Abstract
Oral fluids are convenient alternatives to blood sampling for evaluating significant metabolic components. Two forms of oral fluids, oral mucosal transudates (OMT) and saliva, were collected and compared for content of soluble products of immune activation. The data confirm that OMT and saliva represent distinct body fluids. The concentrations, outputs, and analyte/protein ratios of β-2-microglobulin (β2M), soluble tumor necrosis factor alpha receptor II (sTNFαRII), and neopterin were measured. Both the OMT and the saliva of most of the individuals in the control healthy populations had measurable levels of all three activation markers. When the immune system is activated, as in human immunodeficiency virus (HIV) infection, the levels of β2M and sTNFαRII are increased in both OMT and saliva compared to those in a healthy control population. OMT levels correlated better with levels in serum than did saliva and appear to reflect systemic immune activation in HIV infection. Because acquisition of oral fluids is noninvasive and easily repeatable, measurement of β2M and/or sTNFαRII content in OMT could be useful in the assessment of disease activity in patients with HIV infection or chronic inflammatory diseases.
Oral fluids as test specimens have several advantages over blood and are increasingly being used in diagnosis and assessment of diseases (12, 13). They are easily obtainable and can be collected repeatedly without individuals having to come to medical clinics or offices except to deliver the samples. Two types of oral fluids can be collected: oral mucosal transudates (OMT) and saliva. The former resembles a filtrate of plasma, and the latter contains enzymes and other contributions from the parotid and salivary glands. The method of collection determines the predominance of OMT or saliva. Both types of oral fluids were collected from healthy individuals and also evaluated for use in assessing immune activation markers in human immunodeficiency virus (HIV) infection.
Immune activation is recognized as a major feature of HIV pathogenesis. It has been shown that the level of immune activation is closely related to the course of HIV disease and is a strong prognostic marker (8). The level of immune cell activation in HIV infection is usually assessed by measurement of β2-microglobulin (β2M) and/or neopterin (NPT) (10, 14, 15, 19, 32) or soluble tumor necrosis factor alpha receptor II (sTNFαRII) (3, 5) in serum.
NPT is released by macrophages activated by gamma interferon which has been secreted by stimulated T cells (16). NPT has been detected in human saliva (18). In one study, increased concentration of NPT has been reported in stimulated saliva of HIV-infected subjects (26). However, recent results of Evans and Wansbrough-Jones revealed no significant increase (7). Müller et al. found a lower parotid NPT output and no difference in the NPT concentrations in saliva samples from HIV-infected persons and controls (23). β2M is a product of a variety of activated lymphoid cells. β2M has also been detected in human saliva, and significantly higher levels were found in saliva from patients with juvenile periodontitis (1), adult primary glomerulonephritis (28), and primary Sjögren’s syndrome (20). There are no reports on β2M measurements in the saliva of HIV-infected individuals. Use of oral fluid as a diagnostic medium for several other analytes, including steroid hormones (9, 25), therapeutic drugs (22, 27), drugs of abuse (30), etc., has been discussed as well. There are no reports of measurements of sTNFαRII in oral fluid.
The aims of the present study were (i) to investigate the feasibility of measuring the concentration of immune activation markers such as NPT, β2M, and sTNFαRII in oral fluids, (ii) to compare the analyte output of those markers in OMT and saliva, (iii) to relate the findings on these two oral fluids to those on serum, (iv) to compare the changes in these three markers in the oral fluids of HIV-infected persons who exhibit substantial immune activation versus controls, and (v) to determine the interrelationship of the three different markers in the oral fluids.
MATERIALS AND METHODS
Study population.
Serum, saliva, and/or OMT samples were collected after obtaining informed consent from 39 persons with HIV infection who participate in the Los Angeles cohort of the Multicenter AIDS Cohort Study (17). All patients had serum antibodies to HIV type 1 (HIV-1) as determined by enzyme-linked immunosorbent assay (Genetic Systems, Seattle, Wash.) and confirmed by Western blot analyses (Bio-Rad Laboratories, Hercules, Calif.) (24). Of the HIV-1-seropositive participants, 29 were asymptomatic and are the basis for this report. Three with clinically diagnosed AIDS and oral thrush at about the time of sample collection were compared later with the asymptomatic group. Two groups were selected as controls: (i) 10 healthy heterosexual volunteers and (ii) 16 homosexual HIV-1-seronegative participants from the Multicenter AIDS Cohort Study cohort.
Samples.
Blood was collected by venipuncture, and serum was separated and stored frozen at −80°C until use. Oral fluid samples were collected by laboratory personnel between 9 and 11 a.m. without provocation with any stimulant (i.e., acids or mastication). Two commercially available collection devices were used by following the manufacturers’ instructions. Samples were collected by placing the OraSure collection device (Epitope, Beaverton, Oreg.) between the lower cheek and gum for 2 min. These samples contained mainly OMT (21, 31). Samples were also collected by placing the Omni-Sal device (Saliva Diagnostic Systems, Vancouver, Wash.) under the tongue for 2 min. These samples we call saliva. For study participants who donated both blood and oral fluid, the samples were collected during the same visit. Both OMT and saliva were collected simultaneously from some subjects by placing one OraSure device between the lower cheek and gum and one Omni-Sal device under the tongue for 2 min. After collection, the oral fluid was transferred into a tube containing preservative buffer and thus diluted. The data for each sample were normalized by using a dilution factor (F) that was calculated by using the formula FOMT = (VE + 0.1)/ (VE − 0.63) (for the OraSure device) or FSALIVA = (VE + 0.45)/(VE − 0.55) (for the Omni-Sal device), where VE is the measured volume of liquid extracted from the absorbent pad of the collection device and 0.63 ml is the difference between the volume of preservative buffer (0.73 ml for the specific lot of OraSure devices used) and the volume of liquid (0.1 ml) that remained unextracted from the absorbent pad after centrifugation. The difference between the volume of preservative buffer (1.0 ml for the specific lot of Omni-Sal devices used) and the volume of liquid (0.45 ml) that remained unextracted from the absorbent pad when a serum separator was used for extraction was 0.55 ml. The 0.1- and 0.45-ml volumes were determined in preliminary test tube experiments by using a known volume of oral fluid. The ranges of collected volumes were 0.4 to 1.65 (average, 1.13 ± 0.24) ml of OMT and 0.35 to 2.2 (average, 1.21 ± 0.36) ml of saliva. Oral fluid samples were tested within 3 days of collection or stored at −80°C until use. Preliminary experiments comparing fresh samples and those frozen for 3 months indicated that freezing did not harm the analytes in these samples.
NPT quantification.
NPT in serum was measured by radioimmunoassay (Henning Test Neopterin; B.R.A.H.M.S. Diagnostics GmbH, Berlin, Germany), purchased from Polymedco (New York, N.Y.), by following the manufacturer’s instructions. As oral fluids have lower concentrations of NPT than serum does, a sample volume of 100 μl was used for OMT and saliva instead of the 20 μl used for serum testing. In preliminary experiments, this modification demonstrated that NPT could be measured in oral fluids. In this modified assay, the standards supplied with the kits were used as recommended at 20 μl/well and OMT and saliva samples were used at 100 μl/well. A factor of 5.9 was determined by testing five-times-diluted standards at 100 μl/well and used to transform experimental results to real NPT concentrations.
β2M assay.
β2M in serum and oral fluids was measured by using the IMx automated microparticle enzyme immunoassay system and following the manufacturer’s instructions for IMx β2M (Abbott, Abbott Park, Ill.).
Quantitation of sTNFαRII.
sTNFαRII was measured by using an huTNFαRII enzyme-linked immunosorbent assay kit manufactured by HyCult Biotechnology (Uden, The Netherlands) and purchased from CALTAG Laboratories (San Francisco, Calif.) and following the manufacturer’s instructions. In preliminary recovery studies, no interference of oral fluids with the NPT, β2M, and sTNFαRII assays was found.
Protein assay.
Protein in oral fluids was quantified by the Bradford method (4) using the Bio-Rad protein assay kit with bovine plasma albumin as the standard. To normalize the data for an analyte in every sample tested, the ratio of the experimental value for the analyte to the protein concentration in the same test sample was used.
Oral fluid flow rate.
Oral fluid was collected for 2 min with a collection device. The specimen was extracted from the absorbent pad of the device with a serum separator in the case of the Omni-Sal collection device or by centrifugation at 1,000 × g for 15 min in the case of the OraSure collection device. The volume of the eluate (VE) was measured. The volume of oral fluid (VOMT) or VSALIVA) in the eluate was calculated by using the formula VOMT = VE − 0.63 (for the OraSure device) or VSALIVA = VE − 0.55 (for the Omni-Sal device). The output of a marker in the oral fluid was determined as its concentration was multiplied by the flow rate.
Measurement of CD4+ T-cell numbers.
Whole blood samples were stained with anti-Leu3–phycoerythrin (CD4) and anti-Leu4–fluorescein (CD3) conjugated monoclonal antibodies (Becton Dickinson, Mountain View, Calif.). The percentage of CD4+ T cells was determined by using an EPICS C flow cytometer (Coulter Electronics, Hialeah, Fla.). The CD4+ T-cell numbers were determined by obtaining total and differential leukocyte counts as previously described (11).
Statistical analysis.
The SAS program (29) (SAS Institute, Cary, N.C.) was used for statistical analysis. Data are presented as medians and 25th to 75th percentiles. Comparisons between groups were performed by using the nonparametric t test, the matched paired t test, and/or the Wilcoxon rank sum test. The Pearson rank test was used to assess the correlation between two variables. P values of less than 0.05 were accepted as significant.
RESULTS
Activation marker levels in normal OMT and saliva.
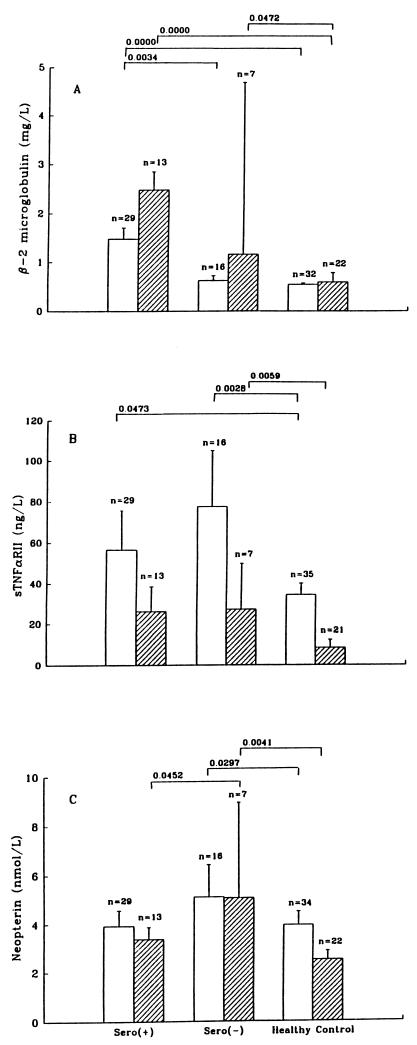
β2M, sTNFαRII, and NPT are detectable in normal OMT and saliva (Fig. 1). Levels of sTNFαRII were similar or higher in OMT. β2M was similar or higher in saliva. Seronegative homosexual men generally had higher levels in both OMT and saliva than the general reference population.
FIG. 1.
Comparison of concentrations of β2M (A), sTNFαRII (B), and NPT (C) in oral fluids of seropositive and seronegative homosexual men and a reference population. For OMT (open bars) and saliva (crossed bars) specimens, the number of samples (n) in each group is shown. Data are medians and standard errors. The P values of differences that are statistically significant are shown at the top.
Levels of immune activation markers in OMT and saliva of HIV-infected persons.
In HIV-positive men without AIDS, the levels of β2M and sTNFαRII were significantly elevated (Fig. 1) in both OMT and saliva. In OMT, the increase in the β2M level was significant compared to the level in both the seronegative and healthy control groups. Correlational analyses showed that the levels of all three analytes were highly correlated (Table 1).
TABLE 1.
Correlation of analyte levels in oral fluids from HIV-seropositive persons
| Sample and parameter | Correlation coefficient (P value)
|
|||
|---|---|---|---|---|
| NPT | β2M | sTNFαRII | Protein | |
| OMTa | ||||
| β2M | 0.6104 (0.0004) | |||
| sTNFαRII | 0.5522 (0.0019) | 0.8214 (0.0001) | ||
| Protein | 0.9234 (0.0001) | 0.5825 (0.0009) | 0.4949 (0.0063) | |
| Flow rate | −0.8193 (0.0001) | −0.4482 (0.0148) | −0.4309 (0.0196) | −0.7193 (0.0001) |
| CD4+ count | −0.1380 (0.4837) | −0.3666 (0.0550) | −0.2481 (0.2031) | −0.0502 (0.7997) |
| Salivab | ||||
| β2M | 0.4900 (0.0891) | |||
| sTNFαRII | 0.5497 (0.0516) | 0.6581 (0.0145) | ||
| Protein | 0.8560 (0.0002) | 0.6215 (0.0234) | 0.8136 (0.0007) | |
| Flow rate | −0.9241 (0.0001) | −0.3665 (0.2180) | −0.4081 (0.1663) | −0.7858 (0.0015) |
| CD4+ count | 0.2162 (0.4781) | −0.0234 (0.9396) | −0.0356 (0.9080) | 0.0388 (0.8998) |
n = 29.
n = 13.
Comparison of levels of activation markers in serum and oral fluid of HIV-seropositive persons.
β2M levels were similar in saliva and serum (Fig. 2) but different in OMT, indicating a difference between these fluids. However, sTNFαRII and NPT concentrations were much higher in serum than in OMT or saliva. Correlation analyses (Table 2) revealed that β2M and sTNFαRII levels in OMT correlated well with the levels in serum. The marker content of saliva, however, did not correlate significantly with the levels in serum. This is another difference between saliva and OMT.
FIG. 2.
Comparison of levels of immune activation markers in oral fluids and sera of HIV-1-infected persons. (A) Box plots of β2M concentrations in OMT (n = 39), saliva (n = 18), and serum (n = 38). (B) Box plots of sTNFαRII concentrations in OMT (n = 39), saliva (n = 18), and serum (n = 30). (C) Box plots of NPT concentrations in OMT (n = 39), saliva (n = 18), and serum (n = 38). The line inside each box represent the median; the lower and upper limits of each box indicate the 25th and 75th percentiles, the vertical lines are the 10th and 90th percentile points, and the lower and upper circles indicate the 5th and 95th percentile points. The P values of statistically significant differences are shown at the top.
TABLE 2.
Correlation of analyte levels in oral fluids with levels in serum during immune activation in HIV-seropositive persons
| Sample and parameter | Correlation coefficient (P value), no. of observations
|
|||
|---|---|---|---|---|
| NPT in serum | β2M in serum | sTNFαRII in serum | CD4+ counts | |
| OMT | ||||
| NPT | 0.2996 (0.0676), 38 | 0.4365 (0.0061), 38 | 0.5509 (0.0016), 30 | −0.1857 (0.2643), 38 |
| β2M | 0.5327 (0.0006), 38 | 0.6474 (0.0001), 38 | 0.6190 (0.0003), 30 | −0.3046 (0.0630), 38 |
| sTNFαRII | 0.2597 (0.1154), 38 | 0.5924 (0.0001), 38 | 0.4072 (0.0255), 30 | −0.2607 (0.1139), 38 |
| CD4+ count | −0.4103 (0.0117), 37 | −0.5393 (0.0006), 37 | −0.5194 (0.0039), 29 | |
| Saliva | ||||
| NPT | 0.0570 (0.8222), 18 | 0.1382 (0.5845), 18 | 0.3400 (0.1675), 18 | −0.2703 (0.2781), 18 |
| β2M | 0.1142 (0.6518), 18 | 0.3195 (0.1963), 18 | 0.4438 (0.0651), 18 | −0.2940 (0.2363), 18 |
| sTNFαRII | 0.0016 (0.9949), 18 | 0.4961 (0.0363), 18 | 0.3578 (0.1449), 18 | −0.1995 (0.4274), 18 |
| CD4+ count | −0.5206 (0.0268), 18 | −0.6360 (0.0046), 18 | −0.5236 (0.0258), 18 | |
Activation marker content of oral fluids considered as output and as ratio to protein content.
The quantity of each marker was related to the flow rate (output) of oral fluid secretion and to the protein content (Table 3). Seropositive men, compared to healthy controls, demonstrated significantly increased β2M and sTNFαRII outputs and ratios in both OMT and saliva. NPT output and analyte/protein ratio were not increased significantly in either OMT or saliva. Healthy controls, HIV-seronegative, and HIV-seropositive persons all had lower β2M and higher sTNFαRII outputs and ratios in OMT than in saliva. These findings are similar to those revealed by direct analyses of the concentrations in OMT and saliva, indicating that the extra measurements (e.g., of flow rate and protein content) may not be necessary.
TABLE 3.
Analyte levels in oral fluids presented in relation to output and protein contenta
| Sample and group (no. of men) | β2M
|
sTNFαR
|
NPT
|
Protein
|
|||
|---|---|---|---|---|---|---|---|
| Output (μg/min) | Ratio (μg/mg) | Output (pg/min) | Ratio (pg/mg) | Output (pmol/min) | Ratio (pmol/mg) | Output (μg/min) | |
| OMT | |||||||
| Healthy controls (35) | 0.30 (0.15) | 0.39 (0.16) | 20.16 (16.29) | 26.57 (18.02) | 1.93 (0.62) | 2.69 (0.86) | 744.7 (199.1) |
| HIV seronegative (16) | 0.24 (0.18) | 0.27 (0.13) | 37.12 (38.76) | 40.17 (31.04) | 1.74 (0.41) | 2.41 (0.72) | 804.0 (393.1) |
| HIV seropositive (29) | 0.77 (0.47)b | 0.81 (0.46)b | 32.96 (29.21)b | 35.50 (30.30) | 2.07 (0.53) | 2.36 (0.67) | 964.4 (439.4)b |
| Saliva | |||||||
| Healthy controls (22) | 0.57 (0.28) | 0.68 (0.33) | 6.69 (8.61) | 7.51 (8.07) | 2.03 (0.87) | 2.36 (0.98) | 920.6 (275.4) |
| HIV seronegative (7) | 1.06 (1.32) | 1.07 (0.60) | 17.68 (26.46) | 25.36 (28.74) | 2.04 (0.65) | 4.35 (4.25) | 788.1 (558.4) |
| HIV seropositive (13) | 1.77 (0.88)b | 1.86 (0.71)b | 16.55 (12.41)b | 17.04 (12.84)b | 2.09 (0.39) | 2.36 (0.54) | 932.9 (271.5) |
Values are means and standard deviations (in parentheses).
P < 0.05 versus controls.
NPT levels in OMT or saliva, however, were not substantially increased in HIV-infected persons, whether measured by concentration (Fig. 1) or in relation to oral fluid secretion flow rate or protein content (Table 3).
Relationship between marker levels in oral fluids and oral infection or stage of HIV disease.
Eight HIV-seropositive persons who had oral thrush at the time of sample collection and three patients who had diagnosed AIDS were compared with the 29 seropositive, thrush- and AIDS-free men reported here. In the HIV-infected participants with AIDS, only NPT output in OMT was significantly different (3.08 ± 1.56 pmol/min, n = 3) from that of the asymptomatic, HIV-seropositive group (2.17 ± 0.56 pmol/min, n = 36) (P = 0.0284). Neither significant differences nor significant correlations between any other marker in OMT versus saliva (measured as a concentration, output, or ratio) and the diagnosis of AIDS were noted. The eight HIV-infected persons with oral thrush showed no significant differences from the thrush-free group. Apparently, oral thrush in HIV infection is not the cause of an increased level of oral fluids. Also, no significant correlations were found between CD4+ T-cell numbers (as a marker for the stage of HIV disease) and the concentration, output, or ratio of NPT, β2M, or sTNFαRII in OMT or saliva (Table 1). This is in accord with previous reports that activation marker levels in serum provide information that differs from that provided by CD4+ T-cell levels, thus indicating independence of these markers of HIV disease.
DISCUSSION
Oral fluids have several advantages over blood and are increasingly being used in the diagnosis and assessment of diseases. The advantages include easy collection, ability to obtain large numbers of specimens within a short time period, safety due to the lack of need for needles and the reported low viral load in oral fluids, safer disposal of waste materials, and possible low overall cost (21, 31). Oral fluid testing has been proposed as the procedure of choice for testing for antibodies to viruses, including HIV (31). Our present findings indicate that oral fluids can also be an alternative to serum or plasma for measurements of β2M and sTNFαRII as markers of immune activation.
Specific features of oral fluid are important to consider when quantitative measurements are sought. These include (i) selection of OMT or saliva as the fluid to measure, (ii) collection of spontaneous versus stimulated fluids, and (iii) ratio of analyte to reference parameters such as oral fluid flow rate and protein content.
Oral fluids are a mixture, with saliva and OMT being the main components. Saliva is a complex mixture of secretions of several salivary glands. OMT (also called gingival crevicular fluid) (21, 31) is the fluid derived from the transport of serum components through the oral mucosa into the mouth. In our present study, efforts were made to collect and analyze OMT separately from saliva. Our analyses emphasize that OMT and saliva are distinct body fluids.
In many saliva studies, samples were collected after stimulation of oral fluid secretion by different techniques (21). It has been shown that variations in stimulation and sample collection methods caused differences in immunological responses (2) and salivary gland function (6). We assume that unstimulated oral fluid better represents the physiological state. Furthermore, as the data indicate, the levels of all of the analytes studied are inversely correlated to the flow rate. In this study, we collected unstimulated samples by using standardized procedures and collection devices, recently developed and commercially available, that include preservative buffers to prevent proteolytic degradations.
Changes in the flow rate influence the concentrations of analytes in oral fluids. Flow rate was inversely correlated with the concentrations of all analytes (Table 1), thus indicating that fluctuations in flow rate will cause differences in marker values. However, when concentrations were normalized with regard to flow rate and analyte measurements were presented as output, variations in output within a short time period were evident. This indicates that changes in analyte concentrations may reflect additional factors. Analyte concentrations in oral secretions, combined with the easy repeatability of oral tests, provide an opportunity for identification of rapid changes and follow-up of short-term changes caused by therapeutic or other interventions.
To normalize analyte concentrations, we used the protein concentration in samples as a reference and present the data as an analyte concentration-to-protein concentration ratio. When total protein is selected as a reference, two facts may be important. The total proteins are a complex mixture. Furthermore, there are large differences among the molecular weights of NPT, β2M, and sTNFαRII. As the results indicate (Table 3), the use of this ratio provides data that are similar to the output data based on flow rates and apply to both β2M and sTNFαRII. The differences between the levels of analytes in HIV-infected persons and normal controls are readily apparent.
Immune activation is an essential feature of HIV pathogenesis. Increased production of cytokines is reflected by elevated levels of the products of cytokine activation, such as β2M, in serum and plasma. The level of β2M (measured as a concentration, output, or ratio) in both OMT and saliva is higher in HIV-infected persons than in healthy controls. In HIV-infected persons, compared to HIV-seronegative controls, the increase in the β2M level is significant for OMT but not for saliva. This difference between OMT and saliva and, more importantly, the lack of correlation between saliva and OMT for the activation parameter indicate that OMT and saliva are distinct body fluids.
In HIV-infected persons, the level of β2M was significantly higher in saliva than in OMT (Fig. 2). Such an elevation could be caused by (i) a selectively higher rate of β2M transport in salivary glands than in OMT or (ii) an increased rate of local synthesis of β2M that reflects higher activation of oral mucosal immune cells. The level of β2M in OMT correlated with the levels of all immune activation markers in serum (Table 2). In contrast, the β2M level in saliva did not correlate with that of any marker in serum. Thus, it seems more likely that β2M in OMT reflects mainly marker levels in serum and generalized immune activation, while β2M in saliva may represent local synthesis. Together, these data strongly indicate that OMT, but not saliva, is the preferred oral fluid alternative for β2M measurement in serum as an indicator of systemic immune activation. sTNFαRII levels in OMT correlated fairly well (P < 0.026) with levels in serum, but saliva did not. Thus, OMT could be an alternative to serum for measurement of sTNFαRII.
We found that NPT levels in the saliva and OMT of the HIV-seropositive group were not significantly increased compared to those in controls. Our NPT level finding in nonstimulated saliva differs from the results of Reibnegger et al., who found significantly higher NPT levels in the stimulated saliva of HIV-infected patients than in that of controls (26). However, our saliva findings agree with that of Müller et al., who have shown that NPT concentration and output in stimulated parotid saliva of HIV-infected persons were neither significantly increased over those of controls nor correlated with NPT levels in serum (23). Similar results for NPT levels in stimulated whole saliva have been reported by Evans and Wansbrough-Jones (7). An important difference between those studies and ours is that we used unstimulated saliva.
Serial assessments of NPT or β2M levels in serum or urine have proved useful in assessing the course of diseases such as multiple sclerosis (11a) and inflammatory bowel disease (24a). The value of measuring markers of immune activation in HIV infection is well known. In the present study, we have documented the capacity to detect generalized immune activation by measurement of the products of immune activation in OMT and saliva. Acquisition of OMT compared to serum or plasma is noninvasive and easily repeatable, and measurements of β2M and/or sTNFαRII could be useful for identification of rapid responses or for follow-up of short-term changes caused by complications or therapeutic interventions in patients with HIV infection or autoimmune disorders or other chronic inflammatory disorders.
ACKNOWLEDGMENTS
We are indebted to all of the persons who donated samples, to Thomas Thieme (Epitope) and Nora Eskes (Saliva Diagnostic System) for generously donating collection devices, to Hripi Nishanian and Matthew McDonald for laboratory support, to Susan Stehn for assistance in data management, and to James Moore and Deborah Mathieson for help with manuscript preparation.
This work was supported by grants AI 36086 and AI 35040 from NIAID, NIH.
REFERENCES
- 1.Akalin F A, Bulut S, Yavuzyilmaz E. Beta2-microglobulin levels in serum and saliva of patients with juvenile periodontitis. J Nihon Univ Sch Dent. 1993;35:230–234. doi: 10.2334/josnusd1959.35.230. [DOI] [PubMed] [Google Scholar]
- 2.Aufricht C, Tenner W, Salzer H R, Khoss A E, Wurst E, Herkner K. Salivary IgA concentration is influenced by the saliva collection method. Eur J Clin Chem Clin Biochem. 1992;30:81–83. [PubMed] [Google Scholar]
- 3.Aukrust P, Liabakk N B, Müller F, Lien E, Espevik T, Froland B S. Serum levels of tumor necrosis factor-α (TNFα) and soluble TNF receptors in human immunodeficiency virus type 1 infection—correlations to clinical, immunologic, and virologic parameters. J Infect Dis. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 5.Diez-Ruiz A, Tilz G P, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumor necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 6.Dodds M, Hsieh S C, Johnson D A. The effect of increased mastication by daily gum-chewing on salivary gland output and dental plaque acidogenicity. J Dent Res. 1991;70:1474–1478. doi: 10.1177/00220345910700120101. [DOI] [PubMed] [Google Scholar]
- 7.Evans M R W, Wansbrough-Jones M H. Saliva and serum neopterin concentrations not significantly correlated in HIV-1 infection. Clin Chem. 1995;41:950–951. [PubMed] [Google Scholar]
- 8.Fahey J L, Taylor J M G, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi J V. The prognostic value of cellular and serologic markers in infection with HIV-1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson D B, editor. Steroid hormones in saliva. S. München, Germany: Karger; 1984. [Google Scholar]
- 10.Fuchs D, Hausen A, Reibnegger G, Werner E R, Dierich M P, Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol Today. 1988;9:150–155. doi: 10.1016/0167-5699(88)91203-0. [DOI] [PubMed] [Google Scholar]
- 11.Giorgi J V, Cheng H-L, Margolick J B, Bauer K D, Ferbas J, Waxdal M, Schmid I, Hultin L E, Jackson A L, Park L, Taylor J M G The Multicenter AIDS Cohort Study Group. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the Multicenter AIDS Cohort Study experience. Clin Immunol Immunopathol. 1990;55:173–186. doi: 10.1016/0090-1229(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 11a.Giovannoni G, Lai M, Kidd D, Thorpe J W, Miller D H, Thompson A J, Keir G, Feldmann M, Thompson E J. Daily urinary neopterin excretion as an immunological marker of disease activity in multiple sclerosis. Brain. 1997;120:1–13. doi: 10.1093/brain/120.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Haeckel R. Report on the workshop conference “The Application of Saliva in Laboratory Medicine.”. J Clin Chem Clin Biochem. 1989;27:221–252. [Google Scholar]
- 13.Haeckel R. Saliva, an alternative specimen in clinical chemistry. J Int Fed Clin Chem. 1990;2:208–216. [Google Scholar]
- 14.Hoekman K, Van-Nieuwkoop J A, Willemze R. The significance of beta2-microglobulin in clinical medicine. Neth J Med. 1985;28:551–557. [PubMed] [Google Scholar]
- 15.Hofmann B, Wang Y, Cumberland W G, Detels R, Bozorgmehri M, Fahey J L. Serum β2-microglobulin level increases in HIV infection: relation to seroconversion, CD4 T-cell fall and prognosis. AIDS. 1990;4:207–214. [PubMed] [Google Scholar]
- 16.Huber C, Batchelor J R, Fuchs D, Hausen A, Lang A, Nierderwieser D. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaslow R A, Ostrow D G, Detels R, Phair J P, Polk B F, Rinaldo C R., Jr The multicenter AIDS cohort study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 18.Katoh S, Sueoka T, Matsuura S, Sugimoto T. Biopterin and neopterin in human saliva. Life Sci. 1989;45:2561–2568. doi: 10.1016/0024-3205(89)90240-3. [DOI] [PubMed] [Google Scholar]
- 19.Lacey J N, Forbes M A, Waugh M A, Cooper E H, Hambling M H. Serum beta2-microglobulin and human immunodeficiency virus infection. AIDS. 1987;1:123–127. [PubMed] [Google Scholar]
- 20.Maddali Bongi S, Campana G, D’Agata A, Palermo C, Bianucci G. The diagnosis value of β2-microglobulin and immunoglobulins in primary Sjögren’s syndrome. Clin Rheumatol. 1995;14:151–156. doi: 10.1007/BF02214934. [DOI] [PubMed] [Google Scholar]
- 21.Mortimer P P, Parry J V. Detection of antibody to HIV in saliva: a brief review. Clin Diagn Virol. 1994;2:231–243. doi: 10.1016/0928-0197(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 22.Mucklow J C. The use of saliva in therapeutic drug monitoring. Ther Drug Monit. 1982;4:229–247. [Google Scholar]
- 23.Müller F, Holberg-Petersen M, Rollag H, Brandtzaeg P, Froland S S. Nonspecific oral immunity in individuals with HIV infection. J Acquired Immune Defic Syndr. 1992;5:46–51. [PubMed] [Google Scholar]
- 24.Nishanian P, Taylor J M G, Korns E, Detels R, Saah A, Fahey J L. Significance of quantitative enzyme-linked immunosorbent assay (ELISA) results in evaluation of three ELISAs and Western blot tests for detection of antibodies to human immunodeficiency virus in a high-risk population. J Clin Microbiol. 1987;25:395–400. doi: 10.1128/jcm.25.2.395-400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Propst A, Propst T, Herold M, Vogel W, Judmaier G. Interleukin-1 receptor antagonist in differential diagnosis of inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 1995;7:1031–1036. doi: 10.1097/00042737-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Read G F. Immunoassays of steroids in saliva. In: Read G F, Riad-Fahmy D, Walker R F, Griffiths K, editors. Proceedings of the Ninth Tenevus Workshop. Cardiff, Wales: Alpha Omega Publishing Ltd.; 1984. pp. 193–201. [Google Scholar]
- 26.Reibnegger G, Fuchs D, Zangerle R, Wachter H. Increased neopterin concentration in saliva of patients with HIV-1 infection. Clin Chem. 1990;36:1379–1380. [PubMed] [Google Scholar]
- 27.Rolinski B, Wintergerst U, Matuschke A, Füessl H, Goebel F D, Roscher A A, Belohradsky B H. Evaluation of saliva as a specimen for monitoring zidovudine therapy in HIV-infected patients. AIDS. 1990;5:885–888. doi: 10.1097/00002030-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Rostoker G, Pech M A, Petit-Phar M, BenMaadi A, Cholin S, Lang P, Dubert J M, Weil B, Lagrue G. Mucosal immunity in adult primary glomerulonephritis. Nephron. 1990;54:42–46. doi: 10.1159/000185808. [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute. SAS user’s guide: statistics version 5 edition. Cary, N.C: SAS Institute; 1985. [Google Scholar]
- 30.Schramm W, Kidwell D A, Smith R H, Craig P A. Drugs of abuse in saliva: a review. J Anal Toxicol. 1992;16:1–9. doi: 10.1093/jat/16.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Tamashiro H, Constantine N T. Serological diagnosis of HIV infection using oral fluid samples. Bull WHO. 1994;72:135–143. [PMC free article] [PubMed] [Google Scholar]
- 32.Wachter H, Fuchs D, Hausen A, Reibnegger G, Werner E R. Neopterin as a marker for activation of cellular immunity: immunologic basis and clinical application. Adv Clin Chem. 1989;27:81–141. doi: 10.1016/s0065-2423(08)60182-1. [DOI] [PubMed] [Google Scholar]