Abstract
Background:
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease. Limited evidence suggests ALS diagnosis may be associated with air pollution exposure and specifically traffic-related pollutants.
Methods:
In this population-based case–control study, we used 3,937 ALS cases from the Danish National Patient Register diagnosed during 1989–2013 and matched on age, sex, year of birth, and vital status to 19,333 population-based controls free of ALS at index date. We used validated predictions of elemental carbon (EC), nitrogen oxides (NOx), carbon monoxide (CO), and fine particles (PM2.5) to assign 1-, 5-, and 10-year average exposures pre-ALS diagnosis at study participants’ present and historical residential addresses. We used an adjusted Bayesian hierarchical conditional logistic model to estimate individual pollutant associations and joint and average associations for traffic-related pollutants (EC, NOx, CO).
Results:
For a standard deviation (SD) increase in 5-year average concentrations, EC (SD=0.42μg/m3) had a high probability of individual association with increased odds of ALS (11.5%; 95% credible interval [CrI] −1.0%, 25.6%; 96.3% posterior probability of positive association), with negative associations for NOx (SD=20μg/m3) (−4.6%; 95% CrI 18.1%, 8.9%; 27.8% posterior probability of positive association), CO (SD=106μg/m3) (−3.2%; 95% CrI 14.4%,10.0%; 26.7% posterior probability of positive association) and a null association for non-EC PM2.5 (SD=2.37μg/m3) (0.7%; 95%CrI 9.2%,12.4%). We found no association between ALS and joint or average traffic pollution concentrations.
Conclusions:
This study found high probability of a positive association between ALS diagnosis and EC concentration. Further work is needed to understand the role of traffic-related air pollution in ALS pathogenesis.
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating and fatal neurodegenerative disease,1 currently without a cure.2 Approximately half of patients die within 3 years of symptom onset.3 Annually, there are nearly 30,000 cases of ALS in Europe and over 200,000 worldwide.4 Known inherited genetic variants only account for 5–10% of ALS cases.5,6 Environmental factors, therefore, are likely important in ALS pathogenesis.7 However, because the disease is relatively rare, it is challenging to conduct large-scale prospective studies. There is a recognized need for more evidence of the environmental contributors of ALS.5,8
Although air pollution is commonly studied in association with respiratory- and cardiovascular-related outcomes, e.g., references9–14, epidemiologic and toxicologic studies also support several plausible biologic mechanisms in association with the nervous system and neurodegeneration, e.g., references15–34. Ambient air pollution, especially urban air pollution, is a ubiquitous exposure that has been associated with several other neurodegenerative disorders, e.g., references16–21,35,36. and is consistently linked to systemic inflammation,22–24 oxidative stress,25–28 and neuroinflammation,15,29 all of which, in turn, have been reported as key pathways to ALS pathogenesis, e.g., references30–34.
Despite the compelling plausibility, few studies to date have evaluated the association between air pollution and ALS.35,37–39 A study in 2021 found that traffic-related air pollutants may be driving observed associations.38 Another study of ALS and PM2.5 in Denmark examining critical windows of exposure found that more recent exposure to PM2.5 (i.e., the previous 1 to 5 years) may be the most important driver of the potential association, though the constituents of PM2.5 were not analyzed, neither together nor separately.40 To our knowledge no study has hitherto attempted to understand the individual, joint, and average associations of the pollutants in a single model. Air pollutants have been consistently associated with adverse health, primarily in single pollutant analyses.13,17,41–43 However, they are highly correlated with one another.41 It is therefore a mixture modeling challenge to infer the association of multiple air pollutants and health outcomes.44 Using three air pollutants commonly used in health studies as traffic-related emissions tracers— elemental carbon (EC), nitrogen oxides (NOx), and carbon monoxide (CO)— we aimed to assess whether exposure to (a) each individual air pollutant is independently associated with ALS diagnosis, and estimate their (b) joint and (c) average traffic-related emissions associations. This study pairs with and complements the work of Nunez et al.40
Methods
Study Population and Outcome Assessment
We used data from the Danish National Patient Register during 1989–2013, through which details on demographic characteristics and certain health outcomes of all Danish residents can be linked based on a unique personal identifier.45 The Register was established in 1977 and is comprehensive, including nationwide clinical and administrative records for all inpatient data, with outpatient data available since 1995.46
We identified ALS cases based on their International Classification of Diseases (ICD) discharge diagnoses, i.e., ICD-8 code 348.0 (ALS) until 1993 and ICD-10 code G12.2 (motor neuron disease) thereafter, using the date of the first relevant code as the diagnosis date. This was the index date. We only included patients who were at least 20 years old when diagnosed because (i) cases younger than 20 years old were at a greater chance of misclassification, since ALS has been predominantly diagnosed in older adults,47 and (ii) the very few juvenile ALS cases have been explained to a much larger degree by genetic mutations (~40%).48 In our validation study, Register data for ALS ascertainment were highly reliable; working with a specialist ALS neurologist to review medical records and comparing to death certificates and hospital discharges, the Danish National Patient Register was found to have an overall predictive value for ALS of 82%.49
We obtained controls through the Danish Civil Registration System, established in 1968 and updated daily, which includes administrative records (e.g., date and place of birth, sex, vital status, and history of civil status and addresses since 1971) on all persons living in Denmark; records are kept even when a person dies or emigrates.50 We randomly matched five controls per case by age, sex, year of birth, and vital status. Controls were alive and free of diagnosed ALS at the ALS diagnosis date of the matched case (index date). The control-sampling scheme followed a risk-set matching pattern, so cases could have served as controls before diagnosis of ALS.51
We obtained all addresses of cases and controls from 1 January 1979 onwards from the Danish Civil Registration System,50 including the dates of moving to and from each address, before the index date. We then obtained the geographical coordinates at the door of each house of the residential history of the participants, with previous evidence of the high accuracy of this method of geocoding of addresses in Denmark.17
This study was approved by the Institutional Review Board Committee at Columbia University and the Danish Data Protection Agency.
Exposure data
We obtained predictions on monthly concentrations of elemental carbon (EC), nitrogen oxides (NOx), carbon monoxide (CO), and fine particles (PM2.5) (as well as ozone, O3, for a sensitivity analysis, usually negatively correlated with other pollutants due to its chemistry52), at residential addresses of study participants from the validated spatio-temporal air pollution modeling system DEHM-UBM-AirGIS that provides full space and time coverage over the study period, described in detail elsewhere.53–56 In brief, DEHM-UBM-AirGIS is a human exposure modeling system for traffic pollution, developed for application in Danish air pollution epidemiologic studies. The modeling system integrates air pollution dispersion models, digital maps, national and local administrative databases, concentrations of air pollutants at regional, urban background and street level, meteorologic data, and a Geographic Information System (GIS). The modeling system is therefore able to generate street configuration and traffic data based on digital maps and national databases, which enables estimation of air quality levels at a large number of addresses in an automatic and effective way. These predicted pollutant concentrations have been extensively used in previous air pollution epidemiologic studies in Denmark.17,40,57–59 The models have good predictive accuracy, with average monthly correlations between measured and modeled results of 0.79 for EC, 0.85 for NOx, 0.91 for CO, 0.92 for O3, and 0.83 for annual concentrations of PM2.5.53,56 Because traffic is a major source of PM2.5 and EC one of the main PM2.5 components in urban environments,60 we removed the EC concentration from the total PM2.5 mass concentration (non-EC PM2.5) by subtraction to avoid overadjustment when including both in the models simultaneously; this was valid since the DEHM-UBM-AirGIS modeling system constructed PM2.5 concentrations by adding from specific species of pollutants, one of which was EC.53–56
Based on the residential history of each case or control, we calculated 1-, 5-, and 10-year average exposure to each pollutant ending at 1 year before the index date, as diagnosis has been shown previously to occur at a median of 12 months after symptoms onset.61 Specifically, each case or control average value (1-, 5- or 10-year) was calculated as the mean of all concentrations recorded across time at the recorded addresses within each time window. A small number of Danish residents lack a complete address history (1.7%; lack of house number). To ensure we were including participants with adequately complete exposure records, we set the following minimum criteria for number of complete exposure record months to include cases and controls: (i) 1-year averages: 9 of 12 months, at least one measurement in each season; (ii) 5-year averages (main exposure): 30 of 60 months; and (iii) 10-year averages: 60 of 120 months.
Covariate data
We included a set of covariates based on as close as possible to index date to account for potential confounding bias, including household socioeconomic status (SES) based on last-reported job title at index date; civil status at index date, last reported place of residence at index date, and place of birth. We used a five-category, individual-level SES definition developed by the Danish Institute of Social Sciences, based on job titles from income tax forms, which has been associated with ALS diagnosis in Denmark,62 as well as how quickly one is identified as having ALS in the Danish Civil Registration System.63 Group 1 (highest status) includes corporate managers and academics; group 2: proprietors, managers of small businesses and teachers; group 3: technicians and nurses; group 4: skilled workers; and group 5: unspecialized workers, such as entry-level positions within food and retail environments. We also included a group for participants whose job title was unknown (group 9). For each married participant, we used the higher of the couple’s individual SES categories, when available. We also used information on civil status (never married, married, divorced, widowed) due to the influence that a spouse may have on visiting a family physician,64 last reported place of residence from postcode (Greater Copenhagen, big cities of Denmark, rest of Denmark, Greenland) to account for various local environmental and behavioral stressors,7 and place of birth (Greater Copenhagen, big cities of Denmark, rest of Denmark, Greenland, foreign, unknown) to adjust for other potential family-specific, location-specific, and early-life confounders, which may have an impact on the probability of developing ALS.65 Ultimately, we were limited by what was available in the Danish Civil Registration System.63 As part of a sensitivity analysis, we also included parish-level SES, measured by percentage of residents with greater than high-school education, in the model. In Denmark, parishes are administrative units with an average population of ~2,500 residents.
Statistical analysis
We analyzed the association between ALS diagnosis (binary) and exposure to traffic-related pollutants by applying a Bayesian formulation of the conditional logistic model, with Bayesian hierarchy on the traffic-related pollutants (EC, NOx, CO).66,67 The conditional approach examines contrasts within matched strata, i.e., groupings of case and matched controls, implicitly adjusting for matching factors (age, sex, year of birth, vital status) within each matched stratum.66 Matching by finer scale than year of birth was not possible. Bayesian inference allows for full distributional estimation of parameters of interest.67 We employed a Bayesian hierarchical formulation because it enables estimates of (a) independent pollutant–outcome associations, (b) a joint association of the three pollutants (i.e., total percentage change in odds of ALS diagnosis with a simultaneous increase in each of EC, NOx, CO), and (c) an average traffic association (i.e., average percentage change in odds of ALS diagnosis with increases in each of EC, NOx, CO), while accounting for the variance–covariance structure between the highly correlated exposures and their coefficients.67 We included a linear term for each included pollutant and adjusted for individual- and parish-level SES, civil status, last reported place of residence, and place of birth.
Specifically, via a logit function, we modeled the log-odds of ALS diagnosis, as follows:
where Yci denotes whether subject i in matched stratum c was diagnosed with ALS, i.e., c represents a case and its matched controls; αc the matched stratum-specific intercepts (not estimated in conditional logistic models); βEC, , βCO, the individual pollutant coefficients (log-odds) per standard deviation (SD) increase in concentration of EC, NOx, CO, non-EC PM2.5 respectively, scaled by their respective SDs and centered at their means, with each β an individual pollutant association adjusted by other terms in the model and the rest as coefficients for subject-specific covariates. Interquartile range (IQR) could equivalently be used to scale pollutant concentrations. If other sources of air pollution are associated with ALS, then including non-EC PM2.5 adjusts for PM2.5 from other sources,68 as well as indicating whether pollution from other sources not explicitly quantified might also have associations with ALS. Therefore, is interpreted as the association with air pollutants not specifically included in our analysis. In urban European environments, traffic-related pollutants typically represent on-average 14% of PM2.5 concentrations.69 In a sensitivity analysis, we included O3 in the model, as O3 concentrations have been associated with many adverse health outcomes,70 and were negatively correlated with traffic-related pollutants, and added , as a natural spline with three degrees of freedom.
In our model, βEC, , and βCO represent the independent individual pollutant associations with ALS diagnosis. In the same model, we estimated the joint association between these three pollutants and ALS diagnosis as:
This sum quantifies the association (log-odds) with ALS of a one-SD increase in the three pollutants simultaneously.
Finally, we assumed that the traffic-related individual pollutant associations arise from a distribution of the average traffic association with ALS diagnosis. We placed a hierarchy on the traffic-specific individual pollutant terms in the model to account for the fact that the traffic-related pollutants, EC, NOx, CO, originate from common sources and primarily traffic in urban environments:
where λ denotes the average one-SD association of traffic-related pollution with variance σλ. Σ, the estimated variance–covariance matrix among individual pollutant estimates, was expressed as a decomposition into a positive-definite correlation matrix Ω and scale matrix τ.71
We used weakly informative priors so that data drove parameter estimation. Hyper-priors for coefficients on non-EC PM2.5 and covariates were N(0,10); for σλ and τ we used Half-Cauchy(0,10), as recommended by Gelman, Polson, and Scott as a weakly-informative prior;72,73 Ω was defined by the weakly-informative prior LKJCorr(1).74 The exception to this was the prior for λ, the average association of traffic-related pollutants, for which estimates became unrealistically high (approaching infinity and not converging with further iterations) with a more weakly-informative prior. We therefore used a prior of N(0,0.1), which did not affect estimates of other parameters. We conducted sensitivity analyses to understand the influence of priors and the robustness of the results.
We present all results as percentage change in odds of ALS diagnosis per SD increase in pollutant concentration (calculated via e.g., , etc. obtained in the modeling process). Due to the risk-set matching pattern of our case–control study, odds ratios are also equivalently incidence ratios (IRs).66 We ran each model with four chains with a sample size of 1,000 each, after a warm-up of 1,000 samples, for 4,000 total samples. We assessed whether the models converged by checking that the Gelman–Rubin potential scale reduction statistic75 was below 1.1 for all estimated model parameters. The reported 95% credible intervals (CrI) are the 2.5th to 97.5th percentiles of each parameter’s posterior marginal distribution. To calculate the probability that an association estimate was greater than null, we used the 4,000 samples of the posterior distribution and took the proportion of samples which were above the null. A 50% probability means that it is as likely as not that the marginal estimate is null, a probability closer to 100% indicates that the association is more likely to be truly positive, with closer to 0% indicating more likely to be truly negative.
We conducted statistical analyses using the R Statistical Software, version 4.1.176 and R-STAN, version 2.21.2.67 All code for analysis, results from analysis, and visualization presented in this manuscript is publicly available via GitHub at https://github.com/rmp15/traffic_air_pollution_als_denmark_epidemiology.
We assessed the sensitivity of our results to hyper-prior assignment; running more iterations and warm-up per chain; inclusion of O3; single traffic-related pollutant models adjusting for non-EC PM2.5; as well as adjusting by parish-level SES. From the parish-level SES sensitivity analysis we excluded those who lived in areas without parish-level SES data, namely: (i) 819 participants for 1-year average exposure; (ii) 826 participants for 5-year average exposure; and (iii) 838 participants for 10-year average exposure.
Results
After filtering the original 4,011 cases and 20,055 controls based on completeness of exposure records, we used information on 3,934 (98.1% of total) cases and 19,298 (96.2% of total) controls for 5-year average exposure. We also used 3,937 cases,19,333 controls for 1-year average exposure and 3,929 cases, 19,250 controls for 10-year average exposure. Descriptive statistics of included cases and controls for 5-year average exposure can be found in Table 1. Descriptive statistics of controls for 5-year exposure by socioeconomic status, civil status, residence, and place of birth are found in eTables 1–4. For the main results, we present 5-year average exposure associations as a balance between representation of most recent exposure as well as long-term concentration.
Table 1.
Demographic characteristics of cases and controls for 5-year average exposure group.
| Characteristic | Overall, N = 23,232 | Case, N = 3,934 | Control, N = 19,298 |
|---|---|---|---|
| Average age (years) | 66 (12) | 66 (12) | 66 (12) |
| Sex, n(%) | |||
| Female | 10,973 (47%) | 1,854 (47%) | 9,119 (47%) |
| Male | 12,259 (53%) | 2,080 (53%) | 10,179 (53%) |
| Socioeconomic status (SES), n(%) | |||
| Group 1 (Highest) | 2,337 (10%) | 451 (11%) | 1,886 (9.8%) |
| Group 2 | 2,839 (12%) | 499 (13%) | 2,340 (12%) |
| Group 3 | 4,360 (19%) | 785 (20%) | 3,575 (19%) |
| Group 4 | 6,598 (28%) | 1,076 (27%) | 5,522 (29%) |
| Group 5 (Lowest) | 4,419 (19%) | 717 (18%) | 3,702 (19%) |
| Group 9 (Unknown) | 2,679 (12%) | 406 (10%) | 2,273 (12%) |
| Place of birth, n(%) | |||
| Greater Copenhagen | 4,858 (21%) | 831 (21%) | 4,027 (21%) |
| Big cities of Denmark | 7,923 (34%) | 1,357 (34%) | 6,566 (34%) |
| Rest of Denmark | 9,009 (39%) | 1,548 (39%) | 7,461 (39%) |
| Greenland | 243 (1.0%) | 53 (1.3%) | 190 (1.0%) |
| Foreign | 1,065 (4.6%) | 122 (3.1%) | 943 (4.9%) |
| Unknown | 134 (0.6%) | 23 (0.6%) | 111 (0.6%) |
| Civil status, n(%) | |||
| Married | 14,158 (61%) | 2,411 (61%) | 11,747 (61%) |
| Divorced | 2,703 (12%) | 433 (11%) | 2,270 (12%) |
| Widowed | 4,224 (18%) | 726 (18%) | 3,498 (18%) |
| Never married | 2,147 (9.2%) | 364 (9.3%) | 1,783 (9.2%) |
| Last reported place of residence, n(%) | |||
| Greater Copenhagen | 7,778 (33%) | 1,328 (34%) | 6,450 (33%) |
| Big cities of Denmark | 3,703 (16%) | 618 (16%) | 3,085 (16%) |
| Rest of Denmark | 11,747 (51%) | 1,988 (51%) | 9,759 (51%) |
| Greenland | 4 (<0.1%) | 0 (0%) | 4 (<0.1%) |
The 5-year average traffic-related pollutant concentrations were 0.85 μg/m3 for EC (SD=0.42 μg/m3), 27 μg/m3 for NOx (SD=20 μg/m3), and 238 μg/m3 for CO (SD=106 μg/m3) (Table 2). Figure 1 shows Spearman correlations between pollutants for 1-, 5-, and 10-year average exposures. Traffic-related pollutants (EC, NOx, CO) were highly correlated in cases, controls and overall, ranging from correlations of 0.91 to 0.96. Otherwise, non-EC PM2.5 was most highly correlated with CO, ranging from 0.67 to 0.7. O3 was negatively correlated with other pollutants, ranging from −0.54 to −0.89. Maps of average concentration of included pollutants (EC, NOx, CO, PM2.5, O3) across Denmark for a representative year (2000; middle of study period 1989–2013) are also available in eFigure 1.
Table 2.
Summary of 1,- 5-, and 10-year average pollutant concentrations of elemental carbon (EC), nitrogen oxides (NOx), carbon monoxide (CO), non-elemental carbon fine particles (non-EC PM2.5), and ozone (O3) (all mean [SD] in μg/m3).
| Pollutant | Overall, N = 23,270 | Case, N = 3,937 | Control, N = 19,333 | |
|---|---|---|---|---|
| 1-year average | EC | 0.81 (0.42) | 0.83 (0.44) | 0.81 (0.42) |
| NOX | 26 (19) | 26 (20) | 26 (19) | |
| CO | 224 (97) | 226 (101) | 224 (96) | |
| non-EC PM2.5 | 11.17 (2.32) | 11.20 (2.34) | 11.17 (2.31) | |
| O3 | 52.6 (6.1) | 52.4 (6.2) | 52.6 (6.1) | |
| Pollutant | Overall, N = 23,232 | Case, N = 3,934 | Control, N = 19,298 | |
| 5-year average | EC | 0.85 (0.42) | 0.86 (0.45) | 0.85 (0.42) |
| NOX | 27 (20) | 28 (21) | 27 (20) | |
| CO | 238 (106) | 239 (112) | 237 (105) | |
| non-EC PM2.5 | 11.76 (2.37) | 11.78 (2.41) | 11.76 (2.37) | |
| O3 | 51.9 (6.0) | 51.9 (6.1) | 52.0 (6.0) | |
| Pollutant | Overall, N = 23,179 | Case, N = 3,929 | Control, N = 19,250 | |
| 10-year average | EC | 0.89 (0.43) | 0.89 (0.46) | 0.88 (0.43) |
| NOX | 29 (20) | 29 (22) | 29 (20) | |
| CO | 253 (115) | 255 (122) | 253 (113) | |
| non-EC PM2.5 | 12.53 (2.55) | 12.55 (2.59) | 12.52 (2.55) | |
| O3 | 51.3 (6.0) | 51.3 (6.1) | 51.4 (6.0) |
Figure 1.
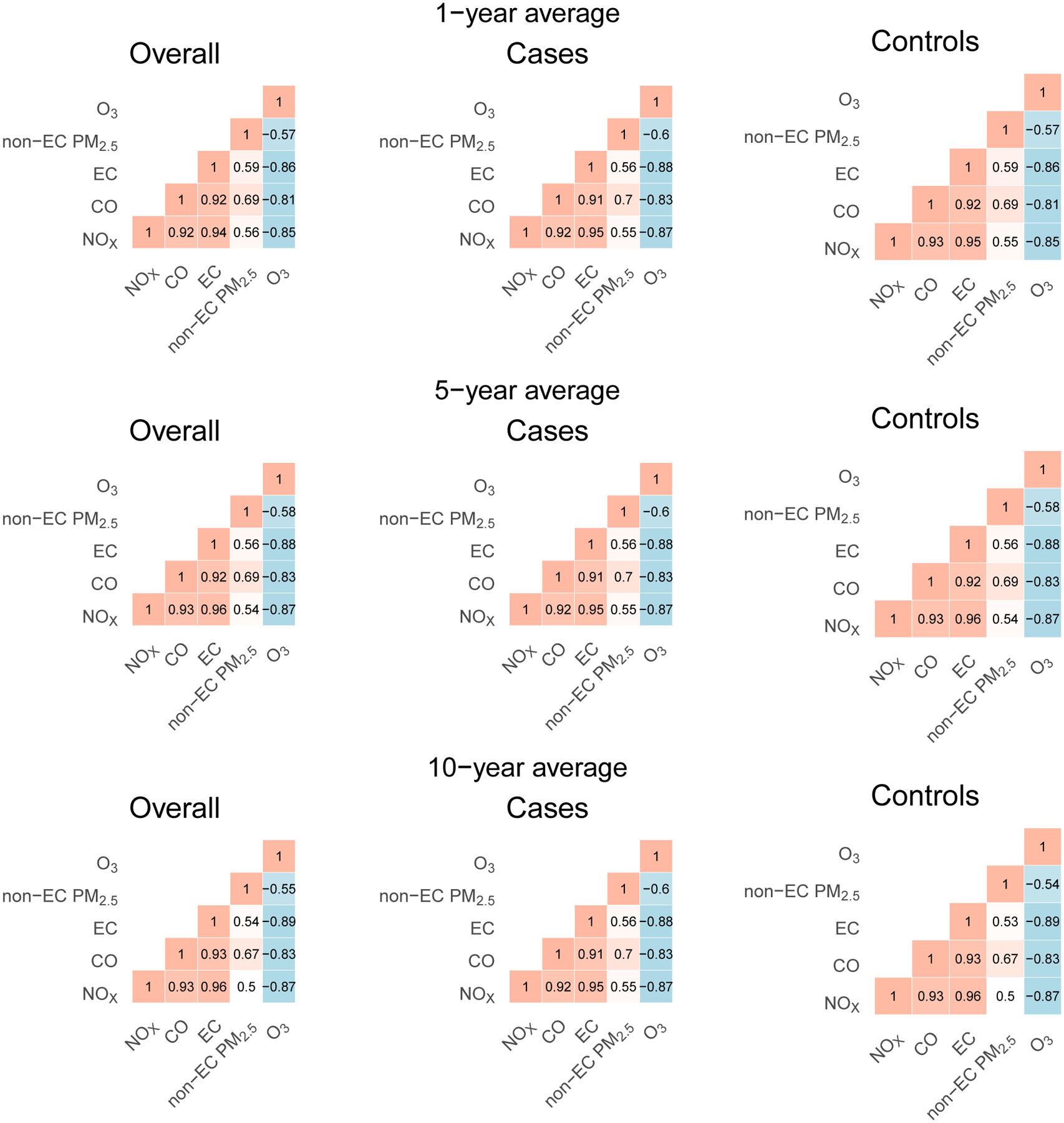
Spearman correlation of 1,- 5-, and 10-year average pollutant concentrations.
For 5-year average pollutant concentrations, we observed the largest overall increase in odds of ALS diagnosis for the individual SD increase in EC (11.5%; 95% CrI: −1.0%, 25.6% per 0.42 μg/m3; 96.3% posterior probability of positive association) (Figure 2). SD increases were associated with a decrease in odds of ALS diagnosis in NOx (−4.6%; 95% CrI: −18.1%, 8.9% per 20 μg/m3; 27.8% posterior probability of positive association) and CO (−3.2%; 95% CrI: −14.4%, 10.0% per 106 μg/m3; 26.7% posterior probability of positive association). Non-EC PM2.5 was not associated with ALS diagnosis (0.7%; 95% CrI: −9.2%, 12.4% per 2.37 μg/m3; 54.1% posterior probability of positive association). One-year EC average exposure was associated with an increase in odds of ALS diagnosis (15.4%; 95% CrI: 1.6%, 25.6% per 0.42 μg/m3; 98.9% posterior probability of positive association). Single-pollutant models for each traffic-related pollutant adjusting for non-EC PM2.5 (eFigure 1; single traffic-related pollutant models D, E and F) resulted in positive associations for each of EC, NOx, CO, with positive associations for non-EC PM2.5 in all but the model with EC. The 95% credible interval for EC in the single-pollutant model (eFigure 1; model F) overlapped with the credible intervals of the EC term in the multi-pollutant models (eFigure 1; models A to C, G to P). The joint association of traffic-related pollutants (EC, NOx, CO) was 2.3% (95% CrI: −3.3%, 7.7%), with an 77.8% posterior probability of a positive association. The average traffic association was null (−0.1%; 95% CrI: −17.4%, 20.8%; 45.5% posterior probability of positive association). Compared to the 1- and 5-year results, the 10-year average exposure results were attenuated, as associations tended further to the null. Results from variations of the main model in the sensitivity analyses were robust to prior choices, inclusion of O3, and inclusion of parish-level SES (eFigure 2).
Figure 2.
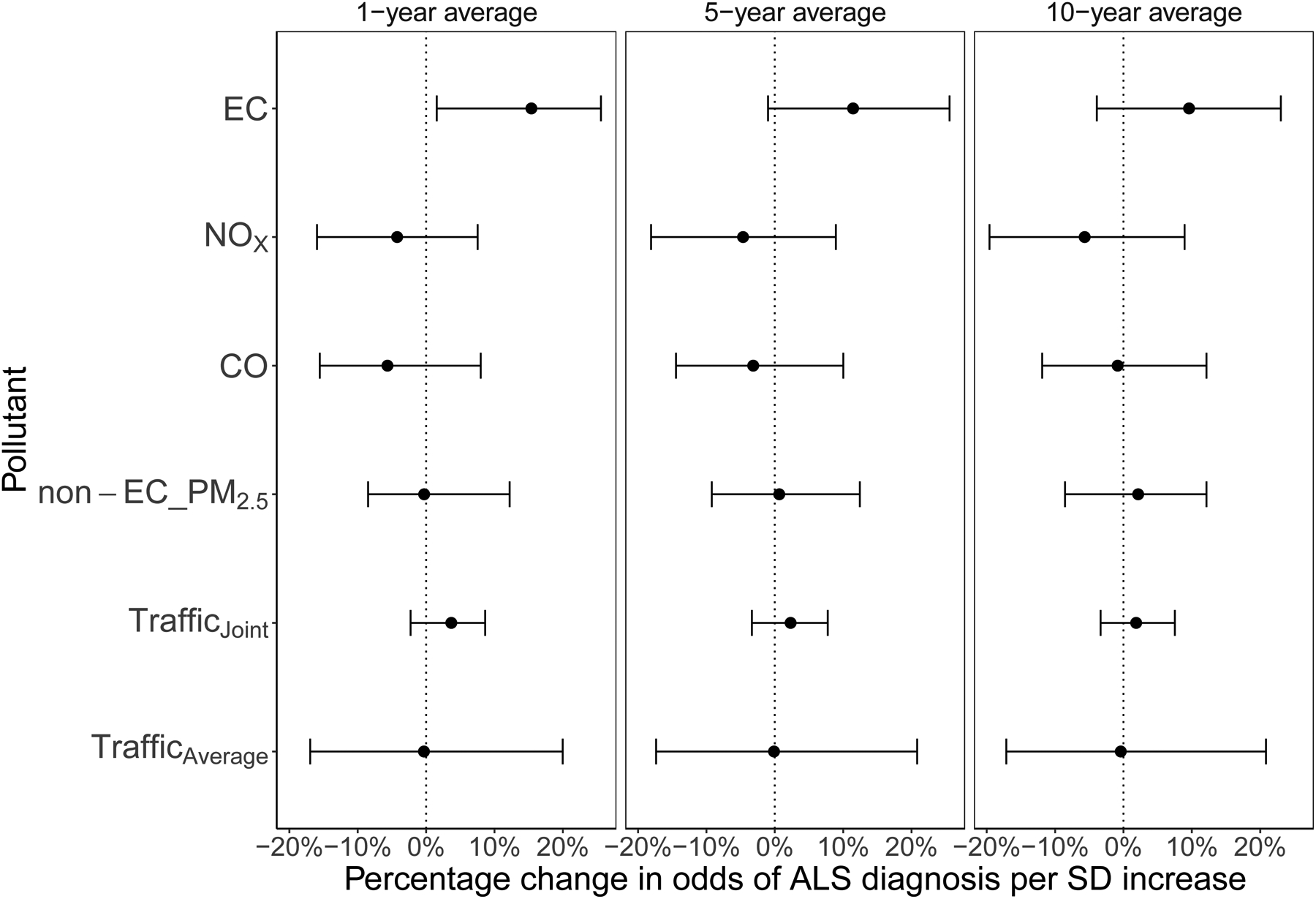
Percentage change in odds of ALS diagnosis per 1-, 5- and 10-year average standard deviation (SD) increase for each pollutant. Results are from the Bayesian hierarchical model including each of elemental carbon (EC), nitrogen oxides (NOx), carbon monoxide (CO), non-elemental carbon fine particles (non-EC PM2.5), and ozone (O3)together, and were additionally adjusted by age, sex, year of birth, vital status, socioeconomic status, civil status, last reported place of residence, and place of birth.
Discussion
In the largest case–control study of ALS and traffic-related air pollution to date (to our knowledge), we found that EC had the largest-in-magnitude independent association with ALS diagnosis, while associations with NOx and CO were negative with credible intervals overlapping the null, and smaller in magnitude. A joint increase in concentrations of traffic-related pollutants had a high probability of being associated with an increase in odds of ALS diagnosis. Sensitivity analyses demonstrated that for single pollutant models, the association for EC was smaller than for our main multi-pollutant model, which took into account the variance–covariance structure of traffic-related pollutants. Overall conclusions for the association between EC and ALS diagnosis were similar from the single- or multi-pollutant models. The inconsistent associations for NOx and CO in the multi- and single-pollutant models and the consistency of the EC association suggest that EC concentrations may have been more relevant than NOx and CO for ALS diagnosis, though further study is required. Our results indicate that traffic-related pollutants, hazardous in many ways,9–21,41–43 may also be associated with ALS diagnosis. Our finding—that increases in EC are potentially positively associated with ALS diagnosis—is plausible. A case–control study in the Netherlands from 2021 reported that ultrafine particles, another traffic emissions-related surrogate, were associated with ALS diagnosis,38 while another based in Catalonia, Spain found ALS cases clustered around key road infrastructure.77 Although we did not find an association with non-EC PM2.5 in our study, our results are not directly comparable to those of the other studies, as our PM2.5 effect estimates capture the PM2.5 components not accounted for by other pollutants in the analysis. A study examining critical windows of exposure of PM2.5 and ALS diagnosis in Denmark found that concentrations 1 to 5 years before exposure may be driving the association with ALS onset,40 consistent with our findings that the most recent 1-year average EC concentration exhibited the largest association.
Our results indicate that EC exposure—a large part of which comes from diesel combustion and small combustion sources (such as wood stoves) in European urban centers, where prevalence of diesel cars is high78—has a high probability of a positive association with ALS diagnosis. In our previous study of ALS and occupational exposures in Denmark we found that those working in agriculture and construction, associated with exposure to diesel engine exhausts, were at higher relative risk than those in other employments.62 Truck drivers, for whom diesel exposure is common, are also at increased risk of sporadic ALS.79 EC exposure has been associated with inflammation,80 mitochondrial dysfunction81 and DNA damage,81,82 all of which are plausible pathways of neurodegeneration. These factors have also previously been identified as particular pathways to pathogenesis of ALS.30–34
We did not find a high probability of a positive association with NOx in our analyses, in contrast with a previous study, though that study did not include EC.38 NOx is also highly correlated with EC (0.94 to 0.96 in our study), which is expected given that they are both combustion products commonly associated with emissions in urban environments. EC exposure was more strongly associated with 1-year than for 5-/10-year average concentrations, which may indicate that the previous year of exposure may be the most relevant exposure window relevant to traffic-related exposures and ALS; this is biologically plausible, as this critical exposure window would be at the pre-symptomatic stage of underlying ALS progression, where traffic-related pollution exposure may add to the ongoing cellular or molecular process of the disease, to the point where the body can no longer compensate and subsequently enters the clinical phase.83–85 We do not expect that these results are attributed to reverse causation, as we have lagged these 1-year exposures by one year already prior to diagnosis, and there was likely little substantial residential movement in the year before ALS diagnosis.86 We do not expect that calendar time was a potential source of confounding, as the controls were matched on age and year of birth. The null joint association, combined with the largest associations from traffic-related pollutant in all models found with EC, further indicates that EC may be driving the association of air pollution with ALS, though further analysis will be necessary to confirm this.
Our study had one of the largest numbers of ALS patients ever included in an environmental health study. Another strength of our study is that we leveraged highly correlated traffic pollutants and Bayesian hierarchical modeling and were able to estimate independent, joint, and average traffic-related pollutant associations. Although we have adjusted implicitly (by matching; age, sex, year of birth, vital status) and explicitly for many common covariates (SES, civil status, residence, place of birth), we cannot rule out residual confounding. Information on individual-level variables, such as body mass index (BMI) and smoking status is not currently available through the Danish Civil Registration System. These variables, while potential risk factors for ALS, are not likely confounders in this analysis as they are not expected to be associated with pollutant concentrations in a manner independent of neighborhood SES. If this information were available, it could be used to further adjust for SES.86 To the extent that the variables we included in our models to adjust for household- and neighborhood-level SES are adequate, we would expect any residual SES-related confounding to be minimal. Exposure measurement error is inevitable, as any modeled exposure will be inaccurate to some degree. However, any error is not likely correlated with ALS diagnosis, and therefore any bias would be towards the null.87 While a previous study found that ALS ascertainment from the Danish National Patient Register was highly reliable,49 outcome misclassification cannot be ruled out, nor can the possibility that date of diagnosis and symptom onset were irregularly aligned. While our analysis adjusted for marital status and household SES, many couples in Denmark cohabitate. This would not be captured by our analysis, and ALS diagnosis in relation to cohabitation status should be further investigated.88
Future research might use larger cohort data to understand the importance of each respective pollutant in a single model. Other mixture model methods, such as Bayesian Kernel Machine Regression89 might be useful in further exploring the robustness of joint associations in a different framework, though this method was not appropriate for our particular research question, since it is currently not available for case–control study applications. The timing of exposure will continue to be an important study route. ALS is projected to increase in prevalence over the next few decades all over the world.4 Understanding ALS pathogenesis and identifying modifiable risk factors is critical for preventive action.
Supplementary Material
Sources of financial support:
Robbie M Parks was supported by the NIEHS K99 ES033742 and the Earth Institute post-doctoral research fellowship at Columbia University. Funding was also provided by the National Institute of Environmental Health Sciences (NIEHS) grants R01 ES030616, R01 ES028805, R01 AG066793, R21 ES028472, P30 ES009089, and P30 ES000002.
Abbreviations:
- ALS
Amyotrophic lateral sclerosis
- BKMR
Bayesian kernel machine regression
- BMI
Body mass index
- CO
Carbon monoxide
- CrI
Credible interval
- DEHM-UBM-AirGIS
Spatio-temporal air pollution modeling system used in study
- EC
Elemental carbon
- ICD
International Classification of Diseases
- IQR
Interquartile range
- IR
Incidence ratio
- Non-EC PM2.5
Non-elemental carbon fine particles
- NOx
Nitrogen oxides
- O3
Ozone
- PM2.5
Fine particles
- SD
Standard deviation
- SES
Socioeconomic status
Footnotes
The authors declare they have no actual or potential competing financial interests.
Description of the process by which someone else could obtain the data and computing code required to replicate the results reported in your submission (or explanation why data or code are not available): Danish patient records are available via the Danish National Patient Register (https://econ.au.dk/the-national-centre-for-register-based-research/danish-registers/the-national-patient-register/browse). Danish population records are available via the Danish Civil Registration System (https://econ.au.dk/the-national-centre-for-register-based-research/danish-registers/the-danish-civil-registration-system-cpr/browse). Exposure data are available via the DEHM-UBM-AirGIS website (https://envs.au.dk/en/research-areas/air-pollution-emissions-and-effects/the-monitoring-program/air-pollution-models/airgis/about-airgis/). All code for analysis, results from analysis, and visualization presented in this manuscript will be publicly available via GitHub at https://github.com/rmp15/multipollutants_and_als_code_review.
References
- 1.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. New England Journal of Medicine. 2001;344(22):1688–1700. [DOI] [PubMed] [Google Scholar]
- 2.Chio A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: A critical review. Amyotrophic Lateral Sclerosis. 2009;10(5–6):310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. The Lancet. 2007;369(9578):2031–2041. [DOI] [PubMed] [Google Scholar]
- 4.Arthur KC, Calvo A, Price TR, Geiger JT, Chio A, Traynor BJ. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nature Communications. 2016;7(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Chalabi A, Hardiman O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nature Reviews Neurology. 2013;9(11):617–628. [DOI] [PubMed] [Google Scholar]
- 6.Hardiman O, Al-Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nature reviews Disease primers. 2017;3(1):1–19. [DOI] [PubMed] [Google Scholar]
- 7.Oskarsson B, Horton DK, Mitsumoto H. Potential environmental factors in amyotrophic lateral sclerosis. Neurologic Clinics. 2015;33(4):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longinetti E, Fang F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Current Opinion In Neurology. 2019;32(5):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett JE, Tamura-Wicks H, Parks RM, et al. Particulate matter air pollution and national and county life expectancy loss in the USA: A spatiotemporal analysis. PLOS Medicine. 2019;16(7):e1002856. doi: 10.1371/journal.pmed.1002856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz J Particulate air pollution and chronic respiratory disease. Environmental Research. 1993;62(1):7–13. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz J The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–326. [DOI] [PubMed] [Google Scholar]
- 13.Brook RD, Rajagopalan S, Pope III CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. [DOI] [PubMed] [Google Scholar]
- 14.Dockery DW, Pope CA, Xu X, et al. An association between air pollution and mortality in six U.S. cities. New England Journal of Medicine. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401 [DOI] [PubMed] [Google Scholar]
- 15.Block ML, Elder A, Auten RL, et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33(5):972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanobetti A, Dominici F, Wang Y, Schwartz JD. A national case-crossover analysis of the short-term effect of PM 2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environmental Health. 2014;13(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritz B, Lee PC, Hansen J, et al. Traffic-related air pollution and Parkinson’s disease in Denmark: A case–control study. Environmental Health Perspectives. 2016;124(3):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, et al. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environmental health perspectives. 2016;124(1):23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levesque S, Surace MJ, McDonald J, Block ML. Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. Journal of Neuroinflammation. 2011;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heusinkveld HJ, Wahle T, Campbell A, et al. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology. 2016;56:94–106. [DOI] [PubMed] [Google Scholar]
- 21.Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro III A, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environmental Health Perspectives. 2011;119(5):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environmental Health Perspectives. 2006;114(7):992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruckerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. American Journal of Respiratory and Critical Care Medicine. 2006;173(4):432–441. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann B, Moebus S, Dragano N, et al. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environmental Health Perspectives. 2009;117(8):1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly FJ. Oxidative stress: Its role in air pollution and adverse health effects. Occupational and Environmental Medicine. 2003;60(8):612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. American journal of respiratory and critical care medicine. 2007;176(4):370–376. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental Health Perspectives. 2003;111(4):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen M, Daneshvar B, Hansen M, et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environmental health perspectives. 2003;111(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Block ML, Calderón-Garcidueñas L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends in neurosciences. 2009;32(9):506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature Reviews Immunology. 2007;7(2):161–167. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron C Oxidative stress: Its role in the pathogenesis of amyotrophic lateral sclerosis. Journal of the neurological sciences. 1995;129:81–84. [DOI] [PubMed] [Google Scholar]
- 32.Mhatre M, Floyd RA, Hensley K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: Common links and potential therapeutic targets. Journal of Alzheimer’s disease. 2004;6(2):147–157. [DOI] [PubMed] [Google Scholar]
- 33.D’Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free radical biology and medicine. 2013;65:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nature Reviews Neurology. 2010;6(4):193–201. [DOI] [PubMed] [Google Scholar]
- 35.Nunez Y, Boehme AK, Weisskopf MG, et al. Fine particle exposure and clinical aggravation in neurodegenerative diseases in New York State. Environmental Health Perspectives. 2021;129(2):027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunez Y, Boehme AK, Li M, et al. Parkinson’s disease aggravation in association with fine particle components in New York State. Environmental Research. 2021;201:111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malek AM, Barchowsky A, Bowser R, et al. Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environmental Pollution. 2015;197:181–186. [DOI] [PubMed] [Google Scholar]
- 38.Yu Z, Peters S, van BL, et al. Long-Term Exposure to Ultrafine Particles and Particulate Matter Constituents and the Risk of Amyotrophic Lateral Sclerosis. Environmental Health Perspectives. 2021;129(9):097702. doi: 10.1289/EHP9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seelen M, Toro CRA, Veldink JH, et al. Long-term air pollution exposure and amyotrophic lateral sclerosis in Netherlands: A population-based case–control study. Environmental Health Perspectives. 2017;125(9):097023. doi: 10.1289/EHP1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunez Y, Balalian A, Parks RM, et al. Exploring relevant time windows in the association between PM2.5 exposure and Amyotrophic Lateral Sclerosis: A case–control study in Denmark. American Journal of Epidemiology. Published online Under Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strak M, Weinmayr G, Rodopoulou S, et al. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: Pooled analysis. BMJ. 2021;374:n1904. doi: 10.1136/bmj.n1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamra GB, Laden F, Cohen AJ, Raaschou-Nielsen O, Brauer M, Loomis D. Lung cancer and exposure to nitrogen dioxide and traffic: A systematic review and meta-analysis. Environmental Health Perspectives. 2015;123(11):1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Kwong JC, Copes R, et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. The Lancet. 2017;389(10070):718–726. [DOI] [PubMed] [Google Scholar]
- 44.Gibson EA, Nunez Y, Abuawad A, et al. An overview of methods to address distinct research questions on environmental mixtures: An application to persistent organic pollutants and leukocyte telomere length. Environmental Health. 2019;18(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank L When an entire country is a cohort. Science. 2000;287(5462):2398–2399. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: A review of content, data quality, and research potential. Clinical epidemiology. 2015;7:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trabjerg BB, Garton FC, van Rheenen W, et al. ALS in Danish registries: heritability and links to psychiatric and cardiovascular disorders. Neurology Genetics. 2020;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathis S, Goizet C, Soulages A, Vallat JM, Le Masson G. Genetics of amyotrophic lateral sclerosis: A review. Journal of the Neurological Sciences. 2019;399:217–226. [DOI] [PubMed] [Google Scholar]
- 49.Kioumourtzoglou MA, Seals RM, Himmerslev L, Gredal O, Hansen J, Weisskopf MG. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2015;16(3–4):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedersen CB. The Danish civil registration system. Scandinavian journal of public health. 2011;39(7_suppl):22–25. [DOI] [PubMed] [Google Scholar]
- 51.Langholz B, Goldstein L. Risk set sampling in epidemiologic cohort studies. Statistical Science. Published online 1996:35–53. [Google Scholar]
- 52.Sillman S The relation between ozone, NOx and hydrocarbons in urban and polluted rural environments. Atmospheric Environment. 1999;33(12):1821–1845. [Google Scholar]
- 53.Khan J, Kakosimos K, Raaschou-Nielsen O, et al. Development and performance evaluation of new AirGIS–a GIS based air pollution and human exposure modelling system. Atmospheric environment. 2019;198:102–121. [Google Scholar]
- 54.Brandt J, Christensen JH, Frohn LM, Palmgren F, Berkowicz R, Zlatev Z. Operational air pollution forecasts from European to local scale. Atmospheric Environment. 2001;35:S91–S98. [Google Scholar]
- 55.Brandt J, Christensen J, Frohn L, Berkowicz R. Air pollution forecasting from regional to urban street scale—-implementation and validation for two cities in Denmark. Physics and Chemistry of the Earth, Parts A/B/C. 2003;28(8):335–344. [Google Scholar]
- 56.Frohn LM, Ketzel M, Christensen JH, et al. Modelling ultrafine particle number concentrations at address resolution in Denmark from 1979–2018–Part 1: Regional and urban scale modelling and evaluation. Atmospheric Environment. 2021;264:118631. [Google Scholar]
- 57.Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, et al. Lung cancer incidence and long-term exposure to air pollution from traffic. Environmental health perspectives. 2011;119(6):860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raaschou-Nielsen O, Sørensen M, Ketzel M, et al. Long-term exposure to traffic-related air pollution and diabetes-associated mortality: A cohort study. Diabetologia. 2013;56(1):36–46. [DOI] [PubMed] [Google Scholar]
- 59.Sørensen M, Hoffmann B, Hvidberg M, et al. Long-term exposure to traffic-related air pollution associated with blood pressure and self-reported hypertension in a Danish cohort. Environmental health perspectives. 2012;120(3):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seinfeld J, Pandis S. Atmospheric chemistry and physics. 1997. New York. Published online 2008. [Google Scholar]
- 61.Galvin M, Gaffney R, Corr B, Mays I, Hardiman O. From first symptoms to diagnosis of amyotrophic lateral sclerosis: Perspectives of an Irish informal caregiver cohort—a thematic analysis. BMJ Open. 2017;7(3). doi: 10.1136/bmjopen-2016-014985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickerson AS, Hansen J, Kioumourtzoglou MA, Specht AJ, Gredal O, Weisskopf MG. Study of occupation and amyotrophic lateral sclerosis in a Danish cohort. Occup Environ Med. 2018;75(9):630–638. doi: 10.1136/oemed-2018-105110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts AL, Johnson NJ, Chen JT, Cudkowicz ME, Weisskopf MG. Race/ethnicity, socioeconomic status, and ALS mortality in the United States. Neurology. 2016;87(22):2300–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bucher BT, Shi J, Pettit RJ, Ferraro J, Chapman WW, Gundlapalli A. Determination of marital status of patients from structured and unstructured electronic healthcare data. In: AMIA Annual Symposium Proceedings. Vol 2019. American Medical Informatics Association; 2019:267. [PMC free article] [PubMed] [Google Scholar]
- 65.Norman RE, Carpenter DO, Scott J, Brune MN, Sly PD. Environmental exposures: an underrecognized contribution to noncommunicable diseases. Reviews on environmental health. 2013;28(1):59–65. [DOI] [PubMed] [Google Scholar]
- 66.Rothman KJ, Greenland S, Lash TL, others. Modern Epidemiology. Vol 3. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia; 2008. [Google Scholar]
- 67.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis, Third Edition. CRC Press; 2013. [Google Scholar]
- 68.Mostofsky E, Schwartz J, Coull BA, et al. Modeling the association between particle constituents of air pollution and health outcomes. American journal of epidemiology. 2012;176(4):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thunis P, Degraeuwe B, Pisoni E, et al. PM2.5 source allocation in European cities: A SHERPA modelling study. Atmospheric Environment. 2018;187:93–106. [Google Scholar]
- 70.Nuvolone D, Petri D, Voller F. The effects of ozone on human health. Environmental Science and Pollution Research. 2018;25(9):8074–8088. [DOI] [PubMed] [Google Scholar]
- 71.Martin R, Peters G, Wilkinson J. Symmetric decomposition of a positive definite matrix. Numerische Mathematik. 1965;7(5):362–383. [Google Scholar]
- 72.Polson NG, Scott JG. On the half-Cauchy prior for a global scale parameter. Bayesian Analysis. 2012;7(4):887–902. [Google Scholar]
- 73.Gelman A Prior distributions for variance parameters in hierarchical models (comment on article by Browne and Draper). Bayesian Anal. 2006;1(3):515–534. doi: 10.1214/06-BA117A [DOI] [Google Scholar]
- 74.Lewandowski D, Kurowicka D, Joe H. Generating random correlation matrices based on vines and extended onion method. Journal of multivariate analysis. 2009;100(9):1989–2001. [Google Scholar]
- 75.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical science. 1992;7(4):457–472. [Google Scholar]
- 76.R Core Team. R: A language and environment for statistical computing. Published online 2013.
- 77.Povedano M, Saez M, Martinez-Matos JA, Barceló MA. Spatial assessment of the association between long-term exposure to environmental factors and the occurrence of amyotrophic lateral sclerosis in Catalonia, Spain: A population-based nested case–control study. Neuroepidemiology. 2018;51(1–2):33–49. [DOI] [PubMed] [Google Scholar]
- 78.von Schneidemesser E, Mar KA, Saar D. Black carbon in Europe: Targeting an air Pollutant and climate forcer. Published online 2017.
- 79.Pamphlett R, Rikard-Bell A. Different occupations associated with amyotrophic lateral sclerosis: Is diesel exhaust the link? PloS One. 2013;8(11):e80993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang R, Dai Y, Zhang X, et al. Reduced pulmonary function and increased pro-inflammatory cytokines in nanoscale carbon black-exposed workers. Part Fibre Toxicol. 2014;11:73. doi: 10.1186/s12989-014-0073-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao X, Xu H, Shang J, et al. Ozonized carbon black induces mitochondrial dysfunction and DNA damage. Environ Toxicol. 2017;32(3):944–955. doi: 10.1002/tox.22295 [DOI] [PubMed] [Google Scholar]
- 82.Kyjovska ZO, Jacobsen NR, Saber AT, et al. DNA damage following pulmonary exposure by instillation to low doses of carbon black (Printex 90) nanoparticles in mice. Environ Mol Mutagen. 2015;56(1):41–49. doi: 10.1002/em.21888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benatar M, Turner MR, Wuu J. Defining pre-symptomatic amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. 2019;20(5–6):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benatar M, Wuu J, McHutchison C, et al. Preventing amyotrophic lateral sclerosis: insights from pre-symptomatic neurodegenerative diseases. Published online 2021. [DOI] [PMC free article] [PubMed]
- 85.Eisen A, Kiernan M, Mitsumoto H, Swash M. Amyotrophic lateral sclerosis: A long preclinical period? Journal of Neurology, Neurosurgery & Psychiatry. 2014;85(11):1232–1238. [DOI] [PubMed] [Google Scholar]
- 86.Weisskopf MG, Webster TF. Trade-offs of personal vs. more proxy exposure measures in environmental epidemiology. Epidemiology (Cambridge, Mass). 2017;28(5):635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement Error in Nonlinear Models: A Modern Perspective. CRC press; 2006. [Google Scholar]
- 88.Frisch M, Simonsen J. Marriage, cohabitation and mortality in Denmark: national cohort study of 6.5 million persons followed for up to three decades (1982–2011). International Journal of Epidemiology. 2013;42(2):559–578. [DOI] [PubMed] [Google Scholar]
- 89.Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


