Abstract
Studies addressing homeostasis of inorganic phosphate (Pi) are mostly restricted to murine models. Data provided by genetically modified mice suggest that renal Pi reabsorption is primarily mediated by the Na+/Pi cotransporter NaPi-IIa/Slc34a1, whereas the contribution of NaPi-IIc/Slc34a3 in adult animals seems negligible. However, mutations in both cotransporters associate with hypophosphatemic syndromes in humans, suggesting major inter-species heterogeneity. Urinary extracellular vesicles (UEV) have been proposed as an alternative source to analyse the intrinsic expression of renal proteins in vivo. Here, we analyse in rats whether the protein abundance of renal Pi transporters in UEV correlates with their renal content. For that, we compared the abundance of NaPi-IIa and NaPi-IIc in paired samples from kidneys and UEV from rats fed acutely and chronically on diets with low or high Pi. In renal brush border membranes (BBM) NaPi-IIa was detected as two fragments corresponding to the full-length protein and to a proteolytic product, whereas NaPi-IIc migrated as a single full-length band. The expression of NaPi-IIa (both fragments) in BBM adapted to acute as well to chronic changes of dietary Pi, whereas adaptation of NaPi-IIc was only detected in response to chronic administration. Both transporters were detected in UEV as well. UEV reflected the renal adaptation of the NaPi-IIa proteolytic fragment (but not the full-length protein) upon chronic but not acute dietary changes, while also reproducing the chronic regulation of NaPi-IIc. Thus, the composition of UEV reflects only partially changes in the expression of NaPi-IIa and NaPi-IIc at the BBM triggered by dietary Pi.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00424-022-02744-1.
Keywords: Phosphate, Urinary extracellular vesicles, Slc34a1, Slc34a3
Introduction
Aberrant low or high plasma phosphate (Pi) possess a health risk as illustrated by the development, among other symptoms, of rickets/osteomalacia and muscle weakness in hypophosphatemic states whereas ectopic calcifications, hyperparathyroidism, and cardiovascular disease associate with hyperphosphatemia (for review see [45]. The kidney is the main organ responsible for maintaining adequate and constant levels of plasma Pi. Thus, renal reabsorption of Pi decreases in response to Pi overload whereas it increases as a reaction to hypophosphatemia. Transport of Pi across renal epithelial cells is mostly mediated by two members of the SLC34 family of Na+/Pi cotransporters that are expressed at the brush border membrane (BBM) of proximal tubules, namely NaPi-IIa/SLC34A1 [9] and NaPi-IIc/SLC34A3 [37], for review see [23]. Their abundance is under hormonal and metabolic control, with the effects of parathyroid hormone (PTH) [1, 8, 10, 18, 19, 31, 32, 39], fibroblast growth factor-23 (FGF23) [15, 20, 42, 46], and dietary Pi [6, 11, 18, 21, 22, 38, 44] among the best studied. Ultimately, high dietary Pi as well as PTH and FGF23 (both hormones produced in states of hyperphosphatemia or upon ingestion of Pi-rich diets) reduce renal reabsorption of Pi by downregulating the expression of cotransporters, whereas low dietary Pi triggers the opposite effects.
Gene ablation studies in mice suggested that NaPi-IIa has a major quantitative role [2], whereas the contribution of NaPi-IIc to renal Pi reabsorption in adult mice seems negligible [26, 40]. Furthermore, several reports in rodents also indicate that the expression NaPi-IIa adapts quickly to changes on Pi-regulating factors (including PTH and dietary Pi), whereas adaptation of NaPi-IIc is far slower [32, 44]. Based on these observations, NaPi-IIa was long considered as the main renal transporter. However, this view may not apply to humans since mutations in NaPi-IIc seem as severe as those in NaPi-IIa. Thus, while mutations of NaPi-IIa associate with idiopathic infantile hypercalcemia (IIH/HCINF2; OMIM: 616,963) [33, 36], mutations affecting NaPi-IIc cause hereditary hypophosphatemic rickets with hypercalciuria (HHRH; OMIM: 241,530) [3, 17, 24]. In both cases, hypophosphatemia caused by urinary Pi loss triggers a compensatory upregulation of 1,25(OH)2 vitamin D3 aimed at stimulating intestinal Pi absorption, that also results in higher absorption of Ca2+, hypercalcemia and hypercalciuria. Understandably, information about the role, regulation and contribution of single transporters to Pi homeostasis in humans is sparse and almost limited to reports describing the discovery of disease-causing mutations.
Urinary extracellular vesicles (UEV) have been proposed as a potential surrogate to analyse the expression of renal proteins in vivo, since several reports suggest that the content of transporters in UEV changes in parallel with their renal expression [14, 27–30, 43, 48, 49] (for review see [12, 35]). However, there are also studies suggesting a lack of correlation of transporter abundances in paired collections of human nephrectomy samples and UEV [34]. Here, were tested the reliability of UEV cargo as an indirect source to study regulation of renal Na+/Pi cotransporters in vivo. As a proof of principle, we have compared the abundance of NaPi-IIa and NaPi-IIc in paired samples of kidneys and UEV from rats acutely and chronically fed high or low Pi-diets.
Material and methods
Animal handling and sample collection
After 2 weeks of adaptation, male Wistar rats 10 weeks old with a body weight of approximately 200 g (Janvier Labs, France) were split into 2 groups of 10 animals each and fed 4 days on diets (Granovit AG, Switzerland) containing either high (H: 1.2% w/w) or low Pi (L: 0.1% w/w). Both diets had similar Ca2+ (1%) and vitamin D3 (800 μ/kg) content. On the fifth day, rats were single placed in metabolic cages (Tecniplast, Italy), and in the evening 5 animals from each group were switched from their initial diet to the opposite diet while the remaining 5 rats stayed on their starting chow. Urine was collected for the next 12 h (8 p.m.–8 a.m.) under mineral oil in the presence of EDTA-free protease inhibitors (Roche, Switzerland). Thus, this study consists of 4 experimental groups each containing 5 animals: rats chronically (5 days) fed on either high Pi (HH) or low Pi (LL) and rats acutely switched (12 h) to either high Pi (LH) or low Pi (HL). Urine samples were centrifuged at 163 × g for 10 min at room temperature and supernatants were stored at -20 °C (ion measurements) or at − 80 °C (isolation of UEV). After urinary collection, rats were anaesthetized with isoflurane and blood was withdrawn from the vena cava with heparinized syringes. Upon centrifugation of blood at 9300 × g for 8 min at 4 °C, plasma was aliquoted and stored at − 80 °C. Rats were sacrificed by cervical dislocation and their kidneys collected and stored at − 80 °C. Animal handling was previously approved by the local veterinary authority (Kantonales Veterinäramt Zürich), according to the Swiss Animal Welfare laws (licence number 156/2016).
Concentration of Pi, Ca2+ and creatinine in plasma and urine
The concentration of Pi was measured with a kit based on the Fiske-Subbarow method (Randox, UK). The concentration of total Ca2+ was quantified with a Quantichrom Calcium Assay kit (Bioassay Systems, USA). The concentration of creatinine in plasma and urine was quantified with an enzymatic-based technique (SYNCHRON Systems) or with the Jaffe method (Wako Chemicals), respectively.
Quantification of PTH, FGF23 and 1,25(OH)2D3 in plasma
Intact PTH and intact FGF23 were both measured using ELISA-based kits (Immutopics, USA). The plasma concentration of 1,25(OH)2D3 was quantified by radioimmunoassay (Immunodiagnostic System, UK).
Isolation of renal BBM
Renal BBM were prepared according to the Mg2+-precipitation technique [5]. In brief, half of the kidneys were homogenized in ice-cold buffer using the Brinkmann Polytron PT 10/35 (Kriens, Switzerland). The homogenization buffer (300 mM D-mannitol, 5 mM EGTA, 12 mM Tris–HCl, pH 7.1) contained EDTA-free protease inhibitors. Upon addition of MgCl2 (final concentration 12 mM) and incubation for 15 min on ice, samples were centrifuged at 2600 × g for 15 min at 4 °C. Supernatants were centrifuged at 39,400 × g for 30 min at 4 °C, and pellets resuspended in experimental buffer (300 mM D-mannitol, 20 mM HEPES-Tris, pH 7.4). Samples were centrifuged again at 39,400 × g for 30 min at 4 °C, and the BBM-containing pellets were resuspended in the experimental buffer and stored at − 20 °C. Protein concentrations were measured with a commercial kit (Bio-Rad, Hercules, CA, USA).
Isolation of UEV
The isolation of UEV was performed using standard procedures as previously described [16]. Briefly, 2 ml urine was centrifuged at 17,000 × g for 15 min at RT. Supernatants were kept at RT, while pellets were resuspended in isolation solution (250 mM sucrose, 10 mM triethanolamine, pH 7.6) containing 1.2 M dithiothreitol (DTT). After incubation for 5 min at 37 °C, pellets were vortexed until dissolved, and centrifuged at 16,000 × g for 15 min at 4 °C. These supernatants were pooled with those obtained after the first centrifugation and pools were subjected to ultracentrifugation at 200,000 × g for 2 h at 4 °C. Pellets containing the UEV were resuspended in 80 µl of 1.5 × Laemmli sample buffer containing 150 mM DTT and stored at − 20 °C.
Western blot
BBM (20 µg) and UEV (amount equivalent to 7.5 µg urinary creatinine) were separated on 9–10% SDS-PAGE and proteins transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Switzerland). Blocking and incubation with primary/secondary antibodies was done in 5% fat-free milk powder/TBS (150 mM NaCl, 25 mM Tris base, pH 7.4) following standard procedures [9]. After exposure to substrate (Immobilon Western Chemiluminescent HRP Substrate, Millipore, Switzerland), signals were detected with the LAS-4000 Luminescent Image Analyzer (Fujifilm, Japan) and quantified with ImageJ. When required, membranes were stripped 15 min at RT in 25 mM glycine, 1% SDS, pH 2. Staining of total proteins (LiCor) was used for loading normalization of BBM (supplementary Figs. 1 and 2). Source and dilutions of primary and secondary antibodies are provided in Table 1.
Table 1.
Source, features and dilutions of used antibodies
| Antibody | Source | Features | Dilution |
|---|---|---|---|
| NaPi-IIa | Home made (Custer M et al., Am J Physiol. 1994) | Rabbit polyclonal Antigen: rat N-terminal 13 aa | 1: 3000 |
| NaPi-IIc | Home made (Nowik M et al., Pflügers Arch. 2008) | Rabbit polyclonal Antigen: rat C-terminal 13 aa | 1: 1500 |
| AQP2 | Santa Cruz sc-515770 | Mouse monoclonal Antigen: human C-terminal aa 232–271 | 1:2000 |
| TSG101 | Santa Cruz sc-7964 | Mouse monoclonal Antigen: full-length mouse TSG101 | 1:500 |
| HRP-a-rabbit | Promega #W4011 | Goat polyclonal | 1:5000 |
| HRP-a-mouse | Promega #W4021 | Goat polyclonal | 1:5000 |
RNA extraction and real-time PCR
Pieces of kidneys were homogenized in RTL buffer and RNA was purified (RNeasy Mini Kit; Qiagen Science, Germany) and reverse transcribed (TaqMan Reverse Transcription kit; ThermoScientific, USA) following the manufacturers’ instructions. The expression of the genes of interest was analysed by semi-quantitative real-time PCR using the KAPA Probe Fast qPCR Universal Master Mix (2x) Kit (Kapa Biosystems, South Africa) in the presence of 0.1 µM FAM/TAMRA-labelled probe and 1 µM primers (Table 2). qPCR was run in the 7500 Fast Real-Time PCR System (Applied Biosystems, Switzerland). Cycle thresholds (Ct) were manually set at the exponential phase of the amplification curve. Ct values of tested genes were normalized to hypoxanthine–guanine phosphoribosyltransferase (HPRT), with relative mRNA expression levels calculated as 2(Ct_HPRT – Ct_gene).
Table 2.
Sequences of primers (forward/reverse) and probes used for qPCR
| Gene | Primers/Probe |
|---|---|
| NaPi-IIa | Forward: GGA ATC ACA GTC TCA TTC GGA TT |
| Reverse: ATG GCC TCT ACC CTG GAC ATA G | |
| Probe: TGT CAA CCA GAG ACA AAA GAG GCT TCC ACT | |
| NaPi-IIc | Forward: GGG ATC GGG ATG AAT TTC AGA |
| Reverse: GGG CCA GCT CAC TCA GTC TCT | |
| Probe: ACG GCA TCT TCA ACT GGC TCA CAG TGT T | |
| HPRT | Forward: GCT GAA GAT TTG GAA AAG GTG TTT A |
| Reverse: ACA CAG AGG GCC ACA ATG TGA | |
| Probe: TTA TGG ACA GGA CTG AAA GAC TTG CTC GAG ATG |
Data are presented as scatter plots together with means ± SEM. Statistical significances were calculated by t-test or one-way ANOVA with Bonferroni’s multiple comparison test, as indicated, using Graph Prism version 8 (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered significant.
Results
Urinary and plasma concentrations of Pi directly correlate with dietary Pi content
As expected, the concentration of Pi in plasma was higher in rats chronically fed on high Pi (HH) compared with the low Pi (LL) group and correlated with dietary Pi also upon acute switches (Fig. 1a). Similarly, the urinary excretion of Pi (measured as Pi/creatinine ratio) was higher in rats chronically fed high Pi than in the group chronically fed low Pi (Fig. 1b). Furthermore, acute switches resulted in the expected higher (LL to LH) or lower (HH to HL) urinary Pi output. Plasma and urinary levels of creatinine (Fig. 1g, h) as well as urinary volume (Fig. 1i) were similar in all groups. The fractional excretion of Pi followed the same trend as the urinary Pi excretion, though the increase induced by the acute switch from low to high Pi did not reach statistical significance (Fig. 1c).
Fig. 1.
Urinary and plasma levels of Pi and Ca2+ correlate with dietary Pi content. (a) Plasma concentration of Pi, (b) urinary excretion of Pi, (c) fractional excretion of Pi, (d) plasma concentration of Ca2+, (e) urinary excretion of Ca2+, (f) fractional excretion of Ca2+, (g) plasma creatinine, (h) urinary excretion of creatinine and (i) urinary volume in samples from rats fed chronically (5 days) with low Pi (LL), acutely changed (12 h) from low to high Pi (LH), chronically fed with high Pi (HH) and acutely changed from high to low Pi (HL). Statistical significances were calculated with one-way ANOVA with Bonferroni’s multiple comparison test. n = 5 for each group, * P < 0.05, **P < 0.01, *** P < 0.001, **** P < 0.0001
Urinary and plasma concentrations of Ca2+ inversely correlate with dietary Pi content
Plasma concentration of Ca2+ was lower in rats acutely and chronically fed high Pi than in the corresponding low Pi groups (Fig. 1d). Consequently, the urinary excretion of Ca2+ was lower in rats chronically fed high Pi (HH) than in the group chronically fed low Pi (LL), and this difference was observed in both Ca2+/creatinine ratio (Fig. 1e) as well as fractional excretion (Fig. 1f). The acute change from low to high Pi (LL to LH) also resulted in lower urinary Ca2+, whereas the acute switch from high to low Pi (HH to HL) had no effect (Fig. 1e, f).
Plasma levels of Pi-regulating hormones differentially adapt to changes on dietary Pi
Plasma levels of intact FGF-23 (Fig. 2a) and PTH (Fig. 2b) were higher in rats fed chronically high Pi (HH) than in those fed chronically low Pi (LL). Both phosphaturic hormones were also regulated in response to acute switches, increasing upon changing from low to high Pi (LL to LH) and decreasing upon switching from high to low (HH to HL) Pi, though the change in PTH induced by the first switch did not reach statistical significance. 1,25(OH)2D3 was lower in animals fed chronically high Pi (HH) compared with the group chronically fed on low Pi (LL) but did not change in response to acute changes (Fig. 2c).
Fig. 2.
Plasma levels of Pi-regulating hormones differentially adapt to changes in dietary Pi. Plasma levels of (a) intact FGF-23, (b) intact PTH and (c) 1,25(OH)2 vitamin D3 in samples from rats fed chronically (5 days) with low Pi (LL), acutely changed (12 h) from low to high Pi (LH), chronically fed with high Pi (HH) and acutely changed from high to low Pi (HL). Statistical significances were calculated with one-way ANOVA with Bonferroni’s multiple comparison test. n = 5 for each group, **** P < 0.0001
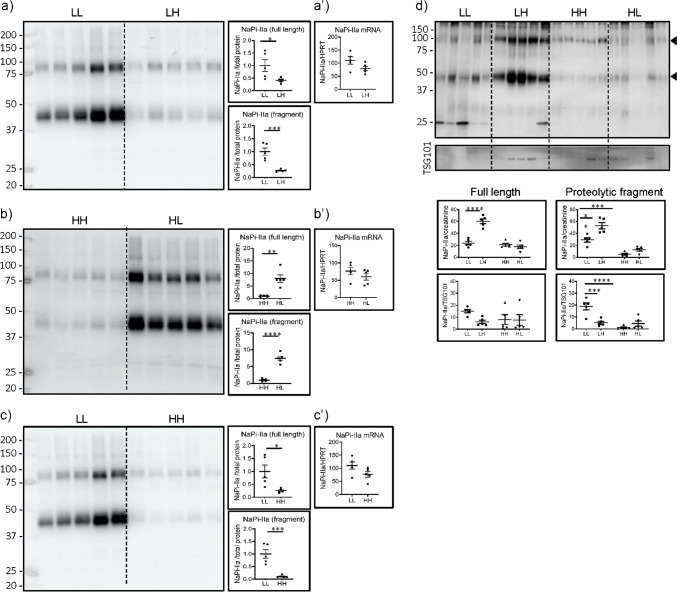
The abundance of the proteolytic fragment of NaPi-IIa in UEV partially correlates with its renal expression
As reported [4], incubation of renal BBM with the NaPi-IIa antibody resulted in the detection of a smear with an apparent molecular weight between 75 and 100 kDa, corresponding to the full length glycosylated protein, together with a lower smear between 37 and 50 kDa known to be a N-terminal proteolytic fragment which is also glycosylated (Fig. 3a–c). As expected [22, 44], the abundance of both bands was lower in BBM from rats acutely switched from low to high Pi (LH) than in rats maintained chronically on low Pi (LL), whereas their expression was higher in rats acutely switched from high to low Pi (HL) than in rats chronically kept on high Pi (HH) (Fig. 3a, b). Also as expected [22, 44], the expression of both fragments was lower in BBM from rats chronically fed on high Pi (HH) than in the chronic low Pi group (LL) (Fig. 3c). In all three cases, no differences on mRNA levels were detected between groups (Fig. 3a’–c’). Together with the mineral and hormonal data described above, these results indicate that the feeding protocols had triggered the expected systemic and renal adaptation.
Fig. 3.
The abundance of the proteolytic fragment of NaPi-IIa in UEV partially correlates with its renal expression. Western blots for NaPi-IIa in renal BBM from rats fed (a) chronically (5 days) low Pi (LL) and acutely changed (12 h) from low to high Pi (LH), (b) chronically fed high Pi (HH) and acutely changed from high to low Pi (HL), (c) chronically fed low (LL) or high Pi (HH), and (d) UEV isolated from all 4 groups. Graphs show the quantifications of the full-length and proteolytic fragment normalized to (a–c) LiCor total protein stain (suppl Fig. 1), (d) urinary creatinine and TSG101. (a’–c’) real-time PCRs on renal RNA samples from the same groups. Statistical significances were calculated by t-test (a–c and a’–c’) or with one-way ANOVA with Bonferroni’s multiple comparison test (d). n = 5 for each group, *P < 0.05, **P < 0.01, *** P < 0.001, **** P < 0.0001. Red asterisks indicate changes in UEV similar to those described in renal BBM
Bands corresponding to the full-length and the proteolytic NaPi-IIa fragment were also detected in UEV (Fig. 3d). However, their quantification heavily relied on the methods used for normalization. Thus, the abundance of both fragments seemed surprisingly higher in UEV isolated from rats acutely switched from low to high Pi (LH) than in samples from rats chronically kept in low Pi (LL) upon normalization to creatinine, whereas no difference or even the opposite change was detected upon normalization to TSG101. However, TSG101 levels fluctuated within and between groups. Regardless of the method for normalization, the acute switch from high to low Pi (HH to HL) did not alter the content of either fragment in UEV. However, both normalization methods indicate that the abundance of the proteolytic product is lower in UEV from rats chronically fed high Pi (HH) than in the chronic low Pi group (LL), a finding in agreement with its expression in renal BBM (Fig. 3c). In contrast, the content of the full-length transporter was similar in UEV from both chronic groups.
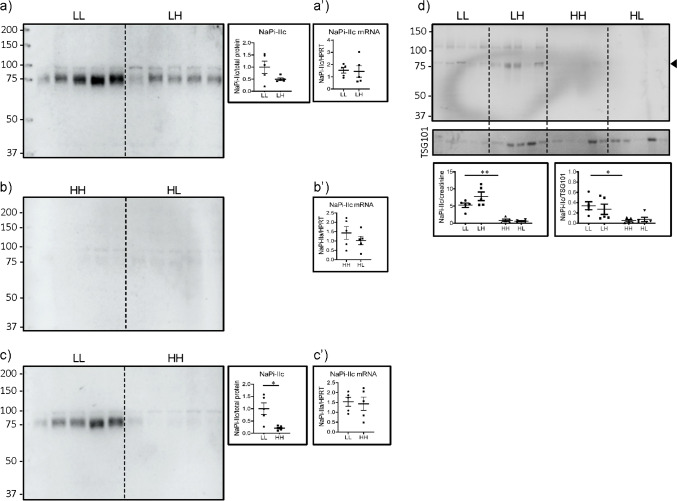
The abundance of NaPi-IIc in UEV partially correlates with its renal expression
The expression of NaPi-IIc, detected only as the full-length protein, tended to be lower in renal BBM from rats acutely switched to high Pi (LH) compared with rats chronically fed on low Pi (LL), though the difference did not reach statistical significance (Fig. 4a). Its expression was very low in kidneys from rats chronically fed on high Pi (HH), and acute switched to low Pi (HL) failed to increase substantially its expression (Fig. 4b). As expected, renal expression of NaPi-IIc was lower in rats chronically fed on high Pi (HH) compared with those kept chronically on low Pi (LL) (Fig. 4c). The chronic change did not involve transcriptional regulation since no differences in the mRNA expression of the cotransporter were found between groups (Fig. 4a’–c’).
Fig. 4.
The abundance of NaPi-IIc in UEV partially correlates with its renal expression. Western blots for NaPi-IIc in renal BBM from rats fed (a) chronically (5 days) low Pi (LL) and acutely changed (12 h) from low to high Pi (LH), (b) chronically fed high Pi (HH) and acutely changed from high to low Pi (HL), (c) chronically fed low (LL) or high Pi (HH), and (d) UEV isolated from all groups. Graphs show the quantifications normalized to (a–c) LiCor total protein stain (supplementary Fig. 1), (d) urinary creatinine and TSG101. (a’–c’) real time PCRs on renal RNA samples from the same groups. Statistical significances were calculated by t-test (a–c and a’–c’) or with one-way ANOVA with Bonferroni’s multiple comparison test (d). n = 5 for each group, *P < 0.05, ** P < 0.01. Asterisks indicate changes in UEV similar to those described in renal BBM
NaPi-IIc was also detected in UEV, though its abundance was at the limit of detection with the available antibody (Fig. 4d). As in renal BBM, the amount of NaPi-IIc in UEV was lower in rats chronically fed on high Pi (HH) compared with animals kept chronically on low Pi (LL) using both normalization criteria.
The abundance of AQP2 in UEV partially correlates with its renal expression
As a negative control for the dietary effect, we analysed the expression of the collecting duct water channel AQP2. This channel is detected as both glycosylated (smear at 37 kDa) and unglycosylated (25 kDa band) proteins [13]. As expected, its renal expression did not change in response to dietary Pi (Fig. 5a–c), except for a small reduction of the glycosylated form in rats acutely changed from high to low phosphate (Fig. 5b). Both forms were also detected in UEV, and their pattern of expression was similar in all dietary conditions (Fig. 5d).
Fig. 5.
The abundance of AQP2 in UEV correlates with its renal expression. Western blots for AQP2 in renal BBM from rats fed (a) chronically (5 days) low Pi (LL) and acutely changed (12 h) from low to high Pi (LH), (b) chronically fed high Pi (HH) and acutely changed from high to low Pi (HL), (c) chronically fed low (LL) or high Pi (HH), and (d) UEV isolated from all 4 groups. Graphs show the quantifications of the unglycosylated and glycosylated channel normalized to (a–c) LiCor total protein stain (supplementary Fig. 2), (d) urinary creatinine and TSG101. Statistical significances were calculated by t-test (A–C) or with one-way ANOVA with Bonferroni’s multiple comparison test (D). n = 5 for each group, *P < 0.05
Discussion
The contribution of the Na+/Pi cotransporters NaPi-IIa/Slc34a1 and NaPi-IIc/Slc34a3 to renal reabsorption of Pi may have some species-specificity, since the consequences of ablation/mutation in mice and humans do not fully overlap [2, 3, 17, 24, 26, 33, 36, 40]. Still, all that is known about the regulation of the transporters in vivo is based on studies in rodent models. UEV, heterogenous vesicular structures that originated from different parts within the urogenital tract, have been proposed as an alternative source to study the expression and regulation of renal proteins (for review see [35]) and a recently published large scale proteomic analysis suggests that UEV cargo reliably reflects changes in renal protein expression [49]. Based on comparative analysis of Western blots of purified UEV and renal samples, the content of several transporters in UEV has indeed been shown to change in parallel to their expression in renal tissue in response to physiological regulators. This includes the abundance and regulation of total and phosphorylated NCC (the thiazide-sensitive NaCl cotransporter located in distal convoluted tubules) [28, 43, 48], ENaC (the amiloride-sensitive epithelial Na+ channel expressed in collecting ducts) [14], pendrin (an anion exchanger in cortical collecting ducts) [27, 30], and the B1 subunit of the V-ATPase (a H+-ATPase expressed in intercalated cell) [29]. However, discrepancies between the protein content of UEV and renal samples have also been described. Thus, no correlation of the levels of NaPi-IIa, the outer medullary potassium channel ROMK, NCC, and the water channel aquaporin 2 (AQP2) was found in paired collections of human nephrectomy samples and UEVs [34].
Here, we explored the possibility of using UEV to study regulation of renal Na+/Pi cotransporters in vivo following standard procedures used by others to study in vivo regulation of renal proteins [27–30, 34, 43, 48]. We choose the same technical approach (purification of UEV followed by Western blot) to better compare our results with published data. For that, and as a proof of principle, we have compared the abundance of NaPi-IIa and NaPi-IIc in paired samples of kidneys and UEV from rats subjected to a procedure known to induce robust changes in the renal expression of both cotransporters, namely feeding with diets with either high or low Pi [6, 11, 18, 21, 22, 38, 44]. In order to test how dynamic changes in renal expression could be reflected in UEV content, diets were provided chronic and acutely. We document that the dietary interventions resulted in the expected systemic changes. Thus, hyperphosphaturia and hyperphosphatemia were observed in the groups acutely and chronically fed with high Pi compared with those fed with low Pi, whereas hypercalciuria and hypercalcemia developed in rats chronically fed on low Pi, the last finding at least partially explained by enhanced intestinal Ca2+ absorption due to less formation of Ca2+-phosphate precipitates and higher circulating levels of 1,25(OH)2 vitamin D3. Plasma levels of FGF-23 and PTH were higher in the groups fed with high Pi than in those fed with low Pi, as expected based on the hyperphosphatemia associated with dietary Pi overload. In agreement with the hormonal data, renal expression of NaPi-IIa and NaPi-IIc was lower in rats chronically fed with high Pi, whereas acute changes resulted in regulation of only NaPi-IIa, consistent with the known slower response of NaPi-IIc to dietary (and PTH) changes [32, 44].
NaPi-IIa and NaPi-IIc were both detected in UEV. However, quantification and comparison of their abundance among the four experimental groups were strongly dependent upon the normalization method. Urinary creatinine has been suggested as one of the most reliable normalization criteria, since it may correct for effects of circadian rhythm and for changes in glomerular filtration rate [12]. Because comparable levels of urinary (and plasma) creatinine as well as urinary output volumes were found in all analysed groups, gels were loaded with quantities of UEV corresponding to equal amounts of urinary creatinine. Normalization to creatinine resulted in a paradoxical higher abundance of the full-length and proteolytic fragment of NaPi-IIa (and a similar tendency of NaPi-IIc) in UEV isolated from rats acutely switched to high Pi (LH) compared with the group chronically fed low Pi (LL). However, it properly reflected the renal reduction of the proteolytic NaPi-IIa fragment as well as of the abundance of NaPi-IIc induced by chronic dietary Pi load (HH vs LL). The product of the tumor susceptibility gene 101 (TSG101), a regulatory component of vesicular trafficking required for sorting of endosomes into multivesicular bodies (MVBs) [25], is one of the proteins often used to normalize UEV samples. Normalization to TSG101, whose abundance was highly heterogeneous in rat UEV, eliminated the apparent increase of full-length NaPi-IIa and even resulted in a reduction of the proteolytic fragment in UEV from acutely Pi-loaded rats. Instead, it still reflected the lower renal expression of the NaPi-IIa proteolytic fragment as well as of NaPi-IIc in rats chronically fed on high Pi as compared with the chronic low Pi group. In this regard, it may be worth to indicate that EVs contain two types of secreted vesicles, namely exosomes (generated by fusion of MVBs with the plasma membrane) and microvesicles (generated by direct budding of the plasma membrane), as well as apoptotic bodies (for review see [12, 41]). Furthermore, the abundance of TSG101 (as well as that of other routinely used UEV biomarkers such as programmed cell death-6 interacting protein PDCD6IP/ALIX and heat shock protein 70/HSP70) seems to be higher in exosomes than other UEV components [7]. On the other hand, it is known that in response to high dietary Pi, NaPi-IIa is internalized into clathrin-coated pits and via endosomes is targeted to lysosomes for degradation [11]. Although speculative, overrepresentation of exosomes in the UEV preparations could explain the paradoxical higher abundance of NaPi-IIa in UEV isolated from rats acutely switched to high Pi observed upon normalization to creatine as well as the elimination of this difference upon normalization to TSG101. Another speculation may be, that removal of cleaved and intact NaPi-IIa may not only occur via internalization and lysosomal degradation but might also involve release of vesicles containing the transporter and its fragments into urine. This possibility will require further investigation beyond the scope of this study.
Two major limitations of our study are that our preparation of bulk UEV did not further distinguish between types of UEVs and that we did not test human urine. The latter would require detailed metabolic analyses of healthy probands which was beyond the scope and possibilities of this study. Various recommendations have been made how to isolate, store and characterize UEVs [12, 47]. Our preparation represents a simplified isolation of bulk UEVs without further separation of small and large UEVs. Of note, similar preparations have been used from various species including humans to study the regulation of transport proteins [27–30, 34, 43, 48].
Taken together, two normalization methods, i.e. creatinine and TSG101 suggest that UEV replicate changes on the renal expression of the NaPi-IIa proteolytic fragment and of NaPi-IIc triggered by chronic changes in dietary Pi, whereas they provide contradictory results regarding the effects of acute dietary switches. This failure of UEV to replicate the acute adaptation of the renal cotransporters may at least partially explain the recently described lack of correlation of NaPi-IIa abundance between UEV and human nephrectomy samples [34]: normal diurnal fluctuations in Pi consumption prior to urinary collection may result in up or downregulation of the renal expression of NaPi-IIa that may not correlate with its UEV abundance. Moreover, it is unclear why the content of the NaPi-IIa proteolytic product in UEV reflects more faithfully than the full-length protein the downregulation of the cotransporter in renal BBM observed upon chronic Pi load. Although the abundance of both fragments was clearly reduced in BBM from rats chronically fed with high Pi compared with animals chronically fed with low Pi, the change was slightly more pronounced for the proteolytic fragment. This, together with potential degradation of the full-length protein during the 12 h of urine collection, could have masked changes at the level of the intact transporter.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Zürich Integrative Rodent Physiology (ZIRP) Core facility for the biochemical analysis and the use of metabolic cages.
Author contribution
N.H and C.A.W designed the study. Z.R, A.D, E.M.P.A and N.H performed the experiment. Z.R, A.D, N.H and C.A.W analysed the data. N.H and C.A.W wrote the manuscript. All authors read and edited the manuscript and approved the final version.
Funding
Open access funding provided by University of Zurich This study was supported by a grant from the Swiss National Science Foundation to C.A.W (176125) and the National Center of Competence in Research NCCR Kidney.CH (project number 183774). CAW received also support from the University of Zurich and University of Zurich Hospital Clinical Research Priority Program “Hyrene”.
Data availability
All data are included, see also supplementary data.
Declarations
Ethics approval
Animal handling was previously approved by the local veterinary authority (Kantonales Veterinäramt Zürich), and was done according to the Swiss Animal Welfare laws (licence number 156/2016).
Consent for publication
All authors approved the publication.
Competing interests
CAW has received honoraria and research grants from Chugai, Kyowa Kirin, Ardelyx, Advicenne, and Medice. The rest of the authors declare no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bacic D, LeHir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006;69:495–503. doi: 10.1038/sj.ki.5000148. [DOI] [PubMed] [Google Scholar]
- 2.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter O, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biber J, Custer M, Magagnin S, Hayes G, Werner A, Lotscher M, Kaissling B, Murer H. Renal Na/Pi-cotransporters. Kidney Int. 1996;49:981–985. doi: 10.1038/ki.1996.139. [DOI] [PubMed] [Google Scholar]
- 5.Biber J, Stieger B, Stange G, Murer H. Isolation of renal proximal tubular brush-border membranes. Nat Protoc. 2007;2:1356–1359. doi: 10.1038/nprot.2007.156. [DOI] [PubMed] [Google Scholar]
- 6.Bourgeois S, Capuano P, Stange G, Muhlemann R, Murer H, Biber J, Wagner CA. The phosphate transporter NaPi-IIa determines the rapid renal adaptation to dietary phosphate intake in mouse irrespective of persistently high FGF23 levels. Pflugers Arch. 2013;465:1557–1572. doi: 10.1007/s00424-013-1298-9. [DOI] [PubMed] [Google Scholar]
- 7.Chutipongtanate S, Greis KD. Multiplex biomarker screening assay for urinary extracellular vesicles study: a targeted label-free proteomic approach. Sci Rep. 2018;8:15039. doi: 10.1038/s41598-018-33280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham R, E X, Steplock D, Shenolikar S, Weinman EJ, Defective PTH regulation of sodium-dependent phosphate transport in NHERF-1-/- renal proximal tubule cells and wild-type cells adapted to low-phosphate media. Am J Physiol Renal Physiol. 2005;289:F933–938. doi: 10.1152/ajprenal.00005.2005. [DOI] [PubMed] [Google Scholar]
- 9.Custer M, Lotscher M, Biber J, Murer H, Kaissling B. Expression of Na-P-I cotransport in rat-kidney - localization by Rt-Pcr and immunohistochemistry. Am J Physiol. 1994;266:F767–F774. doi: 10.1152/ajprenal.1994.266.5.F767. [DOI] [PubMed] [Google Scholar]
- 10.Deliot N, Hernando N, Horst-Liu Z, Gisler SM, Capuano P, Wagner CA, Bacic D, O'Brien S, Biber J, Murer H. Parathyroid hormone treatment induces dissociation of type IIa Na+-P(i) cotransporter-Na+/H+ exchanger regulatory factor-1 complexes. Am J Physiol Cell Physiol. 2005;289:C159–167. doi: 10.1152/ajpcell.00456.2004. [DOI] [PubMed] [Google Scholar]
- 11.Dobrinskikh E, Giral H, Caldas YA, Levi M, Doctor RB. Shank2 redistributes with NaPilla during regulated endocytosis. Am J Physiol Cell Physiol. 2010;299:C1324–1334. doi: 10.1152/ajpcell.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdbrugger U, Blijdorp CJ, Bijnsdorp IV, Borras FE, Burger D, Bussolati B, Byrd JB, Clayton A, Dear JW, Falcon-Perez JM, Grange C, Hill AF, Holthofer H, Hoorn EJ, Jenster G, Jimenez CR, Junker K, Klein J, Knepper MA, Koritzinsky EH, Luther JM, Lenassi M, Leivo J, Mertens I, Musante L, Oeyen E, Puhka M, van Royen ME, Sanchez C, Soekmadji C, Thongboonkerd V, van Steijn V, Verhaegh G, Webber JP, Witwer K, Yuen PST, Zheng L, Llorente A, Martens-Uzunova ES. Urinary extracellular vesicles: a position paper by the Urine Task Force of the International Society for Extracellular Vesicles. Journal of Extracellular Vesicles. 2021;10:e12093. doi: 10.1002/jev2.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frick A, Eriksson UK, de Mattia F, Oberg F, Hedfalk K, Neutze R, de Grip WJ, Deen PM, Tornroth-Horsefield S. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc Natl Acad Sci U S A. 2014;111:6305–6310. doi: 10.1073/pnas.1321406111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frindt G, Gravotta D, Palmer LG. Regulation of ENaC trafficking in rat kidney. J Gen Physiol. 2016;147:217–227. doi: 10.1085/jgp.201511533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297:F282–291. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales PA, Zhou H, Pisitkun T, Wang NS, Star RA, Knepper MA, Yuen PS. Isolation and purification of exosomes in urine. Methods Mol Biol. 2010;641:89–99. doi: 10.1007/978-1-60761-711-2_6. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa S, Sorenson AH, Imel EA, Friedman NE, Gertner JM, Econs MJ. Intronic deletions in the SLC34A3 gene cause hereditary Hypophosphatemic rickets with hypercalciuria. J Clin Endocrinol Metab. 2006;91:4022–4027. doi: 10.1210/jc.2005-2840. [DOI] [PubMed] [Google Scholar]
- 18.Ito M, Iidawa S, Izuka M, Haito S, Segawa H, Kuwahata M, Ohkido I, Ohno H, Miyamoto K. Interaction of a farnesylated protein with renal type IIa Na/Pi co-transporter in response to parathyroid hormone and dietary phosphate. Biochem J. 2004;377:607–616. doi: 10.1042/BJ20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim-Jimenez Z, Hernando N, Biber J, Murer H. A dibasic motif involved in parathyroid hormone-induced down-regulation of the type IIa NaPi cotransporter. Proc Natl Acad Sci U S A. 2000;97:12896–12901. doi: 10.1073/pnas.220394197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kung CJ, Haykir B, Schnitzbauer U, Egli-Spichtig D, Hernando N, Wagner CA. Fibroblast growth factor 23 leads to endolysosomal routing of the renal phosphate cotransporters NaPi-IIa and NaPi-IIc in vivo. Am J Physiol Renal Physiol. 2021;321:F785–F798. doi: 10.1152/ajprenal.00250.2021. [DOI] [PubMed] [Google Scholar]
- 21.Lanaspa MA, Caldas YA, Breusegem SY, Andres-Hernando A, Cicerchi C, Levi M, Sorribas V. Inorganic phosphate modulates the expression of the NaPi-2a transporter in the trans-Golgi network and the interaction with PIST in the proximal tubule. Biomed Res Int. 2013;2013:513932. doi: 10.1155/2013/513932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi M, Lotscher M, Sorribas V, Custer M, Arar M, Kaissling B, Murer H, Biber J. Cellular mechanisms of acute and chronic adaptation of rat renal P(i) transporter to alterations in dietary P(i) Am J Physiol. 1994;267:F900–908. doi: 10.1152/ajprenal.1994.267.5.F900. [DOI] [PubMed] [Google Scholar]
- 23.Levi M, Gratton E, Forster IC, Hernando N, Wagner CA, Biber J, Sorribas V, Murer H. Mechanisms of phosphate transport. Nat Rev Nephrol. 2019;15:482–500. doi: 10.1038/s41581-019-0159-y. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc Natl Acad Sci U S A. 2003;100:7626–7631. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myakala K, Motta S, Murer H, Wagner CA, Koesters R, Biber J, Hernando N. Renal-specific and inducible depletion of NaPi-IIc/Slc34a3, the cotransporter mutated in HHRH, does not affect phosphate or calcium homeostasis in mice. American Journal of Physiology-Renal Physiology. 2014;306:F833–F843. doi: 10.1152/ajprenal.00133.2013. [DOI] [PubMed] [Google Scholar]
- 27.Ochiai-Homma F, Kuribayashi-Okuma E, Tsurutani Y, Ishizawa K, Fujii W, Odajima K, Kawagoe M, Tomomitsu Y, Murakawa M, Asakawa S, Hirohama D, Nagura M, Arai S, Yamazaki O, Tamura Y, Fujigaki Y, Nishikawa T, Shibata S. Characterization of pendrin in urinary extracellular vesicles in a rat model of aldosterone excess and in human primary aldosteronism. Hypertens Res. 2021;44:1557–1567. doi: 10.1038/s41440-021-00710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathare G, Tutakhel OAZ, van der Wel MC, Shelton LM, Deinum J, Lenders JWM, Hoenderop JGJ, Bindels RJM. Hydrochlorothiazide treatment increases the abundance of the NaCl cotransporter in urinary extracellular vesicles of essential hypertensive patients. American Journal of Physiology-Renal Physiology. 2017;312:F1063–F1072. doi: 10.1152/ajprenal.00644.2016. [DOI] [PubMed] [Google Scholar]
- 29.Pathare G, Dhayat NA, Mohebbi N, Wagner CA, Bobulescu IA, Moe OW, Fuster DG. Changes in V-ATPase subunits of human urinary exosomes reflect the renal response to acute acid/alkali loading and the defects in distal renal tubular acidosis. Kidney Int. 2018;93:871–880. doi: 10.1016/j.kint.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathare G, Dhayat N, Mohebbi N, Wagner CA, Cheval L, Neuhaus TJ, Fuster DG. Acute regulated expression of pendrin in human urinary exosomes. Pflugers Archiv-European Journal of Physiology. 2018;470:427–438. doi: 10.1007/s00424-017-2049-0. [DOI] [PubMed] [Google Scholar]
- 31.Pfister MF, Ruf I, Stange G, Ziegler U, Lederer E, Biber J, Murer H. Parathyroid hormone leads to the lysosomal degradation of the renal type II Na/Pi cotransporter. Proc Natl Acad Sci U S A. 1998;95:1909–1914. doi: 10.1073/pnas.95.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picard N, Capuano P, Stange G, Mihailova M, Kaissling B, Murer H, Biber J, Wagner CA. Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflugers Arch. 2010;460:677–687. doi: 10.1007/s00424-010-0841-1. [DOI] [PubMed] [Google Scholar]
- 33.Pronicka E, Ciara E, Halat P, Janiec A, Wojcik M, Rowinska E, Rokicki D, Pludowski P, Wojciechowska E, Wierzbicka A, Ksiazyk JB, Jacoszek A, Konrad M, Schlingmann KP, Litwin M. Biallelic mutations in CYP24A1 or SLC34A1 as a cause of infantile idiopathic hypercalcemia (IIH) with vitamin D hypersensitivity: molecular study of 11 historical IIH cases. J Appl Genet. 2017;58:349–353. doi: 10.1007/s13353-017-0397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabaratnam R, Geertsen L, Skjodt K, Hojlund K, Dimke H, Lund L, Svenningsen P. In human nephrectomy specimens, the kidney level of tubular transport proteins does not correlate with their abundance in urinary extracellular vesicles. Am J Physiol Renal Physiol. 2019;317:F560–F571. doi: 10.1152/ajprenal.00242.2019. [DOI] [PubMed] [Google Scholar]
- 35.Salih M, Fenton RA, Zietse R, Hoorn EJ. Urinary extracellular vesicles as markers to assess kidney sodium transport. Curr Opin Nephrol Hypertens. 2016;25:67–72. doi: 10.1097/Mnh.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 36.Schlingmann KP, Ruminska J, Kaufmann M, Dursun I, Patti M, Kranz B, Pronicka E, Ciara E, Akcay T, Bulus D, Cornelissen EA, Gawlik A, Sikora P, Patzer L, Galiano M, Boyadzhiev V, Dumic M, Vivante A, Kleta R, Dekel B, Levtchenko E, Bindels RJ, Rust S, Forster IC, Hernando N, Jones G, Wagner CA, Konrad M. Autosomal-recessive mutations in SLC34A1 encoding sodium-phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. J Am Soc Nephrol. 2016;27:604–614. doi: 10.1681/ASN.2014101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K. Growth-related renal type II Na/Pi cotransporter. J Biol Chem. 2002;277:19665–19672. doi: 10.1074/jbc.M200943200. [DOI] [PubMed] [Google Scholar]
- 38.Segawa H, Yamanaka S, Ito M, Kuwahata M, Shono M, Yamamoto T, Miyamoto K. Internalization of renal type IIc Na-Pi cotransporter in response to a high-phosphate diet. Am J Physiol Renal Physiol. 2005;288:F587–596. doi: 10.1152/ajprenal.00097.2004. [DOI] [PubMed] [Google Scholar]
- 39.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol. 2007;292:F395–403. doi: 10.1152/ajprenal.00100.2006. [DOI] [PubMed] [Google Scholar]
- 40.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol. 2009;20:104–113. doi: 10.1681/ASN.2008020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl AL, Johansson K, Mossberg M, Kahn R, Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr Nephrol. 2019;34:11–30. doi: 10.1007/s00467-017-3816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomoe Y, Segawa H, Shiozawa K, Kaneko I, Tominaga R, Hanabusa E, Aranami F, Furutani J, Kuwahara S, Tatsumi S, Matsumoto M, Ito M, Miyamoto K. Phosphaturic action of fibroblast growth factor 23 in Npt2 null mice. Am J Physiol Renal Physiol. 2010;298:F1341–1350. doi: 10.1152/ajprenal.00375.2009. [DOI] [PubMed] [Google Scholar]
- 43.Tutakhel OAZ, Moes AD, Valdez-Flores MA, Kortenoeven MLA, von den Vries M, Jelen S, Fenton RA, Zietse R, Hoenderop JGJ, Hoorn EJ, Hilbrands L, Bindels RJM. NaCl cotransporter abundance in urinary vesicles is increased by calcineurin inhibitors and predicts thiazide sensitivity. PLoS ONE. 2017;12:e0176220. doi: 10.1371/journal.pone.0176220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H, Biber J, Forster IC. The Na+-P-i cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary P-i. American Journal of Physiology-Renal Physiology. 2009;296:F691–F699. doi: 10.1152/ajprenal.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner CA, Rubio-Aliaga I, Biber J, Hernando N. Genetic diseases of renal phosphate handling. Nephrol Dial Transplant. 2014;29:45–54. doi: 10.1093/ndt/gfu217. [DOI] [PubMed] [Google Scholar]
- 46.Weinman EJ, Steplock D, Shenolikar S, Biswas R. Fibroblast growth factor-23-mediated inhibition of renal phosphate transport in mice requires sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) and synergizes with parathyroid hormone. J Biol Chem. 2011;286:37216–37221. doi: 10.1074/jbc.M111.288357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Witwer KW, Goberdhan DCI, O'Driscoll L, Thery C, Welsh JA, Blenkiron C, Buzas EI, Di Vizio D, Erdbrugger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lotvall J, Nieuwland R, Ochiya T, Rome S, Sahoo S, Zheng L. Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicles. 2021;10:e12182. doi: 10.1002/jev2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolley MJ, Wu AH, Xu SX, Gordon RD, Fenton RA, Stowasser M. In primary aldosteronism, mineralocorticoids influence exosomal sodium-chloride cotransporter abundance. J Am Soc Nephrol. 2017;28:56–63. doi: 10.1681/Asn.2015111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Q, Poulsen SB, Murali SK, Grimm PR, Su XT, Delpire E, Welling PA, Ellison DH, Fenton RA. Large-scale proteomic assessment of urinary extracellular vesicles highlights their reliability in reflecting protein changes in the kidney. J Am Soc Nephrol. 2021;32:2195–2209. doi: 10.1681/ASN.2020071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included, see also supplementary data.