Abstract
Research studying aging in adults with autism spectrum disorder (ASD) is growing, but longitudinal work is needed. Autistic adults have increased risk of dementia, altered hippocampal volumes and fornix integrity, and verbal memory difficulties compared to neurotypical (NT) adults. This study examined longitudinal aging in middle-age adults with ASD versus a matched NT group, and compared findings to cross-sectional age effects across a broad adult age range.
Participants were 194 adults with (n=106; 74 male) and without (n=88; 52 male) ASD, ages 18–71. Participants (n=45; 40–70 age range) with two visits (2–3 years apart) were included in a longitudinal analysis. Hippocampal volume, fornix fractional anisotropy (FA), and verbal memory were measured via T1-weighted MRI, diffusion tensor imaging, and the Rey Auditory Verbal Learning Test, respectively. Longitudinal mixed models were used for hippocampal system variables and reliable change index categories were used for AVLT analyses. Multi-variate regression was used for cross-sectional analyses.
Middle-age adults with ASD had greater longitudinal hippocampal volume loss and were more likely to show clinically meaningful decline in short-term memory, compared to NT. In contrast, cross-sectional associations between increasing age and worsening short-term memory were identified in NT, but not autistic adults. Reduced fornix FA and long-term memory in ASD were found across the broad cross-sectional age range.
These preliminary longitudinal findings suggest accelerated hippocampal volume loss in ASD and slightly higher rates of clinically-meaningful decline in verbal short-term memory. Contradictory cross-sectional and longitudinal results underscore the importance of longitudinal aging research in autistic adults.
Lay Summary
Autistic adults have increased risk of dementia, differences in brain memory structures, and difficulty with memory compared to neurotypical (NT) adults. However, there are no publications that follow the same middle-age autistic adults over time to see how their brain and memory change. Our preliminary findings in a small middle-age autism sample suggest a key memory brain structure, the hippocampus, may shrink faster over 2–3 years compared to NT, and short-term memory may become more challenging for some. Across a broad adult range, autistic adults also had reduced integrity of connections to the hippocampus and greater challenges with long-term memory. In our larger sample across a broad age range, the results did not hint at this aforementioned pattern of accelerated aging. This underscores the importance of more aging research in autism, and especially research where people are followed over time.
Introduction
Research on aging in adults with autism spectrum disorder (ASD)/autistic adults1 is growing, but longitudinal work in middle-age and older adults has yet to emerge. Importantly, recent evidence suggests that older adults with ASD may be at increased risk of cognitive disorders (Hand et al., 2020) and neurodegenerative disease, including early-onset dementia (Starkstein, Gellar, Parlier, Payne, & Piven, 2015; Vivanti, Shea, Tao, Lyall, & Robins, 2021). These findings raise concern that aging outcomes may be worse for autistic adults. However, studies exploring brain and cognitive aging in ASD to date have shown inconclusive results, which may be due to their cross-sectional nature. Cohort effects in cross-sectional studies of aging in ASD may be particularly strong given differences in diagnostic criteria, resources, and support depending on the decade of diagnosis (Keyes et al., 2012; Mazurek et al., 2014). Longitudinal studies are optimal for studying aging as they control for individual differences in ASD severity and life experience by examining within-subject changes over time. This study sought to provide the first mixed cross-sectional and longitudinal characterization of brain and cognitive aging in autistic adults.
To date, cross-sectional studies suggest accelerated brain aging in ASD across various cortical regions/networks, including cortical thickness (Braden & Riecken, 2019), white matter integrity (Koolschijn, Caan, Teeuw, Olabarriaga, & Geurts, 2017), and a functional brain network implicated in executive functioning (fronto-partietal network; Walsh et al. 2019). However, other studies suggest parallel or less decline in functional network topology of cognitive and sensorimotor networks (Bathelt, Koolschijn, & Geurts, 2020). Few aging studies in ASD have investigated the hippocampus or its structural connections (e.g., fornix), despite their relevance to aging. For example, the hippocampus is the primary structure involved in memory formation, is vulnerable to age-related volume loss, and is significantly affected in Alzheimer’s disease (Foley et al., 2017; Treves & Rolls, 1994; Zhang et al., 2010; Zheng et al., 2018). In ASD, evidence shows atypical hippocampal development trajectories with faster volume loss (Barnea-Goraly et al., 2014) as well as smaller hippocampi (especially the right hippocampus) in young to older autistic adults compared to neurotypical (NT; (Rooij 2018; Braden et al. 2017), again with inconsistencies (Koolschijn & Geurts, 2016). Similarly, the fornix is a white matter track that originates in the hippocampus, arches around the thalamus, and innervates other limbic structures critical for memory consolidation (Markowska, Olton, and Murray 1989; Sutherland and Rodriguez 1989). This structure shows reduced integrity as a function of older age (Brickman et al., 2012; Cabeza, Nyberg, & Park, 2009; Grieve et al., 2007) and memory decline (Gale et al., 1993; Mielke et al., 2012; Rudebeck et al., 2009). Furthermore, cross-sectional studies in adults with ASD suggest accelerated aging may preferentially affect white matter tracts rather than gray matter (Koolschijn & Geurts, 2016; Raznahan et al., 2010). We have also observed reduced fornix FA in middle-age autistic adults (40–64 years old) compared to matched NT participants with a medium-large effect size (Cohen’s d =0.63; Braden et al., 2017). Together, this evidence suggests hippocampal and fornix structure may serve as early biomarkers of atypical aging in ASD, but longitudinal research in mid-to-older adults is needed to characterize trajectories.
The cognitive consequences of brain aging in typical populations have been thoroughly studied and provide a foundation for understanding cognitive aging in ASD. Specifically, in typical aging, early declines are most apparent in memory and executive functioning (Folstein et al., 1975). To date, only one longitudinal study on cognitive function in ASD has been published, showing increases in IQ from childhood to young adulthood (Simonoff et al., 2020). Research on cognitive aging in ASD beyond young adulthood has been cross-sectional, with mixed evidence suggesting either accelerated, parallel, or preserved cognitive functioning in ASD compared to NT groups. For example, one study has shown evidence suggesting exacerbated short-term visual memory decline in autistic adults ages 51–83 (Geurts and Vissers (2012)). Similarly, another study showed evidence suggesting accelerated decline in ASD (ages 30–67) on a global cognitive measure, which included measures of both visual and verbal memory (Powell, Klinger, & Klinger, 2017). In contrast, Lever & Geurts (2016) study found evidence suggesting relatively “preserved” visual memory function with age in ASD vs. NT (ages 20–79). Other studies on verbal memory have shown evidence suggesting parallel declines in ASD compared to NT adults (Tse et al., 2021). Inconsistencies might be explained by sample age differences, the domain of memory/cognition studied, measurement tools, cohort effects or other sources of between-participant variation. While evidence is particularly inconsistent for visual memory, studies of verbal memory have more consistently found this domain is vulnerable to aging in ASD, generally on par with NT groups. Thus, verbal memory may be sensitive to early age-related cognitive changes in ASD. Furthermore, given relationships between declining verbal cognitive abilities with age and reduced quality of life, it is important to better understand if and to what extent verbal memory is impacted by aging in ASD (Singh et al., 2017). Longitudinal designs are warranted to more accurately characterize age-related memory vulnerabilities across the adult lifespan in ASD.
Hippocampal system and memory measures consistently show sex differences in NT samples, and there is some evidence for sex by diagnosis interactions in ASD (Voyer et al., 2021; Braden et al., 2017). In middle-age adults 40–64 years, we previously found smaller bilateral hippocampal volumes in autistic men, compared to a matched NT men (Braden et al., 2017) but no difference was detected by Koolschijn and colleagues (2016) with a mixed sex sample ranging from 30 to 75 years old. Our recent systematic review suggests sex-related biology may uniquely influence brain development and aging trajectories in ASD vs. NT groups (Walsh et al., 2021). For hippocampal volumes, others have shown sex-by-diagnosis interactions with sex differences becoming more apparent in adolescent/young adults compared to children in participants with ASD, but not NT participants (Williams et al., 2020; Zheng et al., 2018). To date, studies of white matter microstructure have not identified distinct fornix sex differences in children/adults with ASD vs NT, although no studies have examined sex differences in mid-to-older adults with ASD (Walsh et al., 2021). In healthy young adults, the fornix shows higher FA in women (Cahn et al., 2021) and age effects in young-to-older adults suggest similar patterns of decline in white matter microstructural integrity (Kodiweera et al., 2016). Finally, sex by diagnosis interactions in memory have been described in verbal autobiographical memory favoring women with ASD in the absence of a NT sex effect (Hull, Mandy, & Petrides, 2017).
In the present study, we conducted a longitudinal analysis over a 2–3 year timeframe to measure age-related changes in hippocampal volume and fornix microstructure (fractional anisotropy; FA) across mid-to-older adulthood in ASD. We hypothesized that autistic adults would show steeper trajectories of decline in hippocampal volume and fornix integrity (FA). We examined reliable change scores for short-term and long-term verbal memory to determine rates of clinically meaningful change. We hypothesized that adults with ASD would demonstrate higher rates of clinically meaningful memory decline. Given mixed findings in cross-sectional studies of cognitive and brain aging in ASD to date, we also conducted a cross-sectional analysis over a wider adult age range for comparison with previous literature and longitudinal results herein. Finally, in the presence of a significant main effect of sex in cross-sectional analyses, we investigated exploratory sex by diagnosis interactions.
Materials and methods
Participants
The study consisted of 194 total participants comprised of 106 adults with ASD (74 male, 32 female; sex was defined as assigned sex at birth) and 88 NT adults (52 male, 36 female) between 18 and 71 years of age that were recruited between the years 2014–2020, partly representative of participants from previous publications (Baxter et al., 2019; Braden & Riecken, 2019; Braden et al., 2017). NT adults were chosen as the comparison group because the lack of aging research in ASD requires characterization in comparison to NT prior to other developmental disorders. Forty-five middle-age and older participants who were contacted via email or phone and completed a follow-up visit within 2–3 years of their initial visit were included in longitudinal analyses. Longitudinal participants consisted of 23 middle-age adults with ASD (19 male, 4 female) and 22 NT participants (18 male, 4 female) between 40 and 66 years of age at baseline. One participant with ASD (aged 71 at baseline), was excluded from longitudinal analyses because they did not have a NT age match. There was an 86% retention rate for longitudinal participants (Fig. 1). Participants in both groups were recruited through flyers posted around Tempe, Arizona and Arizona State University and word of mouth. Some participants were additionally recruited from the Southwest Autism Research & Resource Center (SARRC) database. The SARRC database is voluntary and includes information about people who have been involved in previous clinical or research projects at SARRC. Participants in both groups underwent the same screening and enrollment procedures.
Fig. 1.
Distribution of cross-sectional/longitudinal data as a function of age.
Inclusion/Exclusion Criteria
Participants with ASD had their diagnosis formally verified at SARRC with the Autism Diagnostic Observation Schedule-2, module 4 (ADOS-2; Lord et al., 2012) and a brief psychiatric history interview administered by a research-reliable psychometrist. A score ≥ 7 on the ADOS-2 and an assessment by a psychologist with 25 years of ASD diagnostic experience confirmed DSM-5 criteria were met for their ASD diagnosis. One young adult participant did not meet ADOS-2 criteria for ASD. In this case, the ADOS-2 was overruled based on results from the Autism Diagnostic Interview-Revised (Lord et al., 2000), clinician judgment, and known sensitivity limitations of the standard cut-off criteria for non-intellectually disabled adults with ASD (similar to Bastiaansen et al., 2011). NT participants were excluded if they had a first-degree relative with an ASD diagnosis, were suspected or confirmed to have an ASD diagnosis, or if they had a T-score >66 on the Social Responsiveness Scale-2 Adult Self-Report (SRS-2; Constantino, 2012). Most participants with ASD also completed the SRS-2 during screening, but data were missing from eight young adults in the ASD group. Participants from both groups were excluded if their full-scale IQ score was <70 on the Kaufman Brief Intelligence Test-2 (KBIT-2; Kaufman & Kaufman, 2004), they scored <25 on the Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975), or they self-reported neurological disease such as a stroke or dementia, head injury with loss of consciousness, known genetic disorders, or current use of seizure medications or illicit drugs. Psychiatric conditions were non-exclusionary because of high prevalence in ASD (Lever & Geurts, 2016b). In total, eleven potential participants were excluded from the ASD group: four did not meet criteria on ADOS-2, four reported head trauma with loss of consciousness, and two did not meet criteria on the SRS-2, and one did not meet the IQ criteria. Eight potential participants were excluded for the NT group: two did not meet criteria on the SRS-2, two reported head trauma, two reported concerns for metal contraindications for the MRI, one reported epilepsy with ongoing seizures, and one reported using methamphetamine.
Magnetic Resonance Imaging
MRI data were acquired with a 3-Tesla Philips Ingenia MRI scanner with a maximum gradient strength of 45 mT/m at the Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, AZ. High-resolution, T1-weighted anatomical scans were captured using the following parameters: 3D magnetization prepared rapid acquisition gradient echo [MPRAGE] 256 X 256 in-plane resolution, 240 mm field of view (FOV); 170 sagittal slices 1.2 mm. Additionally, gradient-echo echo-planar (EPI) DTI scans were collected using the following parameters: echo time/repetition time (TE/TR) = 101/7,850 ms, bandwidth = 2,621 Hz/Px with a voxel size of 1.41 X 1.41 X 3 mm thick. Fornix FA values were generated from DTI obtained along 32 directions using a b-value of 2500 s/mm2 in the axial direction with 3 mm slice resolution.
T1-weighted MPRAGE images were used to generate hippocampal volumes in mm3 using FreeSurfer automated parcellation, and longitudinal data was processed in the longitudinal FreeSurfer pipeline (surfer.nmr.mgh.harvard.edu). Left and right hippocampal volumes were divided by total intracranial volumes (TIV) mm3 to correct for individual differences in brain size. Hemisphere volumes were then averaged for primary analyses, and exploratory post-hoc analyses of significant effects were conducted to determine the hemisphere driving significance.
DTI images were processed for eddy current, distortion, b-vec and motion correction and skull stripped. Fractional anisotropy (FA) maps were calculated using custom written MatLab code. FA is a measurement of the restriction of a diffusion process that is thought to quantify axonal integrity and myelination of white matter (Özarslan, Vemuri, & Mareci, 2005; Pierpaoli & Basser, 1996). High FA values indicate directional diffusion and low FA values indicate unrestricted diffusion. FA was chosen as our metric of interest because our previous paper demonstrated a higher effect size for FA differences in middle adulthood ASD vs. NT (Braden et al., 2017). Using Advanced Normalization Tools (ANTs) non-linear transformations, the b0 was registered to the subject’s T1 in native space, then the transformation was applied to FA maps, and finally, volumes were nonlinearly registered to the MNI template. Standard atlases containing the fornix were applied to FA maps. Briefly, the fornix ROI used was from a stereotaxic probabilistic white matter atlas that was generated from 81 normal subjects ranging from the ages of 18 to 59 (Mori et al., 2008). The quality of the dataset was evaluated with FSL quality control tools. This quality control involves using FSL for an automated non-parametric framework that corrects for distortions and signal loss. Motion was extracted from the affine motion and eddy current correction, and quantified by the average volume to volume head motion over each volume. The John’s Hopkin’s University standard atlas containing the fornix body and bilateral cres was applied. For primary analyses, the body/bilateral cres values were averaged, and post-hoc analyses were conducted to explore any significant effects. Five cross-sectional participants with ASD were missing MRI data due to discomfort in the scanning environment or mechanical failure. One cross-sectional NT participant was missing DTI data due to technical errors. One longitudinal NT participant was missing MRI data due to discomfort in the scanning environment and three longitudinal participants with ASD were missing DTI data due to technical errors.
Verbal Learning and Memory
Participants performed the Rey Auditory Verbal Learning Test (AVLT; Schmidt, 1996), typically the same day as the MRI at Barrow Neurological Institute. The AVLT is an auditory verbal learning and memory task consisting of a supra-span word list of 15 words which are repeated five times (A1–), followed by a free recall trial after a 20–30-minute delay (A7). Raw scores for short-term immediate recall (A1) and long-term delayed recall (A7) as short-term memory and long-term memory, respectively, were used for analyses. One NT participant was missing AVLT data due to experimenter error. For longitudinal visits, an alternate form was used at each visit.
Statistical Analysis
Independent two-sample t-tests or chi-squared tests were conducted to examine group differences in age, sex distribution, IQ (KBIT-2), global cognitive function (MMSE), DTI motion, and self-reported autistic traits (SRS-2). The longitudinal marginal models estimating diagnosis differences in hippocampus/fornix trajectories were implemented with SAS 9.4. For primary analyses, models were used to predict total intracranial volume (TIV) corrected hippocampal volume (right/left hemisphere average) and fornix cres/body fractional anisotropy (average of right/left cres and fornix body), with false discovery rate (FDR) correction for these two comparisons (alpha set at p=0.05 after FDR). Post-hoc exploratory models examined hemispheres/sub-regions driving significant diagnosis-by-age effects. For these models, the age variable for each subject was split into between-subject (BS) and within-subject (WS) components, based on recommendations from Guillaume et al. (2014) and Neuhaus & Kalbfleisch (1998). Specifically, we calculated the mean age for each subject across visits. These age-BS values were then mean centered. We then calculated residual age values at each visit relative to the subject’s mean age (across visits) to generate an age-WS variable. All models included the following fixed effects: diagnosis, age-BS, age-WS, the interaction of age-BS and diagnosis, and the interaction of age-WS and diagnosis. Sex was included as a nuisance covariate. An unstructured covariance pattern model was used. Graphs were generated using SAS proc sgplot to display marginal trajectories with 95% confidence intervals. Power for longitudinal analyses was calculated based on the only longitudinal paper in ASD investigating the metrics included herein. Based on effect sizes from Barnea-Goraly and colleagues (2014), we had 96% power to detect diagnosis group by time interactions for accelerated hippocampal volume loss after two-to-three year follow up in participants with ASD, compared to NT participants.
For longitudinal analysis of memory variables (AVLT A1 and A7), we used reliable change index (RCI) for AVLT short-term and long-term memory variables with a clinical cut-off score of 1.64 (Duff, 2012). RCI scores were calculated to account for test-retest/practice effects based on test-retest normative data from Lemay and colleagues (2004), which shows acceptable test-retest reliability for the metrics used in the paper (A1 and A7; 0.71, and 0.81, respectively). Lemay et al. (2004) was chosen because of the similarity in age group to our longitudinal group. The 90% confidence intervals were used to identify participants as declining, stable, or improving in performance. Chi-squared tests were used to compare group distributions in ‘decline’ vs. ‘stable/improving’ categories, with FDR correction for these two comparisons (alpha set at p=0.05 after FDR). As this was not an intervention study, understanding incidence of ‘improvement’ alone was not meaningful or hypothesized. There are no previous reports of longitudinal verbal memory change in ASD to calculate power.
Cross-sectional analysis over a wider adult age range were run for comparison with previous literature and longitudinal results. A multivariate regression was performed on each dependent variable separately using SPSS 25. Hippocampal system variables (average left and right TIV-corrected hippocampi volumes; average fornix body/bilateral cres FA) and AVLT memory variables (short-term memory, and long-term memory) were investigated with the following as the predictors in the model: diagnosis group, centered age, and diagnosis group by centered age, and sex as a covariate. FDR was implemented for each predictor across the dependent variables. Significance was interpreted with alpha set at p=0.05 after FDR. Power analyses based on effect sizes from Powell and collagues (2017) indicated our sample size had 98% and 99% power to detect diagnosis group main effects for short-term verbal memory and verbal learning, respectively (long-term verbal memory was not assessed). Based on effect sizes in our previous paper (Braden et al., 2017), we had 99% power to detect diagnosis group main effects for hippocampal volumes and fornix FA. Since we were primarily powered to detect cross-sectional diagnosis main effects, diagnosis group by age and diagnosis by sex interactions should be viewed as exploratory. Finally, since previous research suggest sex may moderate these measures differently in ASD, exploratory sex by diagnosis interactions were employed in models where sex explained a significant portion of the variance.
Data availability.
Data are available upon reasonable request.
Results
Demographics, DTI Motion, and Autistic Traits
For cross-sectional (Table 1) and longitudinal (Table 2) participants, groups were similar in age, sex distribution, IQ (KBIT-2), global cognitive function (MMSE), and DTI motion, but different on self-reported autistic traits (SRS-2). For the longitudinal (Table 2) cohort, groups were similar with respect to the T1 to T2 interval. Cross-sectional participants over 40 years of age and longitudinal participants, samples were similar in age, IQ (KBIT-2), global cognitive function (MMSE), and self-reported autistic traits (SRS-2), but had different sex distributions as this study initially began in men (Braden et al., 2017; Supplemental Table 1).
Table 1.
Demographics for all participants
| NT (n=89) Mean (±SD) Range |
ASD (n=106) Mean (±SD) Range |
Statistics | |
|---|---|---|---|
| Age (Years) | 40.94 (±16.72) 18–70 |
39.58 (±16.02) 18–71 |
t(193)=0.582, p=0.561 |
| Sex (M/F) | 52/37 | 74/32 | X2(195)=2.742, p=0.098 |
| ADOS-2a Social Affective | n/a | 10.17 (±3.16) 2e-19 |
n/a |
| Age at Diagnosis | n/a | 34.91 (±17.15) 2–66 |
n/a |
| SRS-2b Total t-score | 45.31 (±5.71) 37–66 |
71.81 (±11.47) 43–90 |
t(145.16)=−20.264, p<0.001f |
| MMSE c | 29.47 (±0.88) 25–30 |
29.25 (±1.04) 26–30 |
t(192)=1.573, p=0.117 |
| KBIT-2d Composite | 108.28 (±11.99) 77–141 |
106.71 (±14.48) 70–139 |
t(192.97)=0.830, p=0.408e |
| DTI Motion (mm) | 0.701 (±0.131) 0.50–0.95 |
0.632 (±0.139) 0.44–1.21 |
t(186)=0.148, p=0.881 |
Autism Diagnostic Observation Schedule-2;
Social Responsiveness Scale-2;
Mini Mental State Exam;
Kaufman Brief Intelligence Test-2;
A single case of ADOS-2 below cutoff was over-ruled by Autism Diagnostic Interview-Revised results and clinical judgement;
Levene’s test for equality of variances showed a significant group difference, thus statistics are presented after ‘equal variances not assumed’ adjustment
Table 2.
Baseline demographics for participants included in longitudinal analysis
| NT (n=22) Mean (±SD) Range |
ASD (n=23) Mean (±SD) Range |
Statistics | |
|---|---|---|---|
| Age (Years) | 50.45 (±7.05) 40–64 |
52.57 (±8.46) 40–66 |
t(43)=−0.907, p=0.369 |
| Sex (M/F) | 18/4 | 19/4 | X2(45)=0.005, p=0.945 |
| T1–T2 Interval (months) | 24.86 (±1.67) 23–29 |
26.04 (±2.82) 24–36 |
t(43)=1.698, p=0.097 |
| ADOS-2a Social Affective | n/a | 10.61 (±3.10) 7–19 |
n/a |
| Age at Diagnosis | n/a | 46.43 (±14.12) 6–66 |
n/a |
| SRS-2b Total t-score | 46.41 (±5.89) 38–60 |
72.96 (±10.64) 51–88 |
t(34.65)=−10.415, p<0.001e |
| MMSE c | 29.41 (±0.91) 26–30 |
29.04 (±1.15) 26–30 |
t(43)=1.182, p=0.244 |
| KBIT-2d Composite | 111.18 (±14.14) 89–141 |
110.57 (±14.17) 70–131 |
t(43)=0.146, p=0.885 |
| DTI Motion (mm) | 0.773 (±0.091) 0.66–0.93 |
0.771 (±0.094) 0.61–0.94 |
t(43)=0.071, p=0.943 |
Autism Diagnostic Observation Schedule-2;
Social Responsiveness Scale-2;
Mini Mental State Exam;
Kaufman Brief Intelligence Test-2,
Levene’s test for equality of variances showed a significant group difference, thus statistics are presented after ‘equal variances not assumed’ adjustment
Longitudinal
Hippocampus and Fornix
For average hippocampal volume, there was a significant main effect of diagnosis and a significant age-WS-by-diagnosis interaction (Tables 3–4, Figure 2a). Specifically, participants with ASD, showed smaller average hippocampal volumes than NT groups (t45.2=−3.32, p=.002). Furthermore, the slope of change over time for longitudinal participants with ASD was more negative than NT groups (t44=−2.32, p=.025; Figure 2a). This effect survives FDR correction for multiple comparison across the two dependent measures. Post-hoc exploratory analysis of right and left hippocampal volumes separately showed that the age-WS-by-diagnosis effect was stronger in the right hippocampus (t44=−2.24, p=.030), with a similar but non-significant trend in the left hippocampus (t44=−1.75, p=.087). For fornix body/bilateral cres average FA, there was a significant effects of diagnosis, with the ASD group having reduced FA vs. NT (t41=−2.06, p=.046). There was a significant effect of age-WS (t41=−6.08, p<.0001; Tables 3–4) where longitudinal participants, irrespective of diagnosis, showed significant negative slopes of change over time across study participation (Figure 2b).
Table 3.
Longitudinal group means ± SD
| ASD | NT | |||
|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | |
| Average Hippocampal Volume | .3318 (±.0368) | .3238 (±.0411) | .3654 (±.0397) | .3665 (±.0379) |
|
Average Fornix FA
|
.4217 (±.0385) | .3959 (±.0426) | .4428 (±.0264) | .4153 (±.0323) |
| Short-Term Memory (AVLTa A1) | 6.39 (±2.04) | 5.78 (±1.98) | 5.91 (±2.39) | 6.59 (±2.44) |
|
Long-Term Memory (AVLTa
A7) |
8.87 (±2.96) | 8.30 (±3.40) | 10.95 (±2.95) | 10.82 (±2.61) |
Auditory Verbal Learning Test
Table 4.
Fixed effect estimates for longitudinal models average hippocampal volume
| Estimate | Standard Error | DF | t-value | p-value | ||
|---|---|---|---|---|---|---|
| Average hippocampal volume | Intercept | 0.359 | 0.008 | 44.1 | 43.99 | <.0001 |
| Diagnosis | −0.035 | 0.011 | 44 | −3.32 | 0.002 * | |
| Age-BS | −0.002 | 0.001 | 44 | −2.00 | 0.052 | |
| Age-BS*Diagnosis | 0.002 | 0.001 | 44 | 1.40 | 0.167 | |
| Age-WS | 0.001 | 0.001 | 44 | 0.41 | 0.683 | |
| Age-WS*Diagnosis | −0.004 | 0.002 | 44 | −2.32 | 0.025 * | |
| Sex | 0.026 | 0.014 | 44 | 1.89 | 0.065 | |
| Average fornix body/cres FA | Intercept | 0.434 | 0.006 | 41.8 | 67.77 | <.0001 |
| Diagnosis | −0.018 | .009 | 41 | −2.06 | 0.046 * | |
| Age-BS | −0.0004 | 0.001 | 41 | −0.52 | 0.606 | |
| Age-BS*Diagnosis | −0.002 | 0.001 | 41 | −1.76 | 0.087 | |
| Age-WS | −0.013 | 0.002 | 41 | −6.08 | <.0001 * | |
| Age-WS*Diagnosis | 0.002 | 0.003 | 41.1 | 0.60 | 0.551 | |
| Sex | −0.029 | 0.010 | 41 | −2.79 | 0.008 |
bold indicates p<0.05 after false discovery rate correction
Fig. 2.
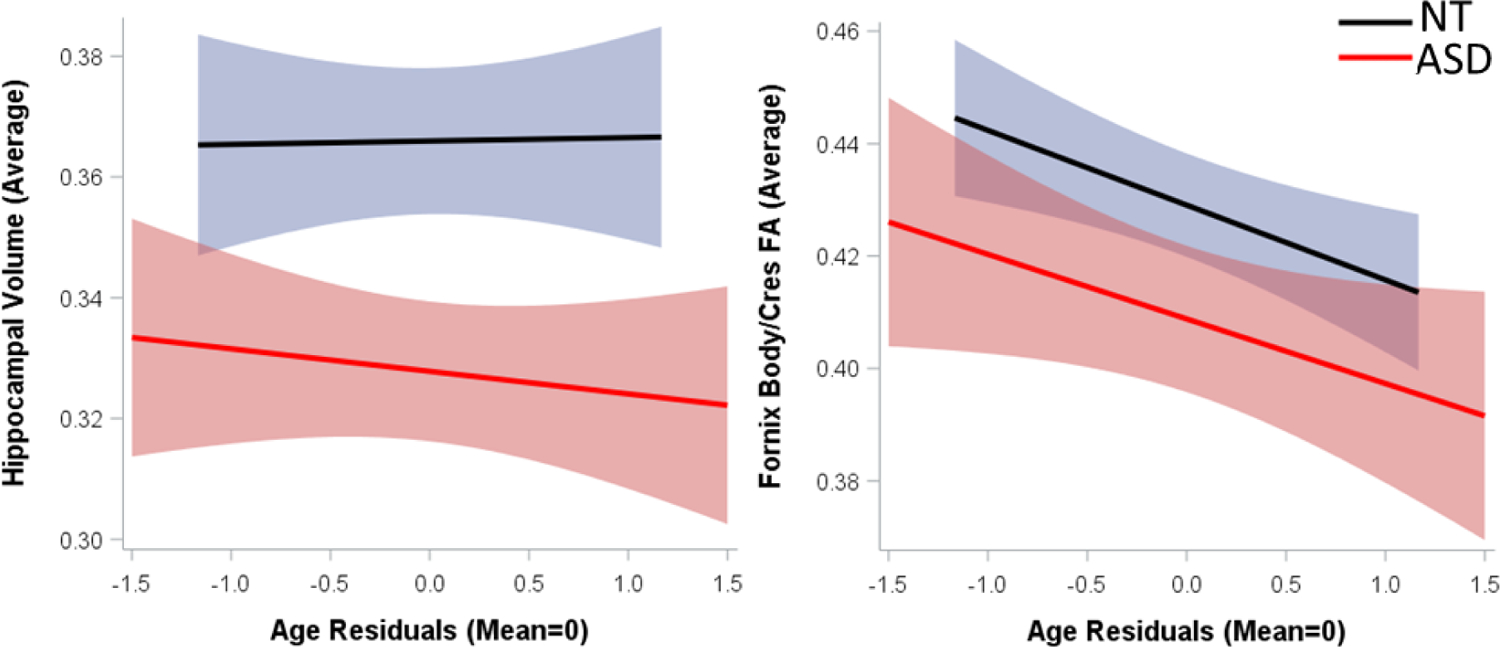
Scatterplot of raw data for average (a; left) hippocampal volume and (b; right) fornix body/cres FA with regression line and 95% confidence intervals for mean effects.
Memory
For short-term verbal memory, RCIs indicated that a greater proportion (33%) of adults with ASD experienced a clinically meaningful decline in short-term verbal memory, compared to NT adults (4%; X245=5.16, p=0.023; Figure 3a), which survived FDR correction for multiple comparisons across the two dependent measures. There were no significant differences between groups for long-term verbal memory. RCI indicated nearly all participants (91–92%) in both groups remained stable in their performance over time (X245=0.31, p=0.577; Figure 3b).
Fig. 3.
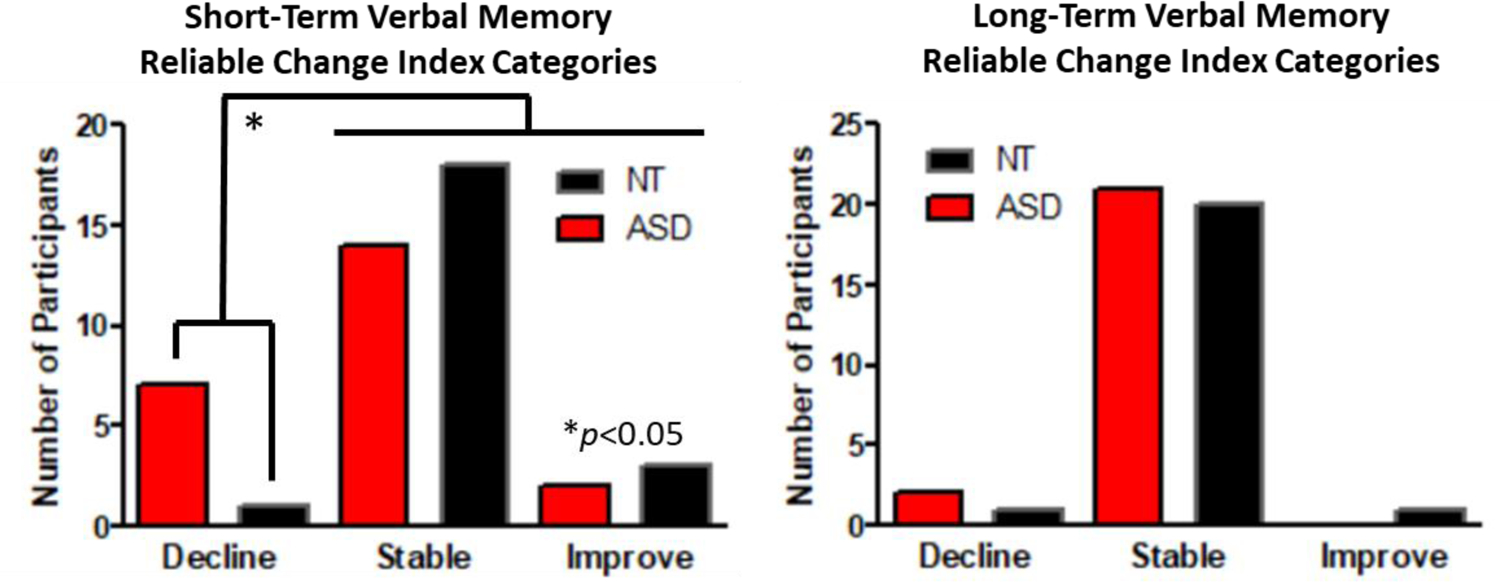
Longitudinal (2–3 year) reliable change index categories by group for (a; left) short-term verbal memory and (b; right) long-term verbal memory on the Auditory Verbal Learning Test (A1 and A7). *p<0.05
Cross-Sectional
For the hippocampus and fornix, all models showed significant main effects of age, such that average bilateral TIV-corrected hippocampal volumes and average fornix body/bilateral cres FA decreased with age, and a main effect of sex (Table 5). There was also a main effect of diagnosis for average fornix FA (NT>ASD), but not for average hippocampal volumes. Since sex explained a significant portion of the variance in each model, we investigated exploratory sex by diagnosis interactions for hippocampal volume and fornix FA, which were not significant (Supplementary Table 2).
Table 5.
Cross-sectional AVLT data and analysis results
| Overall model | Diagnosis group | Age | Age by Diagnosis group | Sex | ASD (mean ±SD) |
NT (mean ±SD) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | β | p | β | p | β | p | β | p | |||
| Average Hippo. volume (%TIV b ) | 4, 184 | 7.85 | <0.001 | −.04 | 0.521 | −.31 | 0.002 * | −.04 | .709 | .19 | 0.006 * | 0.3592 (±0.04) | 0.3632 (±0.04) |
| Average fornix FA c | 4, 183 | 24.39 | <0.001 | −.21 | 0.001 * | −.48 | <0.001 * | −.03 | 0.689 | −.24 | <0.001 * | 0.4196 (±0.04) | 0.4319 (±0.03) |
| Short-Term Memory (AVLTa A1) | 4, 189 | 4.80 | 0.001 | −.11 | 0.134 | −.37 | <0.001 * | .29 | 0.004 * | .13 | 0.076 | 6.26 (±1.93) |
6.69 (±1.90) |
| Long-Term Memory (AVLTa A7) | 4, 189 | 4.82 | 0.001 | −.18 | 0.013 * | −.27 | 0.009 * | .06 | 0.552 | .12 | 0.101 | 9.87 (±3.35) |
11.01 (±2.92) |
Auditory Verbal Learning Test;
Total Intercranial Volume;
Fractional Anisotropy;
bold indicates p<0.05 after false discovery rate correction
For short-term verbal memory, there was a diagnosis by age interaction (Table 5; Fig. 4). Simple effects revealed that increasing age was associated with worsening short-term verbal recall in NT adults only (β=−0.39, p<0.001); there was no age relationship in adults with ASD (β=0.029, p=0.77; Fig. 4). For long-term verbal memory, there was a main effect of diagnosis, with autistic adults recalling fewer items than NT adults, but no interactions (Table 5).
Fig. 4.
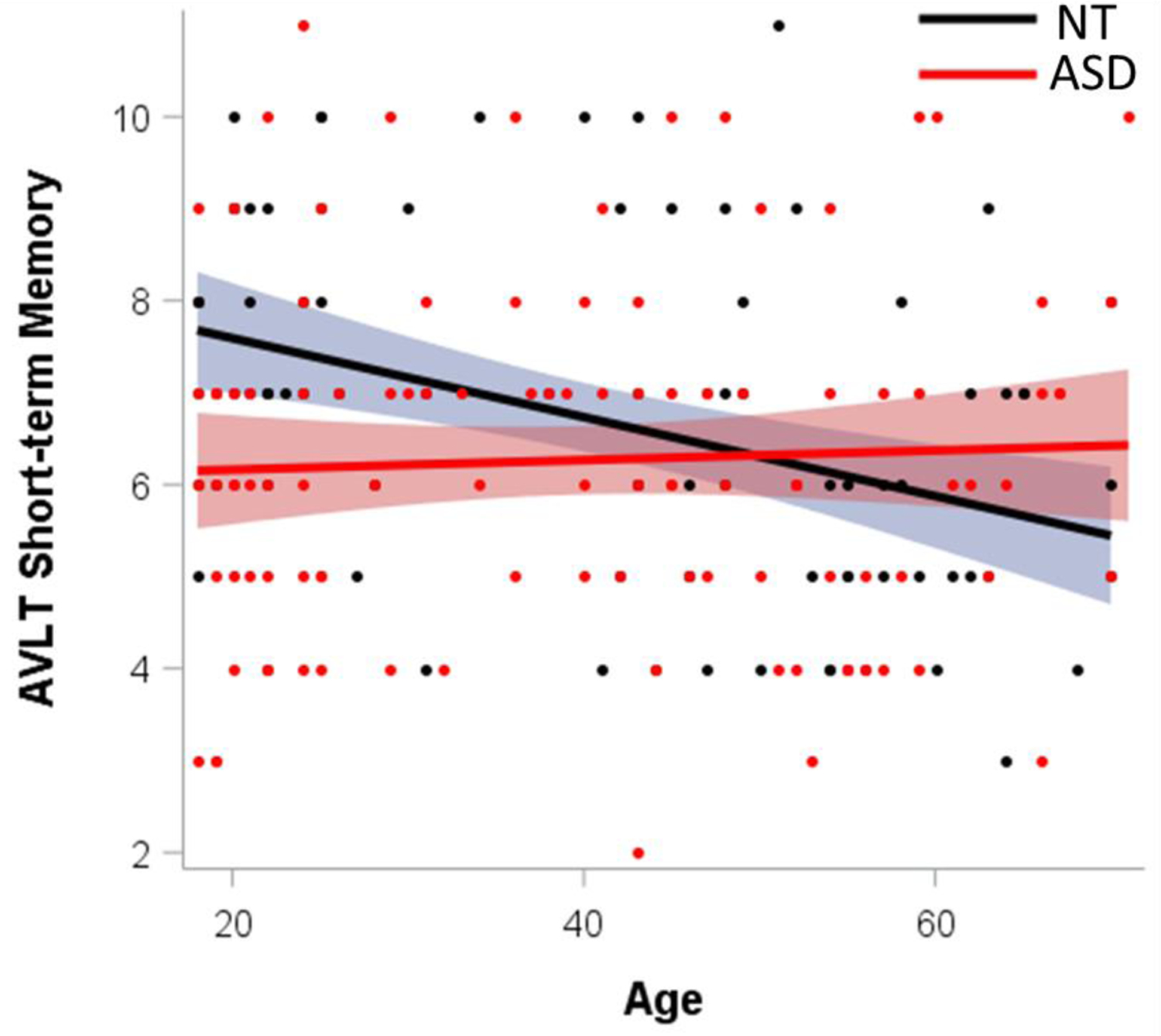
Cross-sectional relationships with age by group on the Auditory Verbal Learning Test (AVLT) short-term memory (A1).
Discussion
This study is the first to examine longitudinal brain aging in ASD, specifically focusing on regions implicated in memory function (hippocampus/fornix). In our longitudinal sample, we found trajectories of accelerated decline in average hippocampal volume in adults with ASD compared to NT. Importantly, we separated cross-sectional and longitudinal effects of age in a single model, and this “accelerated aging” effect was significant for within-subject age (e.g., change over time) only. In order to corroborate these longitudinal brain findings behaviorally, we investigated verbal memory reliable change scores to determine if autistic adults showed patterns suggesting worse aging outcomes. Indeed, we found that mid-to-older adults with ASD showed slightly higher rates of clinically meaningful decline in short-term verbal memory over a two-to-three-year follow-up window. Finally, when comparing these longitudinal effects with exploratory cross-sectional age by diagnosis effects across a wider adult age range, we found that results were inconsistent. Specifically, cross-sectional age relationships suggested a “protection” of verbal memory with age in ASD compared to NT. This pattern of inconsistent results is present in the broader cross-sectional literature on cognitive aging in ASD (Tse et al., 2021), highlighting that cohort effects or difference in age range may confound cross-sectional behavioral analyses of aging in ASD. Lastly, reduced average fornix FA and long-term memory performance in ASD were found across the broad cross-sectional age range and did not interact with age. Together, our preliminary results suggest that a subgroup of autistic adults may experience worse cognitive and brain aging outcomes. However, given our small longitudinal sample with a relatively short follow-up window, further work is needed to validate these findings in larger samples.
Hippocampal System
Our preliminary finding of accelerated average hippocampal volume loss with age in adults with ASD is timely, in light of recent reports that autistic adults are at greater risk for early-onset Alzheimer’s disease and other dementias, compared to NT adults (Vivanti et al., 2021). Hippocampal size is a known early biomarker of dementia (Davda & Corkill, 2020). Our findings are synergistic with prior longitudinal research showing that children and adolescents with ASD have more hippocampal volume loss than NT children (Barnea-Goraly et al., 2014). Further, findings of smaller hippocampi (especially the right hippocampus) is also consistent with the largest ASD brain morphometry study to date across a broad 2–64 year age range. However, no hippocampal difference was detected by Koolschijn and colleagues (2016) with a mixed sex sample ranging from 30 to 75 years old. We hypothesized that sex differences might be contributing to this discrepancy. Indeed, sex was a significant predictor in our cross-sectional analysis, but the sex by diagnosis interaction did not reach significance (p=0.053). Our small longitudinal sample did not have enough women to investigate sex differences in hippocampal change. Subsequent longitudinal follow-up in a larger sample of autistic men and women is warranted.
For fornix FA, there were no differences in age trajectories between adults with ASD and NT adults (neither for within-subject age effects or between-subject age effects). However, there was a significant main effect of diagnosis in both the middle-age and broad adult age cross-sectional sample with autistic adults having reduced average fornix FA. In autistic children, fornix/temporal lobe FA is significantly reduced, compared to NT children (Lee et al., 2007; Poustka et al., 2011). Kleinhans and colleagues (2012) corroborated decreased FA in younger individuals with ASD; however, FA values approached NT levels by young adulthood. Koolschijn and colleagues (2016) identified less favorable cross-sectional age relationships in adults with ASD on various metrics within white matter tracts; however, they didn’t specifically measure the fornix. Perhaps, the two-to-three year follow-up window for longitudinal participants may not be sufficient to detect group differences in fornix FA change over time. Longer longitudinal follow up into elderly age ranges are also warranted for the fornix to rule out further fornix vulnerabilities at old age in ASD.
Verbal Learning and Memory
We present the first preliminary findings of longitudinal verbal memory change in middle-age adults with ASD. We conducted RCI analyses to control for test-retest/practice effects and determine clinically-meaningful change. Our findings in a relatively small sample suggest that autistic adults may experience higher rates of clinically meaningful decline in verbal memory over time compared to NT groups. RCI findings indicated 33% of middle-age adults with ASD experienced a clinically meaningfully decline in performance, compared with 4% of matched NT participants. Those that experienced a meaningful improvement were comparable across groups (ASD: 9%; NT: 13%). Although we identified a slight risk for short-term verbal memory decline in ASD, the majority of participants in both groups remained stable (ASD: 58%; NT: 83%). For verbal long-term memory, nearly all longitudinal participants were stable in their performance. These findings are also consistent with broader research on typical aging, which shows that declines in working and short-term memory precede changes in long-term memory (Folstein et al., 1975). Larger longitudinal studies which include older adults followed for longer durations are needed to validate and further characterize these aging trajectories.
We compared our preliminary longitudinal findings to exploratory cross-sectional age relationships across a broad young to older adult range to determine consistency of results. For short-term verbal memory, cross-sectional findings suggest a relative “protection” of short-term verbal memory in ASD vs. NT. This is synergistic with Lever and Geurts (2016a) where there was a flatter age relationship suggesting less decline in short-term visual memory in adults with ASD vs. NT (although they did not find this effect with short-term verbal memory). These and related cross-sectional findings have been interpreted as suggesting that ASD may confer protection against age-related short-term memory decline. However, our two-to-three-year longitudinal follow-up did not corroborate “protection” from decline, but rather suggests slightly higher rates of clinically meaningful decline in short-term verbal memory compared to NT adults. In the cross-sectional analysis of our broad age range, autistic adults also performed worse over all on long-term verbal memory, compared to NT, which is consistent with other cross-sectional work in ASD (Jones et al., 2011; Powell et al., 2017; Tyson et al., 2014).
Comparing cross-sectional and longitudinal findings
Inconsistencies between cross-sectional and longitudinal cognitive aging findings might be explained by cohort effects or different sample/age-range. For verbal short-term memory, older adults with ASD are presenting with comparable capabilities relative to their young adult counterparts in cross-sectional results, but older NT adults are presenting with worse verbal short-term memory capabilities relative to their young adult counterparts. However, when middle-age autistic adults are tracked over time and compared to a matched NT group, there is a slightly higher rate of clinically meaning decline in short-term memory in ASD. Taken together, these findings shed light on how cohort effects may be influencing cross-sectional age findings in ASD or differences between sample ages and duration to follow up. For cohort effects, there are a multitude of factors that may have differently influenced adults with ASD who were born decades apart and a bias in the characteristics of those who are able to volunteer for research depending on age. ASD research is especially prone to cohort effects because the disorder was only added to the Diagnostic and Statistical Manual of Mental Disorders in the latter half of the 20th century (American Psychiatric Association, 1968). As a result, there are clear differences in age of diagnosis (Tables 1–2), treatment history, family support, etc. between younger and older adults that confound aging inferences in cross-sectional designs (Keyes et al., 2012; Mazurek et al., 2014). There are also differences between our cross-sectional and longitudinal sample age ranges and duration to follow up that could explain inconsistencies. Longitudinal designs are the gold standard for aging research by controlling for cohort and other between-participant factors that could be mistaken for or obfuscate aging effects. However, they are resource and time intensive which makes it difficult to achieve longitudinal durations of follow up that are comparable to cross-sectional sample age ranges. More longitudinal cognitive aging research in ASD over longer durations of follow up is warranted.
Limitations
This study makes important advances in understanding brain and cognitive aging in ASD by presenting novel longitudinal findings in middle-age to older adults. Nevertheless, there are several limitations to address. First, the longitudinal sample size is small and includes mostly middle-aged adults with few older adults. These preliminary findings should be interpreted with caution. However, it should be noted that our analyses of brain aging separated cross-sectional and longitudinal effects of age in a single model, and this “accelerated aging” effect was significant for within-subject age (e.g., change over time) only. The cross-sectional age by group interactions for memory were exploratory as prior findings from Lever and Geurts (2016) would suggest they were under-powered. Nevertheless, we found a similar age by diagnosis pattern that Lever and Geurts (2016) found with short-term visual memory. Another limitation is that the present longitudinal cohort does not contain enough women with ASD to explore sex differences in cognitive and brain aging trajectories. As we follow more middle-age and older autistic women, and their NT matches, we aim to determine the role of sex and hormone exposures/transitions in cognitive and brain aging in ASD. Longer longitudinal follow up will also be necessary to develop mixed models that can provide inference about timeline of changes, which cannot be done with only two timepoints. We were also underpowered to determine the relationship between cognitive and brain changes, so future research is warranted to determine brain mechanisms and biomarkers of cognitive decline in ASD. Lastly, our cohort is restricted to cognitively-able adults. Future research is warranted to determine cognitive and brain aging trajectories in adults with ASD and comorbid intellectual disability.
Conclusions
We present the first longitudinal brain and cognitive aging findings in middle-age adults with ASD, compared to a matched NT cohort. Cross-sectionally we do not find accelerated age-related effects, but longitudinal data does hint at accelerated decline. Our findings, while preliminary, suggest accelerated decline in average hippocampal volume in ASD as well as slightly higher rates of clinically-meaningful decline in verbal short-term memory, measured over a two-to-three year follow-up window. Furthermore, reduced average fornix FA and long-term memory performance in ASD were found across the broad cross-sectional age range. These findings are particularly timely given recent evidence suggesting higher rates of cognitive disorders (Hand et al. 2020) and neurodegenerative conditions, including early-onset dementia in ASD (Vivanti et al., 2021; Starkstein et al., 2015). However, further characterization of trajectories over a longer follow-up window in a larger sample is warranted. Further, our longitudinal verbal memory findings contradicted our cross-sectional results, such that longitudinal findings suggest poorer aging outcomes in some with ASD and cross-sectional findings suggest relative “preservation” of cognitive performance with age in ASD. The broader cross-sectional literature, similarly, contains various studies reporting equivalent or protective aging trajectories in ASD (Tse et al., 2021). These contradictions highlight the importance of further longitudinal research on cognitive and brain aging in ASD and the importance of characterizing how cohort effects influence cross-sectional research in ASD.
Supplementary Material
Acknowledgements
We are grateful to our participants who made this study possible, Drs. Leslie Baxter and Christopher Smith for their contributions establishing our aging with ASD study, and Sharmeen Maze for her dedication to pristine MRI data collection.
Funding
This work was supported by the National Institute of Mental Health [K01MH116098; F31MH122107]; Department of Defense [AR140105]; and Arizona Biomedical Research Commission [ADHS16-162413]. The formal analysis and manuscript preparation was supported by the National Center for Complementary and Integrative Health [F31AT010976]. The funding sources were not involved in the research or preparation of this article.
Grant sponsor:
National Institute of Mental Health, Grant numbers K01MH116098; F31MH122107
Grant sponsor:
Department of Defense, Grant number AR140105.
Grant sponsor:
Arizona Biomedical Research Collaborative, Grant number ADHS16-162413.
Grant sponsor:
National Center for Complementary and Integrative Health, Grant number F31AT010976.
Footnotes
Competing interests
The authors report no competing interests.
Ethics approval
These studies were approved by Arizona State University’s Institutional Review Board.
Consent to participate
All participants provided written consent approved by the Institutional Review Board and in accordance with the Declaration of Helsinki.
Consent for publication
All authors have read and approved the submission.
References
- Barnea-Goraly N, Frazier TW, Piacenza L, Minshew NJ, Keshavan MS, Reiss AL, & Hardan AY (2014). A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 48, 124–128. 10.1016/j.pnpbp.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Nanetti L, Van Der Gaag C, Ketelaars C, Minderaa R, & Keysers C (2011). Age-related increase in inferior frontal gyrus activity and social functioning in autism spectrum disorder. Biological Psychiatry, 69(9), 832–838. 10.1016/j.biopsych.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Bathelt J, Koolschijn PC, & Geurts HM (2020). Age-variant and age-invariant features of functional brain organization in middle- aged and older autistic adults. 1–14. [DOI] [PMC free article] [PubMed]
- Baxter LC, Nespodzany A, Walsh MJM, Wood E, Smith CJ, & Braden BB (2019). The influence of age and ASD on verbal fluency networks. Research in Autism Spectrum Disorders, 63(March), 52–62. 10.1016/j.rasd.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Dassel KB, Bimonte-Nelson HA, O’Rourke HP, Connor DJ, Moorhous S, … Baxter LC (2017). Sex and post-menopause hormone therapy effects on hippocampal volume and verbal memory. Aging, Neuropsychology, and Cognition, 24(3), 227–246. 10.1080/13825585.2016.1182962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Pagni BA, Monahan L, Walsh MJM, Dixon MV, Delaney S, … Ware JE (2021). Quality of life in adults with autism spectrum disorder: influence of age, sex, and a controlled, randomized mindfulness-based stress reduction pilot intervention. Quality of Life Research, (0123456789). 10.1007/s11136-021-03013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Pipe TB, Smith R, Glaspy TK, Deatherage BR, & Baxter LC (2016). Brain and behavior changes associated with an abbreviated 4-week mindfulness-based stress reduction course in back pain patients. Brain and Behavior, 6(3), 1–13. 10.1002/brb3.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, & Riecken C (2019). Thinning faster? Age-related cortical thickness differences in adults with autism spectrum disorder. Research in Autism Spectrum Disorders, 64(February), 31–38. 10.1016/j.rasd.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Smith CJ, Thompson A, Glaspy TK, Wood E, Vatsa D, … Baxter LC (2017). Executive function and functional and structural brain differences in middle-age adults with autism spectrum disorder. Autism Research, 10(12), 1945–1959. 10.1002/aur.1842 [DOI] [PubMed] [Google Scholar]
- Braden BB, Smith CJ, Thompson A, Glaspy TK, Wood E, Vatsa D, … Baxter LC (2017). Executive Function and Functional and Structural Brain Differences in Middle-Age Adults With Autism Spectrum Disorder. 1–15. 10.1002/aur.1842 [DOI] [PubMed] [Google Scholar]
- Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, … Zimmerman ME (2012). Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiology of Aging, 33(8), 1699–1715. 10.1016/j.neurobiolaging.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, & Park DC (2009). Cognitive Neuroscience of Aging: Linking cognitive and cerebral aging. In Cognitive Neuroscience of Aging: Linking cognitive and cerebral aging. 10.1093/acprof:oso/9780195156744.001.0001 [DOI] [Google Scholar]
- Cahn AJ, Little G, Beaulieu C, & Tétreault P (2021). Diffusion properties of the fornix assessed by deterministic tractography shows age, sex, volume, cognitive, hemispheric, and twin relationships in young adults from the Human Connectome Project. Brain Structure and Function, 226(2), 381–395. 10.1007/s00429-020-02181-9 [DOI] [PubMed] [Google Scholar]
- Constantino JN (2012). Social responsiveness scale (2nd editio). Los Angeles: Western Psychological Services. [Google Scholar]
- Davda N, & Corkill R (2020). Biomarkers in the diagnosis and prognosis of Alzheimer’s disease. Journal of Neurology, 267(8), 2475–2477. 10.1007/s00415-020-10037-9 [DOI] [PubMed] [Google Scholar]
- Duff K (2012). Current topics in science and practice evidence-based indicators of neuropsychological change in the individual patient: Relevant concepts and methods. Archives of Clinical Neuropsychology, 27(3), 248–261. 10.1093/arclin/acr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley SF, Tansey KE, Caseras X, Lancaster T, Bracht T, Parker G, … Linden DEJ (2017). Multimodal Brain Imaging Reveals Structural Differences in Alzheimer’s Disease Polygenic Risk Carriers: A Study in Healthy Young Adults. Biological Psychiatry, 81(2), 154–161. 10.1016/j.biopsych.2016.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein Marshal F., Folstein Susan E., McHugh PR (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 2, 189–198. 10.3744/snak.2003.40.2.021 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gale SD, Burr RB, Bigler ED, & Blatter D (1993). Fornix degeneration and memory in traumatic brain injury. Brain Research Bulletin, 32(4), 345–349. 10.1016/0361-9230(93)90198-K [DOI] [PubMed] [Google Scholar]
- Geurts HM, & Vissers ME (2012). Elderly with autism: Executive functions and memory. Journal of Autism and Developmental Disorders, 42(5), 665–675. 10.1007/s10803-011-1291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, & Gordon E (2007). Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. American Journal of Neuroradiology, 28(2), 226–235. [PMC free article] [PubMed] [Google Scholar]
- Guillaume B, Hua X, Thompson PM, Waldorp L, & Nichols TE (2014). Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. NeuroImage, 94, 287–302. 10.1016/j.neuroimage.2014.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand BN, Angell AM, Harris L, & Carpenter LA (2020). Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults. Autism, 24(3), 755–764. 10.1177/1362361319890793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull L, Mandy W, & Petrides KV (2017). Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism, 21(6), 706–727. 10.1177/1362361316669087 [DOI] [PubMed] [Google Scholar]
- Jones CRG, Happé F, Pickles A, Marsden AJS, Tregay J, Baird G, … Charman T (2011). “Everyday memory” impairments in autism spectrum disorders. Journal of Autism and Developmental Disorders, 41(4), 455–464. 10.1007/s10803-010-1067-y [DOI] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). KBIT-2: Kaufman brief intelligence test (2nd ed.). Upper Saddle River, NJ: Pearson Education, Inc. [Google Scholar]
- Keyes KM, Susser E, Cheslack-postava K, Fountain C, Liu K, & Bearman PS (2012). Cohort effects explain the increase in autism diagnosis among children born from 1992 to 2003 in california. International Journal of Epidemiology, 41(2), 495–503. 10.1093/ije/dyr193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Pauley G, Richards T, Neuhaus E, Martin N, Corrigan NM, … Dager SR (2012). Age-related abnormalities in white matter microstructure in autism spectrum disorders. Brain Research, 1479, 1–16. 10.1016/j.brainres.2012.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodiweera C, Alexander AL, Harezlak J, McAllister TW, & Wu YC (2016). Age effects and sex differences in human brain white matter of young to middle-aged adults: A DTI, NODDI, and q-space study. NeuroImage, 128, 180–192. 10.1016/j.neuroimage.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, Caan MWA, Teeuw J, Olabarriaga SD, & Geurts HM (2017). Age-related differences in autism: The case of white matter microstructure. Human Brain Mapping, 38(1), 82–96. 10.1002/hbm.23345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, & Geurts HM (2016). Gray Matter Characteristics in Mid and Old Aged Adults with ASD. Journal of Autism and Developmental Disorders, 46(8), 2666–2678. 10.1007/s10803-016-2810-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, … Lainhart JE (2007). Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters, 424(2), 127–132. 10.1016/j.neulet.2007.07.042 [DOI] [PubMed] [Google Scholar]
- Lemay S, Bédard MA, Rouleau I, & Tremblay PLG (2004). Practice effect and test-retest reliability of attentional and executive tests in middle-aged to elderly subjects. Clinical Neuropsychologist, 18(2), 284–302. 10.1080/13854040490501718 [DOI] [PubMed] [Google Scholar]
- Lever AG, & Geurts HM (2016a). Age-related differences in cognition across the adult lifespan in autism spectrum disorder. Autism Research, 9(6), 666–676. 10.1002/aur.1545 [DOI] [PubMed] [Google Scholar]
- Lever AG, & Geurts HM (2016b). Psychiatric Co-occurring Symptoms and Disorders in Young, Middle-Aged, and Older Adults with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 46(6). 10.1007/s10803-016-2722-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule, (ADOS-2) Modules 1–4. In Los Angeles, California: [Google Scholar]
- Lord Catherine, Risi S, Lambrect L, Cook Edwin H,J, Leventhal BL, DiLavore PC, … Rutter M (2000). The Autism Diagnostic Observation Schedule–Generic. Journal of Autism and Developmental Disorders, 30(3), 205–223. 10.1023/A:1005592401947 [DOI] [PubMed] [Google Scholar]
- Markowska AL, Olton DS, Murray EA, G. DA (1989). A comparative analysis of the role of fornix and cingulate cortex in memory: rats. Exp Brain Res, (74), 187–201. 10.1007/BF00248292 [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Handen BL, Wodka EL, Nowinski L, Butter E, & Engelhardt CR (2014). Age at first autism spectrum disorder diagnosis: The role of birth cohort, demographic factors, and clinical features. Journal of Developmental and Behavioral Pediatrics, 35(9), 561–569. 10.1097/DBP.0000000000000097 [DOI] [PubMed] [Google Scholar]
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, … Lyketsos CG (2012). Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimer’s and Dementia, 8(2), 105–113. 10.1016/j.jalz.2011.05.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus AJM, & Kalbfleisch JD (1998). Between- and Within-Cluster Covariate Effects in the Analysis of Clustered Data Published by : International Biometric Society REFERENCES Linked references are available on JSTOR for this article : You may need to log in to JSTOR to access the linked refe. 54(2), 638–645. [PubMed] [Google Scholar]
- Özarslan E, Vemuri BC, & Mareci TH (2005). Generalized scalar measures for diffusion MRI using trace, variance, and entropy. Magnetic Resonance in Medicine, 53(4), 866–876. 10.1002/mrm.20411 [DOI] [PubMed] [Google Scholar]
- Pevzner. (2017). 乳鼠心肌提取 HHS Public Access. Physiology & Behavior, 176(3), 139–148. 10.1097/WAD.0000000000000262.Relationships28363838 [DOI] [Google Scholar]
- Pierpaoli C, & Basser PJ (1996). Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine, 36(6), 893–906. 10.1002/mrm.1910360612 [DOI] [PubMed] [Google Scholar]
- Poustka L, Jennen-Steinmetz C, Henze R, & Stieltjes B (2011). Fronto-temporal disconnectivity and symptom severity in autism spectrum disorders. European Psychiatry, 26(S2), 1838–1838. 10.1016/S0924-9338(11)73542-8 [DOI] [PubMed] [Google Scholar]
- Powell PS, Klinger LG, & Klinger MR (2017). Patterns of Age-Related Cognitive Differences in Adults with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 47(10), 3204–3219. 10.1007/s10803-017-3238-6 [DOI] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, … Murphy DGM (2010). Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cerebral Cortex, 20(6), 1332–1340. 10.1093/cercor/bhp198 [DOI] [PubMed] [Google Scholar]
- Rooij V (2018). Enigma: Cortical and subcortical Brain Morphometry Differences Between Patients With Autism Spectrum Disorder. Physiology & Behavior, 176(1), 139–148. 10.1176/appi.ajp.2017.17010100.Cortical [DOI] [Google Scholar]
- Rudebeck SR, Scholz J, Millington R, Rohenkohl G, Johansen-Berg H, & Lee ACH (2009). Fornix microstructure correlates with recollection but not familiarity memory. Journal of Neuroscience, 29(47), 14987–14992. 10.1523/JNEUROSCI.4707-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M (1996). Rey auditory verbal learning test: A handbook.
- Simonoff E, Kent R, Stringer D, Lord C, Briskman J, Lukito S, … Baird G (2020). Trajectories in Symptoms of Autism and Cognitive Ability in Autism From Childhood to Adult Life: Findings From a Longitudinal Epidemiological Cohort. Journal of the American Academy of Child and Adolescent Psychiatry, 59(12), 1342–1352. 10.1016/j.jaac.2019.11.020 [DOI] [PubMed] [Google Scholar]
- Singh P, Govil D, Kumar V, & Kumar J (2017). Cognitive Impairment and Quality of Life among Elderly in India. Applied Research in Quality of Life, 12(4), 963–979. 10.1007/s11482-016-9499-y [DOI] [Google Scholar]
- Starkstein S, Gellar S, Parlier M, Payne L, & Piven J (2015). High rates of parkinsonism in adults with autism. Journal of Neurodevelopmental Disorders, 1–11. 10.1186/s11689-015-9125-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, & Rodriguez AJ (1989). The role of the fornix/fimbria and some related subcortical structures in place learning and memory. Behavioural Brain Research, 32(3), 265–277. 10.1016/S0166-4328(89)80059-2 [DOI] [PubMed] [Google Scholar]
- Treves A, & Rolls ET (1994). Computational analysis of the role of the hippocampus in memory. Hippocampus, Vol. 4, pp. 374–391. 10.1002/hipo.450040319 [DOI] [PubMed] [Google Scholar]
- Tse VWS; Lei J; Crabtree J; Mandy W; Stott J (2021). Characteristics of Older Autistic Adults: a Systematic Review of Literature. Journal of Autism and Developmental Disorder. Retrieved from 10.1007/s40489-021-00238-x [DOI] [Google Scholar]
- Tyson K, Kelley E, Fein D, Orinstein A, Troyb E, Barton M, … Rosenthal M (2014). Language and verbal memory in individuals with a history of autism spectrum disorders who have achieved optimal outcomes. Journal of Autism and Developmental Disorders, 44(3), 648–663. 10.1007/s10803-013-1921-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti G, Shea LL, Tao S, Lyall K, & Robins DL (2021). The prevalence and incidence of early-onset dementia among adults with autism spectrum disorder. (April), 1–11. 10.1002/aur.2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D, Aubin J Saint Altman, K., & Gallant G (2021). Sex Differences in Verbal Working Memory : A Systematic Review and. 147(4), 352–398. [DOI] [PubMed] [Google Scholar]
- Walsh MJM, Baxter LC, Smith CJ, & Braden BB (2019). Age group differences in executive network functional connectivity and relationships with social behavior in men with autism spectrum disorder. Research in Autism Spectrum Disorders, 63(December 2017), 63–77. 10.1016/j.rasd.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJM, Wallace GL, Gallegos SM, & Braden BB (2021). Brain-based sex differences in autism spectrum disorder across the lifespan: A systematic review of structural MRI, fMRI, and DTI findings. NeuroImage: Clinical, 31, 102719. 10.1016/j.nicl.2021.102719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Peyre H, Toro R, Beggiato A, & Ramus F (2020). Adjusting for allometric scaling in ABIDE I challenges subcortical volume differences in autism spectrum disorder. Human Brain Mapping, 41(16), 4610–4629. 10.1002/hbm.25145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qiu C, Lindberg O, Bronge L, Aspelin P, Bckman L, … Wahlund LO (2010). Acceleration of hippocampal atrophy in a non-demented elderly population: The SNAC-K study. International Psychogeriatrics, 22(1), 14–25. 10.1017/S1041610209991396 [DOI] [PubMed] [Google Scholar]
- Zheng F, Cui D, Zhang L, Zhang S, Zhao Y, Liu X, … Qiu J (2018). The volume of hippocampal subfields in relation to decline of memory recall across the adult lifespan. Frontiers in Aging Neuroscience, 10(OCT), 1–10. 10.3389/fnagi.2018.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.


