Abstract
A sensitive two-step simultaneous enzyme immunoassay (EIA) for human gamma interferon (IFN-γ) has been developed and used as an in vitro test for human tuberculosis (TB) in comparison with tuberculin skin testing. The EIA was shown to be highly sensitive, detecting less than 0.5 IU of recombinant human IFN-γ per ml within a linear detection range of 0.5 to 150 IU/ml. The assay was highly reproducible and specific for native IFN-γ. In addition, the assay detected chimpanzee, orangutan, gibbon, and squirrel monkey IFN-γs. Cross-reactions with other human cytokines or with IFN-γs derived from mice, cattle, or Old World monkeys were not evident. The assay was used to detect TB infection by incubating whole blood overnight with human, avian, and bovine tuberculin purified protein derivatives (PPDs), as well as positive (mitogen)- and negative-control preparations. The levels of IFN-γ in plasma supernatants were then determined. Blood from 10 tuberculin skin test-positive individuals responded predominantly to the human tuberculin PPD antigen and to a lesser extent to bovine and avian PPD antigens. By contrast, blood from 10 skin test-negative individuals showed minimal responses or no response to any of the tuberculin PPDs. Detectable levels of IFN-γ were present in all blood samples stimulated with mitogen. In vivo tuberculin reactivity was correlated with IFN-γ responsiveness in vitro. These results support the further study of the blood culture–IFN-γ EIA system as an alternative to skin testing for the detection of human TB infection.
Human tuberculosis (TB), which is caused by infection with Mycobacterium tuberculosis, was declared a global emergency by the World Health Organization in 1993 and each year leads to the deaths of 3 million people (24). One essential factor for controlling the spread of this disease is the ability to diagnose infection in its early stages.
TB infection has been detected for more than 100 years by the tuberculin skin test, which measures the cell-mediated immune (CMI) response generated by an intradermal injection of tuberculin purified protein derivative (PPD). However, despite widespread use of the skin test, frequent errors in the administration of PPD and in the reading of results, cross-reactivity with other non-TB mycobacterial infections, the booster phenomenon (19), and anergy in immunocompromised individuals (11, 25) make interpretation difficult and have an effect on determining the test’s true sensitivity and specificity (11).
Therefore, alternate and improved diagnostic assays for TB have been sought. The ability to detect organisms directly in clinical specimens has been greatly improved by the availability of specific mycobacterial DNA amplification techniques. However, these are dependent on the presence of bacteria in the sample and may be hampered by variation in sample quality and by the possibility that the detected DNA may not be from viable organisms (6, 32).
Similarly, a number of immunoassays that detect circulating antibodies or antigen have been developed, and some of these assays appear to have diagnostic utility for patients with clinical TB (1, 7, 14, 27). However, in order to control the spread of TB, it is necessary to identify and treat infected individuals before they become infectious to others through progression to clinical disease. Active TB is associated with a heavy bacterial load and concomitant high levels of circulating antibody resulting from the inability of the immune system to contain bacterial growth (17). M. tuberculosis is an intracellular pathogen that replicates within host macrophages, and host defenses are believed to be largely dependent on T lymphocytes, with antibodies being of only minor importance (15). This suggests that an immunodiagnostic test for TB infection could be based on measuring specific T-cell reactivity.
The delayed-type hypersensitivity response, as measured by the skin test, has been shown to be dependent on the production of cytokines, including gamma interferon (IFN-γ), at the site of tuberculin injection (29). Based on these observations, Wood and colleagues in 1990 described an in vitro assay system for measuring CMI responses and applied this assay to the diagnosis of TB in cattle. The assay was based on stimulation of whole blood with PPDs and subsequent measurement of IFN-γ in a sandwich immunoassay specific for bovine IFN-γ (26). In large field trials, the assay was shown to be more sensitive (93.6%) than the traditional skin test (65.6%) for identification of Mycobacterium bovis-infected cattle (33). In the present study, we describe the development and characteristics of an enzyme immunoassay (EIA) for human IFN-γ and its application, in conjunction with a whole-blood culture system, for the detection of M. tuberculosis infection in humans.
MATERIALS AND METHODS
MAbs.
Two noncompeting monoclonal antibodies (MAbs) specific for human IFN-γ (FA42.1F7 and FA26.7C2) were generated from mice immunized with recombinant IFN-γ (Bachem AG, Bubendorf, Switzerland) by methods described previously by Pietrzykowski et al. (23). MAb IgG was purified from ascites fluid with a ProSep-A (BioProcessing Ltd., Consett, England) column according to the manufacturer’s instructions. FA42.1F7 F(ab′)2 fragments were prepared by pepsin (Sigma-Aldrich Pty Ltd., Castle Hill, New South Wales, Australia) digestion, and FA26.7C2 was conjugated to horseradish peroxidase (HRP) (Sigma-Aldrich) as described by Jones et al. (13).
Cytokines and antigens.
Recombinant human, murine, and bovine IFN-γs were purchased from Boehringer Mannheim (Castle Hill, New South Wales, Australia), Sigma, and Ciba-Geigy (Basel, Switzerland), respectively. The international reference reagent for recombinant human IFN-γ (Gxg01-902-535) was kindly provided by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) (Bethesda, Md.). Squirrel monkey IFN-γ, in the form of culture supernatants of concanavalin A (ConA)-stimulated peripheral blood lymphocytes (PBL), was supplied by R. Macfarlan (CSL Limited, Parkville, Victoria, Australia). Natural human IFN-γ and interleukin-2 (IL-2) were purchased from Boehringer Mannheim, recombinant human IL-5 was purchased from Endogen (Cambridge, Mass.), and recombinant human IL-6, IL-10, and IL-12 were gifts from P. Wood (CSIRO, Parkville, Australia). Human, avian, and bovine PPDs were manufactured by CSL, with their biological potencies standardized to international reference preparations. Phytohemagglutinin P (PHA) was purchased from Difco Laboratories (Detroit, Mich.).
Human IFN-γ EIA.
MAb FA42.1F7 F(ab′)2 fragments were diluted in 50 mM carbonate buffer (pH 9.6) to a concentration of 5 μg/ml and were bound to 96-well EIA plates (MaxiSorp Nunc, Roskilde, Denmark) (100 μl/well) overnight. The plates were postcoated with 150 μl of a 1-mg/ml solution of sodium casein per well in 0.01 M phosphate-buffered saline (PBS; pH 7.2) for 1 h. All traces of postcoating buffer were aspirated, and the plates were dried under vacuum. HRP-conjugated FA26.7C2 diluted in PBS–0.1% casein–20% normal mouse serum (NMS) was added (50 μl/well) to all wells. Then, 50 μl of sample per well was added and mixed, and the mixtures were incubated for 1 h. The plates were subsequently washed six times with PBS containing 0.05% Tween 20, and 100 μl of tetramethylbenzidine substrate per well (13) was added. After 30 min, the production of chromophore was stopped by the addition of 50 μl of 0.5 M H2SO4 per well, and optical densities at 450 nm were read with a 620-nm reference filter. All incubations and manipulations were performed at room temperature (22 ± 5°C).
EIA optical density data were analyzed by generating a linear standard curve from four replicates of known dilutions of recombinant IFN-γ (in international units per milliliter) plotted against their mean optical density values in the EIA. The concentrations of IFN-γ in test samples were calculated from the standard curve with the software package KC-Jr (Bio-Tek Instruments, Inc.).
Participants and skin testing.
The blood samples used in this study were obtained after informed consent from 14 male and 6 female (mean age, 35.1 years; range, 17 to 60 years) volunteers attending a Health and Community Services TB program in Melbourne, Australia. Participants were newly arrived immigrants previously identified by the Australian Immigration Service as being at risk of TB by virtue of country of origin and chest X rays. The study was approved by the Ethics Committee of the Victorian Government Department of Health and Community Services. Skin testing was performed according to the State Guidelines (3) with 10 IU of human tuberculin PPD (CSL). All skin tests were performed immediately after blood samples were taken and were read after 48 to 72 h.
Whole-blood cultures.
Venous blood was collected into Exutainer tubes (Labco Ltd., London, England) containing lithium heparin (15 U/ml). Each blood sample was well mixed immediately prior to whole-blood culture such that 1-ml aliquots of each sample were dispensed into 5 wells of a 24-well tissue culture tray. Then, 120 μl of either sterile PBS (nil antigen control) or optimal concentrations of human PPD, avian PPD, bovine PPD, or PHA were added and thoroughly mixed with each 1-ml aliquot of blood. The 24-well culture plates were incubated for 16 to 24 h at 37°C in a humidified atmosphere. Plasma supernatants were then collected and stored at 2 to 8°C prior to assaying for IFN-γ.
RESULTS
Range and detection limits.
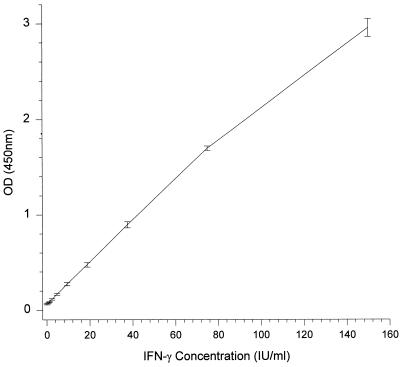
The range and detection limits of the human IFN-γ EIA were determined by testing various recombinant human IFN-γ concentrations (0 to 300 IU/ml) diluted in pooled normal human plasma on two occasions by two operators and with two batches of reagents. The working concentration range of recombinant human IFN-γ detected by the assay was between 0.5 IU/ml (approximately 20 pg/ml) and 150 IU/ml (approximately 5 ng/ml) (Fig. 1). Concentrations of IFN-γ higher than 150 IU/ml, to as high as 100,000 IU/ml (highest tested), were also reactive, but their optical densities exceeded the upper limit of the microplate reader and were not quantifiable without dilution.
FIG. 1.
Standard curve of the human IFN-γ EIA derived from the mean optical densities (OD) (450 nm) ± standard deviations of 16 replicates over four assays.
Specificity for IFN-γ.
To assess the possibility that heterophile antibodies (4, 5, 13, 16) caused interference in the IFN-γ assay, unstimulated human plasma samples from 201 healthy blood donations were tested in sample diluent without NMS. Of these plasma samples, 6% were highly reactive, generating an optical density in the EIA greater than twice that of the negative control (data not shown). Five of these samples were then used to determine the optimum concentration of NMS required in sample diluent to eliminate false reactivity. The addition of 20% (vol/vol) NMS reduced the optical densities of these five plasma samples to less than 0.1 (data not shown). The presence of NMS in the diluent had no effect on the ability of the EIA to detect IFN-γ added into human plasma derived from unstimulated blood (Table 1). The sample diluent for all further assays included 20% NMS.
TABLE 1.
Effect of adding NMS to sample diluent to remove false reactivity of plasma in the human IFN-γ EIA
| Type of normal human plasmaa | Level of added human IFN-γ (IU/ml) | EIA optical density (450 nm)
|
|
|---|---|---|---|
| No NMS | 20% NMS | ||
| Nonreactive | 0 | 0.069 | 0.062 |
| 50 | 1.475 | 1.419 | |
| Falsely reactive | 0 | 2.548 | 0.063 |
| 50 | 2.735 | 1.422 | |
Plasma from unstimulated blood donation.
To investigate the specificity of the assay, recombinant and natural human IFN-γs denatured by heat (56°C, 1 h) or acidic pH (pH 2, 1 h) treatment and various other inducible cytokines were diluted in pooled human plasma and tested. As shown in Table 2, the EIA did not detect denatured human IFN-γ; natural human IL-2 (200 IU/ml); or recombinant human IL-4 (5 ng/ml), IL-5, IL-6, IL-10, or IL-12 (100 ng/ml).
TABLE 2.
Specificity and species cross-reactivity of the human IFN-γ EIA
| Cytokine source and/or concna | Concn (IU/ml)b |
|---|---|
| Human | |
| rIFN-γ (no treatment) | 174 |
| rIFN-γ (pH 2 treatment [HCl for 1 h]) | 4.7 (97.3) |
| rIFN-γ (heat treatment [56°C for 1 h]) | 0 (100) |
| nIFN-γ (no treatment) | 232 |
| nIFN-γ (pH 2 treatment [HCl for 1 h]) | 3.2 (98.6) |
| nIFN-γ (heat treatment [56°C for 1 h]) | 0 (100) |
| nIL-2 (200 IU/ml) | 0 |
| rIL-4 (5 ng/ml) | 0 |
| rIL-5 (100 ng/ml) | 0 |
| rIL-6 (100 ng/ml) | 0 |
| rIL-10 (100 ng/ml) | 0 |
| rIL-12 (100 ng/ml) | 0 |
| Nonhuman primate IFN-γ | |
| Pan troglodytes (chimpanzee)c | 73.1 |
| Pongo pygmaeus (orangutan)c | 35.9 |
| Hylobates spp. (gibbon)c | 247 |
| Saimiri (squirrel monkey)d | 98 |
| Colobus satanas (guerezas)c | 1.8 |
| Papio sphinx (mandrill)c | 0 |
| Papio hamadryas (sacred baboon)c | 0.1 |
| Macaca fascicularis (cynomolgus macaque)c | 0 |
| Murine rIFN-γ (5,000 IU/ml) | 0 |
| Bovine rIFN-γ (100 ng/ml) | 2 |
rIFN-γ and nIFN-γ, recombinant and natural IFN-γ, respectively; rIL and nIL, recombinant and natural interleukin, respectively.
Values in parentheses are percent reduction values.
PHA-stimulated whole blood.
ConA-stimulated PBL.
The species specificity of the IFN-γ EIA was also investigated. Supernatants of either PHA-stimulated whole blood or ConA-stimulated PBL from the nonhuman primate species chimpanzees, orangutans, gibbons (apes), and squirrel monkeys (New World monkeys), but not from colobuses, macaques, mandrills or baboons (Old World monkeys), were reactive in this assay (Table 2). Heat or acid treatment of the reactive samples abrogated this reactivity, supporting the conclusion that EIA reactivity was due to the presence of induced IFN-γ (12, 20). Recombinant bovine IFN-γ (100 ng/ml) was only very weakly reactive in the EIA, and recombinant murine IFN-γ (5,000 IU/ml) was unreactive.
IFN-γ EIA validation.
The linearity of standard curves expressed as a correlation coefficient (r) from 167 assays performed over a 14-month period was used to assess the reproducibility of the IFN-γ EIA. All r values were greater than 0.98, with a mean of 0.996 and a coefficient of variation of 0.3%. The precision of the human IFN-γ EIA was investigated by evaluating 20 plasma samples containing various concentrations of natural human IFN-γ (0 to 122 IU/ml) within the range of the human IFN-γ assay. These samples were tested undiluted as well as at a dilution of 2:1 in pooled human plasma. The mean difference between the IFN-γ concentrations determined for the undiluted and diluted samples was not significant (P = 0.713 [Student’s paired t test]). The accuracy of the human IFN-γ EIA was estimated by assaying four replicates of pooled human plasma spiked with various levels of recombinant human IFN-γ (150, 75, 37.5, 18.8, 9.4, and 4.7 IU/ml) on two occasions by two operators and with two batches of reagents. The average accuracy for the known concentrations was 105.8% ± 11.4%.
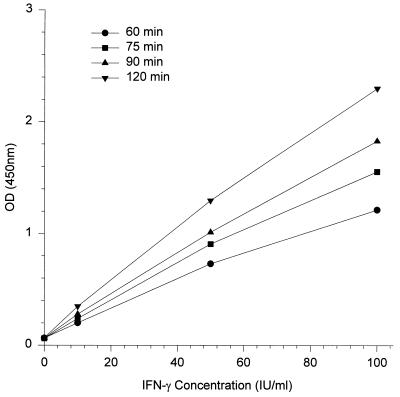
The correlation coefficient of the standard curve is a measure of linearity but does not take into account the slope of the standard curve. The linearity of the assay was examined by varying the sample incubation period. Standard concentrations of human IFN-γ in replicates of six were reacted for 60, 75, 90, and 120 min. All four standard curves produced were linear (r > 0.99), irrespective of assay incubation time (Fig. 2). While the slopes of the four standard curves were different (ranging from 0.012 for 60 min of incubation to 0.022 for 120 min), after log transformation the curves were parallel, indicating that the same IFN-γ concentration would be calculated for a sample regardless of the reaction time. This was confirmed by assaying 35 stimulated plasma samples for IFN-γ and various dilutions of recombinant human IFN-γ with either 60 or 120 min of incubation. The concentrations of IFN-γ in all samples were determined from their respective standard curves, and the mean difference between the two incubations was not significantly different to zero (P = 0.198 [Student’s paired t test]).
FIG. 2.
Human IFN-γ EIA standard curves after 60, 75, 90, and 120 min of incubation of the assay.
To determine the reactivity of the NIH recombinant human IFN-γ reference reagent in the EIA, six replicates of NIH and Boehringer IFN-γ at 150, 125, 100, 75, 50, 20, 10, and 5 IU/ml (in pooled human plasma) were assayed and compared by parallel regression analysis. The relative potency of the NIH IFN-γ reference reagent was 0.83 IU/ml (95% confidence interval, 0.78 to 0.88). The r values for both standard curves were 0.999 and 0.997, respectively.
Detection of TB infection.
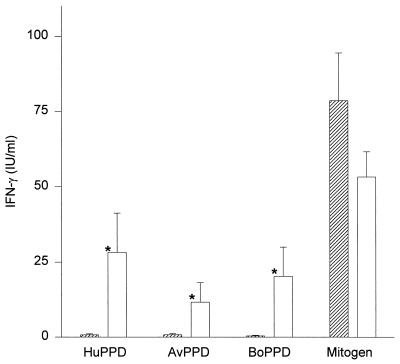
To investigate the utility of the whole-blood culture–IFN-γ assay system for detection of TB infection, blood samples from 10 tuberculin skin test-positive and 10 skin test-negative individuals were tested. The IFN-γ response of each individual to the five different stimulation antigens is listed in Table 3. The mean concentrations (± standard errors of the means) of IFN-γ of blood from tuberculin-positive individuals stimulated with human PPD and bovine PPD were 28.2 ± 12.9 and 20.3 ± 9.7 IU/ml, respectively (Fig. 3). These responses were significantly higher (P = 0.002 [Wilcoxon paired nonparametric signed rank test]) than those produced in response to avian PPD (11.6 ± 6.5 IU/ml). Blood from the skin test-negative individuals showed no or minimal production of IFN-γ in response to human (0.7 ± 0.3 IU/ml), bovine (0.4 ± 0.2 IU/ml), and avian (0.8 ± 0.4 IU/ml) PPDs. The differences in response to the human, avian, and bovine tuberculin PPDs between the skin test-positive group and the skin test-negative group were highly significant (P < 0.0001, P = 0.0011, and P < 0.0001, respectively [Mann-Whitney test]). All skin test-positive individuals produced higher levels of IFN-γ in response to human PPD (5.4 to 142 IU/ml) than did any of the skin test-negative individuals (0 to 2.6 IU/ml). Moreover, the correlation between skin test induration diameter and the magnitude of the IFN-γ response to human PPD was highly significant (r = 0.82 by the Spearman test [P < 0.0001]). All IFN-γ responses to human PPD in the skin test-positive group were greater than their respective responses to avian PPD. There was no IFN-γ detected in the nil antigen control samples, and all blood samples yielded detectable levels of IFN-γ in response to the mitogen (65.8 ± 9.2 IU/ml). The level of response to the mitogen of skin test-positive individuals (53.2 ± 8.4 IU/ml) was not significantly different (P = 0.315 [Mann-Whitney test]) from that of skin test-negative individuals (78.5 ± 15.9 IU/ml).
TABLE 3.
Antigen-stimulated release of IFN-γ in whole-blood cultures from tuberculin skin test-negative and -positive individuals
| Subject | Skin test measurement (response)a | IFN-γ response (IU/ml)
|
||||
|---|---|---|---|---|---|---|
| Nil antigen | Human PPD | Avian PPD | Bovine PPD | Mitogen | ||
| 1 | 0 (−) | 0 | 0 | 0 | 0 | 100 |
| 2 | 0 (−) | 0 | 0 | 0 | 0 | 21.43 |
| 3 | 0 (−) | 0 | 2.61 | 0 | 0 | 79.89 |
| 4 | 0 (−) | 0 | 0.13 | 1.17 | 0 | 37.39 |
| 5 | 0 (−) | 0 | 0.46 | 0.46 | 0.33 | 199 |
| 6 | 0 (−) | 0 | 0 | 0 | 0 | 105 |
| 7 | 0 (−) | 0 | 0 | 0 | 0 | 66.54 |
| 8 | 0 (−) | 0 | 0.87 | 2.14 | 0.7 | 56.68 |
| 9 | 3 (−) | 0 | 0.65 | 0 | 0.52 | 40.00 |
| 10 | 3 (−) | 0 | 2.55 | 3.73 | 2.42 | 78.70 |
| 11 | 15 (+) | 0 | 18.85 | 10.39 | 14.86 | 80.99 |
| 12 | 15 (+) | 0 | 142 | 68.85 | 104 | 50.52 |
| 13 | 25 (+) | 0 | 7.13 | 3.62 | 4.32 | 36.86 |
| 14 | 10 (+) | 0 | 22.78 | 1.77 | 21.08 | 81.51 |
| 15 | 10 (+) | 0 | 16.30 | 4.58 | 8.58 | 89.24 |
| 16 | 15 (+) | 0 | 5.45 | 3.31 | 4.32 | 14.70 |
| 17 | 15 (+) | 0 | 10.19 | 1.89 | 2.54 | 48.03 |
| 18 | 17 (+) | 0 | 20.50 | 1.66 | 10.02 | 15.79 |
| 19 | 15 (+) | 0 | 6.02 | 3.02 | 4.55 | 45.75 |
| 20 | 17 (+) | 0 | 32.95 | 17.05 | 28.57 | 68.74 |
Measurements are in millimeters.
FIG. 3.
Differences between mean (± standard error of the mean) IFN-γ responses (in international units per milliliter) to human PPD (HuPPD), avian PPD (AvPPD), bovine PPD (BoPPD), and mitogen stimulation antigens of tuberculin skin test-negative (▨) and -positive (□) individuals. *, significant difference (P < 0.01).
DISCUSSION
This paper describes the development of a sensitive and specific EIA for human IFN-γ and its application to the detection of TB infection. The assay detected as little as 20 pg of recombinant human IFN-γ per ml, and was linear over a concentration range of 20 pg/ml to 5 ng/ml, and the relative potency of the NIH recombinant IFN-γ reference reagent was 0.83. The assay was shown to be specific for native IFN-γ, since neither denatured IFN-γ nor any of the other cytokines tested reacted in the EIA. The human IFN-γ EIA was found to be highly reproducible and exhibited very low between-assay variation in correlation coefficients for standard curves. The assay was also shown to be both accurate (by consistently generating the expected results for samples of known concentration under various conditions) and precise (by showing no significant difference between potency estimates for samples tested neat and dilute).
It is well-established that two-site sandwich EIAs which employ MAbs as both the solid-phase capture antibody and the HRP-conjugated antibody often suffer from false-positive problems due to the presence of heterophile antibodies in many serum-plasma samples (4, 5, 13, 16). To circumvent this problem, we followed the procedure described by Jones et al. (13) and coated microtiter plates with F(ab′)2 fragments of the solid-phase capture antibody and added 20% NMS to the sample diluent. This effectively eliminated false-positive reactions with plasma samples in the EIA and did not have any effect on the sensitivity of the assay.
IFN-γs from some species of nonhuman primates (chimpanzees, orangutans, gibbons, and squirrel monkeys) were detected by the EIA, whereas bovine, murine, and other Old World monkey IFN-γs were not. The lack of cross-reactivity with Old World monkeys was surprising, given their close phylogenetic relationship and the high degree of amino acid sequence homology that exists between human and macaque (96%) and human and baboon (92%) IFN-γs (8, 31). For example, a similar assay described for the detection of bovine IFN-γ also detected IFN-γs from other members of the family Bovidae (26). Nonetheless, the amino acid sequences of human and Old World monkey IFN-γs are not identical, and, hence, it is possible that the substitutions and deletions in these sequences result in an alteration to at least one of the epitopes recognized by the MAbs employed in this assay. Since we did not test these preparations in a specific bioassay for IFN-γ, we cannot be certain that there was IFN-γ in the samples generated by either ConA or PHA stimulation.
A number of investigators have reported sensitive EIAs employing specific MAbs for human IFN-γ (2, 10, 22, 30). The EIA described in the present study, as well as that described by Gallati et al. (10), was developed as a simultaneous assay in which both the sample and the conjugate were coincubated in the EIA wells. This resulted in a rapid and simple assay system in which only one incubation and washing step was required before the addition of substrate. Development of the EIA in this format did not result in a loss of sensitivity of the assay compared to that performed by using sequential steps (data not shown) (2, 30). Our assay showed sensitivity comparable to that of other previously described human IFN-γ EIAs, and it has the advantage of a large linear dynamic range which eliminated the need to dilute the starting sample. This is an important feature for the application of this assay to TB diagnosis, since there appeared to be wide variation in the levels of IFN-γ (as much as 199 IU/ml) produced in blood stimulated with antigen or mitogen.
Rothel et al. (26) first described the use of a whole-blood culture IFN-γ EIA system for the detection of TB infection in cattle. Having developed a human IFN-γ-specific EIA, we were interested in investigating its utility, in conjunction with whole-blood culture, in detecting TB infection in humans. Heparinized blood was collected from 20 individuals (10 skin test positive and 10 skin test negative), and aliquots were cultured overnight with no (nil) antigen, with human, avian, and bovine PPDs, and with mitogen (PHA). The nil antigen control was included to detect IFN-γ in the circulation which, if present, might mask specific responses and make interpretation difficult. No IFN-γ was detected in nil antigen samples, which is consistent with other studies that have shown that IFN-γ is not normally detectable in human serum (2). However, it is known that some patients have detectable levels of IFN-γ in their serum (18), and, hence, this control preparation should be used routinely. The mitogen antigen was used to show that the blood contained immunologically competent T cells capable of producing IFN-γ. A lack of response in the mitogen control may indicate immunosuppression or deterioration of the blood sample, in which case the blood sample should be considered invalid for interpretation and the test should be repeated with another specimen.
The results from this study demonstrated the ability of the blood culture–IFN-γ EIA system to discriminate among 10 tuberculin skin test-positive and 10 skin test-negative individuals by comparing the levels of IFN-γ produced in vitro in response to human and avian PPDs. Blood from skin test-positive individuals produced larger amounts of IFN-γ when stimulated with human PPD than did that from skin test-negative individuals, and these amounts were always greater than those produced with avian PPD. Since these responses were quantifiable, they could be used to arbitrarily define an assay cutoff. Here, it was evident that an individual might be deemed positive by the assay, indicating probably TB infection (i.e., skin test positivity), when the EIA detected a blood IFN-γ response to human PPD higher than approximately 3 IU/ml, which was also greater than the response to avian PPD. Whether this definition is appropriate for the larger population remains to be determined.
These data support the principle of using this IFN-γ assay as a method for detecting tuberculin reactivity and the need to undertake more extensive studies. Whether the assay is more sensitive for detecting individuals with a false-negative skin test is one potential advantage that should be determined. IFN-γ testing of substantially larger numbers of individuals with known TB infection status will be required to address these issues and to determine the most appropriate cutoff between positive and negative reactivities in this assay. In addition, because the assay system provided a measure of both the quantity and the quality of the antimycobacterial response (i.e., human PPD versus avian PPD), it is likely to be useful in discriminating between M. tuberculosis infection and infection with atypical mycobacteria such as those of the Mycobacterium avium complex. The usefulness of measuring IFN-γ responses in vitro in determining specific CMI responsiveness has also been demonstrated from studies of allergy (9, 28), autoimmunity (34), and AIDS (21). The IFN-γ EIA may also prove useful in investigations into the role of IFN-γ in various immune mechanisms.
This study demonstrates the potential of the blood culture–IFN-γ EIA system for the detection of M. tuberculosis infection in humans. Further studies aimed at assessing its sensitivity and specificity and clinical and epidemiological utility in the wider population are warranted.
ACKNOWLEDGMENTS
We gratefully acknowledge Rod Macfarlan, John Cox, Andrew MacGregor, Anastasia Moisidis, Charles Quinn, and Elizabeth Pietrzykowski for their work in the selection of MAbs. We are grateful to Deborah Bailey, Mary Randall, and the nursing staff at the Department of Health and Community Services TB program at Fairfield Hospital for collection of blood and to the Veterinary staff at Melbourne, Taronga Park (Sydney), and Adelaide Zoological Gardens for provision of the nonhuman primate blood samples. We also acknowledge Jim Rothel for his constructive comments in preparing the manuscript.
REFERENCES
- 1.Al Orainey I O, Gad el Rab M O, al Hajjaj M S, Saeed E S. Detection of mycobacterial antigens in sputum by an enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1992;11:58–61. doi: 10.1007/BF01971274. [DOI] [PubMed] [Google Scholar]
- 2.Andersson G, Ekre H-P T, Alm G, Perlmann P. Monoclonal antibody two-site ELISA for human IFN-γ. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989;125:89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Management, control and prevention of tuberculosis: guidelines for health care providers. Melbourne, Victoria, Australia: Victorian Government Department of Human Services Infectious Diseases Unit; 1996. [Google Scholar]
- 4.Boerman O C, Segers M F G, Poels L G, Kenemans P, Thomas C M G. Heterophilic antibodies in human sera causing falsely increased results in the CA 125 immunofluorometric assay. Clin Chem. 1990;36:888–891. [PubMed] [Google Scholar]
- 5.Boscato L M, Stuart M C. Heterophilic antibodies: a problem for all immunoassays. Clin Chem. 1988;34:27–33. [PubMed] [Google Scholar]
- 6.De Beenhouwer H, Liang Z, de Rijk P, van Eekeren C, Portaels F. Detection and identification of mycobacteria by DNA amplification and oligonucleotide-specific capture plate hybridization. J Clin Microbiol. 1995;33:2994–2998. doi: 10.1128/jcm.33.11.2994-2998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Kantor I N, Barrera L, Ritacco V, Miceli I. Usefulness of the enzyme immunoassay in the diagnosis of tuberculosis. Bol Oficina Sanit Panam. 1991;110:461–470. [PubMed] [Google Scholar]
- 8.Dijkmans R, Tobback M, Beuken E, van Damme J, Billiau A. Molecular cloning of the baboon interferon-gamma cDNA. Lymphokine Res. 1990;9:345–354. [PubMed] [Google Scholar]
- 9.Ellaurie M, Yost S L, Rosenstreich D L. A simplified human whole blood assay for measurement of dust mite-specific gamma interferon production in vitro. Ann Allergy. 1991;66:143–147. [PubMed] [Google Scholar]
- 10.Gallati H, Pracht I, Schmidt J, Haring P, Garotta G. A simple, rapid and large capacity ELISA for biologically active native and recombinant human IFNγ. J Biol Regul Homeost Agents. 1987;1:109–118. [PubMed] [Google Scholar]
- 11.Huebner R E, Schein M F, Bass J B., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 12.Ichimori Y, Suzuki N, Kitada C, Tsukamoto K. Monoclonal antibodies to human interferon-γ II: antibodies with neutralizing activity. Hybridoma. 1987;6:173–181. doi: 10.1089/hyb.1987.6.173. [DOI] [PubMed] [Google Scholar]
- 13.Jones S L, Cox J C, Shepherd J M, Rothel J S, Wood P R, Radford A J. Removal of false-positive reactions from plasma in an enzyme immunoassay for bovine interferon-γ. J Immunol Methods. 1992;155:233–240. doi: 10.1016/0022-1759(92)90290-a. [DOI] [PubMed] [Google Scholar]
- 14.Kansal R, Khuller G K. Detection of mannophosphoinositide antigens in sputum of tuberculosis patients by dot enzyme immunoassay. Med Microbiol Immunol (Berlin) 1991;180:73–78. doi: 10.1007/BF00193848. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann S H E. The interplay between cytokines and T cells in immunity to intracellular bacteria. In: Mustafa A S, al-Attiyah R J, Nath I, Chugh T D, editors. T-cell subsets and cytokines interplay in infectious diseases. Basel, Switzerland: Karger; 1996. pp. 169–180. [Google Scholar]
- 16.Kricka L J, Schmerfeld-Pruss D, Senior M, Goodman D B P, Kaladas P. Interference by human anti-mouse antibody in two-site immunoassays. Clin Chem. 1990;36:892–894. [PubMed] [Google Scholar]
- 17.Lenzini L, Rottoli P, Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977;27:230–237. [PMC free article] [PubMed] [Google Scholar]
- 18.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 19.Menzies R, Vissandjee B, Rocher I, St. Germain Y. The booster effect in two-step tuberculin skin testing among young adults in Montreal. Ann Intern Med. 1994;120:190–198. doi: 10.7326/0003-4819-120-3-199402010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed G S, Taylor C E, Dickinson A M, Craft A W, Kernahan J, Reid M M, Proctor S J, Toms G L. Interferon responses of peripheral blood mononuclear cells from normal and leukaemic children. Br J Haematol. 1986;64:789–798. doi: 10.1111/j.1365-2141.1986.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 21.Murray H W, Hillman J K, Rubin B Y, Kelly C D, Jacobs J L, Tyler L W, Donelly D M, Carriero S M, Godbold J H, Roberts R B. Patients at risk of AIDS-related opportunistic infections. Clinical manifestations and impaired gamma interferon production. N Engl J Med. 1985;313:1504–1510. doi: 10.1056/NEJM198512123132403. [DOI] [PubMed] [Google Scholar]
- 22.Paasch B D, Reed B R, Keck R, Sandlund B K, Gilkerson E, Shalaby R. An evaluation of the accuracy of four ELISA methods for measuring natural and recombinant human interferon-γ. J Immunol Methods. 1996;198:165–176. doi: 10.1016/s0022-1759(96)00155-x. [DOI] [PubMed] [Google Scholar]
- 23.Pietrzykowski E, Cox J, Zachariou M, MacGregor A. Development of an enzyme immunoassay for the detection of Clostridium novyi type B alpha toxin. Biologicals. 1991;19:293–298. doi: 10.1016/s1045-1056(05)80018-1. [DOI] [PubMed] [Google Scholar]
- 24.Raviglione M C, Snider D E, Jr, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 25.Reichman L B. Tuberculin skin testing: the state of the art. Chest. 1979;76:764–770. doi: 10.1378/chest.76.6.764. [DOI] [PubMed] [Google Scholar]
- 26.Rothel J S, Jones S L, Corner L A, Cox J C, Wood P R. A sandwich enzyme immunoassay for bovine interferon-γ and its use for the detection of tuberculosis in cattle. Aust Vet J. 1990;67:134–137. doi: 10.1111/j.1751-0813.1990.tb07730.x. [DOI] [PubMed] [Google Scholar]
- 27.Simonney N, Molina J M, Molimard M, Oksenhendler E, Perronne C, Lagrange P H. Analysis of the immunological humoral response to Mycobacterium tuberculosis glycolipid antigens (DAT, PGLTb1) for diagnosis of tuberculosis in HIV-seropositive and -seronegative patients. Eur J Clin Microbiol Infect Dis. 1995;14:883–891. doi: 10.1007/BF01691495. [DOI] [PubMed] [Google Scholar]
- 28.Tang M, Kemp A, Varigos G. IL-4 and interferon-gamma production in children with atopic disease. Clin Exp Immunol. 1993;92:120–124. doi: 10.1111/j.1365-2249.1993.tb05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsicopoulos A, Hamid Q, Varney V, Ying S, Moqbel R, Durham S R, Kay A B. Preferential messenger RNA expression of Th1-type cells (IFN-γ+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992;148:2058–2061. [PubMed] [Google Scholar]
- 30.Van der Meide P H, Dubbeld M, Schellekens H. Monoclonal antibodies to human immune interferon and their use in a sensitive solid-phase ELISA. J Immunol Methods. 1985;79:293–305. doi: 10.1016/0022-1759(85)90109-7. [DOI] [PubMed] [Google Scholar]
- 31.Villinger F, Brar S S, Mayne A, Chikkala N, Ansari A A. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol. 1995;155:3946–3954. [PubMed] [Google Scholar]
- 32.Wilson S M, McNerney R, Nye P M, Godfrey-Faussett P D, Stoker N G, Voller A. Progress toward a simplified polymerase chain reaction and its application to diagnosis of tuberculosis. J Clin Microbiol. 1993;31:776–782. doi: 10.1128/jcm.31.4.776-782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood P R, Rothel J S. In vitro immunodiagnostic assays for bovine tuberculosis. Vet Microbiol. 1994;40:125–135. doi: 10.1016/0378-1135(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 34.Zangerle P F, de Groote D, Lopez M, Meuleman R J, Vrindts Y, Fauchet F, Dehart I, Jadoul M, Radoux D, Franchimont P. Direct stimulation of cytokines (IL-1β, TNF-α, IL-6, IL-2, IFN-γ and GM-CSF) in whole blood. II. Application to rheumatoid arthritis and osteoarthritis. Cytokine. 1992;4:568–575. doi: 10.1016/1043-4666(92)90021-i. [DOI] [PubMed] [Google Scholar]