Abstract
Two commercially available serologic tests for use in diagnosing Lyme borreliosis were evaluated by using a test panel comprised of sera from patients diagnosed with Lyme borreliosis, non-Lyme disease controls, and healthy subjects. The test methods examined were a Western blot assay and an immunodot assay. The study was initiated to determine how the immunodot assay, which contains purified and recombinant proteins to those borrelial antigens recommended for immunoglobulin M (IgM) detection in the Dearborn criteria, would compare with the Western blot assay as a confirmatory method for serologic diagnosis of Lyme borreliosis. Results obtained showed that the two test methods performed comparably for detecting IgG antibodies. For IgM antibody detection, the immunodot and Western blot assays had similar sensitivities; however, the immunodot assay was more specific and had greater positive predictive value than the Western blot assay. The results obtained indicate that the immunodot assay performs as well as or better than the Western blot assay for diagnosing Lyme borreliosis. Furthermore, because it uses a limited panel (n = 5) of antigens, the immunodot is easier to read and interpret than standard Western blots.
Considerable controversy exists regarding the clinical value of serologic tests for detecting antibodies to Borrelia burgdorferi. Issues of particular concern include sensitivity, specificity, lab-to-lab correlation, and interpretation of results (2, 13). In a previous study, we noted that a majority of physicians who ordered serologic tests for their patients with suspected Lyme borreliosis initiated antibiotic treatment regardless of test results or duration of symptoms at the time of presentation (7, 8). Recommendations resulting from the 1994 meeting on serodiagnosis of Lyme borreliosis in Dearborn, Mich., were designed to alleviate, if not eliminate, some of these problems (1). Recommendations included establishment of a two-tier testing system requiring Western blotting (WB) for confirmation of serologic tests and criteria for interpretation of WB assays. Interpretive criteria were based on publications of Dressler et al. (3) and Engstrom et al. (4). These require identification of immunoglobulin G (IgG) or IgM antibodies to specific antigens on WB. To date, neither monoclonal antibodies nor recombinant proteins are readily available for all the antigens of B. burgdorferi as recommended in the Dearborn criteria and there is no commercially available WB assay that has been approved for confirmatory testing. Furthermore, some recent reports also indicate that the Dearborn criteria for WB may not yield the degree of sensitivity or specificity expected when the criteria were adopted as a recommendation (9, 10). This is particularly true for IgM antibody detection, which reports suggest may be improved by scoring any two bands as positive (12).
The present study was performed to evaluate an immunodot assay for use in diagnosing Lyme borreliosis. Unlike a WB, which is made by electrophoretically separating whole B. burgdorferi organisms, the immunodot utilizes a limited panel of purified and recombinant antigens of B. burgdorferi. A panel of sera containing specimens from patients diagnosed with Lyme disease and non-Lyme disease control sera were tested by using a commercially available WB assay, interpreted by using the Dearborn criteria, and an immunodot assay, interpreted by using the manufacturer’s interpretive criteria. Results obtained indicate that the immunodot assay can provide a useful alternative to the conventional WB assay for aid in diagnosing Lyme borreliosis.
MATERIALS AND METHODS
Serum selection criteria.
Specimens from 28 individuals with clinically diagnosed Lyme disease and 81 individuals with no clinical evidence of Lyme disease were used. The Lyme disease group consisted of 10 patients with early isolated Lyme borreliosis (erythema migrans rash [EM] present at time of sample acquisition), 10 with early disseminated Lyme borreliosis (multiple EM or history of EM with Bell’s palsy), and 8 patients with a diagnosis of Lyme arthritis. Non-Lyme disease sera were obtained from individuals with no history of infection with B. burgdorferi. Medical records of individuals in the non-Lyme disease group were reviewed by a rheumatologist or infectious disease specialist participating in a Lyme disease specialty clinic. Six-month follow-up of this group gave no indication of Lyme disease after our study. Specimens were assigned to non-Lyme disease subgroups as follows: (i) presurgical pediatric orthopedic patients, (ii) pediatric rheumatology patients, (iii) patients with antibody-positive Epstein-Barr virus (EBV), and (iv) patients with seropositive endoscopy-confirmed Helicobacter pylori infection. Serum specimens were aliquoted and stored at −70°C until needed for testing.
Commercial WB.
Patients were tested by use of the MarBlot (Mardx Diagnostics, Inc., Scotch Plains, N.J.) WB kit; the manufacturer’s instructions for running and interpreting WB were followed.
Immunodot blot.
Patients were tested by use of the Borrelia Dot Blot (GenBio, San Diego, Calif.), and the manufacturer’s instructions for the running and interpretation of the test were followed.
Performance of the assays with the patient groups tested was analyzed by use of the following indices: sensitivity = true positives/(true positives + false negatives); specificity = true negatives/(true negatives + false positives); accuracy = (true positives + true negatives)/(true negatives + true positives + false positives + false negatives); positive predictive value = true positives/(true positives + false positives); and negative predictive value = true negatives/(true negatives + false negatives).
RESULTS
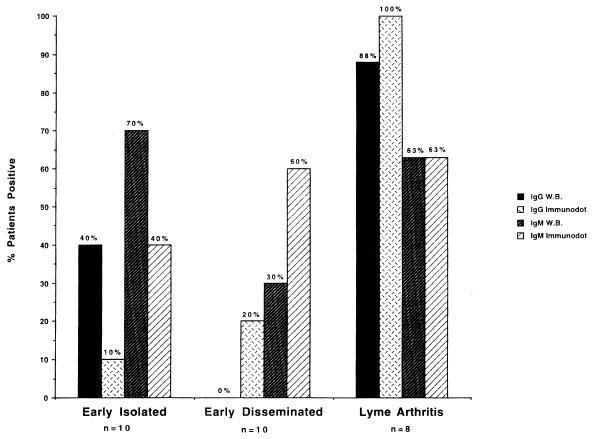
Results obtained when patients diagnosed with Lyme borreliosis were tested for IgG and IgM antibodies to B. burgdorferi are depicted in Fig. 1. Seven of the 10 patients with early isolated Lyme borreliosis (EM present) were positive for IgM antibodies by WB, with four also testing positive for IgG. Immunodot results for this group indicated four patients positive for IgM and one additional patient positive for IgG. In the early disseminated disease group (Bell’s palsy and EM or multiple EM), three patients were positive for IgM and none were positive for IgG antibodies by WB, whereas six were positive by immunodot for IgM and two, including one additional patient, were positive for IgG antibodies. Results for patients with Lyme arthritis showed seven of eight positive for IgG by WB and eight of eight positive by immunodot, with five of eight positive for IgM by both assays.
FIG. 1.
Comparison of the percentages of Lyme borreliosis patients testing positive by WB and immunodot assays. The WB detected a higher percentage of patients with early isolated Lyme borreliosis (EM present at time of sample acquisition) as positive than were positive by immunodot. In contrast, more patients with early disseminated Lyme borreliosis (EM and Bell’s palsy or multiple EM) were positive by immunodot than were positive by WB.
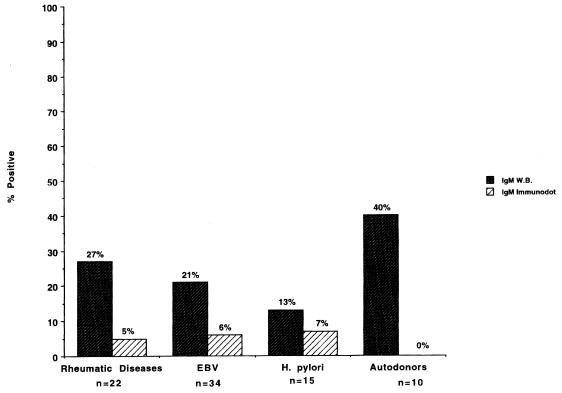
Non-Lyme disease patient serology results are depicted in Fig. 2. None of the non-Lyme disease patients were positive for IgG antibodies by WB or immunodot. False positives in the non-Lyme disease group were detected for both WB and immunodot on testing for IgM antibodies. Results for immunodot showed that 1 of 22 patients with rheumatic diseases, 2 of 34 with EBV, and 1 of 15 with H. pylori tested positive for IgM antibodies to B. burgdorferi. By WB, six patients with rheumatic diseases, seven with EBV, two with H. pylori, and four autodonors were positive for IgM antibodies to B. burgdorferi.
FIG. 2.
Comparison of percentages of non-Lyme borreliosis patients testing positive by WB and immunodot. The results show that the WB assay has a higher false-positive rate than the immunodot assay for all categories of non-Lyme disease patients tested. There were no false-positive results by either assay when testing for IgG antibodies.
Results of calculations for sensitivity, specificity, accuracy, positive predictive value, and negative predictive value are presented in Table 1. The sensitivities and negative predictive values were similar for the immunodot and WB assays; however, specificity, accuracy, and positive predictive value were markedly greater for the immunodot assay than they were for the WB assay.
TABLE 1.
Performance of immunodot and WB assays
| Test method | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| Immunodot | 68 | 95 | 88 | 83 | 89 |
| WB | 64 | 76 | 73 | 49 | 86 |
DISCUSSION
Diagnosing Lyme borreliosis continues to be a contentious area of clinical medicine. This is due in part to the plethora of nonpathognomonic clinical symptoms reported to result from infection with B. burgdorferi (10). In addition, serologic tests for detecting infection are, in general, considered to be unreliable (13).
We have performed serologic tests for detecting antibodies to B. burgdorferi with an in-house-developed enzyme-linked immunosorbent assay (ELISA) and a WB assay since 1988. In our experience, these assays have proven both sensitive and specific in testing of patients seen and monitored at our pediatric Lyme disease clinic (5, 6, 11). However, a large discrepancy exists between our serologic results and those obtained at other testing sites in our service area and reference laboratories. These results, generated by the use of commercially available assays, are often a contributing factor in patients being referred to our specialty clinic. In one previous study, we reported that 93% of positive results by ELISA from one referral source were false positives (8). More recently, we have received referrals and inquiries due to WB results and their interpretations.
A common query regarding WB concerns band identity and location, indicating that some reference labs are reporting WB results as positive or negative or are listing only bands which may correspond to those recommended in the Dearborn criteria. It was a combination of the preceding factors and others which motivated the present study. The commercial WB kit (MarBlot) was selected since it was reported to be used by more labs than any other in College of American Pathologists proficiency reports. Selection of the commercially available immunodot blot was based on its apparent ease of use and greater ability to be standardized since it uses purified and recombinant antigens. A further and perhaps paramount factor in choosing the immunodot was our belief that, with rare exceptions, only patients at an early stage of infection present a challenge to serologic tests. Therefore, the IgM response is critical with regards to assay sensitivity and the immunodot includes all three of the antigens (OspC, p39, and flagellin) indicated as significant for IgM antibody detection by the Dearborn criteria (4). Patients with a prolonged course of infection or Lyme arthritis are almost universally positive by IgG antibody testing (5, 11).
Analysis of the data obtained from this study showed that the immunodot assay, despite having only four separated borrelial antigen preparations, was overall as sensitive as the WB assay for detection of B. burgdorferi-specific antibodies in patient serum. More (seven versus five) patients with early localized Lyme borreliosis tested positive by WB than by the immunodot blot; however, more patients with early disseminated and late Lyme borreliosis tested positive by immunodot than by WB. The one Lyme arthritis patient serum specimen that tested negative by WB actually produced 17 bands on the WB strip; however, only 4 of the 10 bands recognized by the Dearborn criteria were among the 17 detected. As shown in Results, findings obtained from testing of non-Lyme disease sera indicate that the immunodot assay is more specific than the WB assay to which it was compared. The false-positive rate was 23% for WB compared to 5% for immunodot in the non-Lyme disease group. False-positive results obtained in this study occurred only when testing for IgM antibodies. The high false-positive rate for IgM testing by the WB assay obtained with this study population differs from results reported by Sivak et al., who concluded that the IgM WB assay lacked sensitivity, not specificity, and that the Dearborn criteria for IgM should be modified to allow the scoring of a blot as positive if any two bands, as opposed to two of three designated bands, are detected (12).
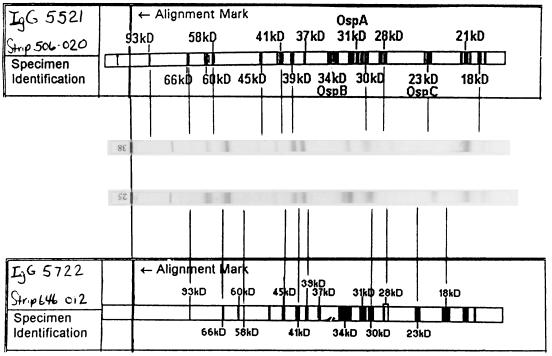
In addition to its superior performance with the panel of test sera used in this study, the immunodot was much easier to read and interpret than the WB. The immunodot test strip is constructed so that six nitrocellulose windows or dots are visible on a plastic strip holder. Each window contains a single antigen preparation (whole B. burgdorferi, a high-molecular-weight recombinant, purified flagellin, recombinant p39, recombinant OspC, and a reagent control). Each dot is scored independently as reactive or not, and results are compared with an algorithm supplied in the kit for interpretation. For some patients, weakly reactive dots or grayness in the dot area caused problems with interpretation. We adopted the following as a guideline. Dot blots are held at 18 in. and examined to see if a distinct rim can be seen around the dot. If a rim is visible, the dot is scored as positive; otherwise, it is negative. In contrast, the WB strips can have many bands, of which 10 must be identified by comparison with a positive control strip, which is calibrated against a template, supplied with each lot of strips. Figure 3 shows two positive control strips with their respective lot templates. Lines drawn from the template-identified bands of the Dearborn criteria to the positive control strips show that the positive control WB strips do not match the template for their lots. One can maneuver the strips about the template to identify significant bands by pattern matching; however, separation by apparent molecular mass on the strips is nonlinear, and we found no single point at which we could simultaneously identify all of the necessary significant bands. This made reading the WB strips time-consuming and subjective, the latter being an issue which implementation of the Dearborn criteria was intended to resolve.
FIG. 3.
Two WB-positive control strips aligned with their respective manufacturers’ templates. The positive control is required to demonstrate reactivity with the 93-, 66-, 41-, 39-, 23-, and 18-kDa bands. Lines have been drawn from the template to the control strip for the 10 bands that are significant for interpretation of patient results. None of the lots used for this study had the necessary bands required for determining reactivity which could be simultaneously aligned with the lot-specific template.
In conclusion, results of this study indicate that the immunodot assay can be used as a substitute for the WB assay when testing for Lyme borreliosis without a loss of sensitivity and with increased specificity. In addition, the immunodot assay appears to be more amenable to routine use since it reduces the subjectivity component associated with blot interpretation and thus the need for substantial technical experience and expertise.
REFERENCES
- 1.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 2.Cutler S J, Wright D J M. Predictive value of serology in diagnosing Lyme borreliosis. J Clin Pathol. 1994;47:344–349. doi: 10.1136/jcp.47.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 4.Engstrom S E, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fawcett P T, Gibney K M, Rose C D, Dubbs S B, Doughty R A. Frequency and specificity of antibodies that cross-react with Borrelia burgdorferi antigens. J Rheumatol. 1992;19:582–587. [PubMed] [Google Scholar]
- 6.Fawcett P T, Gibney K M, Rose C D, Klein J D, Doughty R A. Adsorption with a soluble E. coli antigen fraction improves the specificity of ELISA tests for Lyme disease. J Rheumatol. 1991;18:705–708. [PubMed] [Google Scholar]
- 7.Fawcett P T, Rose C D, Gibney K M, Doughty R A. Correlation of seroreactivity with response to antibiotics in pediatric Lyme borreliosis. Clin Diagn Lab Immunol. 1997;4:85–88. doi: 10.1128/cdli.4.1.85-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawcett P T, Rose C D, Gibney K M. Comparative evaluation of adsorption with E. coli on ELISA tests for Lyme borreliosis. J Rheumatol. 1995;22:684–688. [PubMed] [Google Scholar]
- 9.Ledue T B, Collins M F, Craig W Y. New laboratory guidelines for serologic diagnosis of Lyme disease: evaluation of the two-test protocol. J Clin Microbiol. 1996;34:2343–2350. doi: 10.1128/jcm.34.10.2343-2350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichol G, Dennis D T, Steere A C, Lightfoot R, Wells G, Shea B, Tugwell P. Test-treatment strategies for patients suspected of having Lyme disease: a cost effectiveness analysis. Ann Intern Med. 1998;128:37–48. doi: 10.7326/0003-4819-128-1-199801010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Rose C D, Fawcett P T, Gibney K M, Singsen B H, Dubbs S B, Doughty R A. Use of Western blot and enzyme-linked immunosorbent assays to assist in the diagnosis of Lyme disease. Pediatrics. 1991;88:465–470. [PubMed] [Google Scholar]
- 12.Sivak S L, Aguero-Rosenfeld M E, Nowakowski J, Nadelman R B, Wormser G P. Accuracy of IgM immunoblotting to confirm the clinical diagnosis of early Lyme disease. Arch Intern Med. 1996;156:2105–2109. [PubMed] [Google Scholar]
- 13.Tugwell P, Dennis D T, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere A C. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–1123. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]