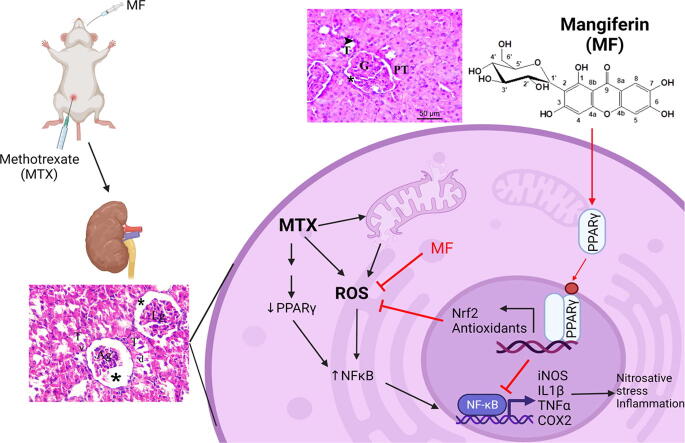
Graphical abstract
Keywords: Mangiferin, Methotrexate, Nephrotoxicity, Peroxisome proliferator-activated receptor-γ (PPAR-γ), Oxidative stress
Abbreviations: ARE, antioxidant responsive element; BADGE, Bisphenol A diglycidyl ether; BUN, Blood urea nitrogen; COX2, cyclooxygenase 2; GSH, glutathione; GST, glutathione-S-transferase; Hmox1, hemoxygenase-1; IkBα, inhibitor of NFκB, alpha; IL-1ß, interleukin-1ß; iNOS, inducible nitric oxide synthase; KEAP1, Kelch-like-ECH-associated protein 1; MDA, malondialdehyde; MF, Mangiferin; MTX, methotrexate; NAG, N-acetyl-ß-D glucosaminidase; NFκB, nuclear factor-κB; NO, nitric oxide; Nrf2, nuclear factor-erythroid factor 2-related factor 2; PPARγ, Peroxisome proliferator-activated receptor-ү; ROS, reactive oxygen species
Abstract
Methotrexate (MTX) is an immunosuppressant used for the treatment of cancer and autoimmune diseases. MTX has a major adverse effect, acute kidney injury, which limits its use. Mangiferin (MF) is a natural bioactive xanthonoid used as a traditional herbal supplement to boost the immune system due to its potent anti-inflammatory and antioxidant activity. The present study evaluates the protective effect of MF against MTX-induced kidney damage. Male Wistar rats received MTX to induce nephrotoxicity or were pretreated with MF for 10 constitutive days before MTX administration. MF dose-dependently improved renal functions of MTX-treated rats and this activity was correlated with increased renal expression of PPARγ, a well-known transcriptional regulator of the immune response. Pretreating rats with PPARγ inhibitor, BADGE, reduced the reno-protective activity of MF. Furthermore, MF treatment significantly reduced MTX-induced upregulation of the pro-inflammatory (NFκB, interleukin-1ß, TNF-α, and COX-2), oxidative stress (Nrf-2, hemoxygenase-1, glutathione, and malondialdehyde), and nitrosative stress (nitric oxide and iNOS) markers in the kidney. Importantly, BADGE treatment significantly reduced the anti-inflammatory and antioxidant activity of MF. Therefore, our data suggest that the reno-protective effect of MF against MTX-induced nephrotoxicity is due to inhibition of inflammation and oxidative stress in a PPAR-γ-dependent manner.
1. Introduction
Mangiferin (MF) is a xanthone derivative found in the leaves of Mangifera indica Linn (Mango) and is traditionally used as an anti-inflammatory and antioxidant to protect against lifestyle and age-related diseases including diabetes, hyperlipidemia, cancer, cardiomyopathy, and nephropathy (Garrido et al., 2004, Khurana et al., 2016, Imran et al., 2017, Ding et al., 2018, Wang et al., 2018). The anti-inflammatory activity of MF is suggested to be mediated by; but not limited to, the downregulation of pro-inflammatory cytokines (IL-1β, TNF-α), suppression of cyclo-oxygenase-2 (COX2) and prostaglandins, along with inactivation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) activity (Imran et al., 2017). As a phenolic compound, MF has a potent antioxidant activity mediated by its iron chelating properties and free radical scavenging activities (Andreu et al., 2005). MF also increases the expression and activation of nuclear factor-erythroid factor 2-related factor 2 (Nrf2) and peroxisome proliferator-activated receptor ү (PPARү), two key players in the antioxidant defense system (Zhang et al., 2015, Gold-Smith et al., 2016).
Nuclear factor-erythroid factor 2-related factor 2 (Nrf2) is a basic leucine-zipper transcription factor which binds and activates the antioxidant responsive element (ARE) in the promotor regions of several cytoprotective genes. Nrf2 activation increases the expression of proteins regulating NADPH regeneration, reactive oxygen species (ROS) detoxification, thioredoxin-based antioxidant system, heme metabolism, and glutathione production (Tonelli et al., 2018). The expression and the activity of Nrf2, which is encoded by the gene nuclear factor erythroid 2 like 2 (NFE2L2), are highly regulated at the transcriptional and post-translational levels to control the cell response to different stressors (He et al., 2020). The promoter region of NFE2L2 gene contains binding sites for different transcription factors such as Nrf2 (positive feedback), aryl hydrocarbon receptor (AHR), and NFκB. Under normal condition, cytoplasmic Nrf2 protein remains inactive by binding to Kelch-like-ECH-associated protein 1 (KEAP1). Under stress condition, Nrf2 dissociates, and the active form translocates to the nucleus to bind and activate antioxidant gene expression (Tonelli et al., 2018).
Peroxisome proliferator-activated receptor-ү is a ligand activated transcription factor which regulates the expression of various genes involved in lipid metabolism, inflammatory response, and oxidative stress (Tyagi et al., 2011). Up on activation with endogenous or exogenous ligands, PPARү heterodimerizes with retinoid X receptors (RXR) transcription factor then binds and activates PPAR response element upstream the target genes. PPARү target genes include Pparg (positive feedback), antioxidant genes such as hemeoxygenase 1 (Hmox1) (Elshazly and Soliman 2019), and inhibitor of NF-kB, alpha (IkBα) (Scirpo et al., 2015), a negative regulator of NFκBactivity. Previous studies suggested a connection between PPARү and Nrf2 pathways against oxidative stress (Polvani et al., 2012, Lee, 2017). PPARү agonists increased the expression of Nrf2 and Nrf2-regulated antioxidant genes such as glutathione-S-transferase (GST), suggesting that at least part of antioxidant activity of PPARү is mediated by Nrf2 (Park et al., 2004).
Methotrexate (MTX) is an antifolate immunosuppressant used for the treatment of cancer and autoimmune diseases (Widemann et al., 2004). MTX is highly cytotoxic and can cause damage to multiple organs due to the inhibition of purine metabolism (Tabassum et al., 2010). Acute kidney injury is the major adverse effect of MTX which limits its use. The mechanism of MTX-induced nephrotoxicity is not fully understood; however, it was suggested to be mediated by MTX crystallization, constriction of the afferent capillary, induction of inflammation, damage of mitochondria, and reduction of the antioxidant capacity in the kidney (Asci et al., 2017, Heidari et al., 2018, Kitamura et al., 2018). Prophylactic treatment is the most acceptable strategy to prevent MTX-induced kidney damage (Kitamura et al., 2018). Several animal studies have shown that natural products with antioxidant and anti-inflammatory properties may protect against MTX-induced kidney damage without affecting the therapeutic activity (Morsy et al., 2013, Yuksel et al., 2017, Younis et al., 2021). Based on its potent antioxidant and anti-inflammatory activities, we sought to determine if MF protects against MTX-induced nephrotoxicity and gain further insights into the underlying mechanism mediating this effect.
2. Materials and methods
2.1. Animals
Adult male albino Wistar rats weighing 180–250 g were used in the current study. Rats were obtained from the faculty of veterinary medicine, Zagazig University, Zagazig, Egypt. Experiments were performed on the rats after one week of acclimatization period. Rats were housed three per cage, food and water were available ad libitum, temperature was maintained at 23 ± 2 °C, and light/dark cycle was kept at 12/12 h.
2.2. Ethical considerations
Animal handling procedures and experimentation were approved by the Ethical Committee for Animal Handling at the Faculty of Pharmacy, Zagazig University, Zagazig, Egypt following the guidelines of the National Institutes of Health Guide for care and use of laboratory animals.
2.3. Chemicals
2.3.1. Drugs
Methotrexate (MTX) was obtained from Baxter Company (Cairo, Egypt). Bisphenol A diglycidyl ether (BADGE), Mangiferin (MF), and Kolliphor ® EL were purchased from Milipore Sigma (St. Louis, USA).
2.4. Drug treatment and experimental design
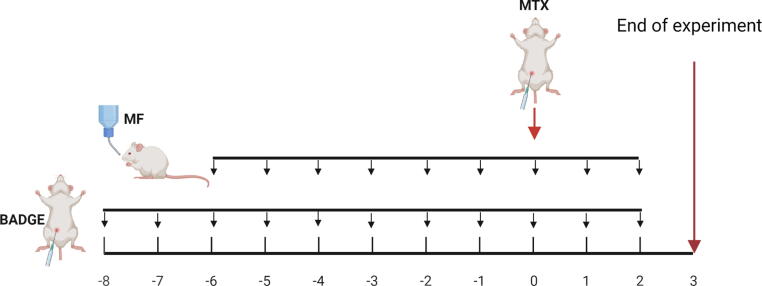
Male Wistar albino rats were randomly divided into six groups. Group1: Control group received the vehicle of the three drugs: MF (daily oral acacia gum solution (10% w/v)), BADGE [daily i.p. injection of Kolliphor ® EL (9%): ethanol (9%): saline (1:1:9)], and MTX (single intraperitoneal (i.p.) injection of saline). Group 2: MTX group was injected i.p. with a single dose of MTX (20 mg/kg) to induce acute renal damage, daily oral acacia gum solution (10% w/v), and daily i.p. injection of Kolliphor ® EL (9%): ethanol (9%): saline (1:1:9) (Abd El-Twab et al., 2016). Groups 3–5: Mangiferin (MF)-treated groups received daily oral doses of MF (10, 20, or 40 mg/kg) suspended in acacia gum solution (10% w/v) for 10 consecutive days starting at day 6 pre-MTX injection. Group 6: Bisphenol A diglycidyl ether (BADGE)-treated group received daily intraperitoneal doses of BADGE (15 mg/kg) dissolved in Kolliphor ® EL (9%): ethanol (9%): saline (1:1:9) (Soliman et al., 2019) for 12 consecutive days starting at day 8 pre-MTX injection and daily oral doses of MF (20 mg/kg) for 10 consecutive days starting at day 6 pre-MTX injection (Fig. 1).
Fig. 1.
Experimental timeline. Acute kidney damage was induced by an I.P. bolus injection of methotrexate (MTX) at day zero (three days before the end of experiment). Mangiferin (MF) was administered daily (P.O.) starting six days before MTX injection until the end of experiment. BADGE was administered daily (I.P.) starting two days before MF treatment until the end of experiment.
2.5. Sampling of blood and tissue
Three days post-MTX treatment, all animals were euthanized by isofluorane inhalation and blood samples were collected by cardiac puncture. Serum was separated by centrifugation of blood samples at 4000 rpm for 10 min and then stored at − 80 °C for biochemical analysis. The two kidneys of each rat were isolated, one kidney was flash frozen for preparation of renal homogenate and the other kidney was fixed in 10% formalin for the histopathological examinations.
2.6. Biochemical assays
2.6.1. Measurement of kidney function parameters
Blood urea nitrogen (BUN) and serum creatinine were measured in blood serum samples using colorimetric assay kits (Biodiagnostic, Giza, Egypt) following the manufacturer’s instructions.
2.6.2. Measurement of urinary excretion of NAG
Urine samples were collected and N-acetyl-ß-D glucosaminidase (NAG) activity was measured using NAG activity colorimetric assay kit (Diazyme Laboratory, San Diego, CA, USA) following the manufacturer’s protocol.
2.6.3. Measurement of renal malondialdehyde and total glutathione levels
Renal malondialdehyde (MDA) and glutathione (GSH) contents were measured in kidney homogenates using quantitative colorimetric assay kits obtained from Bio-diagnostic Co. (Egypt) following the manufacturer's instructions.
2.6.4. Measurement of TNF-α and IL-1β levels
The protein levels of TNF-α and IL-1β were measured in the kidney homogenate using Rat TNF- alpha ELISA kit (RayBiotech, Inc., Norcross, Georgia, USA) and IL-1β ELISA Kit (R&D System, Minneapolis, MN, USA), respectively, according to manufacturer's instructions.
2.6.5. Measurement of phospho- and total NFκB protein levels
The protein levels of phospho- (pNFκB) and total NFκB p65 (tNFκB) levels were measured in the kidney homogenate using Rat NFκB p65 ELISA kit (Cusabio Biotech Co., Ltd., Wuhan, China) and phospho-NFκB p65 ELISA kit (Cell Signaling Technology, Inc., Beverly, MA, USA), respectively, according to manufacturer's instructions.
2.6.6. Serum NO level
Nitric oxide levels were measured in the serum samples using colorimetric NO assay kit (Bio-diagnostic, Egypt) according to manufacturer protocol. This assay is an indirect method for determination of NO by measuring its stable decomposition products nitrite and nitrate using Griess reaction with a mixture of naphthyl ethylenediamine and sulfanilamide (Moshage et al., 1995, Bryan and Grisham, 2007).
2.6.7. Quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted from frozen kidney tissues using Trizol reagent (Invitrogen, Carlsbad, CA) and RNAeasy Mini Kit (Qiagen, USA). cDNA was synthesized using RevertAid Premium Reverse Transcriptase-Kit (Fermentas International Inc., Burlington, Canada) and amplified using Maxima SYBR Green qPCR Kit (Fermentas International Inc., Burlington, Canada), specific primers listed in Table 1, and ABI prism 7500 sequence detector system (Applied Biosystems, Foster City, CA). CT values were normalized to GAPDH control and the fold change was calculated using ΔΔCT method (Livak and Schmittgen 2001).
Table 1.
Primers used for qRT-PCR.
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| NFκB | 5-CTGGCAGCTCTTCTCAAAGC-3 | 5-CCAGGTCATAGAGAGGCTCAA-3′ |
| iNOS | 5′-CGGTTCACAGTCTTGGTGAAAG-3′ | 5′-CAGGTGTTCCCCAGGTAGGTAG-3′ |
| Hmox-1 | 5′-GAG CCA GCC TGA ACT AGC-3′ | 5′-GAT GTG CAC CTC CTT GGT-3′ |
| Nrf2 | 5′-GAGACGGCCATGACTGAT-3′ | 5′-GTGAGGGATCGATGAGTAA-3′ |
| PPARү | 5′-AGACCACTCCCACTCCTTTG-3′ | 5′-AGGTCATACTTGTAATCTGC-3′ |
| COX2 | 5-ACACTCTATCACTGGCATCC-3′ | 5-GAAGGGACACCCTTTCACAT-3′ |
| GAPDH | 5′-TGCTGGTGCTGAGTATGTCG3′ | 5′-TTGAGAGCAATGCCAGCC 3′ |
2.7. Histopathological examination for the kidney
Kidney samples fixed in 10% formalin were processed through the routine histological procedures being dehydrated by immersion in various concentrations of ethanol (70%, 90%, then 100%) 3 times for 30 min each at room temperature, cleared in xylene for 20 min at room temperature, then embedded in paraffin wax at 58 °C. Paraffinized tissues were then cut into 4 μm sections using a microtome (Leica RM 2155, England). Kidney sections were rehydrated gradually by immersion in xylene 2 times for 10 min each, then various ethanol concentrations (100%, 95%, 70%, then 50%) 5 min each. Slides were then rinsed in deionized water, washed with PBS, and stained with hematoxylin and eosin (H&E). Stained slides were blindly examined and scored by expert pathologists.
2.8. Statistical analysis
Data were analyzed by one-way analysis of variants (ANOVA) followed by Tukey’s multiple comparison test using GraphPad Prism 7 (La Jolla, CA, USA). All data are represented as mean ± standard error of the mean (SEM).
3. Results
3.1. Mangiferin improves kidney function of MTX treated rats in a dose-dependent manner
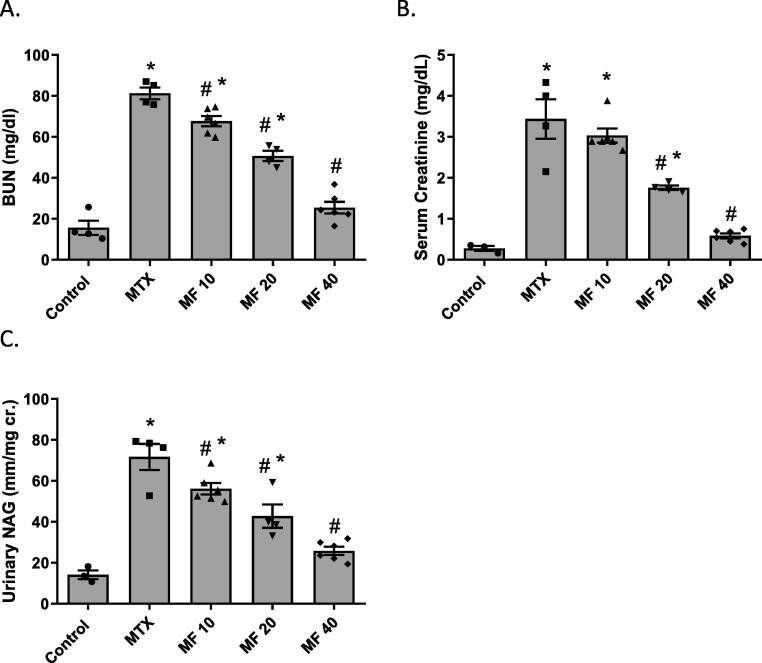
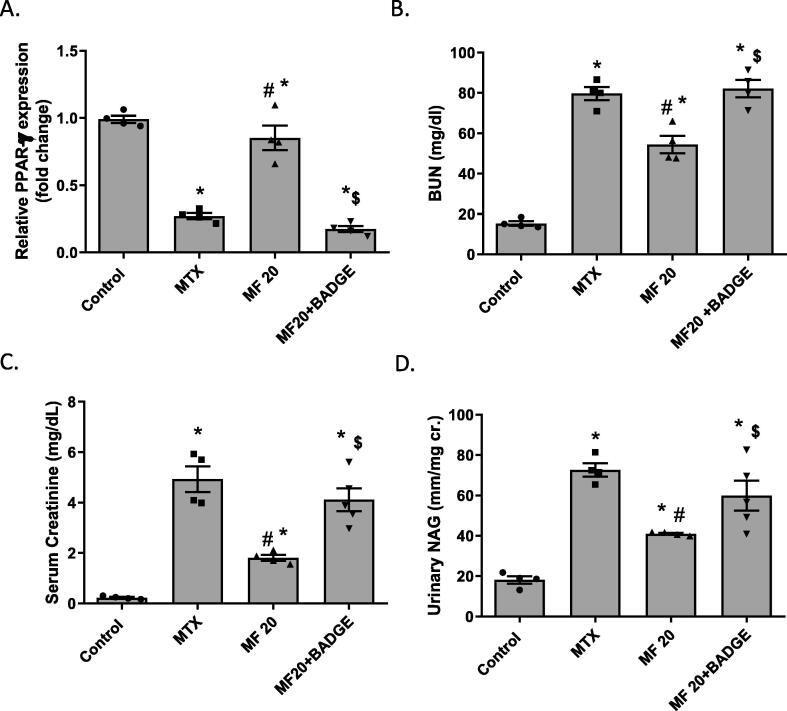
Serum creatinine, blood urea nitrogen (BUN), and urinary N-acetyl-beta-D-glucosaminidase (NAG) were measured to determine the effect of MF on kidney and renal tubular functions. MTX injection significantly increased serum creatinine, BUN, and urinary NAG levels (p < 0.05) when compared to control group; indicating MTX-induced renal damage. Treatment with different doses of MF (10, 20 and 40 mg/kg) improved kidney functions in a dose dependent manner. MF decreased BUN (Fig. 2A, p < 0.05), serum creatinine (Fig. 2B, p < 0.05), and urinary NAG (Fig. 2C, p < 0.05); when compared to the MTX group.
Fig. 2.
Mangiferin (MF) improves kidney function of methotrexate (MTX) treated rats in a dose-dependent manner. The effect of MF (10, 20, and 40 mg/kg) on blood urea nitrogen (BUN) (A), serum creatinine (B), and urinary N-acetyl-beta-D-glucosaminidase (NAG) (C) was determined 3 days post MTX injection. Data is represented as (Mean ± SEM), N ≥ 4, * p ≤ 0.05 compared to control group, #p ≤ 0.05 compared to MTX group.
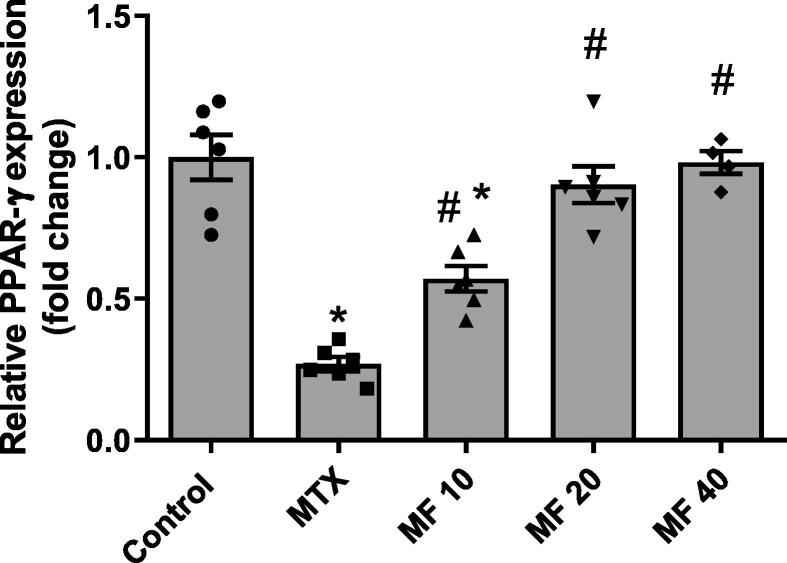
3.2. Mangiferin increases renal PPARϒ expression in MTX treated rats in a dose-dependent manner
PPARϒ is highly expressed in major renal cells and plays a fundamental important role in regulating the inflammatory response in the kidney (Ma et al., 2020). In the present study, MTX-induced nephrotoxicity was associated with a significant reduction in PPARϒ mRNA expression in the kidney. MF treatment showed an elevation in PPARϒ expression in a dose-dependent manner. A dose of 10 mg/kg MF significantly increased PPARϒ expression when compared to MTX group; however, 20 mg/kg and 40 mg/kg doses restored PPARϒ to the normal levels (Fig. 3). No significant difference in PPARϒ expression was observed between 20 and 40 mg/kg; therefore, 20 mg/kg was selected to determine the role of PPARϒ in the reno-protective effect of MF against MTX-induced nephrotoxicity.
Fig. 3.
Mangiferin increases renal PPARϒ expression in MTX treated rats in a dose-dependent manner. The effect of MF (10, 20, and 40 mg/kg) on PPARϒ expression was determined 3 days post MTX injection using qRT-PCR. Data is represented as (Mean ± SEM), N ≥ 4, * p ≤ 0.05 compared to control group, #p ≤ 0.05 compared to MTX group.
3.3. PPARϒ inhibitor reduces the reno-protective effect of mangiferin
To determine if the reno-protective effect of MF is mediated by PPARϒ, animals were pretreated with a selective PPARϒ antagonist, BADGE (15 mg/kg) (Soliman et al., 2019), before MF (20 mg/kg) treatment. BADGE significantly reduced MF-induced upregulation of PPARϒ expression (Fig. 4A) and diminished the effect of MF on renal functions. Fig. 4 showed that BUN (Fig. 4B), serum creatinine (Fig. 4C), and urinary NAG levels (Fig. 4D) were significantly higher in rats treated with both BADGE and MF when compared to MF group. The levels of BUN, serum creatinine, and urinary NAG in the BADGE/MF group were comparable to the untreated MTX group indicating that the reno-protective effect of MF is PPARϒ-dependent.
Fig. 4.
PPARϒ inhibitor (BADGE) reduces the reno-protective effect of mangiferin. Blood urea nitrogen (BUN) (A), serum creatinine (B), and urinary N-acetyl-beta-D-glucosaminidase (NAG) (C) were measured in rats treated with mangiferin (MF, 20 mg/kg) or co-treated with MF (20 mg/kg) and BADGE (15 mg/kg) at 3 days post MTX injection. Data is represented as (Mean ± SEM), N ≥ 4, * p ≤ 0.05 compared to control group, #p ≤ 0.05 compared to MTX group, $p ≤ 0.05 compared to MF group.
3.4. Mangiferin reduced MTX-induced renal inflammation
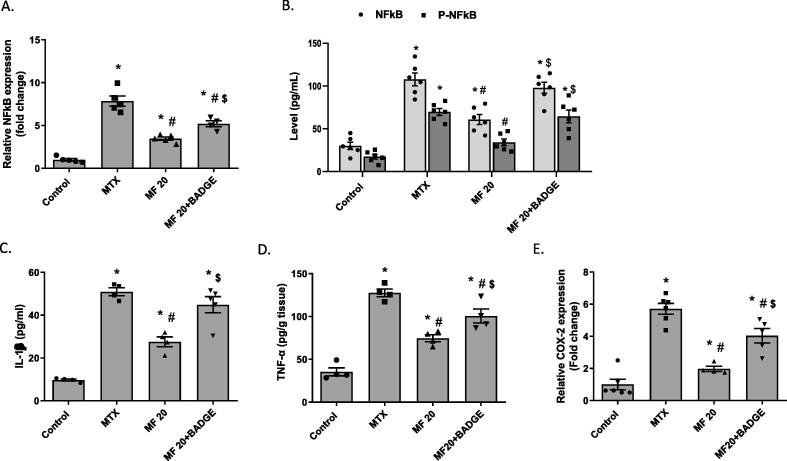
MTX-induced nephrotoxicity was associated with renal inflammation as indicated by increased expression of NFκB (at both mRNA and protein levels), IL-1β, TNF-α, and COX2 in the kidney when compared to control group (Fig. 5A-E). MF treatment (20 mg/kg) significantly reduced the expression of these pro-inflammatory markers when compared to the MTX group. Importantly, concurrent treatment with MF and BADGE significantly increased the expression of NFκB (phosphorylated, pNFκB, and total, tNFκB, forms), IL-1β, TNF-α, and COX2 when compared to MF-treated animals. The level of IL-1β, pNFκB, and tNFκB protein expression in the MF + BADGE group was comparable to the untreated MTX group (Fig. 5B); while the levels of TNF-α, and COX2 were significantly less than MTX group (Fig. 5A, 5C, and 5D, respectively). Our data suggest that the anti-inflammatory activity of MF was mediated, at least in part, by PPARϒ activation.
Fig. 5.
Mangiferin reduced MTX-induced renal inflammation. Inflammatory mediators were measured in the kidney of rats pretreated with MF or MF + BADGE then received MTX. (A) Relative mRNA expression of NFκB was measured by qRT-PCR. (B-D) Total (tNFκB), phosphor (pNFκB), IL-1β and TNF-α protein expression was measured using ELISA techniques, respectively. (E) Relative mRNA expression of NFκB was measured by qRT-PCR. Data is represented as (Mean ± SEM), N ≥ 4, * p ≤ 0.05 compared to control group, #p ≤ 0.05 compared to MTX group, $p ≤ 0.05 compared to MF group.
3.5. Mangiferin enhanced Nrf2 antioxidant defense in a PPARϒ-dependent manner
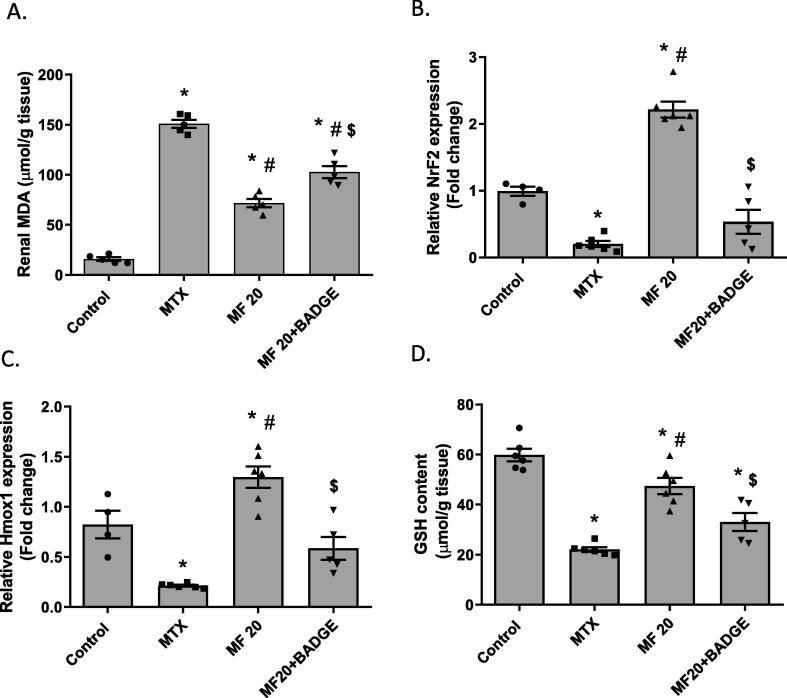
MTX treatment reduced Nrf2 antioxidant response in the kidney as indicated by increased MDA content (a marker of lipid peroxidation), downregulated Nrf2 and Hmox1 expression, and reduced glutathione content (Fig. 6). The MF-treated group showed significant reduction in MDA content and upregulation of both Nrf2 and Nrf2 downstream targets (Hmox1 and GSH) (Fig. 6A-D). Concurrent treatment with MF and BADGE significantly diminished the effect MF on Nrf2 and Hmox1 expression as well as GSH content indicating that the antioxidant activity of MF is PPARϒ-dependent.
Fig. 6.
Mangiferin enhanced Nrf2 antioxidant defense in a PPARϒ-dependent manner. Malondialdehyde (MDA) level (A), relative Nrf2 mRNA expression (B), relative Hmox1 mRNA expression (C), and total glutathione (GSH) content (D) were measured in the kidney of rats pretreated with MF or MF + BADGE then received MTX. Data is represented as (Mean ± SEM), N ≥ 4, * p ≤ 0.05 compared to control group, #p ≤ 0.05 compared to MTX group, $p ≤ 0.05 compared to MF group.
3.6. Mangiferin reduced MTX-induced nitrosative stress in a PPARϒ-dependent manner
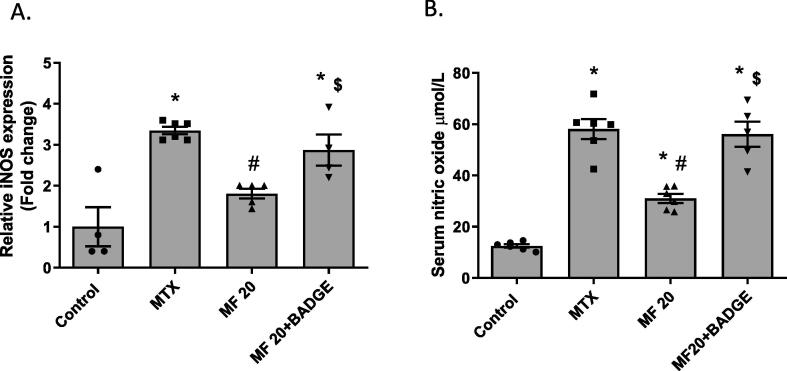
MTX induced nitrosative stress in the kidney as indicated by increased NO production and iNOS expression. The MTX-treated group showed a significant elevation in the levels NO and iNOS mRNA when compared to the control group (Fig. 7A-B). The MF-treated group showed a significant reduction in these nitrosative stress markers when compared to the MTX group. Concurrent treatment with MF and BADGE significantly increased the levels of NO and iNOS (Fig. 7A-B) when compared to the MF group. NO and iNOS expression in the MF + BADGE group was comparable to the untreated MTX group suggesting that the activity of MF against nitrosative stress was PPARϒ-dependent.
Fig. 7.
Mangiferin reduced MTX-induced nitrosative stress in a PPARϒ-dependent manner. Relative iNOS mRNA expression was measured in the kidney (A) and nitric oxide level was measured in the serum (B) of rats pretreated with MF or MF + BADGE then received MTX. Data is represented as (Mean ± SEM), N ≥ 4, * p ≤ 0.05 compared to control group, #p ≤ 0.05 compared to MTX group, $p ≤ 0.05 compared to MF group.
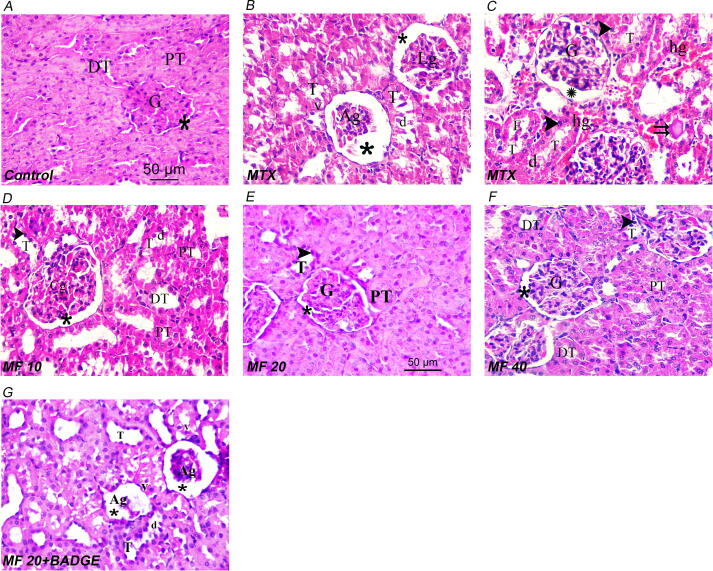
3.7. Histopathological findings
Examination of MTX group renal sections showed lobulated and atrophic glomeruli with desquamated epithelium for the disorganized renal tubules (Fig. 8B, C), compared to the control group with normal glomerular and tubular structure (Fig. 8A). MF treatment at different doses (10, 20, and 40 mg/kg) improved the histopathological alterations elicited by MTX (Fig. 8D-8F). The ameliorative effect of MF (20 mg/kg) on renal pathology was abrogated in animals co-treated with BADGE (Fig. 8G and Table 2.).
Fig. 8.
Representative image of rat kidney in control group (A), MTX-treated group (B and C), MF (10 mg/kg) + MTX group (D), MF (20 mg/kg) + MTX group (E), MF (40 mg/kg) + MTX group (F), and MF (20 mg/kg) + BADGE (15 mg/kg) + MTX group (G). G: normal glomeruli; *: Bowman’s space; PT: Proximal convoluted tubules; DT: distal convoluted tubules; Ag: atrophic glomeruli; Lg: lobulated glomeruli; T: Disorganized tubules; d: desquamated epithelium; v: vacuolated cytoplasm; arrowhead: tubules with dark stained nuclei; double arrows: homogenous acidophilic material; hg: extravasated haemorrhage. Scale bar is 50 μm (40X magnification).
Table 2.
Histopathological finding scoring for kidney in the different experimental groups.
| Histological changes | Control | MTX | MF 10 | MF 20 | MF 40 | MF 20 + BADGE |
|---|---|---|---|---|---|---|
| Damaged glomeruli | − | +++ | ++ | + | + | +++ |
| Luminal casts | − | ++ | − | − | − | ++ |
| Disorganized tubules | − | +++ | + | + | + | +++ |
| Extravasated haemorrhage | − | ++ | + | − | − | ++ |
Histopathological scores were designed as follows: (−, negative; +, weak; ++, moderate; +++, severe).
4. Discussion
Methotrexate (MTX) is one of the most common and effective immunosuppressant used for treatment of autoimmune diseases such as rheumatoid arthritis. MTX has multiple serious adverse effects including acute renal damage. The mechanism of MTX-induced nephrotoxicity involves the induction of oxidative stress and inflammation (Asci et al., 2017, Heidari et al., 2018, Kitamura et al., 2018). MF, a natural C-glucosyl xanthone derivative isolated from different parts of Mangifera indica, is a potent antioxidant and anti-inflammatory traditional medicine used to protect against age and lifestyle-related disorders including nephropathy (Imran et al., 2017, Wang et al., 2018). The present study demonstrated that MF protects against MTX-induced renal damage and this effect was associated with inhibition of oxidative stress and inflammation. Importantly, the use of BADGE, a well-known selective antagonist for the nuclear receptor PPAR-γ, diminished the antioxidant and anti-inflammatory activities of MF in MTX-treated rats. Therefore, our data reveals, for the first time, the reno-protective activity of MF against MTX-induced nephrotoxicity and this effect is PPAR-γ dependent.
Acute kidney injury is one of the major adverse effects of MTX limiting its use. Renal excretion is the primary route of MTX elimination; therefore, kidneys are vulnerable to the toxic effect of MTX and its metabolites. Previous studies have reported the precipitation of MTX and its metabolite, 7-hydroxy-MTX, in the renal tubules resulting in tubular necrosis, inflammation, and histological damage (Abdel-Raheem and Khedr, 2014, El-Sheikh et al., 2015, Erboga et al., 2015). In addition, MTX triggers a renal accumulation of reactive oxygen (ROS) and reactive nitrogen (RNS) species due to the disruption of antioxidant defense system (Morsy et al., 2013, Yuksel et al., 2017). In agreement with these findings, the present study demonstrates that a single dose of MTX induced acute kidney damage which is indicated by histological damage and impaired functions. MTX-mediated nephrotoxicity was accompanied with upregulation of the inflammatory mediators, IL-1β, TNF-α, and COX2, downregulation of the antioxidant defense mediators, Nrf2, Hmox1, and GSH, as well as augmentation of nitrosative stress markers, iNOS and NO. Notably, MTX reduced the expression of PPAR-γ, a key regulator of inflammatory and immune response, and this effect may correlate with the pathological outcomes (Mahmoud et al., 2019).
Previous studies have revealed the reno-protective effect of MF in medical conditions associated with increased risk of renal failure. In diabetic rat model, MF improved the renal structure and function as indicated by the inhibition of glomerular extracellular matrix expansion, amelioration of renal interstitial fibrosis, and reduction of BUN, serum creatinine, and urine protein (Li et al., 2010, Pal et al., 2014, Song et al., 2020). In hyperuricemic nephropathy rat model, MF reduced renal inflammation and apoptosis (Li et al., 2020). In sepsis mouse model, MF attenuated the acute kidney injury induced by cecal-ligation and puncture (He et al., 2014). Moreover, MF protects against drug-induced nephrotoxicity. Sadhukhan and colleagues reported that MF attenuated cisplatin induced nephrotoxicity both in-vivo and in-vitro (Sadhukhan et al., 2018). Consistently, the present study showed that MF ameliorated MTX-induced nephrotoxicity as indicated by the improvement of renal function and histology. This effect was associated with an upregulation of PPAR-γ, a master regulator of the cellular response to oxidative stress and inflammation (Kokeny et al., 2021).
Mangiferin is traditionally used to boost the immune system and reduce the oxidative stress. Previous studies have shown that the reno-protective effect of MF is related to its anti-inflammatory and antioxidant activity (Sadhukhan et al., 2018). In the current study we found that MF reduced the expression of inflammatory cytokines and oxidative stress markers in the kidney of MTX-treated animals. Importantly, this effect was reversed in animals co-treated with BADGE, a well-known selective PPAR-γ antagonist, indicating that the reno-protective effect of MF in MTX-induced kidney damage is PPAR-γ-dependent. Our findings are in line with previous findings showing that the anti-inflammatory effect of MF may be mediated by PPAR-γ. In human osteoarthritis chondrocytes, MF inhibits IL-1β-induced inflammatory response, and this response was abrogated in the presence of PPAR-γ inhibitor (Qu et al., 2017). In addition, molecular docking studies performed by Singh and colleagues suggest a direct interaction between MF and PPAR-γ receptor (Singh et al., 2018). Moreover, several studies have shown that MF modulates PPAR-γ downstream signaling specifically linked to NFκB activation (Mahmoud-Awny et al., 2015, Mei et al., 2021).
NFκB is a transcription regulator activated by ROS and several inflammatory cytokines to promote the pro-oxidant and pro-inflammatory gene expression (Liu et al., 2017). In resting conditions, inactive NFκB is located in the cytoplasm bound to the inhibitory molecule (IκB). Upon activation, IκB is phosphorylated by IκB kinase (IκK) releasing the active form of NFκB which then translocates to the nucleus and increases the expression of pro-oxidant genes such as iNOS and pro-inflammatory genes such as IL-1β, TNF-α, and COX2 (Liu et al., 2017). PPAR-γ is a fundamental repressor for NFκB activity (Ma et al., 2020). Activation of PPAR-γ inhibits the transcriptional activity of NFκB and increases the expression of inhibitor of NFκB, alpha (IkBα), a negative regulator of NFκB activity (Scirpo et al., 2015). Previous studies have shown that MF inhibits NFκB activity in various inflammatory conditions including kidney disorders (Mahmoud-Awny et al., 2015, Sadhukhan et al., 2018, Mei et al., 2021). Nonetheless, the present study reports for the first time that the MF effect on NFκB is mediated by the activation of PPAR-γ since the use of BADGE, PPAR-γ antagonist, reversed MF-induced downregulation of NFκB (both total and phosphorylated forms) and its targets, iNOS, IL-1β, TNF-α, and COX2. Therefore, the anti-inflammatory activity of MF in MTX-induced kidney damage is attributed, at least in part, to PPAR-γ/ NFκB signaling pathway.
PPARγ and Nrf2 are two fundamental synergistic players in establishing the intracellular antioxidative defense system against drug-induced oxidative damage (Lee 2017). Previous studies have suggested the positive feedback loop connecting PPARγ and Nrf2 signaling to reinforce the expression of one another and maintain their activity against oxidative stress. Huang et al (2010) and other researchers reported that PPARγ is a target gene for Nrf2 transcription activity since Nrf2 directly bind to ARE identified in the promoter region of the PPARγ gene (Huang et al., 2010, Lee, 2017). On the other hand, Park et al. reported that PPARγ activation induces the transcription of Nrf2-regulated antioxidant genes such as Hmox1 and GST (Park et al., 2004, Chorley et al., 2012). In the present study, MF reduced MTX-induced oxidative stress in kidney and this effect correlated with upregulation of Nrf2, Hmox1, and GSH. Interestingly, PPARγ antagonist abrogated the antioxidant activity MF and reduced the expression of these antioxidant mediators. Therefore, our data suggests that the antioxidant activity of MF in MTX-induced nephrotoxicity is mediated by PPARγ/Nrf2 signaling pathway.
5. Conclusion
Our study revealed the protective effect of MF against MTX-induced renal damage. This reno-protective effect correlates with the powerful anti-inflammatory and antioxidant activity of MF. Importantly, our results provide some evidence that MF activity is mediated by the activity of PPARγ/Nrf2 signaling. The limitation of our study was that we did not investigate how MF activates PPARγ. Therefore, future studies are still required to determine whether MF directly activates PPARγ by binding the active site or indirectly by increasing the expression of PPARγ endogenous ligands.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors would like to thank Dr. Amira Ibrahim (Pathology department, Faculty of Medicine, Zagazig University, Egypt) for performing the histological analysis.
Consent to Participate
We confirm that all authors have participated, reviewed, approved, and consented to the submission of this manuscript.
Consent to Publish
We confirm that all authors consented to publish this manuscript.
Authors contributions
Authors contributed equally to the manuscript. SME, MMA, SHA, and ES conceived and designed the research. SME, MMA, and SHA conducted experiments and contributed to the research methodology. ES, SHA, SME, and MMA analyzed data. ES and SHA wrote the initial version of the manuscript. All authors read, reviewed, and approved the manuscript.
Funding
There is no financial support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Seba Hassan Attia, Email: SHAttia@medicine.zu.edu.eg.
Eman Soliman, Email: solimane@zu.edu.eg.
References
- Abd El-Twab S.M., Hozayen W.G., Hussein O.E., et al. 18beta-Glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren. Fail. 2016;38:1516–1527. doi: 10.1080/0886022X.2016.1216722. [DOI] [PubMed] [Google Scholar]
- Abdel-Raheem I.T., Khedr N.F. Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexate-induced kidney damage in rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2014;387(4):341–353. doi: 10.1007/s00210-013-0949-x. [DOI] [PubMed] [Google Scholar]
- Andreu G.P., Delgado R., Velho J.A., Curti C., Vercesi A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005;513(1-2):47–55. doi: 10.1016/j.ejphar.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Asci H., Ozmen O., Ellidag H.Y., Aydin B., Bas E., Yilmaz N. The impact of gallic acid on the methotrexate-induced kidney damage in rats. J. Food Drug Anal. 2017;25(4):890–897. doi: 10.1016/j.jfda.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan N.S., Grisham M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43(5):645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorley B.N., Campbell M.R., Wang X., Karaca M., Sambandan D., Bangura F., Xue P., Pi J., Kleeberger S.R., Bell D.A. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40(15):7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L.-Z., Teng X., Zhang Z.-B., Zheng C.-J., Chen S.-H. Mangiferin inhibits apoptosis and oxidative stress via BMP2/Smad-1 signaling in dexamethasone-induced MC3T3-E1 cells. Int. J. Mol. Med. 2018 doi: 10.3892/ijmm.2018.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshazly S., Soliman E. PPAR gamma agonist, pioglitazone, rescues liver damage induced by renal ischemia/reperfusion injury. Toxicol. Appl. Pharmacol. 2019;362:86–94. doi: 10.1016/j.taap.2018.10.022. [DOI] [PubMed] [Google Scholar]
- El-Sheikh A.A.K., Morsy M.A., Abdalla A.M., Hamouda A.H., Alhaider I.A. Mechanisms of Thymoquinone Hepatorenal Protection in Methotrexate-Induced Toxicity in Rats. Mediators Inflamm. 2015;2015:1–12. doi: 10.1155/2015/859383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erboga M., Aktas C., Erboga Z.F., Donmez Y.B., Gurel A. Quercetin ameliorates methotrexate-induced renal damage, apoptosis and oxidative stress in rats. Ren. Fail. 2015;37(9):1492–1497. doi: 10.3109/0886022X.2015.1074521. [DOI] [PubMed] [Google Scholar]
- Garrido G., Gonzalez D., Lemus Y., et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG) Pharmacol. Res. 2004;50:143–149. doi: 10.1016/j.phrs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Gold-Smith F., Fernandez A., Bishop K. Mangiferin and Cancer: Mechanisms of Action. Nutrients. 2016;8(7):396. doi: 10.3390/nu8070396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Peng X., Zhu J., Chen X., Liu H., Tang C., Dong Z., Liu F., Peng Y. Mangiferin attenuate sepsis-induced acute kidney injury via antioxidant and anti-inflammatory effects. Am. J. Nephrol. 2014;40(5):441–450. doi: 10.1159/000369220. [DOI] [PubMed] [Google Scholar]
- He F., Ru X., Wen T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020;21(13):4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari R., Ahmadi A., Mohammadi H., Ommati M.M., Azarpira N., Niknahad H. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed. Pharmacother. 2018;107:834–840. doi: 10.1016/j.biopha.2018.08.050. [DOI] [PubMed] [Google Scholar]
- Huang J., Tabbi-Anneni I., Gunda V., Wang L.i. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299(6):G1211–G1221. doi: 10.1152/ajpgi.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M., Arshad M.S., Butt M.S., Kwon J.-H., Arshad M.U., Sultan M.T. Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017;16(1) doi: 10.1186/s12944-017-0449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana R.K., Kaur R., Lohan S., Singh K.K., Singh B. Mangiferin: a promising anticancer bioactive. Pharm. Pat. Anal. 2016;5(3):169–181. doi: 10.4155/ppa-2016-0003. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Kitamura S., Fujioka M., Kamijo R., Sato S., Sawayama Y., Uramatsu T., Obata Y., Mochizuki Y., Nishikido M., Sakai H., Miyazaki Y., Mukae H., Nishino T. Methotrexate-induced acute kidney injury in patients with hematological malignancies: three case reports with literature review. Renal Replacement Therapy. 2018;4(1) doi: 10.1186/s41100-018-0180-9. [DOI] [Google Scholar]
- Kokeny G., Calvier L., Hansmann G. PPARgamma and TGFbeta-Major Regulators of Metabolism, Inflammation, and Fibrosis in the Lungs and Kidneys. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Collaborative Power of Nrf2 and PPARgamma Activators against Metabolic and Drug-Induced Oxidative Injury. Oxid. Med. Cell Longev. 2017;2017:1378175. doi: 10.1155/2017/1378175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Cui X., Sun X., Li X., Zhu Q., Li W. Mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Phytother. Res. 2010;24(6):893–899. doi: 10.1002/ptr.3045. [DOI] [PubMed] [Google Scholar]
- Li X., Yan Z., Carlström M., Tian J., Zhang X., Zhang W., Wu S., Ye F. Mangiferin Ameliorates Hyperuricemic Nephropathy Which Is Associated With Downregulation of AQP2 and Increased Urinary Uric Acid Excretion. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., et al. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma Y., Shi M., Wang Y., et al. PPARgamma and Its Agonists in Chronic Kidney Disease. Int. J. Nephrol. 2020;2020:2917474. doi: 10.1155/2020/2917474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A.M., Hussein O.E., Abd El-Twab S.M., et al. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARgamma, and suppression of NF-kappaB/NLRP3 inflammasome axis. Food Funct. 2019;10:4593–4607. doi: 10.1039/c9fo00114j. [DOI] [PubMed] [Google Scholar]
- Mahmoud-Awny M., Attia A.S., Abd-Ellah M.F., et al. Mangiferin Mitigates Gastric Ulcer in Ischemia/ Reperfused Rats: Involvement of PPAR-gamma, NF-kappaB and Nrf2/HO-1 Signaling Pathways. PLoS ONE. 2015;10:e0132497. doi: 10.1371/journal.pone.0132497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S., Ma H., Chen X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021;149:111997. doi: 10.1016/j.fct.2021.111997. [DOI] [PubMed] [Google Scholar]
- Morsy M.A., Ibrahim S.A., Amin E.F., Kamel M.Y., Rifaai R.A., Hassan M.K. Curcumin ameliorates methotrexate-induced nephrotoxicity in rats. Adv Pharmacol Sci. 2013;2013:1–7. doi: 10.1155/2013/387071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshage H., Kok B., Huizenga J.R., et al. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- Pal P.B., Sinha K., Sil P.C. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFalpha related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS One. 2014;9:e107220. doi: 10.1371/journal.pone.0107220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.Y., Cho I.J., Kim S.G. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-gamma and retinoid X receptor heterodimer. Cancer Res. 2004;64:3701–3713. doi: 10.1158/0008-5472.CAN-03-3924. [DOI] [PubMed] [Google Scholar]
- Polvani S., Tarocchi M., Galli A. PPARgamma and Oxidative Stress: Con(beta) Catenating NRF2 and FOXO. PPAR Res. 2012;2012 doi: 10.1155/2012/641087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y., Zhou L., Wang C. Mangiferin Inhibits IL-1beta-Induced Inflammatory Response by Activating PPAR-gamma in Human Osteoarthritis Chondrocytes. Inflammation. 2017;40:52–57. doi: 10.1007/s10753-016-0451-y. [DOI] [PubMed] [Google Scholar]
- Sadhukhan P., Saha S., Dutta S., et al. Mangiferin Ameliorates Cisplatin Induced Acute Kidney Injury by Upregulating Nrf-2 via the Activation of PI3K and Exhibits Synergistic Anticancer Activity With Cisplatin. Front. Pharmacol. 2018;9:638. doi: 10.3389/fphar.2018.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scirpo R., Fiorotto R., Villani A., et al. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-gamma limits NF-kappaB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology. 2015;62:1551–1562. doi: 10.1002/hep.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Raj V., Keshari A.K., et al. Isolated mangiferin and naringenin exert antidiabetic effect via PPARgamma/GLUT4 dual agonistic action with strong metabolic regulation. Chem. Biol. Interact. 2018;280:33–44. doi: 10.1016/j.cbi.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Soliman E., Behairy S.F., El-Maraghy N.N., et al. PPAR-gamma agonist, pioglitazone, reduced oxidative and endoplasmic reticulum stress associated with L-NAME-induced hypertension in rats. Life Sci. 2019;239 doi: 10.1016/j.lfs.2019.117047. [DOI] [PubMed] [Google Scholar]
- Song Y., Liu W., Tang K.e., Zang J., Li D., Gao H. Mangiferin Alleviates Renal Interstitial Fibrosis in Streptozotocin-Induced Diabetic Mice through Regulating the PTEN/PI3K/Akt Signaling Pathway. J. Diabetes Res. 2020;2020:1–12. doi: 10.1155/2020/9481720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum H., Parvez S., Pasha S.T., et al. Protective effect of lipoic acid against methotrexate-induced oxidative stress in liver mitochondria. Food Chem. Toxicol. 2010;48:1973–1979. doi: 10.1016/j.fct.2010.04.047. [DOI] [PubMed] [Google Scholar]
- Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018;29(17):1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S., Sharma S., Gupta P., Saini A.S., Kaushal C. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011;2(4):236. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., He X., Lei T., Liu Y., Huai G., Sun M., Deng S., Yang H., Tong R., Wang Y.i. Mangiferin induces islet regeneration in aged mice through regulating p16INK4a. Int. J. Mol. Med. 2018 doi: 10.3892/ijmm.2018.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widemann B.C., Balis F.M., Kempf-Bielack B., et al. High-dose methotrexateinduced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100:2222–2232. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- Younis N.S., Elsewedy H.S., Shehata T.M., et al. Geraniol Averts Methotrexate-Induced Acute Kidney Injury via Keap1/Nrf2/HO-1 and MAPK/NF-kappaB Pathways. Curr Issues Mol Biol. 2021;43:1741–1755. doi: 10.3390/cimb43030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel Y., Yuksel R., Yagmurca M., Haltas H., Erdamar H., Toktas M., Ozcan O. Effects of quercetin on methotrexate-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2017;36(1):51–61. doi: 10.1177/0960327116637414. [DOI] [PubMed] [Google Scholar]
- Zhang B., Zhao J., Li S., Zeng L., Chen Y., Fang J. Mangiferin activates the Nrf2-ARE pathway and reduces etoposide-induced DNA damage in human umbilical cord mononuclear blood cells. Pharm. Biol. 2015;53(4):503–511. doi: 10.3109/13880209.2014.927890. [DOI] [PubMed] [Google Scholar]