those who received both steroids and surgical evacuation (4–12%). However, mortality varied between 0 and 4% in steroids alone group, 0–13% in steroids plus surgery group, and 0–44% in surgery alone group. This study was limited by the heterogeneity of selection criteria among the different series included, but stressed the potential of combined steroid and surgical treatment and the need for further RCTs (Holl et al., 2019). Indeed, a recent RCT on patients receiving surgical treatment randomized into receiving dexamethasone or placebo after the operation, showed that treatment with dexamethasone resulted in fewer favorable outcomes and more adverse events than placebo at 6 months, but fewer repeat operations were performed in the dexamethasone group (Hutchinson et al., 2020). This study also included a small subset of 38 patient who did not receive surgery, showing that 18 of 22 patients (82%) treated with dexamethasone had a favorable outcome whereas 16 of 16 patients (100%) in the placebo group had a favorable outcome. The totality of these results indicated that the benefit of dexamethasone remains uncertain and carries important risks. It should be noted however that the study used a high dose dexamethasone treatment for 2 weeks, and it is unclear if smaller doses may provide benefits of fewer operations while lowering the risk of unfavorable outcomes. Another study, the DECSA trial, is currently underway and will provide further evidence for the role of Dexamathasone (Miah et al., 2018).
A second drug, atorvastatin, has mostly been studied in Chinese cohorts, providing limited evidence that oral atorvastatin may be beneficial in cSDH (Qiu et al., 2017). Moreover, animal studies in mice have shown that atorvastatin can also be pro-inflammatory depending on the dose: a low dose of atorvastatin (3 mg/kg/day) was found to have anti-inflammatory and anti-angiogenic effects (Araújo et al., 2010), while a higher dose of atorvastatin (8 mg/kg/day) led to a significantly increased and persistently high level of the Vascular Endothelial Growth Factor (VEGF) and increased levels of inflammatory factor matrix metalloprotease (Li et al., 2014).
Another drug, tranexamic acid is a potential inhibitor of the fibrinolytic and inflammatory (kinin-kallikrein) systems (Kageyama et al., 2013). In a small series of 21 patients, a good result was seen in 18 patients with the only treatment being tranexamic acid, while Tanweer et al. administered it postoperatively with no reported cSDH recurrence (Tanweer et al., 2016).
Lastly, Angiotensin-Converting Enzyme (ACE) inhibitors are known to decrease the VEGF-production and could therefore reduce inflammation as well as neovascularization and extravasation of fluid into the subdural space. However, there are, to date, no positive results in the literature.
A broad study on the assessment of the correlation between pro- and anti-inflammatory cytokines and cSDH failed to show significance through exploratory and confirmatory factor analysis (Pripp and Stanišić, 2014). The same authors were still able to describe a significant association between increased anti-inflammatory activity in hematoma fluid samples and a lower risk of recurrence, but this relationship was not statistically significant in venous blood samples.
8. Complications and prognosis
Treatment-related complications are largely focused on recurrence after surgical evacuation, however, dexamethasone as a stand-alone treatment or in conjunction with surgery has also been related to drug-related complications. The recurrence rate of cSDHs necessitating re-intervention varies from 9 to 33% (Torihashi et al., 2008; Pahatouridis et al., 2013; Kolias et al., 2014; Nassiri et al., 2020), and seems to be less frequent with adjuvant MMA-embolization (Waqas et al., 2019) and possibly with concomitant administration of dexamethasone (Holl et al., 2019).
Several other factors have been associated with cSDH recurrence, such as diabetes mellitus, preoperative seizure, and preoperative width of hematoma (≥20 mm) (Altaf et al., 2018). The role of antithrombotic medication remains controversial (Torihashi et al., 2008; Chon et al., 2012; Lindvall and Koskinen, 2009; Guha et al., 2016). Another interesting aspect is the higher recurrence in bilateral cSDH (Torihashi et al., 2008; Kung et al., 2012), possibly explained by a poor brain re-expansion or higher rate of pneumocephalus (MORI and MAEDA, 2001) also leading to poorer brain re-expansion, particularly if both sides are performed simultaneously. Septations or membranes also prevent complete hematoma evacuation and irrigation as well as brain re-expansion (Ramachandran and Hegde, 2007; Chon et al., 2012). Lastly, a postoperative midline shift ≥5 mm is a negative prognostic factor. However, it is important to note that the complication rates remain relatively low, and therefore partially explain some variability between reported studies.
With regards to prognosis prediction, there are some retrospective uni- and multivariate analysis of patient- and CT imaging-characteristics with the purpose of predicting recurrence of cSDH after burr-hole evacuation with closed-drainage (Stanišić et al., 2013; Stanišić and Pripp, 2017; Motiei-Langroudi et al., 2017). Factors like the preoperative hematoma volume (>115 ml), presence of loculations, the isodense, hyperdense, laminar and separated CT densities and the residual volume on the first postoperative day after removal of the drainage (>80 ml) were identified as significant radiological predictors of recurrence (Stanišić and Pripp, 2017). On the other hand, patient-related parameters such as clopidogrel or warfarin use also correlated with recurrence needing surgery (Motiei-Langroudi et al., 2017). A clear limitation from such predictive models that prevent their application to clinical practice is the need for a post-operative CT.
A cSDH grading system that predicts recurrence needing surgery further identified that isodense or hyperdense lesions and laminar or separated lesions, and a postoperative cSDH cavity volume >200 ml as the strongest predictors of recurrence (Table 4). (Stanišić and Pripp, 2017)
A recent systematic review of prognostic factors for hematoma recurrence has shown that hyperdense components (hyperdense homogeneous and mixed density) were the strongest prognostic factor of recurrence, providing a relative risk of 2.83(Miah et al., 2021).
Aside from recurrence, other procedure-specific complications may include focal brain contusions (importance of good placement of the burr-holes to avoid more eloquent brain surface, avoidance of subdural drain or at least careful under sight introduction of the drain, and gentle irrigation), seizures, subdural empyema or more superficial surgical site infection, tension pneumocephalus, or postoperative acute subdural or intraparenchymal hematoma (Lega et al., 2010).
Other medical complications include nosocomial infections (respiratory and urinary), deep venous thrombosis or pulmonary embolism, myocardial infarction, stroke or electrolytic imbalances, all of which can be potentially impairing and dangerous in the elderly population (Mehta et al., 2018; Lega et al., 2010).
8.1. Outcomes
Clinical outcomes and average improvement after surgery are poorly documented. The best estimation found in the literature shows an improvement of the modified Rankin scale (between admission and postoperative) in approximately two third of the patients and a worsening in only 13% at 60 days (Glancz et al., 2019). On average, patients seem to improve 1 grade on the mRS scale postoperatively (Brennan et al., 2016). As would be expected, multiple studies have shown that patients with lower mRS scores have a better prognosis than patients higher preoperative scores (Brennan et al., 2016; Ro et al., 2016; Leroy et al., 2015).
Lastly, mortality in patients suffering from cSDH ranges from 0 to 32% (Holl et al., 2018; Mehta et al., 2018; Chon et al., 2012) and depends on clinical condition at presentation (better outcomes with early diagnosis) (van Havenbergh et al., 1996), as well as comorbidities and coagulation status (MORI and MAEDA, 2001; Ramachandran and Hegde, 2007; Gelabert-González et al., 2005).
Efforts to standardize outcomes and data elements in cSDH is underway to help better measure comparative outcomes between various studies (Holl et al., 2021).
9. Areas of ongoing controversy
There remains a number of active areas of research in the field surrounding critical and controversial topics, and these are discussed in further detail below. It is important to note when considering these topics that at the present time, there exists only two studies with level 1 evidence (Hutchinson et al., 2020; Santarius et al., 2009), emphasizing that many questions remain largely unresolved.
9.1. Indications for MMA embolization
RCTs are needed to help better define which patients can benefit the most from MMA-embolization. Specifically, the role of treatment via MMA embolization needs to be assessed as an adjunct to surgery for the prevention of recurrence, and the potential role of MMA embolization to stop progression and possibility induce regression of non or minimally symptomatic cSDH. At the present time, no level 1 evidence exists to support its routine use.
9.2. Staging and head position during cSDH surgical evacuation
No clear evidence exists regarding a single-vs. two-staged procedure for bilateral cSDH. Whereas, a two-staged approach is done with the head horizontally on each side, a single-staged procedure is conducted with the head remaining in a neutral position thereby permitting simultaneous bilateral work. These techniques may result in different rates of postoperative pneumocephalus and residual hematoma volumes. An RCT could bring more insight, as current practice is based mostly on empirical evidence and results from individual perspectives and experiences.
9.3. Location of drainage and suction pressure
Aside from placement of the draining catheter (e.g. subdural vs. subgaleal), application of suction to such a drain is also matter of debate. A partial suction with negative pressure would prevent air moving in whilst allowing the patient to elevate the head without the risk of a concomitant pneumocephalus. Early mobilization may decrease complications like thrombotic events or pulmonary inhalation and infection. On the other hand, bed rest is thought to reduce recurrence rates by facilitating brain re-expansion (Mehta et al., 2018; Santarius et al., 2009).
9.4. Steroids as a treatment of cSDH
Since inflammatory factors may contribute to the formation of neo-membranes and propagate hematoma growth (Glover and Labadie, 1976), several studies have investigated corticosteroids as non-surgical treatment of cSDH. A recent meta-analysis indicated that adding corticosteroids to surgery might be effective, with lower re-intervention rates and mortality in patients, but cautioned that the results are limited by potential bias and the low level of evidence of the studies included (Holl et al., 2019). However, the results of a recent RCT have but these benefits into doubt (Hutchinson et al., 2020). Further, research with perhaps low-dose dexamethasone or shorter duration of dexamethasone treatment would be informative.
9.5. Postoperative antithrombotic medication management
As discussed previously, antithrombotics present a significant risk factor for the development of cSDH. However, the role of anticoagulation in the recurrence of cSDH remains less clear. While some studies have suggested that antithrombotics may represent a risk factor for recurrence (Chon et al., 2012; Lindvall and Koskinen, 2009; Bartek et al., 2017), others have not (Torihashi et al., 2008). An important factor that may explain this discrepancy is the timing of the re-initiation of antithrombotic medication. Recently, it has been shown that resumption of antithrombotics at day 3 postoperatively has no significant effect on recurrence rates (Guha et al., 2016). The subject remains an ongoing area of controversy and several papers have suggested that large studies need to be undertaken (Nassiri et al., 2020).
10. Conclusion
While cSDH is one of the oldest described neurosurgical conditions and remains one of the simplest neurosurgical pathologies to treat, much remains unclear about its pathogenesis and its management remains varied across centers. What is clear, however, is that the number of cases continues to rise, certainly attributable to the aging population, but also due to the increased use of antithrombotic medication, which remains a significant risk factor. A number of interesting advances have been made in the field and ongoing research to better direct treatment strategy is surely to surface in the coming years.
Disclosure
The authors declare no conflicts of interest.
Funding source
No external funding
Research Ethics Board Approval was not required.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bas.2021.100300.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abecassis I.J., Kim L.J. Craniotomy for treatment of chronic subdural hematoma. Neurosurg. Clin. 2017;28(2):229–237. doi: 10.1016/j.nec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Adhiyaman V., Chattopadhyay I., Irshad F., Curran D., Abraham S. Increasing incidence of chronic subdural haematoma in the elderly. QJM: Int. J. Med. 2017;110(6):375–378. doi: 10.1093/qjmed/hcw231. [DOI] [PubMed] [Google Scholar]
- Ahn J.H., Jun H.S., Kim J.H., Oh J.K., Song J.H., Chang I.B. Analysis of risk factor for the development of chronic subdural hematoma in patients with traumatic subdural hygroma. J. Kor. Neurosurg.Soc. 2016;59(6):622. doi: 10.3340/jkns.2016.59.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderazi Y., Brett F. Alcohol and the nervous system. Curr. Diagn. Pathol. 2007;13(3):203–209. [Google Scholar]
- Almenawer S.A., Farrokhyar F., Hong C., et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann. Surg. 2014;259(3):449–457. doi: 10.1097/SLA.0000000000000255. [DOI] [PubMed] [Google Scholar]
- Altaf I., Shams S., Vohra A.H. Radiolological predictors of recurrence of chronic subdural hematoma. Pakistan J. Med. Sci. 2018;34(1):194. doi: 10.12669/pjms.341.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo F.A., Rocha M.A., Mendes J.B., Andrade S.P. Atorvastatin inhibits inflammatory angiogenesis in mice through down regulation of VEGF, TNF-alpha and TGF-beta1. Biomed. Pharmacother. 2010;64(1):29–34. doi: 10.1016/j.biopha.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Asghar M., Adhiyaman V., Greenway M., Bhowmick B.K., Bates A. Chronic subdural haematoma in the elderly—a North Wales experience. J. Roy. Soc. Med. 2002;95(6):290–292. doi: 10.1258/jrsm.95.6.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspegren O.P., Åstrand R., Lundgren M.I., Romner B. Anticoagulation therapy a risk factor for the development of chronic subdural hematoma. Clin. Neurol. Neurosurg. 2013;115(7):981–984. doi: 10.1016/j.clineuro.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Baechli H., Nordmann A., Bucher H., Gratzl O. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg. Rev. 2004;27(4):263–266. doi: 10.1007/s10143-004-0337-6. [DOI] [PubMed] [Google Scholar]
- Ban S.P., Hwang G., Byoun H.S., et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018;286(3):992–999. doi: 10.1148/radiol.2017170053. [DOI] [PubMed] [Google Scholar]
- Bartek J., Jr., Sjåvik K., Kristiansson H., et al. Predictors of recurrence and complications after chronic subdural hematoma surgery: a population-based study. World neurosurgery. 2017;106:609–614. doi: 10.1016/j.wneu.2017.07.044. [DOI] [PubMed] [Google Scholar]
- Brennan P.M., Kolias A.G., Joannides A.J., et al. The management and outcome for patients with chronic subdural hematoma: a prospective, multicenter, observational cohort study in the United Kingdom. J. Neurosurg. 2016;127(4):732–739. doi: 10.3171/2016.8.JNS16134. [DOI] [PubMed] [Google Scholar]
- Castellani R.J., Mojica-Sanchez G., Schwartzbauer G., Hersh D.S. Symptomatic acute-on-chronic subdural hematoma: a clinicopathological study. Am. J. Forensic Med. Pathol. 2017;38(2):126–130. doi: 10.1097/PAF.0000000000000300. [DOI] [PubMed] [Google Scholar]
- Chon K.H., Lee J.M., Koh E.J., Choi H.Y. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012;154(9):1541–1548. doi: 10.1007/s00701-012-1399-9. [DOI] [PubMed] [Google Scholar]
- Cuypers V., Van de Velde M., Devroe S. Intracranial subdural haematoma following neuraxial anaesthesia in the obstetric population: a literature review with analysis of 56 reported cases. Int. J. Obstet. Anesth. 2016;25:58–65. doi: 10.1016/j.ijoa.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Ding H., Liu S., Quan X., Liao S., Liu L. Subperiosteal versus subdural drain after burr hole drainage for chronic subdural hematomas: a systematic review and meta-analysis. World Neurosurg. 2020;136:90–100. doi: 10.1016/j.wneu.2019.12.180. [DOI] [PubMed] [Google Scholar]
- Edlmann E., Giorgi-Coll S., Whitfield P.C., Carpenter K.L.H., Hutchinson P.J. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J. Neuroinflammation. 2017;14(1):108. doi: 10.1186/s12974-017-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes de Oliveira M. Chronic subdural hematomas and pursuit of nonsurgical treatment alternatives. World Neurosurg. 2019;126:481–483. doi: 10.1016/j.wneu.2019.03.151. [DOI] [PubMed] [Google Scholar]
- Frechon P., Emery E., Gaberel T. Is there an interest in performing a systematic CT scan within the first two months after chronic subdural hematoma evacuation? A ten-year single-center retrospective study. Clin. Neurol. Neurosurg. 2020:105682. doi: 10.1016/j.clineuro.2020.105682. [DOI] [PubMed] [Google Scholar]
- Gaist D., Rodríguez L.A.G., Hellfritzsch M., et al. Association of antithrombotic drug use with subdural hematoma risk. Jama. 2017;317(8):836–846. doi: 10.1001/jama.2017.0639. [DOI] [PubMed] [Google Scholar]
- Ganau L., Prisco L., Ganau M. High altitude induced bilateral non-traumatic subdural hematoma. Aviat Space Environ. Med. 2012;83(9):899–901. doi: 10.3357/asem.3331.2012. [DOI] [PubMed] [Google Scholar]
- Gandhoke G.S., Kaif M., Choi L., Williamson R.W., Nakaji P. Histopathological features of the outer membrane of chronic subdural hematoma and correlation with clinical and radiological features. J. Clin. Neurosci. 2013;20(10):1398–1401. doi: 10.1016/j.jocn.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Gelabert-González M., Iglesias-Pais M., García-Allut A., Martínez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin. Neurol. Neurosurg. 2005;107(3):223–229. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Glancz L.J., Poon M.T.C., Coulter I.C., Hutchinson P.J., Kolias A.G., Brennan P.M. Does drain position and duration influence outcomes in patients undergoing burr-hole evacuation of chronic subdural hematoma? Lessons from a UK multicenter prospective cohort study. Neurosurgery. 2019;85(4):486–493. doi: 10.1093/neuros/nyy366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D., Labadie E.L. Physiopathogenesis of subdural hematomas: Part 2: inhibition of growth of experimental hematomas with dexamethasone. J. Neurosurg. 1976;45(4):393–397. doi: 10.3171/jns.1976.45.4.0393. [DOI] [PubMed] [Google Scholar]
- Greuter L., Hejrati N., Soleman J. Type of drain in chronic subdural hematoma-A systematic review and meta-analysis. Front. Neurol. 2020;11:312. doi: 10.3389/fneur.2020.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha D., Coyne S., Macdonald R.L. Timing of the resumption of antithrombotic agents following surgical evacuation of chronic subdural hematomas: a retrospective cohort study. J. Neurosurg. 2016;124(3):750–759. doi: 10.3171/2015.2.JNS141889. [DOI] [PubMed] [Google Scholar]
- Holl D.C., Volovici V., Dirven C.M., et al. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to present to future. World neurosurgery. 2018;116:402–411. doi: 10.1016/j.wneu.2018.05.037. e402. [DOI] [PubMed] [Google Scholar]
- Holl D.C., Volovici V., Dirven C.M., et al. Corticosteroid treatment compared with surgery in chronic subdural hematoma: a systematic review and meta-analysis. Acta Neurochir. 2019;161(6):1231–1242. doi: 10.1007/s00701-019-03881-w. [DOI] [PubMed] [Google Scholar]
- Holl D.C., Chari A., Iorio-Morin C., et al. Study protocol on defining core outcomes and data elements in chronic subdural haematoma. Neurosurgery. 2021;89(4):720–725. doi: 10.1093/neuros/nyab268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson P.J., Edlmann E., Bulters D., Zolnourian A., Holton P., Suttner N., Agyemang K., Thomson S., Anderson I.A., Thomson S., Anderson IA, Al-Tamimi YZ, Henderson D. Trial of dexamethasone for chronic subdural hematoma. New England J. Med. 2020 Dec 31;383(27) doi: 10.1056/NEJMoa2020473. 2616-27. [DOI] [PubMed] [Google Scholar]
- Im T.S., Lee Y.S., Suh S.J., Lee J.H., Ryu K.Y., Kang D.G. The efficacy of titanium burr hole cover for reconstruction of skull defect after burr hole trephination of chronic subdural hematoma. Korean J. Nutr. 2014;10(2):76–81. doi: 10.13004/kjnt.2014.10.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivamoto H.S., Lemos H.P., Jr., Atallah A.N. Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World Neurosurg. 2016;86:399–418. doi: 10.1016/j.wneu.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Kageyama H., Toyooka T., Tsuzuki N., Oka K. Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J. Neurosurg. 2013;119(2):332–337. doi: 10.3171/2013.3.JNS122162. [DOI] [PubMed] [Google Scholar]
- Kanat A., Kayaci S., Yazar U., Kazdal H., Terzi Y. Chronic subdural hematoma in adults: why does it occur more often in males than females? Influence of patient's sexual gender on occurrence. J. Neurosurg. Sci. 2010;54(3):99–103. [PubMed] [Google Scholar]
- Kerr E.E., Prevedello D.M., Jamshidi A., Ditzel Filho L.F., Otto B.A., Carrau R.L. Immediate complications associated with high-flow cerebrospinal fluid egress during endoscopic endonasal skull base surgery: report of 2 cases. Neurosurg. Focus. 2014;37(4):E3. doi: 10.3171/2014.7.FOCUS14294. [DOI] [PubMed] [Google Scholar]
- Khan Q., Wharen R., Grewal S., et al. Overdrainage shunt complications in idiopathic normal-pressure hydrocephalus and lumbar puncture opening pressure. J. Neurosurg. 2013;119(6):1498. doi: 10.3171/2013.7.JNS13484. [DOI] [PubMed] [Google Scholar]
- Kolias A.G., Chari A., Santarius T., Hutchinson P.J. Chronic subdural haematoma: modern management and emerging therapies. Nat. Rev. Neurol. 2014;10(10):570–578. doi: 10.1038/nrneurol.2014.163. [DOI] [PubMed] [Google Scholar]
- Kristof R.A., Grimm J.M., Stoffel-Wagner B. Cerebrospinal fluid leakage into the subdural space: possible influence on the pathogenesis and recurrence frequency of chronic subdural hematoma and subdural hygroma. J. Neurosurg. 2008;108(2):275–280. doi: 10.3171/JNS/2008/108/2/0275. [DOI] [PubMed] [Google Scholar]
- Kudo H., Kuwamura K., Izawa I., Sawa H., Tamaki N. Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol. Med.-Chir. 1992;32(4):207–209. doi: 10.2176/nmc.32.207. [DOI] [PubMed] [Google Scholar]
- Kung W.M., Hung K.S., Chiu W.T., et al. Quantitative assessment of impaired postevacuation brain re-expansion in bilateral chronic subdural haematoma: possible mechanism of the higher recurrence rate. Injury. 2012;43(5):598–602. doi: 10.1016/j.injury.2010.07.240. [DOI] [PubMed] [Google Scholar]
- Lee K. The pathogenesis and clinical significance of traumatic subdural hygroma. Brain Inj. 1998;12(7):595–603. doi: 10.1080/026990598122359. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Bae W.K., Bae H.G., Yun I.G. The fate of traumatic subdural hygroma in serial computed tomographic scans. J. Kor. Med. Sci. 2000;15(5):560–568. doi: 10.3346/jkms.2000.15.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega B.C., Danish S.F., Malhotra N.R., Sonnad S.S., Stein S.C. Choosing the best operation for chronic subdural hematoma: a decision analysis. J. Neurosurg. 2010;113(3):615–621. doi: 10.3171/2009.9.JNS08825. [DOI] [PubMed] [Google Scholar]
- Leroy H.-A., Aboukaïs R., Reyns N., et al. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J. Clin. Neurosci. 2015;22(12):1895–1900. doi: 10.1016/j.jocn.2015.03.064. [DOI] [PubMed] [Google Scholar]
- Li T., Wang D., Tian Y., et al. Effects of atorvastatin on the inflammation regulation and elimination of subdural hematoma in rats. J. Neurol. Sci. 2014;341(1–2):88–96. doi: 10.1016/j.jns.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Lindvall P., Koskinen L.O. Anticoagulants and antiplatelet agents and the risk of development and recurrence of chronic subdural haematomas. J. Clin. Neurosci. 2009;16(10):1287–1290. doi: 10.1016/j.jocn.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Lu H., Wang X.-B., Tang Y. Subdural hematoma associated with high altitude. Chinese Med J. 2015;128(3):407. doi: 10.4103/0366-6999.150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R.L. In: Youmans & Winn Neurological Surgery. seventh ed. Winn R.H., editor. Elsevier; 2017. Pathophysiology of chronic subdural hematoma. [Google Scholar]
- MacFarlane M., Weerakkody Y., Kathiravel Y. Chronic subdural haematomas are more common on the left than on the right. J. Clin. Neurosci. 2009;16(5):642–644. doi: 10.1016/j.jocn.2008.07.074. [DOI] [PubMed] [Google Scholar]
- Markwalder T.-M., Steinsiepe K.F., Rohner M., Reichenbach W., Markwalder H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J. Neurosurg. 1981;55(3):390–396. doi: 10.3171/jns.1981.55.3.0390. [DOI] [PubMed] [Google Scholar]
- Marshman L.A., Manickam A., Carter D. Risk factors for chronic subdural haematoma formation do not account for the established male bias. Clin. Neurol. Neurosurg. 2015;131:1–4. doi: 10.1016/j.clineuro.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Mehta V., Harward S.C., Sankey E.W., Nayar G., Codd P.J. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J. Clin. Neurosci. 2018;50:7–15. doi: 10.1016/j.jocn.2018.01.050. [DOI] [PubMed] [Google Scholar]
- Miah I.P., Holl D.C., Peul W.C., et al. Dexamethasone therapy versus surgery for chronic subdural haematoma (DECSA trial): study protocol for a randomised controlled trial. Trials. 2018;19(1):1–10. doi: 10.1186/s13063-018-2945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miah I.P., Tank Y., Rosendaal F.R., et al. Radiological prognostic factors of chronic subdural hematoma recurrence: a systematic review and meta-analysis. Neuroradiology. 2021;63(1):27–40. doi: 10.1007/s00234-020-02558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missios S. Hippocrates, Galen, and the uses of trepanation in the ancient classical world. Neurosurg. Focus. 2007;23(1):E11. doi: 10.3171/foc.2007.23.1.11. [DOI] [PubMed] [Google Scholar]
- Moen V., Dahlgren N., Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101(4):950–959. doi: 10.1097/00000542-200410000-00021. [DOI] [PubMed] [Google Scholar]
- MORI K., MAEDA M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol. Med.-Chir. 2001;41(8):371–381. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- Motiei-Langroudi R., Stippler M., Shi S., et al. Factors predicting reoperation of chronic subdural hematoma following primary surgical evacuation. 2017;129(5):1143. doi: 10.3171/2017.6.JNS17130. [DOI] [PubMed] [Google Scholar]
- Nagahori T., Nishijima M., Takaku A. Histological study of the outer membrane of chronic subdural hematoma: possible mechanism for expansion of hematoma cavity. No shinkei geka Neurological surgery. 1993;21(8):697–701. [PubMed] [Google Scholar]
- Nakaguchi H., Tanishima T., Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J. Neurosurg. 2001;95(2):256–262. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- Nassiri F., Hachem L.D., Wang J.Z., et al. Reinitiation of anticoagulation after surgical evacuation of subdural hematomas. World Neurosurgery. 2020;135:e616–e622. doi: 10.1016/j.wneu.2019.12.080. [DOI] [PubMed] [Google Scholar]
- Nayil K., Ramzan A., Sajad A., et al. Subdural hematomas: an analysis of 1181 Kashmiri patients. World neurosurgery. 2012;77(1):103–110. doi: 10.1016/j.wneu.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Oishi M., Toyama M., Tamatani S., Kitazawa T., Saito M. Clinical factors of recurrent chronic subdural hematoma. Neurol. Med.-Chir. 2001;41(8):382–386. doi: 10.2176/nmc.41.382. [DOI] [PubMed] [Google Scholar]
- Pahatouridis D., Alexiou G.A., Fotakopoulos G., et al. Chronic subdural haematomas: a comparative study of an enlarged single burr hole versus double burr hole drainage. Neurosurg. Rev. 2013;36(1):151–154. doi: 10.1007/s10143-012-0412-3. ; discussion 154-155. [DOI] [PubMed] [Google Scholar]
- Park S.-H., Lee S.-H., Park J., Hwang J.-H., Hwang S.-K., Hamm I.-S. Chronic subdural hematoma preceded by traumatic subdural hygroma. J. Clin. Neurosci. 2008;15(8):868–872. doi: 10.1016/j.jocn.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Pedersen C.B., Sundbye F., Poulsen F.R. No value of routine brain computed tomography 6 weeks after evacuation of chronic subdural hematoma. Surg. J. 2017;3(4):e174–e176. doi: 10.1055/s-0037-1607215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pripp A.H., Stanišić M. The correlation between pro- and anti-inflammatory cytokines in chronic subdural hematoma patients assessed with factor analysis. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0090149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S., Zhuo W., Sun C., Su Z., Yan A., Shen L. Effects of atorvastatin on chronic subdural hematoma: a systematic review. Medicine (Baltim.) 2017;96(26) doi: 10.1097/MD.0000000000007290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R., Hegde T. Chronic subdural hematomas—causes of morbidity and mortality. Surg. Neurol. 2007;67(4):367–372. doi: 10.1016/j.surneu.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Rauhala M., Luoto T.M., Huhtala H., et al. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J. Neurosurg. 2019;1(aop):1–11. doi: 10.3171/2018.12.JNS183035. [DOI] [PubMed] [Google Scholar]
- Rettenmaier L.A., Park B.J., Holland M.T., et al. Value of targeted epidural blood patch and management of subdural hematoma in spontaneous intracranial hypotension: case report and review of the literature. World neurosurgery. 2017;97:27–38. doi: 10.1016/j.wneu.2016.09.076. [DOI] [PubMed] [Google Scholar]
- Ro H.W., Park S.K., Jang D.K., Yoon W.S., Jang K.S., Han Y.M. Preoperative predictive factors for surgical and functional outcomes in chronic subdural hematoma. Acta Neurochir. 2016;158(1):135–139. doi: 10.1007/s00701-015-2625-z. [DOI] [PubMed] [Google Scholar]
- Rust T., Kiemer N., Erasmus A. Chronic subdural haematomas and anticoagulation or anti-thrombotic therapy. J. Clin. Neurosci. 2006;13(8):823–827. doi: 10.1016/j.jocn.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Sahyouni R., Goshtasbi K., Mahmoodi A., Tran D.K., Chen J.W. Chronic subdural hematoma: a historical and clinical perspective. World neurosurgery. 2017;108:948–953. doi: 10.1016/j.wneu.2017.09.064. [DOI] [PubMed] [Google Scholar]
- Sahyouni R., Mahboubi H., Tran P., Roufail J.S., Chen J.W. Membranectomy in chronic subdural hematoma: meta-analysis. World Neurosurg. 2017;104:418–429. doi: 10.1016/j.wneu.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius T., Kirkpatrick P.J., Ganesan D., et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374(9695):1067–1073. doi: 10.1016/S0140-6736(09)61115-6. [DOI] [PubMed] [Google Scholar]
- Schucht P., Fischer U., Fung C., et al. Follow-up computed tomography after evacuation of chronic subdural hematoma. N. Engl. J. Med. 2019;380:1186–1187. doi: 10.1056/NEJMc1812507. [DOI] [PubMed] [Google Scholar]
- Soleman J., Lutz K., Schaedelin S., et al. Subperiosteal vs subdural drain after burr-hole drainage of chronic subdural hematoma: a randomized clinical trial (cSDH-Drain-Trial) Neurosurgery. 2019;85(5):E825–e834. doi: 10.1093/neuros/nyz095. [DOI] [PubMed] [Google Scholar]
- Sood P., Sinson G.P., Cohen E.P. Subdural hematomas in chronic dialysis patients: significant and increasing. Clin. J. Am. Soc. Nephrol. 2007;2(5):956–959. doi: 10.2215/CJN.03781106. [DOI] [PubMed] [Google Scholar]
- Sousa E.B., Brandão L.F., Tavares C.B., Borges I.B., Neto N.G.F., Kessler I.M. Epidemiological characteristics of 778 patients who underwent surgical drainage of chronic subdural hematomas in Brasília, Brazil. BMC Surg. 2013;13(1):5. doi: 10.1186/1471-2482-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squier W. The “Shaken Baby” syndrome: pathology and mechanisms. Acta Neuropathol. 2011;122(5):519. doi: 10.1007/s00401-011-0875-2. [DOI] [PubMed] [Google Scholar]
- Stanišić M., Pripp A.H. A reliable grading system for prediction of chronic subdural hematoma recurrence requiring reoperation after initial burr-hole surgery. Neurosurgery. 2017;81(5):752–760. doi: 10.1093/neuros/nyx090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisic M., Aasen A.O., Pripp A.H., et al. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: a prospective study. Inflamm. Res. 2012;61(8):845–852. doi: 10.1007/s00011-012-0476-0. [DOI] [PubMed] [Google Scholar]
- Stanišić M., Hald J., Rasmussen I.A., et al. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: a prospective study of 107 operated patients. Acta Neurochir (Wien) 2013;155(2):323–333. doi: 10.1007/s00701-012-1565-0. ; discussion 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanweer O., Frisoli F.A., Bravate C., et al. Tranexamic acid for treatment of residual subdural hematoma after bedside twist-drill evacuation. World Neurosurg. 2016;91:29–33. doi: 10.1016/j.wneu.2016.03.062. [DOI] [PubMed] [Google Scholar]
- Toi H., Kinoshita K., Hirai S., et al. Present epidemiology of chronic subdural hematoma in Japan: analysis of 63,358 cases recorded in a national administrative database. J. Neurosurg. 2017;128(1):222–228. doi: 10.3171/2016.9.JNS16623. [DOI] [PubMed] [Google Scholar]
- Torihashi K., Sadamasa N., Yoshida K., Narumi O., Chin M., Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008;63(6):1125–1129. doi: 10.1227/01.NEU.0000335782.60059.17. [DOI] [PubMed] [Google Scholar]
- Tzioumi D., Oates R.K. Subdural hematomas in children under 2 years. Accidental or inflicted? A 10-year experience. Child Abuse Negl. 1998;22(11):1105–1112. doi: 10.1016/s0145-2134(98)00093-3. [DOI] [PubMed] [Google Scholar]
- Unterhofer C., Freyschlag C.F., Thomé C., Ortler M. Opening the internal hematoma membrane does not alter the recurrence rate of chronic subdural hematomas: a prospective randomized trial. World neurosurgery. 2016;92:31–36. doi: 10.1016/j.wneu.2016.04.081. [DOI] [PubMed] [Google Scholar]
- van Havenbergh T., van Calenbergh F., Goffin J., Plets C. Outcome of chronic subdural haematoma: analysis of prognostic factors. Br. J. Neurosurg. 1996;10(1):35–39. doi: 10.1080/02688699650040502. [DOI] [PubMed] [Google Scholar]
- Waqas M., Vakhari K., Weimer P.V., Hashmi E., Davies J.M., Siddiqui A.H. Safety and effectiveness of embolization for chronic subdural hematoma: systematic review and case series. World Neurosurg. 2019;126:228–236. doi: 10.1016/j.wneu.2019.02.208. [DOI] [PubMed] [Google Scholar]
- Wilms G., Marchal G., Geusens E., et al. Isodense subdural haematomas on CT: MRI findings. Neuroradiology. 1992;34(6):497–499. doi: 10.1007/BF00598959. [DOI] [PubMed] [Google Scholar]
- Yamashima T., Friede R. Why do bridging veins rupture into the virtual subdural space? J. Neurol. Neurosurg. Psychiatr. 1984;47(2):121–127. doi: 10.1136/jnnp.47.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurgery Clinics. 2017;28(2):205–210. doi: 10.1016/j.nec.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Zetterberg H., Tullhög K., Hansson O., Minthon L., Londos E., Blennow K. Low incidence of post-lumbar puncture headache in 1,089 consecutive memory clinic patients. Eur. Neurol. 2010;63(6):326–330. doi: 10.1159/000311703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.