Abstract
Background:
Polycyclic aromatic hydrocarbons (PAHs) are endocrine-disrupting chemicals. Few studies have evaluated the association between pubertal development in girls and PAH exposures quantified by urinary biomarkers.
Methods:
We examined associations of urinary PAH metabolites with pubertal development in 358 girls aged 6–16 years from the San Francisco Bay Area enrolled in a prospective cohort from 2011–2013 and followed until 2020. Using baseline data, we assessed associations of urinary PAH metabolites with pubertal development stage. In prospective analyses limited to girls who at baseline had not yet started breast (N=176) or pubic hair (N=179) development or menstruation (N=267), we used multivariable Cox proportional hazards regression to assess associations of urinary PAH metabolites with onset of breast and pubic hair development, menstruation, and pubertal tempo (interval between onset of breast development and menstruation).
Results:
We detected PAH metabolites in >98% of girls. In cross-sectional analyses using baseline data, PAH metabolites were not associated with pubertal development stage. In prospective analyses, higher concentrations (≥ median) of some PAH metabolites were associated with two-fold higher odds of earlier breast development (2-hydroxynaphthalene, 1-hydroxyphenanthrene, summed hydroxyphenanthrenes, total summed metabolites) or pubic hair development (1-hydroxynaphthalene) among girls overweight at baseline (body mass index (BMI)-for-age percentile ≥85) compared to non-overweight girls with lower metabolites concentrations. PAH metabolites were not associated with age at menarche or pubertal tempo.
Conclusions:
PAH exposures were widespread in our sample. Our results support the hypothesis that, in overweight girls, PAHs impact the timing of pubertal development, an important risk factor for breast cancer.
Keywords: Menarche, Puberty, Polycyclic aromatic hydrocarbons (PAHs), Urinary biomarkers
INTRODUCTION
Age at menarche, after declining in the first half of the 20th century, has stabilized in the last several decades.1 In recent decades, age at onset of breast development (thelarche) has notably declined in the United States (U.S.) and elsewhere,2 lengthening the time between breast development and menarche. Reasons underlying the trend of earlier breast development are not fully understood and may be related to changes in childhood obesity3 or environmental chemical exposures.4
Growing evidence from epidemiologic studies5 supports the hypothesis that endocrine-disrupting chemicals may affect pubertal timing in girls.1,6 Endocrine-disrupting chemicals may affect pubertal timing through multiple mechanisms, including binding with hormone receptors mimicking endogenous hormones such as estrogens and androgens or blocking the functions of endogenous hormones and acting as anti-estrogens and anti-androgens.5 Estrogenic, anti-estrogenic, and anti-androgenic effects have been reported for some PAHs and metabolites.7,8
PAHs are ubiquitous environmental pollutants formed by combustion of organic materials. Common sources of exposure include inhalation of tobacco smoke, vehicle exhaust, or smoke from wood fires, and consumption of charred or smoked meat or fish.9 Data on the relation between specific sources of PAH exposures and pubertal timing, however, are sparse and inconsistent,10–14 and only one prospective study assessed the association of urinary PAH metabolites with timing of breast and pubic hair development.14 Effects of PAH exposures on pubertal development would be highly relevant to breast cancer, as earlier breast development and earlier menarche are associated with increased breast cancer risk.15–17 Furthermore, PAH exposures have been associated with higher risk of breast cancer.18
We examined whether commonly detected urinary PAH metabolites are associated with pubertal timing in girls who participated in the California cohort of the Lessons in Epidemiology and Genetics of Adult Cancer from Youth (LEGACY) Girls Study (2011–2016),19 and subsequently enrolled in the California PAH Study (2017–2020).20
METHODS
The study is based on the LEGACY Girls Study, a multi-center prospective cohort conducted from 2011–2016.19 The California site enrolled 362 girls aged 6–16 years and their mothers from the San Francisco Bay Area. The girls were the daughters of women participating in the Northern California Breast Cancer Family Registry,21 or were recruited through friend referral, community outreach, or social media.19 About half of the enrolled girls had a family history of breast cancer in first- or second-degree relatives. We collected additional data and biospecimens from participants every 6 months. Of the 362 enrolled girls, 320 completed follow-up until 2016. In 2017, 251 of these girls and their mothers were enrolled in the California PAH Study and continued to be followed until 2020.20
Study Sample
The present analysis included 358 girls who provided a urine sample at enrollment in the LEGACY Girls Study. Participating mothers and daughters aged ≥10 years signed informed consent forms for the LEGACY Girls Study and the California PAH Study, and daughters aged 6–9 years signed an assent form for both studies. The Institutional Review Boards of the Cancer Prevention Institute of California and Stanford University and the California Committee for the Protection of Human Subjects approved both studies.
Urine and Data Collection
Every 6 months, a first-void morning urine sample was collected from participating girls on the day of the baseline or follow-up study visit. Samples were collected in medical-grade polypropylene containers. Self-collection kits including a sanitary wipe were mailed to the participants ahead of the visit with detailed instructions, including wiping the vaginal area before urine collection. Samples were transferred to the lab, aliquoted within 48 hours of collection into polypropylene vials, and stored at −80o C.
At enrollment in the LEGACY Girls Study, participating mothers completed a baseline questionnaire on the daughter’s sociodemographic characteristics, family history of breast cancer, medical history, lifestyle, and other factors.19 Trained research staff measured the daughter’s weight and height (two measurements each) using a digital scale and stadiometer, respectively. We collected information on pubertal development from mothers and daughters aged ≥10 years at baseline and every 6 months until 2016, at enrollment in the California PAH Study in 2017, and approximately 14 and 22 months following enrollment. We asked questions about age at first menstruation (in half-year intervals) and Tanner stage of breast and pubic hair development based on validated line drawings showing five stages each with explanatory text.22,23 Tanner staging is routinely used in clinical evaluations of pubertal development. We previously demonstrated substantial agreement between clinical assessments and mothers’ reports for breast Tanner staging.24
Pubertal Outcomes
We examined four pubertal outcomes: breast Tanner stage, pubic hair Tanner stage, menarche status, and pubertal tempo. For girls aged 6–9 years, they were based on mothers’ reports, because girls under age 10 years did not complete questionnaires. For girls aged ≥10 years with missing mother-reported pubertal outcomes data, we used the girls’ self-report. Tanner stage ranges from TS1 (no development, no glandular tissue) to TS5 (full development),22 and TS2 indicates the onset of breast or pubic hair development. Age at onset of breast or pubic hair development was determined as the midpoint between the age at last consistent maternal report of TS1 and age at first consistent maternal report of TS2 or higher (if TS2 was not reported) without regression back to TS1 in subsequent follow-up questionnaires. Pubertal tempo was defined as the interval between age at onset of breast development (TS2) and age at menarche. Girls with missing information on baseline breast TS (N=1) or pubic hair TS (N=2) were excluded from the respective analyses.
Urinary PAH Metabolite Assessment
In baseline urine samples and last collected samples, specific gravity (SG) and PAH metabolites were measured,20 including 1-hydroxy naphthalene (1-NAP), 2-hydroxy naphthalene (2-NAP), 2- and 3-hydroxy fluorene (2&3-FLU), 1-hydroxy phenanthrene (1-PHEN), 2- and 3-hydroxy phenanthrene (2&3-PHEN), 4-hydroxy phenanthrene (4-PHEN), and 1-hydroxy pyrene (1-PYR). The metabolites were measured at the Trace Organic Chemistry laboratory at Lamont-Doherty Earth Observatory, Columbia University, using Sciex Qtrap 6500+ liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS).20,25 SG was measured using the Atago PAL-10-S refractometer. We previously reported that PAH metabolites were detected in nearly all baseline urine samples: ≥98% for 2-NAP, 1-PHEN, 2&3-PHEN, and 1-PYR; 82% for 1-NAP and 2&3-FLU; and 70% for 4-PHEN.20
Statistical Analyses
PAH metabolite concentrations were corrected for SG to account for urine dilution, and values below the limit of detection (LOD) were assigned a value of LOD divided by the square root of 2. Metabolite concentrations were natural-log (ln) transformed for all analyses. We calculated the combined phenanthrene metabolites (∑PHEN) by summing the values for 1-PHEN, 2&3-PHEN, and 4-PHEN, and the sum of all metabolites (∑PAH) except for 1-NAP, because it is a metabolite of both naphthalene and the insecticide carbaryl.26 For 1-NAP, we excluded girls with a 1-NAP1 to 2-NAP ratio > 2.0, as this has been used as an indicator of carbaryl exposure.26 We used Spearman correlation coefficients (SCC) to assess correlations between pairs of PAH metabolites, between individual metabolites and ∑PAH, and between PAH metabolites in baseline and last samples.
In cross-sectional analyses, we used baseline data to compare geometric mean PAH metabolite concentrations between girls at three different pubertal development stages: girls who had not started breast development (TS1) vs. girls with breast TS2 or higher (TS2+); girls who had not started pubic hair development (TS1) vs. girls with pubic hair TS2+; and pre-menarche girls vs. post-menarche girls. We restricted the breast and pubic hair development analyses to 295 and 294 girls aged 6–12 years, respectively, since all older girls had already started breast or pubic hair development per baseline mother’s report. For menarche, we restricted the analyses to 206 girls aged 10–16 years since none of the younger girls had started menstruating per baseline mother’s report. We averaged the two weight and height measurements, and calculated body mass index (BMI) as weight (kg) divided by squared height (m), and categorized by percentile for age using Centers for Disease Control growth charts.27 We classified girls with a BMI percentile ≥85 as overweight.
Because some girls were siblings, we used generalized estimating equations (GEE) to account for expected family correlations in linear and logistic regression models. We used multivariable linear regression models to calculate geometric mean metabolite concentrations and 95% confidence intervals (CIs), adjusting for age at baseline urine collection, race–ethnicity, mother’s education as a proxy for socioeconomic status, and baseline BMI percentile. Missing education was coded as unknown and included in the multivariable models. Categorizing urinary PAH metabolites as high vs. low (at the median or above vs. below the median of all girls combined), we used multivariable logistic regression to estimate odds ratios (ORs) and 95% CIs for the odds of being breast TS2+, pubic hair TS2+, or post-menarche at baseline. We adjusted logistic regression models for breast and pubic hair development stage for age at baseline urine collection, race–ethnicity, and baseline BMI percentile, and additionally adjusted models for menarche for family history of breast cancer and mother’s education.
We based prospective analyses on 176 girls with breast TS1 at baseline, 179 girls with public hair TS1, and 267 pre-menarche girls. We examined associations of PAH metabolite concentrations (high vs. low) with timing of pubertal outcomes using multivariable Cox proportional hazards regression models with attained age (in months) as the time scale, estimating hazard ratios (HRs) and 95% CIs, with an HR >1 indicating earlier pubertal onset. Girls entered the risk set at the age at baseline urine collection, and exited at the age at onset of breast development, pubic hair development, or first menstruation, respectively, or at the age at last follow-up, if they did not experience TS2 or menarche during follow-up. We adjusted Cox models for age at baseline urine collection, race–ethnicity, mother’s education, and baseline BMI percentile. We included the latter because in longitudinal studies, higher BMI several years before onset of puberty was associated with earlier puberty.3
We used the robust variance estimator to account for correlations among siblings. We generated the median age at onset of breast and pubic hair development and age at menarche for girls with high vs. low PAH metabolite concentrations from the estimated baseline survival functions of the multivariable Cox models.28 We assessed the proportional hazards assumption by testing for interactions between the PAH metabolites (high vs. low), covariates, and log-transformed time, and examining Kaplan-Meier survival curves stratified by PAH metabolites and covariates. We found no departures from the proportional hazards assumptions. A previous study found that baseline BMI modified the association between PAH metabolites and timing of breast development.14 We therefore assessed the joint association of baseline PAH metabolites and baseline BMI with pubertal timing using a composite variable of PAH metabolite concentration (low, high) and BMI percentile (<85, ≥85).
We based analysis of pubertal tempo on the 133 girls with data on age at onset of breast development and age at menarche, with tempo greater than or equal to 0. We used linear regression models with GEE accounting for siblings to examine associations of pubertal tempo with PAH metabolite concentrations (high vs. low), adjusting for age at baseline urine collection, race–ethnicity, and mother’s education.
Statistical analyses were conducted using SAS 9.4. (SAS Institute Inc., Cary, NC).
RESULTS
In baseline samples, ∑PAH was strongly correlated with 2-NAP (SCC 0.98), but only weakly with the other metabolites (SCC 0.31–0.37); correlations were strong to very strong (SCC 0.73–0.95) between ∑PHEN and individual phenanthrene metabolites; moderate (SCC 0.45–0.61) between ∑PHEN, 2&3-FLU, and 1-PYR; and weak (SCC 0.18–0.36) between the naphthalene and other metabolites (Supplementary Digital Content eAppendix 1a). We observed similar correlations in last samples (Supplementary Digital Content eAppendix 1b). Correlations between concentrations in baseline and last samples were weak for most metabolites (SCC 0.19–0.35), but moderate for 2-NAP (SCC=0.49) and ∑PAH (SCC=0.47) (Supplementary Digital Content eAppendix 1c).
At baseline, nearly half of the girls had not yet started breast (49%) or pubic hair (50%) development, and 75% had not yet started menstruation. Among girls aged 6–12 years, the proportion of girls who had started pubertal development varied by sociodemographic and other characteristics (Table 1). For both breast and pubic hair development stage, girls with TS2+ were more likely to be older, Hispanic or African American, taller, heavier, and from families with lower maternal education or lower income. Similar patterns were seen for girls aged 10–16 years who were post-menarche compared to those that were pre-menarche at baseline. Mean SG-corrected metabolite concentrations, adjusted for age at baseline urine collection, race–ethnicity, mother’s education, and baseline BMI percentile, were similar by pubertal development stage (Table 2).
TABLE 1.
Characteristics of study participants by pubertal development stage at baseline, California PAH Study, 2011–2020
| All girls N=358 a |
Girls aged 6–12 years Breast Tanner stage N=295 |
Girls aged 6–12 years Pubic hair Tanner stage N=294 |
Girls aged 10–16 years Menarche status N=206 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | TS1 N=176 |
TS2+ N=119 |
TS1 N=179 |
TS2+ N=115 |
Pre-menarche N=115 |
Post-menarche N=91 |
||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Age (years) b | ||||||||||||||
| 6–9 | 152 | 42% | 131 | 74% | 20 | 17% | 133 | 74% | 19 | 17% | 0 | 0% | 0 | 0% |
| 10–12 | 144 | 40% | 45 | 26% | 99 | 83% | 46 | 26% | 96 | 83% | 108 | 94% | 36 | 40% |
| 13–16 | 62 | 17% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 7 | 6% | 55 | 60% |
| Race/ethnicity | ||||||||||||||
| Non-Hispanic White | 172 | 48% | 92 | 52% | 61 | 51% | 97 | 54% | 55 | 48% | 69 | 60% | 30 | 33% |
| African American | 22 | 6% | 7 | 4% | 10 | 8% | 6 | 3% | 11 | 10% | 6 | 5% | 7 | 8% |
| Asian American c | 47 | 13% | 25 | 14% | 10 | 8% | 24 | 13% | 11 | 10% | 15 | 13% | 13 | 14% |
| Hispanic | 104 | 29% | 46 | 26% | 36 | 30% | 45 | 25% | 37 | 32% | 21 | 18% | 37 | 41% |
| Mixed race–ethnicity | 13 | 4% | 6 | 3% | 2 | 2% | 7 | 4% | 1 | 1% | 4 | 3% | 4 | 4% |
| Mother’s education | ||||||||||||||
| Some college or less | 122 | 34% | 46 | 26% | 50 | 42% | 49 | 27% | 46 | 40% | 29 | 25% | 46 | 51% |
| College degree | 90 | 25% | 53 | 30% | 23 | 19% | 52 | 29% | 24 | 21% | 30 | 26% | 14 | 15% |
| Graduate degree | 121 | 34% | 70 | 40% | 39 | 33% | 70 | 39% | 39 | 34% | 52 | 45% | 18 | 20% |
| Unknown | 25 | 7% | 7 | 4% | 7 | 6% | 8 | 4% | 6 | 5% | 4 | 3% | 13 | 14% |
| Family income b | ||||||||||||||
| <$50,000 | 62 | 17% | 24 | 14% | 25 | 21% | 24 | 13% | 25 | 22% | 12 | 10% | 25 | 27% |
| $50,000–99,999 | 65 | 18% | 29 | 16% | 20 | 17% | 33 | 18% | 16 | 14% | 17 | 15% | 19 | 21% |
| $100,000 or more | 186 | 52% | 100 | 57% | 58 | 49% | 98 | 55% | 60 | 52% | 75 | 65% | 36 | 40% |
| Unknown | 45 | 13% | 23 | 13% | 16 | 13% | 24 | 13% | 14 | 12% | 11 | 10% | 11 | 12% |
| Family history of breast cancer b,d | ||||||||||||||
| No | 163 | 46% | 84 | 48% | 57 | 48% | 84 | 47% | 56 | 49% | 56 | 49% | 40 | 44% |
| Yes | 192 | 54% | 91 | 52% | 61 | 51% | 95 | 53% | 57 | 50% | 57 | 50% | 50 | 55% |
| Unknown | 3 | 1% | 1 | 1% | 1 | 1% | 0 | 0% | 2 | 2% | 2 | 2% | 1 | 1% |
| Height (cm) (tertiles) b,e | ||||||||||||||
| 111.4–138.9 | 119 | 33% | 112 | 64% | 6 | 5% | 112 | 63% | 7 | 6% | 10 | 9% | 0 | 0% |
| 139.0–154.9 | 120 | 34% | 56 | 32% | 57 | 48% | 57 | 32% | 54 | 47% | 61 | 53% | 17 | 19% |
| 155–178.5 | 119 | 33% | 8 | 5% | 56 | 47% | 10 | 6% | 54 | 47% | 44 | 38% | 74 | 81% |
| BMI-for-age percentile b | ||||||||||||||
| <85 (not overweight) | 273 | 76% | 151 | 86% | 73 | 61% | 152 | 85% | 72 | 63% | 89 | 77% | 60 | 66% |
| ≥85 (overweight) | 85 | 24% | 25 | 14% | 46 | 39% | 27 | 15% | 43 | 37% | 26 | 23% | 31 | 34% |
Abbreviations: BMI, body mass index; TS, Tanner stage; TS2+, TS2 or higher.
Girls with baseline urinary PAH metabolite measurements.
At baseline urine collection.
Includes 3 Pacific Islander girls.
In first- or second-degree relatives.
Tertiles determined among all girls combined (N=358).
TABLE 2.
Baseline adjusted geometric mean SG-corrected PAH metabolite concentrations (ng/L), by baseline pubertal development stage, California PAH Study, 2011–2020
| Girls aged 6–12 years Breast Tanner Stage N=295 |
Girls aged 6–12 years Pubic hair Tanner Stage N=294 |
Girls aged 10–16 years Menarche Status N=206 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TS1 N=176 |
TS2+ N=119 |
TS1 N=179 |
TS2+ N=115 |
Pre-menarche N=111 |
Post-menarche N=91 |
|||||||
| Urinary PAH metabolite a,b | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) |
| 1-NAP | 1,550 | (901 – 2,680) | 1,540 | (830 – 2,850) | 1,610 | (970 – 2,670) | 1,450 | (778 – 2,700) | 1,990 | (1,160 – 3,420) | 1,610 | (773 – 3,360) |
| 1-NAP c | 813 | (426 – 1,550) | 821 | (417 – 1,620) | 954 | (523 – 1,741) | 678 | (342 – 1,340) | 912 | (465 – 1,790) | 690 | (311 – 1,530) |
| 2-NAP | 2,860 | (2,230 – 3,670) | 3,160 | (2,480 – 4,020) | 2,830 | (2,190 – 3,650) | 3,200 | (2,450 – 4,170) | 3,050 | (2,290 – 4,050) | 3,840 | (2,750 – 5,380) |
| 2&3-FLU | 159 | (100 – 254) | 199 | (126 – 313) | 164 | (107 – 249) | 194 | (121 – 312) | 216 | (143 – 326) | 199 | (117 – 337) |
| 1-PHEN | 126 | (108 – 148) | 119 | (103 – 137) | 121 | (106 – 138) | 125 | (104 – 149) | 125 | (100 – 157) | 146 | (111 – 191) |
| 2&3-PHEN | 104 | (88.7 – 123) | 96.5 | (84.0 – 111) | 96.8 | (85.1 – 110) | 106 | (88.9 – 126) | 107 | (85.4 – 134) | 124 | (95.1 – 162) |
| 4-PHEN | 19.8 | (16.4 – 24.0) | 20.7 | (17.3 – 24.9) | 19.0 | (16.2 – 22.3) | 22.1 | (18.1 – 27.0) | 22.4 | (17.7 – 28.6) | 26.8 | (20.1 – 35.9) |
| ∑PHEN d | 259 | (223 – 301) | 240 | (212 – 273) | 242 | (215 – 271) | 260 | (221 – 306) | 272 | (221 – 336) | 321 | (250 – 412) |
| 1-PYR | 372 | (308 – 448) | 325 | (280 – 377) | 323 | (276 – 378) | 386 | (318 – 469) | 397 | (312 – 506) | 423 | (318 – 563) |
| ∑PAH e | 27.4 | (22.3 – 33.6) | 28.7 | (23.5 – 34.9) | 27.3 | (22.9 – 32.6) | 28.7 | (23.2 – 35.6) | 30.4 | (24.4 – 37.9) | 34.8 | (26.2 – 46.2) |
Abbreviations: 1-NAP, 1-hydroxy naphthalene; 2-NAP, 2-hydroxy naphthalene; 2&3-FLU, 2- and 3-hydroxy fluorene; 1-PHEN, 1-hydroxy phenanthrene; 2&3-PHEN, 2- and 3-hydroxy phenanthrene; 4-PHEN, 4-hydroxy phenanthrene; 1-PYR, 1-hydroxypyrene; ∑PHEN, sum of 1-PHEN, 2&3-PHEN, and 4-PHEN; ∑PAH, sum of all metabolites except 1-NAP; CI, confidence interval; SG, specific gravity; PAH, polycyclic aromatic hydrocarbon; TS, Tanner stage; TS2+, Tanner stage 2 or higher.
Geometric means (ng/L) and 95% CI, adjusted for age at baseline urine collection (continuous), race–ethnicity (non-Hispanic White, African American, Asian American, Hispanic, mixed race–ethnicity), mother’s education (some college or less, college degree, graduate degree, unknown), and baseline BMI-for-age percentile (<85, ≥85).
Differences in mean metabolite concentrations by pubertal development stage were not statistically significant (P <0.05).
Excludes girls with a 1-NAP to 2-NAP ratio > 2 indicative of carbaryl exposure. Exclusions were 77 for breast TS, 75 for pubic hair TS, and 46 for menarche status.
ng/L.
nmol/L.
Associations between PAH metabolite concentrations and pubertal development stage at baseline are presented in Table 3. Categorizing metabolite concentrations as high vs. low (based on the median), in multivariable-adjusted models, PAH metabolites were not associated with the odds of having started breast development (TS2+), pubic hair development (TS2+), or menstruation.
TABLE 3.
Associations of baseline urinary PAH metabolites with pubertal development stage at baseline, California PAH Study, 2011–2020
| Girls aged 6–12 years Breast Tanner stage N=295 |
Girls aged 6–12 years Pubic hair Tanner stage N=294 |
Girls aged 10–16 years Menarche status N=206 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Urinary PAH metabolites d | TS1 N |
TS2+ N |
OR (95% CI) a,b | TS1 N |
TS2+ N |
OR (95% CI) a,b | Pre-Menarche N |
Post-menarche N |
OR (95% CI) a,c |
| 1-NAP | |||||||||
| Low | 91 | 57 | 1.0 | 93 | 56 | 1.0 | 54 | 47 | 1.0 |
| High | 85 | 62 | 1.30 (0.62–2.72) | 86 | 69 | 1.27 (0.63–2.53) | 61 | 44 | 1.07 (0.43–2.70) |
| 1-NAP e | |||||||||
| Low | 64 | 46 | 1.0 | 66 | 45 | 1.0 | 41 | 36 | 1.0 |
| High | 60 | 48 | 1.03 (0.46–2.28) | 64 | 44 | 0.83 (0.39–1.77) | 47 | 36 | 0.82 (0.26–2.62) |
| 2-NAP | |||||||||
| Low | 106 | 48 | 1.0 | 104 | 50 | 1.0 | 59 | 30 | 1.0 |
| High | 70 | 71 | 1.41 (0.71–2.80) | 75 | 65 | 0.95 (0.50–1.81) | 56 | 61 | 1.74 (0.76–3.99) |
| 2&3-FLU | |||||||||
| Low | 93 | 56 | 1.0 | 94 | 54 | 1.0 | 53 | 47 | 1.0 |
| High | 83 | 63 | 0.91 (0.46–1.82) | 85 | 61 | 0.85 (0.45–1.60) | 62 | 44 | 0.53 (0.21–1.34) |
| 1-PHEN | |||||||||
| Low | 93 | 56 | 1.0 | 96 | 54 | 1.0 | 52 | 40 | 1.0 |
| High | 83 | 63 | 0.62 (0.30–1.27) | 83 | 61 | 0.68 (0.35–1.33) | 63 | 51 | 1.01 (0.40–2.57) |
| 2&3-PHEN | |||||||||
| Low | 89 | 63 | 1.0 | 89 | 62 | 1.0 | 55 | 46 | 1.0 |
| High | 87 | 56 | 0.86 (0.42–1.78) | 90 | 53 | 0.72 (0.36–1.41) | 60 | 45 | 0.65 (0.25–1.70) |
| 4-PHEN | |||||||||
| Low | 92 | 57 | 1.0 | 96 | 53 | 1.0 | 57 | 42 | 1.0 |
| High | 84 | 62 | 1.02 (0.52–2.02) | 83 | 62 | 1.11 (0.59–2.09) | 58 | 49 | 0.96 (0.42–2.19) |
| ∑PHEN f | |||||||||
| Low | 92 | 59 | 1.0 | 96 | 56 | 1.0 | 51 | 41 | 1.0 |
| High | 84 | 60 | 0.61 (0.29–1.30) | 83 | 59 | 0.77 (0.40–1.47) | 64 | 50 | 0.72 (0.29–1.83) |
| 1-PYR | |||||||||
| Low | 96 | 53 | 1.0 | 103 | 46 | 1.0 | 59 | 33 | 1.0 |
| High | 80 | 66 | 0.94 (0.46–1.92) | 76 | 69 | 1.57 (0.80–3.08) | 56 | 58 | 2.17 (0.89–5.33) |
| ∑PAH g | |||||||||
| Low | 105 | 52 | 1.0 | 105 | 51 | 1.0 | 60 | 30 | 1.0 |
| High | 71 | 67 | 1.19 (0.60–2.36) | 74 | 64 | 0.98 (0.51–1.87) | 55 | 61 | 1.54 (0.68–3.48) |
Abbreviations: 1-NAP, 1-hydroxy naphthalene; 2-NAP, 2-hydroxy naphthalene; 2&3-FLU, 2- and 3-hydroxy fluorene; 1-PHEN, 1-hydroxy phenanthrene; 2&3-PHEN, 2- and 3-hydroxy phenanthrene; 4-PHEN, 4-hydroxy phenanthrene; 1-PYR, 1-hydroxypyrene; ∑PHEN, sum of 1-PHEN, 2&3-PHEN, and 4-PHEN; ∑PAH, sum of all metabolites except 1-NAP; BMI, body mass index; CI, confidence interval; OR, odds ratio; PAH, polycyclic aromatic hydrocarbon; TS, Tanner stage. TS2+, Tanner stage 2 or higher.
OR estimated using logistic regression with generalized estimating equations (GEE) to account for correlations among siblings from the same family.
OR adjusted for age at baseline urine collection (continuous), race–ethnicity (non-Hispanic White, African American, Asian American, Hispanic, mixed race–ethnicity), and baseline BMI-for-age percentile (<85, ≥85).
OR adjusted for covariates in footnote b, and additionally for family history of breast cancer in first- or second-degree relatives (yes, no or unknown).
High (≥ median) vs. low (< median) PAH metabolite concentration (ng/L). Median concentrations were determined among all girls combined (N=358), concentrations were corrected for specific gravity (SG) and include imputed values for those below the level of detection (LOD).
Excludes girls with a 1-NAP to 2-NAP ratio > 2 indicative of carbaryl exposure. Exclusions were 77 for breast TS, 75 for pubic hair TS, and 46 for menarche status.
ng/L.
nmol/L.
Prospective analyses were based on 176 girls with breast TS1 at baseline urine collection, 179 girls with pubic hair TS1, and 267 pre-menarche girls. In multivariable-adjusted Cox regression models, there were no associations between PAH metabolites and onset of breast or pubic development or menarche (Table 4). Median age at pubertal onset or menarche was similar between girls with high vs. low metabolite concentrations. Similarly, pubertal tempo among 133 girls was not associated with PAH metabolite concentrations in all girls combined or in non-overweight girls (Table 5).
TABLE 4.
Associations of urinary PAH metabolites with pubertal outcomes and median age at pubertal event, California PAH Study, 2011–2020
| Girls with breast Tanner Stage 1 at baseline N=176 a |
Girls with pubic hair Tanner Stage 1 at baseline N=179 b |
Girls with pre-menarche status at baseline N=267 c |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Events | HR (95% CI) d,e | Median age at pubertal event (years) | Difference (months) f | N | Events | HR (95% CI) d,e | Median age at pubertal event (years) | Difference (months) f | N | Events | HR (95% CI) d,e | Median age at pubertal event (years) | Difference (months) f | |
| Urinary PAH metabolites g | |||||||||||||||
| 1-NAP | |||||||||||||||
| Low | 91 | 81 | 1.0 | 10.5 | 93 | 76 | 1.0 | 10.6 | 132 | 105 | 1.0 | 12.5 | |||
| High | 85 | 74 | 0.81 (0.58–1.13) | 10.9 | 3.7 | 86 | 76 | 0.91 (0.65–1.27) | 10.9 | 2.9 | 135 | 108 | 0.87 (0.66–1.15) | 13.0 | 6.0 |
| 1-NAP h | |||||||||||||||
| Low | 64 | 55 | 1.0 | 10.7 | 66 | 54 | 1.0 | 10.8 | 98 | 79 | 1.0 | 12.5 | |||
| High | 60 | 55 | 1.35 (0.90–2.02) | 10.4 | −3.6 | 64 | 57 | 1.22 (0.83–1.78) | 10.9 | 0.8 | 97 | 80 | 1.05 (0.77–1.44) | 12.5 | 0.0 |
| 2-NAP | |||||||||||||||
| Low | 106 | 91 | 1.0 | 10.8 | 104 | 85 | 1.0 | 10.8 | 148 | 117 | 1.0 | 13.0 | |||
| High | 70 | 64 | 1.31 (0.93–1.86) | 10.5 | −4.2 | 75 | 67 | 1.37 (0.95–1.98) | 10.6 | −2.2 | 119 | 96 | 0.97 (0.72–1.30) | 12.8 | −2.0 |
| 2&3-FLU | |||||||||||||||
| Low | 93 | 83 | 1.0 | 10.6 | 94 | 80 | 1.0 | 10.9 | 132 | 103 | 1.0 | 13.0 | |||
| High | 83 | 72 | 0.85 (0.62–1.16) | 10.9 | 2.6 | 85 | 72 | 0.94 (0.67–1.31) | 10.7 | −2.3 | 135 | 110 | 1.07 (0.83–1.37) | 12.9 | −1.0 |
| 1-PHEN | |||||||||||||||
| Low | 93 | 78 | 1.0 | 10.7 | 96 | 75 | 1.0 | 10.8 | 140 | 105 | 1.0 | 13.0 | |||
| High | 83 | 77 | 1.00 (0.74–1.36) | 10.7 | 0.9 | 83 | 77 | 1.10 (0.77–1.55) | 10.8 | −0.7 | 127 | 108 | 1.11 (0.87–1.43) | 13.0 | 0.0 |
| 2&3-PHEN | |||||||||||||||
| Low | 89 | 77 | 1.0 | 10.6 | 89 | 71 | 1.0 | 10.7 | 134 | 106 | 1.0 | 13.0 | |||
| High | 87 | 78 | 0.83 (0.62–1.12) | 10.8 | 1.5 | 80 | 81 | 1.13 (0.81–1.58) | 10.9 | 2.1 | 133 | 107 | 0.99 (0.77–1.27) | 13.0 | 0.0 |
| 4-PHEN | |||||||||||||||
| Low | 92 | 81 | 1.0 | 10.6 | 96 | 79 | 1.0 | 10.6 | 135 | 105 | 1.0 | 13.0 | |||
| High | 84 | 74 | 0.81 (0.59–1.11) | 10.8 | 1.7 | 83 | 73 | 0.84 (0.61–1.18) | 10.9 | 3.3 | 132 | 108 | 1.04 (0.82–1.32) | 13.0 | 0.0 |
| ∑PHEN i | |||||||||||||||
| Low | 92 | 79 | 1.0 | 10.6 | 96 | 77 | 1.0 | 10.6 | 138 | 108 | 1.0 | 12.8 | |||
| High | 84 | 76 | 0.83 (0.61–1.12) | 10.9 | 3.2 | 83 | 75 | 0.89 (0.64–1.26) | 10.9 | 3.6 | 129 | 105 | 0.87 (0.68–1.11) | 13.0 | 3.0 |
| 1-PYR | |||||||||||||||
| Low | 96 | 82 | 1.0 | 10.5 | 103 | 84 | 1.0 | 10.7 | 145 | 112 | 1.0 | 13.0 | |||
| High | 80 | 73 | 0.81 (0.56–1.17) | 10.8 | 3.7 | 76 | 68 | 0.92 (0.65–1.31) | 10.9 | 2.3 | 122 | 101 | 1.07 (0.81–1.42) | 12.8 | −2.0 |
| ∑PAH j | |||||||||||||||
| Low | 105 | 91 | 1.0 | 10.7 | 105 | 87 | 1.0 | 10.8 | 149 | 118 | 1.0 | 13.0 | |||
| High | 71 | 64 | 1.33 (0.95–1.85) | 10.5 | −3.0 | 74 | 65 | 1.27 (0.88–1.83) | 10.6 | −1.6 | 118 | 95 | 0.99 (0.75–1.32) | 12.7 | −4.0 |
Abbreviations: 1-NAP, 1-hydroxy naphthalene; 2-NAP, 2-hydroxy naphthalene; 2&3-FLU, 2- and 3-hydroxy fluorene; 1-PHEN, 1-hydroxy phenanthrene; 2&3-PHEN, 2- and 3-hydroxy phenanthrene; 4-PHEN, 4-hydroxy phenanthrene; 1-PYR, 1-hydroxypyrene; ∑PHEN, sum of 1-PHEN, 2&3-PHEN, and 4-PHEN; ∑PAH, sum of all metabolites except 1-NAP; BMI, body mass index; CI, confidence interval; HR, hazard ratio; PAH, polycyclic aromatic hydrocarbon; TS, Tanner stage; TS2+, TS2 or higher.
Of 176 girls with breast TS1 at baseline urine collection, 155 reached TS2 or higher during follow-up.
Of 179 girls with pubic hair TS1 at baseline urine collection, 152 reached TS2 or higher during follow-up.
Of 267 girls who were pre-menarche at baseline urine collection, 213 reached menarche during follow-up.
HR estimated using Cox proportional hazards regression models and the robust variance estimator to account for correlations among girls from the same family, adjusted for age at baseline urine collection (continuous), race–ethnicity (non-Hispanic White, African American, Asian American, Hispanic, mixed race–ethnicity), mother’s education (some college or less, college degree, graduate degree, unknown), and baseline BMI-for-age percentile (<85, ≥85).
HR >1 indicates earlier pubertal onset.
Positive values indicate older age at pubertal event in girls with high vs. low PAH metabolite concentration; negative values indicate younger age at pubertal event in girls with high vs. low PAH metabolite concentration.
High (≥ median) vs. low (< median) PAH metabolite concentration (ng/L). Median concentrations were determined among all girls combined (N=358), concentrations were corrected for specific gravity (SG) and include imputed values for those below the level of detection (LOD).
Excludes girls with a 1-NAP to 2-NAP ratio > 2 indicative of carbaryl exposure. Exclusions were 52 for breast TS, 49 for pubic hair TS, and 72 for menarche status.
ng/L.
nmol/L.
TABLE 5.
Associations of urinary PAH metabolites with pubertal tempo, California PAH Study, 2011–2020
| Pubertal tempo | ||
|---|---|---|
| All girls N=133 |
BMI percentile <85 a
N=113 |
|
| β and 95% CI b,c | β and 95% CI b,c | |
| Urinary PAH metabolites (high vs. low) d | ||
| 1-NAP | 1.90 (−2.35 – 6.15) | 2.26 (−2.36 – 6.88) |
| 1-NAP e | 1.10 (−3.84 – 6.05) | −0.40 (−5.73 – 4.93) |
| 2-NAP | 1.05 (−3.59 – 5.70) | 0.78 (−4.36 – 5.91) |
| 2&3-FLU | 0.09 (−4.08 – 4.26) | 0.71 (−3.71 – 5.13) |
| 1-PHEN | 1.54 (−2.80 – 5.88) | 1.61 (−2.96 – 6.18) |
| 2&3-PHEN | 3.13 (−0.96 – 7.22) | 2.22 (−2.10 – 6.55) |
| 4-PHEN | 1.52 (−2.63 – 5.68) | 1.33 (−3.03 – 5.69) |
| ∑PHEN f | 2.17 (−2.06 – 6.39) | 1.47 (−3.03 – 5.98) |
| 1-PYR | −0.20 (−4.36 – 3.95) | −0.02 (−4.37 – 4.34) |
| ∑PAH g | 0.37 (−4.14 – 4.88) | −0.13 (−5.11 – 4.85) |
Abbreviations: 1-NAP, 1-hydroxy naphthalene; 2-NAP, 2-hydroxy naphthalene; 2&3-FLU, 2- and 3-hydroxy fluorene; 1-PHEN, 1-hydroxy phenanthrene; 2&3-PHEN, 2- and 3-hydroxy phenanthrene; 4-PHEN, 4-hydroxy phenanthrene; 1-PYR, 1-hydroxypyrene; ∑PHEN, sum of 1-PHEN, 2&3-PHEN, and 4-PHEN; ∑PAH, sum of all metabolites except 1-NAP; BMI, body mass index; CI, confidence interval; PAH, polycyclic aromatic hydrocarbons.
20 girls had a BMI percentile of ≥85, no associations are presented.
β estimated from linear regression models, where the tempo in months is the dependent variable and can be interpreted as the difference (months) between high vs low PAH metabolite concentrations. Models were adjusted for age at baseline urine collection (continuous), race–ethnicity (non-Hispanic White, African American, Asian American, Hispanic, mixed race–ethnicity), and mother’s education (some college or less, college degree, graduate degree, unknown). None of the β was statistically significant.
A positive β indicates a longer interval for girls with high PAH metabolite concentration (i.e., slower pubertal tempo).
High (≥ median) vs. low (< median) PAH metabolite concentration. Median concentrations were determined among all girls combined (N=358), corrected for specific gravity (SG) and including imputed values for those below the level of detection (LOD).
Excludes 35 girls with a 1-NAP to 2-NAP ratio > 2 indicative of carbaryl exposure. Due to small numbers. race–ethnicity was categorized as non-Hispanic White vs. all other.
ng/L.
nmol/L.
Classifying girls jointly by PAH metabolite concentration (low, high) and BMI percentile (<85, ≥85), we found earlier pubertal development among overweight girls with high concentration of some PAH metabolites when compared to non-overweight girls with low concentration (Figure; Supplementary Digital Content eAppendix 2a-c). In overweight girls, high concentrations of 2-NAP, 1-PHEN, ∑PHEN, and ∑PAH were associated with two-fold increased risks of earlier breast development (Supplementary Digital Content eAppendix 2a). In contrast, earlier pubic hair development among overweight girls was found only in those with high 1-NAP. Low concentrations of all other metabolites (2-NAP, 2&3-FLU, 2&3-PHEN, 4-PHEN, ∑PAH) were associated with 2 to 3-fold increased risk of earlier pubic hair development in overweight girls (Supplementary Digital Content eAppendix 2b). In non-overweight girls, high 2-NAP was the only metabolite associated with earlier pubic hair development. For menarche, high metabolite concentrations were not associated with earlier onset in non-overweight or overweight girls (Supplementary Digital Content eAppendix 2c). Earlier menarche was observed in overweight girls with low PAH metabolite concentrations.
Figure.
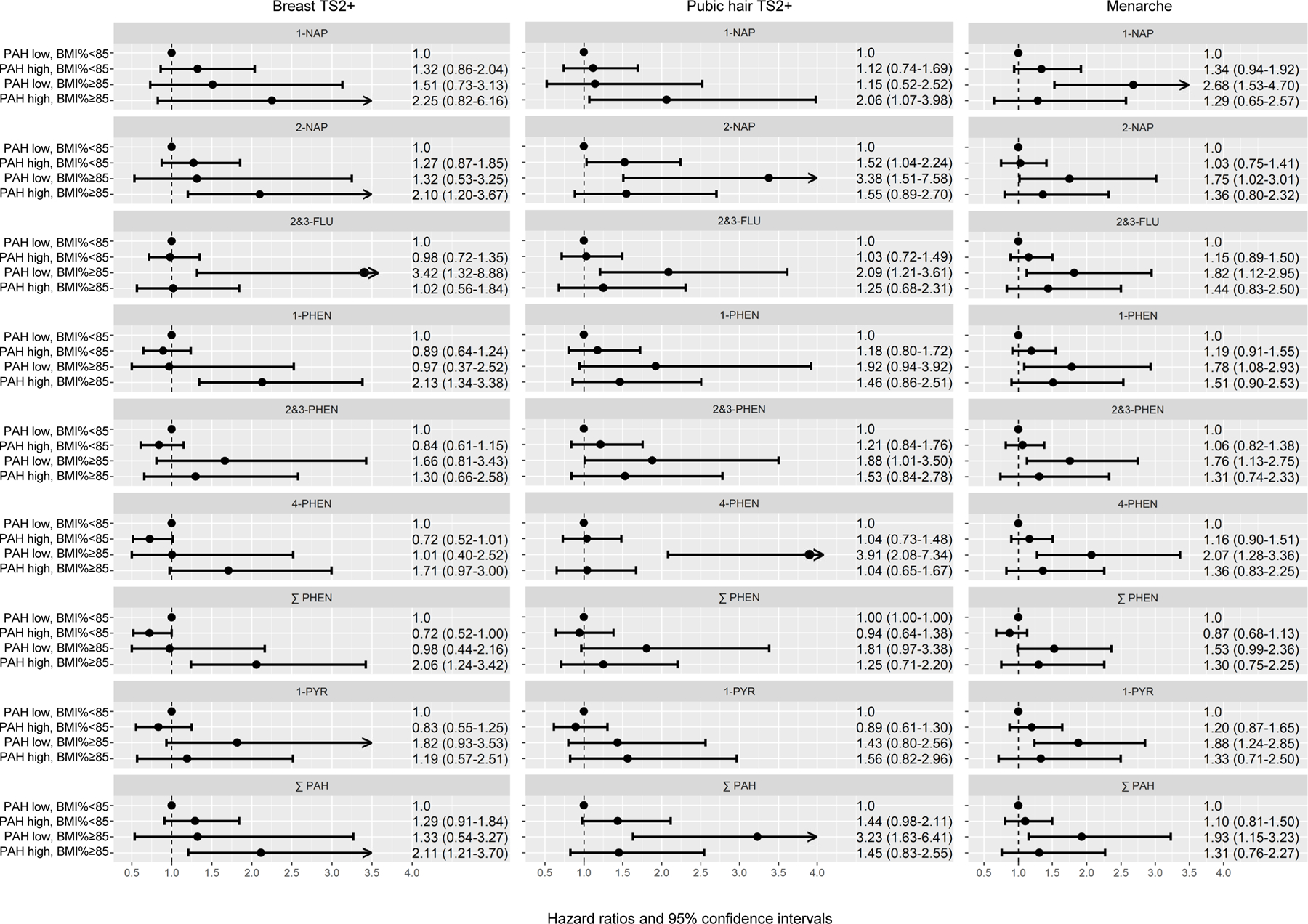
Urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and pubertal development. The figure depicts hazard ratios (HRs) and 95% confidence intervals (CIs) for the joint associations of urinary PAH metabolite concentrations (high vs. low) and BMI-for-age-percentile (<85, ≥85) with pubertal development outcomes from Cox proportional hazards regression models with robust variance estimator, adjusted for age at baseline urine collection (continuous), race–ethnicity (non-Hispanic White, African American, Asian American, Hispanic, mixed race–ethnicity), mother’s education (some college or less, college degree, graduate degree, unknown). The prospective analyses were based on 176 girls with breast Tanner Stage 1 at baseline PAH metabolite measurement, 179 girls with pubic hair Tanner Stage 1 at baseline, and 267 pre-menarche girls at baseline.
DISCUSSION
Follow-up of pre-pubertal girls from the San Francisco Bay Area showed that higher urinary concentrations of selected PAH metabolites (2-NAP, 1-PHEN, ∑PHEN, ∑PAH) were associated with earlier breast development, but only among overweight girls (BMI percentile ≥85) compared to non-overweight girls with low metabolite concentrations. For pubic hair, earlier onset was associated with high 1-NAP among overweight girls and high 2-NAP among non-overweight girls. Urinary PAH metabolites were not associated with timing of menarche or pubertal tempo.
Unlike for other endocrine-disrupting chemicals,5 there is only one prospective study that reported on the relation between urinary PAH metabolites and pubertal timing in girls. The California component of the Breast Cancer and Environment Research Program (BCERP) Puberty Study assessed associations with timing of breast and pubic hair development in 431 San Francisco Bay Area girls aged 6–8 years at study enrollment.14 Among overweight (BMI percentile ≥85) girls, high (vs. low tertile) baseline PAH metabolite concentrations (2-NAP, ∑FLU, ∑PHEN, 1-PYR, ∑PAH) were associated with earlier breast development. Our findings from the prospective analyses are consistent with the findings from the BCERP Study. Breast development was earlier among overweight girls with higher concentrations of 2-NAP, 1-PHEN, ∑PHEN, and ∑PAH. We also found earlier pubic hair development in overweight girls with high 1-NAP and non-overweight girls with high 2-NAP, whereas in the BCERP Study urinary metabolites were not associated with timing of pubic hair development.14 We found no associations between PAH metabolites and timing of menarche or pubertal tempo. The BCERP Study did not evaluate associations with timing of menarche.14
PAHs are lipophilic and accumulate in fat tissue.29 It is therefore plausible that effects of PAH exposures vary by body composition, with chronic long-term exposure to lipophilic PAHs among those with more fat tissue.30 We and others have previously reported higher mean PAH metabolite concentrations among overweight girls compared to non-overweight girls.14,20,31,32 In the BCERP Study, metabolite concentrations were higher in overweight girls as young as 7 years old.14
Urinary PAH metabolites have been detected in large proportions of children and adolescents world-wide, suggesting widespread exposure.33–36 We detected four urinary PAH metabolites in over 98% of samples,37 consistent with high detection rates in other U.S. studies.34,35 In biomarkers studies, the relative contribution of specific sources of exposure is unknown because individuals are typically exposed to multiple PAH sources and complex mixtures of PAH chemicals.38 1-PYR is the most widely used biomarker of PAH exposure and has been linked to cigarette smoke, consumption of charbroiled and smoked meat or fish, and indoor and outdoor air pollution.39 Higher PAH metabolite concentrations have been observed in children exposed to air pollution from traffic and other sources (naphthalene, phenanthrene, 1-pyrene),36,40 tobacco smoke at home (naphthalene, fluorene, and phenanthrene),32,36 and consumption of barbequed food within 48 hrs of urine sample collection (1-NAP, 2-FLU, phenanthrene, and 1-pyrene).36 Thus, in our study the association of earlier breast development in overweight girls associated with higher 2-NAP, 1-PHEN, ∑PHEN, and ∑PAH concentrations likely reflect multiple sources of PAH exposure. Specific exposure sources will vary by population. In our study, only 7% of girls were exposed to smokers in the home and no girls lived in homes heated with coal, oil, or wood,20 compared to much higher proportions of German children and adolescents exposed to smoking in the home (39%) or living in homes heated with oil, coal, pellet or other wood (35%).36 Information on potential sources of PAH exposures was available only for a subset of LEGACY Girls Study participants who enrolled in the California PAH Study.20 Thus, our sample size was too small to evaluate associations between specific PAH exposure sources and pubertal timing.
Few studies have examined the association between PAH exposure and timing of pubic hair development. Earlier pubic hair development has been associated with higher tobacco smoke exposure11 and greater exposure to vehicle traffic.10 Our findings of earlier pubic hair development in girls with higher urinary concentrations of 1-NAP (in overweight girls) and 2-NAP (in non-overweight girls) warrant confirmation in other studies. In vitro studies have reported anti-androgenic activity for select PAHs or metabolites.8 Data on interactions of PAHs with the androgen receptor are limited.41
We found no associations between PAH metabolites and timing of menarche, although variation in age at menarche was limited in our study (91% of girls at ages 11–14 years). Some studies have found an association between childhood tobacco smoke exposure and later menarche,42 although findings have been inconsistent.43 A Korean study reported that high particulate matter concentrations were related with earlier menarche.13 Thus, the impact of PAH exposures on timing of menarche remains uncertain.
Recent reviews have summarized the limited, epidemiologic data on pubertal development and endocrine-disrupting chemicals,5,44,45 with urinary levels of phenols, phthalates, pesticides, and flame retardants being the most extensively studied. Data on urinary PAH metabolites, however, are lacking,5,45 except for the BCERP Study14 and delayed breast development reported in a study of prenatal PAH exposure.46 Findings that certain endocrine-disrupting chemicals are associated with accelerated onset of puberty or menarche, compared with other evidence consistent with delays in pubertal milestones, suggest complex pathways and mechanisms underlying the effects of EDCs on pubertal development.47
The present study has several notable strengths and some limitations that need to be considered when interpreting the results. PAH exposure was assessed by metabolites measured in urine and therefore not subject to inaccurate recall. Furthermore, we have previously reported that PAH metabolite concentrations were associated with self-reported sources of PAH exposure in our study sample.37 The biospecimen collection rate was very high, with 99% of 362 enrolled girls providing a baseline urine sample. The detection rate of urinary metabolites was high, ranging from 82% to 99% for six metabolites studied, comparable to other U.S. studies. Since PAHs are metabolized rapidly, with a half-life of 2.5–6.1 hours following dietary PAH exposure,48 a single urinary exposure measurement may not reflect average PAH exposures during the pre-pubertal window of susceptibility. It is reassuring that we found a moderate correlation in summed metabolite concentrations between baseline and last urine samples measured up to 72 months apart, and substantial agreement in tertile ranking, suggesting longer-term PAH exposures.20
The prospective design allowed us to examine the relation between PAH metabolites and the timing of puberty in girls who had not started pubertal development yet. However, since the participants were aged 6–16 years at study enrollment, some girls had already started pubertal development at the baseline assessment, which limited the sample size for the prospective analyses. The study’s relatively long follow-up allowed us to examine associations with timing of menarche. Pubertal outcomes based on mother reports were assessed using validated methods (i.e., Tanner staging for breast and pubic hair development) that are widely used in clinical settings, but are subject to measurement error. For a subset of girls in the LEGACY Girls Study, we have shown high reliability and validity of mother-reported breast Tanner stage when compared with breast Tanner stage assessed by health professionals.24 Our main findings for overweight girls were based on relatively small sample sizes, and we were able to categorize metabolite concentrations only into high vs. low based on the median. Nevertheless, our results are consistent with a prior study.14 Information on sources of PAH exposure was available only for a subset of girls; thus we could not assess the potential modifying effects of passive smoking or consumption of charred or smoked foods on the association between PAH metabolites and pubertal timing. We did not have information available on air pollutants linked to residential history or other measures of long-term PAH exposures. Larger prospective studies with comprehensive assessment of both biomarkers and sources of PAH exposures and pubertal development are therefore warranted to confirm the present findings in larger subgroups of overweight girls and across more refined exposure categories. PAH biomarkers are likely better integrated exposure measures than self-reported sources of PAH exposure which may be subject to inaccurate recall. Information on specific PAH exposure sources, however, is valuable, as it may direct exposure reduction efforts toward specific exposure sources that are modifiable (e.g., in the home environment).
In conclusion, a deeper understanding of the effects of PAH exposures on pubertal timing will provide new insights on the potential impact of widespread exposure to environmental chemicals early in life on intermediate markers of breast cancer risk in adolescents and risk of breast cancer in adults.49 Earlier pubertal timing is a public health concern because of its association with adverse mental health outcomes in adolescents 50 and higher risks of other cancers,51 cardiovascular disease,52 and type 2 diabetes.53 Changes in pubertal timing may be early indicators of the impact of environmental chemicals on adverse health outcomes,2 warranting careful monitoring.
Supplementary Material
Sources of Funding
The California PAH Study was supported by grants from the California Breast Cancer Research Program (22UB-2308 to E.M.J.) and the National Cancer Institute (CA138638 to E.M.J.). This work was also supported by grants from the National Institute of Environmental Health Sciences (P30ES009089 and 1S10OD020058).
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
Sharing of Data and Computing Code
Upon reasonable request and with Institutional Review Board approval, researchers may request access to study data and computing code by contacting the corresponding author.
References
- 1.Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics 2008;121 Suppl 3:S167–171. [DOI] [PubMed] [Google Scholar]
- 2.Eckert-Lind C, Busch AS, Petersen JH, et al. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr 2020;174(4):e195881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biro FM, Greenspan LC, Galvez MP. Puberty in girls of the 21st century. J Pediatr Adolesc Gynecol 2012;25(5):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck Louis GM, Gray LE Jr., Marcus M, et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics 2008;121 Suppl 3:S192–207. [DOI] [PubMed] [Google Scholar]
- 5.Lee JE, Jung HW, Lee YJ, Lee YA. Early-life exposure to endocrine-disrupting chemicals and pubertal development in girls. Ann Pediatr Endocrinol Metab 2019;24(2):78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouritsen A, Aksglaede L, Sorensen K, et al. Hypothesis: exposure to endocrine-disrupting chemicals may interfere with timing of puberty. Int J Androl 2010;33(2):346–359. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Dong S, Wang H, Tao S, Kiyama R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut 2016;213:809–824. [DOI] [PubMed] [Google Scholar]
- 8.Vinggaard AM, Niemela J, Wedebye EB, Jensen GE. Screening of 397 chemicals and development of a quantitative structure--activity relationship model for androgen receptor antagonism. Chem Res Toxicol 2008;21(4):813–823. [DOI] [PubMed] [Google Scholar]
- 9.ATSDR. Agency for Toxic Substances and Disease Registry. Toxicological profile for polycyclic aromatic hydrocarbons (PAHs) 1995. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp69.pdf [PubMed]
- 10.McGuinn LA, Voss RW, Laurent CA, Greenspan LC, Kushi LH, Windham GC. Residential proximity to traffic and female pubertal development. Environ Int 2016;94:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Windham GC, Lum R, Voss R, et al. Age at Pubertal Onset in Girls and Tobacco Smoke Exposure During Pre- and Postnatal Susceptibility Windows. Epidemiology 2017;28(5):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang JV, Leung GM, Schooling CM. The Association of Air Pollution With Pubertal Development: Evidence From Hong Kong’s “Children of 1997” Birth Cohort. Am J Epidemiol 2017;185(10):914–923. [DOI] [PubMed] [Google Scholar]
- 13.Jung EM, Kim HS, Park H, Ye S, Lee D, Ha EH. Does exposure to PM10 decrease age at menarche? Environ Int 2018;117:16–21. [DOI] [PubMed] [Google Scholar]
- 14.Dobraca D, Laurent CA, Greenspan LC, et al. Urinary polycyclic aromatic hydrocarbons in relation to anthropometric measures and pubertal development in a cohort of Northern California girls. Environ Epidemiol 2020;4(4):e0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012;13(11):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodicoat DH, Schoemaker MJ, Jones ME, et al. Timing of pubertal stages and breast cancer risk: the Breakthrough Generations Study. Breast Cancer Res 2014;16(1):R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg M, D’Aloisio AA, O’Brien KM, Zhao S, Sandler DP. Pubertal timing and breast cancer risk in the Sister Study cohort. Breast Cancer Res 2020;22(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeinomar N, Oskar S, Kehm RD, Sahebzeda S, Terry MB. Environmental exposures and breast cancer risk in the context of underlying susceptibility: A systematic review of the epidemiological literature. Environ Res 2020;187:109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.John EM, Terry MB, Keegan TH, et al. The LEGACY Girls Study: Growth and Development in the Context of Breast Cancer Family History. Epidemiology 2016;27(3):438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John EM, Koo J, Ingles SA, et al. Predictors of urinary polycyclic aromatic hydrocarbon metabolites in girls from the San Francisco Bay Area. Environ Res 2022;205:112534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John EM, Sangaramoorthy M, Koo J, Whittemore AS, West DW. Enrollment and biospecimen collection in a multiethnic family cohort: the Northern California site of the Breast Cancer Family Registry. Cancer Causes Control 2019;30(4):395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 1980;9(3):271–280. [DOI] [PubMed] [Google Scholar]
- 24.Terry MB, Goldberg M, Schechter S, et al. Comparison of Clinical, Maternal, and Self Pubertal Assessments: Implications for Health Studies. Pediatrics 2016;138(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen K, Pitiranggon M, Wu H-C, et al. Improvement on recovery and reproducibility for quantifying urinary mono-hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs) (submitted, under review) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J Expo Sci Environ Epidemiol 2007;17(4):314–320. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). Individual growth charts Available at http://www.cdc.gove/growthcharts/charts.htm.
- 28.Allison PD. Survival analysis using SAS. A practical guide Cary, NC: SAS Institute Inc.;2010. [Google Scholar]
- 29.Obana H, Hori S, Kashimoto T, Kunita N. Polycyclic aromatic hydrocarbons in human fat and liver. Bull Environ Contam Toxicol 1981;27(1):23–27. [DOI] [PubMed] [Google Scholar]
- 30.Niehoff N, White AJ, McCullough LE, et al. Polycyclic aromatic hydrocarbons and postmenopausal breast cancer: An evaluation of effect measure modification by body mass index and weight change. Environ Res 2017;152:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scinicariello F, Buser MC. Urinary polycyclic aromatic hydrocarbons and childhood obesity: NHANES (2001–2006). Environ Health Perspect 2014;122(3):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain RB. Contributions of dietary, demographic, disease, lifestyle and other factors in explaining variabilities in concentrations of selected monohydroxylated polycyclic aromatic hydrocarbons in urine: Data for US children, adolescents, and adults. Environ Pollut 2020;266(Pt 1):115178. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez SF, Pardo O, Hernandez CS, Garlito B, Yusa V, force Bt. Children’s exposure to polycyclic aromatic hydrocarbons in the Valencian Region (Spain): Urinary levels, predictors of exposure and risk assessment. Environ Int 2021;153:106535. [DOI] [PubMed] [Google Scholar]
- 34.Dobraca D, Lum R, Sjodin A, et al. Urinary biomarkers of polycyclic aromatic hydrocarbons in pre- and peri-pubertal girls in Northern California: Predictors of exposure and temporal variability. Environ Res 2018;165:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2021. Available at https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume2_Mar2021-508.pdf.
- 36.Murawski A, Roth A, Schwedler G, et al. Polycyclic aromatic hydrocarbons (PAH) in urine of children and adolescents in Germany - human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Int J Hyg Environ Health 2020;226:113491. [DOI] [PubMed] [Google Scholar]
- 37.John EM, Koo J, Ingles SA, et al. Predictors of urinary polycyclic aromatic hydrocarbon metabolites in girls from the San Francisco Bay Area. Environ Res 2021;205:112534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bostrom CE, Gerde P, Hanberg A, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 2002;110 Suppl 3:451–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strickland P, Kang D. Urinary 1-hydroxypyrene and other PAH metabolites as biomarkers of exposure to environmental PAH in air particulate matter. Toxicol Lett 1999;108(2–3):191–199. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira M, Slezakova K, Delerue-Matos C, Pereira MC, Morais S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ Int 2019;124:180–204. [DOI] [PubMed] [Google Scholar]
- 41.Simeckova P, Pencikova K, Kovac O, et al. In vitro profiling of toxic effects of environmental polycyclic aromatic hydrocarbons on nuclear receptor signaling, disruption of endogenous metabolism and induction of cellular stress. Sci Total Environ 2022;815:151967. [DOI] [PubMed] [Google Scholar]
- 42.Ferris JS, Flom JD, Tehranifar P, Mayne ST, Terry MB. Prenatal and childhood environmental tobacco smoke exposure and age at menarche. Paediatr Perinat Epidemiol 2010;24(6):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Liu Q, Li W, Deng X, Yang B, Huang X. Association of prenatal and childhood environment smoking exposure with puberty timing: a systematic review and meta-analysis. Environ Health Prev Med 2018;23(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenspan LC, Lee MM. Endocrine disrupters and pubertal timing. Curr Opin Endocrinol Diabetes Obes 2018;25(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodgers KM, Udesky JO, Rudel RA, Brody JG. Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms. Environ Res 2018;160:152–182. [DOI] [PubMed] [Google Scholar]
- 46.Kehm RD, Oskar S, Tehranifar P, et al. Associations of prenatal exposure to polycyclic aromatic hydrocarbons with pubertal timing and body composition in adolescent girls: Implications for breast cancer risk. Environ Res 2021;196:110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z, Romanoff L, Bartell S, et al. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol 2012;25(7):1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terry MB, Michels KB, Brody JG, et al. Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res 2019;21(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ullsperger JM, Nikolas MA. A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk? Psychol Bull 2017;143(9):903–938. [DOI] [PubMed] [Google Scholar]
- 51.Fuhrman BJ, Moore SC, Byrne C, et al. Association of the Age at Menarche with Site-Specific Cancer Risks in Pooled Data from Nine Cohorts. Cancer Res 2021;81(8):2246–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lakshman R, Forouhi NG, Sharp SJ, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab 2009;94(12):4953–4960. [DOI] [PubMed] [Google Scholar]
- 53.Lakshman R, Forouhi N, Luben R, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia 2008;51(5):781–786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


