Abstract
The COVID-19 pandemic amplified the need for interventions to support community-dwelling families living with dementia. This study examined the feasibility and acceptability of a remotely delivered weighted blanket intervention for people living with dementia, and the feasibility of collecting outcome measures specific to people with dementia and caregivers. A prospective, within subjects, pre-post design was used; 21 people with dementia and their caregivers participated. Measures of feasibility (days blanket was used for the recommended duration, injuries/adverse events, enrollment and withdrawal rate, time to recruit sample) and acceptability (tolerability, satisfaction, and benefit perceived by participants with dementia and caregivers) were examined. Feasibility of collecting measures was examined through missing data. Results indicated high feasibility and acceptability. Collecting caregiver completed outcome measures was feasible, but measures completed by self-report by people with dementia was not. Weighted blankets are a promising tool for this population that warrant further examination to determine efficacy.
Keywords: Dementia, caregivers, home care, weighted blankets, COVID-19, feasibility studies
Although dementia is commonly considered a memory related disease, behavioral and psychological symptoms of dementia (BPSD) are a key driver of rapidly increasing economic and societal costs (Livingston et al., 2017). BPSD encompass an array of symptoms (e.g., anxiety, apathy, depression, aggression, agitation, mania, psychosis) that virtually all people living with dementia (PwD) experience (Hall & Buckwalter, 1987; Keng et al., 2020). Much of the burden of BPSD (financial, social, and emotional) falls on families (Burley et al., 2020).
Medications to manage BPSD have demonstrated minimal efficacy and high potential for adverse effects (Kales et al., 2019), thus non-pharmacologic interventions are preferred. Efficacious non-pharmacologic interventions for PwD are often complex, multilevel interventions requiring frequent interactions with the healthcare system (e.g., case management, psychoeducation, caregiver training, peer support, cognitive behavioral training) (Trivedi et al., 2019). A major limitation of these interventions is limited uptake in real-world community settings, likely due to low acceptance by families living with dementia and limited feasibility for the home (Gitlin et al., 2020). Simpler interventions with fewer components that place minimal burden on caregivers may be a promising approach to improving the well-being of PwD and their caregivers.
The COVID-19 pandemic has disproportionately affected the physical, psychological, and social health of older adults who experience BPSD and their families (Keng et al., 2020). It has amplified the demand for interventions that do not require in-person interaction (Rising et al., 2021). Exploration of simple non-pharmacologic interventions that can be used in the home, require minimal training, and can be delivered remotely are warranted.
Weighted Blankets as a Non-Pharmacologic Intervention
Weighted blankets are used like comforters and provide deep pressure stimulation to broad areas of the body (Parker & Koscinski, 2016). Deep pressure stimulation increases the arousal of the parasympathetic nervous system and reduces sympathetic arousal, which yield a calming effect (Reynolds et al., 2015). Use of weighted blankets improved several stress-related outcomes (e.g., sleep, fatigue, depression, anxiety, physiologic stress) among non-cognitively impaired adults with mental health conditions (Champagne et al., 2015; Ekholm et al., 2020). Despite the relative simplicity of weighted blankets and benefits demonstrated in other populations, no studies have examined weighted blankets for PwD (Eron et al., 2020).
The conceptual framework guiding this work is the Progressively Lowered Stress Threshold Model, which posits that PwD experience a heightened perception of stress and decreased tolerability of stressful stimuli, which increases their susceptibility to experiencing BPSD (Hall & Buckwalter, 1987). Weighted blankets may mitigate the potential for BPSD by promoting warmth, relaxation, physical comfort, and sleep through tactile stimulation, which together play an important role in the stress process of PwD (Kim & Buschmann, 2004). Caregivers may also experience benefit from weighted blanket use by PwD according to theories of caregiver stress, which postulate that stress reductions among care recipients may indirectly improve stress and well-being of caregivers (Pearlin et al., 1990).
To expand the science of weighted blankets, the purpose of this study was to examine the feasibility and acceptability of weighted blankets for community dwelling PwD, and to examine the feasibility of collecting outcomes to inform future efficacy trials. Study aims were to:
Aim 1: Examine the feasibility and acceptability of a remotely delivered, in-home weighted blanket intervention for community dwelling PwD as perceived by PwD and their family caregivers.
Aim 2: Examine the feasibility of collecting outcome measures in PwD (i.e., BPSD, cognitive function, quality of life) and their family caregivers (i.e., well-being, self-reported health status) to inform future studies to examine efficacy.
Examining feasibility and acceptability are critical preliminary steps in intervention development as described by the National Institute of Health’s Stage Model for Behavioral Intervention Development (National Institute on Aging, 2018). Feasibility studies are necessary to determine if more costly efficacy testing is warranted, inform planning of future trials, identify intervention components in need of refinement, and detect barriers to future implementation (Bowen et al., 2009; Gadke et al., 2021). Efficacious non-pharmacologic interventions have historically had poor uptake by PwD and caregivers (Burley et al., 2020; Gitlin et al., 2020), which heightens the need for early examinations of intervention feasibility and acceptability.
Methods
This study used a prospective, within subjects, pre-post design with a 4-week intervention period (Figure 1). The CONSORT extension for reporting feasibility studies was used to guide reporting of this study (Lancaster & Thabane, 2019).
Figure 1.
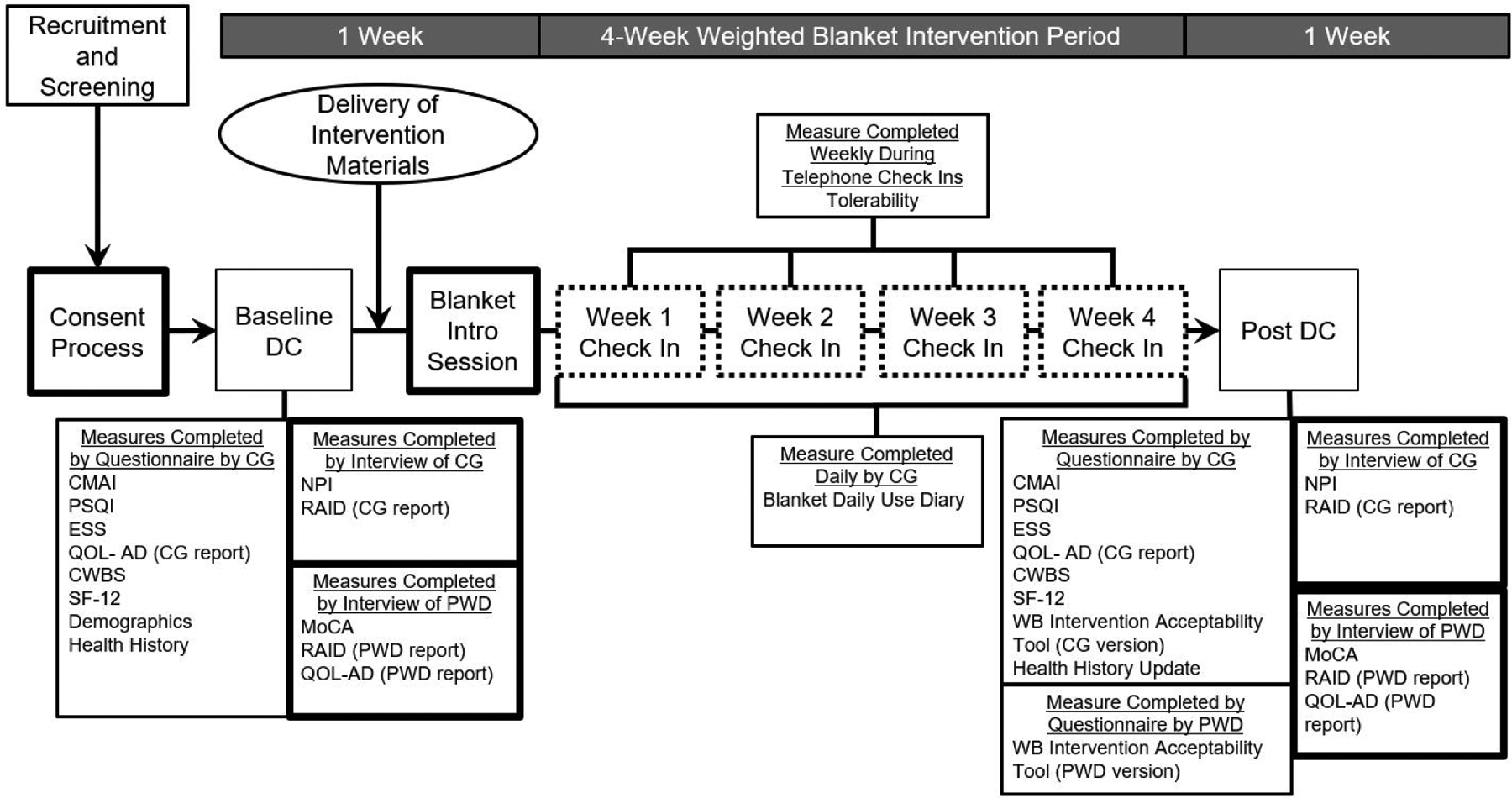
Overview of Weighted Blanket Study Design
 Virtual session vio Zoom or by telephone.
Virtual session vio Zoom or by telephone.
 Weekly telephone check in session.
Weekly telephone check in session.
 Weighted blanket, Weighted Blanket Use Guide and Daily Blanket Use Dairy deliverd to participants’ home by a shipping service.
Weighted blanket, Weighted Blanket Use Guide and Daily Blanket Use Dairy deliverd to participants’ home by a shipping service.
Note. CG Caregiver. CWBS Caregiver Well-Being Scale. CMAI Cohen-Mansfield Agitation Inventory. DC Data collection, ESS Epworth Sleepiness Scale, MoCA Montreal Cognitlve Assessment Test. NPI Neuropsychiatric Inventory. PSQI Pittsburgh Sleep Quality Index, PWD person with dementia, QOL-AD Quality of life in Alzheimer’s Disease Scale. RAID Rating Anxiety in Dementia Scale. WB Weighted blanket
Participants and Setting
The sample included 21 dyads (21 PwD and their family caregivers, 42 people total). Caregivers were screened by telephone to determine eligibility for both members of the dyad using the following inclusion criteria for PwD: 1) age 60 and over with a diagnosis of dementia (of any type), 2) lived in the home with a family caregiver, 3) demonstrated at least 2 BPSD within the past four weeks, 4) weighed 100 pounds or more, and 5) able to lift 10 pounds. Exclusion criteria for PwD were: 1) lived in assisted living or long-term care, 2) a diagnosis of asthma, sleep apnea, or respiratory disorder, 3) paralysis or impaired mobility of limbs, 4) history of claustrophobia, 5) severe open wounds, 6) diabetes, 7) used a weighted blanket in the past month, and 8) unstable medical condition limiting the person’s ability to participate.
Inclusion criteria for family caregivers were: 1) age 21 and over and identified as a primary caregiver of a relative with dementia, 2) lived in the same household as the relative with dementia for at least 1 month, 3) had access and ability to use a telephone, smart phone, tablet, or computer. Caregivers were excluded if unable to read or speak English, or if they had a hearing or visual impairment that limited their ability to complete the telephone screening. Participants were selected by purposive sampling and recruited from October 2020 to February 2021 through dementia support organizations (2 state, 1 national level).
Ethical Considerations
This study was approved by the participating university’s IRB. All participants signed a study consent form electronically. If a person with dementia was unable to sign for themselves, their caregiver signed on their behalf as a proxy.
Weighted Blanket Intervention
A Weighted Blanket Use Guide was developed for this study based on prior research and experiential reports (Eron et al., 2020; Parker & Koscinski, 2016). The guide included: 1) a description of the weighted blanket, 2) directions of when and how to use it, 3) recommended duration of use, 4) safety considerations (See Online Supplementary File 1), and 5) cleaning instructions.
Dyads were mailed the use guide and a weighted blanket. A 10-pound weighted blanket was provided if the person with dementia weighed < 120 pounds, or a 12-pound blanket if they weighed ≥ 120 pounds as recommended by weighted blanket manufacturers and reports on weighted blanket use by older adults (Parker & Koscinski, 2016). Blankets were used in the homes of participants and was individualized based on the dyad’s preferences. They were encouraged to have the person with dementia use the blanket for at least 20 minutes per day.
After receiving the guide and blanket, dyads reviewed materials with the first author during an introduction session (Figure 1). Dyads participated in weekly check-ins by phone to discuss how the blanket was being used, address concerns about use of the blanket, and identify strategies to improve use of the blanket if applicable. All study-related interactions were conducted remotely using Zoom or by telephone.
Outcome Measures and Instruments
Outcomes for Aim 1 were measures of feasibility and acceptability of the weighted blanket intervention, as perceived by PwD and their family caregivers.
Feasibility (Aim 1)
Measures of feasibility were based on feasibility study guidelines, which advise measures focused on feasibility of the intervention and study design (Bowen et al., 2009; Gadke et al., 2021). Feasibility of the intervention was operationalized as 1) average number of days the weighted blanket was used for the recommended duration (at least 20 minutes) across participants, and 2) injuries or adverse events. Feasibility of the study design was operationalized as 1) enrollment rate, 2) number of dyads that declined participation, 3) length of time to recruit the desired sample (20 dyads), and 4) withdrawal rate. Benchmarks of feasibility were defined a priori based on comparable studies (Orgeta et al., 2019; Tamplin et al., 2018): Average number of days that the blanket was used for the minimal recommended 20 minutes per day for 21 days or more; no adverse events or injuries; enrollment rate of at least 50%; declined participation of 10 or fewer dyads; five months or less to recruit the desired sample (20 dyads) and withdrawal rate less than 25%.
A hardcopy Weighted Blanket Daily Use Diary was used to capture the number of days the weighted blanket was used for the recommended duration. Diary items were generated based on prior research on weighted blankets and studies that used daily diaries to measure intervention use (Champagne et al., 2015; Lowery et al., 2014). Total number of minutes the blanket was used by the PWD, their response to the blanket, and challenges encountered to using the blanket were documented each day by caregivers.
Intervention Acceptability (Aim 1)
Intervention acceptability was defined as the 1) extent to which the weighted blanket intervention was tolerated by the person with dementia, 2) degree of satisfaction with the remotely delivered weighted blanket intervention, and 3) benefit of the weighted blanket as reported by PwD and their family caregivers.
Tolerability
Tolerability was operationalized as the extent to which participants with dementia were able to endure the use of the weighted blanket, which was measured using a single item during each of the four-telephone check-ins. Caregivers rated the extent to which the weighted blanket was tolerated by their relatives with dementia over the past week (0=did not tolerate the blanket at all, 10=tolerated the blanket all of the time).
Satisfaction and Benefit
Satisfaction and benefit were measured using a Weighted Blanket Intervention Acceptability Tool. One version was specific to PwD, and a second for caregivers. Items for this tool were modified from a validated tool to measure satisfaction with a dyadic psychoeducational intervention for patients with cancer and their caregivers (Cronbach’s α=0.89 for care recipients with cancer, Cronbach’s α=0.93 for family caregivers) (Titler et al., 2020), and from tools used to measure acceptability of other interventions for PwD (Qiu et al., 2019).
The Weighted Blanket Intervention Acceptability Tool – PwD version is comprised of 6 items pertaining to satisfaction with the intervention rated on a 3-point scale (1=Not at all to 3=A great deal satisfied) that are averaged to yield an individual satisfaction score for the person with dementia. The benefit component is comprised of 1 item pertaining to the degree of relaxation felt while using the blanket (1=not at all to 3=a great deal relaxed), for an individual benefit score. The instrument also assesses what participants liked most and least about using the blanket (open ended responses), if they would recommend a weighted blanket to other PwD (yes, no), and if they would continue to use it themselves (yes, no).
The Weighted Blanket Intervention Acceptability Tool – Caregiver version includes 8 items specific to the caregiver’s satisfaction with the weighted blanket, their satisfaction with the remote delivery, and their perception of their relative’s satisfaction with the weighted blanket rated on a 5-point scale (1=not satisfied to 5=very satisfied). The benefit component is 6 items relating to the caregiver’s perception of the degree of benefit experienced by the dyad (1=not at all to 3=a great deal beneficial). The 8 scores from the satisfaction items, and 6 scores from the benefit items are averaged to yield an individual caregiver satisfaction and benefit score, respectively. The Caregiver version also includes items to assess when using the blanket was most and least beneficial (open ended responses), if they would recommend a weighted blanket to others caring for someone with dementia (yes, no), and if they would continue to encourage their relative to use the blanket (yes, no).
Feasibility of Collecting Outcome Measures (Aim 2)
Aim 2 of this study was to examine the feasibility of collecting outcome measures to inform future studies to examine efficacy. We examined the feasibility of collecting 1) cognitive function, BPSD, and quality of life measures for PwD; 2) caregiver well-being and self-reported health status measures for caregivers. Psychometrically sound instruments were selected to measure each outcome (Table 1). Multiple domains of BPSD were measured including global BPSD, agitation, anxiety, and sleep.
Table 1.
Overview of Person with Dementia and Caregiver Specific Outcome Measures
| Outcome Measures | Instrument | Psychometric Properties of Instrument | Number of Items | Total Score RangeA | Subscale Score RangeA | |
|---|---|---|---|---|---|---|
| PWD specific outcomes | Cognitive function | MoCA | Cronbach’s α range: 0.83 Test-retest reliability: r = 0.92 Construct validity established through CFA (Freitas et al., 2012; Nasreddine et al., 2005) |
16 | 0–30 | N/A |
| BPSD | ||||||
| Global BPSD | NPI | Cronbach’s α range: 0.71–0.88 Percentage agreement between raters: 93.6%–100% Test-retest reliability range (r): 0.79–0.86 (Jackson et al., 2014; Lai, 2014) |
12 | 0–144 | CG Distress Subscale: 0–60 | |
| Agitation | CMAI-Relatives version | Cronbach’s α range: 0.860.91 Inter-rater reliability: 0.41 Construct validity established through CFA (Cohen-Mansfield et al., 1989; Rabinowitz et al., 2005) |
34 | 34–238 | N/A | |
| Anxiety | RAID * | Cronbach’s α: 0.83 Inter-rater reliability k range: 0.51–1 Test-retest reliability k range: 0.53–1 Construct validity established through CFA (Shankar et al., 1999) |
18 | 0–54 | N/A | |
| Sleep disturbances | NPI-Sleep Domain | See NPI instrument above | 18 | 1–12 | Frequency subscale: 1–4 Severity subscale: 1–3 CG distress subscale: 0–5 |
|
| PSQI | Cronbach’s α: 0.85 Test-retest reliability: r = 0.87 (Backhaus et al., 2002) |
9 | 0–21 | N/A | ||
| ESS | Cronbach’s α: 0.73–0.86 Convergent validity established by comparing ESS with PSQI scores (Kendzerska et al., 2014) |
8 | 0–24 | N/A | ||
| Quality of life | QOL-AD* |
PWD report Cronbach’s α: 0.83 CG Proxy Report Cronbach’s α: 0.90 ICC between CR and proxy CG proxy report: r = 0.14–0.39 Construct validity established through CFA (Logsdon et al., 1999; Thorgrimsen et al., 2003) |
13 | 13–52 | N/A | |
| CG specific outcomes | Well-being | CWBS | Cronbach’s α: 0.83 Construct validity established through CFA (Berg-Weger et al., 2000; Tebb et al., 2013) |
16 | 1–5 | Basic needs subscale: 1–5 ADL subscale: 1–5 |
| Self-reported health status | SF-12 | Cronbach’s α range 0.76–0.85 Test-retest reliability range: 0.76–0.89 Construct validity established through CFA (Jakobsson, 2007) |
12 | 0–100 | Physical health composite subscale: 0–100 Mental health composite subscale: 0–100 |
Note. ADL activities of daily living, BPSD, behavioral and psychological symptoms of dementia, CFA confirmatory factor analysis, CG caregiver, CMAI Cohen-Mansfield Agitation Inventory-Relatives version, CWBS Caregiver Well-Being Scale, ESS Epworth Sleepiness Scale, MoCA Montreal Cognitive Assessment Test, NPI Neuropsychiatric Inventory, PWD person with dementia, RAID Rating Anxiety in Dementia Scale, SF-12 Optum SF-12v.2 Health Survey
The RAID and QOL-AD are completed independently by self-report by the person living with dementia, and by proxy report by the caregiver to yield a person with dementia reported total score, and a caregiver reported total score.
- MoCA: Higher scores indicate better cognitive function. ≤9 indicative of moderate dementia, ≤17 indicative of mild dementia, ≤23 indicative of mild cognitive impairment, ≤30 normal cognitive function
- NPI: Higher scores indicative of greater frequency and severity of neuropsychiatric symptoms. Higher caregiver distress scores indicate of greater caregiver distress related to neuropsychiatric symptoms.
- CMAI: Higher scores indicative of greater agitation severity. Score of ≥ 39 suggest clinically significant agitation.
- RAID: Higher scores indicative of greater anxiety. Score of ≥ 11 suggests clinically significant anxiety.
- PSQI: Higher total scores indicative of overall worse sleep quality. Score of ≥ 5 suggests clinically significant sleep disturbances.
- ESS: Higher scores indicate more severe daytime sleepiness.
- QOL-AD: Higher scores are reflective of higher reported quality of life.
- CWBS: Higher total scores indicate greater reported well-being. Higher needs and activities of daily living domain scores indicate that the needs and activities are being met.
The Rating Anxiety in Dementia Scale (RAID) and Quality of Life in Alzheimer’s Disease Scale (QOL-AD) are both completed independently by the person with dementia and by the caregiver as a proxy respondent. All other measures are completed by either the person with the dementia, or the caregiver (Table 2).
Table 2.
Instruments Collected at Baseline and Post-Intervention
| Data Collection Timepoint | Respondent | Data Collection Method * | Instrument |
|---|---|---|---|
| Baseline (1 week prior to intervention period) | Caregiver | Questionnaire | CMAI |
| PSQI | |||
| ESS | |||
| QOL-AD – CG report** | |||
| CWBS | |||
| SF-12 | |||
| Demographics form | |||
| Health History form | |||
| Interview | NPI | ||
| RAID – CG report ** | |||
| PWD | Interview | MoCA | |
| RAID – PWD report ** | |||
| QOL-AD – PWD report ** | |||
| Post-Intervention (1 week after intervention period) | Caregiver | Questionnaire | CMAI |
| PSQI | |||
| ESS | |||
| QOL-AD – CG report ** | |||
| CWBS | |||
| SF-12 | |||
| WBIAT – CG version | |||
| Health History Update | |||
| Interview | NPI | ||
| RAID – CG report ** | |||
| PWD | Interview | MoCA | |
| RAID – PWD report ** | |||
| QOL-AD – PWD report ** | |||
| Questionnaire | WBIAT – PwD version |
Note. CG caregiver, CMAI Cohen-Mansfield Agitation Inventory-Relatives version, CWBS Caregiver Well-Being Scale, ESS Epworth Sleepiness Scale, MoCA Montreal Cognitive Assessment Test, NPI Neuropsychiatric Inventory, PWD person with dementia, RAID Rating Anxiety in Dementia Scale, SF-12 Optum SF-12v.2 Health Survey
Caregivers completed questionnaires electronically or by hardcopy based on their preference. Participants with dementia completed the WBIAT – PwD version by hardcopy questionnaire only. Interviews were conducted virtually or by telephone based on the participant preference.
The RAID and QOL-AD are both completed independently by self-report by the person with dementia and by the caregiver by proxy for the person with dementia. All other measures are completed by either the person with dementia, or the caregiver.
Data Collection
Demographic, Caregiving and Health History Data
A form was completed at baseline by caregivers to assess the dyad’s demographic (i.e., age, gender, race, education, marital status), caregiving (i.e., dyadic relationship, weekly hours of caregiving, duration of having lived together), and health history information for the person with dementia (i.e., dementia type, date of diagnosis, co-morbidities, medications). A health history update form was completed by caregivers post-intervention to capture health changes that occurred among participants with dementia during the study.
Feasibility Data (Aim 1)
The number of days the weighted blanket was used for the recommended duration was collected through the Weighted Blanket Daily Use Diary, which was returned to the research team by mail at the end of the intervention period. A tracking sheet was used to document the number of dyads enrolled and declined participation, length of time to recruit 20 dyads, dyads that withdrew from the study, and injuries or adverse events.
Acceptability Data (Aim 1)
Tolerability data was collected during the four weekly telephone check ins (Figure 1). This data was entered directly into the electronic study database. Satisfaction and benefit data were collected using the Weighted Blanket Intervention Acceptability Tool – PwD and Caregiver versions at the end of the 4-week intervention, which were completed by hardcopy (sent and returned by mail) or electronically (distributed by email) according to participant preference (Table 2).
Outcome Measures (Aim 2)
Outcome measures were collected one week prior to and one week after the 4-week intervention period (Table 2). Data were collected using questionnaires (electronic or hardcopy) and by interview. Measures completed by interview were collected using Zoom, or by telephone. Interview data were entered directly into the electronic database.
Data Analysis
Descriptive statistics [frequencies, percentiles, means, standard deviations (SDs)] were performed to analyze demographic, caregiving, and health history data.
Feasibility (Aim 1)
The mean (SD) were calculated for the group based on the number of days each PWD used the blanket as least 20 minutes per day. Time to recruit 20 dyads was calculated in days since the date of IRB approval. Enrollment and withdrawal rates were calculated (i.e., number of dyads enrolled ÷ number screened, number that withdrew ÷ number enrolled) and reported as percentages. A frequency was calculated for dyads that declined participation.
Acceptability (Aim 1)
Descriptive statistics were used for acceptability. Weekly tolerability scores were averaged over the four weeks for each participant and a mean (SD) was calculated. Mean and SD were calculated for satisfaction and benefit for persons with dementia who completed the Weighted Blanket Intervention Acceptability Tool. Means (SDs) were also calculated for caregiver satisfaction and benefit scores. Content analysis was used for open ended items by clustering similar responses into categories. Frequencies were calculated for dichotomous items.
Feasibility of Collecting Outcome Measures (Aim 2)
The percentage of missing data for each instrument at each timepoint was calculated by adding the number of items missed across participants for each tool at each timepoint, divided by the number of items in the specific scale multiplied by the total number of participants who completed the scale, then multiplying by 100. All outcome measures were scored for each participant at baseline and at post-intervention according to the instrument’s scoring guidelines to further describe the study sample. Means (SDs) for all scales and subscales were calculated across participants at baseline and at post-intervention.
Results
Participant Characteristics
The majority of caregivers were female (n=16), and the majority of those with dementia were male (n=13) and had Alzheimer’s dementia (n=13) (Table 3). Most dyads were white (n=20), married or partnered (n=16), well educated, and over 65 years of age (Table 3). The most common co-morbidities among participants with dementia were hypertension (n=3), high cholesterol (n=3), and other heart conditions (n=4). Most participants with dementia were on a cognition enhancing drug (n=19) and a medication to treat depression or anxiety (n=17). No new conditions or significant medication changes were reported post-intervention.
Table 3.
Sociodemographic, Dementia Diagnosis, and Caregiving Characteristics of Study Sample
| PWD (n=20) | Caregivers (n=20) | |
|---|---|---|
| Female no. (%) | 7 (35) | 16 (80) |
| Mean age (SD) | 77.7 (10.2) | 66.4 (11.2) |
| Race/ethnicity no. (%) | ||
| Non-Hispanic White | 20 (95) | 20 (95) |
| Non-Hispanic Black | 1 (5) | 1 (5) |
| Education no. (%) | ||
| < High school | 2 (10) | 0 |
| High school | 3 (15) | 4 (20) |
| Some college | 4 (20) | 2 (10) |
| College and above | 11 (55) | 14 (70) |
| Relationship between members of dyad no. (%) | ||
| Married or partnered | 16 (80) | |
| Child caring for parent | 4 (20) | |
| Mean duration of dementia diagnosis in months (SD) | 45.7 (28.1) | |
| Participants with dementia type no. | ||
| Alzheimer’s dementia | 13 | |
| Vascular dementia | 1 | |
| Mixed type dementia | 1 | |
| Lewy Body dementia | 1 | |
| Posterior cortical atrophy | 1 | |
| Not specified or unknown | 3 | |
| Mean number of years having lived together (SD) | 35.6 (19.7) | |
| Mean number of hours of care provided by caregiver each day (SD) | 19.0 (32.3) | |
Note. SD standard deviation, PWD people with dementia
Feasibility Results (Aim 1)
All measures of feasibility surpassed predefined benchmarks (Table 4). The weighted blanket was used for 23.8 (SD=6.4) days (Table 4), with an average daily use of 3.7 hours (SD=3.9) per day. Three PwD did not use it at all for 10 or more days, while almost half did not use it for the minimum 20 minutes at least one day throughout the 4-week intervention period. No adverse effects were reported.
Table 4.
Aim 1 Results of Measures of Feasibility and Acceptability
| Operationalized Measures | Predefined Benchmarks of Feasibility * | Results of Study | |
|---|---|---|---|
| Average number of days weighted blanket was used for the recommended duration (SD) | ≥ 21 | 23.8 days (SD=6.4) | |
| Injuries and adverse events | None | None | |
| Enrollment rate | ≥ 50% | 64% | |
| Number of dyads that declined participation | ≤ 10 | 2 | |
| Length of time to recruit desired sample | ≤ 5 months | 3.9 months | |
| Withdrawal rate | < 25% | 5% |
| n | Mean (SD) | Median | Range of Sample Scores | Scale Range | ||
|---|---|---|---|---|---|---|
| Tolerability ** | 20 CRs | 8.9 (2.1) | 10 | 1–10 | 0 did not tolerate the blanket at all to 10 tolerated the blanket all of the time | |
| Satisfaction – CG | 20 CGs | 4.7 (0.4) | 4.9 | 3.6 to 5 | 1 = Not satisfied to 5 = Very satisfied | |
| Satisfaction – PWD | 13 PWD | 2.8 (0.2) | 2.8 | 2.5–3.0 | 1 = Not satisfied to 5 = Very satisfied | |
| Benefit – CG | 20 CGs | 2.5 (0.4) | 2.7 | 1.7 – 3.0 | 1 = Not at all to 3 = A great deal | |
| Benefit – PWD | 13 CRs | 2.8 (0.4) | 3 | 2.0–3.0 | 1 = Not at all to 3 = A great deal |
Note. CG caregiver, PWD people with dementia, SD standard deviation
Benchmarks indicative of feasibility were determined a priori, which were selected based on prior intervention feasibility studies focused on this study’s population (Orgeta et al., 2019; Tamplin et al., 2018).
Tolerability of the weighted blanket by persons living with dementia as reported by caregivers.
Enrollment rate was relatively high (64%). Ten of the 33 dyads screened were ineligible due to: PwD having sleep apnea (n=6), COPD (n=3), or impaired arm mobility (n=1). Two dyads declined participation due to perceived time commitment as being too much. Withdrawal was low (5%) with one dyad withdrawing due to caregiver illness before beginning the intervention period. The sample was recruited in under four months (Table 4).
Acceptability Results
Tolerability
The tolerability of weighted blankets by participants with dementia was relatively high (M=8.9/10, SD=2.1) (Table 4). Ten participants tolerated the blanket “all of the time”.
Satisfaction and Benefit – PwD
Thirteen of 20 participants with dementia completed the Weighted Blanket Intervention Acceptability Tool and reported high satisfaction (M=2.8/3, SD=0.2) and benefit (M=2.8/3, SD=0.4 (Table 3). Satisfaction items with the highest rating were related to the freedom to choose frequency of use (M=2.9, SD=0.3) and comfort when using the blanket (M=2.9, SD=0.3). Twelve of the 13 participants reported they would continue to use the blanket and all 13 reported they would recommend use of a weighted blanket to other PwD. Open-ended responses clustered into three categories pertaining to what they liked most about using the blanket: it provided comfort, it helped them sleep, and it provided warmth. Responses to what they liked the least about using the blanket included it was too heavy or too hot (at times), the weighted beads clumped together, and it could be softer.
Satisfaction and Benefit – Caregivers
Caregivers’ (n=20) satisfaction with the weighted blanket was 4.7/5 (SD=0.4), and benefit was 2.5/3 (SD=0.4; Table 3). Satisfaction items with the highest ratings were delivery of study materials to their home (M=5.0, SD=0), explanation about the weighted blanket (M=2.9, SD=0.4), and how their questions were addressed (M=4.9, SD=0.4). Most caregivers were satisfied or very satisfied with the remote delivery of the intervention (n=15), and with participating in weekly telephone check ins (n=20). Most (n=19) would encourage continued use of the blanket and would recommend use by others caring for someone with dementia. Caregivers noted that weighted blankets were most helpful during afternoon naps and in the evening. Two caregivers reported the blanket was least helpful when their relative was restless or agitated.
Missing Data and Results of Outcome Measures
Missing data for caregiver outcome measures were low across timepoints and ranged from 0 to 1.5% (Table 5). There were no missing data for the Montreal Cognitive Assessment Test (MoCA) completed by interview of participants with dementia. Only 10 people with dementia were able to complete the Rating Anxiety in Dementia Scale (RAID) and Quality of Life in Alzheimer’s Disease Scale (QOL-AD) by self-report, resulting in high degrees of missing data for these instruments (≥50%) (Table 5).
Table 5.
Aim 2 Results of Missing Data and Scored Outcome Measures Collected at Baseline and at Post-Intervention
| Measure | Instrument | % Missing Data | Scales and Subscales | Scale Ranges A | Baseline (n=20)* | Post-Intervention (n=20) | |
|---|---|---|---|---|---|---|---|
| Baseline (n=21)* | Post-Intervention (n=20) | Mean (SD) | Mean (SD) | ||||
| Cognitive Function | MoCA ▲ | 0 | 0 | MoCA | 0–30 | 9.2 (8.0) | 9.1 (8.2) |
| BPSD | |||||||
| Global BPSD | NPI ■ | 0 | 0 | NPI Total | 0–144 | 25.0 (16.0) | 22.8 (20.1) |
| CG Distress Subscale | 0–60 | 13.2 (7.6) | 11.3 (9.8) | ||||
| Agitation | CMAI ● | 0 | 0.3 | CMAI Total | 34–238 | 59.1 (13.4) | 55.8 (14.4) |
| Anxiety | RAID – PWD ▲ | 52.3 | 50.0 | RAID – PWD (n=10)** | 0–54 | 5.7 (5.8) | 5.4 (4.8) |
| RAID – CG ■ | 0 | 0 | RAID – CG | 0–54 | 9.7 (6.0) | 7.7 (5.0) | |
| Sleep | NPI-Sleep Domain ■ | 0 | 0 | NPI-Sleep Domain Total | 0–12 | 3.9 (2.9) | 4.3 (3.7) |
| Frequency | 0–4 | 1.9 (1.0) | 2.0 (1.1) | ||||
| Severity | 0–3 | 1.8 (0.7) | 1.9 (0.8) | ||||
| CG Distress | 0–5 | 2.8 (1.4) | 2.8 (1.4) | ||||
| PSQI ● | 0.5 | 0 | PSQI | 0–21 | 6.3 (3.1) | 6.3 (3.5) | |
| ESS ● | 0 | 0 | ESS | 0–24 | 9.9 (5.7) | 9.3 (6.3) | |
| PWD Quality of Life | QOL-AD – PWD ▲ | 61.1 | 35.0 | QOL-AD – PWD (n=10)** | 13–52 | 41.3 (5.1) | 42.4 (3.0) |
| QOL-AD – CG ● | 1.5 | 1.5 | QOL-AD – CG | 13–52 | 33.3 (5.7) | 33.7 (5.3) | |
| Caregiver Well-Being | CWBS ● | 0 | 0 | CWBS Total | 1–5 | 3.9 (0.6) | 4.0 (0.6) |
| Basic Needs Subscale | 1–5 | 4.2 (0.7) | 4.2 (0.6) | ||||
| ADL Subscale | 1–5 | 3.8 (0.7) | 3.8 (0.7) | ||||
| CG Self-Reported Health Status | SF-12 ● | 0 | 0 | SF-12 Total | 0–100 | 50.2 (5.5) | 48.3 (6.1) |
| PCS | 0–100 | 56.9 (6.7) | 52.8 (9.2) | ||||
| MCS | 0–100 | 43.1 (12.6) | 43.9 (11.1) | ||||
Note. ADL activities of daily living, BPSD, behavioral and psychological symptoms of dementia, CG caregiver, CMAI Cohen-Mansfield Agitation Inventory-Relatives version, CWBS Caregiver Well-Being Scale, ESS Epworth Sleepiness Scale, MCS Mental Health Composite Score, MoCA Montreal Cognitive Assessment Test, NPI Neuropsychiatric Inventory, PCS Physical Health Composite Score, PWD person with dementia, RAID Rating Anxiety in Dementia Scale, SD standard deviation, SF-12 Optum SF-12v.2 Health Survey
Sample size differs from baseline to post-intervention as 1 dyad withdrew after baseline data collection, but prior to beginning the intervention period. Only the 20 dyads that completed the intervention were included in the calculations for scored measures.
Sample size differs for the RAID – PWD and QOL-AD – PWD as only 10 participants with dementia were able to complete these measures.
▲Completed by interview of participant with dementia ■Completed by interview of caregiver ● Completed by questionnaire by caregiver
Scored measure results support that the cognitive impairment severity of the sample was moderate (MoCA score ≤ 9) (Saczynski et al., 2015). Participants with dementia experienced clinically significant sleep disturbances (PSQI ≥ 5) (Buysse et al., 1989) and agitation (Husebo et al., 2011) (CMAI ≥ 39), but not anxiety (RAID ≥ 11) (Shankar et al., 1999). RAID scores of PwD tended to be lower than scores reported by caregivers, with similar results for the QOL-AD (Table 5). Compared to prior community-based samples of caregivers, Caregiver Well-Being scores were higher in this study (Tebb et al., 2013); physical health scores on the SF-12 were higher and mental health scores were lower in this study (Farina et al., 2017).
Discussion
This study explored the feasibility and acceptability of weighted blankets for community dwelling PwD, and the feasibility of collecting several outcomes. The major findings of this study include: 1) weighted blankets were feasible and used safely by PwD with support from family caregivers, 2) high degrees of intervention acceptability were reported by PwD and their caregivers, 3) collecting multiple types of outcomes specific to PwD and caregivers was feasible, but collecting measures completed by self-report of PwD was not. These findings provide foundational information to plan and execute future studies to test the efficacy of weighted blankets for improving outcomes among PwD and their caregivers.
In alignment with prior weighted blanket research (Eron et al., 2020), this study indicated no side effects with use of weighted blankets; however, this is the first study to demonstrate safety with use by PwD (Eron et al., 2020). Most participants used the blanket for more than the recommended 20 minutes per day. This suggests that the blanket fit within their daily routines, but circumstances that made using the blanket a challenge (e.g., too heavy or too hot, limited benefit during times of restlessness/agitation) warrant further examination. Safety and feasibility are essential components of successful, widely adopted community-based interventions (Gadke et al., 2021), thus findings of this study support testing the efficacy of weighted blankets among PwD living at home with an adult caregiver.
Overall high scores of tolerability, satisfaction, and benefit with the weighted blanket are meaningful indicators of intervention acceptability - a key factor in promoting widespread, sustained use of interventions (Gadke et al., 2021). Remote delivery of the intervention was also acceptable to caregivers, which is significant as the need for remotely delivered interventions was intensified by the COVID-19 pandemic. By involving key stakeholders and by determining acceptability at the onset of development, this intervention, if found to be efficacious in a future study, has an increased likelihood of successful implementation and greater capacity to impact families in the future (Gitlin et al., 2020; Qiu et al., 2019).
This study provides valuable information to inform measurement selection in a future efficacy study. Concordant with prior research (Perfect et al., 2021), this study demonstrated limitations in collecting measures completed by self-report by PwD, which highlights a key area for future research (described below). Notwithstanding, this study supports that several other outcome measures completed by caregivers can be collected with minimal missing data, which can be included in future studies.
Strengths of this study were the use of psychometrically sound outcome measures, a standardized intervention protocol, measure of adverse events, and predefined benchmarks to determine feasibility. Despite these strengths, this study did have four key limitations. First, the sample lacked diversity in sociodemographic and caregiving characteristics, which limits the understanding of feasibility and acceptability of weighted blankets among diverse groups of PwD and their caregivers. Second, outcomes completed by self-report of PwD had significant amounts of missing data, which limits interpretation of scored measures. Third, satisfaction surveys carry a risk of participants providing socially acceptable answers, so it is possible participants provided more positive ratings; however, this is the first study to examine satisfaction and benefit of weighted blankets. Fourth, the Weighted Blanket Use Diary may not be an accurate indicator of actual blanket use, particularly for dyads who did not regularly spend time together in the same physical space. Prospective measures for blanket use need to be explored in future studies (e.g., real-time logging, sensor technology).
Implications for Future Research
There are two important implications for future research. First, given the overall positive findings shown in this study, efficacy testing is warranted and will be essential to promote weighted blankets as an evidence-based intervention for PwD. Next steps are a pilot study to determine effect size for a large efficacy study using a randomized design to examine the efficacy of weighted blankets on BPSD. Findings pertaining to feasibility can inform the design of the pilot study in two key ways. First, the limited variability of this study’s sample supports that alternative recruitment strategies need to be used in the pilot study to recruit a more diverse sample in terms of sociodemographic and caregiving characteristics. Second, different measures need to be used to reliably capture the perspectives of PwD. Alternatively, cognitive screening may be useful to identify PwD in earlier stages of cognitive decline who may have an increased likelihood of completing selected measures.
The second implication is additional research is needed to develop new and/or refine current measures of quality of life and anxiety in dementia that people with varying degrees of cognitive impairment can complete. Measures may be improved by limiting the number of items, using dichotomous response options, allowing flexibility in administration protocols, measuring “in the moment” feelings, and rigorous field testing (Perfect et al., 2021). Improving self-report measures is essential to empowering the voice of PwD and ensuring interventions are developed based on evidence from multiple perspectives.
Implications for Clinical Practice
Nurses and family healthcare providers play a critical role in encouraging use of non-pharmacologic interventions by PwD and their families. Weighted blankets are already used in some clinical settings, yet there is limited evidence to support them. No formal technical safety standards have been published regarding weighted blankets for PwD. Demonstration of efficacy is needed prior to making practice recommendations for use by PwD.
Conclusion
This study found weighted blankets to be a feasible and acceptable in-home nonpharmacological intervention for families living with dementia. The remote delivery of the intervention was feasible, as well as collection of several outcome measures specific to PwD and their caregivers; however, collection of self-report measures completed by PwD was not. Key findings will inform efficacy trials focused on weighted blankets for reducing BPSD among community dwelling PwD. As families living with dementia needed in-home care strategies even before the COVID-19 pandemic began, weighted blankets represent a non-pharmacologic intervention that warrant additional research to determine efficacy in this population.
Supplementary Material
What this paper adds
Demonstrates the safety, feasibility, and acceptability of weighted blanket use by older adults living with dementia, a population excluded from prior weighted blanket studies.
Provides preliminary data to refine the development and inform future testing of a remotely delivered weighted blanket intervention.
Highlights several outcome measures that can feasibly be collected among community-dwelling people living with dementia and their caregivers.
Applications of study findings
Safety guidelines can inform clinical practice and long-term care providers who may already be using weighted blankets in practice.
Findings pertaining to intervention and study design feasibility can inform the planning and conduct of a future efficacy study.
Findings pertaining to the feasibility of collecting outcome measures can be used to substantiate the need for the development and/or refinement of measurement tools that people living with dementia can feasibly complete on their own.
Funding:
This work was funded by the Predoctoral Fellowship Training Grant (T32 NR016914. Program Director: Titler) Complexity: Innovations in Promoting Health and Safety. 7/1/2017 – 6/30/2022.
Footnotes
Conflicts of Interest: We have no conflicts of interest to disclose.
University of Michigan IRB Approval Number: HUM00186832
Contributor Information
Melissa L. Harris, Duke University.
Marita G. Titler, University of Michigan.
References
- Backhaus J, Junghanns K, Broocks A, Riemann D, & Hohagen F (2002). Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research, 53(3), 737–740. [DOI] [PubMed] [Google Scholar]
- Berg-Weger M, Rubio DM, & Tebb SS (2000). The caregiver well-being scale revisited. Health & Social Work, 25(4), 255–263. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, Bakken S, Kaplan CP, Squiers L, Fabrizio C, & Fernandez M (2009). How we design feasibility studies. American Journal of Preventive Medicine, 36(5), 452–457. 10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley CV, Livingston G, Knapp MRJ, Wimo A, Norman R, & Brodaty H (2020). Time to invest in nonpharmacological interventions for behaviours and psychological symptoms associated with dementia. Alzheimer’s & Dementia, 16(S10), e042281. https://doi.org/ 10.1002/alz.042281 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: New instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Champagne T, Mullen B, Dickson D, & Krishnamurty S (2015). Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occupational Therapy in Mental Health, 31(3), 211–233. [Google Scholar]
- Cohen-Mansfield J, Marx MS, & Rosenthal AS (1989). A description of agitation in a nursing home. Journal of Gerontology, 44(3), M77–M84. [DOI] [PubMed] [Google Scholar]
- Ekholm B, Spulber S, & Adler M (2020). A randomized controlled study of weighted chain blankets for insomnia in psychiatric disorders. Journal of Clinical Sleep Medicine, 16(9), 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron K, Kohnert L, Watters A, Logan C, Weisner-Rose M, & Mehler PS (2020). Weighted blanket use: A systematic review. The American Journal of Occupational Therapy, 74(2), 7402205010p7402205011–7402205010p7402205014. 10.5014/ajot.2020.037358 [DOI] [PubMed] [Google Scholar]
- Farina N, Page TE, Daley S, Brown A, Bowling A, Basset T, Livingston G, Knapp M, Murray J, & Banerjee S (2017). Factors associated with the quality of life of family carers of people with dementia: A systematic review. Alzheimer’s & Dementia, 13(5), 572–581. [DOI] [PubMed] [Google Scholar]
- Freitas S, Simoes MR, Alves L, Vicente M, & Santana I (2012). Montreal Cognitive Assessment (MoCA): validation study for vascular dementia. Journal of International Neuropsychological Society, 18(6), 1031–1040. [DOI] [PubMed] [Google Scholar]
- Gadke DL, Kratochwill TR, & Gettinger M (2021). Incorporating feasibility protocols in intervention research. Journal School Psychology, 84, 1–18. 10.1016/j.jsp.2020.11.004 [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Baier RR, Jutkowitz E, Baker ZG, Gustavson AM, Sefcik JS, Hodgson NA, Koeuth S, & Gaugler JE (2020). Dissemination and implementation of evidence-based dementia care using embedded pragmatic trials. Journal of the American Geriatrics Society, 68, S28–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GR, & Buckwalter KC (1987). Progressively lowered stress threshold: A conceptual model for care of adults with Alzheimer’s disease. Archives of Psychiatric Nursing, 1(6), 399–406. [PubMed] [Google Scholar]
- Husebo BS, Ballard C, Sandvik R, Nilsen OB, & Aarsland D (2011). Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: Cluster randomised clinical trial. BMJ, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MA, Fauth EB, & Geiser C (2014). Comparing the neuropsychiatric inventory and the revised memory and behavior problems checklist for associations with caregiver burden and depressive symptoms. International Psychogeriatrics, 26(6), 1021–1031. [DOI] [PubMed] [Google Scholar]
- Jakobsson U (2007). Using the 12-item Short Form health survey (SF-12) to measure quality of life among older people. Aging Clinical and Experimental Research, 19(6), 457–464. [DOI] [PubMed] [Google Scholar]
- Kales HC, Lyketsos CG, Miller EM, & Ballard C (2019). Management of behavioral and psychological symptoms in people with Alzheimer’s disease: An international Delphi consensus. International Psychogeriatrics, 31(1), 83–90. [DOI] [PubMed] [Google Scholar]
- Kendzerska TB, Smith PM, Brignardello-Petersen R, Leung RS, & Tomlinson GA (2014). Evaluation of the measurement properties of the Epworth sleepiness scale: A systematic review. Sleep Medicine Reviews, 18(4), 321–331. [DOI] [PubMed] [Google Scholar]
- Keng A, Brown EE, Rostas A, Rajji TK, Pollock BG, Mulsant BH, & Kumar S (2020). Effectively caring for individuals with behavioral and psychological symptoms of dementia during the COVID-19 pandemic. Frontiers in Psychiatry, 11, 573367–573367. 10.3389/fpsyt.2020.573367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, & Buschmann MT (2004). Touch-stress model and Alzheimer’s disease: Using touch intervention to alleviate patients’ stress. Journal of Gerontological Nursing, 30(12), 33–39. 10.3928/0098-9134-20041201-08 [DOI] [PubMed] [Google Scholar]
- Lai CK (2014). The merits and problems of Neuropsychiatric Inventory as an assessment tool in people with dementia and other neurological disorders. Clinical Interventions in Aging, 9, 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster GA, & Thabane L (2019). Guidelines for reporting non-randomised pilot and feasibility studies. In (Vol. 5, pp. 1–6): BioMed Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, & Cohen-Mansfield J (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673–2734. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, Teri L, Weiner MF, Gibbons LE, Raskind M, Peskind E, Grundman M, Koss E, Thomas RG, & Thai LJ (1999). Assessment of agitation in Alzheimer’s disease: the agitated behavior in dementia scale. Journal of the American Geriatrics Society, 47(11), 1354–1358. [DOI] [PubMed] [Google Scholar]
- Lowery D, Cerga Pashoja A, Iliffe S, Thuné-Boyle I, Griffin M, Lee J, Bailey A, Bhattacharya R & Warner J (2014). The effect of exercise on behavioural and psychological symptoms of dementia: the EVIDEM-E randomised controlled clinical trial. International Journal of Geriatric Psychiatry, 29(8), 819–827. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, & Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- National Institute on Aging. (2018). NIH Stage Model for Behavioral Intervention Development. National Institute of Health. https://www.nia.nih.gov/research/dbsr/nih-stage-model-behavioral-intervention-development [Google Scholar]
- Orgeta V, Tuijt R, Leung P, Verdaguer ES, Gould RL, Jones R, & Livingston G (2019). Behavioral activation for promoting well-being in mild dementia: feasibility and outcomes of a pilot randomized controlled trial. Journal of Alzheimer’s Disease, 72(2), 563–574. [DOI] [PubMed] [Google Scholar]
- Parker E, & Koscinski C (2016). The weighted blanket guide: Everything you need to know about weighted blankets and deep pressure for autism, chronic pain, and other conditions. Jessica Kingsley Publishers. [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, & Skaff MM (1990). Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist, 30(5), 583–594. [DOI] [PubMed] [Google Scholar]
- Perfect D, Griffiths AW, Vasconcelos Da Silva M, Lemos Dekker N, McDermid J, & Surr CA (2021). Collecting self-report research data with people with dementia within care home clinical trials: Benefits, challenges and best practice. Dementia, 20(1), 148–160. [DOI] [PubMed] [Google Scholar]
- Qiu D, Hu M, Yu Y, Tang B, & Xiao S (2019). Acceptability of psychosocial interventions for dementia caregivers: A systematic review. BMC Psychiatry, 19(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J, Davidson M, De Deyn PP, Katz I, Brodaty H, & Cohen-Mansfield J (2005). Factor analysis of the Cohen-Mansfield Agitation Inventory in three large samples of nursing home patients with dementia and behavioral disturbance. The American Journal of Geriatric Psychiatry, 13(11), 991–998. [DOI] [PubMed] [Google Scholar]
- Reynolds S, Lane SJ, & Mullen B (2015). Effects of deep pressure stimulation on physiological arousal. The American Journal of Occupational Therapy, 69(3), 6903350010p6903350011–6903350010p6903350015. [DOI] [PubMed] [Google Scholar]
- Rising KL, Salcedo VJ, Amadio G, Casten R, Chang A, Gentsch A, O’Hayer CV, Sarpoulaki N, Worster B, & Gerolamo AM (2021). Living Through the Pandemic: The Voices of Persons With Dementia and Their Caregivers. Journal of Applied Gerontology, 41(1), 30–35. 10.1177/07334648211036399 [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Inouye SK, Guess J, Jones RN, Fong TG, Nemeth E, Hodara A, Ngo L, & Marcantonio ER (2015). The Montreal cognitive assessment: Creating a crosswalk with the mini-mental state examination. Journal of the American Geriatrics Society, 63(11), 2370–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K, Walker M, Frost D, & Orrell M (1999). The development of a valid and reliable scale for rating anxiety in dementia (RAID). Aging & Mental Health, 3(1), 39–49. [Google Scholar]
- Tamplin J, Clark IN, Lee Y-EC, & Baker FA (2018). Remini-sing: A feasibility study of therapeutic group singing to support relationship quality and wellbeing for community-dwelling people living with dementia and their family caregivers. Frontiers in Medicine, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebb SS, Berg-Weger M, & Rubio DM (2013). The Caregiver Well-Being Scale: Developing a short-form rapid assessment instrument. Health & Social Work, 38(4), 222–230. [DOI] [PubMed] [Google Scholar]
- Thorgrimsen L, Selwood A, Spector A, Royan L, de Madariaga Lopez M, Woods R, & Orrell M (2003). Whose quality of life is it anyway?: The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Disease & Associated Disorders, 17(4), 201–208. [DOI] [PubMed] [Google Scholar]
- Titler MG, Shuman C, Dockham B, Harris M, & Northouse L (2020). Acceptability of a dyadic psychoeducational intervention for patients and caregivers. Oncology Nursing Forum, 47(3), 342–351. 10.1188/20.ONF.342-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi DP, Braun A, Dickinson A, Gage H, Hamilton L, Goodman C, Ashaye K, Iliffe S, & Manthorpe J (2019). Managing behavioural and psychological symptoms in community dwelling older people with dementia: 1. A systematic review of the effectiveness of interventions. Dementia, 18(7–8), 2925–2949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


