Abstract
Biofilms are biological viscoelastic gels composed of bacterial cells embedded in a self-secreted polymeric extracellular matrix (ECM). In environmental settings, such as in the rhizosphere and phyllosphere, biofilm colonization occurs at the solid–air interface. The biofilms’ ability to colonize and expand over these surfaces depends on the formation of osmotic gradients and ECM viscoelastic properties. In this work, we study the influence of biofilm ECM components on its viscoelasticity and expansion, using the model organism Bacillus subtilis and deletion mutants of its three major ECM components, TasA, EPS and BslA. Using a multi-scale approach, we quantified macro-scale viscoelasticity and expansion dynamics. Furthermore, we used a microsphere assay to visualize the micro-scale expansion patterns. We find that the viscoelastic phase angle is likely the best viscoelastic parameter correlating to biofilm expansion dynamics. Moreover, we quantify the sensitivity of the biofilm to changes in substrate water potential as a function of ECM composition. Finally, we find that the deletion of ECM components significantly increases the coherence of micro-scale colony expansion patterns. These results demonstrate the influence of ECM viscoelasticity and substrate water potential on the expansion of biofilm colonies on wet surfaces at the air–solid interface, commonly found in natural environments.
Keywords: biofilm, extracellular matrix, viscoelasticity, morphogenesis, biofilm mechanics, gel expansion
1. Introduction
Bacterial biofilms are surface-attached bacterial communities embedded in a self-secreted polymeric extracellular matrix (ECM). The majority of biofilms form in aqueous environments, either submerged in liquid or on wet surfaces at the solid–air interface [1]. The ECM is crucial for the survival of the bacterial community: it provides hydration, nutrient storage and protection from external environmental conditions, including mechanical forces, osmolarity fluctuations and antimicrobial treatments [1]. Biofilms self-assemble into a structure analogous to a colloidal gel that exhibits viscoelastic mechanical behaviour [2,3]. The viscoelastic behaviour of biofilms confers resistance to fluctuating shear stresses, and it enables the morphogenesis of higher-order biofilm structures such as bacterial streamers and channel networks [4–6].
The biofilms’ viscoelastic response is governed by the ECM composition, which is extremely varied across bacterial species [7]. The primary ECM components are polysaccharides, proteins and extracellular DNA (eDNA). These components structure the ECM through physicochemical interactions such as hydrogen bonding, polymer entanglement and ionic cross-linking to determine the biofilms’ viscoelastic behaviour [8,9]. Yet, as with colloidal gels, environmental variables influence biofilm rheology and in turn control morphogenesis [3,10].
Biofilm morphogenesis is determined by the interplay between the biofilms’ viscoelastic properties and internally generated physical forces. In water-saturated conditions, biofilms are known to display characteristic three-dimensional geometries such as mushrooms, streamers and channels [5,11,12]. While at solid–air interfaces, like upon solid agar, biofilms grow as colonies and display myriad distinctive morphologies depending upon the physical characteristics of the biofilm and the substrate. In this diffusion-dominated condition, the spreading is driven by cell growth, surfactant production, and the balance between osmotic pressure gradients and elastic stresses conferred by the ECM [13–16]. In turn, the surface on which bacterial colonies form influences colony morphology and expansion behaviour through its water availability [16], mechanical stiffness [17] and adhesive properties [13].
The ECM generates osmotic gradients that drive liquid from the agar matrix into the biofilm matrix [15]. Furthermore, these osmotic gradients are the driving force behind the radial expansion of the colonies and can lead to the indentation of soft surfaces [14,15]. The radial expansion of colonies generates growth-induced compressive stress, which leads to mechanical instabilities causing buckling of the biofilm and the emergence of buckling patterns [4,18,19]. As the biofilms’ viscoelastic properties influence mechanical instabilities, the ECM plays a key role in their occurrence. However, the spatial variations in biofilm micro-scale expansion as a function of ECM composition remain understudied. Concomitantly, the link between macro-scale viscoelastic properties and micro-scale expansion behaviour is yet to be fully understood.
The model organism Bacillus subtilis is a rod-shaped, soil-dwelling, gram-positive bacterium commonly found in the rhizosphere of plants. The ECM of wild-type B. subtilis NCIB 3610 is well characterized [20]. The main components of the B. subtilis ECM are the proteins TasA and BslA, the exo-polysaccharide EPS and eDNA [20,21]. TasA forms amyloid fibres that promote bacterial cell–cell attachment and influence the biofilms’ internal cohesion [22]. The BslA protein is a hydrophobin that forms a hydrophobic layer acting as a surface coating for the ECM [23], which binds to the major polysaccharide component EPS [24]. The morphology of plate-grown B. subtilis biofilms transitions from cell chaining [19] through micro-scale cell bundles [4] to macro-scale wrinkles [12,19]. Controlling the ECM composition of the biofilm matrix by using well-studied deletion mutants allows a systematic study of the influence of the ECM components on the micro-scale morphogenesis and macro-scale viscoelastic properties.
To investigate the influence of the ECM components and osmotic pressure on biofilm mechanical properties and colony expansion behaviour, we use a library of deletion mutants of the ECM of B. subtilis biofilms (ΔbslA, ΔtasA and ΔepsO) and its wild-type (WT). We use a multi-scale approach to link the macro-scale viscoelastic properties of the WT and mutants with the micro-scale biofilm colony expansion behaviour. We reveal how micro-scale biofilm expansion behaviours are influenced by ECM composition and relate characteristic expansion behaviours of the colonies to their viscoelastic properties.
2. Methods
2.1. Culture conditions and growth
Experiments were performed using B. subtilis strain NCIB 3610 WT (purchased from American Type Culture Collection, ATCCⓇ 6051), the exo-polysaccharide deletion mutant ΔepsO, the TasA deletion mutant ΔtasA and the BslA deletion mutant ΔbslA. The deletion mutants carried the following fluorescent reporters: mCherry for ΔtasA and GFP for ΔepsO and ΔbslA. Single colonies were grown from frozen stocks on Luria broth (LB) agar plates at 30°C for 24 h. B. subtilis suspensions were grown overnight after inoculating 3 ml of Luria broth glycerol manganese (LBGM) media (20 g LB, 0.1 mM MnSO4 and 1% v/v glycerol) with cells from a single colony and incubated at 30°C while shaking at 200 r.p.m. Subcultures were prepared by diluting the overnight culture 1 : 100 and incubated under the same conditions for 5 h. The following antibiotics were added to the culture medium of the deletion mutants to maintain the mutation: 3 μg ml−1 tetracycline and 100 μg ml−1 spectinomycin to ΔepsO, 50 μg ml−1 kanamycin and 100 μg ml−1 spectinomycin to ΔtasA, and 10 μg ml−1 chloramphenicol and 25 μg ml−1 kanamycin to ΔbslA.
The growth curves for the WT and the mutants were characterized by measuring the OD600 in a plate reader (Synergy HTX, BioTek Instruments). The bacterial suspensions were obtained by diluting the overnight culture 1 : 200 in 200 μl LBGM medium in 96-well plates. The OD600 measurements were performed in triplicate for 12 h at 30°C with continuous shaking.
2.2. Macro-scale characterization of the colony expansion
Colony expansion experiments were performed on 1% (w/v) LB and LBGM agar pads in glass-bottomed 12-well plates. LB and LBGM agar pads were fabricated in moulds (height = 1 mm, diameter = 22 mm) using glass coverslips on both top and bottom surfaces to ensure planar surfaces of the agar pads. Both glass coverslips were treated with Sigmacote (Sigma) to reduce the adhesion strength of the agar to glass. After fabrication, the agar pads were transferred to the glass-bottomed black 12-well plate with sterilized tweezers. The space between the wells was filled with 5 ml DH2O to minimize evaporation throughout the experiment. The agar pads were inoculated by adding a drop of 0.5 μl mid-exponential phase culture (OD600 = 0.5) in the centre of the pad. The inoculated agar pads were dried for 15 min in a sterile environment.
The 12-well plates were imaged on an inverted microscope (Ti-Eclipse 2, Nikon, Japan) equipped with a digital camera (Photometrics, Kinetics). The macro-scale radial expansion of biofilm colonies was imaged in bright-field configuration with a 2× objective and time-lapse imaging (1 frame per 2 min for 12 h) at 30°C. We measured a minimum of 3 replicates per conditions. Higher magnification images of the colony were acquired with a 10× objective in phase contrast for the WT and epifluorescence configuration for the mutants (mCherry for ΔtasA; GFP for ΔepsO and ΔbslA).
A custom Python script was used to calculate the radial expansion of the biofilm colonies. Images were preprocessed using averaged background subtraction and median filtering. The colony boundary was obtained through holistically nested edge detection (HED) [25]. HED transformed images were binarized using the Otsu threshold and the findContours function was applied in combination with the convexHull function to obtain the colony radius.
To further characterize the biofilms’ physical parameters, the contact angle, as well as the solid content of the biofilm, was measured. Rheological measurements were performed to obtain the biofilms’ rheological properties. The water potential of the agar plates was obtained by potentiometer measurements. The details of the characterization are described in the electronic supplementary material.
2.3. Micro-scale characterization of the colony expansion
To perform the micro-scale characterization of colony expansion, 12-well plates were prepared with 1% (w/v) LBGM agar pads, which were fabricated using the same mould and inoculation procedure as described in §2.2. In order to image the micro-scale expansion behaviour within the biofilm colony, we added of 1 μm neutrally charged polystyrene microsphere (GFP or mCherry) solution (Fluorospheres, Sigma) at a concentration of 0.002% (w/v). We added mCherry microspheres for ΔepsO and ΔbslA mutants and GFP microspheres for ΔtasA. The microsphere suspension was sonicated for 15 min to eliminate aggregates. The microsphere volumes were deposited 15 min after the bacterial inoculation with a spatial offset from the centre of the bacterial suspension of 250 μm using a microscope-mounted micro-manipulator system. The well plates were then placed on the microscope stage. After 1 h of acclimation, imaging was performed using an inverted microscope (Ti-Eclipse 2, Nikon, Japan) with a 10× objective at 30°C. We acquired images of each agar pad every minute for 14 h, using phase contrast and epifluorescence imaging (mCherry and GFP). For all conditions, we performed the experiments for at least three replicates.
Particle tracks were obtained on median filtered images using the Python package Trackpy. Tracks were masked using the initial seeding geometry and then the final colony morphology. This was to ensure particles that remained outside of the biofilm colony throughout the experiment were excluded from the analysis. Unmoved particles were defined as particles whose convex hull was lower than a 4 μm2 threshold over the duration of their track. To calculate the orientation order parameter and orientation maps, we used the aligned by Fourier transform (AFT) Python module [26]. AFT applies a kernel Fourier transform to the trajectory maps generated from the Trackpy analysis. Finally, we calculated the probability density function of the particle velocities using an in house Matlab script.
3. Results
3.1. Macro-scale colony expansion
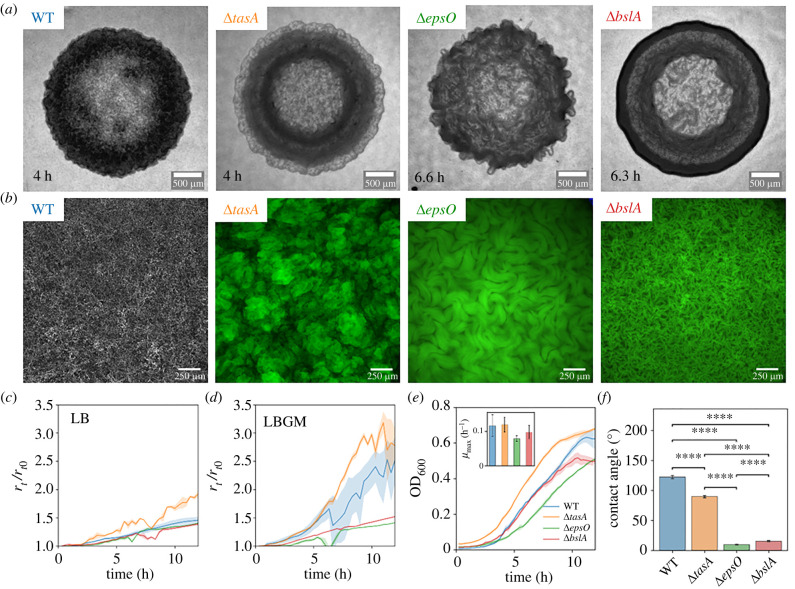
The ECM composition has a significant influence on the macro-scale morphology of the early-stage biofilm colony structure when grown on agar pads. Bacillus subtilis NCIB 3610 WT and the three mutants ΔepsO, ΔtasA and ΔbslA form circular colonies with different morphologies (figure 1a). The main structural feature in early-stage colonies (4–6 h) were rings in the expansion direction, stemming from differences in the density of the biomass, and wrinkles, corresponding to three-dimensional structures with different characteristic sizes. Imaging of colonies formed during several experiments allowed the recognition of distinctive features in the different mutant strains, whose typical images are reported in figure 1. The WT (figure 1a, left-hand panel) formed a compact colony with no defined structure, where no rings and wrinkles could be distinguished. The ΔtasA mutant formed colonies with distinct rings and wrinkles of different sizes and spatial densities. The wrinkles observed in ΔepsO displayed a nematic structure, suggesting the wrinkles exhibited local collective ordering. The ΔepsO colony was more homogeneous with larger wrinkles and a rugose appearance, whereas the ΔbslA colony was characterized by a dense outer ring. At the end of the experiment, the 15 h old biofilm colonies of WT and ΔtasA displayed a rugose perimeter while ΔbslA and ΔepsO showed a smooth perimeter (electronic supplementary material, figure S1). In the conditions we used in our experiments, no swarming behaviour caused by flagella-induced motility was observed.
Figure 1.
Characterization of the biofilm colonies formed by B. subtilis NCIB 3610 WT (blue), ΔtasA (orange), ΔepsO (green) and ΔbslA (red). (a) Bright-field images of the biofilm colonies formed on 1% (w/v) LBGM agar pads imaged at 2× magnification. We report images of colonies that reached a radius of 1.4 mm. The ages of the colonies are staggered and are indicated in the panels. The scale bar is 500 μm. (b) Representative images acquired at 10× of the central region of the biofilm colonies taken at the biofilm–agar interface in (a). The WT was imaged in phase contrast. The fluorescent mutants were imaged in an epifluorescence configuration (mCherry for ΔtasA; GFP for ΔepsO and ΔbslA). (c) Temporal evolution of the radius of the colonies, rt, normalized to the initial radius, r0, grown on LB agar pads. The line represents the mean while the shading represents the standard deviation of the mean. The same applies for the data shown in (d,e). (d) Normalized radius, rt/r0, as in (c), for colonies grown on LBGM agar pads. (e) Growth curves in LBGM medium of the planktonic cultures of the WT and the mutants. The inset shows the mean maximal growth rate μmax measured on the growth curves. The error bars show the standard deviation of the mean. Statistical analysis revealed that none of the mean maximal growth rates are significantly different. (f) Contact angle of deionized water measured on the biofilm surface after 18 h of growth. The bars represent the mean and the error bars show the standard deviation of the mean. The statistical difference was computed with a t-test (ns, not significant; ****p-value 0.0001).
The ECM composition has a large influence on the morphogenesis of colony structures. The macro-scale differences in morphology observed in figure 1a between the WT and the mutant strains correspond to differences at the micro-scale in the biofilm structure (figure 1b). The WT displayed a relatively homogeneous micro-scale network, while the ΔtasA exhibited a more patchy structure. Cell chaining and nematic ordering of cell bundles were found on a larger length scale (of the order of 300 μm) for the ΔepsO and on a smaller length scale (of the order of 50 μm) for the ΔbslA. The structuring of the ΔbslA mutant was less clearly defined compared to the ΔepsO mutant. Further, the ΔepsO and ΔbslA mutants show a more defined two-dimensional structure, while the ΔtasA shows a structure similar to vertical lift-off in the third dimension, corroborating the observations reported in [19].
The composition of the ECM components controls the expansion rate of the biofilm colony. In order to compare the colony expansion of the WT and the mutants, we computed the radius of the biofilm colonies grown on LB medium (figure 1c) and LBGM, a biofilm enhancing growth medium [27] (figure 1d). For B. subtilis LBGM medium activates the histine kinase KinD, which then initiates a signalling cascade to activate the SpoOA operon responsible for matrix secretion [28]. The comparison between the two media shows that LBGM enhances biofilm formation as the radial expansion for colonies grown on LBGM was faster compared to ones grown on LB. At t = 10 h the ratio of the temporal colony radius rt to initial colony radius r0 for WT was 66% higher on LBGM than LB, ΔtasA increased by 79%, whilst ΔbslA only marginally increased by 8% and ΔepsO showed no difference. For both growth media, the ΔtasA mutant (figure 1c,d) colonies expanded fastest with a mean expansion rate of 1.55 ± 0.13 μm min−1 (data format: mean ± standard deviation of the mean) between 5 and 10 h of growth and also shown in the highest ratio of rt/r0. The WT expanded slightly slower than ΔtasA mutant with a mean expansion rate of 1.29 ± 0.15 μm min−1 while the ΔepsO (expansion rate: 0.25 ± 0.05 μm min−1) and ΔbslA (expansion rate: 0.27 ± 0.02 μm min−1) mutants expanded slowest not showing a large difference in colony expansion between the two mutants. Based on the enhanced expansion difference between the WT and the mutants on LBGM, we decided to use LBGM as the growth medium for the following experiments.
Radial expansion differences of colonies between the WT and the mutants are due to differences in ECM composition and not differences in growth rate. We conducted a planktonic growth experiment measuring the OD600 (figure 1e). Similar to the radial colony expansion, we found that ΔtasA grew fastest (≈10% higher OD600 compared to the WT at t = 10 h), followed by the WT. Note that, in bulk, ΔbslA seems to grow ≈25% faster at t = 10 h than ΔepsO, which was not observed for the colony expansion. Though, when comparing the maximal growth rates (inset of figure 1e), there were only minor differences between the WT and the mutants in bulk growth.
The hydrophobicity of the biofilm colony surface changes as a result of the deletion of ECM components for B. subtilis biofilms. We measured the contact angle of a deionized water droplet on the surface of biofilm colonies grown on an LBGM agar plate (figure 1; electronic supplementary material). The droplet was placed on smooth areas of the colonies with little wrinkles, as excessive roughness due to wrinkles could cause a transition to the Cassie–Baxter wetting regime [29]. We found that the contact angle for the WT and the ΔtasA were approximately 120° and 90°, respectively, indicating a hydrophobic biofilm surface. Contrary, the contact angles of the ΔepsO and ΔbslA (approx. 16° and 10°, respectively) indicated a hydrophilic biofilm surface. The findings for ΔbslA and ΔepsO were in line with literature which describes how the binding of the hydrophobin BslA to the EPS at the exterior of the ECM confers hydrophobicity [24].
3.2. Rheological characterization
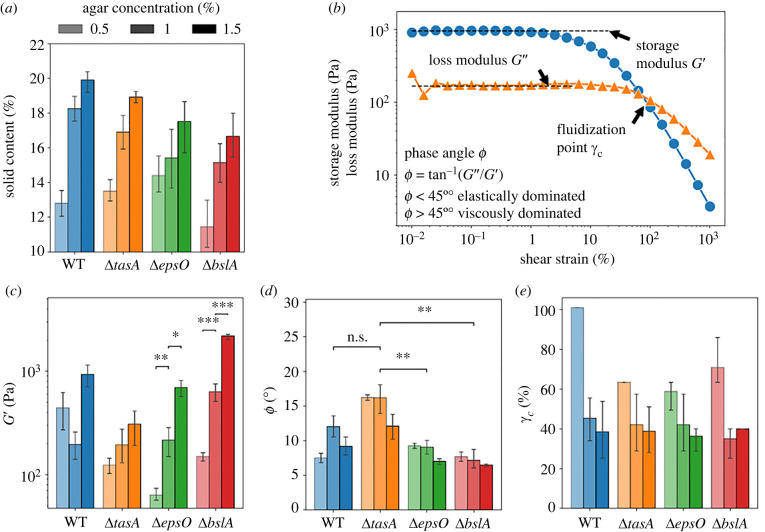
Different ECM components and the water potential of the substrate impact the rheological properties of the B. subtilis WT and the mutants’ biofilms, as shown by the characterization through solid content and rheological parameter measurements. We measured the solid content of the biofilms by dry mass measurements and three rheological parameters by performing shear strain sweeps (figure 2). The storage or elastic modulus G′ represents the elastic solid-like properties which can be interpreted as the stiffness of a sample. The phase angle indicates the ratio of elastic to viscous contribution to the overall viscoelastic response, computed as . We obtained both G′ and G″ from the plateau region (shear strain, ) (figure 2b). Finally, the cross-over strain or fluidization point γc describes the point at which the viscous response dominates the elastic response indicative of a microstructural breakdown. The rheological measurements were performed by scraping the biofilm off the agar plates and transferring it to the rheometer. The procedure potentially affects the biofilms’ structure. However, the rheological measurements remain valid as shown in [30], especially when used to compare different growth conditions and mutants.
Figure 2.
Rheological characterization of the biofilm colonies. (a) Solid content of the colonies formed by B. subtilis NCIB 3610 WT (blue), ΔtasA (orange), ΔepsO (green) and ΔbslA (red). The agar concentration of the agar plates is indicated by the shading of the columns. Colour-code and shading apply to all panels in this figure. (b) Representative plot of a shear strain sweep of a biofilm sample showing the storage modulus computed from the plateau region G′ (blue line) and the loss modulus computed from the plateau region G″ (orange line) as well as the fluidization point γc. (c) The storage modulus, G′, obtained for the WT and the mutants. The statistical difference was computed between G′ at different agar concentration for ΔepsO and ΔbslA with a t-test and is indicated in the panel (ns: not significant; *p-value = 0.05, **p-value 0.01, ***p-value 0.001). (d) The phase angle, , obtained for the WT and the mutants. The statistical difference was computed between ΔtasA, the highest phase angle, and the other strains at 1% (w/v) agar concentration and indicated in the panel (ns: not significant; *p-value = 0.05, **p-value 0.01, ***p-value 0.001). (e) The cross-over strain, γc, computed for the WT and the mutants.
The average solid content of the biofilms for all the biofilms tested increases with increasing water potential of the substrate. These results were obtained by firstly measuring the water potential of each agar concentration (for details, see electronic supplementary material, figure S2). The water potential describes how freely water molecules can move within a system and is measured in MPa [31]. The water potential decreases with agar concentration and was as follows: , and . We then measured the solid content of the biofilms and found that for the WT and all of the mutants, the average solid content lies between 11% and 19% and increases with the agar concentration (figure 2a). This trend is expected as the 0.5% (w/v) agar pads have the highest water potential, meaning the water molecules are least constricted and thus are more easily transferred into the biofilm.
The storage modulus G′ is highest for biofilm grown on 1.5% (w/v) agar plates, meaning that a stiffer material is formed at the lower water potential of the substrate (figure 2c; electronic supplementary material, figure S2b). Comparing G′ between the different mutants, we found that the absolute values of G′ varied strongly. ΔbslA showed the highest G′ (≈2450 ± 180 Pa) followed by the WT (≈1000 ± 190 Pa), when grown on 1.5% (w/v) agar. For ΔepsO and ΔtasA, we obtained a G′ approximately 25% and 63% lower than the WT respectively indicating a weaker biofilm structure. The sensitivity of G′ to the water potential of the substrate varied with ECM component deletion. ΔbslA and ΔepsO were the most sensitive to the water potential of the substrate, with G’ changing by a factor of 14.6 and 11, respectively, from 0.5% (w/v) to 1.5% (w/v) agar, whereas ΔtasA displayed the lowest sensitivity of the mutants with a factor of 2.5 from highest to lowest water potential of the substrate. The differences in G′ between the mutants and the WT were less pronounced for biofilms grown on the 1% (w/v) agar plates with the exception of ΔbslA. Here, WT, ΔtasA and ΔepsO showed negligible difference whereas the ΔbslA is three times larger. The elasticity of the WT contrasted with the mutants by not correlating strongly with the water potential of the substrate, a finding seen within Vibrio cholerae [32].
Low phase angles are obtained for all the biofilms tested (with ranging from 7° to 16°). This indicates that each biofilm deformed viscoelastically with elastically dominant behaviour (figure 2d). The phase angle did not display a clear dependence on the agar concentrations. Therefore, the impact of the water potential of the substrate on the phase angle could be considered negligible. For the ΔtasA mutant, the highest phase angles were obtained meaning the viscoelastic behaviour was the most fluid-like.
The cross-over strain γc is highest at lower agar concentrations for all studied strains (figure 2e). This is due to the biofilm structure dissipating more energy through viscous dissipation at lower agar concentrations, therefore displaying a higher fluidization point. Differences between the mutants at the agar concentrations 1% (w/v) and 1.5% (w/v) showed no significant difference within the error bar indicating a similar ‘fluidization’ behaviour of biofilms grown under these conditions.
3.3. Micro-scale expansion behaviour as a function of extracellular matrix deletion
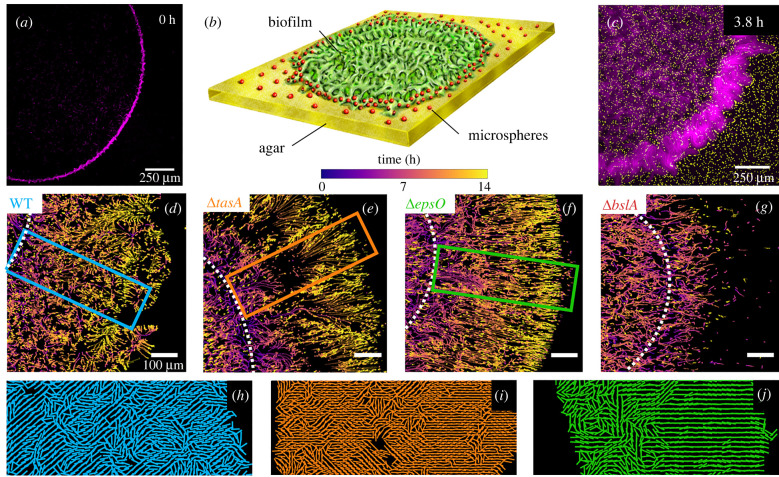
The composition of the ECM influences the micro-scale expansion behaviour of the biofilm. We studied the biofilms’ micro-scale expansion behaviour by tracking the planar transport of microspheres during the development of the biofilms. We used the coffee ring effect to obtain a controlled initial distribution of bacterial cells. When bacteria were deposited on the agar pad and the droplet of the bacterial suspension was dried in a sterile environment, the coffee ring effect created an initial distribution in the form of an annulus (figure 3a). Our tracking method allowed the simultaneous visualization of the micro-scale expansion structures and macro-scale structural development (figure 3b,c).
Figure 3.
Micro-scale expansion behaviour characterized through particle tracking. (a) Representative fluorescent image at t = 0 h showing the coffee ring effect with bacterial cells of ΔtasA (purple) accumulating at the outer edge of the droplet. (b) Schematic of the experimental setup illustrating a biofilm colony (green) surrounded by microspheres (red) distributed on the agar surface (yellow), which are picked up and advected by the biofilm colony upon expansion. (c) Representative image showing a fluorescent overlay of the ΔtasA (purple) and the microspheres (yellow). (d–g) Particle tracks of moving particles colour-coded by time for the WT and mutant strains. The white dotted line shows the location of the original bacterial ‘coffee’ ring. The highlighting boxes indicate the region of inspection for the corresponding orientation plots shown in (h–j). Zoomed-in view of the local orientation of the particle tracks for (h) WT, (i) ΔtasA and (j) ΔepsO.
Deletion of singular ECM components leads to distinct micro-scale expansion behaviour of the biofilm colonies upon expansion. The WT colony expanded in a heterogeneous manner with distinct edge regions as time progressed (figure 3d; electronic supplementary material, figure S3a). Deletion of the amyloid protein TasA resulted in a more coherently directed outward motion compared to the WT (figure 3e; electronic supplementary material, figure S3b). In the outer region, we observed divergent and convergent trajectories resembling a collective fountain-like flow. This resulted in higher and lower density areas in the form of ridges (figure 3e). This formation of ridges was attributed to the growth of bundles which were seen in the fluorescent images of the bacterial colony (figure 3c). The ΔepsO showed a uniform front upon expansion from the initial annular structure without diverging and converging streamlines (figure 3f; electronic supplementary material, figure S3c). However, in the ΔbslA, we observed an absence of outer particle movement, indicating that particles may not have been advected by the expanding biofilm structure (figure 3g; electronic supplementary material, figure S3d).
Major differences in orientation of particle trajectories between the WT and the mutants are found during micro-scale expansion. We observed distinct formation patterns in the orientation of expansion (figure 3h–j; for details, see §2.3). The orientation of the WT trajectories showed a generally disordered structure, defined by the absence of regions of aligned trajectories (figure 3h). For ΔtasA, the trajectories showed regions of both coherent and incoherent orientation (figure 3i). The orientation of the trajectories of the ΔepsO confirmed the earlier observations which indicated an increasingly coherent outward expansion with time (figure 3j). For ΔtasA, we also noted that the orientation of trajectories became increasingly coherent as time progressed.
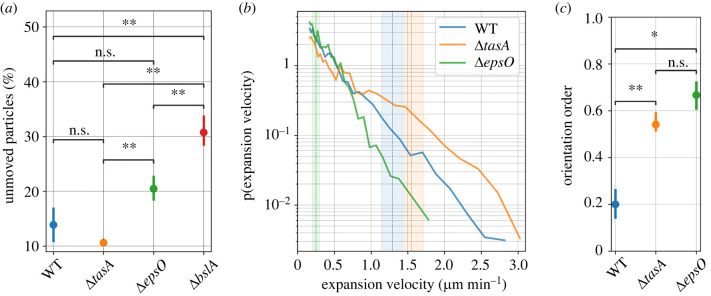
ECM components influence the efficiency in particle advection in expanding colonies. We found that the WT and the mutant colonies were able to pick up different amounts of particles during radial expansion (figure 4a). The fraction of unmoved particles in the outer expansion zone was for the WT. The ΔtasA displayed a higher particle pick-up, with only unmoved particles in comparison to the WT, indicating that the TasA protein may negatively influence particle pick-up. However, deletion of the EPS and BslA from the biofilm ECM resulted in a reduction to particle pick-up. Particle pick-up for ΔepsO was approximately two times lower than ΔtasA, while ΔbslA was three times lower than ΔtasA. The latter indicates that the presence of BslA promotes the effective transport of particles during biofilm expansion.
Figure 4.
Quantification of the micro-scale expansion behaviour characterized through particle tracking. (a) Percentage of unmoved particles for the WT, ΔtasA, ΔepsO and ΔbslA mutant strains. The statistical difference was computed with a t-test (ns: not significant; **p-value 0.01). (b) Probability density function of velocities of the moved particles. The dashed line represents the mean radial expansion of the colonies between time t = 5 h and t = 10 h (see figure 1d) with the shaded area representing the standard deviation of the mean. (c) Orientation order of the particle tracks (figure 3h–j) shown for the WT, ΔtasA and ΔepsO.
Micro-scale expansion velocity distributions vary as a function of ECM composition. We measured the velocity distribution of the particles within the expanding colony (figure 4b). For ΔtasA, we found the highest probability for high maximal expansion velocities of approx. 3 μm min−1 whilst ΔepsO displayed a maximal expansion velocity of about 1.75 μm min−1. The WT had a velocity distribution located between ΔtasA and ΔepsO. We found that the average macro-scale expansion rates, calculated between t = 5 h and t = 10 h (dashed lines in figure 4b), were within range of the measured micro-scale particle velocities. Computing the orientation order of the particle trajectories, a relative measure of coherence ranging between 0 and 1 (figure 4c), we found that ΔepsO had the highest orientation order, while WT was the least ordered, supporting our qualitative observations (figure 3h–j).
4. Discussion
This work relates the micro- and macro-structure of bacterial biofilm colonies to ECM composition using a library of ECM mutants. To complement our macro-scale observations and rheological measurements, we developed a simple technique to track the planar micro-scale expansion behaviour of bacterial colonies. Our technique is insensitive to local cell density and provides highly resolved expansion patterns. Similar techniques which have been used to track colony maturation include traction force microscopy, and single-cell tracking [17,33,34]. These methods have revealed how substrate stiffness influences transient stresses associated with colony expansion [17]. However, these techniques result in average deformation fields or are limited to smaller inspection areas. Here, we discuss our findings in the context of macro-scale parameters relating the contribution of ECM components to micro-scale colony expansion.
In our study, we assume the production of surfactant remains invariant between mutants as each colony displayed a surfactant ring (see electronic supplementary material, figure S4). The availability of surfactant reduces agar surface tension and facilitates colony expansion [34] (see electronic supplementary material, figure S5). According to current literature, the macro-scale expansion is regulated by the ECM elastic modulus G′ and osmotic potentials [32]. We found that the expansion behaviour of ΔepsO and ΔbslA on 1% (w/v) LBGM agar was similar despite their stiffness G′ differing by a factor of three. Whilst ΔepsO and ΔtasA had a similar stiffness G′, but ΔtasA expanded five times faster. From a purely rheological perspective, our results indicate that the phase angle , which describes the ratio of viscous to elastic modulus of the biofilm, provides a better correlation with colony expansion rate than the elastic modulus G′. The phase angle was similar for ΔbslA and ΔepsO. However, ΔtasA had a significantly higher phase angle, likely due to the lack of the cross-linking protein TasA. Both the WTs’ expansion rate and phase angle fell between ΔtasA and the other two mutants, which can be explained by the mixed contribution of all ECM components.
Furthermore, our macro-scale structural observations demonstrated that ECM composition affects the patterning behaviour of expanding colonies. Our results demonstrated that ECM components influence micro-scale patterning with three distinct morphologies displayed within the central region of the biofilm colonies (figure 1b). The conformation of cell bundling and patterning has been attributed to mechanical instabilities, the occurrence of which depends upon cell elongation, ECM viscoelastic properties and cell surface cohesion [4,19,35]. Therefore, the differences in biofilm hydrophobicity should mediate the biofilm–agar cohesion, thus altering the buckling pattern [36]. In this work, we measured the ECM viscoelastic properties, while cell elongation could not be probed and relative cell surface adhesion was inferred from the colony hydrophobicity. We note how the biofilm hydrophobicity alters with deletion of hydrophobin BslA and polysaccharide EPS, both of which in theory should influence the osmotic pressure of the biofilm ECM [37].
Our results indicate the role of ECM components in buffering the dependence of biofilm viscoelasticity on water potential of the substrate. Suppressing EPS and BslA results in an increased dependence of biofilm viscoelasticity to agar water potential, which primarily influences the biofilm elastic modulus G′ in comparison to the other viscoelastic properties. We explain the latter by the contribution of BslA and EPS to the ECM osmotic potential, which determines the water potential of the biofilm (see electronic supplementary material, water potential equation). An analogous system is found in gels, where viscoelastic behaviour is known to systematically vary with water potential of the substrate [38]. For ΔtasA, we saw a lower dependence in stiffness G′ on the water potential of the substrate. Therefore, the crosslinking function of TasA does not seem to contribute significantly to the sensitivity of biofilm elasticity towards substrate water potential. Similarly, the slower expansion rate of ΔbslA and ΔepsO colonies in comparison to WT can be explained by a reduction in osmotic potential, as the relative concentration of these polymeric components in the ECM are expected to be reduced. These variables can be linked through the Van’t Hoff relation, which describes biofilm osmotic pressure as a function of polymer concentration [15].
Our macro-scale mechanical measurements of the biofilms can be used to inform on micro-scale expansion behaviour, which contributes to the biofilm morphogenesis. Colony expansion is typically considered as a balance between ECM elastic moduli, G′, and osmotically induced expansion. However, within biofilm pellicles, expansion has been shown to be mediated by viscosity [39]. We find that the phase angle, , of the plate-grown biofilms can inform on the differences in expansion behaviour within the mutant colonies. In particular, ΔtasA, which had the highest phase angle, displayed microsphere trajectories with converging and diverging fountain-like flows, hinting at a liquid-like behaviour. Whereas ΔepsO, which displayed a lower phase angle, expanded in a uniform manner which we interpreted as a more elastic-like flow. Their differences were quantified via the orientation order parameter, which was highest for the ΔepsO. The WT, which had an intermediate phase angle, displayed the least coherent micro-scale expansion behaviour. We hypothesize that the incoherent micro-scale expansion of the WT could be due to the occurrence of regions of bacterial subpopulations with a differing expression of ECM components [40].
Our set-up revealed the dependence of ECM components on advecting neutrally charged particles. Deletion of BslA strongly reduced advection efficiency, whereas ΔtasA exhibited the most efficient particle pick-up. BslA is known to coat the biofilm with a hydrophobic layer [23] and we have demonstrated that this layer also influences transport of external matter. However, we hypothesize this interaction is dependent on the charge of the ECM component and particles. Therefore, it would be interesting to perform this assay with both negatively and positively charged particles to assess the dependence of ECM charge on particle advection.
In this study, we studied the influence of two proteins and a polysaccharide component of the B. subtilis ECM on colony expansion at different scales. Our results indicated that the viscoelastic phase angle is likely a more reliable parameter than elastic modulus G′ in describing the colony expansion behaviour. Furthermore, our micro-scale characterization revealed that singular deletion mutants had a higher coherence in the expansion pattern than the WT. Furthermore, we demonstrated the effect of hydrophobin on the advection of external particles. However, there are additional primary ECM components that are abundant in biofilms [5,41]. The influence of other ECM components could be investigated with the particle assay to rapidly characterize the colony micro-scale expansion behaviour and particle pick-up efficiencies. Our approach could give insight on the incorporation of bacterial cells, microspheres or other environmental debris in the biofilm structure during the radial expansion of biofilm colonies. Furthermore, the study of osmotic pressure and water potential of the substrate on biofilm rheological properties is especially relevant in environmental settings, where biofilms grow on wet surfaces at the solid–air interface such as in the rhizophere and the phyllosphere.
Acknowledgements
The deletion mutants ΔepsO and ΔtasA were kindly provided by the Kovács Lab, DTU, Denmark. The ΔbslA was kindly provided by Kobayashi Lab, Nara Institute of Science and Technology, Japan.
Data accessibility
Representative imaging data and figure data are available in a publicly accessible database: http://doi.org/10.3929/ethz-b-000569192 [42]. The data are provided in electronic supplementary material [43].
Authors' contributions
S.G.V.C and D.L.K. contributed equally to this work. S.G.V.C.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, software, writing—original draft, writing—review and editing; D.L.K.: conceptualization, data curation, formal analysis, investigation, methodology, software, writing—original draft, writing—review and editing; S.G.: data curation, formal analysis, methodology, writing—review and editing; J.J.-M.: methodology, resources, supervision, writing—review and editing; E.S.: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The authors acknowledge support from Marie Sklodowska Curie Action (MSCA) fellowship ID: 101033169 (to S.G.V.C.), discretionary funding from ETH and discretionary funding from Eawag (to J.J.-M.) and the SNSF PRIMA grant no. 179834 (to E.S.).
References
- 1.Flemming H-C, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8, 623-633. ( 10.1038/nrmicro2415) [DOI] [PubMed] [Google Scholar]
- 2.Wilking JN, Angelini TE, Seminara A, Brenner MP, Weitz DA. 2011. Biofilms as complex fluids. MRS Bull. 36, 385-391. ( 10.1557/mrs.2011.71) [DOI] [Google Scholar]
- 3.Ido N, Lybman A, Hayet S, Azulay DN, Ghrayeb M, Liddawieh S, Chai L. 2020. Bacillus subtilis biofilms characterized as hydrogels. Insights on water uptake and water binding in biofilms. Soft Matter 16, 6180-6190. ( 10.1039/D0SM00581A) [DOI] [PubMed] [Google Scholar]
- 4.van Gestel J. 2015. From cell differentiation to cell collectives: Bacillus subtilis uses division of labor to migrate. PLoS Biol. 13, e1002141. ( 10.1371/journal.pbio.1002141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Secchi E, Savorana G, Vitale A, Eberl L, Stocker R, Rusconi R. 2022. The structural role of bacterial eDNA in the formation of biofilm streamers. Proc. Natl Acad. Sci. USA 119, e2113723119. ( 10.1073/pnas.2113723119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin B, Fei C, Wang B, Stone HA, Wingreen NS, Bassler BL. 2021. Hierarchical transitions and fractal wrinkling drive bacterial pellicle morphogenesis. Proc. Natl Acad. Sci. USA 118, e2023504118. ( 10.1073/pnas.2023504118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wimpenny J, Manz W, Szewzyk U. 2000. Heterogeneity in biofilms. FEMS Microbiol. Rev. 24, 661-671. ( 10.1111/j.1574-6976.2000.tb00565.x) [DOI] [PubMed] [Google Scholar]
- 8.Grumbein S, Opitz M, Lieleg O. 2014. Selected metal ions protect Bacillus subtilis biofilms from erosion. Metallomics 6, 1441-1450. ( 10.1039/C4MT00049H) [DOI] [PubMed] [Google Scholar]
- 9.Jana S, Charlton S, Eland L, Burgess J, Wipat A, Curtis T, Chen J. 2020. Nonlinear rheological characteristics of single species bacterial biofilms. npj Biofilms Microbiomes 6, 19. ( 10.1038/s41522-020-0126-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones WL, Sutton MP, McKittrick L, Stewart PS. 2011. Chemical and antimicrobial treatments change the viscoelastic properties of bacterial biofilms. Biofouling 27, 207-215. ( 10.1080/08927014.2011.554977) [DOI] [PubMed] [Google Scholar]
- 11.Jennings LK, et al. 2015. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl Acad. Sci. USA 112, 11 353-11 358. ( 10.1073/pnas.1503058112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilking JN, Zaburdaev V, Volder MD, Losick R, Brenner MP, Weitz DA. 2013. Liquid transport facilitated by channels in Bacillus subtilis biofilms. Proc. Natl Acad. Sci. USA 110, 848-852. ( 10.1073/pnas.1216376110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fei C, Mao S, Yan J, Alert R, Stone HA, Bassler BL, Wingreen NS, Košmrlj A. 2020. Nonuniform growth and surface friction determine bacterial biofilm morphology on soft substrates. Proc. Natl Acad. Sci. USA 117, 7622-7632. ( 10.1073/pnas.1919607117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seminara A, Angelini TE, Wilking JN, Vlamakis H, Ebrahim S, Kolter R, Weitz DA, Brenner MP. 2012. Osmotic spreading of Bacillus subtilis biofilms driven by an extracellular matrix. Proc. Natl Acad. Sci. USA 109, 1116-1121. ( 10.1073/pnas.1109261108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Dai W, Tsai S-M, Zehnder SM, Sarntinoranont M, Angelini TE. 2015. Surface indentation and fluid intake generated by the polymer matrix of Bacillus subtilis biofilms. Soft Matter 11, 3612-3617. ( 10.1039/C5SM00148J) [DOI] [PubMed] [Google Scholar]
- 16.Rhodeland B, Hoeger K, Ursell T. 2020. Bacterial surface motility is modulated by colony-scale flow and granular jamming. J. R. Soc. Interface 17, 20200147. ( 10.1098/rsif.2020.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asp ME, Thanh M-TH, Germann DA, Carroll RJ, Franceski A, Welch RD, Gopinath A, Patteson AE. 2022. Spreading rates of bacterial colonies depend on substrate stiffness and permeability. PNAS Nexus 1, pgac025. ( 10.1093/pnasnexus/pgac025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cont A, Rossy T, Al-Mayyah Z, Persat A. 2020. Biofilms deform soft surfaces and disrupt epithelia. eLife 9, e56533. ( 10.7554/eLife.56533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaman YI, Demir E, Vetter R, Kocabas A. 2019. Emergence of active nematics in chaining bacterial biofilms. Nat. Commun. 10, 2285. ( 10.1038/s41467-019-10311-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnaouteli S, Bamford NC, Stanley-Wall NR, Kovács ÁT. 2021. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 19, 600-614. ( 10.1038/s41579-021-00540-9) [DOI] [PubMed] [Google Scholar]
- 21.Branda SS, Chu F, Kearns DB, Losick R, Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59, 1229-1238. ( 10.1111/j.1365-2958.2005.05020.x) [DOI] [PubMed] [Google Scholar]
- 22.Romero D, Aguilar C, Losick R, Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl Acad. Sci. USA 107, 2230-2234. ( 10.1073/pnas.0910560107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobley L, Ostrowski A, Rao FV, Bromley KM, Porter M, Prescott AR, MacPhee CE, van Aalten DMF. 2013. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc. Natl Acad. Sci. USA 110, 13 600-13 605. ( 10.1073/pnas.1306390110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K, Iwano M. 2012. BslA(YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol. Microbiol. 85, 51-66. ( 10.1111/j.1365-2958.2012.08094.x) [DOI] [PubMed] [Google Scholar]
- 25.Xie S, Tu Z. 2015. Holistically-nested edge detection. In 2015 IEEE Int. Conf. on Computer Vision (ICCV), Santiago, Chile, 7–13 December 2015, pp. 1395–1403. ( 10.1109/ICCV.2015.164) [DOI]
- 26.Marcotti S, de Freitas DB, Troughton LD, Kenny FN, Shaw TJ, Stramer BM, Oakes PW. 2021. A workflow for rapid unbiased quantification of fibrillar feature alignment in biological images. Front. Comput. Sci. 3, 745831. ( 10.3389/fcomp.2021.745831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gingichashvili S, Duanis-Assaf D, Shemesh M, Featherstone JDB, Feuerstein O, Steinberg D. 2017. Bacillus subtilis biofilm development—a computerized study of morphology and kinetics. Front. Microbiol. 8, 2072. ( 10.3389/fmicb.2017.02072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shemesh M, Chai Y. 2013. A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via histidine kinase KinD signaling. J. Bacteriol. 195, 2747-2754. ( 10.1128/JB.00028-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marmur A. 2003. Wetting on hydrophobic rough surfaces: to be heterogeneous or not to be? Langmuir 19, 8343-8348. ( 10.1021/la0344682) [DOI] [Google Scholar]
- 30.Geisel S, Secchi E, Vermant J. 2022. Experimental challenges in determining the rheological properties of bacterial biofilms. Interface Focus 12, 20220032. ( 10.1098/rsfs.2022.0032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papendick RI, Campbell GS. 1981. Theory and measurement of water potential. In Water potential relations in soil microbiology (eds Parr JF, Gardner WR, Elliott LF). SSSA Special Publication no. 9, pp. 1-22. Madison, WI: Soil Science Society of America. [Google Scholar]
- 32.Yan J, et al. 2018. Bacterial biofilm material properties enable removal and transfer by capillary peeling. Adv. Mater. 30, 1804153. ( 10.1002/adma.201804153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabass B, Koch MD, Liu G, Stone HA, Shaevitz JW. 2017. Force generation by groups of migrating bacteria. Proc. Natl Acad. Sci. USA 114, 7266-7271. ( 10.1073/pnas.1621469114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Be’er A, Smith RS, Zhang HP, Florin E-L, Payne SM, Swinney HL. 2009. Paenibacillus dendritiformis bacterial colony growth depends on surfactant but not on bacterial motion. J. Bacteriol. 191, 5758-5764. ( 10.1128/JB.00660-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dervaux J, Magniez JC, Libchaber A. 2014. On growth and form of Bacillus subtilis biofilms. Interface Focus 4, 20130051. ( 10.1098/rsfs.2013.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan J, Fei C, Mao S, Moreau A, Wingreen NS, Košmrlj A, Stone HA, Bassler BL. 2019. Mechanical instability and interfacial energy drive biofilm morphogenesis. eLife 8, e43920. ( 10.7554/eLife.43920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J, Nadell C, Stone H, Wingreen N, Bassler B. 2017. Extracellular-matrix-mediated osmotic pressure drives Vibrio cholerae biofilm expansion and cheater exclusion. Nat. Commun. 8, 327. ( 10.1038/s41467-017-00401-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mason TG, Lacasse M-D, Grest GS, Levine D, Bibette J, Weitz DA. 1997. Osmotic pressure and viscoelastic shear moduli of concentrated emulsions. Phys. Rev. E 56, 3150-3166. ( 10.1103/PhysRevE.56.3150) [DOI] [Google Scholar]
- 39.Angelini TE, Roper M, Kolter R, Weitz DA, Brenner MP. 2009. Bacillus subtilis spreads by surfing on waves of surfactant. Proc. Natl Acad. Sci. USA 106, 18 109-18 113. ( 10.1073/pnas.0905890106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dragoš A, et al. 2018. Division of labor during biofilm matrix production. Curr. Biol. 28, 1903-1913.e5. ( 10.1073/pnas.0905890106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng N, Cai P, Mortimer M, Chunhui Gao YW, Huang Q. 2020. The exopolysaccharide–eDNA interaction modulates 3D architecture of Bacillus subtilis biofilm. BMC Microbiol. 20, 115. ( 10.1186/s12866-020-01789-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charlton S, Kurz D. 2022. The role of biofilm matrix composition in controlling colony expansion and morphology. ETH Zurich. ( 10.3929/ethz-b-000569192) [DOI] [PMC free article] [PubMed]
- 43.Charlton SGV, Kurz DL, Geisel S, Jimenez-Martinez J, Secchi E. 2022. The role of biofilm matrix composition in controlling colony expansion and morphology. Figshare. ( 10.6084/m9.figshare.c.6204488) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Charlton S, Kurz D. 2022. The role of biofilm matrix composition in controlling colony expansion and morphology. ETH Zurich. ( 10.3929/ethz-b-000569192) [DOI] [PMC free article] [PubMed]
- Charlton SGV, Kurz DL, Geisel S, Jimenez-Martinez J, Secchi E. 2022. The role of biofilm matrix composition in controlling colony expansion and morphology. Figshare. ( 10.6084/m9.figshare.c.6204488) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Representative imaging data and figure data are available in a publicly accessible database: http://doi.org/10.3929/ethz-b-000569192 [42]. The data are provided in electronic supplementary material [43].