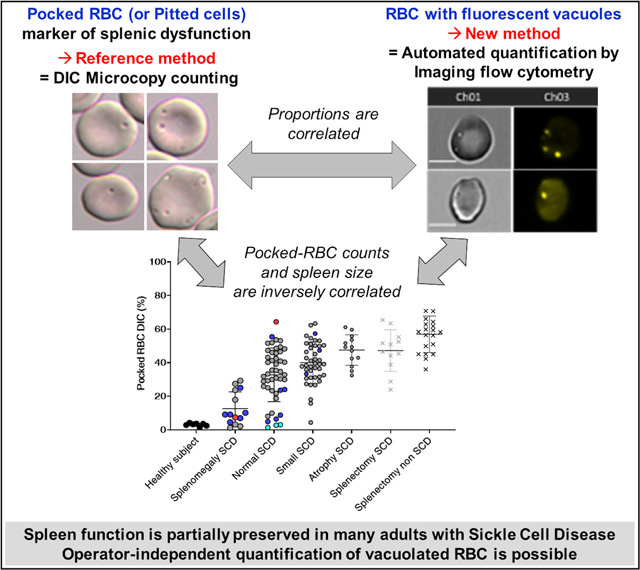
Graphical Abstract
To the Editor:
The human spleen protects against blood-borne infections and filters red blood cells (RBC). Defective spleen function (hyposplenism) is mostly caused by splenectomy or by immunological or hematological diseases.1 Hyposplenism occurs during infancy in sickle cell disease (SCD) and shows no clear correlation with spleen size.2 Spleen function is assessed by scintigraphy, and by quantifying circulating blood cells, such as marginal zone B lymphocytes, or RBC containing Howell-Jolly bodies (HJB-RBC) or vacuoles (pocked-RBC). These RBC subpopulations are cleared of their inclusions by the spleen in a process called pitting. Circulating pocked-RBCs represent ≥4.5% of total RBC in hyposplenic patients and over 20% in splenectomized subjects.1 Pocked-RBC counts display better diagnostic performance than counting HJB-RBC (Figure S1E), correlate with spleen signal intensity on scintigraphy3 but require differential interference contrast (DIC) microscopy performed by an expert reader. We uncovered a new fluorescence-based method to count pocked-RBC, and assessed its accuracy in healthy subjects, patients with SCD (splenectomized or not), and in subjects splenectomized without underlying RBC-related disease.
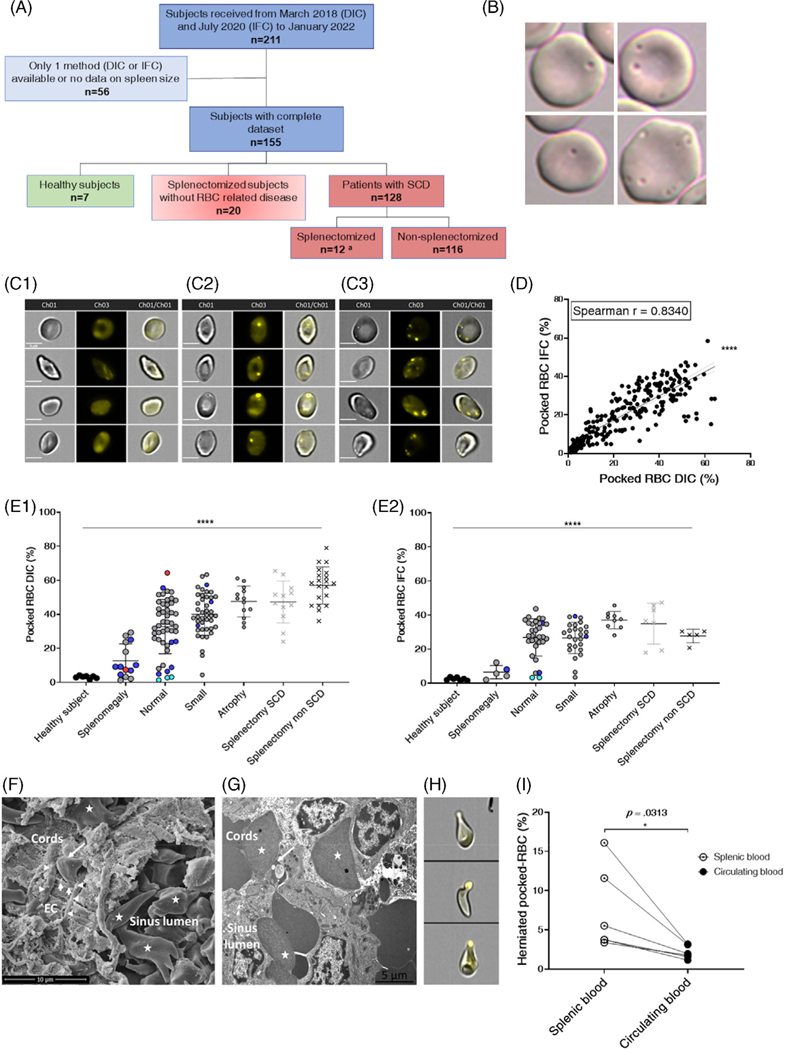
From March 2018 through January 2022, we enrolled 7 healthy subjects, 128 adults with SCD, 6 children living with SCD, and 20 splenectomized subjects without underlying RBC-related disease at 2 hospitals of the Assistance Publique-Hôpitaux de Paris network (AP-HP) in a study entitled “Pathophysiological Explorations of Red Blood Cells” (ClinicalTrials.gov reference NCT03541525). Twelve of the adult SCD patients were splenectomized. Of the 128 patients with SCD, 112 received a simple transfusion or were involved in a transfusion program during the study. All samples were collected just prior to the transfusion, along with routine sampling for medical follow-up. Based on clinical and imaging data, spleen size was graded by two team members (LJ, SM) unaware of pocked-RBC counts, into four categories: splenomegaly, normal, small, and atrophic. The children with SCD had partial splenectomy for hypersplenism. The 128 adults with SCD include 108, 15, 3 and 2 patients with HbSS, HbSC, HbS/β+ and HbS/β0 genotypes, respectively (Figure 1E1,E2).
FIGURE 1.
Proportion of Pocked RBC in the circulation: Inter-method consistency, correlation with spleen size, progressive pathological processes, RBC filtration, and pitting in SCD spleens. (A) Flow chart of subjects and patients. Quantification of pocked-RBC by IFC was performed in 7 of 12 splenectomized patients with SCD (A). (B) Typical aspects of single- and multiple-pocked-RBC by DIC. (C) Typical aspects of spotless RBC (C1), single- (C2) and multiple-spotted RBC (C3) by IFC. Scale bars = 5 μM. (D) Linear regression relationship and nonparametric Pearson correlation between % pocked-RBC by DIC and IFC in 221 blood samples (Y = 0.6277*X + 4.777), (****: p < .0001). In 63 instances, patients were sampled pre- and post-transfusion (126 samples), 15 patients were sampled twice and 1 patient was sampled 4 times during follow-up. (E) Proportion of pocked-RBC in peripheral blood samples collected before transfusion, assessed by DIC (E1) and IFC (E2) in different subgroups of adult patients with SCD and healthy individuals defined by spleen size. Each dot represents a single measure or mean of measures when the patient had been sampled multiple times. Genotypes and status regarding splenectomy are differentiated as follows: HbSS (gray dots, gray crosses for SCD splenectomized subjects), HbS/beta-zero (red dots), HbSC (blue dots), HbS/beta-plus (cyan dots), and splenectomized subjects without underlying RBC disease (black crosses). Kruskal-Wallis and Dunn’s multiple comparison tests were used for both the whole cohort and HbSS separately (****: p < .0001). Black bar represents the statistical analysis including all genotypes. (F) Scanning electron microscopy of spleen sample from an SCD child showing packed RBC, many of which are sickled (star) and a typical sinus structure with the cordal side on the left and the luminal side on the right, elongated endothelial cells (EC), basal helicoid fibers (horizontal arrowheads), both delimiting an inter-endothelial slit (vertical arrowheads) into which an RBC is engaged (only its cordal part is visible, oblique arrow) (F). (G) Transmission electron microscopy shows a pocked-RBC crossing an inter-endothelial slit, the vacuole-containing part of the RBC lying upstream from the slit (oblique arrow). Sickled RBC are visible in the sinus lumen and cords (stars). (H) Typical images of “herniated” pocked-RBC, and their proportions in circulating blood and spleen blood from 6 children with SCD (I). Wilcoxon matched-pairs signed rank test was used (*: p < .0332)
After buffy coat removal, RBC were stained with Cell Trace Yellow (CTY; Thermo Fisher Scientific, France) according to the manufacturer’s recommendations with minor modifications. Briefly, 5 μl of the post-centrifugation (1250 g, 5 min) pellet were washed twice (400 g, 5 min) and incubated for 20 min in phosphate-buffered saline (PBS) with CTY 1/500 v/v. The staining reaction was stopped with PBS containing 1% Albumax (Gibco, France) for 5 min. RBC were washed twice and resuspended in 500 μl 1% Albumax-PBS at 1% hematocrit.
Imaging flow cytometry (IFC; ImageStream X Mark II, AMNIS, Luminex) was performed to acquire (INSPIRE software, Luminex) brightfield (channel 01) and fluorescence images (Laser 561 nm, 20 mW, Channel 03) at 60x magnification. At least 50 000 RBC were acquired for each sample. Images were processed with the IDEAS v6.2 software (Luminex). Focused RBC were selected using gradient root mean square values, and single cells were gated using area and aspect ratio functions. The IDEAS “Spot Count” wizard was used to discriminate fluorescent spot-containing from spotless RBC. As per software recommendations, 282 and 165 images were tagged respectively, generating a classification of channel 3 images according to the association of the feature “Spot Count” with the software-defined Mask “Spot (M03, Channel 3, Bright, 23, 1)”. A template file (.ast file) was generated and used to deduce the proportion of pocked-RBC for each sample.
We counted pocked-RBC with DIC as previously described2 and by IFC in 221 samples (Figure 1B–E). The IDEAS script efficiently discriminated CTY-spotted from spotless RBC on IFC images. As opposed to conventional epifluorescence microscopy, where the even staining of RBC cytoplasm by CTY results in suboptimal discrimination, the IDEAS software offers fine adjustment of fluorescence intensity and thus reproducible discrimination between background cytoplasmic fluorescence and the slightly more intense vacuole fluorescence. RBC with one or more fluorescent vacuoles were counted as pocked-RBC (Figure 1C2,C3). Fluorescence-based and manual counts were correlated (Spearman r = .8340, p < .0001, Figure 1D), although pocked-RBC counts were 20%–30% lower by IFC than by DIC. The deficit in sensitivity by IFC may result from the lower definition of images generated at x600 magnification without immersion, as opposed to the DIC which is at x1000 magnification with immersion. Alternatively, a proportion of vacuoles visualized by DIC may not be fluorescent. To assess the stability of pocked-RBC counts upon sample storage, we stored 44 samples at 4°C in their original heparincontaining sampling tubes and quantified pocked-RBC by IFC on days 0, 1, 2, 3, 4, and 7. The median proportion of pocked-RBC changed by <10% during the first 48 hours of storage (Figure S1.A,D0–D2), but then progressively declined with a median variation >10% compared to counts at D0 (Figure S1.A,D3–D7). Samples can thus be stored and shipped for a centralized analysis in the 2 days following sampling. We also analyzed the impact of transfusion on pocked RBC counts. Exchange transfusion was associated with a 55.8% dilutional fall in pocked RBC counts (Figure S1.B). In a separate cohort of 116 patients followed prospectively post-transfusion, pocked-RBC counts rose rapidly and stabilized 20–24 days after transfusion (Figure S1.C,D), suggesting that counts performed just prior to a monthly exchange transfusion accurately reflect spleen function, as further indicated by the observed correlation with spleen size. Because pocked-RBC quantification also correlated with splenic scintigraphy results in a previous study,3 this new fluorescence-based counting method is a strong candidate marker of spleen function that can be automated on 20 μl of blood. Of note, the IDEAS software also quantifies RBC morphological features4 on brightfield IFC images, a potentially useful asset in medical hematology. In theory, circulating malaria-infected RBC that retain CTY (not shown) may be mistakenly counted as Pocked RBC by IFC, but because the prevalence of malaria in SCD children is <1%, usually with low parasitemia, this does not jeopardize the potential use of the new marker in malaria-endemic areas.
Some experts consider that adults with SCD are almost constantly asplenic with an atrophic spleen. However, among our 128 adults living with SCD, 108 (84%) of whom carrying the HbSS genotype (Figure 1E1,E2), we observed large variations in spleen size, including approximately 50% of normally-sized or enlarged spleens. Pocked-RBC counts with either DIC or IFC inversely correlated with spleen size, not only in the whole cohort (p < .0001), but also when data from patients carrying the HbSS were analyzed separately (p < .0001, Figure 1E1,E2). Pocked-RBC counts in SCD patients with either atrophic or removed spleens were similarly high, suggesting that splenic atrophy corresponds to complete asplenia. Conversely, patients with either enlarged, normal, or small spleens had more variable counts, suggesting that splenic filtering function is often preserved in SCD (including Sickle Cell Anemia), at least partially, even in adulthood. Management of SCD at reference centers, including the early use of hydroxyurea and transfusion programs, may contribute to this preservation of spleen function.2
To explore the underlying mechanism(s) of vacuoles removal by the spleen, we retrieved spleen fragments from a child living with Sickle Cell Anemia, partially splenectomized for hypersplenism. This child had a normal pocked-RBC count (2.3%). Histological analysis of this fragment showed a congestive spleen without widespread sickling or fibrosis (not shown). Electronic microscopy was performed as previously described.5 This analysis showed red pulp congestion (not shown), mild to intense sickling in sinuses (Figure 1F,G), and normal sinus wall, with typical images of RBCs crossing an intact sinus wall in scan electron microscopy (Figure 1F) and transmission electron microscopy (Figure 1G). Because electron microscopy suggested the persistence of pitting in the SCD spleen, we quantified elongated RBC harboring a peripheral, “herniated” vacuole, a morphology highly suggestive of ongoing pitting (Figure 1G). Peripheral blood samples taken per-operatively and splenic blood6 from the six SCD children undergoing splenectomy were CTY-labeled and the percentage of herniated pocked-RBC (Figure 1H) was manually quantified against 350 to 500 CTY-spot-positive RBC “pocked-RBC”. Compared to circulating blood, the splenic blood showed a 1.9 to 5-fold enrichment in these “herniated” pocked-RBC, strongly suggesting that RBC with CTY-positive vacuoles are indeed pitted in the spleen (Figure 1L).
We show that the new fluorescence-based quantification of pocked-RBC, employing the spontaneous accumulation of CTY dye in vacuoles, correlates with the reference DIC counts. It thus lays a path to robust, operator-independent quantification of spleen function, which in our opinion justifies the investment in an imaging flow cytometer, expensive equipment currently available only at reference centers. We also showed that spleen function is partially preserved in many adults with SCD and that the fluorescent vacuoles that accumulate in RBC from SCD patients are likely expelled by the spleen-specific pitting process, as long as sinus structures persist in the preserved or partially damaged SCD spleens. The unexpected spleen function preservation in adult patients with Sickle Cell Anemia may have been previously overlooked or may be a recent, additional benefit of the increasing use of hydroxyurea and transfusion programs. Because the spleen is generally protective not only against infections but also against pulmonary thrombosis/embolism, assessing spleen function may provide a useful prognosis indication in patients with SCD. Not least, a residual filtering spleen may remove pathogenic subpopulations of RBC, protecting patients from other SCD-related complications. Therefore, measuring residual spleen function may help guide major therapeutic decisions in SCD, such as splenectomy, hematopoietic stem cell transplantation, or gene therapy. Future studies will prospectively determine the prognostic and therapeutic value of this new marker.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jean-Yves Rinckel and Fabienne Proamer (EFS-GEST) for their excellent expert technical assistance in electronic microscopy.
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
CONFLICT OF INTEREST
No conflict of interest is to disclose.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. The Lancet. 2011;378(9785):86–97. [DOI] [PubMed] [Google Scholar]
- 2.Rogers ZR, Wang WC, Luo Z, et al. Biomarkers of splenic function in infants with sickle cell anemia: baseline data from the BABY HUG trial. Blood. 2011;117(9):2614–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson HA, Gallagher D, Chilcote R, et al. Developmental pattern of splenic dysfunction in sickle cell disorders. Pediatrics. 1985;76(3): 392–397. [PubMed] [Google Scholar]
- 4.El Hoss S, Dussiot M, Renaud O, Brousse V, El Nemer W. A novel noninvasive method to measure splenic filtration function in humans. Haematologica. 2018;103(10):e436–e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safeukui I, Correas J-M, Brousse V, et al. Retention of plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood. 2008;112(6):2520–2528. [DOI] [PubMed] [Google Scholar]
- 6.Kho S, Qotrunnada L, Leonardo L, et al. Evaluation of splenic accumulation and colocalization of immature reticulocytes and plasmodium vivax in asymptomatic malaria: a prospective human splenectomy study. PLoS Med. 2021;18(5):e1003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.