Abstract
Background
Reduced lipid content in the stratum corneum is a major cause of skin-barrier dysfunction in various pathological conditions. Promoting lipid production is a potential strategy to improve skin-barrier function. Recent evidence supports the beneficial effects of adiponectin on lipid metabolism and senescence in keratinocytes.
Objective
This study aimed to investigate whether plant extracts can enhance skin-barrier function.
Methods
We screened fruit and herb extracts that enhance the lipid synthesis of keratinocytes via AMP-activated protein kinase (AMPK) activation and SIRT1 signaling in the adiponectin pathway. The levels of major lipid synthesis enzymes and transcription factors as well as epidermal barrier lipids involved in adiponectin-associated epidermal barrier formation were evaluated in the herbal extracts- or adiponectin-treated human epidermal keratinocyte and equivalent models. The mRNA expression of major lipid synthesis enzymes increased following treatment with Lycii Fructus, Prunus tomentosa, and Melia toosendan extracts.
Results
The expression of transcription factors SIRT1, liver X receptor α, peroxisome proliferator-activated receptors (PPARs), and sterol regulatory element-binding proteins (SREBPs) were upregulated. Levels of free fatty acids, cholesterol, and ceramides were elevated. The expression of keratinocyte differentiation markers increased. In particular, among fruit extracts with a detectable effect, Melia toosendan induced the highest expression of lipid synthase.
Conclusion
These results indicate that Melia toosendan is a promising candidate for improving skin-barrier function.
Keywords: Adiponectin, Herbal, Keratinocytes, Lipids, Skin
INTRODUCTION
The basic concept of the skin barrier is two-compartment organization: the “bricks and mortar” model, in which corneocytes (“the bricks”) and intercellular lipid lamellae (“the mortar”) play a major role1. The skin-barrier function is weakened due to reduction in lipid contents in the stratum corneum (SC) in dry or aging skin and in chronic inflammatory disorders such as atopic dermatitis2,3,4,5,6. Therefore, replenishing intercellular lipid lamellae is key to strengthening skin-barrier function, and attempts to discover new effective barrier potentiators that can be used in cosmetics materials are required.
Adiponectin is an anti-aging adipokine known to improve fat and insulin metabolism7,8. Recently, adiponectin was shown to effectively enhance skin-barrier function by increasing keratinocyte lipid synthesis through nuclear hormone receptors (NHR) such as SIRT1, a nicotinamide adenine dinucleotide-dependent deacetylase, and peroxisome proliferator-activated receptors (PPARs)9,10. These functions of adiponectin are mediated by adiponectin receptors—AdipoR1 and AdipoR211; they induce AMP-activated protein kinase (AMPK) and the SIRT1 pathway12,13.
Many natural extracts are used in cosmetics, but it is often uncertain how they affect keratinocyte lipid synthesis and differentiation contributing to epidermal barrier formation. Therefore, we screened plant-derived natural extracts that can be used in cosmetics and can enhance barrier function by comparing their effects based on the relative mRNA expression of SIRT1 and NHR in keratinocytes and compared their efficacy with respect to the induction of lipid synthesis and differentiation with that of adiponectin.
MATERIALS AND METHODS
Screening process from herbal extract library
Initially, about 300 plant extracts extracted with 70% ethanol were screened (herbal extract library). To investigate the effect of herbal extracts on lipid production of keratinocytes, keratinocyte viability was checked by first treating with herbal extract library at 50 ppm for 24 hours. Of these, 168 herbal extracts with more than 80% viability were selected (primary screening), and 12 possible candidate herbal extracts were selected through a literature review (secondary screening). Among the 12 candidates, six herbal extracts with higher or similar AMPK activation (increasing p-AMPK expression) than adiponectin were selected (tertiary screening) (Supplementary Fig. 1). Among these six candidates, we analyzed the expression of adiponectin receptors, enzymes—including fatty acid synthase (FAS), HMG-CoA reductase (HMGCR), and serine palmitoyltransferase (SPT)—and transcription factors—including liver X receptor α (LXRα), PPARs, and SIRT1 which were associated with lipid synthesis—using quantitative real-time reverse transcription PCR (qRT-PCR) and selected Lycii fructus (LF; Lycium barbarum L. fructus), Prunus tomentosa (PT) fructus, and Melia toosendan (MT) fructus as candidates capable of reinforcing the skin barrier in this comparative study (4th screening) (Supplementary Table 1). This study was approved by the Institutional Review Board of Yonsei University Wonju College of Medicine (approval no. CR321371).
Reagents and antibodies
Recombinant human full-length adiponectin was obtained from Biobud (Seongnam, Korea). We obtained antibodies against LXRα, PPARα, PPARβ, PPARγ, sterol regulatory element-binding proteins (SREBPs), and b-actin from Santa Cruz Biotechnology (Santa Cruz, CA, USA); filaggrin, involucrin, cytokeratin 14, PPARβ, and SIRT1 from Abcam (Cambridge, UK). sphingosine-1-phosphate (S1P), sphingosine, sphinganine, and ceramide were obtained from Avanti Polar Lipid (Alabaster, AL, USA). Organic solvents for sphingolipid extraction and liquid chromatography tandem-mass spectrometry analysis were purchased from Merck (Darmstadt, Germany).
Cell cultures
Normal human epidermal keratinocytes (HEKs) were purchased from Gibco BRL, Life Technologies (Grand Island, NY, USA). HEKs were maintained in 0.06 mM Ca2þ EpiLife medium (Thermo-Scientific, Waltham, MA, USA) supplemented with EDGS (EpiLife Defined Growth Supplement; Life Technologies, Carlsbad, CA, USA) at 37℃ in a humidified atmosphere containing 5% CO2. All experiments were performed using cells on their third passage. After reaching 80% confluence, cells were treated with adiponectin (10 µg/ml) or herbal extracts (10 ppm) for 24 hours and then harvested with TrypLE Select (Gibco BRL).
Human skin equivalent preparation
Human epidermal equivalent (HEE) (Neoderm-E) and culture medium were purchased from TEGO Science, Inc., (Seoul, Korea) and incubated at 37℃ in a 5% CO2 atmosphere. Briefly, to construct epidermal equivalents, keratinocytes were seeded into culture inserts. After culturing cells for three days, the constructs were cultured at the air-liquid interface. After two weeks of exposure to air, the HEE was treated with adiponectin (10 µg/ml) or herbal extracts (10 ppm) for 24 hours. The medium was changed every day during incubation.
Sphingolipid analysis
To assess cellular sphingolipid levels, cells were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer, followed by extraction of sphingolipids, as reported previously14,15. The extracted lipids were dried using a vacuum system (Vision, Seoul, Korea), redissolved in methanol, and analyzed by liquid chromatography electrospray ionization-tandemmass spectrometry (LC-ESI-MS/MS, API 3200 QTRAP mass; AB/SCIEX, Concord, ON, Canada) in the selective ion monitoring mode. Ceramide tandem-mass spectrometry transitions (m/z) were 510/264 for C14-ceramide, 538/264 for C16-ceramide, 552/264 for C17-ceramide, 566/264 for C18-ceramide, 594/264 for C20-ceramide, 648/264 for C24:1-ceramide, and 650/264 for C24-ceramide. The sphingoid base tandem-mass spectrometry transitions (m/z) were 286/238 for C17 sphingosine as an internal standard, 300/252 for C18 sphingosine, and 302/60 for C18 sphinganine. Data were acquired using Analyst 1.4.2 software (Applied Biosystems, Foster City, CA, USA).
Free cholesterol and free fatty acid analysis
To quantify free cholesterol and free fatty acids (FAs), lipid was extracted using the Folch method, with minor modifications16,17,18. Briefly, the SC tissues from the HEKs and HEEs were lysed and sonicated in methanol/chloroform (1:2, v/v) containing butylated hydroxytoluene (500 µg/ml), followed by the addition of 500 pmol of docosahexaenoic acid and d6-cholesterol as an internal standard. The extracted lipids were dried using a vacuum system (Vision), redissolved in methanol, and analyzed by LC-ESI-MS/MS (API 3200 QTRAP mass; AB/SCIEX) in the selective ion monitoring mode. First, both free cholesterol and FAs were separated by reverse phase high performance liquid chromatography (HPLC, NANOSPACE SI-2 HPLC equipped with an HTS autosampler Z; Shiseido, Tokyo, Japan) using a KINETEX C18 column (2.1 mm×50 mm, ID: 2.6 µm; Phenomenex, St. Louis, MO, USA), as previously described18,19. The MS/MS transitions (m/z) of the FAs were 227→183 for C14:0 FA, 253→209 for C16:1 FA, 255→211 for C16:0 FA, 277→233 for C18:3 FA, 279→235 for C18:2 FA, 281→237 for C18:1 FA, 283→239 for C18:0 FA, 303→259 for C20:4 FA, 311→267 for C20:0 FA, 337→293 for C22:1 FA, 339→295 for C22:0 FA, 365→321 for C24:1 FA, and 367→323 for C24 FA. The MS/MS transitions (m/z) of cholesterol were 369.3→161.5, 369.3→147.1, and 374.4→152.7 for d6-cholesterol. All data were acquired using Analyst 1.5.1 software (Applied Biosystems).
qRT-PCR and PCR analysis
Total RNA from HEK was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and quantified using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Carlsbad, CA, USA). Total RNA (1 mg) was reverse transcribed using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, Madison, WI, USA). qRT-PCR was performed using SYBR Green PCR master mix (Applied Biosystems), using Quantstudio 3 (PCR Instrument System; Thermo-Scientific). PCR gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase expression. Quantification was performed using the Ct method.
Western blotting
Cells were harvested and lysed in RIPA buffer and supernatants were recovered by centrifugation. Sample protein concentrations were determined using bicinchoninic acid, and equal amounts of protein were used between samples during western blotting. Proteins were resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes, which were blocked using 3% bovine serum albumin (BSA) and then probed with appropriate antibodies. Protein-antibody complexes were visualized on X-ray films after reacting them with the enhanced chemiluminescence detection reagent (Santa Cruz Biotechnology).
Immunofluorescence
Immunofluorescent staining of 5 µm frozen sections was performed following standard protocols. Frozen sections were air-dried for 30 minutes and permeabilized in 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 15 minutes. Sections were washed in PBS and blocked with blocking serum solution (DAKO, Carpinteria, CA, USA). Thereafter, adjacent sections were incubated with the following primary antibodies at 4℃ overnight: filaggrin (diluted 1:200), involucrin (diluted 1:200), and cytokeratin 14 (dilution 1:100). After washing, the cells were incubated with the relevant Alexa Fluor secondary antibodies (Molecular Probes, Eugene, OR, USA) before being washed and mounted in Shandon Immumount (Thermo Electron Corporation, Waltham, MA, USA) containing 10 µg/ml DAPI. Cells were imaged using a fluorescent microscope (Olympus Corp., Tokyo, Japan).
Statistical analysis
Data were expressed as the mean±standard deviation and analyzed using the paired Student t-test and analysis of variance with Bonferroni’s post-hoc correction for comparison between the mean of two numeric values. Statistical significance was defined at p<0.05. Statistical analysis was performed using PASW statistics software (version 23.0; IBM Corp., Armonk, NY, USA).
RESULTS
Screening a plant extract library for effects of lipid synthesis on human epidermal keratinocytes
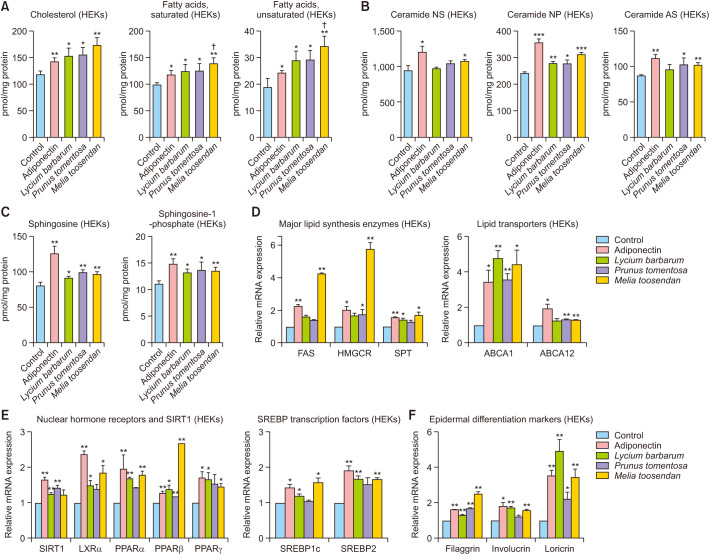
Three candidates—LF, PT fructus, and MT fructus—were selected by screening a plant extract library composed of 300 herbal extracts. HEKs were treated with three candidates or adiponectin for 24 hours, and then, the epidermal barrier related lipid content was measured. Levels of cholesterol and FAs (saturated and unsaturated) were significantly increased in LF (p<0.05), PT (p<0.05), and MT (p<0.01) extracts. In particular, MT (p<0.05) showed a higher increase than adiponectin (Fig. 1A). For ceramide, LF showed a significant increase only in ceramide non-hydroxy phytosphingosine (NP) (p<0.01) compared to the control, whereas PT showed significant differences both in ceramide NP (p<0.05) and alpha-hydroxy sphingosine (AS) (p<0.05). MT, in contrast, showed significant differences in ceramide non-hydroxy sphingosine (NS) (p<0.05), NP (p<0.001), and AS (p<0.01) but showed a lesser increase than adiponectin (Fig. 1B). Levels of other major sphingolipids—sphingosine and S1P—were significantly upregulated in the LF (p<0.05), PT (p<0.05), and MT (p<0.01) treatment groups compared with those only in the control group but not the adiponectin group (Fig. 1C). To identify the mechanism of lipid content production by each herbal extract, the mRNA expression of FAS, HMGCR, and SPT as the rate-limiting enzymes for the synthesis of major epidermal barrier lipids (free FA, cholesterol, and ceramide, respectively) and lipid transporters in keratinocytes (ABCA1 and ABCA12) were evaluated following herbal extract treatment of primary HEK cells for 24 hours by qRT-PCR. Similar to adiponectin, MT significantly upregulated levels of all major lipid synthesis enzymes and lipid transporters compared with those of control (Fig. 1D). In particular, mRNA levels of FAS and HMGCR showed the greatest increase after MT treatment. In contrast, the other two plant extracts (LF and PT) showed rather complicated results, increasing the levels of only some enzymes and transporters. To determine the effect of plant extracts on major transcription factors for epidermal differentiation and SC lipid barrier formation, keratinocyte mRNA expression of SIRT1 and NHRs, including LXRs and PPARs, was analyzed. An overall increase in the transcription factors after 24-hour treatment with adiponectin or three plant extracts was observed (Fig. 1E). This increase was also observed in SREBP1c and SREBP2, which regulate the expression of keratinocyte lipid synthetic enzyme (Fig. 1E).
Fig. 1. Screened herbal extracts increased lipid synthesis via the adiponectin signaling pathway in keratinocytes. (A~C) Lipid quantification (cholesterol, fatty acids, ceramides, sphingosine, and sphingosine-1-phosphate) was performed by high-performance liquid chromatography in HEKs after 24 hours of treatment with adiponectin (10 µg/ml) or herbal extracts (10 ppm). (D~E) Quantitative real-time PCR of major lipid synthesis enzymes and their regulatory factors in adiponectin or herbal extract treated HEKs. (F) PCR results of the expression of epidermal differentiation markers (filaggrin, involucrin, and loricrin). Values are presented as mean±standard deviation (*p<0.05, **p<0.01, ***p<0.001 versus control; †p<0.05 vs. adiponectin). HEK: human epidermal keratinocyte, NS: non-hydroxy sphingosine, NP: non-hydroxy phytosphingosine, AS: alpha-hydroxy sphingosine, SIRT: sirtuin, SREBP: sterol regulatory element-binding protein.
Finally, the expression of the epidermal differentiation marker proteins—filaggrin, involucrin, and loricrin—was also identified. Significant upregulation of the differentiation markers was observed in all LF, PT, and MT (Fig. 1F).
So far, all three herbal extracts showed good overall effects; especially, MT showed a consistent increase in expression of the transcription factors for epidermal barrier formation, lipid synthetic enzymes, and differentiation markers and had a similar effect to adiponectin as the positive control.
Plant extracts also increased the lipid content in 3-dimensional epidermal equivalent
The HEE model was further treated with LF, PT, or MT extracts to investigate whether these effects of herbal extracts are also similar in differentiated epidermal equivalents, similar to the HEK model. There was no significant difference in the total ceramide content other than that of adiponectin, but levels of sphingosine significantly increased in the PT (p<0.05) and MT (p<0.05) extract treatment groups, and S1P showed a significant increase in the LF treatment group (p<0.05) (Fig. 2A).
Fig. 2. Herbal extracts increased sphingolipid content in 3D epidermal equivalent. (A) Sphingolipid contents quantified by high-performance liquid chromatography in human epidermal equivalents after 24 hours of treatment with adiponectin (10 µg/ml) or herbal extracts (10 ppm). (B) mRNA levels of rate-limiting lipid synthesis enzymes (FAS, HMGCR, and SPT) and lipid transport genes (ABCA1 and ABCA12) were evaluated by real-time PCR, after 24 hours of treatment of human epidermal equivalent with adiponectin (10 µg/ml) or herbal extracts (10 ppm). Values are presented as mean±standard deviation (*p<0.05, **p<0.01 vs. control). ABCA: ATP-binding cassette transporter A, HEE: human epidermal equivalent, FAS: fatty acid synthase, HMGCR: HMG-CoA reductase, SPT: serine palmitoyltransferase.
On the other hand, in terms of the expression of major lipid synthetic enzymes for epidermal lipid barrier, MT (p<0.01) showed a significant increase in the expression of all three enzymes, and the other two fruit extracts (LF and PT) showed a tendency to increase the expression of these lipid synthetic enzymes. Both lipid transporters were significantly upregulated following MT and LF treatment; PT (p<0.05) upregulated only ABCA12 (Fig. 2B).
Plant extracts upregulated SIRT1, nuclear hormone receptors and SREBPs
Treatment of HEEs with LF, PT, and MT extracts showed significant increases in mRNA expression of SIRT1, LXRα, PPARα, and PPARβ; in PPARγ, only LF (p<0.05) showed a significant increase. Similarly, for lipogenesis transcription factors ‘SREBP’, SREBP2 showed significant increases following treatment with all three plant extracts, while SREBP1c showed significant increases following LF (p<0.01) and MT (p<0.01) treatment (Fig. 3A). The protein levels of these regulatory factors were additionally confirmed using western blotting. Consistent with the PCR results, the other two extracts also showed good effects, but MT extract treatment showed the strongest expression (Fig. 3B).
Fig. 3. Herbal extracts induced the expression of NHR, SIRT1, and SREBPs in the 3D epidermal equivalent. HEEs were treated with adiponectin (10 µg/ml) or herbal extracts (10 ppm) for 24 hours, and the expression of each factor was analyzed by real-time PCR (A) and western blotting (B). Values are presented as mean±standard deviation (*p<0.05, **p<0.01 vs. control). NHR: nuclear hormone receptor, HEE: human epidermal equivalent, SIRT: sirtuin, LXRα: liver X receptor-α, PPAR: peroxisome proliferator-activated receptor, SREBP: sterol regulatory element-binding protein.
Summarizing the results on HEE, all three plant extracts increased the expression of lipid synthesis enzymes and transcription factors to some extent, and like the keratinocyte experiment, the effect of MT seems to be consistently the best.
Plant extracts increased the expression of epidermal differentiation marker protein
The mRNA expression of filaggrin, involucrin, and loricrin—which are epidermal differentiation marker proteins—were confirmed in HEEs after treatment with plant extracts. After LF (p<0.01) and PT (p<0.05) treatment, involucrin and loricrin were significantly increased. In contrast, MT (p<0.01) treatment significant increases levels of all three marker proteins, and the expression of involucrin was particularly high (Fig. 4A). Immunofluorescence, which was implemented to confirm this result directly, showed a greater increase in the expression of filaggrin and involucrin compared with that in the control group after treatment with plant extracts, and the most pronounced increase was observed after MT treatment (Fig. 4B).
Fig. 4. Herbal extracts increased expression of epidermal differentiation marker proteins in the 3D epidermal equivalent. (A) Expression of filaggrin, involucrin, and loricrin quantified by real-time PCR in human epidermal equivalents (HEEs) after 24 hours of treatment with adiponectin (10 µg/ml) or herbal extracts (10 ppm). (B) Expression of filaggrin, involucrin, and keratin-14 visualized by immunofluorescence in HEEs after 24 hours of treatment with adiponectin (10 µg/ml) or herbal extracts (10 ppm). Values are presented as mean±standard deviation (*p<0.05, **p<0.01 vs. control).
DISCUSSION
Our results confirmed that the natural plant extracts of LF, PT, and MT enhance lipid synthesis and differentiation activity in both keratinocyte and the HEE. Increasing the lipid content of SC is an important strategy for restoring skin-barrier function because reducing the lipid content of SC is associated with decreased skin-barrier function. Therefore, this study identified the possibility of employing three plant extracts as barrier potentiators. Among the three candidates, MT exhibited the most pronounced efficacy in general; thus, skin-barrier enhancement function is expected to be the best when MT is employed.
LF, also called goji berry or Wolfberry, is the fruit of Lycium barbarum and is mainly consumed in the cooked or dried form in Asia20. It is rich in carotenoids, including zeaxanthin palmitate (pasalien), and therefore has become popular as an antioxidant phytochemical20,21. In addition, polysaccharides, phenolic acid, and flavonoids also contribute to these antioxidant and anti-inflammatory properties20,22. Recently, it has been confirmed that LF has a cardiovascular protective effect along with hypoglycemic and hypolipidemic effects22,23. In the skin, the expression of filaggrin, claudin-1 mRNA, and aquaporin 3 significantly increases after treating keratinocytes with traditional Chinese medicine containing LF24.
PT is one of the four cherry species and is widely distributed in Korea, Japan. And China. It has higher total phenolic content than other Prunus spps and has superior antioxidant activity25,26. Six flavonoids in PT seed extract are known to inhibit nitric oxide and prostaglandin E2 production26. However, unlike LF, the effect on the skin-barrier function has not yet been studied.
MT is one of the traditional Chinese medicines used as a potential neuroactive compound27. MT contains various limonoids and is known to have insecticidal and anti-inflammatory activities. Among them, toosendanin (TSN), a triterpenoid derivative extract, acts as a presynaptic blocker and is involved in cell signaling, transcriptional regulation, apoptosis, and cell-cycle regulation28. Recently, TSN has been known to have an antitumor effect and to inhibit protein kinase C activity, thereby reducing melanin synthesis by melanocytes29,30; In another study, it was confirmed that limonoids such as TSN enhance ceramide production in keratinocytes31. However, as this study only involved experiments conducted on HEKs (Epilife, Kurabo, Tokyo, Japan), the effect on 3D equivalents such as HEEs has not been revealed yet.
In this study, adiponectin was treated as a positive control and was compared with three natural plant extracts that showed similar effects to that observed on adiponectin treatment. Adiponectin is a cell-signaling molecule secreted from adipocytes7 and is known to have beneficial effects on lipid and insulin metabolism, wound healing, and cellular senescence8. Additionally, it was recently discovered that adiponectin enhances lipid synthesis of human keratinocytes through SIRT1 and nuclear hormone receptor signaling10; these plant extracts in our experiment were found to work through AMPK activation, the main adiponectin signal pathway, and upregulation of SIRT1 and PPARs.
In this study, we did not determine whether these plant extracts directly activated adiponectin receptors; however, we showed that the main downstream signals of adiponectin were activated and showed similar effects in keratinocytes, showing potential as adiponectin signal activators. Therefore, there is room to confirm whether it acts as an adiponectin receptor agonist through further studies. In addition, further research is required on which specific ingredients within the aforementioned natural compound have a strengthening effect on skin-barrier function and whether they are still effective even for external application.
There was some discrepancy in the results, such as the differences in the levels of sphingosine and S1P in HEKs and HEEs. Basically, this seems to be due to the difference between HEKs and HEEs. HEEs are a 3D model, and epidermal differentiation is more evolved in these cell lines than in HEKs. Next, there is a possibility that the difference in the experimental techniques employed for HEKs and HEEs, such as the media or reaction time, may also have influenced the results. However, even after considering these differences, the overall trend appears to be consistent.
In conclusion, three plant extracts (LF, PT fructus, and MT fructus) were found to enhance lipid synthesis and differentiation for SC barrier formation in both keratinocyte and HEE, confirming its potential as a barrier potentiator, similar to adiponectin. Among the three, MT, in particular, demonstrated consistent good efficacy for enhancing the overall lipid production, the expression of lipid synthesis enzyme and its transcription factors, and skin differentiation enhancement. Therefore, MT is expected to have a strong skin-barrier reinforcement function and is considered the most promising material of functional moisturizers for enhancing barrier function.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (grant number 2018R1C1B5085484) and from the Yonsei University Wonju Campus Future-Leading Research Initiative of 2019 (#2019-52-0062). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-21-288-s001.pdf.
Selection of herbal extracts that increased phospho-AMPK (tertiary screening). Using ELISA (A) and western blotting (B), 6 herbal extracts (orange color) that increased expression of p-AMPK above that of adiponectin were selected. AMPK: AMP-activated protein kinase.
Selection of herbal extracts increased mRNA expression of transcription factors involved in lipid synthesis
References
- 1.Choi EH. Aging of the skin barrier. Clin Dermatol. 2019;37:336–345. doi: 10.1016/j.clindermatol.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Denda M, Sato J, Masuda Y, Tsuchiya T, Koyama J, Kuramoto M, et al. Exposure to a dry environment enhances epidermal permeability barrier function. J Invest Dermatol. 1998;111:858–863. doi: 10.1046/j.1523-1747.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- 3.Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest. 1995;95:2281–2290. doi: 10.1172/JCI117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi EH, Man MQ, Xu P, Xin S, Liu Z, Crumrine DA, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847–2856. doi: 10.1038/sj.jid.5700913. [DOI] [PubMed] [Google Scholar]
- 5.Baurecht H, Rühlemann MC, Rodríguez E, Thielking F, Harder I, Erkens AS, et al. Epidermal lipid composition, barrier integrity, and eczematous inflammation are associated with skin microbiome configuration. J Allergy Clin Immunol. 2018;141:1668–1676.e16. doi: 10.1016/j.jaci.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Madison KC. Barrier function of the skin: “la raison d’être” of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 8.Kadowaki T, Yamauchi T, Okada-Iwabu M, Iwabu M. Adiponectin and its receptors: implications for obesity-associated diseases and longevity. Lancet Diabetes Endocrinol. 2014;2:8–9. doi: 10.1016/S2213-8587(13)70120-7. [DOI] [PubMed] [Google Scholar]
- 9.Blander G, Bhimavarapu A, Mammone T, Maes D, Elliston K, Reich C, et al. SIRT1 promotes differentiation of normal human keratinocytes. J Invest Dermatol. 2009;129:41–49. doi: 10.1038/jid.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong SP, Seo HS, Shin KO, Park K, Park BC, Kim MH, et al. Adiponectin enhances human keratinocyte lipid synthesis via SIRT1 and nuclear hormone receptor signaling. J Invest Dermatol. 2019;139:573–582. doi: 10.1016/j.jid.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 13.Mei Z, Zhang X, Yi J, Huang J, He J, Tao Y. Sirtuins in metabolism, DNA repair and cancer. J Exp Clin Cancer Res. 2016;35:182. doi: 10.1186/s13046-016-0461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci U S A. 2016;113:E1334–E1342. doi: 10.1073/pnas.1504555113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin KO, Lim CJ, Park HY, Kim S, Kim B, Lee Y, et al. Activation of SIRT1 enhances epidermal permeability barrier formation through ceramide synthase 2- and 3-dependent mechanisms. J Invest Dermatol. 2020;140:1435–1438.e5. doi: 10.1016/j.jid.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Della Corte A, Chitarrini G, Di Gangi IM, Masuero D, Soini E, Mattivi F, et al. A rapid LC-MS/MS method for quantitative profiling of fatty acids, sterols, glycerolipids, glycerophospholipids and sphingolipids in grapes. Talanta. 2015;140:52–61. doi: 10.1016/j.talanta.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Pérez-Navarro J, Da Ros A, Masuero D, Izquierdo-Cañas PM, Hermosín-Gutiérrez I, Gómez-Alonso S, et al. LC-MS/MS analysis of free fatty acid composition and other lipids in skins and seeds of Vitis vinifera grape cultivars. Food Res Int. 2019;125:108556. doi: 10.1016/j.foodres.2019.108556. [DOI] [PubMed] [Google Scholar]
- 19.Hasan M, Siegmund W, Oswald S. Rapid LC-MS/MS method for the determination of 4-hydroxycholesterol/cholesterol ratio in serum as endogenous biomarker for CYP3A activity in human and foals. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1033-1034:193–199. doi: 10.1016/j.jchromb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Ma ZF, Zhang H, Teh SS, Wang CW, Zhang Y, Hayford F, et al. Goji berries as a potential natural antioxidant medicine: an insight into their molecular mechanisms of action. Oxid Med Cell Longev. 2019;2019:2437397. doi: 10.1155/2019/2437397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patsilinakos A, Ragno R, Carradori S, Petralito S, Cesa S. Carotenoid content of Goji berries: CIELAB, HPLC-DAD analyses and quantitative correlation. Food Chem. 2018;268:49–56. doi: 10.1016/j.foodchem.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Toh DWK, Xia X, Sutanto CN, Low JHM, Poh KK, Wang JW, et al. Enhancing the cardiovascular protective effects of a healthy dietary pattern with wolfberry (Lycium barbarum): a randomized controlled trial. Am J Clin Nutr. 2021;114:80–89. doi: 10.1093/ajcn/nqab062. Erratum in: Am J Clin Nutr 2021;114:397. [DOI] [PubMed] [Google Scholar]
- 23.Masci A, Carradori S, Casadei MA, Paolicelli P, Petralito S, Ragno R, et al. Lycium barbarum polysaccharides: extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018;254:377–389. doi: 10.1016/j.foodchem.2018.01.176. [DOI] [PubMed] [Google Scholar]
- 24.Meng H, Li J, Dong Y, He Y, Ren H, Liu Y, et al. Poly traditional Chinese medicine formulation prepared with skin moisturizing properties. Dermatol Ther. 2020;33:e14105. doi: 10.1111/dth.14105. [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Jiang Q, Lin J, Li X, Sun C, Chen K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015;173:855–863. doi: 10.1016/j.foodchem.2014.10.094. [DOI] [PubMed] [Google Scholar]
- 26.Kim SK, Kim HJ, Choi SE, Park KH, Choi HK, Lee MW. Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg. Arch Pharm Res. 2008;31:424–428. doi: 10.1007/s12272-001-1174-9. [DOI] [PubMed] [Google Scholar]
- 27.Inada A, Yamada M, Murata H, Kobayashi M, Toya H, Kato Y, et al. Phytochemical studies of seeds of medicinal plants. I. Two sulfated triterpenoid glycosides, sulfapatrinosides I and II, from seeds of Patrinia scabiosaefolia FISCHER. Chem Pharm Bull (Tokyo) 1988;36:4269–4274. doi: 10.1248/cpb.36.4269. [DOI] [PubMed] [Google Scholar]
- 28.Fan H, Chen W, Zhu J, Zhang J, Peng S. Toosendanin alleviates dextran sulfate sodium-induced colitis by inhibiting M1 macrophage polarization and regulating NLRP3 inflammasome and Nrf2/HO-1 signaling. Int Immunopharmacol. 2019;76:105909. doi: 10.1016/j.intimp.2019.105909. [DOI] [PubMed] [Google Scholar]
- 29.Wang G, Huang YX, Zhang R, Hou LD, Liu H, Chen XY, et al. Toosendanin suppresses oncogenic phenotypes of human gastric carcinoma SGC-7901 cells partly via miR-200a-mediated downregulation of β-catenin pathway. Int J Oncol. 2017;51:1563–1573. doi: 10.3892/ijo.2017.4139. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima H, Wakabayashi Y, Wakamatsu K, Imokawa G. An extract of Melia toosendan attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Arch Dermatol Res. 2011;303:263–276. doi: 10.1007/s00403-011-1143-y. Erratum in: Arch Dermatol Res 2013;305:463-465. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki A, Hashimoto H, Shimotoyodome Y, Ito S, Ishikawa J, Sugai Y, et al. Limonoids and unsaturated fatty acids present in Melia toosendan increase ceramide production in keratinocytes. Fitoterapia. 2021;155:105058. doi: 10.1016/j.fitote.2021.105058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selection of herbal extracts that increased phospho-AMPK (tertiary screening). Using ELISA (A) and western blotting (B), 6 herbal extracts (orange color) that increased expression of p-AMPK above that of adiponectin were selected. AMPK: AMP-activated protein kinase.
Selection of herbal extracts increased mRNA expression of transcription factors involved in lipid synthesis
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.