Abstract
Pre-clinical studies demonstrate opposing effects of long-chain polyunsaturated fatty acid (PUFA) metabolites on inflammation and nociception. Omega-6 (n-6) PUFAs amplify both processes while omega-3 (n-3) PUFAs inhibit them. This cross-sectional study examined relationships between PUFAs in circulating erythrocytes and two chronic idiopathic pain conditions: temporomandibular disorder (TMD) and low back pain in a community-based sample of 503 U.S. adults. Presence or absence of TMD and low back pain, respectively, were determined by clinical examination and by responses to established screening questions. Liquid chromatography tandem mass spectrometry quantified PUFAs. In multivariable logistic regression models, a higher ratio of n-6/n-3 long-chain PUFAs was associated with greater odds of TMD (odds ratio ((OR)=1.75, 95% confidence limits (CL): 1.16, 2.64) and low back pain (OR=1.63, 95% CL: 1.07, 2.49). Higher levels of the pronociceptive n-6 long-chain arachidonic acid were associated with greater probability of both pain conditions for women, but not men. Higher levels of the antinociceptive long-chain n-3 PUFAs eicosapentaenoic and docosahexaenoic acids were associated with lower probability of both pain conditions for men, but not women. As systemic inflammation is not a hallmark of these conditions, PUFAs may influence idiopathic pain through other mechanisms.
Keywords: Epidemiology, lipidomics, omega-6/omega-3 long-chain PUFA ratio, idiopathic pain, pain intensity
INTRODUCTION
Twentieth century developments in agribusiness and medicine were catalysts for major change to the American diet. Techniques to extract oil from seed, such as the soybean seed, led to wide scale replacement of saturated fats, such as butter and lard, with polyunsaturated fats. When these vegetable oils were shown to lower serum cholesterol concentration,18 the first Dietary Goals for the United States39 heavily promoted their use. What ensued was an increased consumption of linoleic acid (LA)—the omega-6 (n-6) essential fatty acid—and a decreased consumption of α-linolenic acid (ALA)—the omega-3 (n-3) essential fatty acid. Today’s American diet is excessively high in n-6 polyunsaturated fatty acids (PUFAs) and relatively deficient in n-3 PUFAs.2
Both n-6 LA and n-3 ALA are precursors of signaling molecules with opposing roles in inflammation and pain regulation. LA is converted through a series of linked desaturation and elongation reactions to long-chain arachidonic acid (AA,). In turn, AA is a precursor to bioactive oxylipins, such as prostaglandins and leukotrienes. In addition to inducing a proinflammatory response, these oxylipins heighten neuronal excitability, contributing to pain exacerbation.3 The n-3 ALA is converted, albeit inefficiently—up to 10% conversion—by the same series of desaturation and elongation reactions to long-chain n-3 eicosapentaenoic acid (EPA) and long-chain n-3 docosahexaenoic acid (DHA). See Figure 1 for an illustration of these metabolic pathways. Oxylipin derivatives, such as resolvins derived from EPA and DHA exert potent antinociceptive and analgesic effects in addition to well-established anti-inflammatory, proresolving effects.11
Figure 1.
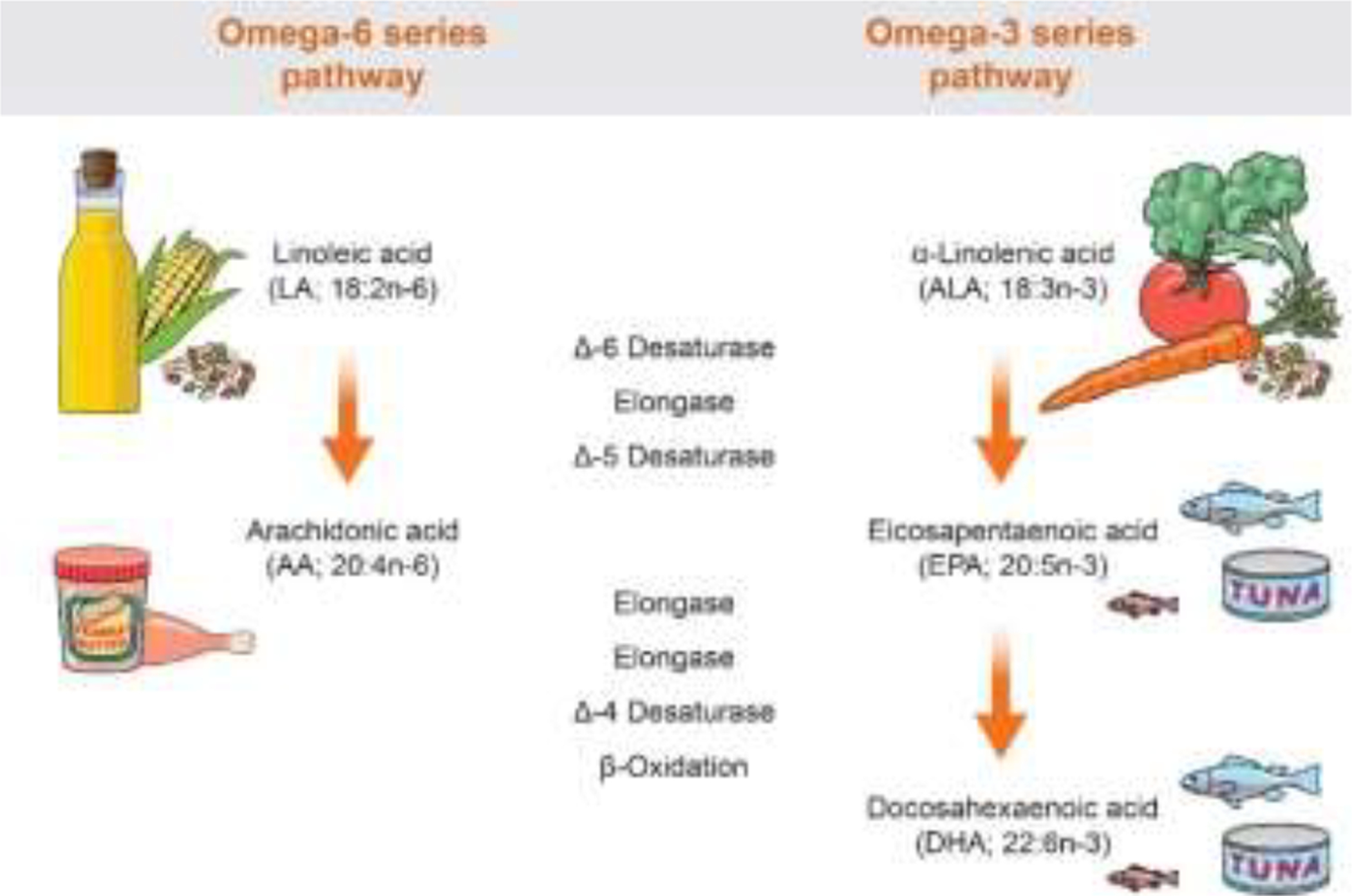
Schematic presentation of the metabolic pathways and dietary sources of omega-6 and omega-3 polyunsaturated fatty acids
Because LA and ALA compete for the same desaturation and elongation enzymes, a diet high in LA limits the conversion of ALA and inhibits incorporation of DHA and EPA into tissues, promoting a proinflammatory, pronociceptive state.32 Conversely, a diet low in LA allows greater conversion of ALA to long‐chain n‐3 PUFA and permits better incorporation of n‐3 PUFAs into tissues.31 Studying the n-6/n-3 PUFA ratio captures the impact of this interdependence and potential imbalance on health outcomes.
Clinical studies of PUFAs and chronic pain are mostly limited to known pain conditions where inflammation is a hallmark, such as rheumatoid arthritis, inflammatory bowel disease, and dysmenorrhea.14 It remains unclear whether PUFAs play a role in idiopathic pain conditions that are not characterized by systemic inflammation.
Painful temporomandibular disorder (TMD) typically presents clinically as both myalgia of the masticatory muscles and arthralgia of the jaw joint/s. Painful TMD affects 3% of men and 6% of women in the U.S.16 The disorder is idiopathic,9 meaning that the pain has no apparent pathological or traumatic origin. TMDs respond poorly to pharmacotherapy, and therapy often relies on use of occlusal appliances that prove largely unsatisfactory, providing limited and transient pain relief.40
Like painful TMD, chronic low back pain lacks identifiable pathology or tissue injury sufficient to account for the pain. In the absence of inflammation and other clinical findings, about 80% of low back pain is diagnosed “nonspecific”8 Point prevalence is 10% among men and 12% among women,52 making low back pain the most common chronic pain condition in the U.S.
We measured PUFA concentrations in phospholipid membranes of circulating erythrocytes of adults to test the hypothesis that a high ratio of n-6 long-chain PUFAs to n-3 long-chain PUFAs was associated with greater odds of chronic painful TMD and of low back pain. Pain prevalence is greater in women than men and men and women differ in their experience of pain and pain processing. Compared with men, women have higher prevalence of clinical pain, lower thresholds to experimental pain stimuli and greater analgesic response to opioids.13, 24 We therefore determined whether the relationship between long-chain PUFAs and pain differed for men and women. Finally, we examined whether PUFAs were associated with self-reported intensity of facial pain, an endpoint used widely in clinical trials pain therapy.
METHODS
Study Design, Setting, Participants
This study used cross-sectional data from OPPERA-II, the second phase of the Orofacial Pain Prospective Evaluation and Research Assessment (OPPERA) study. In its first phase, OPPERA recruited 1,008 painful TMD cases and 3,258 TMD-free controls to identify risk factors for TMD. Cases and controls were a community-based sample of volunteers aged 18 to 44 years from communities near academic health centers in Baltimore, MD; Buffalo, NY; Chapel Hill, NC; and Gainesville, FL between 2006 and 2013. The second phase of OPPERA (OPPERA-II) took place between 2014 and 2016 and entailed follow-up of 543 people originally enrolled in OPPERA-I along with an additional 127 adults aged 18–74 years with recent-onset TMD who were recruited from the same four communities. Both groups of OPPERA-II participants underwent clinical examinations and quantitative sensory testing. They completed standardized questionnaires and a venous non-fasting blood draw. This current study used data and biospecimens from these 670 OPPERA-II participants.
Chronic temporomandibular disorder
In the parent study, painful TMD was main pain outcome of interest and serves as the primary outcome in this analysis. Examiners verified painful TMD using Diagnostic Criteria for Temporomandibular Disorder.38 In brief, trained examiners assessed presence of pain reported in the cheeks, jaw muscles, temples, or jaw joints to classify presence or absence of TMD. To be classified as cases, subjects had to have all four of the following findings: a) history of orofacial pain in examiner-verified locations of masseter, temporalis, submandibular or temporomandibular joint area; b) evoked pain in the same muscles and/or temporomandibular joint(s) following palpation of those structures or jaw maneuver; c) reported familiarity of evoked pain to facial pain symptoms during the preceding 30 days; and d) pain that was modified by jaw function (i.e., chewing, opening the mouth, or jaw habits).
Intensity of facial pain
A composite measure of facial pain intensity was computed using responses to the Characteristic Pain Intensity scale in the Graded Chronic Pain Scale.50 Ohrbach et al.,27 described its scoring in OPPERA. In brief, the mean score was derived from responses to three questions that asked about current pain intensity; and the worst and the average pain intensity experienced in the past 3 months. All responses were recorded on a numeric rating scale anchored at zero (no pain) and 10 (pain as bad could be). This mean value was multiplied by 10, to yield a 0–100 characteristic facial pain intensity score.
Chronic low back pain
Inclusion of chronic low back pain in this analysis was to determine if findings were replicated in this highly prevalent condition. Low back pain was classified using responses to screening questions designed for face-to-face interviews and paper or online questionnaires recommended for studies of back pain prevalence by Dionne and colleagues.10 Participants were classified with low back pain if they reported pain that occurred in the lower back (as indicated with a shaded manikin drawing) during the preceding 3 months that was bad enough to limit usual activities or change their routine for more than one day and that was not related to fever or menstruation.
Main exposure
Circulating concentrations of essential fatty acids (LA, ALA) and long-chain PUFAs (AA, EPA, DHA) are reported in μg/mL. We specified the n-6/n-3 PUFA ratio using long-chain PUFAs, i.e., those with 20 or 22-carbon PUFAs, computed as AA/(EPA+DHA). This is because mediators and regulators of inflammation and pain signaling, are generated from these 20- and 22-carbon PUFAs.5, 19
Covariates
OPPERA study site was a covariate because recruitment communities differed on key characteristics. Sex was included because men and women differ in pain prevalence and because sex differences occur in the conversion of ALA to EPA and DHA.4, 7 Age was modelled in four categories (18–29, 30–39, 40–49, 50–74 years) because of its nonlinear relationship with TMD. In the National Health Interview Survey, TMD prevalence is significantly higher in white race than in African American race.16 We compared associations for white and African American race and pooled other racial groups with Hispanic ethnicity because of similarities in the strength of associations with pain and because of low enrollment numbers. Associations between socioeconomic indicators and TMD were null or weak in OPPERA, so socioeconomic variables were not included. Body mass index (BMI) was included because a high n-6/n-3 PUFA ratio increases risk for obesity,43 and because comorbid obesity is common in low back pain.28 Standardized equipment measured weight and height during clinical examinations. BMI was calculated by dividing weight in kilograms by the square of height in meters and analyzed as a binary variable, using the World Health Organization classification scheme to distinguish non-obese (BMI <30.0) from obese, (BMI ≥30.0). The essential fatty acids n-6 LA and n-3 ALA were modelled because they are precursors to highly bioactive derivatives.
Blood sample
At the OPPERA-II study visit, a non-fasting 20 ml sample of circulating blood was obtained by venipuncture and collected into tubes containing EDTA that were promptly centrifuged for 10 min at 4°C. After removing the supernatant plasma, erythrocytes were washed with sodium perborate, vortexed and again centrifuged. After removing the sodium perborate supernatant, erythrocytes were aliquoted into 400 uL cryotubes prior to storage at −80oC.
Sample preparation
Erythrocyte samples were obtained and stored in −80°C until extraction. At that time, 150 μL was extracted with 1 mL of 90:10 methanol to water. Samples were vortexed then centrifuged at 20,000 rcf for 10 minutes. The supernatant was dried down and reconstituted in 150 μL of 90:10 methanol to water containing 50 ng/mL of deuterated internal standards for analysis. Two quality control standards with known concentrations (20 and 500 ng/mL) as well as the deuterated internal standard mixture were analyzed at least twice in each batch of 96 samples to ensure the results were reproducible and as predicted. The calibration curve was analyzed twice per batch of 96 samples and averaged to include error across the analysis, and error bars were created using standard deviation.
Instrument methods
Samples were analyzed using a Waters Acquity Ultra-Performance Liquid Chromatography system tandem to a ThermoScientific TSQ Vantage. Separations were performed on a 150 mm × 2.1 mm BEH C18 with a flow rate of 0.25 mL/min and an injection volume of 10 μL. Initial mobile phase composition was 65% A (water with 30 mM ammonium formate) and 35% B (80% acetonitrile 20% methanol). A linear decrease was performed to 45% A at 2 min with a hold for 1 min. Another linear decrease to 20% A was performed at 7 min followed by a decrease to 5% A at 8 min and another at 10 min to 0% A. The gradient was returned to starting conditions at 13 min and held for 3 min for a total run time of 16 min. Single reaction monitoring (SRM) was performed in negative mode. The peak width was set to 0.7 Da with a scan time of 0.05 sec per transition. The transitions are provided in Supplementary Table 1. The source conditions were as follows: spray voltage 3200 V, sheath gas 50 units, auxiliary gas 15 units, and capillary temp 270°C. Analytes with their class, limit of detection (LOD), and limit of quantitation (LOQ) are reported in Supplementary Table 2.
Power calculation
When planning the study, statistical power was calculated for the fixed sample size OPPERA-II participants who had stored blood samples registered in the study database (n=670).
The hypothesized effect size was a difference between TMD-cases and TMD-non-cases of 0.33 standardized mean concentration of n-6 AA. That effect size was approximately one third of the effect size observed in our pilot study of 48 OPPERA participants, where mean concentrations were approximately 1,200 ng/mL in TMD cases and 900 ng/mL in controls, with standard deviation of 300 ng/mL (i.e., standardized mean difference=1.0). We elected an effect size of 0.33 because effect sizes observed in pilot studies typically over-estimate effect sizes in the population. Using the SAS POWER procedure, with alpha set at P<0.001, we calculated that there is 80% power to detect a standardized mean difference of 0.33 between TMD cases and controls with non-equivalent sample sizes of 240 cases versus 430 controls (e.g., TMD cases and controls). The calculation was repeated, post hoc, for the smaller sample of 170 cases and 333 controls in the complete case analysis, and the minimum detectable effect size was determined to be 0.39.
Missing values
Of the 670 study participants, 8 had unreported race/ethnicity, and 13 had unrecorded BMI. Seven biospecimens produced no signal for LA, AA, EPA and DHA and two biospecimens had levels of LA or DHA below the limit of quantitation. A further 150 biospecimens had no signal for ALA. We explored the nature of missing data and chose to conduct complete case analysis for 503 participants with no missing data. To assess the impact of bias, we conducted multiple imputation to replace the high number of missing ALA values. We fit the desired model separately on each of the 50 imputed datasets and combined the results, based on the concepts of Rubin36 and Schafer.37 A total of 114 of the 150 missing values for ALA were imputed, reflecting missingness in several predictor variables. We repeated the multivariable logistic regression analyses testing the association between the n-6/n-3 PUFA ratio and the two pain conditions (Supplementary Tables 3, 4). As a different strategy, we imputed a value of 0.5 for all missing ALA values, chosen arbitrarily as a midpoint between zero and 0.97 ng/mL, which was the laboratory limit of detection, on the assumption that ALA was present in samples but only at very low levels.
Statistical analysis
Statistical analyses were conducted in Stata/SE 14.2 (Stata Corporation, College Station, TX). We detected outliers using spike plots. Spike plots are a frequency plot for a continuous variable in which the frequencies are depicted as vertical lines from zero. Any spike located a considerable distance above other values in the distribution indicated the presence of an outlier. There were no outliers for ALA or EPA. Each of LA, AA, EPA, DHA had a single outlying value which was recoded to the nearest value that was not extreme. PUFA distributions were assessed for normality numerically using the Shapiro-Wilk test, and given the large sample size, were analyzed using parametric tests. We computed means and standard errors to describe the distribution of PUFA concentrations in demographic and BMI subgroups. Group differences in mean values were tested for statistical significance using linear regression. Associations of PUFA concentrations with TMD pain and low back pain case status (case, non-case) for were reported as means and 95% confidence limits (CL) with their differences tested for significance using linear regression. In unadjusted analysis, the n-6/n-3 PUFA ratio was multiplied by 100 to improve interpretation of the estimates.
Multivariable binary logistic regression estimated covariate-adjusted odds ratios (OR) and 95% CL for the relationship of n-6/n-3 PUFA ratio with the dependent variable TMD pain. The analysis was repeated for low back pain. In each analysis, the minimally adjusted model took account of study site, age group and sex. The partially adjusted model added race/ethnicity and obesity, and the fully adjusted model added the essential fatty acids LA and ALA modelled as standardized Z scores, so that their coefficients were larger than 1.0 and hence easier to interpret.
To explore whether the relationship between long-chain PUFAs (AA, EPA, and DHA) and pain differed for men and women, a multiplicative term (e.g., sex*AA) was added to multivariable regression models and tested for significance using the Wald test. Sex-specific effects were calculated with a post-estimation command and the odds ratio expressed as the relative increase in odds of TMD pain (and low back pain) per standard deviation increase of the PUFA.
Marginal effects, defined as the change in predicted probability of TMD pain (or low back pain) per unit change in the exposure of interest, were plotted to visually depict the incremental probability of TMD pain and low back pain associated with increases in circulating PUFAs. We examined associations of the individual long-chain PUFAs with pain, in addition to examining their ratio.
Multivariable linear regression estimated covariate-adjusted beta coefficients and 95% CI for the relationship between the n-6/n-3 PUFA ratio and facial pain intensity, quantified as a continuous measure. We also computed tertiles of the continuous measure to test for dose response association.
The value of p<0.05 was used to define statistical significance, with the exception that for effect modification p<0.10 was used.
Ethical conduct of research with humans
The study was reviewed and approved by the UNC Office of Human Research Ethics (study 13–2232). All study participants verbally agreed to a screening interview done by telephone and provided informed, signed consent for all other study procedures.
RESULTS
The 503 participants were aged 18 to 74 years with a mean age of 39. The sample comprised 64.8% women, 65.8% white race, 23.1% African American race and 34.6% met criteria for obesity.. Overall, 170 participants had TMD pain and 333 did not. In addition, 122 participants had low back pain and 381 participants did not.
We examined unadjusted associations between covariates and PUFA concentrations (Table 1). Concentrations of AA and EPA were higher for men than women. Concentrations of PUFAs tended to increase with age, although increases were non-linear for ALA. For ALA and DHA, age-related increases fell short of the threshold for statistical significance. Differences in PUFA concentrations between racial groups were statistically non-significant but appeared higher for African Americans than for other racial/ethnic groups. Obesity was associated with higher concentrations of AA and EPA.
Table 1.
Unadjusted means and standard errors for circulating concentrations (ng/mL) of omega-6 (n-6) and omega-3 (n-3) essential fatty acids and long-chain polyunsaturated fatty acids in erythrocyte membranes
| Linoleic acid 18:2n-6 | α-linolenic acid 18:3n-3 | Arachidonic acid 20:4n-6 | Eicosapentaenoic acid 20:5n-3 | Docosahexaenoic acid 22:6n-3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | S.E. | Mean | S.E. | Mean | S.E. | Mean | S.E. | Mean | S.E. | |
| Total | 503 | 1116.9 | 45.3 | 78.5 | 2.9 | 79.1 | 2.8 | 99.4 | 4.7 | 18.7 | 0.9 |
| Sex | |||||||||||
| Men | 177 | 1126.3 | 76.9 | 81.6 | 5.3 | 86.9 | 5.4 | 120.4 | 9.6 | 17.8 | 1.6 |
| Women | 326 | 1111.9 | 56.2 | 76.9 | 3.5 | 74.8 | 3.2 | 88.0 | 4.9 | 19.3 | 1.1 |
| P-value | 0.879 | 0.439 | 0.039 | 0.001 | 0.425 | ||||||
| Age, y | |||||||||||
| 18–29 | 131 | 982.0 | 88.4 | 72.4 | 5.6 | 67.1 | 4.1 | 72.2 | 5.9 | 15.2 | 1.2 |
| 30–39 | 138 | 1001.9 | 66.9 | 70.3 | 4.1 | 71.7 | 4.9 | 97.1 | 9.6 | 18.2 | 1.6 |
| 40–49 | 149 | 1234.6 | 93.8 | 86.0 | 5.9 | 88.4 | 5.7 | 112.9 | 9.6 | 20.5 | 2.0 |
| 50–74 | 85 | 1305.5 | 116.9 | 88.3 | 8.2 | 93.3 | 8.0 | 121.2 | 11.7 | 22.0 | 2.2 |
| P-value | 0.029 | 0.065 | 0.002 | 0.002 | 0.051 | ||||||
| Race (a) | |||||||||||
| White | 331 | 1050.7 | 49.7 | 74.2 | 3.2 | 75.5 | 3.2 | 97.9 | 5.6 | 17.6 | 1.0 |
| Black/AA | 116 | 1285.9 | 119.8 | 87.3 | 7.2 | 91.2 | 7.1 | 110.6 | 11.3 | 21.7 | 2.3 |
| Other | 56 | 1158.3 | 130.3 | 86.2 | 9.5 | 75.1 | 7.0 | 84.5 | 11.1 | 19.3 | 2.5 |
| P-value | 0.095 | 0.113 | 0.061 | 0.283 | 0.162 | ||||||
| Obesity (b) | |||||||||||
| Not obese | 329 | 1100.2 | 57.8 | 77.4 | 3.7 | 73.1 | 3.2 | 92.2 | 5.5 | 19.6 | 1.2 |
| Obese | 174 | 1148.6 | 72.3 | 80.8 | 4.7 | 90.5 | 5.4 | 112.9 | 8.6 | 17.1 | 1.2 |
| P-value | 0.612 | 0.575 | 0.003 | 0.036 | 0.095 | ||||||
Racial groups self-identified as White; Black/African American; others were pooled (Asian; Native American; other or multiple races; Hispanic ethnicity, not stated).
Not obese refers to a body mass index <30 kg/m2. Obese refers to a body mass index ≥30 kg/m2
We compared PUFAs concentrations, and the n-6/n-3 PUFA ratio, in pain cases and non-cases (Table 2). Although no individual PUFA concentrations differed to a statistically significant degree between painful TMD cases and non-cases, the n-6/n-3 PUFA ratio was higher in painful TMD cases than non-cases. Similarly, the n-6/n-3 PUFA ratio was higher in low back pain cases than non-cases. In addition, n-3 DHA concentration was lower in low back pain cases than for non-cases.
Table 2.
Unadjusted means and 95% confidence limits (CL) of circulating concentrations (ng/mL) of omega-3 (n-6) and omega-6 (n-3) essential fatty acids and long-chain polyunsaturated fatty acids in erythrocyte membranes, stratified by TMD and low back pain case status
| Painful TMD non-case (n=333) | Painful TMD case (n=170) | ||||
|---|---|---|---|---|---|
| Mean | 95% CL | Mean | 95% CL | P | |
| Linoleic acid 18:2n-6 | 1088.6 | 979.1, 1198.0 | 1172.5 | 1019.3, 1325.7 | 0.381 |
| α-linolenic acid 18:3n-3 | 77.1 | 70.0, 84.1 | 81.4 | 71.6, 91.3 | 0.478 |
| Arachidonic acid 20:4n-6 | 76.6 | 69.9, 83.4 | 83.9 | 74.5, 93.3 | 0.219 |
| Eicosapentaenoic acid 20:5n-3 | 101.5 | 90.2, 112.8 | 95.1 | 79.3, 111.0 | 0.520 |
| Docosahexaenoic acid 22:6n-3 | 19.2 | 17.0, 21.3 | 17.9 | 14.9, 20.9 | 0.493 |
| Low back pain non-case (n=381) | Low back pain case (n=122) | ||||
| Mean | 95% CL | Mean | 95% CL | P | |
| Linoleic acid 18:2n-6 | 1140.2 | 1037.9, 1242.5 | 1044.3 | 863.5, 1225.1 | 0.365 |
| α-linolenic acid 18:3n-3 | 79.3 | 72.8, 85.9 | 76.1 | 64.5, 87.7 | 0.634 |
| Arachidonic acid 20:4n-6 | 76.5 | 70.2, 82.8 | 87.2 | 76.1, 98.4 | 0.099 |
| Eicosapentaenoic acid 20:5n-3 | 99.8 | 89.2, 110.4 | 98.0 | 79.3, 116.7 | 0.872 |
| Docosahexaenoic acid 22:6n-3 | 19.7 | 17.7, 21.7 | 15.6 | 12.1, 19.1 | 0.043 |
| n-6/n-3 PUFA ratio (×100) | 87.9 | 82.8, 93.1 | 101.4 | 92.4, 110.5 | 0.011 |
In covariate-adjusted multivariable logistic regression, a higher n-6/n-3 PUFA ratio was associated with greater odds of TMD pain (Table 3). In the fully adjusted model, odds of TMD increased 1.75 times (95% CL: 1.16, 2.64) per unit increase in n-6/n-3 PUFA ratio. The essential fatty acids LA and ALA were not significantly associated with TMD.
Table 3.
Multivariable-adjusted odds ratios (OR) with 95% confidence limits (CL) for painful temporomandibular disorder associated with n-6/n-3 PUFA ratio, n=503
| Minimally adjusted model(a) | Partially adjusted model(b) | Fully adjusted model(c) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CL | P | OR | 95% CL | P | OR | 95% CL | P | |
| n-6/n-3 PUFA ratio | 1.48 | 1.01, 2.16 | 0.046 | 1.58 | 1.07, 2.34 | 0.021 | 1.75 | 1.16, 2.64 | 0.007 |
| Age, y | |||||||||
| 18–29 | Ref | Ref | Ref | ||||||
| 30–39 | 0.57 | 0.34, 0.96 | 0.036 | 0.56 | 0.33, 0.95 | 0.032 | 0.56 | 0.33, 0.97 | 0.037 |
| 40–49 | 0.75 | 0.45, 1.24 | 0.260 | 0.82 | 0.48, 1.38 | 0.447 | 0.83 | 0.49, 1.40 | 0.476 |
| 50–74 | 0.96 | 0.54, 1.72 | 0.894 | 0.98 | 0.54, 1.79 | 0.948 | 0.97 | 0.53, 1.78 | 0.922 |
| Sex | |||||||||
| Men | Ref | Ref | Ref | ||||||
| Women | 1.42 | 0.95, 2.14 | 0.090 | 1.43 | 0.94, 2.17 | 0.096 | 1.41 | 0.92, 2.14 | 0.111 |
| Race(d) | |||||||||
| White | Ref | Ref | |||||||
| Black/African American | 0.40 | 0.23, 0.70 | 0.001 | 0.38 | 0.22, 0.68 | 0.001 | |||
| Other | 1.89 | 1.03, 3.45 | 0.040 | 1.86 | 1.02, 3.42 | 0.044 | |||
| Obesity | |||||||||
| Not obese (BMI <30 kg/m2) | Ref | Ref | |||||||
| Obese (BMI ≥30 kg/m2) | 1.28 | 0.84, 1.95 | 0.241 | 1.27 | 0.83, 1.93 | 0.272 | |||
| Linoleic acid 18:2n-6 (z-score) | 1.36 | 0.88, 2.10 | 0.165 | ||||||
| α-linolenic acid 18:3n-3 (z-score) | 0.94 | 0.62, 1.42 | 0.780 | ||||||
| Intercept | 0.33 | 0.18, 0.62 | 0.001 | 0.33 | 0.17, 0.63 | 0.001 | 0.26 | 0.13, 0.53 | <0.001 |
Model adjusted for study site (not shown), age and sex
Model additionally adjusted race/ethnicity and obesity
Model additionally adjusted for the essential fatty acids linoleic acid and α-linolenic acid
Racial groups are White; Black/African American; or Other (Asian; Native American; Other or multiple races; Not stated. Includes Hispanic ethnicity).
Likewise, a higher n-6/n-3 PUFA ratio was associated with greater odds of low back pain (Table 4). In the fully adjusted model, odds of low back pain increased 1.63 times (95% CL: 1.07, 2.49) per unit increase in n-6/n-3 PUFA ratio. As with TMD, the essential fatty acids LA and ALA were not significantly associated with low back pain.
Table 4.
Multivariable-adjusted odds ratios (OR) with 95% confidence limits (CL) for low back pain associated with n-6/n-3 PUFA ratio, n=503
| Minimally adjusted model(a) | Partially adjusted model(b) | Fully adjusted model(c) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CL | P | OR | 95% CL | P | OR | 95% CL | P | |
| n-6/n-3 PUFA ratio | 1.64 | 1.11, 2.44 | 0.013 | 1.67 | 1.11, 2.52 | 0.014 | 1.63 | 1.07, 2.49 | 0.022 |
| Age, y | |||||||||
| 18–29 | Ref | Ref | Ref | ||||||
| 30–39 | 1.52 | 0.82, 2.82 | 0.183 | 1.45 | 0.78, 2.70 | 0.242 | 1.45 | 0.78, 2.71 | 0.239 |
| 40–49 | 2.26 | 1.24, 4.09 | 0.007 | 2.04 | 1.11, 3.75 | 0.022 | 2.02 | 1.10, 3.72 | 0.024 |
| 50–74 | 2.24 | 1.13, 4.43 | 0.020 | 2.00 | 1.00, 3.99 | 0.050 | 2.00 | 1.00, 4.00 | 0.051 |
| Sex | |||||||||
| Men | Ref | Ref | Ref | ||||||
| Women | 0.81 | 0.52, 1.26 | 0.358 | 0.78 | 0.50, 1.22 | 0.275 | 0.79 | 0.51, 1.24 | 0.311 |
| Race(d) | |||||||||
| White | Ref | Ref | |||||||
| Black/African American | 0.94 | 0.54, 1.64 | 0.840 | 0.95 | 0.55, 1.66 | 0.866 | |||
| Other | 0.93 | 0.45, 1.90 | 0.841 | 0.92 | 0.45, 1.89 | 0.828 | |||
| Obesity | |||||||||
| Not obese (BMI <30 kg/m2) | Ref | Ref | |||||||
| Obese (BMI ≥30 kg/m2) | 1.71 | 1.10, 2.66 | 0.017 | 1.72 | 1.10, 2.68 | 0.017 | |||
| Linoleic acid 18:2n-6 (z-score) | 0.76 | 0.46, 1.25 | 0.278 | ||||||
| α-linolenic acid 18:3n-3 (z-score) | 1.26 | 0.79, 1.99 | 0.333 | ||||||
| Intercept | 0.12 | 0.06, 0.25 | <0.001 | 0.10 | 0.05, 0.22 | <0.001 | 0.10 | 0.05, 0.24 | <0.001 |
Model adjusted for study site (not shown), age and sex
Model additionally adjusted race/ethnicity and obesity
Model additionally adjusted for the essential fatty acids linoleic acid and α-linolenic acid
Racial groups are White; Black/African American; or Other (Asian; Native American; Other or multiple races; Not stated. Includes Hispanic ethnicity).
Tables 3 and 4 highlight differences between the two pain conditions. Specifically, age had a U-shape relationship with TMD pain, with odds greatest in youngest and oldest age groups. In contrast, age was positively associated with odds of low back pain. In addition, obesity was not associated with TMD, but was positively associated with low back pain.
Of note, no individual PUFA was associated with either pain condition (data not shown). However, associations became evident when exploring effect modification by sex (Figures 2A–2H). A greater concentration of n-6 AA was associated with TMD (p effect modification = 0.065) and low back pain (p effect modification = 0.071) for women but not for men (Figs 2A, 2B). In contrast, a greater circulating concentration n-3 EPA, thought to be antinociceptive, was associated with lower probabilities of TMD (p effect modification = 0.051) and low back pain (p effect modification = 0.003) for men but not for women (Figs 2C, 2D). Similarly, although higher concentration of n-3 DHA was associated with lower probabilities of TMD pain and low back pain for both sexes, the protective influence was more pronounced for men (Figs 2E, 2F). Men and women had similar probability of TMD (p effect modification = 0.173) with a higher n-6/n-3 PUFA ratio (Fig 2G), but a higher n-6/n-3 PUFA ratio was associated with substantially greater probability of low back pain (p effect modification = 0.001) in men than women (Fig 2H). With this one exception, women fared worse than men with respect to the presumed putative and protective associations of long-chain PUFAs with TMD and low back pain. Sex-specific main effects from these covariate-adjusted logistic regression models are reported in Supplementary Table 3.
Figure 2.
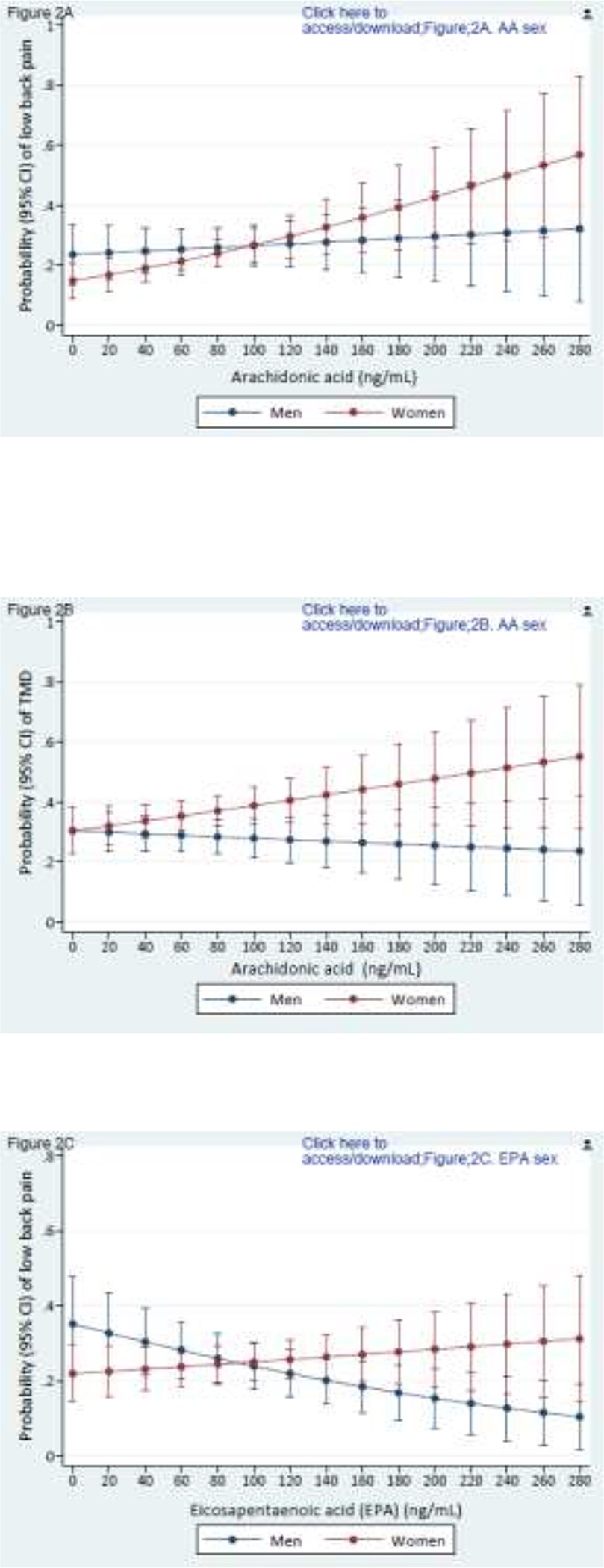
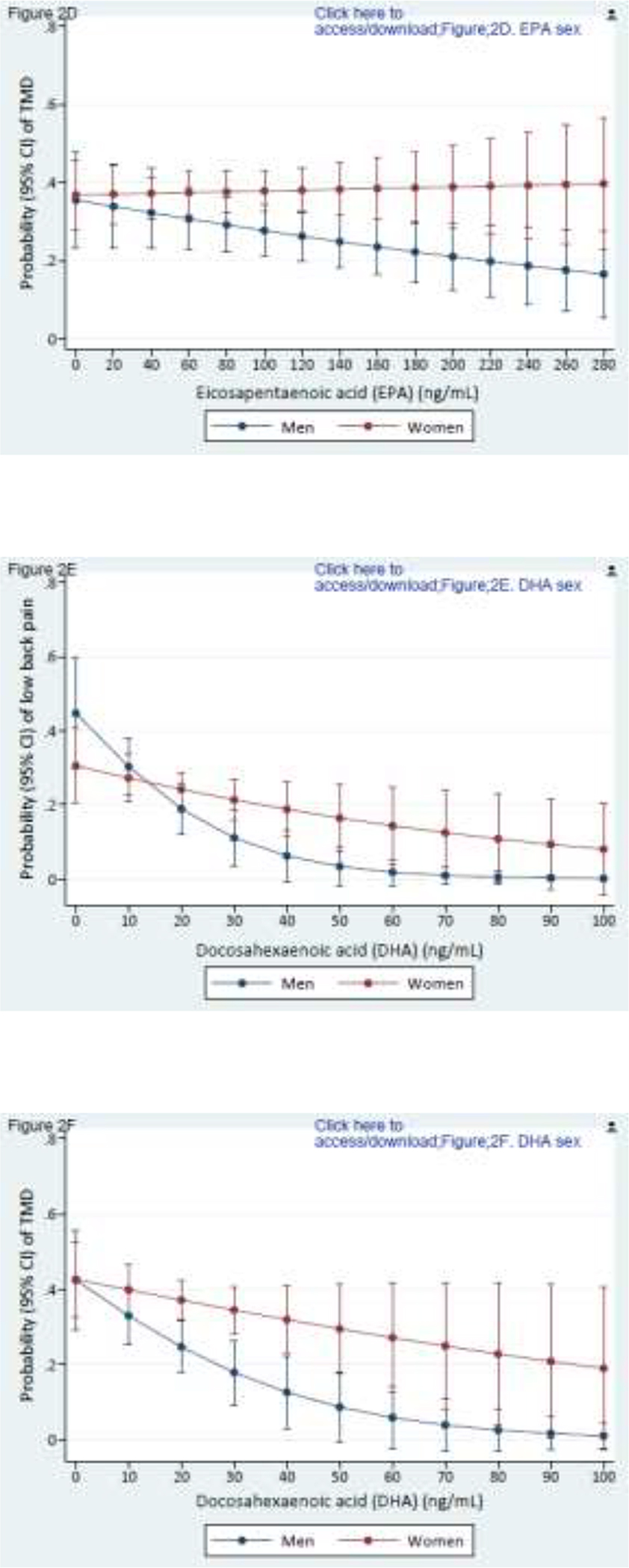
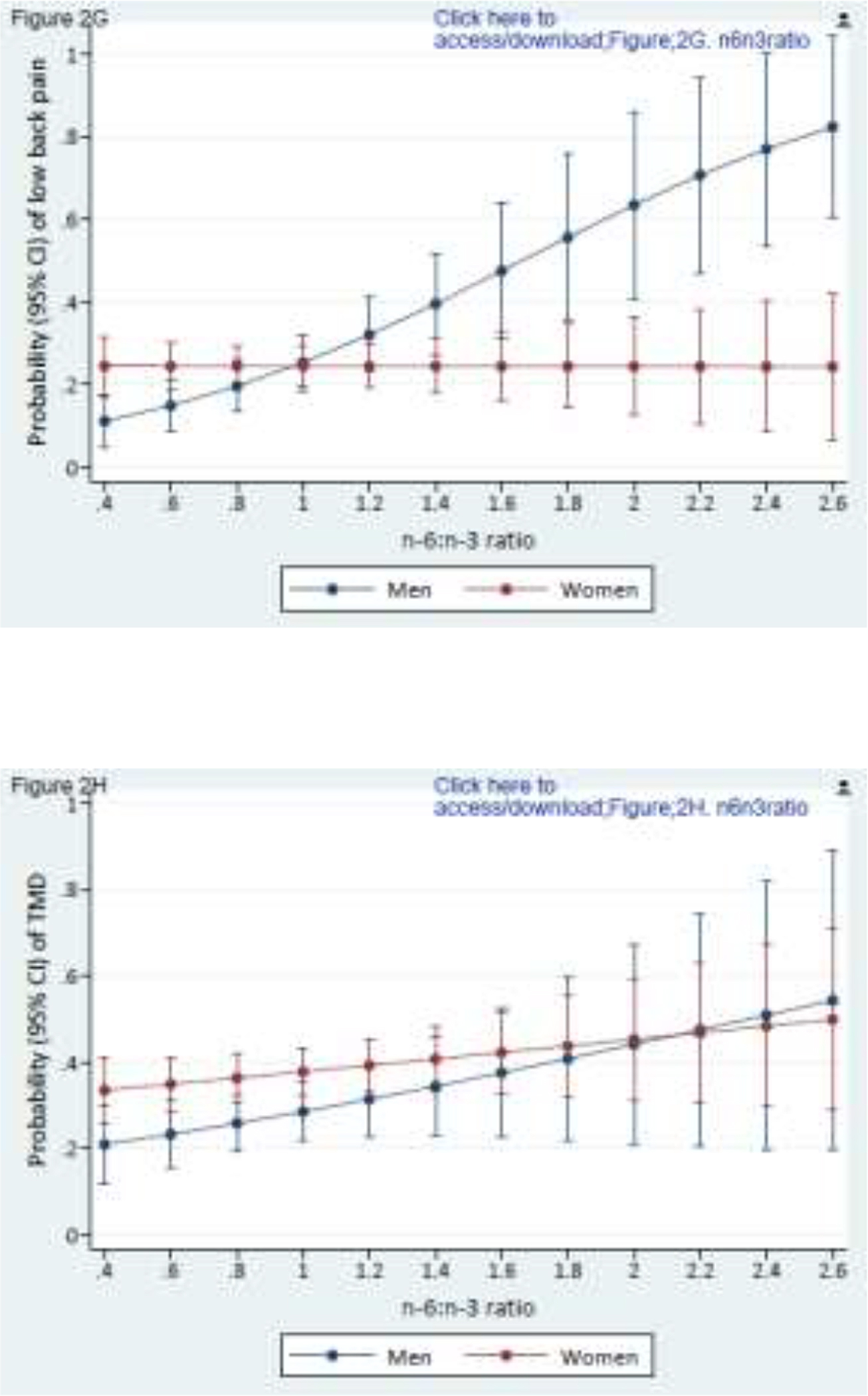
Composite Figures 2A to 2H report sex differences in the relationships between PUFAs and pain conditions. Values are predicted probabilities of painful temporomandibular disorder (TMD) and low back pain (low back pain) taken from a multivariable logistic regression model, adjusted for linoleic acid and α-linolenic acid (n=503 in all figures).
Figures 2A and 2B depict the relationship of arachidonic acid with TMD pain and low back pain for men and women (P effect modification = 0.065 for TMD; and 0.071 for low back pain, respectively).
Figures 2C and 2D depict the relationship of eicosapentaenoic acid and TMD pain and low back pain for men and women (p effect modification = 0.051 for TMD; and 0.003 for low back pain, respectively).
Figures 2E and 2F depict the relationship of docosahexaenoic acid and TMD pain and low back pain for men and women (p effect modification = 0.048 for TMD; and 0.019 for low back pain, respectively).
Figures 2G and 2H depict the relationship of n-6/n-3 PUFA ratio and TMD pain and low back pain for men and women (p effect modification = 0.173 for TMD; and 0.001 for low back pain, respectively).
Multivariable logistic regression models were fit with ALA values computed by multiple imputation (Supplementary Table4). This showed that the n-6/n-3 PUFA ratio remained positively associated with TMD (OR= 1.42, 95% CL: 1.05, 1.92) with a narrower confidence interval as expected. In the imputed model, the effect size for the association between n-6/n-3 PUFA ratio and low back pain was smaller (OR=1.37, 95% CL: 0.98, 1.91, Supplementary Table 5). We repeated two multivariable logistic regression analyses again, this time imputing a value of 0.5 ug/mL for missing ALA value. This value is the midpoint between zero and the laboratory limit of detection (0.97 ng/mL), on the assumption that ALA was present in samples but only at very low levels. Again, the results were not appreciably altered (data not shown). These sensitivity analyses revealed that complete case analysis did not meaningfully bias the results.
In multivariable linear regression, the n-6/n-3 PUFA ratio was associated with greater facial pain intensity (β=4.57, 95% CL: 0.62, 8.52, P=0.023), results not tabulated. When tertiles of the n-6/n-3 PUFA ratio were modelled in the same analysis, significantly greater intensity of facial pain was observed in the highest tertile compared with the lowest tertile (Figure3).
Figure 3.
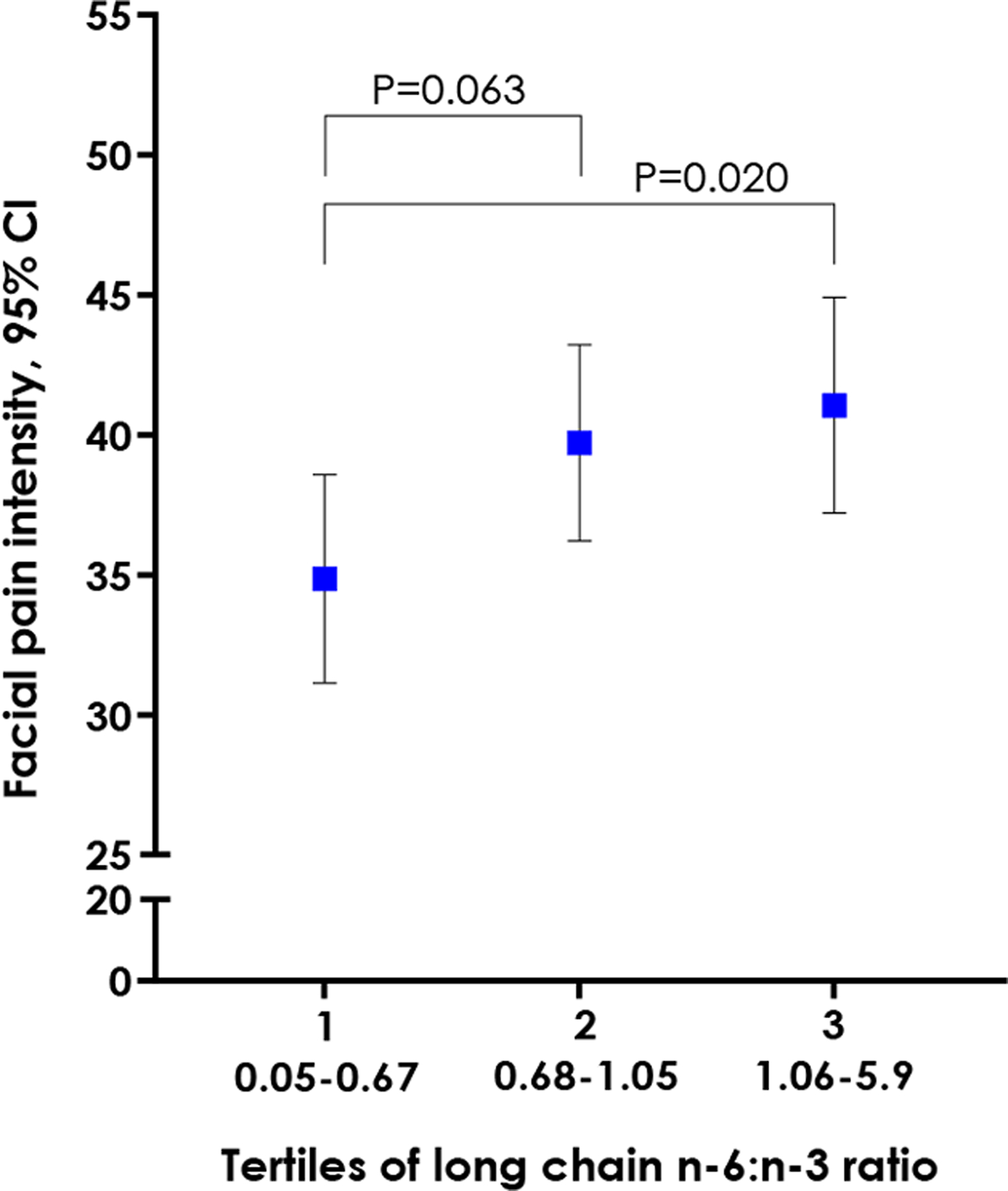
Predicted TMD pain intensity across tertiles of the LC n-6/n-3 PUFA ratio. Values are covariate-adjusted marginal estimates taken from a linear regression analysis where the dependent variable is reported facial pain intensity rated on a 0 to 100 numeric rating scale. Covariates are study site, age, sex, linoleic acid, and α-linolenic acid.
DISCUSSION
In this study, a high ratio of n-6 long-chain AA to n-3 long-chain PUFAs was associated with greater odds of painful TMD, greater intensity of facial pain, and greater odds of low back pain. No individual long-chain PUFA was associated with TMD pain or low back pain in the study population as a whole. That was because the associations were obscured by sex-specific differences in these relationships. Closer examination showed that higher levels of n-6 AA were associated with greater probability of both pain conditions for women, but not for men. Further, higher levels of n-3 EPA and DHA were associated with lower probability of both pain conditions for men, but not for women.
These findings highlight dual disadvantages for women in that the putative pronociceptive associations of n-6 were stronger for women and the protective antinociceptive associations of n-3 were weaker for women. Because idiopathic pain prevalence is higher in women, a better understanding is needed of sex-specific differences in molecular pathways linking PUFAs to pain. Previous reports of sex differences in conversion of essential fatty acids to their longer-chain metabolites20 fall short of elucidating health consequences of these differences. These sex-specific differences also raise questions for planning randomized clinical trials about whether men and women differ in the potential therapeutic efficacy of EPA and DHA.
The strength of evidence for sex differences in these associations is weak from this single observational study. It is important that future studies determine whether the women and men respond differently to levels of circulating PUFAs. Randomized clinical trials will provide stronger evidence. Our findings have clinical relevance in as much as they address the important unanswered question of why pain prevalence is greater in women than men. While the n-6/n-3 PUFA ratio was associated with both pain conditions in the full sample, none of the individual constituents of the PUFA ratio were associated with either pain condition in the full sample. One explanation is that the imbalance between n-6 and n-3 PUFAs matters more for TMD pain and low back pain that the absolute levels of the individual constituents.
The parent study, OPPERA-II, defined pain outcomes as TMD vs no TMD and low back pain vs no low back pain. Each classification was independent of the other, and hence some TMD controls had low back pain as did some TMD cases. Likewise, some low back pain controls had TMD, as did some low back pain cases. The objective of the case-control study design is to determine if an exposure is associated an outcome. Controls must be free of the outcome of interest, and yet be capable of getting it. There is no requirement that controls, or cases, be free of other outcomes. In fact, in this study, only 22% of TMD cases were free of the other four pain conditions studied.45 To restrict TMD cases and controls to people free of back pain conditions would seriously limit the generalizability of findings to the general population. That is because in “real world” conditions, substantial overlap exists between certain pain conditions. TMD and low back pain belong to a set of pain conditions labelled “chronic overlapping pain disorders”, because of the high level of their coexistence.
Clinical studies of PUFAs and pain are dominated by inflammatory pain disorders, such as rheumatoid arthritis,23 inflammatory bowel disease,1 and dysmenorrhea.30 One exception was a cross-sectional study of 167 adults with knee osteoarthritis. In that study, adults with a high n-6/n-3 PUFA ratio, relative to a low ratio, had greater experimental pain sensitivity, greater clinical pain, and more functional limitations.42 Stronger evidence comes from a randomized controlled trial (RCT) in adults with frequent migraine,33 in which the role of inflammation is unclear.12 The RCT compared two dietary PUFA interventions: one that increased n-3 intake while maintaining n-6 intake, and the other that increased n-3 and decreased n-6 intake, against a control diet that maintained n-3 and n-6 PUFA intake at levels of the typical American diet. Both interventional diets reduced headache frequency and intensity compared with the control diet.33
Evidence for systemic inflammation in chronic TMD pain is weak. Our group evaluated systemic levels of 22 pro- and anti-inflammatory cytokines in 344 women in a case-control study of chronic TMD pain. It found that cases differed from controls in only two of the 22 markers, and differences were in opposing directions: cases had elevated levels of the proinflammatory cytokine MCP-1 and elevated levels of the anti-inflammatory cytokine IL-1ra.44 Evidence from other clinical studies of systemic inflammation in TMD pain is limited by small sample sizes, a restricted age range, and the exclusion of men, which precludes examination of sex differences inflammatory responses in chronic pain.21, 29, 34, 46 If systemic inflammation is not a major feature of idiopathic pain disorders, then any effect of PUFAs on these disorders must operate through other pathways. One likely mechanism is a direct effect in modulating nociceptive signaling independently of inflammatory processes.
Preclinical studies show that n-6 PUFAs, in addition to their mostly inflammatory actions, are precursors of oxylipins with pronociceptive effects.3 Animal models have demonstrated functional-behavioral links connecting n-6 derived oxylipins, and their molecular biosynthetic pathways, with pain and nociception.11 Likewise, n-3 EPA and DHA are precursors for bioactive oxylipins with potent antinociceptive and analgesic properties.11
Most notable is a metabolite class of specialized proresolving mediators (SPMs), comprised of resolvins, protectins, maresins and lipoxins. In an exploratory single-arm, non-randomized clinical trial, 44 adults with chronic moderate to severe pain were administered SPM precursor-containing oral marine oil supplements, standardized to 17-HDHA and 18-HEPE. After four weeks, their PROMIS-43 scores for pain interference and pain intensity decreased significantly from baseline levels, while no change was detected in biomarkers of inflammation.6
Studies specify the n-6/n-3 PUFA ratio in different ways. The most inclusive method is to divide the sum of all short- and long-chain n-6 PUFAs by the sum of all short- and long-chain n-3 PUFAs. However, that method is criticized because the shorter 18-carbon fatty acids have different physiological effects on biological parameters than the long-chain PUFAs,47 and, in aggregating all PUFAs into a single index, it is difficult to disentangle the influential PUFAs from the less important ones. The nonsignificant association of LA and ALA with TMD and low back in our study would be obscured had those PUFAs been included in the computed PUFA ratio. Another approach, as used in this study, is to calculate the ratio using only long-chain PUFAs.. We specified this ratio as AA/EPA+DHA. This ratio was applied in an earlier study examining PUFAs and breast cancer cell lines.22
A simpler version specification of AA/EPA has been used in studies of depression,35, 41 metastatic colorectal cancer,49 and non-alcoholic fatty liver disease.48 The ratio AA/DHA was studied with cystic fibrosis25 and the protective ratios DHA/AA and EPA/AA were studied with acute coronary syndrome.26
This variation in PUFA ratio specification across studies means that effect sizes are not directly comparable. For example, a commonly cited paper by Simopoulos43 points out that the n-6/n-3 PUFA ratio increased from 1:1 during evolution to 20:1 today or even higher. Because computation of those ratios drew on all PUFAs in the LA and ALA series, they are quite different from ratios based on a subset of these PUFAs. Papers sometimes recommend an optimal ratio of around 2:1, but this is dependent on how the ratio is specified. We also make the case that not all PUFAs are equally potent. For example, we found that the precursor fatty acids LA and ALA had very weak and non-significant associations with pain. When the ratio is computed from all available PUFAs it obscures detection of which PUFAs are associated with pain and which are not. A strength of our study was its biochemical assessment of PUFAs. Since circulating PUFA concentrations take account of variability in metabolism and absorption, they more accurately assess bioavailability than dietary intake measures. Another strength was that TMD presence or absence was verified by examiner following a rigorous protocol. Limitations should be considered in the interpretation of our results. As an observational study, no definitive conclusions can be made regarding causality. Although we adjusted for known confounding variables, the possibility remains of residual confounding. OPPERA-II participants were not asked to fast before blood collection. However non-fasting samples are unlikely to bias findings because erythrocytes are insensitive to fasting status.15 Erythrocytes have lower within-person variability than plasma,51 are less affected by an acute dose of n-3 PUFAs,17 have a much longer half-life than plasma and are a better indicator of diet over weeks because plasma concentration reflect intake in the previous 24 hours.17
We see three directions for future studies. One is to determine whether other studies replicate our observed sex differences in associations between long-chain PUFAs and pain. If future studies corroborate these sex-specific differences, dosing schedules of fish-oil supplementation in randomized clinical trials may differ for men and women with chronic pain. Another direction is to elucidate mechanistic pathways of EPA- and DHA-derived metabolites in idiopathic pain conditions. Third, we recommend a phase 2 RCT to explore potential analgesic or antinociceptive effect of n-3 oxylipins in people with chronic idiopathic pain.
Supplementary Material
Highlights.
A higher n-6/n-3 PUFA ratio was associated with greater odds of chronic pain
A higher n-6/n-3 PUFA ratio was associated with greater facial pain intensity
A higher n-6 AA level was associated with greater odds of pain in women but not men
Higher n-3 PUFA levels were associated with lower odds of pain in men but not women
PUFAs likely influence these pain conditions through non-inflammatory pathways
Perspective.
Perspective: This cross-sectional clinical study found that a higher ratio of circulating n-6/n-3 long-chain PUFAs was associated with greater odds of two common chronic overlapping pain conditions. This suggests that that the pro- and anti-nociceptive properties of n-6 and n-3 PUFAs, respectively, influence pain independently of their well-established inflammatory pathways.
Research Funding
Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Number R21DE029746. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. It was also supported by the NIH Clinical and Translational Science Award (CTSA) # 550KR171714.
Disclosures
Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Number R21DE029746. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflict of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bjorkkjaer T, Brun JG, Valen M, Arslan G, Lind R, Brunborg LA, Berstad A, Froyland L. Short-term duodenal seal oil administration normalised n-6 to n-3 fatty acid ratio in rectal mucosa and ameliorated bodily pain in patients with inflammatory bowel disease. Lipids Health Dis. 5:6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 93:950–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd JT, LoCoco PM, Furr AR, Bendele MR, Tram M, Li Q, Chang FM, Colley ME, Samenuk GM, Arris DA, Locke EE, Bach SBH, Tobon A, Ruparel SB, Hargreaves KM. Elevated dietary omega-6 polyunsaturated fatty acids induce reversible peripheral nerve dysfunction that exacerbates comorbid pain conditions. Nat Metab. 3:762–773, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 88:411–420, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 83:1505S–1519S, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Callan N, Hanes D, Bradley R. Early evidence of efficacy for orally administered SPM-enriched marine lipid fraction on quality of life and pain in a sample of adults with chronic pain. J Transl Med. 18:401, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 94:1914S–1919S, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA. Diagnostic evaluation of LBP: reaching a specific diagnosis is often impossible. Arch Intern Med. 162:1444–1447; discussion 1447–1448, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 123:226–230, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, Wyatt M, Cassidy JD, Rossignol M, Leboeuf-Yde C, Hartvigsen J, Leino-Arjas P, Latza U, Reis S, Gil Del Real MT, Kovacs FM, Oberg B, Cedraschi C, Bouter LM, Koes BW, Picavet HS, van Tulder MW, Burton K, Foster NE, Macfarlane GJ, Thomas E, Underwood M, Waddell G, Shekelle P, Volinn E, Von Korff M. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976). 33:95–103, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Domenichiello AF, Sapio MR, Loydpierson AJ, Maric D, Goto T, Horowitz MS, Keyes GS, Yuan ZX, Majchrzak-Hong SF, Mannes AJ, Iadarola MJ, Ramsden CE. Molecular Pathways Linking Oxylipins to Nociception in Rats. J Pain. 22:275–299, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol. 15:483–490, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Fillingim RB. Sex differences in analgesic responses: evidence from experimental pain models. Eur J Anaesthesiol Suppl. 26:16–24, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 129:210–223, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Harris WS, Varvel SA, Pottala JV, Warnick GR, McConnell JP. Comparative effects of an acute dose of fish oil on omega-3 fatty acid levels in red blood cells versus plasma: implications for clinical utility. J Clin Lipidol. 7:433–440, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Isong U, Gansky SA, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain. 22:317–322, 2008 [PMC free article] [PubMed] [Google Scholar]
- 17.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 38:2012–2022, 1997 [PubMed] [Google Scholar]
- 18.Laine DC, Snodgrass CM, Dawson EA, Ener MA, Kuba K, Frantz ID Jr. Lightly hydrogenated soy oil versus other vegetable oils as a lipid-lowering dietary constituent. Am J Clin Nutr. 35:683–690, 1982 [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Lee H, Kang S, Park WJ. Fatty Acid Desaturases, Polyunsaturated Fatty Acid Regulation, and Biotechnological Advances. Nutrients. 8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohner S, Fekete K, Marosvolgyi T, Decsi T. Gender differences in the long-chain polyunsaturated fatty acid status: systematic review of 51 publications. Ann Nutr Metab. 62:98–112, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Louca Jounger S, Christidis N, Svensson P, List T, Ernberg M. Increased levels of intramuscular cytokines in patients with jaw muscle pain. J Headache Pain. 18:30, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansara PP, Deshpande RA, Vaidya MM, Kaul-Ghanekar R. Differential Ratios of Omega Fatty Acids (AA/EPA+DHA) Modulate Growth, Lipid Peroxidation and Expression of Tumor Regulatory MARBPs in Breast Cancer Cell Lines MCF7 and MDA-MB-231. PLoS One. 10:e0136542, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 107 Suppl 2:S171–184, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Mogil JS. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci. 21:353–365, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Morin C, Cantin AM, Vezina FA, Fortin S. The Efficacy of MAG-DHA for Correcting AA/DHA Imbalance of Cystic Fibrosis Patients. Mar Drugs. 16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizaki Y, Shimada K, Tani S, Ogawa T, Ando J, Takahashi M, Yamamoto M, Shinozaki T, Miyazaki T, Miyauchi K, Nagao K, Hirayama A, Yoshimura M, Komuro I, Nagai R, Daida H. Association between the ratio of serum n-3 to n-6 polyunsaturated fatty acids and acute coronary syndrome in non-obese patients with coronary risk factor: a multicenter cross-sectional study. BMC Cardiovasc Disord. 20:160, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, Lim PF, Ribeiro-Dasilva M, Greenspan JD, Knott C, Maixner W, Slade G. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 12:T27–45, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 8:399–408, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JW, Chung JW. Inflammatory Cytokines and Sleep Disturbance in Patients with Temporomandibular Disorders. J Oral Facial Pain Headache. 30:27–33, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Rahbar N, Asgharzadeh N, Ghorbani R. Effect of omega-3 fatty acids on intensity of primary dysmenorrhea. Int J Gynaecol Obstet. 117:45–47, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung YM, Berk M, Mann JD. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 87:135–141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsden CE, Ringel A, Majchrzak-Hong SF, Yang J, Blanchard H, Zamora D, Loewke JD, Rapoport SI, Hibbeln JR, Davis JM, Hammock BD, Taha AY. Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids: Implications for idiopathic pain syndromes? Mol Pain. 12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsden CE, Zamora D, Faurot KR, MacIntosh B, Horowitz M, Keyes GS, Yuan ZX, Miller V, Lynch C, Honvoh G, Park J, Levy R, Domenichiello AF, Johnston A, Majchrzak-Hong S, Hibbeln JR, Barrow DA, Loewke J, Davis JM, Mannes A, Palsson OS, Suchindran CM, Gaylord SA, Mann JD. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: randomized controlled trial. BMJ. 374:n1448, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro-Dasilva MC, Fillingim RB, Wallet SM. Estrogen-Induced Monocytic Response Correlates with TMD Pain: A Case Control Study. J Dent Res. 96:285–291, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzo AM, Corsetto PA, Montorfano G, Opizzi A, Faliva M, Giacosa A, Ricevuti G, Pelucchi C, Berra B, Rondanelli M. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutr J. 11:82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin DBAn-iaflseomviaaovdJotRSS, Series C 21: 136–141.
- 37.Schafer JLAoIMDBR, FL: Chapman & Hall/CRC. [Google Scholar]
- 38.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF, International Rdc/Tmd Consortium Network IafDR, Orofacial Pain Special Interest Group IAftSoP. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache. 28:6–27, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Select Committee on Nutrition and Human Needs. Dietary Goals for the United States. Washington: DUSGPO. [Google Scholar]
- 40.Sessle BJ. Why are the diagnosis and management of orofacial pain so challenging? J Can Dent Assoc. 75:275–277, 2009 [PubMed] [Google Scholar]
- 41.Shibata M, Ohara T, Yoshida D, Hata J, Mukai N, Kawano H, Kanba S, Kitazono T, Ninomiya T. Association between the ratio of serum arachidonic acid to eicosapentaenoic acid and the presence of depressive symptoms in a general Japanese population: the Hisayama Study. J Affect Disord. 237:73–79, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Sibille KT, King C, Garrett TJ, Glover TL, Zhang H, Chen H, Reddy D, Goodin BR, Sotolongo A, Petrov ME, Cruz-Almeida Y, Herbert M, Bartley EJ, Edberg JC, Staud R, Redden DT, Bradley LA, Fillingim RB. Omega-6: Omega-3 PUFA Ratio, Pain, Functioning, and Distress in Adults With Knee Pain. Clin J Pain. 34:182–189, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 8:128, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W, Nackley AG. Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 152:2802–2812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slade GD, Greenspan JD, Fillingim RB, Maixner W, Sharma S, Ohrbach R. Overlap of Five Chronic Pain Conditions: Temporomandibular Disorders, Headache, Back Pain, Irritable Bowel Syndrome, and Fibromyalgia. J Oral Facial Pain Headache. 34:s15–s28, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Son C, Park YK, Park JW. Long-term evaluation of temporomandibular disorders in association with cytokine and autoantibody status in young women. Cytokine. 144:155551, 2021 [DOI] [PubMed] [Google Scholar]
- 47.Stanley JC, Elsom RL, Calder PC, Griffin BA, Harris WS, Jebb SA, Lovegrove JA, Moore CS, Riemersma RA, Sanders TA. UK Food Standards Agency Workshop Report: the effects of the dietary n-6:n-3 fatty acid ratio on cardiovascular health. Br J Nutr. 98:1305–1310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tutino V, De Nunzio V, Caruso MG, Bonfiglio C, Franco I, Mirizzi A, De Leonardis G, Cozzolongo R, Giannuzzi V, Giannelli G, Notarnicola M, Osella AR. Aerobic Physical Activity and a Low Glycemic Diet Reduce the AA/EPA Ratio in Red Blood Cell Membranes of Patients with NAFLD. Nutrients. 10, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tutino V, De Nunzio V, Caruso MG, Veronese N, Lorusso D, Di Masi M, Benedetto ML, Notarnicola M. Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients. Int J Mol Sci. 20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 50:133–149, 1992 [DOI] [PubMed] [Google Scholar]
- 51.H. WS Assessing fatty acid biostatus: Red blood cells or plasma?. Lipid Technology. 25:79–81., 2013 [Google Scholar]
- 52.Wu A, March L, Zheng X, Huang J, Wang X, Zhao J, Blyth FM, Smith E, Buchbinder R, Hoy D. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 8:299, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


