Abstract
Background:
Despite curative hepatectomy, most colorectal liver metastasis (CRLM) patients relapse locally within two years. Genomic predictors for hepatic recurrence are poorly understood. This study aims to identify genomic signatures for recurrence in resected CRLM patients treated with adjuvant hepatic artery infusion (HAI) and/or systemic (SYS) chemotherapy.
Methods:
Patients undergoing curative hepatectomy and adjuvant HAI+SYS or SYS between January 2000-October 2017 with next-generation sequencing data were catalogued. Gene and signaling-level alterations were checked for association with time to any (AR), liver (LR), and extrahepatic recurrence (ER) using Kaplan-Meier analysis.
Results:
Of 172 receiving HAI+SYS, 100 patients recurred, with 69 LR and 83 ER. Five and ten-year LR rates were 57%(95%CI=48-65%) and 51%(95%CI=41-60%), respectively. Five and 10-year ER rates were 51%(95%CI=43-58%) and 45%(95%CI=36-54%), respectively. More ER was observed with tumors harboring altered KRAS(38%[95%CI=25-50%] vs 63%[95%CI=53-71%], p-adj=0.003) and RAS/RAF(36%[95%CI=25-48%] vs 66%[95%CI=56-74%], p-adj<0.001) than wild-type. Co-altered RAS/RAF-TP53 was associated with worse AR(26%[95%CI=14-40%] vs 48%[95%CI=39-57%], p-unadj<0.001), ER(30%[95%CI=17-45%] vs 62%[95%CI=53-70%], p-unadj<0.001), and LR rate(40%[95%CI=24-57%] vs 70%[95%CI=60-77%], p-unadj=0.002). On multivariable analysis, controlling for clinical risk score, ablation, margin status, and primary T-stage, co-altered RAS/RAF-TP53 was associated with increased risk for AR(HR=2.14, 95%CI=1.38-3.31, p-unadj<0.001), LR(HR=1.79, 95%CI=1.06-3.02, p-unadj=0.029), and ER(HR=2.81, 95%CI=1.78-4.44, p-unadj<0.001).
Conclusions:
Altered KRAS, RAS/RAF, and RAS/RAF-TP53 associated with earlier local and distant recurrence in resected CRLM patients receiving adjuvant HAI+SYS. Co-altered RAS/RAF-TP53 was a novel predictor of LR warranting investigation of whether genomic cooperativity is associated with this relapsing phenotype. Systemic therapies tailored to high-risk tumor biology are needed to reduce distant relapse after hepatectomy.
INTRODUCTION
Colorectal cancer is the second most common cause of cancer death in the United States.1 Most patients with resectable colorectal liver metastases (CRLM) relapse after hepatectomy and these recurrences frequently involve the liver remnant.2,3 Among patients with completely resected CRLM, it is unclear which populations are at greater risk for hepatic or extrahepatic recurrence making it difficult to tailor appropriate adjuvant treatments and surveillance strategies.
Since liver recurrence is common after CRLM resection, adjuvant hepatic artery infusion (HAI) chemotherapy has been utilized to reduce the risk of hepatic relapse. Adjuvant HAI chemotherapy has been shown in randomized trials to significantly reduce intrahepatic recurrence and prolong overall recurrence-free survival.4,5 The local impact of adjuvant HAI chemotherapy on CRLM patients harboring different genomic profiles, however, remains poorly understood. Prior reports suggest that treatment of CRLM with some systemic agents may induce selective expansion of tumor cells with more aggressive genomic profiles,6 however, these studies have not identified specific profiles associated with hepatic recurrence after resection. Alterations in the RAS (e.g. KRAS, NRAS, BRAF) pathway, have been shown to be associated with a greater risk for lung, peritoneal, and brain recurrence after hepatectomy.7-9
Prior reports have investigated clinical and pathologic predictors of recurrence patterns in resected CRLM cohorts treated with adjuvant systemic chemotherapy, however, genomic predictors of recurrence patterns in resected cohorts treated with combined adjuvant HAI and systemic chemotherapy have not been studied. Investigation of cohorts that recur despite complete resection and combined HAI and systemic chemotherapy may unmask unique genomic signals associated with overall recurrence and/or specific recurrence patterns. In this study, we sought to identify genomic correlates of recurrence patterns in resected CRLM patients treated with adjuvant systemic and HAI chemotherapy.
METHODS
Patient and Tissue Selection
All sequenced CRLM patients undergoing complete resection followed by adjuvant HAI and systemic chemotherapy (HAI-CRLM) between January 1, 2000 and October 31, 2017 were identified from a prospectively maintained hepatectomy database. Race and ethnicity data were not recorded into this database during the study period. Patients with preoperatively identified extrahepatic disease, mismatch repair mutations, no HAI chemotherapy administration (i.e., floxuridine), ablation without resection, or with insufficient follow up (<3 months) were excluded. Patients with recurrent disease within 3 months of hepatectomy were presumed to have occult synchronous disease and were also excluded. For comparison of clinicopathologic and genomic profiles to HAI-CRLM patients, a sequenced cohort of completely resected CRLM patients treated with adjuvant systemic chemotherapy alone (SYS-CRLM) between January 1, 2000 and October 31, 2017 without extrahepatic disease, mismatch repair mutations, ablation without resection, or insufficient follow up (<3 months) were identified.
Clinicopathologic characteristics, surgical history, perioperative outcomes, survival, and recurrence patterns were collected. The clinical risk score (CRS), a composite metric of 5 parameters predicting higher risk of disease recurrence following curative-intent hepatectomy, was calculated as defined previously.10 High CRS patients were defined as having a score of 3 or higher. Right colon tumors were defined as arising from the cecum to the distal transverse colon and left colon tumors originating from the splenic flexure to the distal sigmoid colon. Rectal tumors were defined as originating at or below the rectosigmoid junction. Preoperative chemotherapy was considered treatment with any systemic agent in anticipation of curative-intent hepatectomy. Adjuvant systemic chemotherapy was considered any therapy given after hepatectomy without evidence of active disease. Postoperatively, patients were monitored every 3-6 months for five years and then typically annually thereafter. Follow up included tumor marker serology and contrast-enhanced computed tomography and/or magnetic resonance imaging. Recurrent disease was established by identifying a new lesion on cross-sectional imaging and/or biopsy. This study was approved by our Institutional Review Board.
Genomic Analysis
Paired DNA from tumor specimens and matched normal tissue underwent targeted next generation sequencing using the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) assay–a platform that detects point mutations, copy number alterations, and gene fusions from 341 (early iteration) to 468 (modern iteration) cancer-associated genes.11 Sequenced genomic data was stored for analysis on a secure server for large-scale cancer genomics data (cBioPortal for Cancer Genomics).12,13 Actionable gene targets were defined by the OncoKb database as somatic alterations reported to confer some heightened response or resistance to therapy relative to the wild-type (wt) configuration. Driver gene alterations resulted in some selective advantage for tumorigenesis such as gain-of-function for proto-oncogenes or loss-of-function for tumor suppression genes.14 When both primary colorectal and CRLM tumor sequencing data were available, the latter was used given its proximity to the date of hepatectomy. Genomic profiles of primary colorectal tumors were considered eligible for inclusion based on reports noting high genomic concordance between matched primary and metastatic liver lesions.15,16 Select mutations from genes and signaling pathways were examined for associations with recurrence patterns if at least 10 patients had a mutation (mt) present.
Statistical Analysis
Time to any recurrence (AR), any liver recurrence (LR), and any extrahepatic recurrence (ER) were assessed from hepatectomy until detection of that specific recurrence. Regardless of whether recurrent disease occurred at an extrahepatic site or in the liver previously, detection of any LR or any ER were documented, respectively. Kaplan-Meier methods and the log-rank test assessed relationships between outcomes and genomic variables. Patients who died or were alive without recurrence at last follow up were censored. On univariable analyses, false discovery rate corrections were applied within each outcome for with possible genomic correlates.
Given the potential prognostic relevance of co-altered TP53 and RAS/RAF pathway mutation (KRAS, ARAF, BRAF, NRAS, HRAS),17,18 the relationship between co-altered TP53 and RAS/RAF with recurrence was assessed. First, a dependent or multiplicative effect between RAS/RAF and TP53 was checked using an interaction term in a logistic or Cox model, where appropriate. Next, an additive component to RAS/RAF-TP53 co-occurrence was assessed with bivariable (TP53 and RAS/RAF) Cox or logistic models as positive for both TP53 and RAS/RAF (mt-mt) versus all other options in univariable analyses. Classification and regression tree (CART) analyses explored subgroups that could be masked through standard modeling procedures. For CART analyses, an exponential distribution, ten-fold cross validation, and cross-complexity pruning were used. Effect sizes were represented by the lambda (λ) parameter estimate (PE). P-values for co-occurrence analyses were not adjusted (p-unadj).
Cox regression methods assessed the relationship between clinicopathologic characteristics and outcomes. The composite CRS was utilized herein rather than its individual components. Multivariable models were built for significant clinicopathologic factors with altered RAS/RAF, TP53, or co-altered RAS/RAF-TP53 for the investigated outcomes. Clinical characteristics and mutation rates were compared between the HAI-CRLM and SYS-CRLM cohorts with Fisher's Exact test and Wilcoxon Rank Sum test where appropriate. Univariable KM analyses were repeated in the SYS-CRLM patients.
Unless otherwise stated, unadjusted two-sided p-values less than 0.05 were considered statistically significant. Recursive partitioning was performed with the rpart package (version 4.1-13, 2018) in Cran R (R Core Team, Vienna Austria) and all other analyses were performed with SAS 9.4 TS1M5 (The SAS Institute, Cary, NC).
RESULTS
Clinical Characteristics and Recurrence Patterns of Resected HAI and SYS CRLM Patients
Of 230 sequenced CRLM patients operated upon during the study period, 172 (75%) underwent adjuvant HAI/SYS and 58 (25%) underwent only adjuvant SYS. Of the 172 HAI-CRLM patients, 56% (n=96/172) were male and the median age at hepatectomy was 54 years (range=28-77). At resection, 19% (n=32/172) underwent ablation combined with resection and 6% (n=10/172) had positive margins. Compared to 58 SYS-CRLM patients, the HAI-CRLM cohort was younger, had more liver metastases, more commonly received preoperative chemotherapy, underwent major hepatectomy more frequently, and had fewer positive margins (Table 1).
Table 1:
Clinicopathologic characteristics of all colorectal liver metastasis (CRLM) patients completely resected with hepatic artery infusion (HAI) and systemic chemotherapy and systemic chemotherapy alone (SYS).
| All patients | HAI-CRLM | SYS-CRLM | p-value | ||
|---|---|---|---|---|---|
| Number of patients | 230 | 172 | 58 | ||
| Gender | Male | 129 (56) | 96 (56) | 33 (57) | >0.95 |
| Female | 101 (44) | 76 (44) | 25 (43) | ||
| Age at Surgery, years | Median (range) | 55 (28-84) | 54 (28-77) | 59 (28-84) | 0.004 |
| Primary Tumor Location | Right Colon | 59 (26) | 39 (23) | 20 (34) | 0.26 |
| Left Colon | 108 (47) | 86 (50) | 22 (38) | ||
| Rectum | 59 (26) | 44 (26) | 15 (26) | ||
| Colon, NOS | 4 (2) | 3 (2) | 1 (2) | ||
| Primary Pathologic T-classification | T0-T2 | 36 (16) | 31 (18) | 5 (9) | 0.14 |
| T3-T4 | 193 (84) | 141 (82) | 52 (90) | ||
| Primary Pathologic Nodal Status | N0 | 71 (31) | 57 (33) | 14 (24) | 0.25 |
| N+ | 159 (69) | 115 (67) | 44 (76) | ||
| Disease Free Interval, months | Median (range) | 0.6 (0.0-118.9) | 1.0 (0-118.9) | 0.4 (0.0-90.5) | 0.22 |
| Preoperative CEA, ng/mL | Median (range) | 7.9 (0.5-5285.7) | 7.7 (0.5-5285.7) | 8.0 (1.6-983.8) | 0.78 |
| N Missing | (6) | (4) | (2) | ||
| Largest CRLM Size, cm | Median (range) | 2.5 (0.3-16.1) | 2.5 (0.3-14.5) | 2.4 (0.5-16.1) | >0.95 |
| Number of CRLM | Median (range) | 3 (1-15) | 3 (1-15) | 2 (1-15) | <0.001 |
| Solitary | 71 (31) | 42 (24) | 29 (50) | <0.001 | |
| Multifocal | 159 (69) | 130 (76) | 29 (50) | ||
| Clinical Risk Score (CRS) | Low Risk (0-2) | 117 (51) | 85 (49) | 32 (55) | 0.54 |
| High Risk (3-5) | 113 (49) | 87 (51) | 26 (45) | ||
| Preoperative Chemotherapy | Yes | 177 (77) | 142 (83) | 35 (60) | 0.001 |
| No | 53 (23) | 30 (17) | 23 (4) | ||
| Extent of Hepatectomy | Minor | 152 (66) | 107 (62) | 45 (78) | 0.037 |
| Major | 78 (34) | 65 (38) | 13 (22) | ||
| Liver Margin | R0 | 210 (91) | 162 (94) | 48 (83) | 0.013 |
| R1 | 20 (9) | 10 (6) | 10 (17) | ||
| Ablation at Hepatectomy | Yes | 37 (16) | 32 (19) | 5 (9) | 0.10 |
| No | 193 (84) | 140 (81) | 53 (91) |
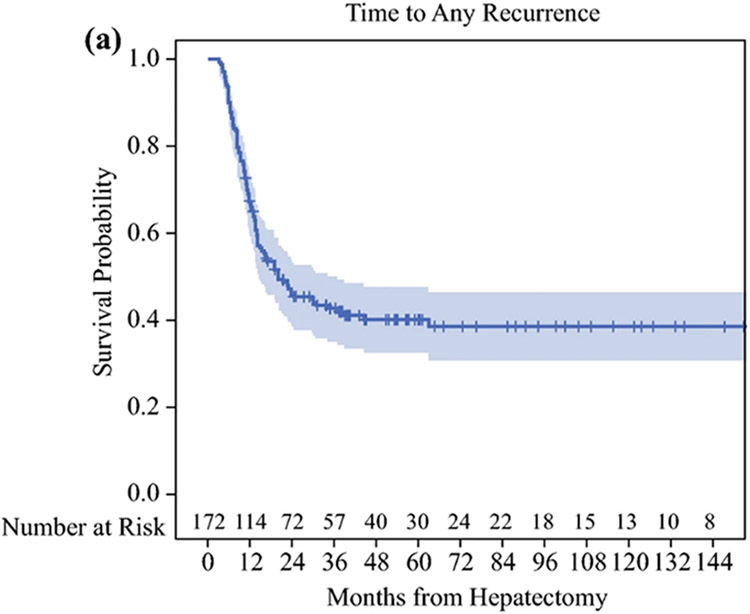
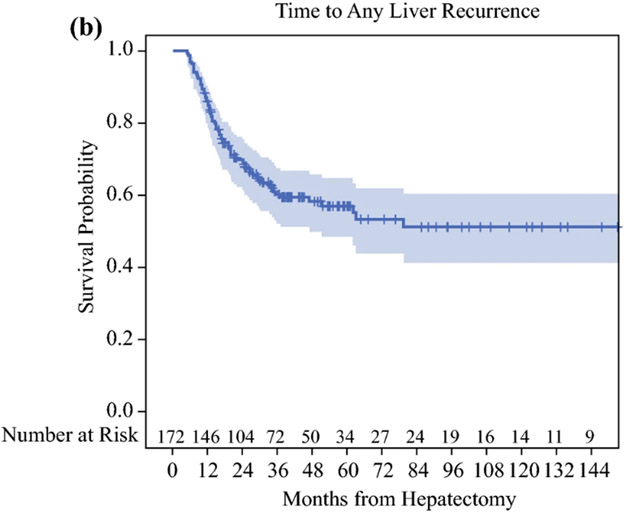
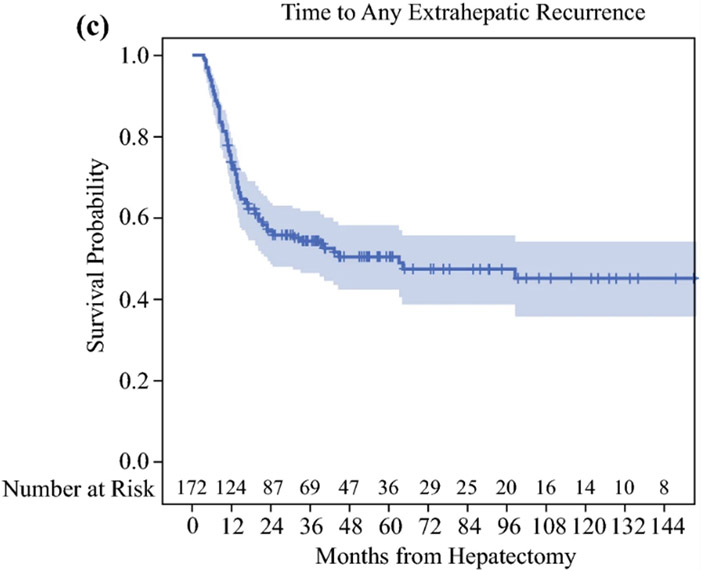
Median follow up among HAI-CRLM survivors (n=152) was 41 months (range=11-197) and median time to AR was 20 months (95%CI=15-37). Five and ten-year rates free of AR were 40% (95%CI=32-48%) and 39% (95%CI=31-46%), respectively (Figure 1A). Accounting for all recurrences, 69 patients developed LR and 83 ER. By last follow up, 17 recurred only in the liver, 31 recurred at an extrahepatic site only, 52 recurred in both the liver and an extrahepatic site, and 72 had no recurrences detected. Five and ten-year rates free of LR were 57% (95%CI=48-65%) and 51% (95%CI=41-60%), respectively (Figure 1B), and 5 and 10-year rates free of ER were 51% (95%CI=43-58%) and 45% (95%CI=36-54%), respectively (Figure 1C).
Figure 1.
A: Time to Any Recurrence, B: Time to Any Liver Recurrence, C: Time to Any Extrahepatic Recurrence in colorectal liver metastasis patients after complete resection with adjuvant local and systemic chemotherapy (HAI-CRLM).
Median follow up among SYS-CRLM survivors (n=43) was 32 months (range=10-131) and median time to AR was 14 months (95%CI=11-22). Accounting for all recurrences, 37 patients developed LR and 37 ER. By last follow up, only 7 recurred in the liver, only 7 recurred at an extrahepatic site, 30 recurred in both the liver and an extrahepatic site, and 14 had no recurrences detected.
Genomic Profiles Associated with Recurrence
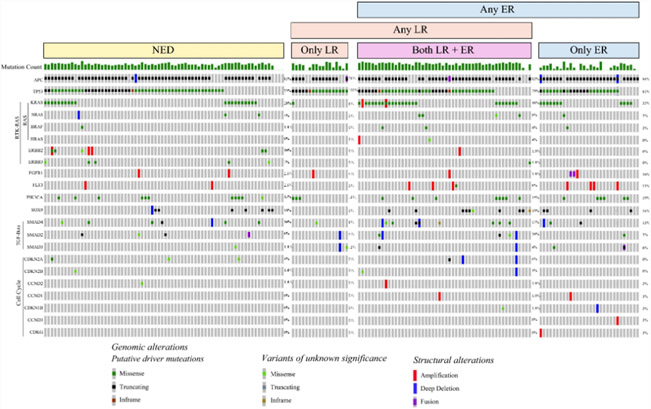
The most commonly altered genes among the HAI-CRLM patients were APC (85%, n=146/172), TP53 (80%, n=137/172), and KRAS (33%, n=57/172, Figure 2). The most commonly altered signaling pathways were Wnt (92%, n=159/172), p53 (84%, n=144/172), RTK-RAS (55%, n=94/172), PI3K (31%, n=54/172), Notch (23%, n=40/172), TGF-beta (14%, n=24/172), HIPPO (12%, n=21/172), and Cell Cycle (6%, n=10/172).
Figure 2:
Oncoprint of Genomic Alterations Stratified by Recurrence Patterns; NED: No evidence of disease, LR: Liver recurrence, ER: Extrahepatic recurrence. Timing of and censor events are not reflected, so inference about difference in recurrence patterns cannot be derived from this figure.
No specific gene or signaling pathway alterations were significantly associated with worse AR and LR rates in HAI-CRLM patients. Compared to the wild-type, HAI-CRLM patients had worse ER rates with tumors altered at FLT3 (3yr. 12% [95%CI=1-41%] vs 57% [95%CI=49-64%], p-adj=0.037), KRAS (3yr. 38% [95%CI=25-50%] vs 63% [95%CI=53-71%], p-adj=0.003), and SMAD4 (3yr. 31% [95%CI=11-54%] vs 57% [95%CI=49-64%], p-adj=0.029, Supplemental Figure 1). At the signaling pathway level, alterations in the RTK-RAS (3yr. 41% [95%CI=31-51%] vs 71% [95%CI=59-80%], p-adj<0.001), RAS/RAF (3yr. 36% [95%CI=25-48%] vs 66% [95%CI=56-74%], p-adj<0.001), and Cell cycle pathways (3yr. 13% [95%CI=1-43%] vs 38% [95%CI=19-56%], p-adj=0.010) were significantly associated with worse ER rates (Supplemental Figure 2).
The SYS-CRLM cohort had a higher proportion of KRAS (55% vs 33%, p-unadj=0.005), BRAF (10% vs 2%, p-unadj=0.018), and SMAD4 alterations (21% vs 9%, p-unadj=0.034) compared to the HAI-CRLM cohort. Additionally, SYS-CRLM patients had more RTK-RAS (76% vs 55%, p-unadj=0.005), RAS/RAF (69% vs 38%, p-unadj<0.001), and TGF-Beta pathway alterations (26% vs 14%, p-unadj=0.044). For this cohort, no genes or signaling pathways were significantly associated with AR, LR, or ER.
Additive and Interactive Analyses of TP53 and RAS
Co-alteration of RAS/RAF-TP53 in HAI-CRLM patients was associated with worse AR (3yr. 26% [95%CI=14-40%] vs 48% [95%CI=39-57%], p-unadj<0.001), LR (3yr. 40% [95%CI=24-57%] vs 70% [95%CI=60-77%], p-unadj=0.002), and ER (3yr. 30% [95%CI=17-45%] vs 62% [95%CI=53-70%], p-unadj<0.001). In bivariable analyses, RAS/RAF remained significantly associated with worse AR (HR=1.96, 95%CI=1.29-2.96, p-unadj=0.002), LR (HR=1.80, 95%CI=1.09-2.97, p-unadj=0.021), and ER (HR=2.63, 95%CI=1.67-4.13, p-unadj<0.001). Altered TP53 in bivariable analyses, however, was not found to have a significant association with AR (p-unadj=0.07), LR (p-unadj=0.08), or ER (p-unadj=0.23). No significant interactions were found between RAS/RAF and TP53 for AR (p-unadj=0.68), LR (p-unadj=0.74), or ER (p-unadj=0.39).
When RAS/RAF-TP53 co-alteration was tested among SYS-CRLM patients, no significant association was found with AR (3yr. 19% vs 26%, p-unadj=0.51), LR (3yr. 38% vs 43%, p-unadj=0.60), or ER (3yr. 31% vs 38%, p-unadj=0.26).
Classification and Regression Tree Analysis of Genomic Correlates for Recurrence
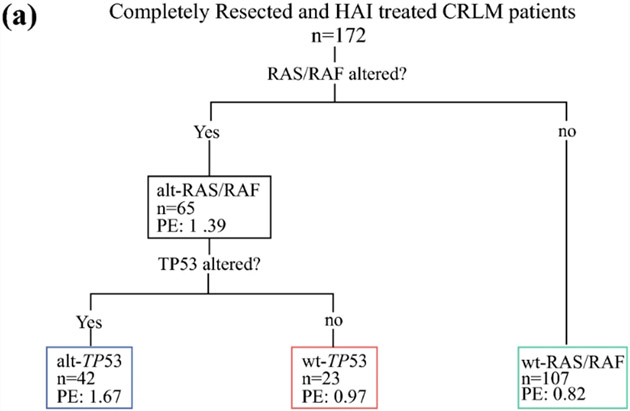
In the model for AR, an initial split occurred on RAS/RAF alterations with PE=0.82 for wt-RAS/RAF and PE=1.39 for mt-RAS/RAF indicating greater risk of recurrence (Figure 3). A subsequent split occurred on mt-RAS/RAF with TP53 alterations where PE=0.97 for wt-TP53 and PE=1.67 for mt-TP53. The three terminal nodes had the following 5-year rates free of AR: wt-RAS/RAF (48%, 95%CI=38-58%), mt-RAS/RAF+wt-TP53 (47%, 95%CI=26-66%), and mt-RAS/RAF+mt-TP53 (26%, 95%CI=14-40%). Attempts to build trees for ER and LR failed, as no splits remained after pruning.
Figure 3.
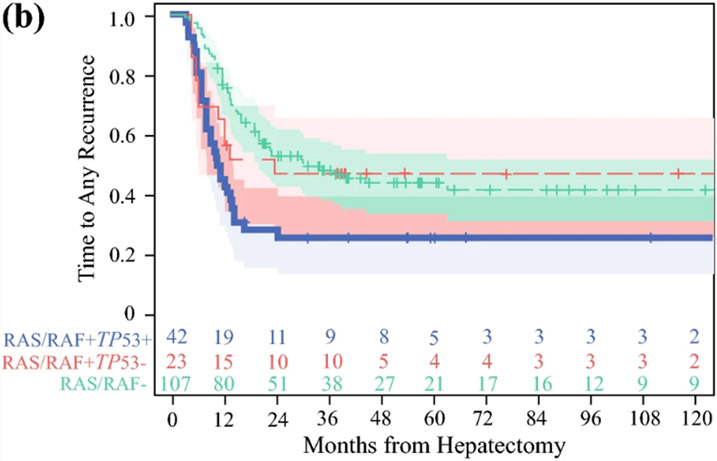
A: Classification and regression tree analysis of patients with completely resected colorectal liver metastases and hepatic artery infusion chemotherapy demonstrating three terminal nodes of genomic risk for recurrence (n=172); PE: parameter estimate; B: Stratification of recurrence-free survival by the three genomic risk groups; alt-RAS-alt-TP53, alt-RAS-wt-TP53, wt-RAS. Number at risk over time indicated in the adjoining risk table.
Clinicopathologic and Genomic Factors Associated with Recurrence
On univariable analysis, advanced primary T-stage, high CRS, positive margins, and ablation with hepatectomy were associated with worse AR. Younger age at hepatectomy, high CRS, minor hepatectomy, positive margins, and ablation were associated with worse LR. Younger age at hepatectomy, advanced primary T-stage, and high CRS were associated with worse ER (Table 2).
Table 2:
Cox proportional hazards regression model for unadjusted recurrence, liver recurrence, and extrahepatic recurrence-free survival in patients following complete resection of colorectal liver metastases with adjuvant hepatic artery infusion and systemic chemotherapy.
| Any Recurrence | Any Liver Recurrence | Any Extrahepatic Recurrence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | ||||||||
| Variable | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age at resection | 0.98 (0.97-1.00) | 0.12 | 0.97 (0.95-0.99) | 0.007 | 0.97 (0.95-0.99) | 0.005 | 0.98 (0.96-1.00) | 0.043 | 0.98 (0.96-1.00) | 0.07 | |||
| Primary T-Stage | T3-T4 | 2.29 (1.22-4.30) | 0.010 | 2.01 (1.06-3.82) | 0.032 | 1.97 (0.94-4.13) | 0.07 | 2.22 (1.11-4.43) | 0.024 | 2.06 (1.02-4.16) | 0.044 | ||
| T0-T2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Clinical Risk Score | High (3-5) | 1.66 (1.11-2.46) | 0.013 | 1.45 (0.96-2.19) | 0.08 | 1.73 (1.07-2.80) | 0.026 | 1.39 (0.83-2.31) | 0.21 | 1.79 (1.15-2.77) | 0.010 | 1.70 (1.08-2.67) | 0.021 |
| Low (0-2) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Preoperative Chemotherapy | Yes | 1.51 (0.84-2.71) | 0.16 | 1.48 (0.74-2.99) | 0.20 | 1.23 (0.67-2.28) | 0.50 | ||||||
| No | 1.00 | 1.00 | 1.00 | ||||||||||
| Extent of Hepatectomy | Major | 0.80 (0.53-1.20) | 0.28 | 0.58 (0.34-0.97) | 0.038 | 0.51 (0.30-0.87) | 0.014 | 0.93 (0.60-1.46) | 0.76 | ||||
| Minor | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| Liver Margin | R1 | 2.62 (1.31-5.24) | 0.006 | 2.32 (1.13-4.74) | 0.022 | 3.25 (1.47-7.19) | 0.004 | 2.38 (1.03-5.49) | 0.042 | 1.27 (0.51-3.13) | 0.61 | ||
| R0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Ablation at Hepatectomy | Yes | 1.80 (1.13-2.85) | 0.013 | 1.44 (0.90-2.31) | 0.13 | 2.28 (1.35-3.84) | 0.002 | 2.04 (1.17-3.53) | 0.012 | 1.39 (0.82-2.34) | 0.22 | ||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| RAS-TP53 | Co-Altered | 2.05 (1.34-3.13) | <0.001 | 2.14 (1.38-3.31) | <0.001 | 1.95 (1.17-3.24) | 0.010 | 1.79 (1.06-3.01) | 0.029 | 2.57 (1.64-4.04) | <0.001 | 2.81 (1.78-4.44) | <0.001 |
| All others | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
Multivariable analyses were used to investigate the association of co-altered RAS/RAF-TP53 with recurrence patterns while controlling for significant clinical covariates. Co-altered RAS/RAF-TP53 (HR=2.14, 95%CI=1.38-3.31, p-unadj<0.001), advanced primary T-stage (HR=2.01, 95%CI=1.06-3.82, p-unadj=0.032), and positive margins (HR=2.32, 95%CI=1.13-4.74, p-unadj=0.022) remained significantly associated with worse AR. Additionally, co-altered RAS/RAF-TP53 (HR=1.79, 95%CI=1.06-3.01, p-unadj=0.029), age at hepatectomy (HR=0.97, 95%CI=0.95-0.99, p-unadj=0.005), ablation (HR=2.04, 95%CI=1.17-3.53, p-unadj=0.012), minor hepatectomy (HR=0.51, 95%CI=0.30-0.87, p-unadj=0.014), and positive margins (HR=2.38, 95%CI=1.03-5.49, p-unadj=0.042) remained significantly associated with LR. Co-altered RAS/RAF-TP53 (HR=2.81, 95%CI=1.78-4.44, p-unadj<0.001), advanced primary T-stage (HR=2.06, 95%CI=1.02-4.16, p-unadj=0.044), and high CRS (HR=1.70, 95%CI=1.08-2.67, p-unadj=0.021) remained significantly associated with worse ER.
DISCUSSION
Although hepatectomy is the only curative option,19,20 most CRLM patients relapse within two years of resection.21 The liver is the most common site of recurrence and, as a result, hepatic metastases are the most common cause of death.21 Novel therapies are necessary to reduce the risk for liver relapse after hepatectomy. Decreased liver recurrence has been reported with adjuvant HAI and systemic chemotherapy after complete resection of CRLM across four randomized trials.4,22-24 Consequently, a unique opportunity arises to study predictors of recurrence patterns after curative hepatectomy, adjuvant HAI, and systemic chemotherapy. In this report, altered RAS/RAF and co-altered RAS/RAF-TP53 were associated with worse AR, LR, and to a greater extent, ER for CRLM patients after resection with adjuvant HAI and systemic chemotherapy, but not systemic chemotherapy alone.
Predictably, advanced primary T-stage and high CRS were associated with worse ER reflecting more advanced initial tumors in HAI-CRLM patients. Independent risk factors for worse LR represented variables associated with patients at risk for having residual microscopic disease including minor hepatectomy, ablation with resection, and positive margins. Younger age at hepatectomy was also associated with worse LR, however, this likely reflects that younger patients have more advanced disease at presentation as noted in prior work and this cohort.25,26
The genomic profile of the HAI-CRLM cohort resembles multiple other reports on resected CRLM patients; for example, BRAF and KRAS were altered in less than 5% and a third of patients, respectively.27-29 Altered KRAS was associated with worse ER, recapitulating findings from earlier work.30,31 Although KRAS alteration has been linked to worse outcomes,32-35 HAI chemotherapy after hepatectomy has been associated with prolonged survival regardless of KRAS status.36 Interestingly, the SYS-CRLM cohort in this study had significantly more KRAS and RAS/RAF-altered patients; however, no significant associations with AR, LR, or ER were found. The lack of an identifiable association could be due to limited sampling. Alternatively, this may suggest that an additional trigger for relapse may be attributable to patient characteristics, the tumor, or selection of HAI in the first place. Additionally, HAI-CRLM patients can receive less systemic doses due to the added regional regimen, which may impact the risk for recurrence, however, this study was not designed to investigate this association. Given the increased risk for ER with RAS/RAF and RAS/RAF-TP53 altered tumors, improved systemic chemotherapy options are necessary to improve distant control.
Tumors with altered RAS/RAF are notably resistant to anti-epidermal growth factor receptor chemotherapy37,38 and are also associated with more aggressive features like poor pathologic response to chemotherapy,7,39,40 diaphragm invasion,41 and positive margins.42,43 As a result, some have argued that hepatectomy in the context of RAS alteration may not be justified44 due to its association with worse overall survival,7-9,39-45 recurrence-free survival,7,39,40,43,46 and post-recurrence survival.47,48 A report from Passot et al found no RAS-altered survivors four years out from resection,44 however, other reports have indicated that RAS alteration does not lead to a substantial decline in survival until co-altered with TP53.49 Moreover, 5-year survival for the RAS/RAF-altered HAI-CRLM cohort herein was 70%, potentially from achieving regional hepatic control, as local recurrence after hepatectomy is a poor predictor of survival.3 Given the possibility of long-term survival, RAS alteration should not preclude hepatectomy. Other reports have described associations between RAS alteration with lung and peritoneal recurrence after resection of CRLM,7-9 however, this study is the first to show an association with aggregate ER. Notably, RAS alteration was not associated with LR in prior reports7-9, however on bivariable analysis, an association was detected. Altered TP53 was not associated with LR on univariable or bivariable analyses. Interestingly, co-altered RAS/RAF-TP53 was associated with worse AR, LR, and ER suggesting that TP53 alteration could play a collaborative role in promoting relapse. Preclinical evidence for the interplay between loss-of-function p53 alteration and RAS activation has been reported.50,51 Emerging data also suggests that TP53 alteration negatively correlates with cytolytic immune cell activity suggesting that liver recurrence after hepatectomy may leverage immune evasion to flourish.
Limitations
Interpretation of these findings should be done cautiously due to the limited number of recurrence events in patients altered for FLT3, SMAD4, and Cell Cycle pathway genes. Additionally, inclusion of patients with available sequencing data or those treated with adjuvant local and systemic chemotherapy created a selection bias. Patients with available sequencing data likely represent those with higher risk biology, which may have prompted sequencing in the first place. This is reflected by the relatively fewer patients in the SYS-CRLM cohort during the same study period. However, this patient sample was a unique cohort to study drivers of recurrence that emerge following immense selective pressure. Although the genomic profile of the HAI-CRLM cohort differed from the SYS-CRLM patients, tumor profiles from published work were more consistent with our HAI cohort. Regardless, studying a larger SYS-CRLM cohort for comparison would be beneficial. Finally, it is unclear if sequencing a single tumor sample is the best strategy to profile the cancer genome given that CRLM patients have, by definition, systemic disease. Although high concordance between the genomic profiles of primary and metastatic liver tumors has been noted,52 recent reports call into question whether newer, non-invasive biomarkers such as circulating tumor cells or DNA would make for more reliable tumor markers.53 Nonetheless, high concordance has been reported between CRLM and the primary colorectal tumor16,52,54 but not with extrahepatic metastases9 supporting the use of the primary and liver tumors and exclusion of extrahepatic samples.
CONCLUSIONS
Altered RAS/RAF and RAS/RAF-TP53 CRLM tumors were associated with worse AR, LR, and ER for resected patients after adjuvant HAI and systemic chemotherapy, but not systemic chemotherapy alone. Future investigations into genomic profiles of recurrence should incorporate RAS/RAF-TP53 co-alteration and seek other unexplored drivers of local and distant recurrence arising in locally treated patients. Furthermore, better systemic chemotherapy options are necessary to improve distant control after complete hepatectomy.
Supplementary Material
Supplemental Figure 1A: Time to Any Liver Recurrence (LR) by Alteration in TP53, B: FLT3, C: KRAS, D: SMAD4; E: Time to Any Extrahepatic Recurrence (ER) by Alteration in TP53, F: FLT3, G: KRAS, H: SMAD4.
Supplemental Figure 2A: Time to Any Liver Recurrence (LR) by Alteration in RTK/RAS, B: RAS, C: Cell Cycle Pathway; D: Time to Any Extrahepatic Recurrence (ER) by Alteration in RTK/RAS, E: RAS, F: Cell Cycle Pathway.
SYNOPSIS.
Despite curative hepatectomy, most colorectal liver metastasis patients recur locally within two years of resection. Genomic predictors for hepatic recurrence are poorly understood but may hold implications for therapeutic devision making and are thus explored herein.
FUNDING SOURCES:
This work was supported in part by the NIH/NCI P30 CA008748 Cancer Center Support Grant.
Footnotes
DISCLOSURES: The authors have no relevant conflicts of interest to report.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020. [DOI] [PubMed] [Google Scholar]
- 2.D'Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096–1103. [DOI] [PubMed] [Google Scholar]
- 3.Lee AJ, Loyer EM, Kang HC, et al. Intrahepatic Recurrence Patterns Predict Survival After Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2019;26(1):275–281. [DOI] [PubMed] [Google Scholar]
- 4.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341(27):2039–2048. [DOI] [PubMed] [Google Scholar]
- 5.Kemeny NE, Chou JF, Boucher TM, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol. 2016;113(5):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreou A, Kopetz S, Maru DM, et al. Adjuvant chemotherapy with FOLFOX for primary colorectal cancer is associated with increased somatic gene mutations and inferior survival in patients undergoing hepatectomy for metachronous liver metastases. Ann Surg. 2012;256(4):642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258(4):619–626; discussion 626-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaeger R, Cowell E, Chou JF, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121(8):1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passot G, Kim BJ, Glehen O, et al. Impact of RAS Mutations in Metastatic Colorectal Cancer After Potentially Curative Resection: Does Site of Metastases Matter? Ann Surg Oncol. 2018;25(1):179–187. [DOI] [PubMed] [Google Scholar]
- 10.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318; discussion 318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vakiani E, Janakiraman M, Shen R, et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol. 2012;30(24):2956–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brannon AR, Vakiani E, Sylvester BE, et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014;15(8):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi Y, Kopetz S, Newhook TE, et al. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin Cancer Res. 2019;25(19):5843–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta J, Narayan RR, Goldman DA, et al. Distinct Genomic Profiles are Associated With Conversion to Resection and Survival in Patients With Initially Unresectable Colorectal Liver Metastases Treated With Systemic and Hepatic Artery Chemotherapy. Ann Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–4580. [DOI] [PubMed] [Google Scholar]
- 20.Cucchetti A, Ferrero A, Cescon M, et al. Cure model survival analysis after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2015;22(6):1908–1914. [DOI] [PubMed] [Google Scholar]
- 21.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–448. [DOI] [PubMed] [Google Scholar]
- 22.Kemeny N, Jarnagin W, Gonen M, et al. Phase I/II study of hepatic arterial therapy with floxuridine and dexamethasone in combination with intravenous irinotecan as adjuvant treatment after resection of hepatic metastases from colorectal cancer. J Clin Oncol. 2003;21(17):3303–3309. [DOI] [PubMed] [Google Scholar]
- 23.Kemeny N, Capanu M, D'Angelica M, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol. 2009;20(7):1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemeny NE, Jarnagin WR, Capanu M, et al. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol. 2011;29(7):884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Haas RJ, Wicherts DA, Salloum C, et al. Long-term outcomes after hepatic resection for colorectal metastases in young patients. Cancer. 2010;116(3):647–658. [DOI] [PubMed] [Google Scholar]
- 26.Lieu CH, Renfro LA, de Gramont A, et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD Clinical Trials Program. J Clin Oncol. 2014;32(27):2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karagkounis G, Torbenson MS, Daniel HD, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119(23):4137–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margonis GA, Spolverato G, Kim Y, Karagkounis G, Choti MA, Pawlik TM. Effect of KRAS Mutation on Long-Term Outcomes of Patients Undergoing Hepatic Resection for Colorectal Liver Metastases. Ann Surg Oncol. 2015;22(13):4158–4165. [DOI] [PubMed] [Google Scholar]
- 29.Frankel TL, Vakiani E, Nathan H, et al. Mutation location on the RAS oncogene affects pathologic features and survival after resection of colorectal liver metastases. Cancer. 2017;123(4):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira AA, Rego JF, Morris V, et al. Association between KRAS mutation and lung metastasis in advanced colorectal cancer. Br J Cancer. 2015;112(3):424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margonis GA, Kim Y, Sasaki K, Samaha M, Amini N, Pawlik TM. Codon 13 KRAS mutation predicts patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Cancer. 2016;122(17):2698–2707. [DOI] [PubMed] [Google Scholar]
- 32.Nash GM, Gimbel M, Shia J, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17(2):572–578. [DOI] [PubMed] [Google Scholar]
- 33.Margonis GA, Kim Y, Spolverato G, et al. Association Between Specific Mutations in KRAS Codon 12 and Colorectal Liver Metastasis. JAMA Surg. 2015;150(8):722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goffredo P, Utria AF, Beck AC, et al. The Prognostic Impact of KRAS Mutation in Patients Having Curative Resection of Synchronous Colorectal Liver Metastases. J Gastrointest Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 35.Margonis GA, Buettner S, Andreatos N, et al. Prognostic Factors Change Over Time After Hepatectomy for Colorectal Liver Metastases: A Multi-institutional, International Analysis of 1099 Patients. Ann Surg. 2019;269(6):1129–1137. [DOI] [PubMed] [Google Scholar]
- 36.Gholami S, Kemeny NE, Boucher TM, et al. Adjuvant Hepatic Artery Infusion Chemotherapy is Associated With Improved Survival Regardless of KRAS Mutation Status in Patients With Resected Colorectal Liver Metastases: A Retrospective Analysis of 674 Patients. Ann Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995. [DOI] [PubMed] [Google Scholar]
- 38.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita S, Chun YS, Kopetz SE, et al. APC and PIK3CA Mutational Cooperativity Predicts Pathologic Response and Survival in Patients Undergoing Resection for Colorectal Liver Metastases. Ann Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 40.Zimmitti G, Shindoh J, Mise Y, et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22(3):834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okuno M, Gourmard C, Mizuno T, et al. Pathological diaphragmatic invasion by colorectal liver metastases is associated with RAS mutation, peritoneal recurrence and worse survival. HPB (Oxford). 2018;20(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brudvik KW, Mise Y, Chung MH, et al. RAS Mutation Predicts Positive Resection Margins and Narrower Resection Margins in Patients Undergoing Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2016;23(8):2635–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passot G, Chun YS, Kopetz SE, et al. Prognostic factors after resection of colorectal liver metastases following preoperative second-line chemotherapy: Impact of RAS mutations. Eur J Surg Oncol. 2016;42(9):1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Passot G, Denbo JW, Yamashita S, et al. Is hepatectomy justified for patients with RAS mutant colorectal liver metastases? An analysis of 524 patients undergoing curative liver resection. Surgery. 2017;161(2):332–340. [DOI] [PubMed] [Google Scholar]
- 45.Mizuno T, Cloyd JM, Vicente D, et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. Eur J Surg Oncol. 2018;44(5):684–692. [DOI] [PubMed] [Google Scholar]
- 46.Aloia TA, Kim BJ, Segraves-Chun YS, et al. A Randomized Controlled Trial of Postoperative Thoracic Epidural Analgesia Versus Intravenous Patient-controlled Analgesia After Major Hepatopancreatobiliary Surgery. Ann Surg. 2017;266(3):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denbo JW, Yamashita S, Passot G, et al. RAS Mutation Is Associated with Decreased Survival in Patients Undergoing Repeat Hepatectomy for Colorectal Liver Metastases. J Gastrointest Surg. 2017;21(1):68–77. [DOI] [PubMed] [Google Scholar]
- 48.Okuno M, Goumard C, Kopetz S, et al. RAS Mutation is Associated with Unsalvageable Recurrence Following Hepatectomy for Colorectal Cancer Liver Metastases. Ann Surg Oncol. 2018;25(8):2457–2466. [DOI] [PubMed] [Google Scholar]
- 49.Datta J, Smith JJ, Chatila WK, et al. Coaltered Ras/B-raf and TP53 Is Associated with Extremes of Survivorship and Distinct Patterns of Metastasis in Patients with Metastatic Colorectal Cancer. Clin Cancer Res. 2020;26(5):1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parada LF, Land H, Weinberg RA, Wolf D, Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984;312(5995):649–651. [DOI] [PubMed] [Google Scholar]
- 51.McMurray HR, Sampson ER, Compitello G, et al. Synergistic response to oncogenic mutations defines gene class critical to cancer phenotype. Nature. 2008;453(7198):1112–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell. 2018;33(1):125–136 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narayan RR, Goldman DA, Gonen M, et al. Peripheral Circulating Tumor DNA Detection Predicts Poor Outcomes After Liver Resection for Metastatic Colorectal Cancer. Ann Surg Oncol. 2019;26(6):1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tie J, Lipton L, Desai J, et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res. 2011;17(5):1122–1130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1A: Time to Any Liver Recurrence (LR) by Alteration in TP53, B: FLT3, C: KRAS, D: SMAD4; E: Time to Any Extrahepatic Recurrence (ER) by Alteration in TP53, F: FLT3, G: KRAS, H: SMAD4.
Supplemental Figure 2A: Time to Any Liver Recurrence (LR) by Alteration in RTK/RAS, B: RAS, C: Cell Cycle Pathway; D: Time to Any Extrahepatic Recurrence (ER) by Alteration in RTK/RAS, E: RAS, F: Cell Cycle Pathway.