Abstract
Autism spectrum disorder (ASD) is characterized by impaired social communication and poor adaptation to change; thus, pubertal development may be precarious. Pubertal timing and tempo were measured in 244 youth (7.9% Black, 83.3% White, 8.7% multiracial) with ASD (N=140) and typical development (N=104). Pubertal development was measured using Tanner Staging of Genital (G, males), Breast (B, females) and Pubic Hair (PH) in both sexes at Year 1 (10–13-years), Year 2 (11–14-years), and Year 3 (12–15 years). Nonlinear mixed effects models analysed interindividual differences in timing and tempo. For both sexes, ASD and higher BMI were associated with earlier pubertal timing. Males generally exhibited faster tempo than females. Linear regression models did not show associations between pubertal timing and internalizing symptoms at Time 3. Findings showing advanced pubertal maturation in ASD youth suggest greater risk of psychological, social, and physiological challenges.
Keywords: autism, puberty, adolescence, female, development
Lay Summary
Youth with autism spectrum disorder (ASD) have difficulty in social communication and adaption to change, thus puberty may be a challenging transition. The study examined onset (timing) and progression (tempo) of puberty over three years, using physical exam, in 244 adolescents with and without ASD, enrolled at ages 10–13. ASD youth started puberty earlier, while males generally progressed at a faster pace. Further examination of puberty in ASD should identify impact on social, behavioral, and mental health outcomes.
Introduction
Adolescence formally refers to the developmental transition of juvenile social and cognitive processes to their adult forms and is directly associated with chronological age as well as psychological and social experience (Spear, 2000; Steinberg, 2005). It is a time when peer relationships become more salient and complex (Graber & Brooks-Gunn, 1996). It can be conceptualized across periods; namely, early adolescence (10–14 years), middle adolescence (15–17 years) and late adolescence/young adulthood (17– 24). The progression from childhood to adulthood is formed by experience-dependent brain reorganization (Andersen, 2003), rendering it a time of novel psychological opportunities as well as challenges (Dahl, 2004). Among the challenges is a significant increase in psychiatric disorders (Breslau et al., 2017), as half of people who suffer from mental illness have their onset during adolescence (Kessler et al., 2005).
Puberty: Definition and Measurement
Generally co-occurring during adolescence, puberty refers to biological maturation leading to striking developmental changes in cognitive, emotional, and physiological development (e.g., (Chrousos et al., 1998; Spear, 2000; Steinberg, 2005). The biological changes related to puberty are initiated after a period of childhood quiescence via activation of the hypothalamic-pituitary-gonadal (HPG) axis driven by gonadotropin-releasing hormone stimulating the secretion of luteinizing hormone and follicle-stimulating hormone. Subsequently, interrelated neuroendocrine processes and hormones are initiated: adrenarche (e.g., dehydroepiandrosterone; DHEAS), gonadarche (e.g., estradiol and testosterone), and rapid physical growth (Buck Louis et al., 2008; Dahl, 2004). The surge of gonadotrophins produced in and released from the anterior pituitary, increases estrogen in females (e.g., ovulation, menstruation and breast development) and testosterone in males (e.g., phallic growth, voice changes). The activation of these pubertal hormones facilitates the motivation for social and sexual relationships with peers in both sexes (Forbes & Dahl, 2010; Sisk & Foster, 2004).
Patterns in pubertal stages were first conceptualized and standardized by Marshal and Tanner for girls (Marshall & Tanner, 1969) and boys (Marshall & Tanner, 1970), separately characterizing physical development along two dimensions and five stages described below. Subsequently, Tanner growth stages have become a gold standard to characterize pubertal progression. While menses has often been used as a proxy for puberty, it occurs in the later stages of puberty (Tanner stage 4 or 5; (Marshall & Tanner, 1969) and only moderately correlates with the onset of puberty (Biro et al., 2006). The parameters used to examine physical maturation include timing (onset), stage (sequence), and tempo (pace).
There is significant interindividual variation in maturation that can be impacted by biobehavioral (e.g., body mass index), demographic (e.g., race, ethnicity), and environmental (e.g., social economic status, family composition) factors contributing to early, normative, or delayed onset (Mendle et al., 2019). Rising rates of overweight and obesity in the general population (Hales et al., 2018) and ASD (Corbett, Muscatello, et al., 2020; Whiteley et al., 2004) has been linked to significant physical and mental health conditions (Hill et al., 2015; Phillips et al., 2014; Zuckerman et al., 2014), thus BMI must be carefully considered in research on pubertal onset and progression.
The way in which puberty is assessed (e.g., (Beltz et al., 2014; Corbett et al., 2019; Koopman-Verhoeff et al., 2020; Shirtcliff et al., 2009) or analyzed (e.g., linear vs. nonlinear methods; (Beltz et al., 2014)) can result in significant differences in findings (Biro et al., 2010; Marceau et al., 2011). Pubertal development, like many biological growth trajectories, is not linear and theorized as a sigmoid shape in which there is a definitive start and end point with significant heterogeneity in the growth trajectory (Grimm & Ram, 2009; Marceau et al., 2011). The way in which puberty is measured can influence the precise determination of onset (timing) and trajectory (tempo).
Pubertal Timing
Pubertal timing generally provides a more robust explanation for the physical and psychosocial changes that occur during adolescence than chronological age (Biro et al., 2006). While there is a broad developmental range, age of pubertal onset has been steadily dropping in the United States for females (Herman-Giddens et al., 1997), with mean onset of 9.6 and 10.2 years for Black and White girls, respectively (Biro et al., 2006). The first physical sign of puberty in girls is the development of glandular breast tissue (thelarche) with early reports suggesting a mean onset of 11.15 years (Marshall & Tanner, 1969, 1970). A recent systematic review indicates the pubertal onset for girls in the United States ranges from 8.8 to 10.3 years (Eckert-Lind et al., 2020). Boys mature somewhat later than girls. Early reports indicated a mean onset of genitalia development between 9.5 to 13.5 years (Marshall & Tanner, 1969, 1970). Currently, the mean age for genital development is 10.14, 9.14, and 10.04 years for White, African American, and Hispanic boys, respectively (Herman-Giddens et al., 2012). Earlier sexual maturity in females and males have been demonstrated worldwide (e.g., (Eckert-Lind et al., 2020; Goldstein, 2011). To date, most of the research on pubertal development has focused on timing, yet the progression over the course of puberty is also important.
Pubertal Tempo
Pubertal tempo refers to how rapidly or slowly an individual progresses from the onset of puberty until full sexual maturation. While logical to assume timing and tempo would be associated, a lack of association has been found (Huang et al., 2009) including dissociable sex-based patterns. Marceau (2011) found that timing and tempo were correlated in males, but not in females suggesting different underlying mechanisms. Other studies have shown that earlier timing in females was associated with faster tempo (Biro et al., 2001; Mendle et al., 2010) such that girls with earlier pubertal onset tend to reach menarche faster (Apter & Vihko, 1983; Pantsiotou et al., 2008). It should be noted that girls tend to progress through puberty more quickly (e.g., faster tempo) than boys (e.g., (Beltz et al., 2014)). Early onset in combination with more rapid pubertal maturation (tempo) is predictive of poor psychological outcomes (Marceau et al., 2011).
While less studied than timing, the pace of growth from prepubertal (Tanner Stage 1) to sexual maturity (Tanner Stage 5) has important developmental implications for understanding individual differences (Marshall & Tanner, 1969, 1970). Progression from Tanner Stage 2 to 5 in female breast development ranges from 1.51 to 9 years (mean 4 years), whereas male genital development ranges from 1.86 to 4.72 years (mean 3 years). The precision of pubertal measurement is important for studying factors associated with atypical development.
Psychological Consequences of Deviations in Timing and Tempo
Differences in the timing and tempo of puberty can have significant psychological, social, and physiological consequences. Incongruence between psychological and physical maturation can increase the risk for mental health problems (Graber et al., 1997; Kaltiala-Heino et al., 2003; Waylen & Wolke, 2004). Several negative psychological outcomes have been associated with early pubertal timing in adolescent females when compared to normative or later maturing females (Mendle et al., 2007), including depression (Angold, 2003; Conley & Rudolph, 2009; Ge et al., 2001; Llewellyn et al., 2012; Rierdan & Koff, 1991), suicidality (Graber et al., 1997) and anxiety (Patton et al., 1996). However, puberty has been less well studied in many neurodevelopmental disorders.
Autism Spectrum Disorder1
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by impairments in reciprocal social communication and a repertoire of restricted, repetitive interests and behaviors (APA, 2013). Frequently, a male bias is reported showing a 4:1 male-to-female ratio (Maenner et al., 2020); however, other evidence suggests the ratio may be closer to 2:1 or 3:1 due to under-diagnosis (Kim et al., 2011; Loomes et al., 2017) and a unique female phenotype (Corbett, Vandekar, et al., 2020; Kreiser & White, 2014; Mandy et al., 2012; Uljarevic et al., 2020). The constellation of symptoms in ASD contributes in poor adaptation to change including developmental transitions (Taylor et al., 2017; Taylor & Seltzer, 2010); therefore, adolescence may be particularly challenging (Picci & Scherf, 2015) although research for youth with ASD is mixed. There is evidence of improvement in ASD symptom presentation (Seltzer et al., 2004), social cognition (Anderson et al., 2007; Anderson et al., 2009) and behavior regulation (Anderson et al., 2011; Brown, 1969; Eisenberg, 1956; Gillberg & Schaumann, 1981; Rutter, 1970). However, social withdrawal and psychosocial problems can intensify during adolescence (Anderson et al., 2011; Billstedt et al., 2005; Gillberg & Steffenburg, 1987) and sex-based differences can be magnified. For example, in females with ASD, the onset of menses can present significant difficulties with mood and emotion regulation but is understudied (Burke et al., 2010; Hamilton et al., 2011; Obaydi & Puri, 2008).
Pubertal Measurement, Timing, Menses in ASD
There has been limited research on pubertal timing in ASD and most of it has been primarily focused on females largely because it has been easier to detect physical changes (e.g., breast development, onset of menses); yet, such studies have been lacking in methodological rigor (e.g., retrospective and online reporting, based strictly on menarche). Case reports suggest precocious puberty (onset of secondary sexual characteristics before age 8) occurs in some females with ASD (Mouridsen, 1989; Pohl et al., 2014; Yoshirmura et al., 2005), accompanied by deterioration of functioning following pubertal onset (Ayres & Mailloux, 1983; Gillberg & Schaumann, 1981). However, delayed puberty was reported in a clinically referred sample (Harper & Collins, 1979), and from retrospective self-reports (Herguner & Herguner, 2016; Knickmeyer et al., 2006; Whitehouse et al., 2011). In one study, May and colleagues (2017) found no significant differences in pubertal timing between ASD males and females compared to TD peers based on parent- and self-report measures (May et al., 2017). One study that focused on males with ASD reported precocious puberty in a small, clinically referred sample (Tordjman et al., 1997).
Although many studies examining puberty rely on parent- and self-report measures, which may not be reliable indices of precise pubertal timing (Koopman-Verhoeff et al., 2020). In a comprehensive study of early adolescents with and without ASD and their parents, pubertal level based on self- and parent-report showed only slight to fair (κ=.17-.32) and slight to moderate (κ=.21-.44) concordance, respectively when compared to Tanner staging (Corbett et al., 2019). Using such reports may be adequate for general estimates of classification (Koopman-Verhoeff et al., 2020), yet there is low agreement gold standard measures.
Recent research examining early adolescence reported earlier pubertal development in females with ASD, relative to ASD males and TD females (Corbett, Vandekar, et al., 2020). Specifically, females with ASD had significantly earlier breast development and menses by an average of 9.5 months compared to TD females. While results were compelling, the data were cross-sectional and focused strictly on pubertal timing.
The purpose of the current study was to examine interindividual differences in pubertal timing and tempo (progression) in a large sample of early-to-middle adolescents at three time points, one year apart, based on biological sex (female vs. male) and group (ASD vs. TD). The study had two primary aims and hypotheses. Aim 1 estimated interindividual differences in Male External Genitalia (G), Female Breast (B), Pubic Hair (PH) Tanner stage of timing and tempo. The first hypothesis (Hyp) 1a. was that males and females would demonstrate significant interindividual differences in pubertal timing (based on Tanner staging) (Marceau et al., 2011). Hyp 1b. was that males would exhibit later pubertal development compared to females. Aim 2 examined the relation between diagnosis and biological sex by estimating timing and tempo separately in males and females comparing ASD with TD. Based on previous findings, Hyp. 2 stated females with ASD would demonstrate earlier pubertal onset and faster tempo compared to TD females, whereas males with ASD will show comparable timing and tempo compared to TD males. An exploratory aim examined the extent to which pubertal timing predicted internalizing symptoms between the groups.
Methods
The research was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Vanderbilt Institutional Review Board approved the study. Informed written consent and assent were obtained from all parents and study participants, respectively, prior to inclusion in the study.
Participants
Data were collected as part of a longitudinal study on pubertal development and stress (Corbett, 2017). The current study includes data from the first three assessments: Year 1 (Y1) enrollment when the children were between 10-years-0-months to 13-years-11-months of age, Year 2 (Y2), 11 to 14 years and Year 3 (Y3) when the children were between 12 to 15 years. The diagnostic procedures were completed in Y1; however, the physical exam and psychological measures were completed annually.
In Y1, the sample included 244 total youth, with 239 participants that completed the physical exam described below. The ASD group consisted of 140 participants (median age 11.2) including 36 females and 104 males. The TD group consisted of 104 participants (median age 11.6) including 46 females and 58 males. Of the 239 participants to complete the physical exam, one ASD male was missing measurement for G stage, and one TD female was missing measurement for PH stage, resulting in 238 non-missing measurements for GB and PH stage at Y1.
For Year 1, the racial and ethnic characterization of the sample was comprised of 7.9% Black, 83.3% White, 8.7% multiracial, and less than 1% Asian or Pacific Islander. Participants in the ASD and TD groups were recruited from a broad community sample in the southern United States covering a 200-mile radius that targeted medical and health-related services, clinics, research registries, regional autism/disability organizations, schools, and social media platforms. Inclusion for both groups required an intelligence quotient (IQ) score ≥ 70 due to task demands in the source longitudinal study. Children were excluded if taking medications that alter the Hypothalamic-Pituitary-Adrenal (HPA) axis (e.g., corticosteroids; see (Granger et al., 2009)) or HPG axis (e.g., growth hormone), or medical condition known to impact pubertal development (e.g., Cushing’s Disease). Demographic information for each group is presented in Table 1.
Table 1.
Demographic Statistics
| TD | ASD | Test Statistic | p-value | ||||
|---|---|---|---|---|---|---|---|
| (N=104) | (N=140) | ||||||
| N | Md | IQR | Md | IQR | |||
| Age at Y1 | 244 | 11.6 | (10.6, 12.6) | 11.2 | (10.5, 12.2) | F1,242=2.71 | 0.1013 |
| Percentile BMI Y1 | 239 | 53 | (30, 88) | 69 | (39, 96) | F1,237=6.12 | 0.0143 |
| GB stage at Y1 | 238 | 2 | (1, 3) | 2 | (1, 3) | F1,236=0.04 | 0.8353 |
| PH stage at Y1 | 238 | 2 | (1, 3) | 1 | (1, 3) | F1,236=0.12 | 0.7333 |
| PDS algorithm Y1 | 239 | 2 | (1, 3) | 2 | (1, 3) | F1,237=0.13 | 0.7193 |
| CBCL Affective Y1 | 242 | 52 | (50, 59) | 67 | (60, 73) | F1,240=114.50 | <0.0013 |
| CBCL Anxiety Y1 | 242 | 51 | (50, 59) | 70 | (60, 72) | F1,240=133.25 | <0.0013 |
| Age at Y2 | 174 | 12.7 | (11.8, 13.8) | 12.5 | (11.7, 13.6) | F1,172=1.27 | 0.2613 |
| Percentile BMI Y2 | 129 | 50 | (29, 89) | 85 | (58, 98) | F1,127=15.38 | <0.0013 |
| GB stage at Y2 | 132 | 2 | (2, 4) | 3 | (2, 4) | F1,130=1.33 | 0.2503 |
| PH stage at Y2 | 133 | 2 | (2, 4) | 3 | (2, 4) | F1,131=2.42 | 0.1223 |
| PDS algorithm Y2 | 171 | 3 | (2, 4) | 3 | (2, 4) | F1,169=0.23 | 0.6333 |
| CBCL Affective Y2 | 171 | 51 | (50, 56) | 63 | (55,72) | F1,169=55.14 | <0.0013 |
| CBCL Anxiety Y2 | 171 | 51 | (50, 59) | 66 | (58, 71) | F1,169=74.42 | <0.0013 |
| Age at Y3 | 163 | 13.8 | (12.8, 14.8) | 13.3 | (12.6, 14.4) | F1,161=3.30 | 0.0713 |
| Percentile BMI Y3 | 104 | 51 | (31, 86) | 77 | (42, 95) | F1,102=4.72 | 0.0323 |
| GB stage at Y3 | 103 | 4 | (2, 5) | 4 | (3, 5) | F1,101=0.00 | 0.9843 |
| PH stage at Y3 | 104 | 4 | (3, 5) | 4 | (3, 5) | F1,102=0.00 | 0.9763 |
| PDS algorithm Y3 | 163 | 3 | (3, 4) | 3 | (3, 4) | F1,161=0.13 | 0.7233 |
| CBCL Affective Y3 | 163 | 51 | (50, 63) | 61 | (51, 70) | F1,161=19.24 | <0.0013 |
| CBCL Anxiety Y3 | 163 | 51 | (50, 58) | 62 | (58, 70) | F1,161=46.36 | <0.0013 |
| N | Proportion | Proportion | Test Statistic | ||||
| Sex: Female | 244 | 0.442 46/104 | 0.257 36/140 | X2=9.17 | 0.0022 | ||
| Race | 244 | X2=12.06 | 0.0072 | ||||
| White | 0.856 (89/104) | 0.814 (114/140) | |||||
| Black | 0.019 (2/104) | 0.121 (17/140) | |||||
| Asian/Pacific Islander | 0.000 (0/104) | 0.007 (1/140) | |||||
| Multiracial | 0.125 (13/104) | 0.057 (8/140) | |||||
Note: N is the number of non-missing values.
Kruskal-Wallis.
Pearson.
Wilcoxon.
TD, typical development; ASD, autism spectrum disorder; Md, median; IQR, interquartile range; BMI, body mass index; CBCL, Child Behavior Checklist; GB, genital/breast; PH, pubic hair; PDS, pubertal development scale; Y, Year.
In Y2, 174 participants were retained (ASD = 89, TD = 89). The ASD group had a median age of 12.5 years, and the TD group had a median age of 12.7 years. The overall attrition rate was 28%, which was comparable to other longitudinal studies after the initial enrollment (Negriff et al., 2015). In Y3 there were 163 participants (ASD = 83, TD = 80). The ASD group had a median age of 13.3 years, and the TD group had a median age of 13.8 years. The overall attrition rate was 33% after the initial enrollment. At Y2 and Y3, some participants were unable to complete the full physical examination due to restrictions on in-person lab visits resulting from the COVID-19 pandemic (Y2 N= 43; Y3 N= 59). For comprehensive characterization of attrition across several demographic variables and by sex and diagnosis see Supplementary Tables 1 and 2. Importantly, the significant drop in numbers between Y1 and Y2 for the ASD group was often the result of dropping out after eligibility testing was completed such that families chose to no longer participate in the study despite receiving ASD diagnostic confirmation and documentation.
Diagnostic Procedures
The diagnosis of ASD was based on the Diagnostic and Statistical Manual-5 (APA, 2013) and confirmed by an established diagnosis by a psychologist, psychiatrist, or behavioral pediatrician with autism expertise, current clinical judgment by a study team member, and corroborated by the Autism Diagnostic Observation Schedule (ADOS-2; (Lord et al., 2012). In Y1, all participants with suspected or confirmed autism diagnosis completed a comprehensive assessment at an initial eligibility visit. Following the visit, all participants received a research letter containing the diagnostic results from the measures outlined below.
Autism Diagnostic Observation Schedule-Second Edition
Autism Diagnostic Observation Schedule-Second Edition (ADOS-2; (Lord et al., 2012) is a semi-structured interactive play and interview-based instrument used to support the diagnosis of ASD. The ADOS was administered by research-reliable personnel.
Social Communication Questionnaire
Social Communication Questionnaire (SCQ; (Rutter et al., 2003) is a screening instrument for symptoms of ASD. A score of 15 is suggestive of ASD. Recent research reported lower sensitivity and specificity (Barnard-Brak et al., 2016); therefore, TD children with a score ≥ 10 were excluded.
Wechsler Abbreviated Scale of Intelligence, Second Edition
Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II, (Wechsler, 2011) is a measure of cognitive ability which was used to obtain an estimate of the youth’s intellectual functioning (IQ ≥ 70 required to participate in the study).
After an initial eligibility visit, study participants with confirmed classification as ASD or TD, and meeting inclusion criteria, participated in the study visit at Y1 and annually for Y2 and Y3.
Dependent Measures
To measure pubertal development rigorously and comprehensively, the source study employed three different approaches: a physical exam, a parent-report measure, and self-report based on visual representation of Tanner stages (Marshall & Tanner, 1969, 1970). The current study used physical exam as the primary dependent variable for pubertal development, as recent research demonstrated that physical exam is the optimal approach for accurate pubertal measurement rather than parent- and self-reports (Corbett et al., 2019; Koopman-Verhoeff et al., 2020).
Physical Examination (PE).
The PE was completed at each annual visit to reliably identify pubertal development and assign Tanner stage (Marshall & Tanner, 1969, 1970). The exam ascertained two measures with 5 stages for Male External Genitalia (G1-G5 for males) and Female Breast (B1-B5 for females) (G/B stage) and Pubic hair (P1-P5 for both genders) (PH stage). The exam consisted of visual inspection and categorization of pubertal and genital maturation. To be consistent with the original Tanner staging and to maximize participation, palpation of breasts or measurement of testes was not conducted.
Pubertal assessment consisted of a brief, standardized physical exam conducted by trained, licensed study physicians. A male physician conducted most of the exams, but a female physician provided same-gender exams as requested. The sex of the physician is usually not as important as the participant’s comfort level and the competence of the physician (Dorn et al., 2006). Physicians spent approximately 5-minutes to establish rapport, explain the rationale for the exam and address any questions or concerns, which helped to normalize the experience. During the exam, the adolescent was requested to loosen clothing to fully expose breast and lower genital region, rather than disrobing, which aided in the level of comfort for the participants. A companion (e.g., parent or same-gender research member) was offered to accompany the participant in the exam room.
Physicians were blinded to parental- and self-reports. Inter-rater reliability was established between study physicians on 10 randomly selected participants. Cohen’s Kappa (κ) was calculated between study physicians to assess the degree to which raters were able to identify Tanner stages for G/B and PH markers. Inter-rater reliability for markers ranged from κ= 0.62 to 0.75 (all p <0.001; substantial agreement). Absolute agreement was .75. Kappa was also calculated to assess the extent to which physicians were able to reliably and independently identify when participants had initiated pubertal maturation (Stage 2) for each marker. Kappa ranged from 0.62 to 1.00 (good to very good). In cases of disagreement, physician ratings were never greater than one stage difference.
Pubertal Development Scale
Pubertal Development Scale (PDS; (Petersen et al., 1988) is a widely used parent- or self-report measure of pubertal status which has satisfactory reliability and validity as an alternative to PE. The PDS responses range from 1 (has not begun) to 4 (is complete) examining growth, skin changes, pubic hair and breast across gender, and girls were asked if they have begun menstruating. The PDS has been employed in studies in autism (Corbett et al., 2012; Corbett et al., 2010; Corbett et al., 2013). To convert the 4-point scale to the Tanner 5-scale stages, the Puberty Category Scores were calculated according to previously established criteria (Carskadon & Acebo, 1993; Crockett, 1988; Shirtcliff et al., 2009). While PE was the primary dependent variable, the PDS was collected for comparison.
The Child Behavior Checklist
The Child Behavior Checklist (CBCL; (Achenbach, 2001) is a broad-based parent report form used to provide children’s competencies and behavioral/emotional problems from 6 to 18 years of age. The CBCL Anxiety and Affective (Depression) Domains from Year 3 were used in regression data analysis. However, data from all three years are provided for completeness.
Body Mass Index (BMI):
BMI was calculated using the standard formula (lb./in2) × 703 for use with the CDC growth charts for children and adolescents (2 through 19 years; https://www.cdc.gov/healthyweight/bmi/calculator.html). Percentiles were calculated based on sex and age.
Statistical Approach
The analytic approach used to model puberty can produce varying results especially for tempo (Beltz et al., 2014). To examine pubertal timing and tempo, it was essential to use an analytic approach that considers the interindividual heterogeneity of the child to adolescent transition while measuring when participants enter puberty and how quickly they take to reach subsequent developmental stages. A nonlinear mixed effects model (NLME) was used to estimate individual differences in G/B Tanner stage timing and tempo (Marceau et al., 2011). This model uses a sigmoid curve to estimate subject specific parameters corresponding to timing (the approximate age of Tanner Stage 3).
A nonlinear mixed effects model (NLME) was used to fit Tanner stage (G/B or PH stage) as a function of covariates using a sigmoid model (Marceau et al., 2011).
| (1) |
where stageit is the Tanner stage for subject i at time point t, and dxi denotes diagnosis. The alpha terms describe the association between each covariate and pubertal tempo and control the steepness of the sigmoid curve, whereas the lambda terms describe how each variable is associated with pubertal timing and affect the age at which a child reaches Tanner stage 3. We modeled associations between Tanner stage and diagnosis, BMI, and sex. We also allowed interindividual differences in timing by including the subject random effect λi. We fit the same model using the parent report to compare results to physician staging.
To test Hyp. 1a for interindividual differences in G/B and PH Tanner stage timing the variance component of the random effect term was tested using a parametric bootstrap to simulate the data under the null hypothesis, that there is no subject-specific intercept, and then fit the NLME model to each bootstrap sample. We used the distribution of 1000 bootstrap samples to compute p-values for the random effect variance component.
To test Hyp. 1b for sex differences in pubertal timing, the λ3 parameter was tested and the effect of sex on pubertal tempo was tested using the α3 parameter. Because estimation in NLME models can be challenging, parameter estimates were first by nonlinear least squares, and those values were used as starting values for the NLME model.
To study Hyp. 2, which compared diagnostic differences in timing/tempo within each sex, the model in equation (1) was fit in each sex, separately, without the terms including sex. Within the male and female models, the diagnosis terms were tested to investigate how timing and tempo differ between the diagnostics groups for each sex.
To examine the exploratory aim, linear regression models using the random effects extracted from the non-linear models were used to predict internalizing symptoms from Year 3. These models included age at Year 3, and Year 3 BMI as covariates.
Results
Genital/Breast (G/B) Stage
All Participants:
In order to test interindividual and sex differences in pubertal timing and tempo, separate NLME models were fit with G/B stage and PH stage as the outcome. The test of the random effects term was significant in both models indicating substantial interindividual differences in pubertal timing (both bootstrap p<0.001). The standard deviation of the random effect term was 0.99 years, suggesting substantial variability in the age at which an individual reaches Tanner stage 3. Tests of fixed effects terms indicated that diagnosis and higher BMI were independently associated with earlier pubertal onset for G/B (Diagnosis, t=−2.71, p=0.007; BMI, t=−3.56, p=0.001, Table 2) and PH stage (Table 3) across both sexes. Therefore, Diagnosis and BMI were strong predictors of G/B stage. Specifically, ASD diagnosis is characterized by nearly 5.0 months (0.43 years) earlier G/B stage (Figure 1) and approximately 4.0 months (0.34 years) earlier PH stage. Regarding BMI, an increase in 1 BMI percentile was associated with earlier pubertal onset in G/B stage by approximately 2.96 days and 3.60 days in PH stage. There was no effect of sex on pubertal timing for G/B stage (t=−1.04, p=0.298), but there was evidence for earlier onset for females in PH stage (Table 3).
Table 2.
Fixed Effects Parameter Estimates for GB Stage Model
| Parameter | Estimate | SE | DF | t-value | p-value | |
|---|---|---|---|---|---|---|
| Tempo | ||||||
| Intercept | 1.133 | 0.135 | 222 | 8.38 | <0.0001 | |
| Diagnosis | 0.058 | 0.124 | 222 | 0.47 | 0.642 | |
| BMI | 0.006 | 0.002 | 222 | 3.33 | 0.001 | |
| Sex | −0.411 | 0.124 | 222 | −3.31 | 0.001 | |
| Timing | ||||||
| Intercept | 13.591 | 0.188 | 222 | 72.09 | <0.0001 | |
| Diagnosis | −0.425 | 0.157 | 222 | −2.71 | 0.007 | |
| BMI | −0.008 | 0.002 | 222 | −3.56 | 0.001 | |
| Sex | −0.171 | 0.164 | 222 | −1.04 | 0.298 |
Note: Tempo parameters control the rate of progression through puberty, with positive values being faster. Timing parameters control the age at which an individual reaches stage 3 in years. SE, standard error; DF, degrees of freedom; BMI, body mass index
Table 3.
Fixed Effects Parameter Estimates for the PH Stage Model
| Parameter | Estimate | SE | DF | t-value | p-value | |
|---|---|---|---|---|---|---|
| Tempo | ||||||
| Intercept | 1.867 | 0.166 | 223 | 11.22 | <0.0001 | |
| Diagnosis | 0.080 | 0.123 | 223 | 0.65 | 0.517 | |
| BMI | 0.001 | 0.002 | 223 | 0.64 | 0.523 | |
| Sex | −0.892 | 0.135 | 223 | −6.62 | <0.0001 | |
| Timing | ||||||
| Intercept | 13.702 | 0.172 | 223 | 79.53 | <0.0001 | |
| Diagnosis | −0.335 | 0.145 | 223 | −2.31 | 0.022 | |
| BMI | −0.010 | 0.002 | 223 | −4.71 | <0.001 | |
| Sex | −0.482 | 0.150 | 223 | −3.21 | 0.002 |
Note: Tempo parameters control the rate of progression through puberty, with positive values being faster. Timing parameters control the shift of the curve along the X-axis in years. SE, standard error; DF, degrees of freedom; BMI, body mass index
Figure 1.
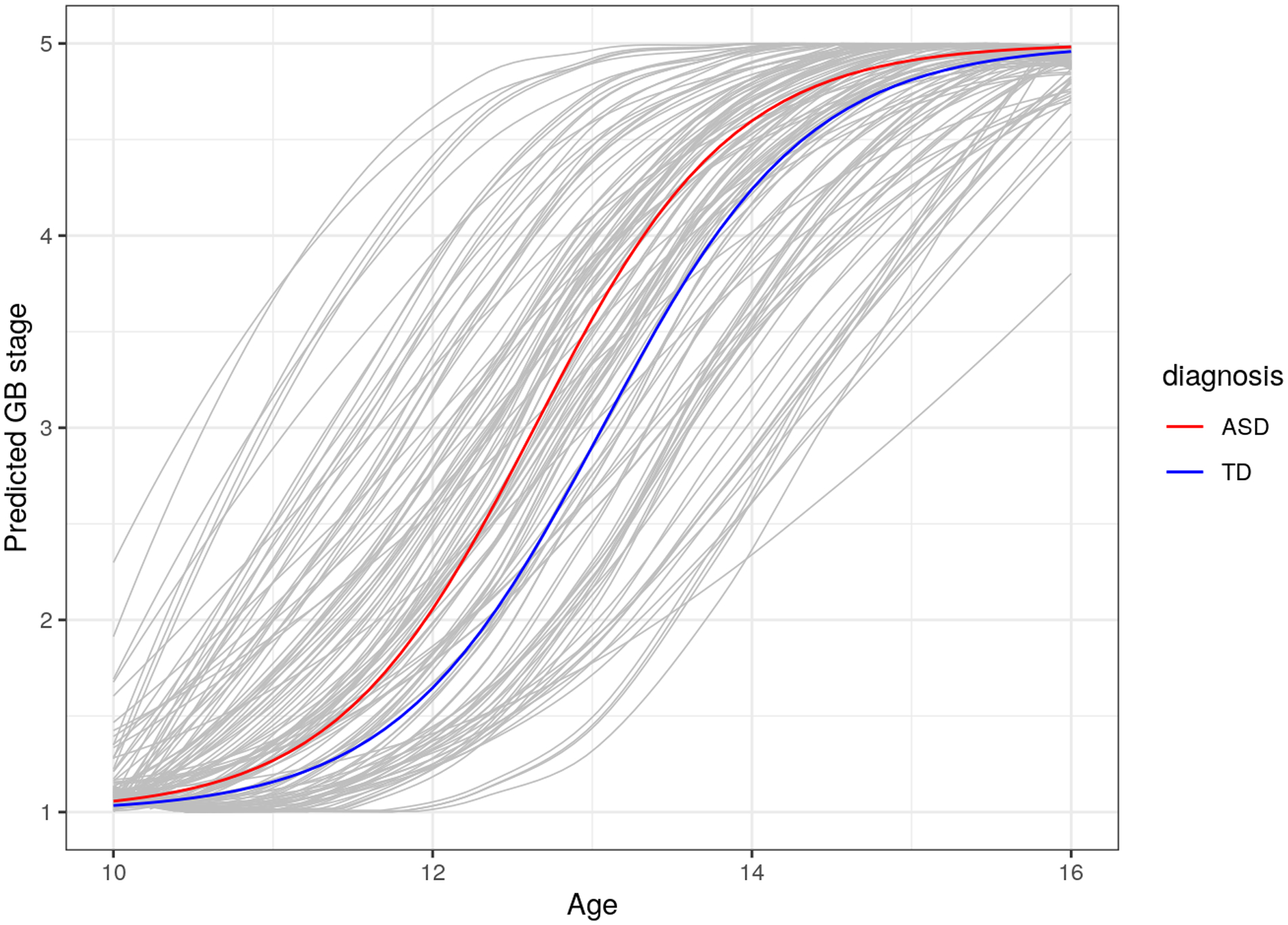
Participants with ASD Show Advanced Pubertal Onset in Genital (G) and Breast (B) Development Compared to TD Youth.
In the total sample, there was evidence for tempo differences in G/B stage associated with BMI (t=3.33, p=0.001), or sex (t=−3.31, p=0.001), but not diagnosis (t=0.47, p=0.64) (Table 2). For PH stage, there was only evidence for an effect of sex (Table 3).
Males:
We fit the G/B and PH stage models separately in males and females to investigate diagnostic differences specific to each sex. Male participants with ASD showed earlier G stage by approximately 3.43 months (Table 4; Figure 2A) and earlier PH stage by 2.88 months but neither was significantly different than TD males (Table 5; Figure 3A). In addition, we found evidence that BMI was associated with earlier timing in G/B stage and PH stage (Table 4 & 5; Supplementary Figures 1 and 2).
Table 4.
Fixed Effects Parameter Estimates for GB Stage Model by Sex
| Parameter | Estimate | SE | DF | t-value | p-value | |
|---|---|---|---|---|---|---|
| Males | ||||||
| Tempo | ||||||
| Intercept | 1.039 | 0.152 | 155 | 6.82 | <0.0001 | |
| Diagnosis | 0.018 | 0.165 | 155 | 0.11 | 0.915 | |
| BMI | 0.009 | 0.003 | 155 | 3.64 | 0.0004 | |
| Timing | ||||||
| Intercept | 13.337 | 0.202 | 155 | 66.03 | <0.0001 | |
| Diagnosis | −0.286 | 0.182 | 155 | −1.57 | 0.119 | |
| BMI | −0.006 | 0.002 | 155 | −2.25 | 0.026 | |
| Females | ||||||
| Tempo | ||||||
| Intercept | 0.856 | 0.217 | 64 | 3.94 | 0.0002 | |
| Diagnosis | 0.049 | 0.195 | 64 | 0.25 | 0.804 | |
| BMI | 0.003 | 0.003 | 64 | 1.07 | 0.287 | |
| Timing | ||||||
| Intercept | 14.035 | 0.360 | 64 | 38.94 | <0.0001 | |
| Diagnosis | −0.693 | 0.290 | 64 | −2.39 | 0.020 | |
| BMI | −0.016 | 0.005 | 64 | −3.32 | 0.002 |
Note: Tempo parameters control the rate of progression through puberty, with positive values being faster. Timing parameters control the shift of the curve along the X-axis in years. SE, standard error; DF, degrees of freedom; BMI, body mass index
Figure 2.
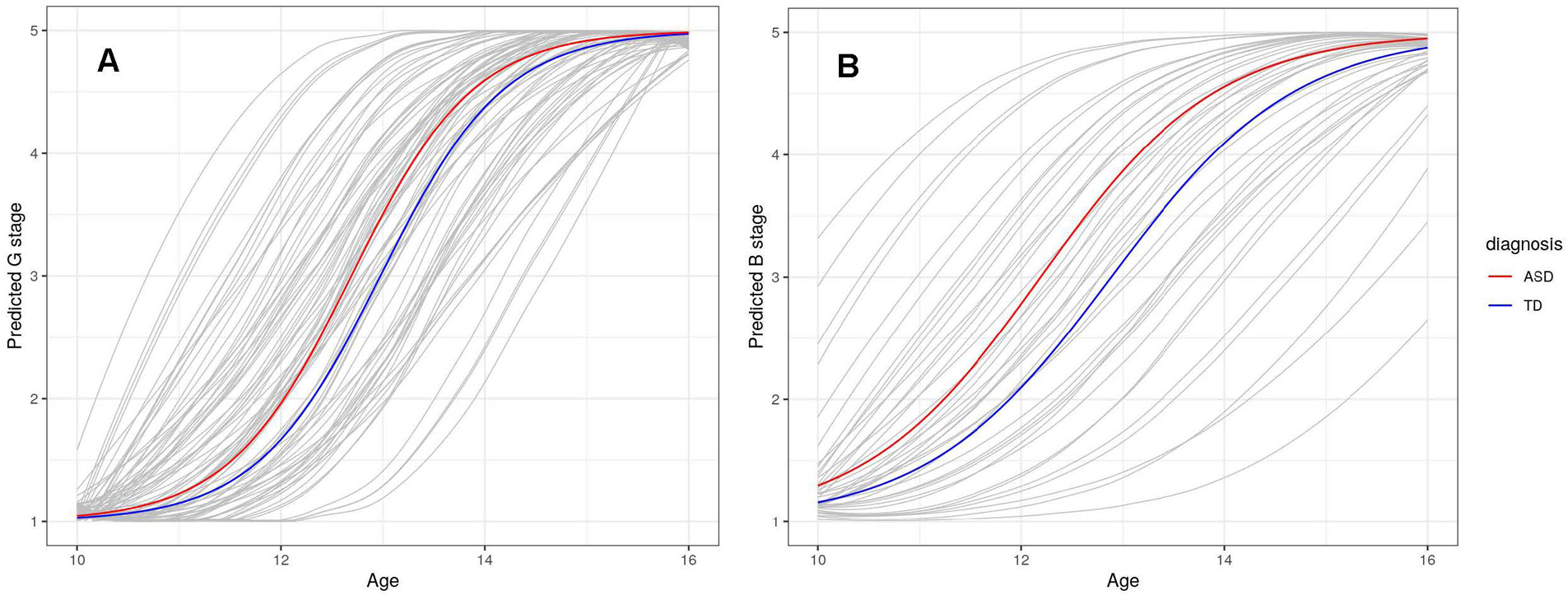
(A) Males with ASD Show Advanced Genital Development Compared to Typically Developing Males. (B) Females with ASD show advanced Breast Development Compared to Typically Developing Females.
Table 5.
Fixed Effects Parameter Estimates for PH Stage Model by Sex
| Parameter | Estimate | SE | DF | t-value | p-value | |
|---|---|---|---|---|---|---|
| Males | ||||||
| Tempo | ||||||
| Intercept | 1.786 | 0.203 | 157 | 8.78 | <0.0001 | |
| Diagnosis | −0.147 | 0.200 | 157 | −0.74 | 0.462 | |
| BMI | 0.004 | 0.003 | 157 | 1.58 | 0.115 | |
| Timing | ||||||
| Intercept | 13.566 | 0.182 | 157 | 74.60 | <0.0001 | |
| Diagnosis | −0.244 | 0.166 | 157 | −1.47 | 0.143 | |
| BMI | −0.008 | 0.002 | 157 | −3.74 | 0.0003 | |
| Females | ||||||
| Tempo | ||||||
| Intercept | 1.002 | 0.220 | 63 | 4.55 | <0.001 | |
| Diagnosis | 0.131 | 0.182 | 63 | 0.72 | 0.473 | |
| BMI | 0.0004 | 0.003 | 63 | 0.15 | 0.880 | |
| Timing | ||||||
| Intercept | 13.461 | 0.325 | 63 | 41.39 | <0.0001 | |
| Diagnosis | −0.476 | 0.280 | 63 | −1.70 | 0.094 | |
| BMI | −0.013 | 0.004 | 63 | −2.88 | 0.005 |
Note: Tempo parameters control the rate of progression through puberty, with positive values being faster. Timing parameters control the shift of the curve along the X-axis in years. SE, standard error; DF, degrees of freedom; BMI, body mass index
Figure 3.
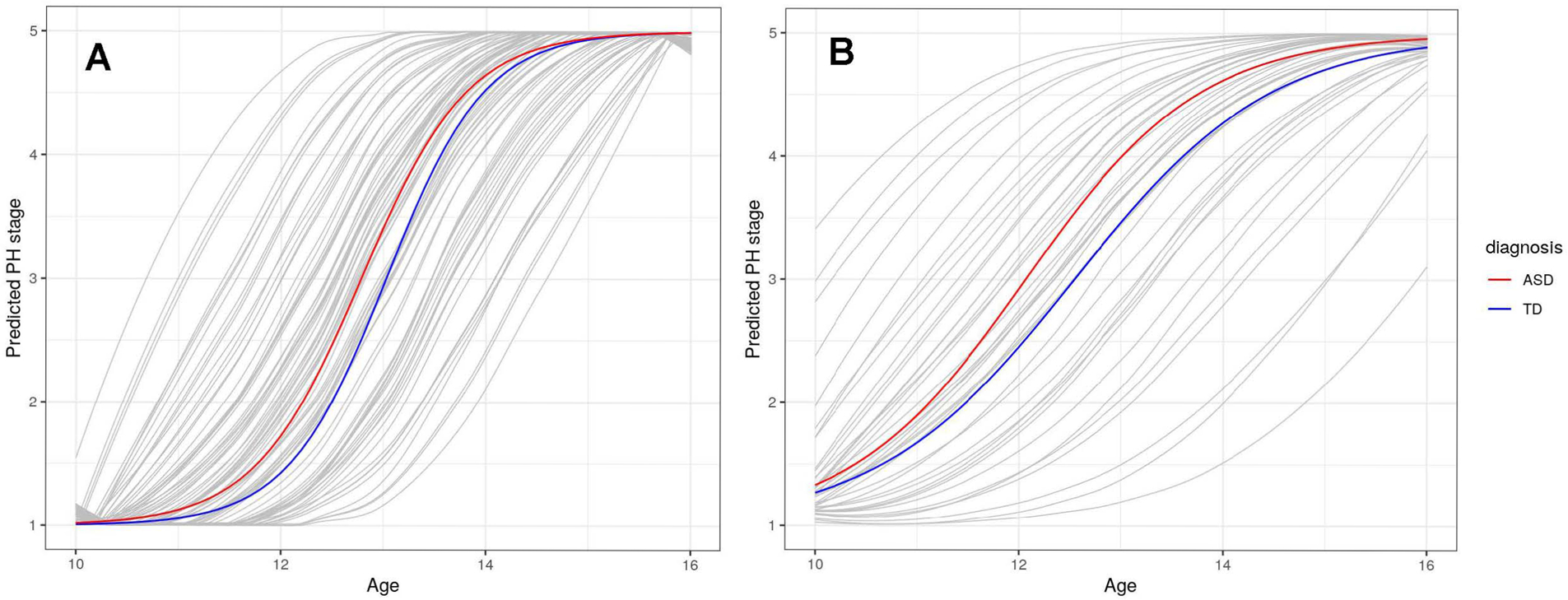
(A) No Diagnostic Differences in Pubic Hair (PH) Development Between Males with ASD or Typically Development. (B) Females with ASD show advanced Pubic Hair (PH) Development Compared to Typically Developing Females
Females:
For the females, participants with ASD showed earlier B stage by approximately 8.28 months (Table 4; Figure 2B) and earlier PH stage by nearly 6.0 months (Table 5; Figure 3B). However, there was little evidence for differences in tempo for B or PH stage. An increase in 1-unit of percentile BMI was associated with earlier GB by 5.8 days and PH stage by roughly 4.6 days (Supplementary Figures 3 and 4).
PDS Algorithm
All Participants:
Results for the PDS model align with the PH stage model, with strong effects for timing in diagnosis, BMI, and sex. As such, ASD is associated with earlier PDS by nearly 6.0 months (0.46 years) (Supplementary Table 3). An increase of one BMI percentile is associated with earlier PDS values by nearly 2.9 days. Female sex is associated with more advanced perceived development on the PDS by nearly 1.75 years.
Pubertal Timing and Internalizing Symptoms
The random effects terms from the nonlinear models were extracted and used as a predictor in exploratory linear models with internalizing symptoms at Year 3 (CBCL Anxiety and Affective domains) as the outcome in order to understand how timing affects internalizing symptoms in males and females separately. Age at Year 1 and Year 1 BMI were included as covariates. Models with random effects for pubertal timing for GB stage and PH stage were evaluated. Parameter estimates for GB and PH models in males and females are provided in Supplementary Tables 4 and 5.
Females:
For females, pubertal timing was not significantly associated with anxiety (p=0.25) or affective problems (p=0.054) at Year 1. Similarly, models with the PH Stage timing random effect were not associated with anxiety (p=0.27) or affective problems (p=0.19). No other terms in these models were significant.
Males:
For the model using the GB stage random effect, in males, only BMI at Year 1 was a significant predictor of anxiety (p=0.016) but not for affective problems (p=0.19). Pubertal timing was not predictive for both anxiety (p=0.16) and affective problems (p=0.70). In males, PH stage pubertal timing was not associated with anxiety (p=0.16) or affective problems (p=0.41). No other terms in this model were significant.
Discussion
The overarching goal of the study was to examine pubertal timing and tempo in a large, well characterized sample of youth with ASD and TD across three time-points as part of a longitudinal study. The aims examined interindividual differences based on sex (males and females), diagnosis (ASD and TD) and the relationship to physical characteristics (BMI). This was accomplished with the use of sophisticated statistical models and rigorous characterization of pubertal development.
Regarding sex-based differences, females entered puberty earlier than males, which is consistent with extensive research and established developmental norms (e.g., (Lee, 1980; Marceau et al., 2011; Susman et al., 2010). While pubertal tempo is less studied and established in the field for typically developing children, prior studies have revealed a faster tempo in males compared to females, which is predicted by BMI. However, in the current study in which we include TD and ASD children, there were no diagnostic differences based on tempo.
Nevertheless, based on the extant literature, the relation between diagnosis and biological sex was examined by estimating timing and tempo separately in males and females with ASD compared to TD males and females. For males, the results showed that males with ASD enter puberty 3.43 months earlier than TD males, though this effect for diagnosis was not statistically significant. In contrast BMI was a strong predictor of genital stage. The result is largely consistent with hypotheses and previous reports showing only females with ASD evidenced earlier puberty based on breast development (Corbett, Vandekar, et al., 2020). It may be the case that due to males in general having later development, the diagnostic distinction is not observed until the later waves of the study when the boys were relatively older. The results are not consistent with one of the few studies exclusively looking at males with ASD, which reported earlier pubertal timing (Tordjman et al., 1997); although they are partially consistent with another study showing no onset differences for males or females with ASD compared to TD youth (May et al., 2017).
Similarly to genital stage, there were no diagnostic differences in pubic hair stage between TD and ASD males, underscoring the physiologically distinct hormonal changes that underlie genital vs. pubic hair development. Overall, while females showed earlier onset compared to males, the males progressed through puberty at an accelerated rate; therefore, the faster tempo may intensify the impact of early timing (Marceau et al., 2011). Males with ASD with atypical physical maturation may be particularly vulnerable due to incongruence between psychosocial and physical development. As predicted in the Maturational Deviance hypothesis, such divergence in normative development in both timing and tempo could contribute to enhanced psychological and emotional distress (Petersen & Taylor, 1980).
For females, the results corroborate previous findings of advanced pubertal onset in females with ASD (Corbett, Vandekar, et al., 2020; Mouridsen, 1989; Pohl et al., 2014; Yoshirmura et al., 2005). Breast development showed a nearly 9-month difference in onset for females on the autism spectrum compared to TD peers. In addition, PH differences emerged by the third year of data collection revealing earlier emergence of pubic hair in adolescent females with ASD by nearly 6 months. While the underlying mechanism of early entry into puberty remains unclear, prior studies have suggested that the age of onset of puberty is driven by genetic factors (Palmert & Hirschhorn, 2003). There are well-described associations of early or precocious puberty in various syndromes (e.g., Cohen syndrome, Williams syndrome, Angelman syndrome; and neurological disorders (e.g., Rett syndrome, epilepsy) (Winter et al., 2019). It is theorized that the complex genetic landscape of these disease processes plays a pivotal role in the determination of pubertal timing and may involve alterations in the finely tuned hypothalamic-pituitary-gonadal axis. For example, in Rett syndrome, a neurodevelopmental disorder that is most commonly caused by a mutation in the Methyl-CpG-binding protein 2 gene (MECP2), earlier onset of puberty has been described (Killian et al., 2014). Data from mouse models suggest that MECP2-null mice have modified gene expression in the hypothalamus (Ben-Shachar et al., 2009) and altered estrogen receptor expression (Westberry et al., 2010). Although studies are needed, differences in the HPG axis form the basis of a possible mechanistic explanation for pubertal differences in children with Rett syndrome. Findings in other neurodevelopmental disorders underscore the importance of thoroughly describing pubertal timing in the ASD population in order to more fully elucidate the role of genetic determinants of pubertal progression in all children.
The tempo or rate of change was similar in females. The disassociation between timing and tempo appears consistent with some studies (Huang et al., 2009; Marceau et al., 2011), but in contrast to others that have found inverse relationships in females (Biro et al., 2001; Mendle et al., 2010; Pantsiotou et al., 2008) or males (Marceau et al., 2011). Collectively, findings underscore the notion that distinct underlying endocrine mechanisms influence timing and tempo development for genital and breast development (e.g., gonadotropin-releasing hormone) and pubic hair (e.g., androgens) and therefore should be studied together, when possible. In general, adrenarche precedes and is independent of gonadarche (Marceau et al., 2015; Palmert et al., 2001).
Several models have been proposed to explain how deviations in pubertal timing and/or tempo can lead to developmental psychopathology. For example, the Maturational Deviance hypothesis proposes that differences in normative development in timing (early, delayed) or tempo (fast, slow) lead to greater psychological distress and behavioral issues (Petersen & Taylor, 1980). On the other hand, the Accentuation hypothesis posits that life transitions accentuate underlying emotional and behavioral predispositions during these periods of heightened novelty and uncertainty (Caspi & Moffitt, 1991). Whether cause or effect, it seems that youth who mature early (especially with faster tempo) are at greater psychological, social and physiological risk due to their lack of preparedness for the increased demands of adolescence (Ge et al., 2002). Such discrepancy may be magnified in ASD, a condition marked by social communication difficulties and poor adaptation to change (APA, 2013). However, in the current study pubertal timing did not predict internalizing symptoms in Year 3. It is possible that we did not have the sample size to identify potential differences. Also, we used a proxy for anxiety and depression symptoms based on parent report, but we did not use clinical interviews or similar approaches to formally identify symptomology or diagnose participants.
Furthermore, pubertal onset and progression across biological sex and diagnostic groups are highly influenced by BMI, which appears stronger in females than males. Yet in relation to predicting internalizing symptoms, BMI was a strong predictor albeit in males only. Recently it was reported that children and adolescents with ASD are more overweight and obese compared to their peers (Corbett et al., 2021), a finding consistent with other previous studies (e.g., (Criado et al., 2018; Curtin et al., 2010; Healy et al., 2019; Zuckerman et al., 2014). This concerning trend is within the context of increasing numbers of youth with weight-related health concerns in the general public, which warrants clinical and research attention to identify the antecedents, predictors, and treatment targets specific to ASD.
In the current study, Tanner stages using the gold standard physical exam served as the primary dependent variable. For comparison, the often-used PDS (parent questionnaire) was assessed for comparison. Analysis of the timing and tempo of puberty according to the PDS revealed that diagnosis and BMI were strong predictors. There was also a significant effect of sex showing that females enter puberty earlier. However, the PDS estimated pubertal entry to be around 1.75 years earlier in females than males, whereas the physical exam was closer to one half year earlier. The difference of an entire year suggests that the PDS may have overestimated pubertal onset in the current sample. Given such findings, the PDS is able to broadly characterize pubertal development; however, it has been shown to be less precise than more robust measures such as physical exam (Corbett et al., 2019; Koopman-Verhoeff et al., 2020), so estimates of differences in timing or tempo may be biased.
To our knowledge, this is the first study examining pubertal tempo in youth with ASD and among only a few rigorously examining tempo in TD participants. The initial findings underscore the importance of considering timing and tempo in adolescents with and without ASD. Insights gained from carefully exploring pubertal changes with regards to interindividual progression offers insights for psychological, social, and physiological development, especially for those experiencing atypical patterns. Youth with ASD are less socially mature than peers and often experience difficulty during the heightened social demands of adolescence; therefore, deviations in pubertal development may intensify such challenges amidst discordance between social and physical development.
Limitations and Future Directions
While there are several strengths of the study (e.g., focus on pubertal timing and tempo, comprehensive examination, well-characterized large sample), there are limitations to acknowledge. There was significant attrition from Y1 to Y3, largely between Y1 and Y2, which is a challenge with longitudinal studies. It is plausible that the inclusion of physical exam may have contributed to participant drops after initial participation. Another limitation is the annual vs. bi-annual or more frequent study visits. Due to the non-linear nature of pubertal progression, a more frequent collection of data may be optimal. The decision to include physical exams as a more precise albeit potentially invasive procedure was balanced with the idea of more frequent visits. As noted by others, there is an ongoing tension in longitudinal research between the rigor of methodology and attrition outcomes (Marceau et al., 2011). Finally, the initial wave of the study occurred when the participants were between 10 to 13 years of age; however, as previously reported (Corbett, Vandekar, et al., 2020) and shown in Figures 2 and 3, many of the females had already entered puberty; therefore, the absolute onset and progression of puberty cannot be determined. Lest children are measured before the onset of puberty and followed until full sexual maturation, children who are more developed will have less growth to acquire thereby limiting the measurement of timing and tempo (Negriff et al., 2015). Finally, an exploratory aim examined the extent to which pubertal timing predicted internalizing symptoms between the groups when the majority of the sample reached early-to-mid puberty (Y3). It is plausible that pubertal onset impacted internalizing symptoms at different developmental stages; nevertheless, comparing timing to internalizing symptoms across all years was beyond the scope of the current study. Future studies are planned to address these aforementioned concerns.
To our knowledge, this is the first study examining pubertal tempo in youth with ASD and among only a few rigorously examining timing. Findings showing advanced and faster pubertal maturation in ASD youth suggest greater risk of psychological, social, and physiological challenges due to their lack of preparedness for the increased demands of adolescence. The findings underscore the importance of considering developmental trajectories in adolescents with and without ASD and the associations with important physical (BMI) profiles. Insights gained from carefully exploring pubertal changes may offer insights for psychological, social, and physiological development especially for those experiencing atypical patterns.
Supplementary Material
Acknowledgment:
This study was funded by the National Institute of Mental Health (MH111599 PI: Corbett) with core support from the National Center for Advancing Translational Sciences (CTSA UL1 TR000445). Funders had no role in the conduct of the research or preparation of the article. We dedicate this study to Lucas Heckers, a dedicated peer research assistant, who made a significant and meaningful impact on youth with autism in this study and many others.
Footnotes
Conflict of Interest: The authors declare no conflict of interests.
At the time of writing this manuscript there is controversy regarding the use of terminology and whether person-first language in which the individual (e.g., adolescent) is referenced before the condition (e.g., autism) or whether identity-first language (e.g., autistic adolescent) should be used. Because such issues have not been resolved, we have opted to take a mixed terminology approach. Similarly, we will use the terms autism, autism spectrum disorder and autistic interchangeably.
References
- Achenbach TM (2001). Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Andersen SL (2003). Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev, 27(1–2), 3–18. http://www.ncbi.nlm.nih.gov/pubmed/12732219 [DOI] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, … Pickles A (2007). Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol, 75(4), 594–604. 10.1037/0022-006X.75.4.594 [DOI] [PubMed] [Google Scholar]
- Anderson DK, Maye MP, & Lord C (2011). Changes in maladaptive behaviors from midchildhood to young adulthood in autism spectrum disorder. Am J Intellect Dev Disabil, 116(5), 381–397. 10.1352/1944-7558-116.5.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Oti RS, Lord C, & Welch K (2009). Patterns of growth in adaptive social abilities among children with autism spectrum disorders. J Abnorm Child Psychol, 37(7), 1019–1034. 10.1007/s10802-009-9326-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A (2003). Adolescent depression, cortisol and DHEA. Psychol Med, 33(4), 573–581. 10.1017/s003329170300775x [DOI] [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders, Fifth Edition (DSM-5). American Psychiatric Association. [Google Scholar]
- Apter D, & Vihko R (1983). Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab, 57(1), 82–86. 10.1210/jcem-57-1-82 [DOI] [PubMed] [Google Scholar]
- Ayres AJ, & Mailloux ZK (1983). Possible pubertal effect on therapeutic gains in an autistic girl. Am J Occup Ther, 37(8), 535–540. 10.5014/ajot.37.8.535 [DOI] [PubMed] [Google Scholar]
- Barnard-Brak L, Brewer A, Chesnut S, Richman D, & Schaeffer AM (2016). The sensitivity and specificity of the social communication questionnaire for autism spectrum with respect to age. Autism Res, 9(8), 838–845. 10.1002/aur.1584 [DOI] [PubMed] [Google Scholar]
- Beltz AM, Corley RP, Bricker JB, Wadsworth SJ, & Berenbaum SA (2014). Modeling pubertal timing and tempo and examining links to behavior problems. Dev Psychol, 50(12), 2715–2726. 10.1037/a0038096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, & Zoghbi HY (2009). Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet, 18(13), 2431–2442. 10.1093/hmg/ddp181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstedt E, Gillberg IC, & Gillberg C (2005). Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord, 35(3), 351–360. https://www.ncbi.nlm.nih.gov/pubmed/16119476 [DOI] [PubMed] [Google Scholar]
- Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, … Wolff MS (2010). Pubertal Assessment Method and Baseline Characteristics in a Mixed Longitudinal Study of Girls. Pediatrics, 126(3), E583–E590. 10.1542/peds.2009-3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM, Huang B, Crawford PB, Lucky AW, Striegel-Moore R, Barton BA, & Daniels S (2006). Pubertal correlates in black and white girls. J Pediatr, 148(2), 234–240. 10.1016/j.jpeds.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Biro FM, McMahon RP, Striegel-Moore R, Crawford PB, Obarzanek E, Morrison JA, … Falkner F (2001). Impact of timing of pubertal maturation on growth in black and white female adolescents: The National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr, 138(5), 636–643. 10.1067/mpd.2001.114476 [DOI] [PubMed] [Google Scholar]
- Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, & Miller E (2017). Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry, 7(5), e1139. 10.1038/tp.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL (1969). Adolescent Development of Children with Infantile Psychosis. Seminars in Psychiatry, 1(1), 79-&. [Google Scholar]
- Buck Louis GM, Gray LE Jr., Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, … Euling SY (2008). Environmental factors and puberty timing: expert panel research needs. Pediatrics, 121 Suppl 3, S192–207. 10.1542/peds.1813E [DOI] [PubMed] [Google Scholar]
- Burke LM, Kalpakian CZ, Smith YR, & Quint EH (2010). Gynecologic issues of adolescents with Down syndrome, autism, and cerebral Palsy. Journal of Pediatric Adolescent Gynecology, 23(1), 11–15. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, & Acebo C (1993). A Self-Administered Rating-Scale for Pubertal Development. Journal of Adolescent Health, 14(3), 190–195. https://doi.org/Doi 10.1016/1054-139x(93)90004-9 [DOI] [PubMed] [Google Scholar]
- Caspi A, & Moffitt TE (1991). Individual differences are accentuated during periods of social change: the sample case of girls at puberty. J Pers Soc Psychol, 61(1), 157–168. 10.1037//0022-3514.61.1.157 [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, & Gold PW (1998). Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med, 129(3), 229–240. http://www.ncbi.nlm.nih.gov/pubmed/9696732 [DOI] [PubMed] [Google Scholar]
- Conley CS, & Rudolph KD (2009). The emerging sex difference in adolescent depression: interacting contributions of puberty and peer stress. Dev Psychopathol, 21(2), 593–620. 10.1017/S0954579409000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA (2017). Examining stress and arousal across pubertal development in ASD [Grant]. [Google Scholar]
- Corbett BA, Muscatello RA, Horrocks BK, Klemencic ME, & Tanguturi Y (2020). Differences in Body Mass Index (BMI) in Early Adolescents with Autism Spectrum Disorder Compared to Youth with Typical Development. J Autism Dev Disord. 10.1007/s10803-020-04749-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Muscatello RA, Horrocks BK, Klemencic ME, & Tanguturi Y (2021). Differences in Body Mass Index (BMI) in Early Adolescents with Autism Spectrum Disorder Compared to Youth with Typical Development. J Autism Dev Disord, 51(8), 2790–2799. 10.1007/s10803-020-04749-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Muscatello RA, Tanguturi Y, McGinn E, & Ioannou S (2019). Pubertal Development Measurement in Children With and Without Autism Spectrum Disorder: A Comparison Between Physical Exam, Parent- and Self-Report. J Autism Dev Disord. 10.1007/s10803-019-04192-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, & Lanni KE (2012). Comparing biobehavioral profiles across two social stress paradigms in children with and without autism spectrum disorders. Mol Autism, 3(1), 13. 10.1186/2040-2392-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Simon D, Ryan N, & Mendoza S (2010). Elevated cortisol during play is associated with age and social engagement in children with autism. Mol Autism, 1(1), 13. 10.1186/2040-2392-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Swain DM, Newsom C, Wang L, Song Y, & Edgerton D (2013). Biobehavioral profiles of arousal and social motivation in autism spectrum disorders. J Child Psychol Psychiatry. 10.1111/jcpp.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Vandekar S, Muscatello RA, & Tanguturi Y (2020). Pubertal Timing During Early Adolescence: Advanced Pubertal Onset in Females with Autism Spectrum Disorder. Autism Res. 10.1002/aur.2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado KK, Sharp WG, McCracken CE, De Vinck-Baroody O, Dong L, Aman MG, … Scahill L (2018). Overweight and obese status in children with autism spectrum disorder and disruptive behavior. Autism, 22(4), 450–459. 10.1177/1362361316683888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett LJ (1988). Pubertal Development Scale: Pubertal categories. [Google Scholar]
- Curtin C, Anderson SE, Must A, & Bandini L (2010). The prevalence of obesity in children with autism: a secondary data analysis using nationally representative data from the National Survey of Children’s Health. BMC Pediatr, 10, 11. 10.1186/1471-2431-10-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE (2004). Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci, 1021, 1–22. 10.1196/annals.1308.001 [DOI] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, & Biro F (2006). Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science, 10(1), 30–56. [Google Scholar]
- Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Brauner EV, & Juul A (2020). Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr, e195881. 10.1001/jamapediatrics.2019.5881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg L (1956). The Autistic-Child in Adolescence. American Journal of Psychiatry, 112(8), 607–612. https://doi.org/DOI 10.1176/ajp.112.8.607 [DOI] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2010). Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cognition, 71(1), 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Brody GH, Conger RD, Simons RL, & Murry VM (2002). Contextual amplification of pubertal transition effects on deviant peer affiliation and externalizing behavior among African American children. Dev Psychol, 38(1), 42–54. 10.1037//0012-1649.38.1.42 [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, & Elder GH Jr. (2001). Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol, 37(3), 404–417. https://www.ncbi.nlm.nih.gov/pubmed/11370915 [DOI] [PubMed] [Google Scholar]
- Gillberg C, & Schaumann H (1981). Infantile autism and puberty. J Autism Dev Disord, 11(4), 365–371. http://www.ncbi.nlm.nih.gov/pubmed/7052813 [DOI] [PubMed] [Google Scholar]
- Gillberg C, & Steffenburg S (1987). Outcome and prognostic factors in infantile autism and similar conditions: a population-based study of 46 cases followed through puberty. Journal of Autism and Developmental Disorders, 2, 273–287. [DOI] [PubMed] [Google Scholar]
- Goldstein JR (2011). A secular trend toward earlier male sexual maturity: evidence from shifting ages of male young adult mortality. PLoS One, 6(8), e14826. 10.1371/journal.pone.0014826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA, & Brooks-Gunn J (1996). Expectations for and precursors to leaving home in young women. New Dir Child Dev(71), 21–38. http://www.ncbi.nlm.nih.gov/pubmed/8684662 [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, & Brooks-Gunn J (1997). Is psychopathology associated with the timing of pubertal development? J Am Acad Child Adolesc Psychiatry, 36(12), 1768–1776. 10.1097/00004583-199712000-00026 [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, & Kapelewski CH (2009). Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34(10), 1437–1448. 10.1016/j.psyneuen.2009.06.017 [DOI] [PubMed] [Google Scholar]
- Grimm KJ, & Ram N (2009). Nonlinear Growth Models in Mplus and SAS. Structural Equation Modeling-a Multidisciplinary Journal, 16(4), 676–701. 10.1080/10705510903206055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, & Ogden CL (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. NCHS Data Brief, 67(6), 1–28. https://www.cdc.gov/mmwr/volumes/67/ss/ss6706a1.htm?s_cid=ss6706a1_w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A, Marshal MP, & Murray PJ (2011). Autism spectrum disorders and menstruation. J Adolesc Health, 49(4), 443–445. 10.1016/j.jadohealth.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Harper JF, & Collins JK (1979). Physical growth and development in a sample of autistic girls from New South Wales. Aust Paediatr J, 15(2), 110–112. https://www.ncbi.nlm.nih.gov/pubmed/485990 [DOI] [PubMed] [Google Scholar]
- Healy S, Aigner CJ, & Haegele JA (2019). Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism, 23(4), 1046–1050. 10.1177/1362361318791817 [DOI] [PubMed] [Google Scholar]
- Herguner A, & Herguner S (2016). Association Between Age at Menarche and Autistic Traits in Turkish University Students. American Journal of Human Biology, 28(1), 44–47. 10.1002/ajhb.22739 [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, & Hasemeier CM (1997). Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics, 99(4), 505–512. 10.1542/peds.99.4.505 [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, … Reiter EO (2012). Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics, 130(5), e1058–1068. 10.1542/peds.2011-3291 [DOI] [PubMed] [Google Scholar]
- Hill AP, Zuckerman KE, & Fombonne E (2015). Obesity and Autism. Pediatrics, 136(6), 1051–1061. 10.1542/peds.2015-1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Biro FM, & Dorn LD (2009). Determination of relative timing of pubertal maturation through ordinal logistic modeling: evaluation of growth and timing parameters. J Adolesc Health, 45(4), 383–388. 10.1016/j.jadohealth.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Marttunen M, Rantanen P, & Rimpela M (2003). Early puberty is associated with mental health problems in middle adolescence. Soc Sci Med, 57(6), 1055–1064. https://www.ncbi.nlm.nih.gov/pubmed/12878105 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62(6), 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Killian JT, Lane JB, Cutter GR, Skinner SA, Kaufmann WE, Tarquinio DC, … Percy AK (2014). Pubertal development in Rett syndrome deviates from typical females. Pediatr Neurol, 51(6), 769–775. 10.1016/j.pediatrneurol.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, … Grinker RR (2011). Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry, 168(9), 904–912. 10.1176/appi.ajp.2011.10101532 [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Wheelwright S, Hoekstra R, & Baron-Cohen S (2006). Age of menarche in females with autism spectrum conditions. Dev Med Child Neurol, 48(12), 1007–1008. 10.1017/S0012162206222229 [DOI] [PubMed] [Google Scholar]
- Koopman-Verhoeff ME, Gredvig-Ardito C, Barker DH, Saletin JM, & Carskadon MA (2020). Classifying Pubertal Development Using Child and Parent Report: Comparing the Pubertal Development Scales to Tanner Staging. J Adolesc Health, 66(5), 597–602. 10.1016/j.jadohealth.2019.11.308 [DOI] [PubMed] [Google Scholar]
- Kreiser NL, & White SW (2014). ASD in Females: Are We Overstating the Gender Difference in Diagnosis? Clinical child and family psychology review, 17(1), 67–84. 10.1007/s10567-013-0148-9 [DOI] [PubMed] [Google Scholar]
- Lee PA (1980). Normal ages of pubertal events among American males and females. J Adolesc Health Care, 1(1), 26–29. https://www.ncbi.nlm.nih.gov/pubmed/6458588 [DOI] [PubMed] [Google Scholar]
- Llewellyn N, Rudolph KD, & Roisman GI (2012). Other-Sex Relationship Stress and Sex Differences in the Contribution of Puberty to Depression. J Early Adolesc, 32(6), 824–850. 10.1177/0272431611429945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry, 56(6), 466–474. 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule (ADOS-2) (2nd ed.). Western Psychological Services. [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, EdS, Washington A, Patrick M, … Dietz PM (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ, 69(4), 1–12. 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, & Skuse D (2012). Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord, 42(7), 1304–1313. 10.1007/s10803-011-1356-0 [DOI] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, & Susman EJ (2011). Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models. Dev Psychol, 47(5), 1389–1409. 10.1037/a0023838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, & Susman EJ (2015). Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Dev Psychobiol, 57(6), 742–768. 10.1002/dev.21214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM (1969). Variations in pattern of puberal development in girls. Archives of Disease in Childhood, 44(235), 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45(239), 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T, Pang KC, O’Connell MA, & Williams K (2017). Typical Pubertal Timing in an Australian Population of Girls and Boys with Autism Spectrum Disorder. J Autism Dev Disord, 47(12), 3983–3993. 10.1007/s10803-017-3281-3 [DOI] [PubMed] [Google Scholar]
- Mendle J, Beltz AM, Carter R, & Dorn LD (2019). Understanding Puberty and Its Measurement: Ideas for Research in a New Generation. J Res Adolesc, 29(1), 82–95. 10.1111/jora.12371 [DOI] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, & Graber JA (2010). Development’s tortoise and hare: pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Dev Psychol, 46(5), 1341–1353. 10.1037/a0020205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, & Emery RE (2007). Detrimental Psychological Outcomes Associated with Early Pubertal Timing in Adolescent Girls. Dev Rev, 27(2), 151–171. 10.1016/j.dr.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouridsen SE (1989). Pervasive developmental disorder and idiopathic precocious puberty in a 5-year-old girl. J Autism Dev Disord, 19(2), 351–353. https://www.ncbi.nlm.nih.gov/pubmed/2745399 [DOI] [PubMed] [Google Scholar]
- Negriff S, Blankson AN, & Trickett PK (2015). Pubertal Timing and Tempo: Associations With Childhood Maltreatment. J Res Adolesc, 25(2), 201–213. 10.1111/jora.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaydi H, & Puri BK (2008). Prevalence of premenstrual syndrome in autism: a prospective observer-rated study. J Int Med Res, 36(2), 268–272. 10.1177/147323000803600208 [DOI] [PubMed] [Google Scholar]
- Palmert MR, Hayden DL, Mansfield MJ, Crigler JF Jr., Crowley WF Jr., Chandler DW, & Boepple PA (2001). The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab, 86(9), 4536–4542. 10.1210/jcem.86.9.7863 [DOI] [PubMed] [Google Scholar]
- Palmert MR, & Hirschhorn JN (2003). Genetic approaches to stature, pubertal timing, and other complex traits. Mol Genet Metab, 80(1–2), 1–10. 10.1016/s1096-7192(03)00107-0 [DOI] [PubMed] [Google Scholar]
- Pantsiotou S, Papadimitriou A, Douros K, Priftis K, Nicolaidou P, & Fretzayas A (2008). Maturational tempo differences in relation to the timing of the onset of puberty in girls. Acta Paediatr, 97(2), 217–220. 10.1111/j.1651-2227.2007.00598.x [DOI] [PubMed] [Google Scholar]
- Patton GC, Hibbert ME, Carlin J, Shao Q, Rosier M, Caust J, & Bowes G (1996). Menarche and the onset of depression and anxiety in Victoria, Australia. Journal of Epidemiology and Community Health, 50(6), 661–666. https://doi.org/DOI 10.1136/jech.50.6.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: reliability, validity and initial norms. Journal of Youth and Adolescence, 17(2), 117–131. [DOI] [PubMed] [Google Scholar]
- Petersen AC, & Taylor B (1980). The biological approach to adolescence: Biological change and psychological adaptation. . In Edelson J (Ed.), Handbook of adolescent psychology. Wiley. [Google Scholar]
- Phillips KL, Schieve LA, Visser S, Boulet S, Sharma AJ, Kogan MD, … Yeargin-Allsopp M (2014). Prevalence and impact of unhealthy weight in a national sample of US adolescents with autism and other learning and behavioral disabilities. Matern Child Health J, 18(8), 1964–1975. 10.1007/s10995-014-1442-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picci G, & Scherf KS (2015). A Two-Hit Model of Autism: Adolescence as the Second Hit. Clin Psychol Sci, 3(3), 349–371. 10.1177/2167702614540646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl A, Cassidy S, Auyeung B, & Baron-Cohen S (2014). Uncovering steroidopathy in women with autism: a latent class analysis. Molecular Autism, 5. https://doi.org/Artn 27 10.1186/2040-2392-5-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rierdan J, & Koff E (1991). Depressive symptomatology among very early maturing girls. J Youth Adolesc, 20(4), 415–425. 10.1007/BF01537183 [DOI] [PubMed] [Google Scholar]
- Rutter M (1970). Autistic Children - Infancy to Adulthood. Seminars in Psychiatry, 2(4), 435-&. <Go to ISI>://WOS:A1970H855700006 [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The Social Communication Questionnaire. Western Psychological Services. [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, & Greenberg JS (2004). Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev, 10(4), 234–247. 10.1002/mrdd.20038 [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, & Pollak SD (2009). Pubertal development: correspondence between hormonal and physical development. Child Dev, 80(2), 327–337. 10.1111/j.1467-8624.2009.01263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, & Foster DL (2004). The neural basis of puberty and adolescence. Nat Neurosci, 7(10), 1040–1047. 10.1038/nn1326 [DOI] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev, 24(4), 417–463. https://doi.org/S0149-7634(00)00014-2 [pii] [DOI] [PubMed] [Google Scholar]
- Steinberg L (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9(2), 69–74. 10.1016/j.tics.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, & Dorn LD (2010). Cortisol and alpha amylase reactivity and timing of puberty: vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology, 35(4), 557–569. 10.1016/j.psyneuen.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Adams RE, & Bishop SL (2017). Social participation and its relation to internalizing symptoms among youth with autism spectrum disorder as they transition from high school. Autism Research, 10(4), 663–672. 10.1002/aur.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, & Seltzer MM (2010). Changes in the autism behavioral phenotype during the transition to adulthood. J Autism Dev Disord, 40(12), 1431–1446. 10.1007/s10803-010-1005-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Ferrari P, Sulmont V, Duyme M, & Roubertoux P (1997). Androgenic activity in autism. Am J Psychiatry, 154(11), 1626–1627. https://www.ncbi.nlm.nih.gov/pubmed/9356582 [DOI] [PubMed] [Google Scholar]
- Uljarevic M, Cooper MN, Bebbington K, Glasson EJ, Maybery MT, Varcin K, … Whitehouse AJO (2020). Deconstructing the repetitive behaviour phenotype in autism spectrum disorder through a large population-based analysis. J Child Psychol Psychiatry. 10.1111/jcpp.13203 [DOI] [PubMed] [Google Scholar]
- Waylen A, & Wolke D (2004). Sex ‘n’ drugs ‘n’ rock ‘n’ roll: the meaning and social consequences of pubertal timing. Eur J Endocrinol, 151 Suppl 3, U151–159. https://www.ncbi.nlm.nih.gov/pubmed/15554900 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence II (Vol. Second Edition). PsychCorp. [Google Scholar]
- Westberry JM, Trout AL, & Wilson ME (2010). Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology, 151(2), 731–740. 10.1210/en.2009-0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Maybery MT, Hickey M, & Sloboda DM (2011). Brief report: autistic-like traits in childhood predict later age at menarche in girls. J Autism Dev Disord, 41(8), 1125–1130. 10.1007/s10803-010-1129-1 [DOI] [PubMed] [Google Scholar]
- Whiteley P, Dodou K, Todd L, & Shattock P (2004). Body mass index of children from the United Kingdom diagnosed with pervasive developmental disorders. Pediatr Int, 46(5), 531–533. 10.1111/j.1442-200x.2004.01946.x [DOI] [PubMed] [Google Scholar]
- Winter S, Durand A, & Brauner R (2019). Precocious and Early Central Puberty in Children With Pre-existing Medical Conditions: A Single Center Study. Front Pediatr, 7, 35. 10.3389/fped.2019.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshirmura K, Naiki Y, Horikawa R, & Tanaka T (2005). Three patients with autism and central precocious puberty. Clinical Pediatrics Endocrinology, 14(Suppl 24), 55–57. [Google Scholar]
- Zuckerman KE, Hill AP, Guion K, Voltolina L, & Fombonne E (2014). Overweight and obesity: prevalence and correlates in a large clinical sample of children with autism spectrum disorder. J Autism Dev Disord, 44(7), 1708–1719. 10.1007/s10803-014-2050-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


