Abstract
Background:
Light at night (LAN) may alter estrogen regulation through circadian disruption. High levels of outdoor LAN may increase breast cancer risk, but studies have largely not considered possible residual confounding from correlated environmental exposures. We evaluated the association between indoor and outdoor LAN and incident breast cancer.
Methods:
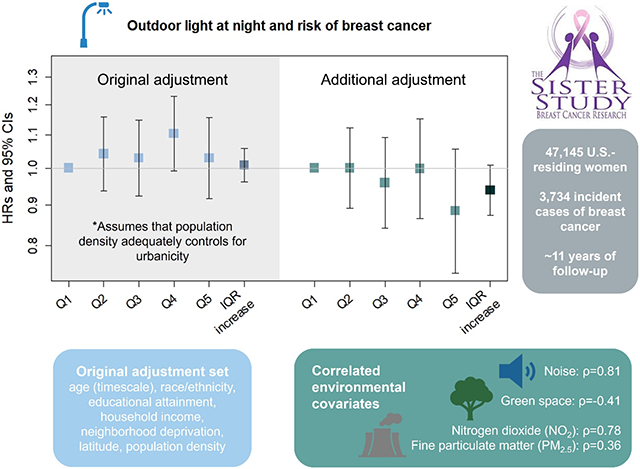
In 47,145 participants in the prospective Sister Study cohort living in the contiguous U.S., exposure to outdoor LAN was determined using satellite-measured residential data and indoor LAN was self-reported (light/TV on, light from outside the room, nightlight, no light). We used Cox proportional hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between outdoor and indoor LAN and breast cancer risk. Models were adjusted for age, race/ethnicity, educational attainment, annual household income, neighborhood disadvantage, latitude, and population density as a proxy for urbanicity. To evaluate the potential for residual confounding of the outdoor LAN and breast cancer relationship by factors associated with urbanicity, we considered further adjustment for exposures correlated with outdoor LAN including NO2 [Spearman correlation coefficient, rho (ρ)=0.78], PM2.5 (ρ=0.36), green space (ρ=−0.41), and noise (ρ=0.81).
Results:
During 11 years of follow-up, 3,734 breast cancer cases were identified. Outdoor LAN was modestly, but non-monotonically, associated with a higher risk of breast cancer (Quintile 4 vs. 1: HR=1.10, 95% CI: 0.99-1.22; Quintile 5 vs 1: HR=1.04, 95% CI: 0.93-1.16); however, no association was evident after adjustment for correlated ambient exposures (Quintile 4 vs. 1: HR=0.99, 95% CI: 0.86-1.14; Quintile 5 vs. 1: HR=0.89, 95% CI: 0.74-1.06). Compared to those with no indoor LAN exposure, sleeping with a light or TV on was associated with a HR=1.09 (95% CI: 0.97-1.23) in the adjusted model.
Conclusions:
Outdoor LAN does not appear to increase the risk of breast cancer after adjustment for correlated environmental exposures.
Keywords: Breast cancer, light at night, environmental epidemiology, prospective cohort
Graphical Abstract
1. Introduction
Light at night is a pervasive and rapidly growing form of environmental pollution,1 and exposure to light at night (LAN) has become a substantial public health concern. Animal models and studies of shift workers suggest that exposure to LAN may play a role in the development of cardiovascular disease, cancer, metabolic disorders, and cognition and mood disorders.2 Individuals are exposed to outdoor LAN from streetlights, buildings, stadiums, and other sources and indoor LAN through the use of lights and electronic devices in the home and sleeping environment. High levels of LAN have the potential to disrupt normal circadian functioning, including melatonin secretion, gene expression, cell cycle regulation, metabolic function, and sleep-wake patterns, which may in turn affect chronic disease risk, including carcinogenesis.3
Among women in the United States (U.S.), breast cancer is the most common malignancy and the second-leading cause of cancer-related mortality.4 Established lifestyle, reproductive, and genetic risk factors for breast cancer are estimated to explain only half of incident cases,5 underscoring the importance of identifying new breast cancer risk factors. Numerous ecologic studies have assessed the relationship between satellite-measured outdoor LAN and breast cancer, with most reporting positive associations.6-9 Similarly, individual-level studies also support a modest positive association between outdoor LAN and breast cancer,10-14 as does a recent meta-analysis of 8 cohort/case-cohort studies [highest vs. lowest category: risk ratio (RR)=1.11, 95% CI: 1.07-1.16].15 Studies of self-reported indoor LAN provide weaker evidence: of the several studies conducted to date,11,16-22 only one has observed a positive association (OR=1.22, 95% CI: 1.12-1.31).19 In the Sister Study, we previously reported negligible associations between indoor LAN exposure and breast cancer risk.22 The present work expands on the previous Sister Study analysis with the inclusion of outdoor LAN exposure estimates and an additional ~1,000 incident breast cancer cases over an additional ~4 years of follow-up.
Exposure to outdoor LAN increases with increasing population density and urbanicity.23 Therefore, outdoor LAN could be a proxy for exposure to other environmental exposures that are also associated with the urban environment, such as traffic-related pollution. However, few studies have considered whether these correlated environmental exposures may act as potential confounders of the outdoor LAN and breast cancer relationship.24 In this prospective study, we evaluated the associations between outdoor LAN and breast cancer risk, with consideration of adjustment for other ambient environmental exposures and include an update on our indoor LAN findings with additional follow-up time and case accrual. We hypothesize that exposure to outdoor LAN will be associated with a higher risk of breast cancer, even after adjustment for additional environmental covariates.
2. Methods
2.1. Study population
The Sister Study is a U.S.-based prospective cohort of 50,884 women from all 50 states and Puerto Rico and was designed to assess environmental risk factors for breast cancer.25 Participants were aged 35 to 74 years and had a full or half-sister who had been previously diagnosed with breast cancer but were breast cancer-free themselves at the time of enrollment (2003-2009). At study entry, participants completed mailed questionnaires and computer-assisted telephone interviews to assess sociodemographic characteristics, lifestyle factors, residential history and current address, and environmental as well as occupational risk factors. Women complete additional short questionnaires annually and detailed questionnaires every 2 to 3 years. The Institutional Review Board of the National Institute of Environmental Health Sciences approved the Sister Study. All participants provided written informed consent. For this study, we have included follow-up through September 30, 2019 (Data Release 9.1).
2.2. Outcome assessment
Incident breast cancer diagnoses were self-reported on follow-up questionnaires or via phone calls to the study’s hotline. Pathology reports and access to medical records were requested to confirm diagnoses and to ascertain additional information about the tumor, including estrogen receptor (ER) and progesterone receptor (PR) status and tumor type (e.g., lobular, ductal) and invasiveness. Medical records were obtained for over 80% of diagnoses. Given the high accuracy of several breast cancer characteristics between self-report and medical records,26 self-reported breast cancer information was included when medical reports were not available. Menopausal status at enrollment and throughout follow-up was self-reported and used to determine menopausal status at the time of breast cancer diagnosis.
2.3. Exposure assessment
Annual average outdoor LAN was assessed using satellite images obtained from the Operational Linescan System of the U.S. Defense Meteorological Satellite Program (DMSP), which is maintained by the National Oceanic and Atmospheric Administration’s Earth Observation Group (https://ngdc.noaa.gov/eog, 2019). Daily global coverage was provided, and annual composites were made after excluding sun and moon luminance, glare, clouds, atmospheric lighting, and ephemeral events such as fires. Processed images were then georectified to a 30 arc-second grid (equivalent to ~1 km-sq).13 The DMSP Global Radiance Calibrated Nighttime Lights high-dynamic range data was used as it provides better variability of LAN measures in urban areas compared to the 6-bit low-dynamic range data.13 The high-dynamic images were generated using three different gain settings (low, medium, high), and provide relative levels of illumination.13 Outdoor LAN was available in units of radiance (nW/cm2/sr) and was linked to participants’ geocoded enrollment addresses using ArcGIS (ESRI, Redlands, CA). High-dynamic DMSP satellite images are available for 1996, 1999, 2000, 2003, 2004, 2006, and 2010, and data was linked to the closest corresponding prior enrollment year (e.g., 2006 data was linked to enrollment years 2006-2009). Nine women were excluded due to not completing the residential history questionnaire. Enrollment address geocoding has been completed for >99% of the study population, and exact street addresses for current residences were geocoded for 93.2% of women. For 5.1% of the women, the geocoding was available for a nearby intersection, and for 1.4%, it was available for the centroid of the ZIP code. Women with any geocode status were assigned a value for outdoor LAN and included in the analysis. Outdoor LAN was assessed per IQR increase and as quintiles.
Information on indoor LAN was self-reported at enrollment and included use of a sleep mask and specific light sources in the bedroom while sleeping (select all that apply: daylight; one or more lights on in the room; light from a television on in the room for most or all of the night; light from other rooms; light from outside shining in; light from a small nightlight or clock radio). For this analysis, indoor LAN was assessed as a four-level variable reflecting highest to lowest level of assumed exposure: 1) one or more lights or light from a TV, 2) light from outside the room (either from other rooms or through the windows, as these sources are both likely to be dimmer than lights on in the room itself), 3) light from a small nightlight or clock radio, and 4) none (including all women who reported using a sleep mask, regardless of other light sources reported).22 For women who reported multiple sources of light in the bedroom, they were classified based on the highest level of light reported unless they reported using a sleep mask.
2.4. Covariate assessment
On the baseline questionnaire, women reported their year of birth, race (American Indian/Alaska Native, Asian, Black/African American, Native Hawaiian/Pacific Islander, white; with the option to select multiple), ethnicity (Hispanic/Latina, with the option to provide country/region of origin), parity, educational attainment, annual household income, and primary residential address. Race/ethnicity is associated both with differences in breast cancer incidence27 and with residential location and neighborhood characteristics (and consequently outdoor light at night exposure) due to systemic discriminatory practices such as redlining.28 For women missing information on household income (n=1,993, <4%), multiple imputation by fully conditional specification was used to impute household income.29
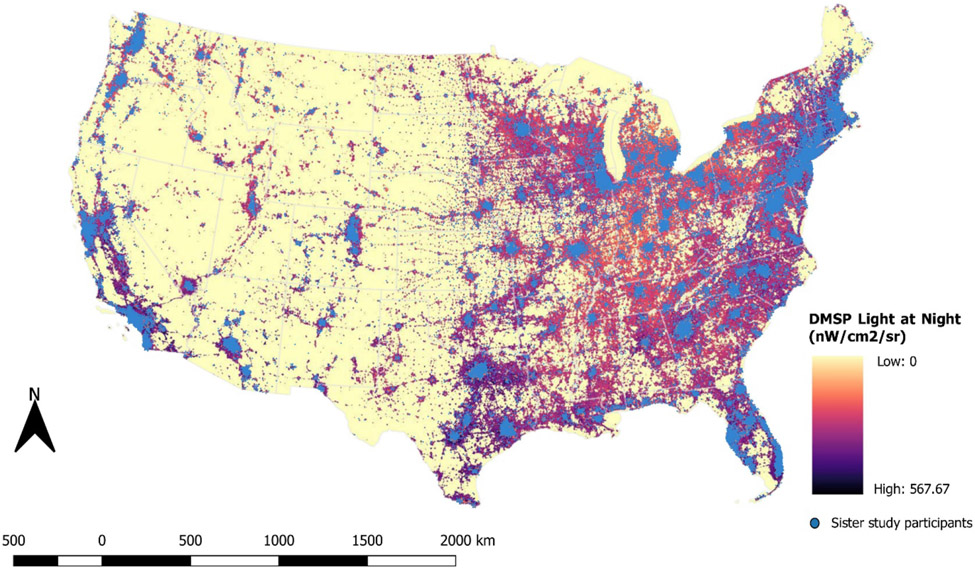
Geocoded primary enrollment residential addresses (Figure 1) were used to determine several covariate values. Latitude of primary enrollment residence was dichotomized as Northern (≥37°N) or Southern (<37°N) to account for differences in length of night associated with latitude and because incidence of breast cancer varies by latitude.30 Population density was determined at the Census tract level for the year 2000 using publicly available data from the U.S. Census Bureau (http://www.census.gov) by dividing the tract population size by the tract area in square miles (N/miles2). Area Deprivation Index (ADI) is based on 17 U.S. Census indicators of poverty, education, housing, and employment from the 2000 long-form Census, which are weighted to create Census block group-level measurements of neighborhood disadvantage.31 Values range from 1 (low deprivation) to 100 (high deprivation). Annual average concentrations of nitrogen dioxide (NO2) and particulate matter less than 2.5 microns in diameter (PM2.5) for 2006 were determined at the residence location using a validated regionalized universal kriging model with spatial smoothing, which incorporated regulatory monitoring information and a range of geographical covariates, including some satellite-derived measures.32,33 Measures of green space were determined at the residence location using NASA’s Moderate Resolution Imaging Spectroradiometer (MODIS) method to capture the normalized difference vegetation index (NDVI) (https://modis.gsfc.nasa.gov/data/dataprod/mod13.php) at approximately a 439.5 m resolution. NDVI values from July were matched to the year of enrollment in the study and range from −1 to 1. A-weighted 24-hour equivalent sound levels, or the average sound level for the day in decibels (dB), were determined based on a land-use regression model with 270-m resolution developed using noise data from the National Park Service and monitors in cities and at airports for the years 2000 to 2014.34
Figure 1.
Outdoor light at night for 2006, as captured by the U.S. Defense Meteorological Satellite Program. Blue dots represent Sister Study participants’ primary enrollment residences.
2.5. Statistical analysis
We excluded women who were diagnosed with breast cancer (invasive or ductal carcinoma in situ (DCIS)) prior to completing the enrollment interview, who had unknown timing of breast cancer diagnosis, did not contribute any follow-up time, or had an unknown year of enrollment (n=402). To focus on environmental (i.e., non-occupational) LAN exposure, we excluded women who reported working at night (n=1,477) or as part of a flight crew (flight attendant, pilot, or flight engineer; n=22) at enrollment. Women who were blind (n=6), women who reported daylight as the only light source while sleeping (n=200), and those who were missing outdoor (n=135, due to missing geocode) or indoor (n=16) LAN were also excluded (Supplemental figure 1).
Descriptive statistics are presented for the total analytic sample and by quintile of outdoor LAN. The Spearman correlation coefficient, ρ, was determined for the relationship between continuous outdoor LAN, population density, ADI, NO2, PM2.5, green space, and noise, as well as for outdoor LAN over time (1996, 1999, 2000, 2003, 2004, 2006, 2010). Correlations were evaluated in R (version 4.2.1; R Project for Statistical Computing) and presented using the package corrplot (https://www.rdocumentation.org/packages/corrplot).
We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between indoor and outdoor LAN and breast cancer using Cox proportional hazards regression models. Age was used as the time scale: women entered the risk set at their age at study enrollment and left the risk set at their age at diagnosis of invasive breast cancer or DCIS, at the end of the follow-up period (September 30, 2019), or age of a censoring event (death or loss-to-follow-up). In addition to assessing all breast cancers combined, separate analyses considered whether associations varied based on menopausal status at time of diagnosis, hormone receptor status, and invasiveness. We assessed heterogeneity by tumor characteristics using the likelihood ratio test (LRT) p-for-interaction for the interaction term between LAN (indoor, outdoor) and menopausal status, estrogen receptor (ER) status, progesterone receptor (PR) status, or invasiveness.
Confounders were selected using a directed acyclic graph.35 For both indoor and outdoor LAN models, we adjusted for age (as the timescale), self-reported race/ethnicity [non-Hispanic white, non-Hispanic Black/African American, Hispanic, “other” (collapsed due to small numbers; includes women who self-identified as Asian, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native)], educational attainment (high school diploma or equivalent, some college or a technical degree, Bachelor’s degree or higher), annual household income (<$50,000, $50,000-$99,999, $100,000-$200,000, >$200,000), neighborhood disadvantage (ADI, continuous; 1-100), latitude (<37°N, ≥37°N) and population density (continuous) (Model 1). This model assumes that population density adequately controls for urbanicity (i.e., all factors related to living in an urban area). Reproductive factors (parity, age at first birth) were determined to not be confounders based on our directed acyclic graph. For outdoor LAN, we considered a second adjustment set that assumes some amount of residual confounding by urbanicity due to correlations between outdoor LAN and other environmental exposures; this model includes the covariates in Model 1 as well as PM2.5, NO2, green space, and noise (Model 2). To assess the individual impact of adjusting for these additional variables, each was separately added to the Model 1 for outdoor LAN. We also evaluated the possibility of collinearity using linear regression models with quintiles of outdoor LAN or the IQR increase in outdoor LAN as the dependent variable and the Model 2 covariates as the predictors. From these regression models, we used R2 to calculate the variance inflation factor [VIF = 1/(1 − R2)]. A VIF of >5 suggests collinearity.36
Women who were missing information on either exposure or confounders for either model (n=1,632) were excluded from all analyses, which included residents of Puerto Rico, Hawaii, or Alaska, for whom information on NO2, PM2.5, green space, or noise were not available (Supplemental figure 1). Women who were excluded from the analysis due to missing covariates were similar to women in the analytic sample for most characteristics but tended to live in areas with lower deprivation and were more likely to be Hispanic and have a lower annual household income and lower educational attainment.
In a subgroup analysis, we investigated potential effect measure modification of associations between LAN and breast cancer by race (non-Hispanic white, non-Hispanic Black/African American). The purpose of this subgroup analysis was to identify differences in HRs by race, which is a social construct and, in a racialized country such as the U.S., contributes to individual socioeconomic status. Black and other minoritized women have poorer health outcomes compared to non-Hispanic white women,37 emphasizing the need for disaggregated data to be presented where possible. Interaction between race and LAN (indoor, outdoor) was assessed using multiplicative interaction terms, and statistical significance was determined using the LRT p-for-interaction.
Several sensitivity analyses were conducted. To address the possibility that outdoor LAN exposure only matters if it is actually entering the bedroom, we analyzed the association between outdoor LAN and breast cancer risk limiting to participants who reported having light from outside entering the bedroom while sleeping. To account for not having historical outdoor or indoor LAN, we restricted to women who lived at their primary enrollment residence for at least 10 years and for at least 20 years. We also considered analyses restricted to women who did not move outside of their enrollment residence census tract during follow-up (prior to March 15, 2018) to account for the lack of LAN data throughout the follow-up period. We further excluded women who were diagnosed with breast cancer during the first 2 years of follow-up to address the possibility of pre-clinical symptoms influencing self-reported indoor LAN exposures.
Statistical significance was determined using two-sided tests with p-values of 0.05. All analyses, unless otherwise noted, were conducted using SAS statistical software version 9.4 (SAS Institute, Cary, NC).
3. Results
Among the 47,145 women in our final analytic sample, a total of 3,734 cases were diagnosed (2,921 invasive, 793 DCIS) during an average of 11 ± 3 years of follow-up. At enrollment, the average age of the study participants was 56 years, and two thirds of the women were postmenopausal (Table 1). Most women were non-Hispanic white (85%), married or cohabitating (75%), and lived in the Northern half of the U.S. (66%). About half had at least a bachelor’s degree (51%).
Table 1.
Enrollment characteristics of Sister Study participants (N=47,145) over 514,771 person-years of follow-up (2003-2019), overall and by quintile of outdoor LAN exposure
| Outdoor light at night, nW/cm2/sr, median (IQR) | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Overall | Quintile 1 11.4 (6.9, 18.4) |
Quintile 2 54.4 (39.8, 71.7) |
Quintile 3 132.5 (112.0, 154.1) |
Quintile 4 221.1 (197.8, 247.7) |
Quintile 5 382.6 (321.0, 488.8) |
| Age at baseline, years (mean ± SD) | 55.8 ± 9.0 | 56.3 ± 8.8 | 55.7 ± 8.9 | 55.6 ± 9.1 | 55.6 ± 9 | 55.6 ± 9 |
| Population density, per square mile (mean ± SD) | 3345.6 ± 9138.0 | 158.3 ± 308.0 | 974.9 ± 1216.1 | 2224.1 ± 1990.5 | 3566.6 ± 2856.3 | 9804.3 ± 18575.5 |
| ADI (2000) (mean ± SD) | 34.3 ± 24.4 | 46.7 ± 23.8 | 33.4 ± 23.1 | 29.0 ± 22.4 | 29.4 ± 22.5 | 33.0 ± 25.9 |
| PM2.5, μg/m3 (2006) (mean ± SD) | 10.5 ± 2.4 | 9.0 ± 2.6 | 10.1 ± 2.2 | 10.8 ± 2.1 | 11.2 ± 2.1 | 11.5 ± 2.1 |
| NO2, ppb (2006) (mean ± SD) | 10.1 ± 4.9 | 5.3 ± 1.7 | 7.7 ± 2.3 | 9.8 ± 2.9 | 11.8 ± 3.4 | 15.7 ± 5.5 |
| Green space, MODIS NDVI (mean ± SD) | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 |
| Noise, dB (2000-2014) (mean ± SD) | 52.3 ± 4.0 | 47.5 ± 3.4 | 50.8 ± 2.5 | 52.6 ± 2.2 | 54.1 ± 2.2 | 56.4 ± 2.5 |
| Indoor LAN (%) | ||||||
| No light | 18 | 19 | 18 | 18 | 18 | 19 |
| Small nightlight | 39 | 43 | 43 | 40 | 38 | 33 |
| Light outside room | 31 | 29 | 29 | 30 | 32 | 33 |
| Light/TV on in room | 12 | 9 | 11 | 12 | 13 | 15 |
| Breast cancer, yes (%) | 8 | 7 | 8 | 8 | 8 | 8 |
| Race/ethnicity (%) | ||||||
| Non-Hispanic white | 85 | 92 | 89 | 85 | 84 | 76 |
| Non-Hispanic Black | 9 | 3 | 6 | 10 | 10 | 15 |
| Hispanic/Latina | 3 | 1 | 2 | 3 | 3 | 7 |
| “Other”1 | 3 | 3 | 3 | 2 | 2 | 2 |
| Educational attainment (%) | ||||||
| HS or less | 15 | 21 | 15 | 14 | 13 | 12 |
| Some coll./technical | 34 | 39 | 36 | 32 | 32 | 31 |
| Bachelor’s or higher | 51 | 40 | 49 | 53 | 55 | 57 |
| Household income (%) | ||||||
| <$50,000 | 25 | 30 | 22 | 22 | 23 | 27 |
| $50,000-$99,999 | 41 | 45 | 42 | 40 | 41 | 39 |
| $100,000-$200,000 | 27 | 21 | 29 | 29 | 28 | 26 |
| >$200,000 | 7 | 4 | 7 | 8 | 8 | 8 |
| Marital status (%)2 | ||||||
| Never married/single | 5 | 2 | 3 | 5 | 6 | 10 |
| Married/cohabitating | 75 | 85 | 80 | 75 | 72 | 64 |
| Widowed, divorced, or separated | 20 | 13 | 17 | 20 | 22 | 26 |
| BMI, kg/m2 (%)3 | ||||||
| <25 | 39 | 36 | 39 | 40 | 40 | 40 |
| 25-<30 | 32 | 33 | 33 | 31 | 30 | 31 |
| ≥30 | 30 | 31 | 29 | 29 | 30 | 30 |
| Parity (%)4 | ||||||
| Nulliparous | 18 | 14 | 16 | 17 | 18 | 24 |
| 1 | 14 | 13 | 14 | 14 | 15 | 16 |
| 2 | 37 | 37 | 39 | 39 | 37 | 33 |
| ≥3 | 31 | 35 | 31 | 30 | 30 | 27 |
| Postmenopausal at enrollment (%)5 | 67 | 70 | 67 | 66 | 65 | 66 |
| Latitude (%) | ||||||
| <37° N | 34 | 28 | 32 | 32 | 33 | 45 |
| ≥37° N | 66 | 72 | 68 | 68 | 67 | 55 |
Note: LAN, light at night; IQR, interquartile range; SD, standard deviation; ADI, Area Deprivation Index; PM2.5, fine particulate matter with diameter <2.5 μm; NO2, nitrogen dioxide; BMI, body mass index
“Other” category includes women who self-identified as Asian, Native Hawaiian/Pacific Islander, or American Indian/Alaska Native
n=6 women were missing marital status
n=17 women were missing BMI at enrollment
n=34 women were missing parity at enrollment
n=18 women were missing menopausal status at enrollment
Population density, PM2.5, NO2, and noise increased with increasing outdoor LAN whereas green space was inversely related to outdoor LAN. ADI was highest for women exposed to the lowest level of outdoor LAN but did not demonstrate a linear association across quintiles. Women living in areas with high outdoor LAN exposure were more likely to be non-Hispanic Black (Quintile 5: 15%) than in areas of low exposure (Quintile 1: 3%). Women with the highest outdoor LAN exposure were also more likely to have attained a bachelor’s degree or higher, to have never married or be single, to be nulliparous, to live in the Southern half of the U.S., and to report sleeping with a light or TV on in the room.
Continuous outdoor LAN was strongly correlated with population density (ρ = 0.82), NO2 (ρ = 0.78), and noise (ρ = 0.81; Supplemental figure 2a). NO2 and noise were also highly correlated with each other (ρ = 0.75). From 1999 to 2010, relative values of outdoor LAN values were extremely consistent, with correlation coefficients ranging from 0.92 to 0.98 (Supplemental figure 2b).
In our statistical model that did not adjust for the correlated environmental exposures (Model 1), the HR associated with outdoor LAN was elevated in the fourth (HR=1.10, 95% CI: 0.99-1.22; Table 2), but not the fifth quintile (HR=1.04, 95% CI: 0.93-1.16). However, after additional adjustment for NO2, PM2, green space and noise (Model 2), the elevated HR seen in Q4 was no longer apparent (HR=0.99, 95% CI: 0.86-1.14), and the HR for Q5 (HR=0.89, 95% CI: 0.74-1.06) and an IQR increase (HR=0.94, 95% CI: 0.87-1.01) both suggested an inverse relationship. Compared to having no light in the bedroom, sleeping with a light or TV on was associated with a HR=1.09 (95% CI: 0.97-1.23; Table 2).
Table 2.
Association between outdoor and indoor LAN and breast cancer among Sister Study participants (N=47,145)
| Total # (%) |
# cases |
Person- years |
Age-adjusted1 HR (95% CI) |
Model 12 HR (95% CI) |
Model 23 HR (95% CI) |
|
|---|---|---|---|---|---|---|
| Outdoor LAN | ||||||
| Q1 ≤27.55 | 9429 (20) | 703 | 102402 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Q2 ≤90.85 | 9429 (20) | 736 | 102525 | 1.06 (0.95-1.17) | 1.03 (0.93-1.15) | 0.99 (0.88-1.11) |
| Q3 ≤176.20 | 9429 (20) | 752 | 102874 | 1.08 (0.97-1.20) | 1.05 (0.94-1.17) | 0.97 (0.85-1.10) |
| Q4 ≤278.50 | 9429 (20) | 796 | 103643 | 1.13 (1.02-1.25) | 1.10 (0.99-1.22) | 0.99 (0.86-1.14) |
| Q5 ≤2776.52 | 9429 (20) | 747 | 103328 | 1.07 (0.96-1.18) | 1.04 (0.93-1.16) | 0.89 (0.74-1.06) |
| IQR increase4 | 47145 (100) | 3734 | 514771 | 1.02 (0.98-1.06) | 1.01 (0.96-1.06) | 0.94 (0.87-1.01) |
| Indoor LAN | ||||||
| No light/sleep mask | 8529 (18) | 679 | 92445 | 1.0 (ref) | 1.0 (ref) | - |
| Small nightlight | 18527 (39) | 1429 | 204591 | 0.96 (0.87-1.05) | 0.96 (0.87-1.05) | - |
| Light from outside room | 14452 (31) | 1179 | 158907 | 1.02 (0.92-1.12) | 1.02 (0.93-1.13) | |
| Light/TV in room | 5637 (12) | 447 | 58828 | 1.07 (0.95-1.21) | 1.09 (0.97-1.23) |
Note: LAN, light at night; HR, hazard ratio; CI, confidence interval; IQR, interquartile range; ADI, Area Deprivation Index; PM2.5, fine particulate matter with diameter <2.5 μm; NO2, nitrogen dioxide
Age used as the timescale
Additionally adjusted for race/ethnicity, educational attainment, household income, ADI, latitude, and population density
Additionally adjusted for PM2.5, NO2, green space, and noise
IQR increase = 207.88 nW/cm2/sr
When added separately to Model 1, adjustment for NO2 (Quintile 4 vs. 1: HR=1.02, 95% CI: 0.91-1.16) and noise (Quintile 4 vs. 1: HR=1.04, 95% CI: 0.91-1.19) attenuated the outdoor LAN estimate the most (Table 3). Outdoor LAN results adjusted for the correlated environmental exposures (Model 2) are presented for the remainder of the analyses. The VIFs of 3.16 for quintiles of outdoor LAN and 3.31 for an IQR increase in outdoor LAN are both below 5.00 and therefore do not suggest collinearity.
Table 3.
Association between outdoor LAN and breast cancer risk in the Sister Study comparing models with each of the environmental covariates from Model 2 separately added to Model 1
| Model 11 HR (95% CI) |
Model 11 +NO2 HR (95% CI) |
Model 11 +noise HR (95% CI) |
Model 11 +green space HR (95% CI) |
Model 11 +PM2.5 HR (95% CI) |
|
|---|---|---|---|---|---|
| Outdoor LAN | |||||
| Q1 ≤27.55 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Q2 ≤90.85 | 1.03 (0.93-1.15) | 1.01 (0.91-1.12) | 1.01 (0.90-1.13) | 1.02 (0.92-1.14) | 1.03 (0.92-1.14) |
| Q3 ≤176.20 | 1.05 (0.94-1.17) | 1.00 (0.89-1.12) | 1.00 (0.89-1.14) | 1.03 (0.93-1.15) | 1.04 (0.93-1.16) |
| Q4 ≤278.50 | 1.10 (0.99-1.22) | 1.02 (0.91-1.16) | 1.04 (0.91-1.19) | 1.08 (0.97-1.20) | 1.08 (0.97-1.21) |
| Q5 ≤2776.52 | 1.04 (0.93-1.16) | 0.93 (0.80-1.08) | 0.97 (0.83-1.13) | 1.00 (0.89-1.13) | 1.02 (0.91-1.15) |
| IQR increase2 | 1.01 (0.96-1.06) | 1.01 (0.96-1.06) | 0.98 (0.92-1.04) | 1.00 (0.95-1.05) | 1.00 (0.95-1.05) |
Note: LAN, light at night; HR, hazard ratio; CI, confidence interval; IQR, interquartile range; ADI, Area Deprivation Index; PM2.5, fine particulate matter with diameter <2.5 μm; NO2, nitrogen dioxide
Adjusted for race/ethnicity, educational attainment, household income, ADI, latitude, and population density
IQR increase = 207.88 nW/cm2/sr
When outdoor LAN was assessed only among women who reported light entering the bedroom while sleeping, the HRs for both quintile 4 (HR=1.13, 95% CI: 0.85-1.51) and quintile 5 (HR=1.13, 95% CI: 0.79-1.60) were elevated, although the wide confidence intervals are reflective of the small number of women included in this analysis (n=11,573; Table 4).
Table 4.
Association between outdoor LAN and breast cancer among women in the Sister Study who reported light entering the bedroom from outside the residence (N=11,573)
| Total # (%) | # cases |
Person- years |
Adjusted HR1 (95% CI) |
|
|---|---|---|---|---|
| Outdoor LAN | ||||
| Q1 ≤27.55 | 1846 (16) | 138 | 19995 | 1.0 (ref) |
| Q2 ≤90.85 | 2133 (18) | 183 | 23281 | 1.14 (0.90-1.45) |
| Q3 ≤176.20 | 2341 (20) | 171 | 25767 | 0.96 (0.74-1.26) |
| Q4 ≤278.50 | 2463 (21) | 208 | 27098 | 1.13 (0.85-1.51) |
| Q5 ≤2776.52 | 2790 (24) | 228 | 30634 | 1.13 (0.79-1.60) |
| IQR increase2 | 11573 (100) | 928 | 126775 | 1.04 (0.91-1.19) |
Note: LAN, light at night; HR, hazard ratio; CI, confidence interval; IQR, interquartile range; ADI, Area Deprivation Index; PM2.5, fine particulate matter with diameter <2.5 μm; NO2, nitrogen dioxide
Adjusted for race/ethnicity, educational attainment, household income, ADI, latitude, population density, PM2.5, NO2, green space, and noise
IQR increase = 207.88 nW/cm2/sr
Estimates were similar for sleeping with a light or TV on in the room when stratified by menopausal status (pre: HR=1.09, 95% CI: 0.81-1.48; post: HR=1.05, 95% CI: 0.91-1.21), as were estimates for an IQR increase in outdoor LAN (pre: HR=0.91, 95% CI: 0.76, 1.10; post: HR=0.93, 95% CI: 0.85-1.01; Supplemental table 1). Associations also did not meaningfully differ by ER and PR status for indoor (e.g., sleeping with a light/TV on – ER+: HR=1.06, 95% CI: 0.91-1.22; ER−: HR=1.01, 95% CI: 1.01, 95% CI: 0.73-1.41) or outdoor LAN (Quintile 5 vs. 1 – ER+: HR=0.89, 95% CI: 0.72-1.09; ER−: HR=0.96, 95% CI: 0.59-1.57; Supplemental table 2). Although the outdoor LAN HRs differed somewhat for PR status (Quintile 5 vs. 1 – PR+: 0.84, 95% CI: 0.67-1.06; PR−: HR=1.03, 95% CI: 0.71-1.50), the confidence intervals are imprecise and have considerable overlap. Estimates for outdoor LAN in relation to DCIS were elevated (Quintile 4 vs. 1: HR=1.17, 95% CI: 0.85-1.60) compared to invasive breast cancer (Quintile 4 vs. 1: HR=0.97, 95% CI: 0.82-1.12), although we had reduced power to detect an association due to the small number of DCIS cases (n=793; Supplemental table 3). Non-Hispanic Black women had an elevated risk of breast cancer for indoor LAN (light from outside the room: HR=1.32, 95% CI: 0.91-1.91; light/TV in the room: HR=1.28, 95% CI: 0.91-1.80) compared to non-Hispanic white women (light from outside the room: HR=1.03, 95% CI: 0.93-1.14; light/TV in the room: HR=1.09, 95% CI: 0.94-1.25), although these analyses were also limited by the limited number of non-Hispanic Black cases (n=293; Supplemental table 4).
Associations for all measures of LAN were generally consistent among long-term residents (women who reported living at their enrollment address for at least 10 years or at least 20 years prior to enrollment), non-movers (women who did not move outside their enrollment residence census tract prior to March 15, 2018) and the 2-year lagged analysis (Supplemental table 5).
4. Discussion
In this large, prospective U.S.-based cohort study, we observed little evidence that either indoor or outdoor LAN was related to breast cancer risk. After adjustment for factors correlated with urbanicity (NO2, noise, PM2.5, and green space), no association was observed between outdoor LAN and the risk of breast cancer. When separately adding these four covariates to the model, NO2, followed by noise, led to the largest attenuation of the association between higher outdoor LAN and breast cancer risk, compared to the lowest quintile of outdoor LAN.
As described by Stevens and Davis (1996), there is biologic plausibility for an association between exposure to LAN and the risk of breast cancer, referred to as “the melatonin hypothesis.”38 The light-dark cycle is the primary regulator of human circadian rhythms.2 Melatonin is a neurotransmitter that typically peaks between midnight and 4:00 a.m. and is both a product of and signal for circadian rhythms.2 Relevant for breast carcinogenesis, the functions of melatonin include the suppression of estrogen, as well as antioxidant, apoptotic, and anti-proliferative effects in cancer cells.39 Exposure to light, when detected by specialized cells in the eyes, has been demonstrated to suppress melatonin secretion.40 Thus, a decrease in melatonin is hypothesized to lead to an increase in estrogen and a concomitant increase in breast cancer risk.38
Our finding of no association between outdoor LAN, either categorically or continuously, and breast cancer risk in our full study population does not provide evidence in favor of “the melatonin hypothesis” and is not consistent with the results of other large, U.S.-based cohort studies that have evaluated this association, despite the use of the same outdoor LAN assessment method. In the Nurses’ Health Study II, women exposed to the highest quintile of outdoor LAN had 1.14 times the risk of breast cancer of women exposed to the lowest quintile (95% CI: 1.01-1.29), and a small positive association was also observed for continuous outdoor LAN (HR=1.05, 95% CI: 1.00-1.11).12 Similar estimates were observed in the California Teachers Study (Quintile 5 vs. 1: HR=1.12, 95% CI: 1.00-1.26),11 the NIH-AARP Diet and Health Study (Quintile 5 vs. 1: HR=1.10, 95% CI: 1.02-1.18),13 and the Southern Communities Cohort (Quintile 5 vs. 1: HR=1.27, 95% CI: 1.00-1.60).14 However, none of these studies adjusted for NO2 or noise, which attenuated the weak associations we observed for outdoor LAN in our first model that did not adjust for these correlated exposures. It is possible that confounding by NO2 exposure, which has been associated with breast cancer risk in a recent meta-analysis,41 and in this cohort specifically,42 was responsible for the non-null associations observed between outdoor LAN and breast cancer in previous cohort studies. Noise, which further reduced our observed estimates when added to the model with NO2, has also been associated with breast cancer risk in a few studies,43,44 although none of these studies adjusted for NO2 exposure. NO2 and noise, both frequently resulting from road traffic,45,46 were highly correlated at participant residence locations.
Our results for outdoor LAN are, however, consistent with those of three case-control studies. No associations between outdoor LAN and breast cancer were observed among Danish nurses (Tertile 3 vs. 1: OR=0.97, 95% CI: 0.77-1.23)47 or Canadian women (Tertile 3 vs. 1: OR=0.83, 95% CI: 0.63-1.09).48 The MCC-Spain Study used images of Madrid and Barcelona taken from the International Space Station (ISS) in 2012 and 2013, respectively, to capture outdoor LAN; it was also the only analysis to adjust for indoor LAN in their outdoor LAN model.17 No association was observed for overall visual light (Tertile 3 vs. 1: OR=0.81, 95% CI: 0.54-1.20), although the odds of breast cancer were elevated among women who were exposed to the highest tertile of blue light (OR=1.47, 95% CI: 1.00-2.17).
An important limitation of all but one of the prior studies is the lack of information regarding the indoor environment. High exposure to outdoor LAN is irrelevant if black out shades or sleep masks are used to block outdoor light from entering the bedroom during the sleeping period. When we limited our analysis to women who reported having light enter the bedroom from the outside, we did note elevated effect estimates in our sensitivity analysis, although our analysis was underpowered. Future analyses with larger sample sizes assessing outdoor LAN in context of the indoor environment are needed to confirm our suggested associations.
We observed a small, but elevated, association for sleeping with a light or TV on and the risk of breast cancer, which is consistent with the previous literature. The California Teachers Study, which compared “heavy users” of indoor LAN (defined as ≥10 months of use, ≥5 days per week for ≥7 hours per night) to “non-users” users, found an elevated estimate (HR=1.13, 95% CI: 0.84-1.52).11 A similar-magnitude association was observed in a case-control study in Seattle (continuous across 6 reported levels: OR=1.1, 95% CI: 0.9-1.2).16 In an Israeli case-control study, women reporting the highest level of bedroom light had an OR of 1.22 (95% CI: 1.12-1.31) after adjustment for sleeping with the shutters open or with a television on.19 Other studies that did not observe elevated risks for indoor LAN and breast cancer include the MCC-Spain Study (“quite illuminated” compared to “total darkness”: OR=0.77, 95% CI: 0.39-1.51)17 and the UK Generations Study (“light enough to see across the room, but not to read” or “light enough to read” compared to “too dark to see your hand, or you wear a mask”: HR=1.01, 95% CI: 0.88-1.15).18 Despite having an additional 1,000 cases, our estimates for indoor LAN (HR=1.09, 95% CI: 0.97-1.23) were only marginally more precise than the previous analysis in the Sister Study (HR=1.09, 95% CI: 0.93-1.26).22 To date, no study has assessed objectively measured indoor LAN and breast cancer risk, which could further illuminate whether an association truly does or does not exist between indoor LAN and breast cancer.
The Sister Study is a large, prospective cohort with over 10 years of follow-up and over 3,700 breast cancer diagnoses. Participants included in this analysis lived in all 48 states of the contiguous U.S. at enrollment and represented a range of ages and occupations. Extensive questionnaire data was available, and we were able to link multiple environmental exposures to participants through the use of geocoded enrollment addresses. However, indoor and outdoor LAN exposures were only available for the enrollment time period. Women who moved during follow-up may have altered their indoor LAN habits or moved to an area with significantly more or less outdoor LAN. However, when restricting to women who had lived at their enrollment residence for at least 10 or 20 years, we did not see a difference in our estimated associations for indoor or outdoor LAN, nor did we see differences in the relative values of outdoor LAN over the available years of data. However, it is possible that we did not capture the etiologically relevant time window in our analysis49-51 or did not have sufficient follow-up time.
Since the enrollment period of our study population (2003-2009), two other methods of evaluating outdoor LAN exposure have become available. ISS images provide high spatial resolution with the added benefit of allowing for an assessment of blue light exposure. However, these types of images are only available for a select number of cities in the U.S. and were taken after the enrollment period of our study (e.g., Houston in 2014, Phoenix in 2013; https://eol.jsc.nasa.gov/). Visible Infrared Imaging Radiometer Suite Day-Night Band (DNB) is another satellite-derived method of ascertaining outdoor LAN exposure that has a higher resolution (15 arc-second grid vs. 30 arc-second grid) and a greater ability to detect lower levels of light (0.02 nW/cm2/sr vs. 0.5 nW/cm2/sr) compared to the DMSP method52 that we used for our analysis. We did not use DNB because the first year of availability (2012) is three years after the end of our enrollment period, which would have restricted our analytic sample to women who were still living at their enrollment residence in 2012 and limited the amount of follow-up time and case accrual. The previously-mentioned Canadian case-control study assessed outdoor LAN using both DNB and DMSP, and did not observe an association with either method (DNB, Tertile 3 vs. 1: OR=0.95, 95% CI: 0.70-1.27),48 suggesting that the resolution difference between DMSP and DNB is unlikely to explain our null finding. It is likely that our use of DMSP data for determining outdoor LAN exposure resulted in some amount of exposure misclassification. However, several large cohort studies have previously found positive associations between DMSP outdoor LAN and breast cancer, as noted above, but used a different set of adjustment variables, further suggesting that confounding by other environmental exposures, rather than exposure measurement error, more plausibly explains our negligible findings. Future studies could consider whether more spatially-refined measures of outdoor LAN are associated with breast cancer risk, after accounting for correlated environmental exposures.
Indoor LAN data was self-reported by study participants at enrollment. Participants were asked about light sources but not asked about the types of bulbs used, the brightness level of indoor LAN sources, or the use of electronic devices before falling asleep. This lack of specificity could have resulted in misclassification of indoor LAN. Additionally, light emitting diodes, or LEDs, have grown in popularity since our study enrollment period, and this type of lighting emits predominantly blue wavelength light.53 Blue light more strongly impacts circadian functioning and, consequently, melatonin concentrations in humans compared to longer-wavelength light (e.g., red).3 Our indoor LAN assessment may therefore be an underestimate of true exposure and may not be generalizable to present day indoor LAN exposures.
While we attempted to account for residual confounding by additionally adjusting for NO2, PM2.5, green space, and noise, it is possible that our residual confounding model resulted in bias amplification. Bias amplification can occur when covariates that are correlated with the exposure because of an upstream, unmeasured variable (e.g., living in an urban/more developed area beyond a simple measurement of population density) are also associated with the outcome along with other confounders of the exposure-outcome relationship (e.g., socioeconomic status) and are included in the model.54 The challenge becomes determining which is worse: the amplification of bias from including correlated variables in the model (represented by Model 2) or not adjusting for all possible sources of confounding (represented by Model 1). This trade-off is difficult to quantify although unlikely to be meaningful for our analysis, given the small difference between our two model effect estimates for outdoor LAN. Of note, we did not find collinearity to be impacting our model 2, when including the additional environmental exposures. Supplemental figure 1 depicts the relationship between these variables in a simplified directed acyclic graph. While our analysis attempted to isolate the impact of LAN on breast cancer risk, future studies could consider using a mixtures methods approach (e.g., quantile g-computation55) to address the impact of LAN in conjunction with other exposures.
5. Conclusion
In this large, prospective U.S. cohort, outdoor LAN does not appear related to breast cancer risk after adjustment for correlated environmental exposures. To our knowledge, this analysis is the first study of outdoor LAN and breast cancer to investigate a range of ambient environmental exposures as potential confounders. In our population, NO2 and noise were highly correlated with outdoor LAN and appeared to confound a modest suggestive association between outdoor LAN and breast cancer risk, although it is possible that adjusting for correlated variables led to bias amplification. These findings underscore the importance of carefully considering how multiple, correlated environmental exposures may impact observed associations. We did observe slightly elevated, but imprecise, estimates for high outdoor LAN among women reporting light from the outside entering the sleep environment and for the highest category of indoor LAN. If LAN, which serves many important functions in society, does impact the risk of breast cancer, the increase in risk is likely to be very small.
Supplementary Material
Acknowledgements
The authors would like to thank Joseph Engeda of DLH Corp for providing the map used for Figure 1.
Funding sources
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z1AES103332, Z1AES044005) and the Extramural Research Program of the NIH, National Institute of Environmental Health Sciences (F31ES033062, T32ES007018).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyba CCM, Kuester T, Sánchez de Miguel A, Baugh K, Jechow A, Hölker F, Bennie J, Elvidge CD, Gaston KJ, Guanter L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv 2017;3:e1701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, Stevens RG, Turek FW, et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ 2017;607–608:1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world: Breast Cancer and Circadian Disruption. CA Cancer J Clin 2014;64:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 5.Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K, for the Breast Cancer Surveillance Consortium. Population-Attributable Risk Proportion of Clinical Risk Factors for Breast Cancer. JAMA Oncol 2017;3:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YJ, Park MS, Lee E, Choi JW. High Incidence of Breast Cancer in Light-Polluted Areas with Spatial Effects in Korea. Asian Pac J Cancer Prev 2016;17:361–7. [DOI] [PubMed] [Google Scholar]
- 7.Kloog I, Haim A, Stevens RG, Barchana M, Portnov BA. Light at Night Co-distributes with Incident Breast but not Lung Cancer in the Female Population of Israel. Chronobiol Int 2008;25:65–81. [DOI] [PubMed] [Google Scholar]
- 8.Portnov BA, Stevens RG, Samociuk H, Wakefield D, Gregorio DI. Light at night and breast cancer incidence in Connecticut: An ecological study of age group effects. Sci Total Environ 2016;572:1020–4. [DOI] [PubMed] [Google Scholar]
- 9.Rybnikova N, Haim A, Portnov BA. Artificial Light at Night (ALAN) and breast cancer incidence worldwide: A revisit of earlier findings with analysis of current trends. Chronobiol Int 2015;32:757–73. [DOI] [PubMed] [Google Scholar]
- 10.Bauer SE, Wagner SE, Burch J, Bayakly R, Vena JE. A case-referent study: light at night and breast cancer risk in Georgia. Int J Health Geogr 2013;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, Reynolds P. Light at Night and Breast Cancer Risk Among California Teachers: Epidemiology 2014;25:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F. Outdoor Light at Night and Breast Cancer Incidence in the Nurses’ Health Study II. Environ Health Perspect 2017;125:087010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao Q, James P, Breheny P, Jia P, Park Y, Zhang D, Fisher JA, Ward MH, Jones RR. Outdoor light at night and postmenopausal breast cancer risk in the NIH-AARP diet and health study. Int J Cancer 2020;ijc.33016. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Q, Gierach GL, Bauer C, Blot WJ, James P, Jones RR. The Association between Outdoor Artificial Light at Night and Breast Cancer Risk in Black and White Women in the Southern Community Cohort Study. Environ Health Perspect 2021;129:087701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbano T, Vinceti M, Wise LA, Filippini T. Light at night and risk of breast cancer: a systematic review and dose–response meta-analysis. Int J Health Geogr 2021;20:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis S, Mirick DK, Stevens RG. Night Shift Work, Light at Night, and Risk of Breast Cancer. JNCI J Natl Cancer Inst 2001;93:1557–62. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Saenz A, Sánchez de Miguel A, Espinosa A, Valentin A, Aragonés N, Llorca J, Amiano P, Martín Sánchez V, Guevara M, Capelo R, Tardón A, Peiró-Perez R, et al. Evaluating the Association between Artificial Light-at-Night Exposure and Breast and Prostate Cancer Risk in Spain (MCC-Spain Study). Environ Health Perspect 2018;126:047011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johns LE, Jones ME, Schoemaker MJ, McFadden E, Ashworth A, Swerdlow AJ. Domestic light at night and breast cancer risk: a prospective analysis of 105 000 UK women in the Generations Study. Br J Cancer 2018;118:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloog I, Portnov BA, Rennert HS, Haim A. Does the Modern Urbanized Sleeping Habitat Pose a Breast Cancer Risk? Chronobiol Int 2011;28:76–80. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Zheng T, Holford TR, Boyle P, Zhang Y, Dai M. Light at night and breast cancer risk: results from a population-based case–control study in Connecticut, USA. Cancer Causes Control 2010;21:2281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Leary ES, Schoenfeld ER, Stevens RG, Kabat GC, Henderson K, Grimson R, Gammon MD, Leske MC. Shift Work, Light at Night, and Breast Cancer on Long Island, New York. Am J Epidemiol 2006;164:358–66. [DOI] [PubMed] [Google Scholar]
- 22.White AJ, Weinberg CR, Park Y, D’Aloisio AA, Vogtmann E, Nichols HB, Sandler DP. Sleep characteristics, light at night and breast cancer risk in a prospective cohort. Int J Cancer 2017;141:2204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huss A, van Wel L, Bogaards L, Vrijkotte T, Wolf L, Hoek G, Vermeulen R. Shedding Some Light in the Dark—A Comparison of Personal Measurements with Satellite-Based Estimates of Exposure to Light at Night among Children in the Netherlands. Environ Health Perspect 2019;127:067001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones RR. Exposure to artificial light at night and risk of cancer: where do we go from here? Br J Cancer 2021;124:1467–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, Kleeberger CA, Shore DL, DeRoo LA, Taylor JA, Weinberg CR, the Sister Study Research Team. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environ Health Perspect 2017;125:127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Aloisio AA, Nichols HB, Hodgson ME, Deming-Halverson SL, Sandler DP. Validity of self-reported breast cancer characteristics in a nationwide cohort of women with a family history of breast cancer. BMC Cancer 2017;17:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state: Breast Cancer Statistics, 2017. CA Cancer J Clin 2017;67:439–48. [DOI] [PubMed] [Google Scholar]
- 28.Greer J. The Home Owners’ Loan Corporation and the Development of the Residential Security Maps. J Urban Hist 2013;39:275–96. [Google Scholar]
- 29.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw [Internet] 2011. [cited 2021 Aug 6];45. Available from: http://www.jstatsoft.org/v45/i03/ [Google Scholar]
- 30.Oh E-Y, Ansell C, Nawaz H, Yang C-H, Wood PA, Hrushesky WJM. Global breast cancer seasonality. Breast Cancer Res Treat 2010;123:233–43. [DOI] [PubMed] [Google Scholar]
- 31.Kind AJH, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. Neighborhood Socioeconomic Disadvantage and 30-Day Rehospitalization: A Retrospective Cohort Study. Ann Intern Med 2014;161:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampson PD, Richards M, Szpiro AA, Bergen S, Sheppard L, Larson TV, Kaufman JD. A regionalized national universal kriging model using Partial Least Squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ 2013;75:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young MT, Bechle MJ, Sampson PD, Szpiro AA, Marshall JD, Sheppard L, Kaufman JD. Satellite-Based NO 2 and Model Validation in a National Prediction Model Based on Universal Kriging and Land-Use Regression. Environ Sci Technol 2016;50:3686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mennitt DJ, Fristrup KM. Influence factors and spatiotemporal patterns of environmental sound levels in the contiguous United States. Noise Control Eng J 2016;64:342–53. [Google Scholar]
- 35.Greenland S, Pearl J, Robins JM. Causal Diagrams for Epidemiologic Research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 36.Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol 2019;72:558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams DR, Mohammed SA. Racism and Health I: Pathways and Scientific Evidence. Am Behav Sci 2013;57:1152–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens RG, Davis S. The Melatonin Hypothesis: Electric Power and Breast Cancer. Environ Health Perspect 1996;104:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, Frasch T, Blask DE. Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer 2015;22:R183–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tähkämö L, Partonen T, Pesonen A-K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int 2019;36:151–70. [DOI] [PubMed] [Google Scholar]
- 41.Gabet S, Lemarchand C, Guénel P, Slama R. Breast Cancer Risk in Association with Atmospheric Pollution Exposure: A Meta-Analysis of Effect Estimates Followed by a Health Impact Assessment. Environ Health Perspect 2021;129:057012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White AJ, Keller JP, Zhao S, Carroll R, Kaufman JD, Sandler DP. Air Pollution, Clustering of Particulate Matter Components, and Breast Cancer in the Sister Study: A U.S.-Wide Cohort. Environ Health Perspect 2019;127:107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersen ZJ, Jørgensen JT, Elsborg L, Lophaven SN, Backalarz C, Laursen JE, Pedersen TH, Simonsen MK, Bräuner EV, Lynge E. Long-term exposure to road traffic noise and incidence of breast cancer: a cohort study. Breast Cancer Res 2018;20:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sørensen M, Poulsen AH, Kroman N, Hvidtfeldt UA, Thacher JD, Roswall N, Brandt J, Frohn LM, Jensen SS, Levin G, Raaschou-Nielsen O. Road and railway noise and risk for breast cancer: A nationwide study covering Denmark. Environ Res 2021;195:110739. [DOI] [PubMed] [Google Scholar]
- 45.Forehead H, Huynh N. Review of modelling air pollution from traffic at street-level - The state of the science. Environ Pollut 2018;241:775–86. [DOI] [PubMed] [Google Scholar]
- 46.Goines L, Hagler L. Noise Pollution: A Modern Plague: South Med J 2007;100:287–94. [DOI] [PubMed] [Google Scholar]
- 47.Clarke RB, Amini H, James P, von Euler-Chelpin M, Jørgensen JT, Mehta A, Cole-Hunter T, Westendorp R, Mortensen LH, Loft S, Brandt J, Hertel O, et al. Outdoor light at night and breast cancer incidence in the Danish Nurse Cohort. Environ Res 2021;194:110631. [DOI] [PubMed] [Google Scholar]
- 48.Ritonja J, McIsaac MA, Sanders E, Kyba CCM, Grundy A, Cordina-Duverger E, Spinelli JJ, Aronson KJ. Outdoor light at night at residences and breast cancer risk in Canada. Eur J Epidemiol [Internet] 2020. [cited 2020 Jun 23];Available from: http://link.springer.com/10.1007/s10654-020-00610-x [DOI] [PubMed] [Google Scholar]
- 49.Terry MB, Michels KB, Brody JG, Byrne C, Chen S, Jerry DJ, Malecki KMC, Martin MB, Miller RL, Neuhausen SL, Silk K, Trentham-Dietz A. Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res 2019;21:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papantoniou K, Castaño-Vinyals G, Espinosa A, Aragonés N, Pérez-Gómez B, Ardanaz E, Altzibar JM, Sanchez VM, Gómez-Acebo I, Llorca J, Muñoz D, Tardón A, et al. Breast cancer risk and night shift work in a case–control study in a Spanish population. Eur J Epidemiol 2016;31:867–78. [DOI] [PubMed] [Google Scholar]
- 51.Menegaux F, Truong T, Anger A, Cordina-Duverger E, Lamkarkach F, Arveux P, Kerbrat P, Févotte J, Guénel P. Night work and breast cancer: A population-based case-control study in France (the CECILE study). Int J Cancer 2013;132:924–31. [DOI] [PubMed] [Google Scholar]
- 52.Rybnikova NA, Portnov BA. Outdoor light and breast cancer incidence: a comparative analysis of DMSP and VIIRS-DNB satellite data. Int J Remote Sens 2017;38:5952–61. [Google Scholar]
- 53.Hatori M, Gronfier C, Van Gelder RN, Bernstein PS, Carreras J, Panda S, Marks F, Sliney D, Hunt CE, Hirota T, Furukawa T, Tsubota K. Global rise of potential health hazards caused by blue light-induced circadian disruption in modern aging societies. Npj Aging Mech Dis 2017;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weisskopf MG, Seals RM, Webster TF. Bias Amplification in Epidemiologic Analysis of Exposure to Mixtures. Environ Health Perspect 2018;126:047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect 2020;128:047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.