Abstract
Background:
Adolescents and young adults (AYAs) who have undergone liver transplantations often struggle to adhere to their post-transplant immunosuppressant medications, which can lead to serious health complications. The objective of this pilot study is to examine the acceptability and feasibility of a brief mobile health (mHealth) intervention and its impact on medication adherence among AYA liver transplant recipients.
Methods:
Thirty-five AYAs (13–21 years old) were randomized to either 1) receive praise text messages whenever laboratory results indicated immunosuppressant medications within the expected range or 2) usual care. Motivation for adherence and adherence were assessed via self-report, and a medication level variability index (MLVI) was calculated based on values abstracted from the electronic health record.
Results:
Multilevel, multivariate models showed significant associations between group assignment and some self-reported motivation and adherence outcomes, but not MLVI. Specifically, AYA receiving the praise text messages were significantly more likely to report taking their prescribed doses (OR = 2.49, p = .03), taking their medicine according to the directions (OR = 2.39, p = .04), and being highly confident in taking their medication (OR = 2.46, p = .04), compared to the usual services group. Qualitative responses indicated praise texts were mostly helpful but could be improved.
Conclusions:
The results suggest texting patients about positive health indicators was acceptable, and with refinement, might promote AYA illness self-management.
Keywords: adolescents, liver transplant, adherence, text message, mobile health
Although medical advancements have enabled children receiving liver transplants to live longer, medical management post-transplant is complex. Youth are required to take immunosuppressive drugs among other medications, usually for the rest of their lives, to decrease risks of graft rejection and other complications. When liver transplant recipients do not follow their medication regimen, they are at increased risk for various health complications, including late acute rejection, death, and re-transplantation due to chronic rejection.1–4 Despite these severe consequences, medication nonadherence remains a significant problem.5 Even if children and their families develop a successful adherence routine initially after transplant, adherence challenges may arise as more time elapses.6
Adolescents and young adults (AYA) are particularly at risk for nonadherence post liver-transplant,7–9 although Shemesh and colleagues did not find any associations of age with adherence within their sample of children and adolescents between 1 and 17 years old.10. Some possible reasons for these difficulties may include fear of friends finding out about their medical illness, family stressors, striving for normality, forgetting, and concerns regarding aversive side effects.11 Among AYA, nonadherence may be greater in older compared to younger adolescents,1 and exacerbated when late AYA transition to adult care.12 Focusing efforts to increase treatment adherence among the AYA population is therefore critical.
Demographic risk factors have also been identified for nonadherence. The literature consistently suggests that low socioeconomic status is associated with more adherence difficulties among pediatric liver transplant recipients.1,10 For example, lower family income and government funded medical insurance (versus private insurance) predicts non-adherence.9 Research on links between race, ethnicity, and post-transplant medical adherence is more inconsistent.9 However, there are reasons to consider ethnic differences when examining medication adherence among transplant recipients. First, mortality rates and graft failure tend to be higher in minoritized youth,13 and missing immunosuppressant medication doses may be a contributing factor. Adherence difficulties and health disparities have also been identified in minoritized youth with other medical conditions.14
Despite research highlighting risk factors for AYA medication nonadherence, little is known about effective interventions for improving these concerns.15 Some have suggested utilizing technology to address adherence barriers among transplant recipients.16 There have been promising reports of improvements in symptoms, medication adherence and self-management through telemedicine interventions in AYAs with chronic medical conditions such as sickle cell disease, childhood cancer, asthma and obesity.17–22 The majority of technology-based interventions aiming to promote health behaviors among AYA with chronic health conditions have used text messaging.16 To date, there have only been two studies evaluating the use of text messages to promote adherence behaviors among AYA with liver transplants. One study found that text message reminders resulted in significant improvements in medication adherence and reduced rejection episodes.23 A separate study found that AYA with liver transplants receiving automated two-way reminder text reminders showed higher participation rates in laboratory testing.24
This emerging body of evidence supports further evaluation of text message interventions to promote adherence among AYA with liver transplants. However, both studies focused on this population have investigated sending reminders which only targets one adherence barrier—forgetting. This is not surprising, as the most the most common application of text messages to promote adherence across different health behaviors and conditions is sending reminders.25 Yet, adherence is a complex behavior impacted by multiple factors. The Information-Motivation-Behavioral Skills Model provides a comprehensive framework for understanding adherence,26 and suggests that interventions could improve AYA medication-taking through several routes, by providing informational feedback, enhancing motivation, and leveraging the principles of behavioral reinforcement.27 Guided by this framework the current study expands on the science of adherence promotion by evaluating a brief text-message intervention providing patients with positive feedback when their labs indicate good adherence.
Treatment guidelines recommend that pediatric liver transplant recipients undergo laboratory tests to measure immunosuppressant levels in their blood, which provides physicians with a rough marker of medication adherence.28 A common practice across health care systems is to use a “no news is good news” model, such that only patients whose labs indicate nonadherence are contacted and provided with additional supportive services.29 When labs indicate satisfactory adherence, physicians do not regularly contact patients. This represents a missed opportunity for promoting adherence and could even be detrimental to patients who may feel uncertainty or distress from the lack of feedback.30 This ambiguity could negatively impact patient motivation and the patient-provider relationship. Further, from a Social Learning Theory perspective,31 calling attention to positive behaviors increases the likelihood of future occurrence.
Using verbal praise to reinforce disease management strategies can be an effective communication technique.32 In adult populations, studies suggest that providing patients with positive feedback (delivered verbally by a physician, through interactive voice response calls, or letters from insurance companies) promotes medication adherence.33,34 However, some studies testing the impact of verbal praise on medical adherence have not found effects.35 No study to date has examined using praise to reinforce optimal adherence among AYA transplant recipients. Delivering praise through text messages may be appealing and feasible for today’s youth who have high rates of mobile phone use.36
This mixed-methods pilot study explored the feasibility and impact of a brief text-messaging intervention, during which AYA patients were praised for their efforts via text messaging when their lab results reflected sufficient immunosuppressant medication adherence. We had two primary aims: 1) Study patient satisfaction with and utilization of this intervention; and 2) Investigate the effects of this intervention on medication adherence and adherence motivation. We hypothesized that AYA assigned to receive text message praise would report higher medication adherence and motivation for adherence, and that their immunosuppressant blood levels from the medical record would demonstrate greater consistency over the course of the study.
Method
Participant Characteristics
This study took place within Children’s Hospital Los Angeles (CHLA) Division of Gastroenterology, Hepatology, and Nutrition (NCT04995770). The sample included (N = 35) AYA 13–21 years old. Inclusion criteria were having a liver transplant, receiving post-transplant care at CHLA, having access to a working cell phone which could receive text messages, and speaking English. There were no additional exclusion criteria. See Figure 1 for CONSORT diagram.
Figure 1.
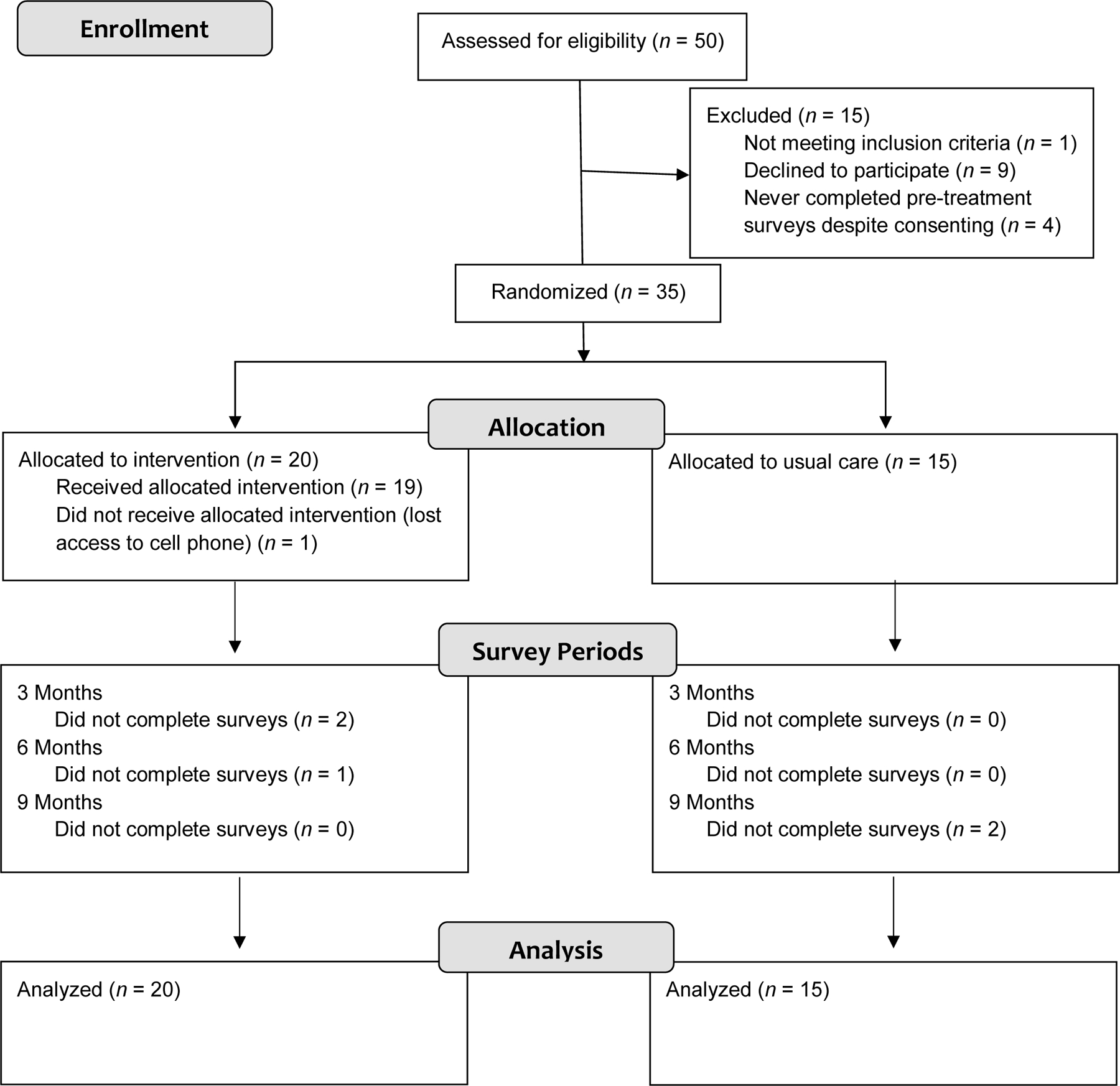
CONSORT diagram.
Design
The liver transplant team social worker maintains a list of all active patients followed in the clinic. At the initiation of the study, the social worker extracted all the patients from this list who met age-based criteria for participation on the study, along with their phone numbers and mailing addresses. Between August and December of 2019, we mailed letters and placed up to three phone calls to each eligible patient, and also had study personnel directly share flyers and make in-person verbal invitations to participate after medical visits. The last participant was enrolled in December 2019, and followed for one year. Researchers obtained consent from adult participants, and parental permission and assent from minor participants. Based on pre-hoc analyses aiming to achieve statistical power > .80 for detecting group differences using repeated measures, we selected a recruitment target of 50 AYA. However, we did not reach this target, enrolling only 35 AYA (n = 20 allocated to praise text messages, n = 15 allocated to usual care).
Once participants were consented, they were texted a link to a pre-treatment survey via REDCap, which was programmed to randomly allocate participants who completed the pre-treatment survey to one of the two conditions based on a list of group assignments created using the Excel rand( ) function. Participants received an automated text message via REDCap informing them of their assigned condition. All participants were texted links to complete surveys again at 3 months (mid-treatment), 6 months (post-treatment), and 9 months (follow-up). At the conclusion of the study, we randomly selected 20 participants to invite for a qualitative phone interview. Fifteen (75.0%) agreed to be interviewed (n = 10 from the intervention; n = 5 from the usual care). Participants could earn up to $90 in gift cards for completing all procedures. Researchers also obtained consent to abstract data from participants’ electronic health records for the year prior to and post study enrollment. Procedures were approved by the CHLA Institutional Review Board.
Conditions
The text message praise intervention lasted for 6 months. Each week, transplant coordinators, masked from treatment condition, prepared a list of all patients between 13–21 years whose laboratory tests indicated immunosuppressant medications within the expected range. A researcher then sent a praise text message praise via REDCap to patients on that list who were currently assigned to the intervention. We rotated through 14 standardized text messages each week, such as, “Your labs look very good. Super job taking your meds!” The co-authors developed the praise messages based on their combined training in evidence-based child therapy which emphasizes using praise to promote desired behaviors, and clinical experience encouraging patient adherence. After initially brainstorming 20 messages, the co-authors discussed them collaboratively, ultimately selecting 14 to use in the study. Researchers piloted the text message delivery several times to different study personnel cell phones and checked that text messages continued to be delivered to these cell phones periodically throughout the study period. Participants in usual care did not receive praise text messages. All participants continued to receive usual care from the multidisciplinary liver transplant team, including phone calls and follow-up care when laboratory tests indicated immunosuppressant medications outside of the expected range.
Measures
Demographics and socioeconomic status.
Participants reported their gender identity, ethnicity, race, and highest level of education on the pre-treatment survey. Participants also reported which caregivers they lived with and their parents’ highest level of education. In addition, participants’ socioeconomic statuses were measured using the Family Affluence Scale II (FAS II).37
Medication adherence.
In this study, adherence was measured by a 3-item visual analogue scale (VAS).38 On the VAS, participants mark a point on a line scaled from 0% to 100% to estimate their percentage of adherence to medications over the past 3 months. At all survey points, participants were asked to indicate their adherence over the past three months according to three items: 1) the percentage of the time patients took their medicine, 2) the percentage of the time patients took all the doses for the day, and 3) the percentage of the time patients took their medicine according to the directions.38 For this study, we dichotomized responses to each VAS item as imperfect adherence (<100%) or perfect adherence (=100%) to account for patients’ tendency to overreport adherence.39 VAS scores were highly negatively skewed and leptokurkik. Therefore, medians and interquartile ranges were reported.
Motivation for adherence.
Adherence motivation was measured by the Rollnick’s Readiness Ruler, a 3-item questionnaire assessing participants’ attitudes toward a specified target behavior.40 Each question asks participants to mark a point on a line scaled from 0 to 10 to indicate their motivation level.40 An example item is: “How important is it to you to take your medication exactly as your doctor has told you?” In addition to importance, we assessed “confidence” and “readiness” to take medication. Like VAS responses, motivation scores were highly negatively skewed and leptokurkik, so medians and interquartile ranges were reported. We also dichotomized motivation into imperfect motivation (<10 out of 10) or perfect motivation (10 out of 10) because participants reported exceedingly high motivation.
Immunosuppressant levels.
As part of regular care, patients have laboratory blood draws to measure trough levels of immunosuppressant medications such as tacrolimus. The clinic policy is to collect labs at least every 4 weeks in the first year post-transplant, and then ideally every 8–12 weeks after one year. For this study, we abstracted all the tacrolimus lab values from one year prior to study enrollment to one year post study enrollment. One coder abstracted data from all 35 participants using REDCap, and a second coder independently abstracted data from 20 randomly selected participants to allow for calculation of reliability statistics. The tacrolimus values were abstracted with excellent reliability (ICC = .999). We calculated the medication level variability index (MLVI), a validated objective measure of the degree of fluctuation of tacrolimus blood levels over time.41 MLVI is calculated as the standard deviation (SD) of at least 3 tacrolimus trough blood levels for each patient. A higher MLVI denotes more fluctuation in levels. For our analyses, we dichotomized participants using the MLVI>2.0 cutoff which indicates erratic adherence.10
Qualitative exit survey and interview.
As part of the post-treatment surveys, participants assigned to receive text message praise were asked open-ended questions assessing the feasibility and acceptability of the intervention. Phone interviews lasting between 20 and 30 minutes were conducted with a randomly selected subset of participants from both the text message praise and usual care conditions by the first author, a researcher who is not part of the liver transplant clinical team. Interviews were conducted using a semi-structured guide with questions focused on participants’ experiences in the study and views of the praise text message intervention. Interviews were audio recorded and transcribed. Transcripts were checked against the original recordings.
Analysis
Using mixed-methods, we assessed feasibility, acceptability, and preliminary efficacy of the intervention. This study was conceptualized as a pilot study, aiming to estimate effect sizes to inform a future clinical trial with greater statistical power. We began by testing for significant differences in pre-enrollment characteristics between the intervention and usual care conditions, using independent samples t-tests for continuous variables and chi-square tests for categorical variables with at least five observations in each cell. We also probed for statistical differences in outcomes at each time period, using chi-square tests, as well.
We used multivariate, mixed-effect logistic models to test for differences in self-reported medication adherence using the VAS38 and motivation for adherence using Rollnick’s Readiness Ruler40 over the four time points assessed (pre-enrollment, mid-intervention, post-intervention, and follow-up). Due to skewed responses on the adherence and motivation for adherence measures, we dichotomized these outcomes based on perfect adherence and perfect motivation versus imperfect adherence and imperfect motivation. We also used mixed-effect models to test for differences in MLVI>2.0 (a binary outcome) from the year prior to the year post study enrollment.
Mixed-effect models are helpful when examining data from relatively small samples, such as in pilot studies, because researchers can leverage repeated measures to increase statistical power.42 Further, mixed-effects models can be preferable to alternatives such as repeated analysis of variance texts because they do not require listwise deletion if some observations are missing.43 Age, time living with a transplant (graft age), gender, Latinx ethnicity, FAS II, and maternal education were included as covariates as these factors have been associated with adherence outcomes in the literature.1,9–14,44 Continuous or ordinal variables were mean-centered to facilitate interpretation. We analyzed data using the SPSS Version 27 GLMM command, specifying an autoregressive moving average random effect covariance type. All statistical tests were based on two-tailed alternatives, and p < 0.05 was considered significant.
Mixed-effects models produce adjusted odds ratios (OR) which estimate the relationship between each individual independent variable and the average level of the dependent variable across all observed time points. To interpret effect sizes related to the intervention, we inspected the ORs characterizing the relations between assignment to the praise text messages and adherence, motivation, and MLVI outcomes. We considered OR = 1.7 to be small, OR = 3.5 to be medium, and OR = 6.7 to be large.45 Two authors independently coded all qualitative exit survey responses and phone intervention transcripts using Dedoose software, resolving any disagreements by consensus. Using inductive coding and following Braun and Clarke’s (2006) thematic analysis guidelines,46 the authors collaboratively developed themes and subthemes, iteratively merging, adding, and removing redundant themes.
Results
Descriptive data
See Table 1 for descriptive statistics of pre-enrollment characteristics. There were no statistically significant differences in pre-enrollment characters by group assignment (p < .05). Participants assigned to the text intervention received a mean of 2.95 (SD = 2.28) praise texts, ranging from 1 to 11. The median was 2 (IQR = 2, 3). Descriptive data for outcomes are reported in Table 2. Participants had on average 7.77 (SD = 13.84) immunosuppressant labs in the year prior to study enrollment and 9.94 (SD = 11.30) in the year post study enrollment. The number of labs ranged from a minimum of 0 to a maximum of 71 in the year prior to study enrollment, and a minimum of 0 to a maximum of 48 in the year post study enrollment. There were no statistically significant differences between outcomes by condition at any individual timepoint (Table 3).
Table 1.
Baseline Participant Characteristics
| Characteristic | Total Sample | Intervention Condition | Usual Care Condition | p |
|---|---|---|---|---|
| n (%) | ||||
|
| ||||
| Age at Study Entry, years | 16.00 (2.31) | 16.00 (2.65) | 16.00 (1.85) | .50 |
| Time Living with Current Graft, months | 129.09 (65.90) | 129.85 (66.97) | 128.07 (66.76) | .47 |
| Socioeconomic Status (FAS II, 0–9) | 4.94 (2.01) | 4.65 (2.21) | 5.33 (1.72) | .16 |
| M (SD) | ||||
|
| ||||
| Gender Identity | ||||
| Cisgender Female | 17 (48.57) | 8 (40.00) | 9 (60.00) | .32 |
| Cisgender Male | 16 (45.71) | 11 (55.00) | 5 (33.33) | .31 |
| Transgender/Not Reported | 2 (5.71) | 1 (5.00) | 1 (6.67) | -- |
| Race | ||||
| American Indian/Alaska Native | 1 (2.86) | 0 (0.00) | 1 (6.67) | -- |
| Asian/Pacific Islander | 2 (5.71) | 1 (5.00) | 0 (0.00) | -- |
| Black/African American | 1 (2.86) | 0 (0.00) | 1 (6.67) | -- |
| White | 9 (25.71) | 7 (35.00) | 2 (13.33) | -- |
| More Than One Race | 6 (17.14) | 3 (15.00) | 3 (20.00) | -- |
| Not Reported | 16 (45.71) | 4 (20.00) | 4 (26.67) | -- |
| Latinx Ethnicity | 26 (74.29) | 13 (65.00) | 12 (80.00) | -- |
| Patient’s Highest Level of Education | ||||
| 8th Grade | 12 (34.29) | 9 (45.00) | 3 (20.00) | -- |
| 9th Grade | 2 (5.71) | 0 (0.00) | 2 (13.33) | -- |
| 10th Grade | 4 (11.43) | 2 (10.00) | 2 (13.33) | -- |
| 11th Grade | 3 (8.57) | 2 (10.00) | 1 (6.67) | -- |
| 12th Grade/Diploma/Equivalent | 10 (28.57) | 4 (20.00) | 6 (40.00) | -- |
| Some College/Vocational School | 4 (11.43) | 3 (15.00) | 1 (6.67) | -- |
| Which Caregivers Do You Live With? | ||||
| Mother and Father | 18 (51.43) | 12 (60.00) | 6 (40.00) | .32 |
| Mother Only | 15 (42.86) | 6 (30.00) | 9 (60.00) | .10 |
| Father Only | 1 (2.86) | 1 (5.00) | 0 (0.00) | -- |
| Father and Other Relative | 1 (2.86) | 1 (5.00) | 0 (0.00) | -- |
| Maternal Education | ||||
| Less Than High School | 8 (22.86) | 6 (30.00) | 4 (26.67) | -- |
| High School Diploma/Equivalent | 8 (22.86) | 2 (10.00) | 6 (40.00) | -- |
| Some College/Vocational School | 8 (22.86) | 6 (30.00) | 2 (13.33) | -- |
| Completed College/Vocational School | 6 (17.14) | 4 (20.00) | 2 (13.33) | -- |
| Some Graduate School | 3 (8.57) | 1 (5.00) | 1 (6.67) | -- |
| Paternal Education | ||||
| Less Than High School | 4 (11.43) | 3 (15.00) | 1 (6.67) | -- |
| High School Diploma/Equivalent | 3 (8.57) | 3 (15.00) | 0 (0.00) | -- |
| Some College/Vocational School | 6 (17.14) | 3 (15.00) | 3 (20.00) | -- |
| Completed College/Vocational School | 4 (11.43) | 4 (20.00) | 0 (0.00) | -- |
| Some Graduate School | 1 (2.86) | 0 (0.00) | 1 (6.67) | -- |
| Completed Graduate School | 1 (2.86) | 1 (5.00) | 0 (0.00) | -- |
| Not Reported | 1 (2.86) | 0 (0.00) | 1 (6.67) | -- |
Note. Chi-square tests were conducted only for variables with at least five observations in each cell.
Table 2.
Study Outcomes over Time by Condition
| Intervention Condition (n = 20) | Usual Care Condition (n = 15) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Self-Report | 0 Months | 3 Months | 6 Months | 9 Months | 0 Months | 3 Months | 6 Months | 9 Months |
| VAS 1a | n = 19 | n = 18 | n = 19 | n = 19 | n = 15 | n = 15 | n = 14 | n = 13 |
| Mdn | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 90.00 |
| IQR | 90.00–100.00 | 89.75–100.00 | 95.00–100.00 | 95.00–100.00 | 84.00–100.00 | 90.00–100.00 | 97.00–100.00 | 86.50–100.00 |
| n (%)d | 10 (52.6) | 10 (55.6) | 13 (68.4) | 13 (68.4) | 8 (53.3) | 8 (53.3) | 9 (64.3) | 5 (38.5) |
| VAS 2b | n = 19 | n = 18 | n = 19 | n = 20 | n = 15 | n = 15 | n = 14 | n = 13 |
| Mdn | 100.00 | 100.00 | 100.00 | 100.00 | 99.00 | 98.00 | 100.00 | 92.00 |
| IQR | 90.00–100.00 | 93.75–100.00 | 96.00–100.00 | 98.00–100.00 | 83.00–100.00 | 90.00–100.00 | 94.00–100.00 | 89.00–100.00 |
| n (%)d | 10 (52.6) | 11 (61.1) | 12 (63.2) | 13 (65.0) | 7 (46.7) | 5 (33.3) | 10 (71.4) | 5 (38.5) |
| VAS 3c | n = 19 | n = 18 | n = 19 | n = 20 | n = 15 | n = 15 | n = 13 | n = 13 |
| Mdn | 96.00 | 99.50 | 100.00 | 100.00 | 97.00 | 96.00 | 98.00 | 96.00 |
| IQR | 85.00–100.00 | 90.00–100.00 | 97.00–100.00 | 85.00–100.00 | 81.00–100.00 | 83.00–100.00 | 84.50–100.00 | 90.00–100.00 |
| n (%)d | 7 (36.7) | 9 (50.0) | 12 (63.2) | 11 (55.0) | 6 (40.0) | 7 (46.7) | 5 (38.5) | 4 (30.8) |
| Importance | n = 20 | n = 18 | n = 19 | n = 20 | n = 15 | n = 15 | n = 14 | n = 13 |
| Mdn | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| IQR | 100.00–100.00 | 100.00–100.00 | 100.00–100.00 | 100.00–100.00 | 99.00–100.00 | 100.00–100.00 | 99.75–100.00 | 100.00–100.00 |
| n (%)d | 17 (85.0) | 16 (88.9) | 15 (79.0) | 19 (95.0) | 11 (73.3) | 12 (80.0) | 11 (78.6) | 11 (84.6) |
| Confidence | n = 20 | n = 18 | n = 19 | n = 20 | n = 14 | n = 15 | n = 14 | n = 13 |
| Mdn | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 98.00 |
| IQR | 91.50–100.00 | 98.25–100.00 | 100.00–100.00 | 98.50–100.00 | 91.50–100.00 | 90.00–100.00 | 95.00–100.00 | 92.50–100.00 |
| n (%)d | 12 (60.0) | 13 (72.2) | 16 (84.2) | 15 (75.0) | 9 (64.3) | 10 (66.7) | 9 (64.3) | 6 (46.2) |
| Readiness | n = 20 | n = 18 | n = 19 | n = 20 | n = 15 | n = 15 | n = 14 | n = 13 |
| Mdn | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| IQR | 85.75–100.00 | 100.00–100.00 | 100.00–100.00 | 94.00–100.00 | 93.50–100.00 | 92.00–100.00 | 99.00–100.00 | 97.00–100.00 |
| n (%)d | 11 (55.0) | 16 (88.9) | 17 (89.5) | 12 (60.0) | 9 (60.0) | 10 (66.7) | 11 (78.6) | 8 (61.5) |
|
| ||||||||
| EHR Abstracted | Year Prior | Year Post | Year Prior | Year Post | ||||
|
| ||||||||
| MLVI | n = 14 | n = 16 | n = 12 | n = 13 | ||||
| Mdn (IQR) | 1.74 (0.75, 3.09) | 1.55 (0.84, 1.79) | 1.05 (0.65, 2.91) | 1.72 (0.98, 2.73) | ||||
| >2.0, n (%)d | 6 (42.9) | 3 (15.0) | 4 (33.3) | 6 (46.2) | ||||
VAS 1 = the percentage of the time patients took their medicine.
VAS 2 = the percentage of the time patients took all the doses for the day.
VAS 3 = the percentage of the time patients took their medicine according to the directions.
The counts and percentages describe the proportion of individuals reporting 100% adherence for each VAS and Ruler item.
Table 3.
Tests of Statistical Difference between Outcomes by Condition
| Intervention Condition (n = 20) | |||||
|---|---|---|---|---|---|
|
| |||||
| Self-Report | 0 Months | 3 Months | 6 Months | 9 Months | 12 Months |
| VAS 1a | |||||
| χ2 | 0.01 | 0.02 | 0.06 | 2.82 | -- |
| p | 1.000 | 1.000 | 1.000 | 0.149 | -- |
| VAS 2b | |||||
| χ2 | 0.12 | 2.53 | 0.25 | 2.24 | -- |
| p | 1.000 | 0.166 | 0.719 | 0.169 | -- |
| VAS 3c | |||||
| χ2 | 1.80 | 0.04 | 1.89 | 1.87 | -- |
| p | 0.300 | 1.000 | 0.280 | 0.284 | -- |
| Importance | |||||
| χ2 | 0.73 | 0.50 | 0.01 | 1.03 | -- |
| p | 0.430 | 0.639 | 1.000 | 0.547 | -- |
| Confidence | |||||
| χ2 | 0.64 | 0.12 | 1.74 | 2.83 | -- |
| p | 1.000 | 1.000 | 0.238 | 0.142 | -- |
| Readiness | |||||
| χ2 | 0.87 | 2.42 | 0.75 | 0.01 | -- |
| p | 1.000 | 0.203 | 0.628 | 1.000 | -- |
| MLVI | |||||
| χ2 | 0.25 | -- | -- | -- | 0.43 |
| p | 0.701 | -- | -- | -- | 0.688 |
VAS 1 = the percentage of the time patients took their medicine.
VAS 2 = the percentage of the time patients took all the doses for the day.
VAS 3 = the percentage of the time patients took their medicine according to the directions.
Feasibility Indicators
In terms of feasibility, implementing the intervention put some minor additional demands on clinic personnel, as transplant coordinators put in extra time identifying which AYA had labs in the expected range. Sending text messages via REDCap each week took fewer than 10 minutes of study personnel time and cost less than 1 cent per participant. Most participants reported they received the text messages easily. However, some remarked on feasibility barriers, such as not having consistent access to their phone throughout the study period.
Mixed-Effects Logistic Regression Models
Mixed-effects logistic regression models predicting VAS and Ruler items estimated the independent effect of each predictor on the average level of each outcome across all four measurement periods. Individual ORs indicated that participants assigned to the text message condition were significantly more likely to report taking 100% of their prescribed doses (OR = 2.49, p = .03) and taking their medicine according to the directions 100% of the time (OR = 2.39, p = .04), adjusting for other predictors in the model. In addition, participants in the text message condition were significantly more likely to rate their confidence in taking their medication a 10 out of 10 (OR = 2.46, p = .04), adjusting for other predictors in the model. These ORs are small.45 See Tables 4 and 5 for the fixed effect coefficients and significance tests. In at least one of the six models, older age, more months living with a graft, Latinx ethnicity, and higher maternal education were associated with lower adherence or motivation. Cisgender female identity frequently was associated with higher adherence and motivation.
Table 4.
Logistic Mixed-Effects Models Predicting Self-Reported Perfect Adherence or MLVI>2.0
| Model Coefficients | β | SE | Odds Ratio | 95% CI | p |
|---|---|---|---|---|---|
| DV = VAS 1 (Nn = 129) | |||||
| Intercept | −0.12 | 1.43 | 0.89 | 0.05–15.20 | 0.934 |
| Time | 0.10 | 0.18 | 1.11 | 0.78–1.59 | 0.563 |
| Text Message Praisea | 0.64 | 0.43 | 1.90 | 0.81–4.42 | 0.137 |
| Age | −0.18 | 0.10 | 0.83 | 0.68–1.01 | 0.064 |
| Graft Age | −0.11 | 0.04 | 0.89 | 0.82–0.97 | 0.006* |
| Latinxb | −1.04 | 0.51 | 0.35 | 0.13–0.96 | 0.041* |
| Cisgender Femalec | 1.19 | 0.45 | 3.29 | 1.36–7.96 | 0.009* |
| FAS II | 0.03 | 0.12 | 1.03 | 0.81–1.32 | 0.803 |
| Maternal Education | −0.42 | 0.17 | 0.66 | 0.47–0.92 | 0.014* |
| DV = VAS 2 (Nn = 130) | |||||
| Intercept | −1.01 | 1.77 | 0.36 | 0.01–12.04 | 0.568 |
| Time | 0.10 | 0.18 | 1.11 | 0.77–1.58 | 0.577 |
| Text Message Praise | 0.91 | 0.42 | 2.49 | 1.08–5.75 | 0.033* |
| Age | −0.23 | 0.10 | 0.80 | 0.66–0.97 | 0.022* |
| Graft Age | −0.09 | 0.04 | 0.91 | 0.85–0.98 | 0.016* |
| Latinx | −0.02 | 0.48 | 0.98 | 0.37–2.55 | 0.961 |
| Cisgender Female | 1.03 | 0.43 | 2.79 | 1.18–6.60 | 0.020* |
| FAS II | 0.01 | 0.12 | 1.01 | 0.79–1.28 | 0.947 |
| Maternal Education | −0.26 | 0.17 | 0.77 | 0.55–1.07 | 0.119 |
| DV = VAS 3 (Nn = 129) | |||||
| Intercept | −0.87 | 1.79 | 0.42 | 0.01–14.58 | 0.628 |
| Time | 0.07 | 0.18 | 1.08 | 0.75–1.54 | 0.691 |
| Text Message Praise | 0.87 | 0.43 | 2.39 | 1.03–5.56 | 0.043* |
| Age | −0.23 | 0.10 | 0.80 | 0.66–0.97 | 0.021* |
| Graft Age | 0.05 | 0.04 | 1.05 | 0.97–1.13 | 0.227 |
| Latinx | −0.75 | 0.49 | 0.47 | 0.18–1.24 | 0.125 |
| Cisgender Female | 1.23 | 0.44 | 3.42 | 1.43–8.16 | 0.006* |
| FAS II | −0.13 | 0.12 | 0.88 | 0.69–1.12 | 0.295 |
| Maternal Education | −0.38 | 0.17 | 0.68 | 0.49–0.95 | 0.025* |
| DV = MLVI>2.0 (Nn = 54) | |||||
| Intercept | 1.06 | 1.99 | 2.87 | −2.96–5.07 | 0.599 |
| Time | −0.08 | 0.16 | 0.93 | −0.40–0.24 | 0.626 |
| Text Message Praise | −0.63 | 0.67 | 0.53 | −1.97–0.71 | 0.351 |
| Age | 0.23 | 0.14 | 1.26 | −0.06–0.52 | 0.116 |
| Graft Age | −0.07 | 0.06 | 0.94 | −0.18–0.05 | 0.244 |
| Latinx | −0.80 | 0.82 | 0.45 | −2.46–0.85 | 0.334 |
| Cisgender Female | −1.05 | 0.67 | 0.35 | −2.40–0.31 | 0.126 |
| FAS II | 0.21 | 0.24 | 1.23 | −0.27–0.69 | 0.385 |
| Maternal Education | 0.22 | 0.31 | 1.25 | −0.41–0.85 | 0.482 |
Reference group = Usual care condition.
Reference group = Non-Latinx participants.
Reference group = Non-cisgender female participants.
Table 5.
Logistic Mixed-Effects Models Predicting Self-Reported Perfect Motivation for Adherence
| Model Coefficients | β | SE | Odds Ratio | 95% CI | p |
|---|---|---|---|---|---|
| DV = Importance (Nn = 131) | |||||
| Intercept | 1.38 | 1.43 | 3.99 | 0.24–67.61 | 0.335 |
| Time | 0.14 | 0.18 | 1.16 | 0.81–1.65 | 0.424 |
| Text Message Praisea | 0.39 | 0.42 | 1.47 | 0.64–3.40 | 0.362 |
| Age | −0.02 | 0.10 | 0.98 | 0.81–1.18 | 0.797 |
| Graft Age | −0.08 | 0.04 | 0.92 | 0.86–0.99 | 0.032* |
| Latinxb | −0.18 | 0.48 | 0.83 | 0.32–2.16 | 0.703 |
| Cisgender Femalec | −0.35 | 0.43 | 0.70 | 0.30–1.66 | 0.415 |
| FAS II | 0.06 | 0.12 | 1.06 | 0.84–1.35 | 0.605 |
| Maternal Education | −0.07 | 0.17 | 0.94 | 0.67–1.30 | 0.694 |
| DV = Confidence (Nn = 130) | |||||
| Intercept | 0.64 | 2.21 | 1.90 | 0.02–151.87 | 0.772 |
| Time | 0.00 | 0.18 | 1.00 | 0.70–1.44 | 0.981 |
| Text Message Praise | 0.90 | 0.44 | 2.46 | 1.02–5.90 | 0.044* |
| Age | −0.15 | 0.10 | 0.86 | 0.70–1.05 | 0.142 |
| Graft Age | −0.06 | 0.04 | 0.94 | 0.87–1.02 | 0.120 |
| Latinx | −0.47 | 0.49 | 0.62 | 0.24–1.65 | 0.339 |
| Cisgender Female | 0.23 | 0.44 | 1.26 | 0.53–3.00 | 0.603 |
| FAS II | 0.00 | 0.12 | 1.00 | 0.78–1.28 | 0.999 |
| Maternal Education | −0.64 | 0.18 | 0.53 | 0.37-.076 | 0.001* |
| DV = Readiness (Nn = 131) | |||||
| Intercept | 0.49 | 1.68 | 1.63 | 0.06–45.17 | 0.773 |
| Time | 0.05 | 0.18 | 1.06 | 0.74–1.51 | 0.763 |
| Text Message Praise | 0.60 | 0.42 | 1.82 | 0.79–4.19 | 0.160 |
| Age | −0.09 | 0.10 | 0.91 | 0.76–1.10 | 0.344 |
| Graft Age | −0.01 | 0.04 | 0.99 | 0.92–1.07 | 0.770 |
| Latinx | −0.56 | 0.48 | 0.57 | 0.22–1.49 | 0.253 |
| Cisgender Female | 0.99 | 0.43 | 2.68 | 1.14–6.34 | 0.025* |
| FAS II | −0.06 | 0.12 | 0.94 | 0.74–1.19 | 0.616 |
| Maternal Education | −0.47 | 0.17 | 0.63 | 0.45–0.87 | 0.006* |
Reference group = Usual care condition.
Reference group = Non-Latinx participants.
Reference group = Non-cisgender female participants.
Models predicting MLVI did not include any significant predictors (Table 4). These models included fewer observations, because there were only two time periods, the year prior and the year post enrollment, rather than four repeated measures in the models examining self-reported outcomes. The OR associated with condition assignment suggested those in the text message group were about half as likely to have erratic adherence, defined as MLVI > 2.0 (OR = 0.53, p = 0.351), but this was not significant.
Qualitative Feedback
We generated several themes from the data, including ways participants found praise text messages to be helpful. The key themes describing participants experiences of the intervention are summarized in Table 6. We also identified themes of Recommendations for Improving Praise Texts (e.g., participants suggested sending messages more quickly after labs were drawn, providing options to contact your doctor with questions, and including prizes) and Feasibility Barriers (e.g., losing phone privileges, having phone stolen). In addition, we generated a theme of Delivering Praise Messages via the Patient Portal. Most participants reported not knowing what a portal was, and only two reported currently using the portal. Once learning about the portal, many reported it would have benefits of appearing more “professional,” prevent praise messages from “getting lost like texts,” and would allow messages to be “coordinated” with other functions like reviewing lab results and messaging doctors. However, they also described barriers to using the portal, such as lack of space to install applications on their phone, difficulties remembering passwords, and the extra effort it required.
Table 6.
Participants’ Subjective Experience of the Praise Text Intervention
| Theme | Illustrative Quotations |
|---|---|
| Praise Texts Were Encouraging | “It would encourage me to try harder and keep the momentum going of good labs.” “It gave more like a motivational type of focus.” |
| Praise Texts Were Reassuring | “It calmed my nerves while I was waiting for a call.” “I would stop worrying about if the labs are good or not.” |
| Praise Texts Were Informative | “You would be able to see your body, your health is doing alright.” “It was good to keep me updated and knowing how I’m doing.” |
| Praise Texts Were Easy to Receive | “Kids always have their phones so they could see them automatically.” “Well, it’s just like on your phone, you know? You wouldn’t have to download an app. They just directly message you about it.” |
Discussion
This pilot study investigated the feasibility, acceptability, and potential impact of sending praise text messages to AYA with liver transplants when their lab results reflected proper medication adherence. We identified good adherence whenever regularly taken immunosuppressant laboratory tests were within the expected range and sent text messages praising medication adherence as the desired behavior. This tactic is a shift away from the traditional “no news is good news” approach. The primary outcome was self-reported adherence, and additionally we examined the impact of the text messages on motivation for adherence and MLVI, an indicator of ongoing good adherence derived from the medical chart.
Acceptability was fairly strong based on qualitative feedback. Participants described praise messages as being encouraging, reassuring, and informative. Furthermore, the majority of AYA in this study identified text messages as their preferred mode for receiving praise about their labs because they generally had their phone accessible, felt comfortable texting, and found it easy. Their feedback was consistent with recent trends in technology use.47 They also provided feedback for ways to enhance acceptability, such as by considering other technological models of patient communication, increasing the coordination of the messages, or adding tangible rewards. This study took place from August 2019 through December 2020, spanning the historical period before and during the COVID-19 pandemic. During this time, patients and providers became much more familiar with using technology to deliver care, which could also make interventions like text message praise even more feasible and acceptable within the culture and structure of health care systems. Recent reviews suggest the increasing use of technology in health care delivery during the pandemic may have possible therapeutic benefits for children.48,49 For the current study, this may have made text messages from the liver transplant clinic even more welcome for AYA patients, if they were experiencing pandemic-related social isolation, heightened health anxiety, family stress and reduced physical and psychosocial support which have all been reported in recent literature.48,49The statistical evidence for intervention effects on motivation, adherence, and consistency of lab values over time was mixed. There were significant effects of praise messages on the average level of self-reported medication adherence and participants’ confidence in taking their medications across all four observation periods, which is promising. However, there was no significant relationship between praise messages and MLVI>2.0, a biochemical measure indicating erratic adherence. Among participants with sufficient tacrolimus values to calculate MLVI, only 15.0% of intervention participants displayed erratic adherence in the year after study randomization in contrast to 46.2% of usual care participants. Moreover, although non-significant, adjusted OR results suggested that intervention participants were half as likely to display erratic adherence compared to usual care participants, controlling for other known adherence risk factors. All intervention ORs were also in the hypothesized direction. Combining these data observations offers support and rationale for conducting a larger randomized controlled trial with sufficient statistical power to detect small effects. Additionally, as there are few published economic evaluations to support mHealth interventions,50,51 future studies of adherence-promoting text message interventions should collect economic data to determine whether health care systems should invest in mHealth options for patients.
The results of this study suggest that the praise text message intervention is promising but could be refined to be more impactful. For example, AYA might benefit from receiving feedback more immediately or with a clearer, more personalized praise message (e.g., “We just reviewed your labs from Monday November 10th. It looks great! Wonderful job staying on top of your medications.”). Participant suggestions are consistent with evidence-based behavioral therapies for youth that promote using immediate, specific praise.52 Also, incorporating two-way text message communication might help ensure that participants are consistently receiving text messages (e.g., inviting a confirmation response from AYA) or by giving AYA opportunities to respond with questions or comments. In support of this idea, a meta-analysis of text message interventions promoting medication adherence found significantly better results with two-way versus one-way texting capabilities.53 Furthermore, integrating the text message intervention into the existing electronic health record platform could better match the existing workflow for clinic personnel. For example, a more convenient process could give transplant coordinators the option to click a box in the patient’s chart to automatically send a praise message when documenting lab review activities.
In 2009, the United States congress passed the Health Information Technology for Economic and Clinical Health (HITECH) Act, which incentivized providers to adopt electronic health records and make them accessible to patients, such as through portals.54 Considering the increasing prevalence of patient portals, as well as their relative security to other forms of electronic communication, it would be reasonable to consider delivering praise messages via a patient portal instead of text messaging.55 However, the feedback from the participants suggests portals may not be engaging or appealing to AYA. Although a few participants reported they would see benefits to receiving the messages in a portal, almost no participants had enrolled in the existing patient portal and many described substantial barriers to using it. The qualitative results of this study support delivering motivational messages to AYA via text messaging, rather than patient portals.
This study expanded the literature of text message interventions for promoting medication adherence by examining the impact of praise. This strategy takes advantage of a low-cost, developmentally appropriate communication technology to promote health behaviors, and expands the empirical support for such interventions beyond medication or appointment reminders. Our findings build upon the small body of research investigating the impact of text messages on AYA with liver transplants. Like Miloh and colleagues,23 the results of this study support the hypothesis that text message interventions could promote medication adherence among AYA with liver transplants. Our intervention differs from the two previously published studies by evaluating praise messages rather than reminders.23,24 Adherence is a complex behavior involving more than simply remembering or forgetting to take an action. Future researchers should develop and investigate other text message strategies guided by the Information-Motivation-Behavioral Skills Model26, such as providing personalized feedback, psychoeducation, or translating interventions from Motivational Interviewing or cognitive-behavioral therapy to be delivered via mobile technology.
Strengths and Limitations
This study had several limitations. First, the sample size was small, limiting statistical power to find significant effects. Second, as a single site study, there is limited generalizability to other populations that differ demographically from ours. In addition, participants self-reported very high adherence and motivation throughout the study which may reflect social desirability bias. However, dichotomizing these outcomes as imperfect or perfect may help distinguish between AYA with optimal versus lower motivation or adherence, even if the actual percentage they report may be inflated. In addition, we did not directly assess adherence to the lab draw schedule, which makes MLVI data more difficult to interpret. However, prior research has found no impact of missing immunosuppressant data on the relationship between MLVI and poor health outcomes.56 Future studies would benefit from including standardized lab visits as part of participation to collect biochemical measures on a more consistent schedule. Including additional adherence measures such as electronic monitors or pharmacy possession ratios may strengthen future studies. Further, participants were aware of the study condition to which they were assigned which could have influenced the outcomes. However, the team determined that patient safety would be greater if AYA understood whether they should expect text messages or not, so they would not be confused about the results of their laboratory tests. Finally, we did not have access to backend data from REDCap that would have confirmed whether text messages were successfully delivered or received, which would have been valuable for assessing the feasibility and implementation of the intervention.
These limitations should be considered in light of the study’s strengths. First, using random assignment and piloting the intervention in a real-world setting enhanced the internal and external validity of this study. Second, using MLVI to evaluate the impact of the intervention on a biochemically measured outcome helped strengthen the study through multimethod measurement. In addition, using both qualitative and quantitative methods allowed for a richer examination of the potential impact of text message praise on AYA with liver transplants.
Conclusion
This pilot study evaluated the feasibility, acceptability, and potential effects of sending praise text messages to AYA for immunosuppressant labs within the expected range. Overall, participants found the intervention encouraging, reassuring, informative, and easy to use. The results of this study suggest that text message praise could promote AYA confidence in taking medication and medication adherence; however, the intervention requires further refinement to improve feasibility, acceptability, and efficacy. Future research with larger samples should investigate how text message interventions like this might promote AYA adherence and health, as they traverse a difficult developmental period.
Funding:
This work was supported by grants (UL1TR001855 and UL1TR000130) from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health, awarded to the Southern California Clinical and Translational Science Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We appreciate the assistance from our colleagues, Colleen Borkovec, Holly Nagasugi, and Ashton Pollreisz, as well as undergraduate research students, Yujie Chen and Owen Friend, in conducting this study.
Abbreviations:
- AYA
adolescent and young adult
- MLVI
medication level variability index
- VAS
visual analogue scale
- FAS
Family Affluence Scale
Data Availability Statement:
A de-identified dataset is available upon request to the first author.
References
- 1.Berquist R, Berquist WE, Esquivel CO, et al. Adolescent non‐adherence: prevalence and consequences in liver transplant recipients. Pediatr Transplant 2006;10(3):304–10. DOI: 10.1111/j.1399-3046.2005.00451.x [DOI] [PubMed] [Google Scholar]
- 2.Fredericks EM, Lopez MJ, Magee JC, et al. Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. Am J Transplant 2007;7:1974–1983. DOI: 10.1111/j.1600-6143.2007.01878.x [DOI] [PubMed] [Google Scholar]
- 3.Morrissey PE, Flynn ML, Lin S. Medication noncompliance and its implications in transplant recipients. Drugs 2007;67(10):1463–81. DOI: 10.2165/00003495-200767100-00007 [DOI] [PubMed] [Google Scholar]
- 4.Shemesh E, Shneider BL, Savitzky JK, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics 2004; 113:825–832. DOI: 10.1542/peds.113.4.825 [DOI] [PubMed] [Google Scholar]
- 5.Lurie S, Shemesh E, Sheiner PA, et al. Non‐adherence in pediatric liver transplant recipients− an assessment of risk factors and natural history. Pediatr Transplant 2000;4(3):200–6. DOI: 10.1034/j.1399-3046.2000.00110.x [DOI] [PubMed] [Google Scholar]
- 6.Loiselle KA, Gutierrez‐Colina AM, Eaton CK, et al. Longitudinal stability of medication adherence among adolescent solid organ transplant recipients. Pediatr Transplant 2015;19(4):428–35. DOI: 10.1111/petr.12480 [DOI] [PubMed] [Google Scholar]
- 7.Burra P, Germani G, Gnoato F, et al. Adherence in liver transplant recipients. Liver Transpl 2011;17(7):760–70. DOI: 10.1002/lt.22294 [DOI] [PubMed] [Google Scholar]
- 8.Dobbels F, Damme‐Lombaert RV, Vanhaecke J, Geest SD. Growing pains: Non‐adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatr Transplant 2005;9(3):381–90. DOI: 10.1111/j.1399-3046.2005.00356.x [DOI] [PubMed] [Google Scholar]
- 9.Killian MO, Schuman DL, Mayersohn GS, Triplett KN. Psychosocial predictors of medication non‐adherence in pediatric organ transplantation: a systematic review. Pediatr Transplant 2018;22(4):e13188. DOI: 10.1111/petr.13188 [DOI] [PubMed] [Google Scholar]
- 10.Shemesh E, Duncan S, Anand R, et al. Trajectory of adherence behavior in pediatric and adolescent liver transplant recipients: The medication adherence in children who had a liver transplant cohort. Liver Transpl 2018;24(1):80–8. DOI: 10.1002/lt.24837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanghøj S, Boisen KA. Self-reported barriers to medication adherence among chronically ill adolescents: a systematic review. J Adolesc Health 2014;54(2):121–38. DOI: 10.1016/j.jadohealth.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 12.Annunziato RA, Emre S, Shneider B, et al. Adherence and medical outcomes in pediatric liver transplant recipients who transition to adult services. Pediatr Transplant 2007;11(6):608–14. DOI: 10.1111/j.1399-3046.2007.00689.x [DOI] [PubMed] [Google Scholar]
- 13.Thammana RV, Knechtle SJ, Romero R, et al. Racial and socioeconomic disparities in pediatric and young adult liver transplant outcomes. Liver Transpl 2014;20(1):100–15. DOI: 10.1002/lt.23769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQuaid EL, Landier W. Cultural issues in medication adherence: disparities and directions. J Gen Intern Med 2018;33(2):200–6. DOI: 10.1007/s11606-017-4199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredericks EM, Dore‐Stites D, Lopez MJ, et al. Transition of pediatric liver transplant recipients to adult care: patient and parent perspectives. Pediatr Transplant 2011;15(4):414–24. DOI: 10.1111/j.1399-3046.2011.01499.x [DOI] [PubMed] [Google Scholar]
- 16.Mehta P, Steinberg EA, Kelly SL, et al. Medication adherence among adolescent solid‐organ transplant recipients: a survey of healthcare providers. Pediatr Transplant 2017;21(7):e13018. DOI: 10.1111/petr.13018 [DOI] [PubMed] [Google Scholar]
- 17.Badawy SM, Barrera L, Sinno MG, Kaviany S, O’dwyer LC, Kuhns LM. Text messaging and mobile phone apps as interventions to improve adherence in adolescents with chronic health conditions: a systematic review. JMIR mHealth uHealth 2017; 15;5(5):e7798. DOI: 10.2196/mhealth.7798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badawy SM, Kuhns LM. Texting and mobile phone app interventions for improving adherence to preventive behavior in adolescents: a systematic review. JMIR mHealth uHealth 2017;5(4):e6837. DOI: 10.2196/mhealth.6837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radovic A, Badawy SM. Technology use for adolescent health and wellness. Pediatrics 2020;145(Supplement_2):S186–94. DOI: 10.1542/peds.2019-2056G [DOI] [PubMed] [Google Scholar]
- 20.Ramsey WA, Heidelberg RE, Gilbert AM, Heneghan MB, Badawy SM, Alberts NM. eHealth and mHealth interventions in pediatric cancer: a systematic review of interventions across the cancer continuum. Psycho‐oncol 2020;29(1):17–37. DOI: 10.1002/pon.5280 [DOI] [PubMed] [Google Scholar]
- 21.Badawy SM, Cronin RM, Hankins J, Crosby L, DeBaun M, Thompson AA, Shah N. Patient-centered eHealth interventions for children, adolescents, and adults with sickle cell disease: systematic review. JMIR 2018;20(7):e10940. DOI: 10.2196/10940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah AC, Badawy SM. Telemedicine in pediatrics: systematic review of randomized controlled trials. JMIR Pediatr Parent 2021;4(1):e22696. DOI: 10.2196/22696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miloh T, Annunziato R, Arnon R, et al. Improved adherence and outcomes for pediatric liver transplant recipients by using text messaging. Pediatrics 2009;124(5):e844–50. DOI: 10.1542/peds.2009-0415 [DOI] [PubMed] [Google Scholar]
- 24.McKenzie RB, Berquist WE, Foley MA, et al. Text messaging improves participation in laboratory testing in adolescent liver transplant patients. J Particip Med 2015;7:e7. [PMC free article] [PubMed] [Google Scholar]
- 25.Schwebel FJ, Larimer ME. Using text message reminders in health care services: A narrative literature review. Internet Interv 2018;13:82–104. DOI: 10.1016/j.invent.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher WA, Fisher JD, Harman J. The information-motivation-behavioral skills model: A general social psychological approach to understanding and promoting health behavior. Social psychological foundations of health and illness 2003;22(4):82–106. [Google Scholar]
- 27.Fredericks EM, Dore-Stites D. Adherence to immunosuppressants: how can it be improved in adolescent organ transplant recipients?. Curr Opin Organ Transplant 2010;15(5):614. DOI: 10.1097/MOT.0b013e32833d3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christina S, Annunziato RA, Schiano TD, et al. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl 2014;20(10):1168–77. DOI: 10.1002/lt.23930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwan JL, Cram P. Do not assume that no news is good news: test result management and communication in primary care. BMJ Qual Saf 2015;24:664–666. DOI: 10.1136/bmjqs-2015-004645 [DOI] [PubMed] [Google Scholar]
- 30.Sweeny K. On the experience of awaiting uncertain news. Curr Dir Psychol Sci 2018;27(4):281–5. DOI: 10.1177/0963721417754197 [DOI] [Google Scholar]
- 31.Parcel GS, Baranowski T. Social learning theory and health education. Health Educ J 1981;12(3):14–8. DOI: 10.1080/00970050.1981.10618149 [DOI] [PubMed] [Google Scholar]
- 32.Clark NM, Gong M. Management of chronic disease by practitioners and patients: are we teaching the wrong things?. BMJ 2000;320(7234):572–5. DOI: 10.1136/bmj.320.7234.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA 2002;288(22):2880–3. DOI: 10.1001/jama.288.22.2880 [DOI] [PubMed] [Google Scholar]
- 34.Stacy JN, Schwartz SM, Ershoff D, Shreve MS. Incorporating tailored interactive patient solutions using interactive voice response technology to improve statin adherence: results of a randomized clinical trial in a managed care setting. Popul Health Manag 2009;12(5):241–54. DOI: 10.1089/pop.2008.0046 [DOI] [PubMed] [Google Scholar]
- 35.Gaude GS, Hattiholi J, Chaudhury A. Role of health education and self-action plan in improving the drug compliance in bronchial asthma. J Family Med Prim Care 2014;3(1):33. DOI: 10.4103/2249-4863.130269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsh-Yechezkel G, Mandelzweig L, Novikov I, Bar-Yosef N, Livneh I, Oren M, Waysberg R, Sadetzki S. Mobile phone-use habits among adolescents: Predictors of intensive use. Cyberpsychol Behav Soc Netw 2019;22(3):212–9. DOI: 10.1089/cyber.2018.0177 [DOI] [PubMed] [Google Scholar]
- 37.Boyce W, Torsheim T, Currie C, Zambon A. The family affluence scale as a measure of national wealth: validation of an adolescent self-report measure. Soc Indic Res 2006;78(3):473–87. DOI: 10.1007/s11205-005-1607-6 [DOI] [Google Scholar]
- 38.Walsh JC, Dalton M, Gazzard BG. Adherence to combination antiretroviral therapy assessed by anonymous patient self-report. AIDS 1998;12(17):2361–3. [PubMed] [Google Scholar]
- 39.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 2015;5(4):470–82. DOI: 10.1007/s13142-015-0315-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rollnick S, Mason P, Butler C. Health behavior change: A guide for practitioners Edinburgh, UK: Churchill Livingstone; 2001. [Google Scholar]
- 41.Shemesh E, Fine RN. Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant 2010;14(8):940–3. DOI: 10.1111/j.1399-3046.2010.01396.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridenour TA, Hall DL, Bost JE. A small sample randomized clinical trial methodology using N-of-1 designs and mixed model analysis. Am J Drug Alcohol Abuse 2009; 35(4):260–6. DOI: 10.1080/00952990903005916 [DOI] [PubMed] [Google Scholar]
- 43.Edwards LJ. Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatr Pulmonol 2000; 30(4):330–44. DOI: [DOI] [PubMed] [Google Scholar]
- 44.Boucquemont J, Pai AL, Dharnidharka VR, Hebert D, Furth SL, Foster BJ. Gender differences in medication adherence among adolescent and young adult kidney transplant recipients. Transplant 2019;103(4):798–806. DOI: 10.1097/TP.0000000000002359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput 2010;39(4):860–4. DOI: 10.1080/03610911003650383 [DOI] [Google Scholar]
- 46.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3(2):77–101. DOI: 10.1191/1478088706QP063OA [DOI] [Google Scholar]
- 47.Anderson M, Jiang J. Teens, social media & technology 2018. Pew Research Center 2018;31(2018):1673–89. Retrieved from https://www.pewresearch.org/internet/2018/05/31/teens-social-media-technology-2018/ [Google Scholar]
- 48.Badawy SM, Radovic A. Digital approaches to remote pediatric health care delivery during the COVID-19 pandemic: existing evidence and a call for further research. JMIR Pediatr Parent 2020;3(1):e20049. DOI: 10.2196/20049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serlachius A, Badawy SM, Thabrew H. Psychosocial challenges and opportunities for youth with chronic health conditions during the COVID-19 pandemic. JMIR Pediatr Parent 2020;3(2):e23057. DOI: 10.2196/23057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badawy SM, Kuhns LM. Economic evaluation of text-messaging and smartphone-based interventions to improve medication adherence in adolescents with chronic health conditions: a systematic review. JMIR mHealth uHealth 2016;4(4):e6425. DOI: 10.2196/mhealth.6425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iribarren SJ, Cato K, Falzon L, Stone PW. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PloS one 2017;12(2):e0170581. DOI: 10.1371/journal.pone.0170581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leijten P, Gardner F, Melendez-Torres GJ, et al. Meta-analyses: Key parenting program components for disruptive child behavior. J Am Acad Child Adolesc Psychiatry 2019;58(2):180–90. DOI: 10.1016/j.jaac.2018.07.900 [DOI] [PubMed] [Google Scholar]
- 53.Wald DS, Butt S, Bestwick JP. One-way versus two-way text messaging on improving medication adherence: meta-analysis of randomized trials. Am J Med 2015;128(10):1139.e1–1139.e5. DOI: 10.1016/j.amjmed.2015.05.035 [DOI] [PubMed] [Google Scholar]
- 54.Murphy-Abdouch K. Patient access to personal health information: regulation vs. reality. Perspect Health Inf Manag 2015;12,1c. eCollection. [PMC free article] [PubMed] [Google Scholar]
- 55.Goldzweig CL, Orshansky G, Paige NM, et al. Electronic patient portals: evidence on health outcomes, satisfaction, efficiency, and attitudes a systematic review. Ann Intern Med 2013;159:677–87. doi: 10.7326/0003-4819-159-10-201311190-00006 [DOI] [PubMed] [Google Scholar]
- 56.Shemesh E, Bucuvalas JC, Anand R, Mazariegos GV, Alonso EM, Venick RS, Reyes‐Mugica M, Annunziato RA, Shneider BL. The Medication Level Variability Index (MLVI) predicts poor liver transplant outcomes: a prospective multi‐site study. Am J Transplant 2017;17(10):2668–78. DOI: 10.1111/ajt.14276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A de-identified dataset is available upon request to the first author.


